This study provides new mechanistic insight into the impact of suppressor of cytokine signaling 3 (SOCS3) on the vasculature, including divergent effects depending on the source of angiotensin II (Ang II) (local vs. systemic). Bone marrow-derived cells deficient in SOCS3 protect against systemic Ang II-induced vascular dysfunction.
Keywords: endothelial dysfunction, endothelium, carotid artery disease, cerebral arteries, suppressor of cytokine signaling 3
Abstract
Carotid artery disease is a major contributor to stroke and cognitive deficits. Angiotensin II (Ang II) promotes vascular dysfunction and disease through mechanisms that include the IL-6/STAT3 pathway. Here, we investigated the importance of suppressor of cytokine signaling 3 (SOCS3) in models of Ang II-induced vascular dysfunction. We examined direct effects of Ang II on carotid arteries from SOCS3-deficient (SOCS3+/−) mice and wild-type (WT) littermates using organ culture and then tested endothelial function with acetylcholine (ACh). A low concentration of Ang II (1 nmol/l) did not affect ACh-induced vasodilation in WT but reduced that of SOCS3+/− mice by ∼50% (P < 0.05). In relation to mechanisms, effects of Ang II in SOCS3+/− mice were prevented by inhibitors of STAT3, IL-6, NF-κB, or superoxide. Systemic Ang II (1.4 mg/kg per day for 14 days) also reduced vasodilation to ACh in WT. Surprisingly, SOCS3 deficiency prevented most of the endothelial dysfunction. To examine potential underlying mechanisms, we performed bone marrow transplantation. WT mice reconstituted with SOCS3+/− bone marrow were protected from Ang II-induced endothelial dysfunction, whereas reconstitution of SOCS3+/− mice with WT bone marrow exacerbated Ang II-induced effects. The SOCS3 genotype of bone marrow-derived cells did not influence direct effects of Ang II on vascular function. These data provide new mechanistic insight into the influence of SOCS3 on the vasculature, including divergent effects depending on the source of Ang II. Bone marrow-derived cells deficient in SOCS3 protect against systemic Ang II-induced vascular dysfunction.
NEW & NOTEWORTHY
This study provides new mechanistic insight into the impact of suppressor of cytokine signaling 3 (SOCS3) on the vasculature, including divergent effects depending on the source of angiotensin II (Ang II) (local vs. systemic). Bone marrow-derived cells deficient in SOCS3 protect against systemic Ang II-induced vascular dysfunction.
carotid artery disease contributes to stroke and cognitive deficits. Angiotensin II (Ang II) is a major effector of the renin-angiotensin system, known to play diverse roles in various forms of vascular disease and hypertension, including carotid artery disease (21, 42). Clinical and experimental studies indicate that Ang II, via activation of the AT1 receptor (AT1-R), impairs vascular function, contributing to progression of vascular disease (42). Two major interrelated effects of Ang II are oxidative stress and inflammation (8). Ang II increases vascular oxidative stress via mechanisms that include activation of NADPH oxidases, a major source of superoxide (22, 29, 45). Superoxide impairs endothelial function through interactions with endothelium-derived nitric oxide (NO) and other mechanisms. Endothelial dysfunction is a key event in the onset and pathogenesis of vascular diseases of diverse etiology (55). Genetic or pharmacological interventions that inhibit formation or effects of reactive oxygen species (ROS) protect against Ang II-induced endothelial dysfunction and hypertension (27, 34).
Low-grade inflammation is a common feature of vascular disease. For example, activation of NF-κB, a key integrator of inflammatory molecule expression, occurs in response to Ang II (25). Ang II-induced oxidative stress, endothelial dysfunction, and hypertension require expression of IL-6 and activation of JAK/STAT3 (28, 51). A negative regulator of IL-6/JAK/STAT3 signaling is suppressor of cytokine signaling 3 (SOCS3), which suppresses this signaling pathway by multiple mechanisms (58). Although a role for SOCS3 in the immune system and cancer is known, the functional importance of SOCS3 in vascular disease and hypertension has not been defined.
Because both circulating and local (tissue) sources of Ang II are well described (30), we used models that tested both effects of Ang II on the vasculature. We examined the hypothesis that SOCS3 protects against Ang II-induced vascular dysfunction and defined mechanisms involved. For most experiments, we studied carotid arteries, a segment of the circulation where the clinical consequences of vascular disease commonly emerge. As part of our effort, we also examined a resistance artery. Overall, the findings obtained support the concept that SOCS3 has functionally important effects in both Ang II-induced vascular dysfunction and hypertension.
METHODS
Experimental animals.
We used a model with partial genetic deficiency in Socs3 (SOCS3+/−). SOCS3-deficient mice were generated previously by deleting the exon containing the entire coding region of the gene (36). Whereas complete genetic deficiency in SOCS3 is lethal, SOCS3+/− mice are phenotypically normal under unstressed conditions (36). We obtained breeding pairs of these mice from Dr. Paul Rothman. Animals for study were obtained by breeding SOCS3+/− with C57BL6/J mice. Both male and female SOCS3+/− mice and SOCS3+/+ littermates (wild-type, WT) (∼4–6 mo of age) were used. A small series of studies were also performed in male C57BL6/J mice. Mice were fed regular chow and water and maintained under standard housing conditions. All studies followed the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Direct effects of Ang II on the vasculature.
Mice were euthanized with pentobarbital (100 mg/kg ip). Carotid arteries and aorta were removed, cleaned, cut into segments ∼5 mm in length, and placed in oxygenated Krebs solution containing 118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25.0 mM NaHCO3, and 11.0 mM glucose. As described previously (11, 12, 28, 51), vessels were then incubated for 22 h in DMEM growth medium (containing 5 mM glucose, 120 U/ml penicillin, 120 μg/ml streptomycin, and 50 μg/ml polymixin B at 37°C, gassed with 95% O2-5% CO2) with vehicle (DMSO or saline) or Ang II (1 or 10 nM) or a combination of Ang II with S3I-201 (10 μM), or losartan (1 μM), or NF-κB essential modulator (NEMO)-binding domain (NBD) peptides (10 μM), or an anti-IL-6 antibody (5 μg/ml).
Following incubation, arteries were suspended in organ baths (20). To evaluate endothelial function, responses to cumulative addition of acetylcholine (10−10-10−4 M) were measured following precontraction (∼50–60% of maximum) using the thromboxane A2 analog U46619. We found previously that vasodilation to acetylcholine is mediated by endothelial NO synthase (eNOS) in this artery (20). Nitroprusside (10−10-10−4 M) was used to assess endothelium-independent relaxation (20). Tempol (1 mM), a superoxide scavenger, was used to determine whether vascular dysfunction was mediated by superoxide.
Ang II-dependent hypertension.
Following anesthesia with ketamine/xylazine (87.5 and 12.5 mg/kg ip, respectively), osmotic minipumps (model 1002, Alzet) were implanted subcutaneously to deliver saline or a pressor dose of human Ang II (Sigma-Aldrich, 1.4 mg/kg per day) for 14 days (28). In a subset of animals, a nonpressor dose of Ang II was used (0.28 mg/kg per day).
For studies of a resistance vessel, basilar arteries were isolated, cannulated, and pressurized to 60 mmHg, so lumen diameter could be measured as described (18, 19, 28). To examine dilator responses, arteries were first constricted by ∼30% using U46619.
Blood pressure measurements.
Systolic blood pressure was measured in conscious mice as performed previously (1, 12, 13, 28, 48). Mice were trained for 5 days before pump implantation, and blood pressure was measured daily in the morning after pump implantation. Using this approach, we have found a good correlation between measurement of arterial pressure with tail cuff and direct measurements with indwelling catheters in awake mice (13, 47, 48).
Bone marrow chimeric mice.
CD45.1 mice, a C57BL/6J congenic strain used wildly in transplant studies (54), were obtained from the National Cancer Institute. This strain carries the differential pan leukocyte marker CD45.1, whereas WT C57BL/6 express CD45.2. CD45.1 and CD45.2 mice are phenotypically identical (41) but are different genetically at the single locus of the ptprc gene, which encodes an antigen found in all leukocytes (56). As described (26), the contributions of CD45.1 and CD45.2 to hematopoiesis were detected using plasma samples and flow cytometry after staining with their specific antibodies, respectively.
As described (26), WT (WT CD45.1), SOCS3+/−, and SOCS3+/+ mice were irradiated with two doses (500 and 450 rad) of whole body X-ray irradiation. To isolate bone marrow from donors, femur, hips/pelvis, and tibia were removed and stored in sterile RP10 media (Gibco) supplemented with 10% fetal calf serum, 100 U of penicillin, 100 μg of streptomycin, and 50 μg of gentamicin per ml, 10 mM HEPES, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol until bone marrow removal. After removal of bone tips, bone marrow was flushed out using a needle and syringe. Isolated bone marrow was then spun down, and red blood cells were lysed for 30 s followed by multiple washings. Irradiated recipient mice were reconstituted with five million total cells per mouse via tail vein injection. Bone marrow chimeras were provided with drinking H2O containing ampicillin (2 mg/ml) for 6 wk and were housed for a minimum of 8 wk to allow reconstitution of the peripheral immune system.
Fluorescence-activated cell sorting.
Peripheral blood samples were collected from the retroorbital vein. After depletion of RBCs with the use of ACK lysis buffer (dd H2O containing 0.15 M of NH4Cl, 1.00 mM of KHCO3, and 0.10 mM of Na2EDTA), cells were resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS containing 3% fetal calf serum) and incubated with an antibody mixture (anti-mouse CD16/32 for preventing nonspecific Ab binding, anti-mouse CD45.1, and anti-mouse CD45.2) for 30 min in a 96-well flat-bottom plate. After being washed with FACS buffer twice, cells were fixed with Cytofix/Cytoperm (BD Biosciences). Cells were analyzed by flow cytometry (BD Biosciences), and collected data were analyzed using FlowJo (TreeStar).
Quantitative real-time RT-PCR.
RNA from aorta, isolated from the same mice in which carotid or cerebral arteries were used for functional studies, was prepared using the RNAeasy (Qiagen) method following extraction with TRIzol reagent (Invitrogen) (6, 28). RNA concentrations were determined using a NanoDrop spectrophotometer, with an OD260/OD280 ratio of greater than 1.9 (indicating very high-quality RNA) (6, 28). Identical amounts of RNA (300 ng) were used for RT. Identical amounts of RT product were used for real-time PCR with a single well of a 96-well plate containing both TaqMan probes/primers (Applied Biosystems) for genes of interest (with FAM fluorophore) and β-actin (with VIC fluorophore) as a housekeeping gene. Expression levels were normalized to β-actin. Expression of eNOS (Mm00435204_m1, Life Technologies) and AT1a receptors for Ang II (AT1-R, Mm01166161_m1, Life Technologies), superoxide dismutase 1 (SOD1) (Mm01344233_g1, Life Technologies), SOD2 (Mm01313000_m1, Life Technologies), SOCS1 (Mm06782550_s1, Life Technologies), SOCS3 (Mm01249143_g1, Life Technologies), and IL-6 (Mm 00446190_m1, Life Technologies) were determined by quantitative real-time RT-PCR using the TaqMan method.
Drugs.
Acetylcholine, nitroprusside, losartan, and tempol were obtained from Sigma-Aldrich. U46619 (Cayman Chemical) was dissolved in ethanol with subsequent dilutions made in saline. Other chemicals were S3I-201 (Calbiochem), NBD peptides (Imgenex), and an anti-IL-6 antibody (R&D Systems).
Statistical analysis.
All data are expressed as means ± SE. Relaxation responses are presented as percentage of relaxation relative to the level of precontraction induced by U46619. Absolute contractions were presented as grams of tension. In cerebral arteries, changes in vessel diameter were expressed as percentage of change. Results were expressed as the means ± SE and compared by Student's t-test or ANOVA followed by Student-Newman-Keuls post hoc test as appropriate. Differences were considered significant at P ≤ 0.05.
RESULTS
Partial SOCS3 deficiency augments direct effects of Ang II on endothelial function.
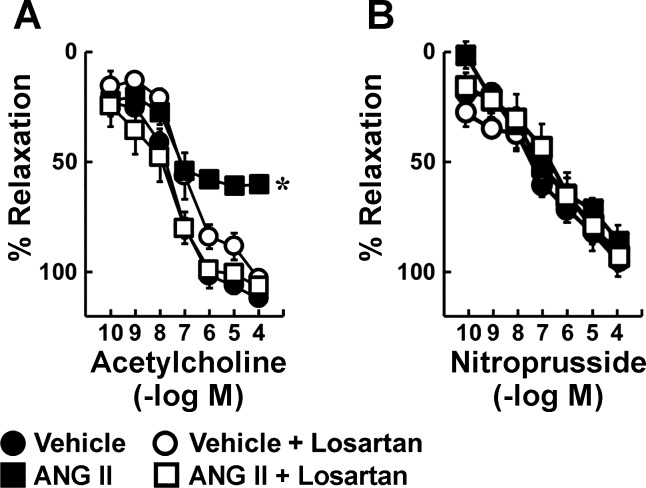
Acetylcholine produced concentration-dependent relaxation in carotid arteries from C57BL6/J mice incubated with vehicle (Fig. 1). Ang II (10 nM) reduced the maximal response by approximately one-half, and these inhibitory effects were prevented by losartan, an AT1-R antagonist (Fig. 1). Responses to nitroprusside were not affected by Ang II or losartan (Fig. 1).
Fig. 1.
Effects of angiotensin II (Ang II) on endothelial function are mediated by AT1 receptors. Vasodilation was induced by acetylcholine (A) and nitroprusside (B) in carotid arteries from C57BL/6 mice incubated with vehicle or Ang II (10 nM) in the presence and absence of losartan for 22 h; n = 6, *P < 0.05 vs. vehicle.
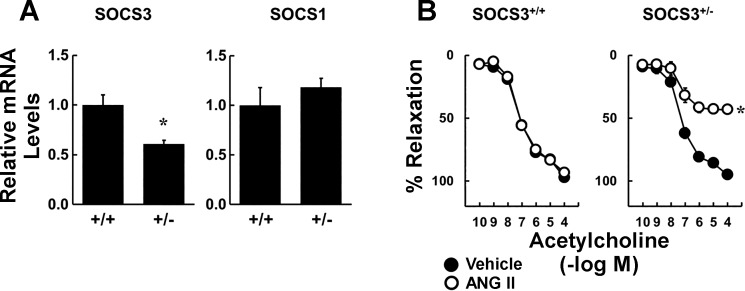
Levels of SOCS3 mRNA in the vasculature were reduced by ∼50% in SOCS3+/− mice (compared with WT SOCS3+/+) either when vessels were simply harvested or in mice that were infused with vehicle for 2 wk (Fig. 2A). In contrast, mRNA levels for SOCS1 were not altered in SOCS3+/− mice (Fig. 2A). These results confirm partial genetic deficiency of SOCS3 and suggest that there was no compensatory increase in expression of a closely related SOCS isoform.
Fig. 2.
Effects of suppressor of cytokine signaling 3 (SOCS3) deficiency on local Ang II-induced endothelial dysfunction. A: SOCS3 and SOCS1 gene expression in aorta from SOCS3+/+ and SOCS3+/− mice (n = 6). *P < 0.05 vs. SOCS3+/+. B: acetylcholine-induced relaxation in carotid arteries from SOCS3+/+ and SOCS3+/− mice incubated with vehicle or Ang II (1 nM) (n = 6). *P < 0.05 vs. vehicle.
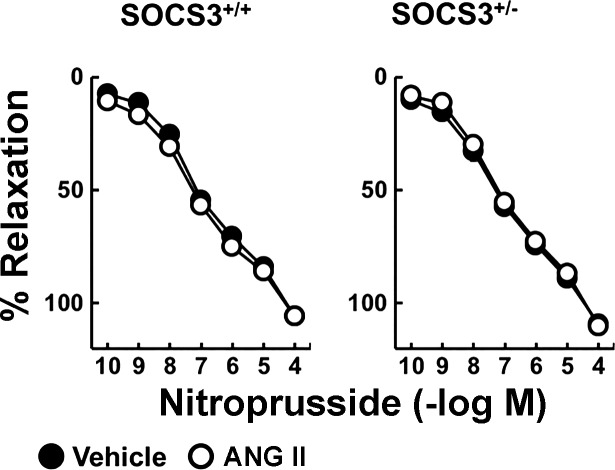
Because we hypothesized that direct effects of Ang II would be enhanced in SOCS3+/− mice, we tested effects of a lower concentration of Ang II (1 nM) in subsequent experiments. In arteries from SOCS3+/+ and SOCS3+/− mice, relaxation to acetylcholine was similar after incubation with vehicle. Consistent with previous findings, this low concentration of Ang II did not affect acetylcholine-induced responses in arteries from WT mice (Fig. 2B) (12). In contrast, 1 nM Ang II reduced responses in arteries from SOCS3+/− mice by ∼50% (Fig. 2B). Vascular responses to nitroprusside (Fig. 3) and U46619 (data not shown) were similar in these groups, indicating that effects of Ang II were selective for endothelial cells. These data suggested that partial deficiency in SOCS3 does not alter vascular function under baseline conditions but predisposes to Ang II-induced endothelial dysfunction.
Fig. 3.
Endothelium-independent relaxation in carotid arteries was not affected by Ang II or SOCS3 deficiency. Nitroprusside induced relaxation in arteries from SOCS3+/+ and SOCS3+/− mice incubated with vehicle or Ang II (1 nM); n = 6, *P < 0.05 vs. vehicle.
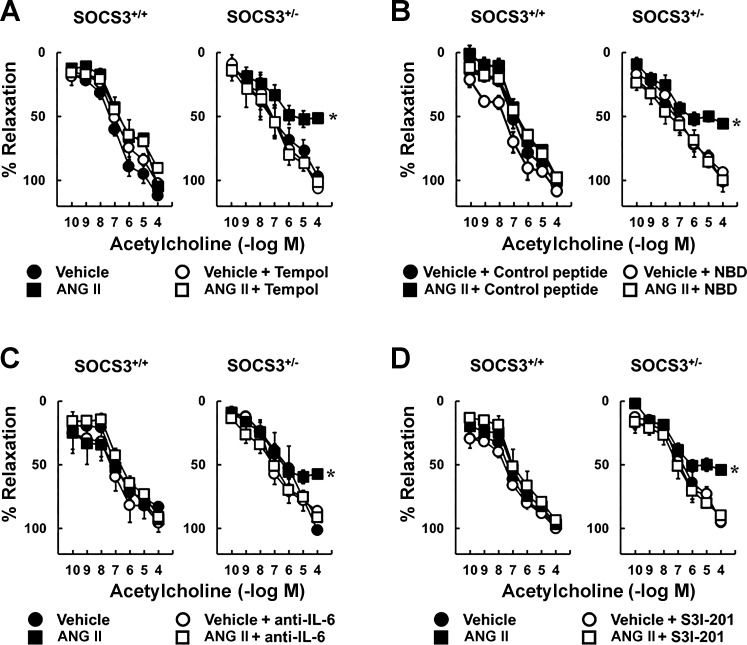
Endothelial dysfunction in SOCS3+/− mice requires oxidative- and inflammatory-related signaling.
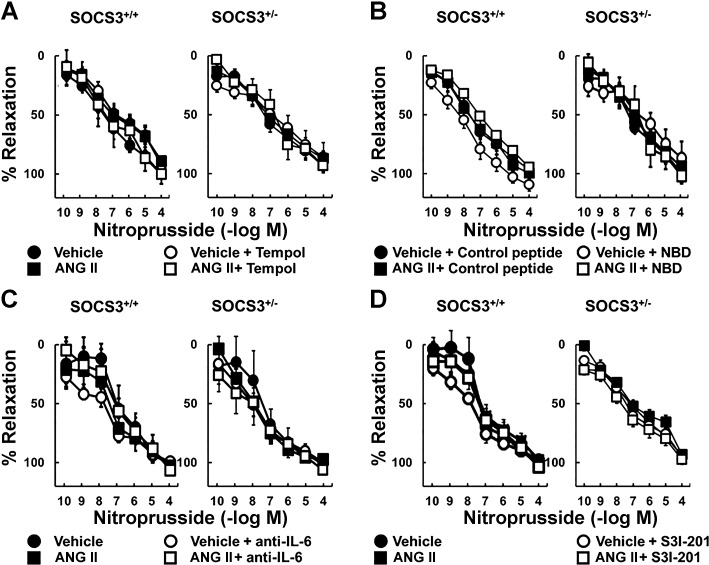
To test whether Ang II-induced endothelial dysfunction in SOCS3+/− mice was mediated by superoxide, responses were examined in the presence of tempol. Ang II-induced impairment of responses to acetylcholine in arteries from SOCS3+/− mice was reversed by tempol (Fig. 4A), whereas relaxation in arteries treated with vehicle was not affected (Fig. 4A). In addition, nitroprusside-induced vasodilation was similar in these groups (Fig. 5A).
Fig. 4.
Ang II-induced endothelial dysfunction in SOCS3+/− arteries was mediated by oxidative stress and inflammation. Acetylcholine induced relaxation in carotid arteries from SOCS3+/+ and SOCS3+/− mice incubated with vehicle or Ang II (1 nM) with or without a superoxide scavenger (tempol) (A), NF-κB essential modulator (NEMO)-binding domain (NBD), which is an inhibitory peptide of NF-κB (B), an IL-6-neutralizing antibody (anti-IL-6) (C), or a small-molecule inhibitor of STAT3 (S3I-201) (D); n = 6.
Fig. 5.
Endothelium-independent relaxation was not affected by Ang II or other treatments. Nitroprusside induced relaxation in carotid arteries from SOCS3+/+ and SOCS3+/− mice incubated with vehicle or Ang II in the presence and absence of tempol (1 mM) (A), an inhibitory peptide of NF-κB (NBD, 10 μM) (B), an IL-6-neutralizing antibody (anti-IL-6, 10 μg/ml) (C), or a small-molecule inhibitor of STAT3 (S3I-201, 10 μM) (D); n = 6.
To test whether Ang II-induced endothelial dysfunction in SOCS3+/− mice is dependent on activation of NF-κB, a cell-penetrating NBD peptide was used. This NBD peptide inhibits NF-κB activation in models of inflammation (39). Acetylcholine-induced relaxation was similar in WT arteries treated with vehicle or Ang II, as well as vehicle-treated SOCS3+/− arteries (Fig. 4B). NBD peptide did not alter endothelial function in WT mice or vehicle-treated SOCS3+/− mice (Fig. 4B) but prevented Ang II-induced endothelial dysfunction in SOCS3+/− mice (Fig. 4B). The inactive control peptide had no effect on acetylcholine-induced relaxation (Fig. 4B). Nitroprusside-induced vasodilation was also similar in these groups (Fig. 5B).
To determine whether effects of Ang II were mediated by IL-6 and/or STAT3, arteries were incubated with an anti-IL-6 antibody or a STAT3 inhibitor (S3I-201). Ang II-induced endothelial dysfunction in SOCS3+/− mice was prevented by these inhibitors (Fig. 4, C and D). Neither the antibody nor S3I-201 altered acetylcholine-induced relaxation in arteries from WT mice or vessels from SOCS3+/− mice treated with vehicle (Fig. 4, C and D). Vascular responses to nitroprusside were similar in these groups (Fig. 5, C and D).
SOCS3 deficiency protects against endothelial dysfunction in Ang II-dependent hypertension.
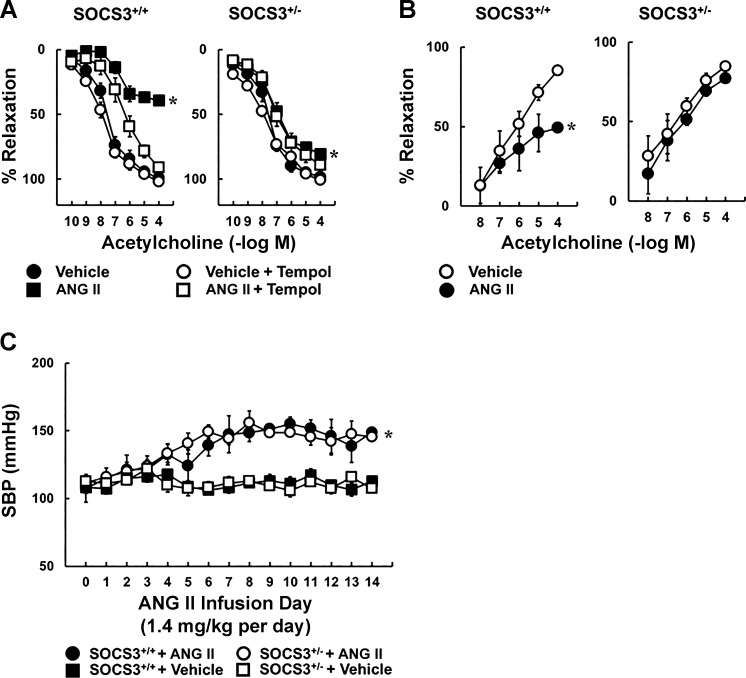
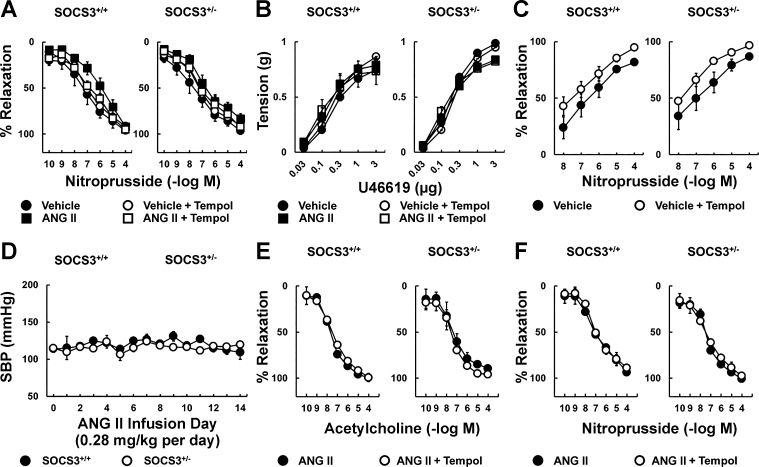
To examine the impact of SOCS3 in vivo, we produced hypertension by infusing a pressor dose of Ang II chronically. Ang II infusion reduced the response to acetylcholine in arteries from SOCS3+/+ mice by more than 50%, an effect that was largely prevented by tempol (Fig. 6A), and was similar in magnitude to that seen in the incubation model (Fig. 1). Unexpectedly, Ang II had little effect on endothelial function in SOCS3+/− mice (Fig. 6A). Relaxation of arteries to acetylcholine was similar in saline-treated groups with or without tempol (Fig. 6A). Responses to nitroprusside and U46619 were similar in these groups as well (Fig. 7, A and B).
Fig. 6.
Role of SOCS3 in Ang II-dependent hypertension. A: acetylcholine-induced relaxation in carotid arteries from SOCS3+/+ and SOCS3+/− mice systemically administrated with vehicle or Ang II (1.4 mg/kg per day) for 14 days. The role of oxidative stress was examined by acute administration of tempol; n = 6, *P < 0.05 vs. vehicle. B: vasodilation induced by acetylcholine in basilar arteries from SOCS3+/+ and SOCS3+/− mice systemically administrated with either vehicle or Ang II (1.4 mg/kg per day) for 14 days; n = 4, *P < 0.05 vs. vehicle. C: systolic blood pressure in SOCS3+/+ and SOCS3+/− mice systemically administrated with either vehicle or Ang II for 14 days. *P < 0.05 vs. vehicle.
Fig. 7.
Vascular function following systemic administration of Ang II. A and B: nitroprusside-induced relaxation (A) and U46619-induced contraction (B) in carotid arteries from SOCS3+/+ and SOCS3+/− mice treated with vehicle or Ang II (1.4 mg/kg per day) for 14 days; n = 6. C: nitroprusside-induced vasodilation in basilar arteries from SOCS3+/+ and SOCS3+/− mice treated with vehicle or Ang II (1.4 mg/kg per day) for 14 days; n = 4. D: systolic blood pressure measured using tail-cuff in SOCS3+/+ and SOCS3+/− mice systemically administrated with a nonpressor dose of Ang II for 14 days; n = 3. E and F: endothelium-dependent (E) and endothelium-independent (F) relaxation in carotid arteries from SOCS3+/+ and SOCS3+/− mice treated with the nonpressor dose of Ang II for 14 days; n = 3. The role of oxidative stress in each group was examined by acute administration of a superoxide scavenger tempol (1 mM).
Protective effects of SOCS3 deficiency extend to resistance arteries.
To examine the functional importance of SOCS3 in a resistance vessel, basilar arteries were isolated from saline- and Ang II-infused mice. Dilation of basilar arteries from SOCS3+/+ mice to acetylcholine was reduced by approximately one-half in the Ang II-treated group compared with mice treated with saline (Fig. 6B). Consistent with carotid arteries, SOCS3+/− mice were protected against Ang II-induced endothelial dysfunction in basilar arteries (Fig. 6B). Responses to nitroprusside were only modestly affected (Fig. 7C).
SOCS3 deficiency did not alter the pressor response to Ang II.
To investigate effects related to blood pressure, arterial pressure was evaluated in SOCS3+/+ and SOCS3+/− mice. Blood pressure was similar in both strains treated with saline or Ang II (Fig. 6C). Thus global SOCS3 deficiency did not affect the pressor response to Ang II.
To define the importance of SOCS3 during infusion of a nonpressor dose of Ang II, mice were treated with 0.28 mg/kg Ang II per day for 14 days. This dose of Ang II did not alter blood pressure in either strain of mice (Fig. 7D). In addition, acetylcholine- and nitroprusside-induced responses in carotid arteries were similar (Fig. 7, E and F).
Vascular expression of select genes.
In an effort to obtain additional insight into mechanisms, we measured mRNA levels of select genes previously implicated in vascular dysfunction (Table 1). SOCS3 deficiency was associated with reduced expression of eNOS, SOD1, and SOD2. In contrast, we detected an increase in AT1-R expression in SOCS3+/− mice. IL-6 expression was similar between genotypes and was significantly increased following Ang II infusion.
Table 1.
Expression of select genes implicated in vascular disease
| SOCS3+/+ |
SOCS3+/+ |
SOCS3+/− |
SOCS3+/− |
|
|---|---|---|---|---|
| Gene | + Vehicle | + Ang II | + Vehicle | + Ang II |
| eNOS | 1 ± 0.21 | 1.49 ± 0.33 | 0.33 ± 0.17* | 0.54 ± 0.17 |
| SOD1 | 1 ± 0.14 | 0.42 ± 0.13* | 0.56 ± 0.12* | 0.40 ± 0.14* |
| SOD2 | 1 ± 0.22 | 0.27 ± 0.07* | 0.85 ± 0.05 | 0.34 ± 0.16 |
| AT1-R | 1 ± 0.27 | 3.44 ± 0.96 | 2.18 ± 0.36* | 2.32 ± 0.35 |
| IL-6 | 1 ± 0.28 | 23.97 ± 9.87* | 0.92 ± 0.55 | 15.34 ± 6.06*† |
Values are means ± SE. Gene expression in aorta from SOCS3+/+ and SOCS3+/− mice systemically treated with vehicle or angiotensin II (Ang II) for 14 days; n = 6,
P < 0.05 vs. vehicle.
P < 0.05 vs. SOCS3+/− plus vehicle.
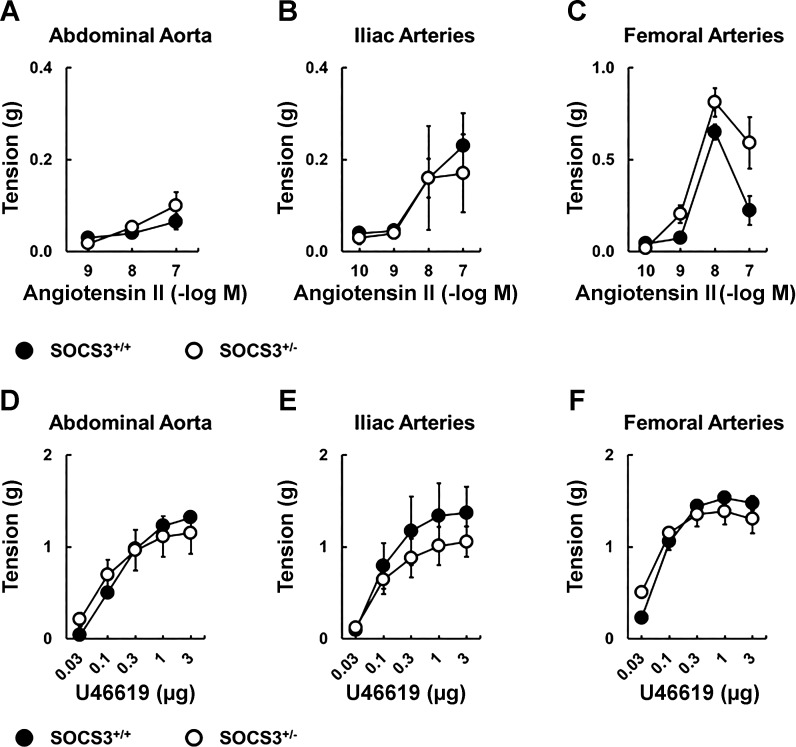
To determine whether observed increases in AT1-R expression translated to functional changes, we examined constrictor effects of Ang II in aorta and peripheral arteries. Effects of Ang II on vascular tone were similar in WT and SOCS3+/− mice (Fig. 8). Because expression of AT1-R was relatively low in aorta (Ct values of ∼32), these results suggest that small differences in AT1-R mRNA levels between strains did not translate to functional effects on vascular tone.
Fig. 8.
Effects of SOCS3 deficiency on vasoconstriction. Ang II induced contraction in abdominal aorta (A), iliac arteries (B), and femoral arteries (C) from SOCS3+/+ (n = 5) and SOCS3+/− mice (n = 7). U46619 induced contraction in abdominal aorta (D), iliac arteries (E), and femoral arteries (F) from SOCS3+/+ (n = 5) and SOCS3+/− mice (n = 7).
Bone marrow-derived cells deficient in SOCS3 protect against systemic effects of Ang II.
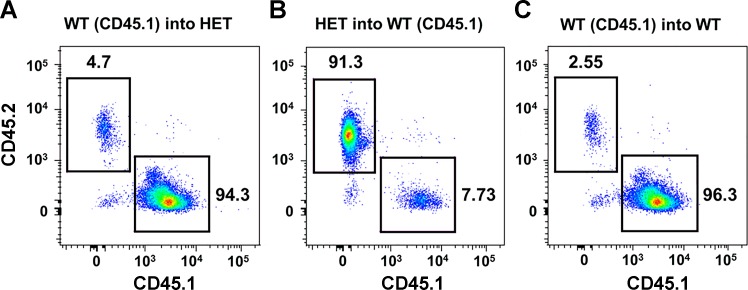
To this point, our findings suggest that the impact of SOCS3 depends on whether Ang II was administrated locally or systemically. In considering mechanisms that might account for these differences, we considered the emerging role for hematopoietic cells in hypertension (49, 55). Thus we tested the hypothesis that deficiency in SOCS3 in bone marrow-derived cells would protect against Ang II-induced endothelial function. After mouse recovery from bone marrow transplantation, FACS analysis confirmed effective reconstitution of cells in the various groups (Fig. 9). For example, 94% of bone marrow-derived cells in SOCS3+/− mice were reconstituted with WT (CD45.1) bone marrow (Fig. 9). In previous studies, a reconstitution rate of ∼95% was considered successful for standard bone marrow transplantation (4, 10).
Fig. 9.
Fluorescence-activated cell sorting. A: reconstitution of irradiated SOCS3+/− mice with WT (CD45.1) bone marrow. X-axis represents WT (CD45.1) bone marrow. Y-axis represents SOCS3+/− bone marrow. B: reconstitution of irradiated WT (CD45.1) mice with SOCS3+/− bone marrow. X-axis represents WT (CD45.1) bone marrow. Y-axis represents SOCS3+/− bone marrow. C: reconstitution of irradiated SOCS3+/+ mice with WT (CD45.1) bone marrow. X-axis represents WT (CD45.1) bone marrow. Y-axis represents SOCS3+/+ bone marrow.
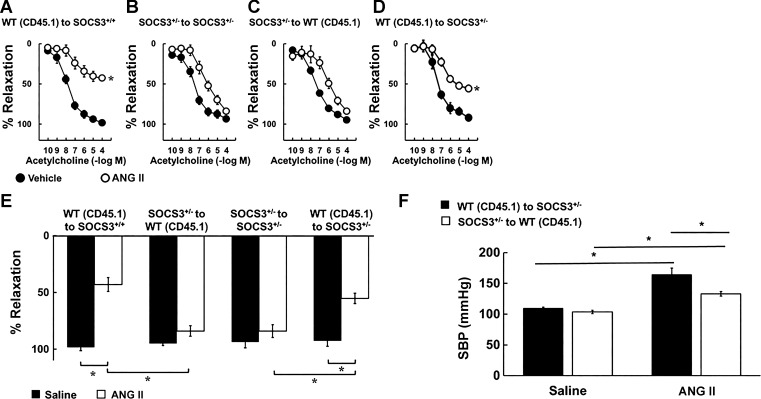
Mice were then infused with either saline or Ang II (1.4 mg/kg per day) for 14 days followed by examination of vascular function. WT (CD45.1) into SOCS3+/+ and SOCS3+/− into SOCS3+/− bone marrow chimeras exhibited vascular function consistent with nonirradiated controls (Fig. 10, A and B). For example, acetylcholine-induced relaxation in arteries from the WT (CD45.1) into SOCS3+/+ group was reduced by ∼60% (P < 0.05) (Fig. 10A), whereas the majority of Ang II-induced endothelial dysfunction was prevented in the SOCS3+/− into SOCS3+/− group (Fig. 10B). Vascular responses to nitroprusside and U46619 were similar in these respective groups (data not shown).
Fig. 10.
Endothelial function and blood pressure of bone marrow chimeras in chronic Ang II-dependent hypertension. Acetylcholine induced relaxation in carotid arteries from WT (CD45.1) to SOCS3+/+ (A), SOCS3+/− to SOCS3+/− (B), SOCS3+/− to WT (CD45.1) (C), and WT (CD45.1) to SOCS3+/− (D) bone marrow chimeras systemically treated with vehicle or Ang II (1.4 mg/kg per day) for 14 days. *P < 0.05 vs. vehicle. E: acetylcholine-induced maximum relaxation in carotid arteries from WT (CD45.1) to SOCS3+/+, SOCS3+/− to WT (CD45.1), SOCS3+/− to SOCS3+/−, and SOCS3+/− to WT (CD45.1) bone marrow chimeras treated systemically with vehicle or Ang II (1.4 mg/kg per day) for 14 days; n = 6, *P < 0.05 vs. vehicle. F: blood pressure of WT (CD45.1) to SOCS3+/−, SOCS3+/− to WT (CD45.1) bone marrow chimeras was measured on day 14 of Ang II (1.4 mg/kg per day) infusion; n = 6, *P < 0.05 vs. vehicle.
Reconstitution of lethally irradiated WT (CD45.1) mice with SOCS3+/− bone marrow protected against detrimental effects of Ang II on endothelial dysfunction (Fig. 10C). In contrast, irradiated SOCS3+/− mice reconstituted with WT (CD45.1) bone marrow exhibited exacerbated Ang II-induced vascular dysfunction (Fig. 10D). For ease of comparison, maximum vascular effects of acetylcholine are presented in Fig. 10E. Nitroprusside-induced relaxation and U46619-induced contraction of carotid arteries was similar in all groups (data not shown).
Bone marrow-derived cells deficient in SOCS3 reduced the pressor response to Ang II.
Blood pressure was similar in groups infused with saline (Fig. 10F), but the pressor response to Ang II was reduced in WT (CD45.1) mice reconstituted with bone marrow from SOCS3+/− mice [compared with SOCS3+/− mice reconstituted with WT (CD45.1) bone marrow] (Fig. 10F). These data suggest that deficiency of SOCS3 in bone marrow-derived cells has beneficial effects on blood pressure during hypertension.
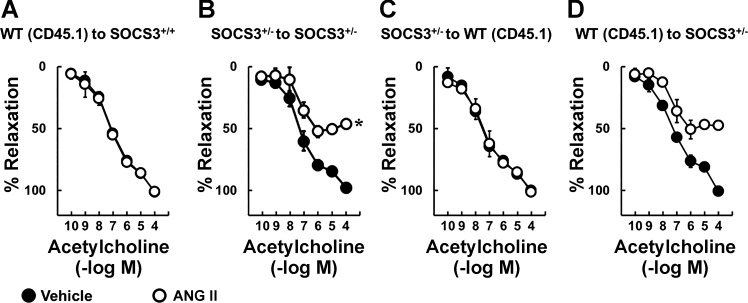
Direct vascular effects of Ang II are not affected by the SOCS3 genotype of bone marrow-derived cells.
To test whether SOCS3 in bone marrow-derived cells alters direct effects of Ang II on endothelial function, arteries were isolated from each group of mice after bone marrow transplantation, incubated with saline or Ang II (1 nM) overnight, followed by evaluation of vascular function. Consistent with previous results, Ang II did not alter endothelial function in the WT (CD45.1) into SOCS3+/+ group (Fig. 11A). Effects of Ang II on acetylcholine-induced responses in the SOCS3+/− into SOCS3+/− group were similar to that seen in nonirradiated SOCS3+/− mice (Fig. 11B). Vascular responses to acetylcholine in SOCS3+/− into WT (CD45.1) and WT (CD45.1) into SOCS3+/− groups were also similar to those in nonirradiated SOCS3+/+ and SOCS3+/− mice (Fig. 11, C and D). Responses of arteries to nitroprusside and U46619 were similar in these groups (data not shown). These data suggest that the SOCS3 genotype of bone marrow-derived cells does not influence direct effects of Ang II on the vessel wall.
Fig. 11.
Endothelial function in bone marrow chimeras in response to local effects of Ang II. Acetylcholine induced relaxation in carotid arteries from WT (CD45.1) to SOCS3+/+ (A), SOCS3+/− to SOCS3+/− (B), SOCS3+/− to WT (CD45.1) (C), and WT (CD45.1) to SOCS3+/− (D) bone marrow chimeras incubated with either vehicle or Ang II; n = 3, *P < 0.05 vs. vehicle.
DISCUSSION
There are several major findings in this study. First, partial genetic deficiency in SOCS3 had no effect on vascular responses under normal conditions but augmented direct effects of Ang II on endothelial function. These findings suggest that SOCS3 protects against local effects of Ang II on the vessel wall. Second, in relation to mechanisms, local effects of Ang II on vascular function in SOCS3+/− mice were prevented by inhibition of NF-κB, IL-6, STAT3, or superoxide, suggesting that SOCS3 inhibits prooxidant and proinflammatory elements. Third, baseline arterial pressure and the pressor response to Ang II were not altered in SOCS3+/− mice, suggesting that a global reduction in SOCS3 does not protect against pressor effects of Ang II. Fourth, systemic administration of Ang II impaired endothelial function in WT mice but had surprisingly little effect on endothelial function in SOCS3+/− mice. This protective effect of SOCS3 deficiency on the vasculature extended to a resistance artery supplying the brain. Fifth, bone marrow derived-cells deficient in SOCS3 protected against endothelial dysfunction and increases in arterial pressure produced by systemic Ang II. This conclusion is based on the observations that reconstitution with SOCS3+/− bone marrow protected WT mice against endothelial dysfunction, whereas SOCS3+/− mice reconstituted with WT bone marrow exhibited enhanced endothelial dysfunction. Collectively, these data provide the first evidence that SOCS3 exerts divergent effects on vascular function depending on whether the source of Ang II is local or circulating. Bone marrow-derived cells deficient in SOCS3 protect against Ang II-induced vascular dysfunction and hypertension.
Local vascular effects of Ang II are augmented by genetic deficiency in SOCS3: Role of oxidant- and immune-related mechanisms.
Both systemic (circulating) and locally derived Ang II contribute to effects of the peptide on vascular function (16). One of our models tested direct effects of Ang II on the vessel wall, without activating cells in other organ systems or compartments. This approach avoids potential confounding effects of increases in arterial pressure when Ang II is administered systemically or centrally.
With the use of this vessel-culture model, Ang II produces concentration-dependent effects on endothelial function (28). We confirmed that a low concentration of Ang II (1 nM) does not alter endothelial function in vessels from normal mice (12). Interestingly, the degree of impairment of endothelial function in arteries treated with 1 nM Ang II in SOCS3+/− mice was similar in magnitude to that produced by a 10-fold higher concentration of Ang II in SOCS3+/+ mice. Thus loss of only one copy of the SOCS3 gene substantially increased sensitivity of vessels to direct effects of Ang II.
IL-6 and STAT3 signaling contribute to Ang II-induced endothelial dysfunction (28, 51). SOCS3 inhibits STAT3- and IL-6-dependent effects in other models (52). In relation to vascular disease and hypertension, vascular expression of SOCS3 increases during chronic systemic administration of Ang II (28). Although such findings raised questions regarding its role, the functional importance of SOCS3 had not been previously evaluated. To our knowledge, the present experiments provide the first insight into the functional importance of SOCS3 in intact vessels.
Ang II promotes oxidative stress (40). Pharmacological or genetic approaches that target superoxide or NADPH oxidase protect the vasculature from effects of Ang II (15, 37). A key mechanism underlying Ang II-induced endothelial dysfunction involves decreased bioavailability of NO resulting from the interaction between NO and superoxide (43). Consistent with these concepts, tempol reversed Ang II-induced endothelial dysfunction in SOCS3+/− mice.
Expression of IL-6 and activation of STAT3 are required for Ang II to increase vascular superoxide (28, 51). Oxidative stress and inflammation interact through mutual feed-forward mechanisms (53). Ang II activates NF-κB and increases expression of IL-6 (2). A new finding was that endothelial dysfunction in SOCS3+/− mice was prevented by inhibitors of IL-6 or STAT3.
NF-κB is a ubiquitously expressed transcription factor, involved in regulation of an array of genes, including ones important in vascular pathophysiology (23). Despite the fact that Ang II activates NF-κB in vascular cells and vessels (14, 46), few studies have examined its impact in relation to endothelial function. In the present studies, the local effect of Ang II on endothelial function in SOCS3+/− mice was prevented by an inhibitor of NF-κB.
Role of SOCS3 in hypertension.
Systemic delivery of Ang II is one of the most common approaches to experimentally produce hypertension. In the present study, the pressor dose of Ang II increased arterial pressure by ∼40 mmHg compared with vehicle in both WT and SOCS3+/− mice. Although these results in WT are consistent with previous studies using radiotelemetry or tail-cuff methods to measure arterial pressure, we cannot exclude the possibility that small differences in blood pressure between strains were missed with the present approach. Because Ang II can induce vascular dysfunction through both blood pressure-dependent and -independent mechanisms (5), we also tested effects of a nonpressor dose of Ang II. Consistent with previous studies, infusion of Ang II at 0.28 mg/kg per day did not alter blood pressure or endothelial dysfunction in either strain of mice (31).
Deficiency in SOCS3 protects against systemic Ang II-induced endothelial dysfunction.
The pressor dose of Ang II reduced endothelial function in carotid arteries from WT mice. Consistent with previous work using scavengers of superoxide and Nox2-deficient animals (12, 28, 51), this loss of endothelial function was reversed by a scavenger of superoxide. Unexpectedly, systemic Ang II had little effect on endothelial function in arteries from SOCS3+/− mice. This protective effect of SOCS3 deficiency extended to a resistance vessel supplying brain. Thus SOCS3 deficiency augmented local effects of Ang II but protected the vasculature in response to systemic Ang II.
Vessel-incubation and cell-culture models have been used to examine direct effects of Ang II previously (3, 28, 51). In studies evaluating the role of ROS, Nox2, IL-6, IL-10, and STAT3, endothelial function was qualitatively similar whether Ang II was administered locally to isolated vessels or systemically (28, 51). In our experience, the present experiments are the first where the two models differed in relation to effects of Ang II on endothelial function. Such results raised questions regarding the possible role of SOCS3 in nonvascular cells, particularly in bone marrow-derived cells. A limitation of this approach is that, like studies done in cell culture, the duration of the vessel incubation studies was not as long as the more chronic studies in which osmotic minipumps were used to deliver Ang II. As noted, however, this in vitro approach has been predictive of effects in vivo in the past and for a large literature that examined effects of Ang II using vascular cells in culture.
Hematopoietic SOCS3+/− cells protect against Ang II-dependent endothelial dysfunction and hypertension.
After confirming the efficacy of transplantation, we found that SOCS3+/− bone marrow derived-cells protected against endothelial dysfunction and increases in arterial pressure produced by systemic administration of Ang II. Ang II-induced elevation in blood pressure was significantly attenuated in irradiated WT (CD45.1) mice reconstituted with SOCS3+/− bone marrow. These results provide direct evidence that the beneficial effects of SOCS3+/− bone marrow extend to blood pressure. SOCS3+/− to WT (CD45.1) bone marrow chimeras have SOCS3+/− bone marrow, which was protective, but a SOCS3+/+ vasculature and were not predisposed to the detrimental effects of Ang II. Thus the reduced blood pressure seen in SOCS3+/− to WT (CD45.1) bone marrow chimeras may result from lacking Ang II detrimental effects on the vessel wall in combination with protective effects from SOCS3 deficiency in other cells.
Bone marrow-derived stem cells contain two populations of cells, hematopoietic and mesenchymal stem cells (24). Hematopoietic cells are precursors to all types of blood cells, whereas mesenchymal stem cells can differentiate into other cell types, including endothelial cells (44), which provide a microenvironment supporting hematogenesis (24). With standard protocols for bone marrow transplantation (26), mesenchymal stem cells are not transplanted with hematopoietic stem cells (33, 35).
The role of SOCS3 can vary in different models and cell types (7, 32, 57). Although SOCS3 is often considered a suppressor of proinflammatory signaling (7), deficiency in SOCS3 can result in anti-inflammatory effects (32, 57). With consideration that IL-6 can elicit both pro- and anti-inflammatory effects in other models (50),, the present results support the possibility that SOCS3 functions as a determinant of pro- or anti-inflammatory effects of Ang II and perhaps downstream IL-6 signaling. In relation to these concepts, it is interesting that Ang II-induced hypertension is exaggerated in bone marrow-specific AT1-R-deficient mice (9), which suggests a protective effect of Ang II signaling in immune cells. We speculate that this protective effect may involve activating IL-6 anti-inflammatory signaling. Future studies would need to be designed to explore this concept further.
Although studies to date have not defined the exact cell subtype(s) that underlies protective effects of bone marrow transfer, we anticipate that beneficial effects most likely result from cells within the leukocyte lineage. A contribution by red blood cells is highly unlikely, as those cells were lysed during preparation for transplantation. On the basis of results from previous studies, macrophages may be promising candidates for beneficial effects of SOCS3 deficiency (38, 57). Additional studies would be needed to test this possibility.
Local vascular effects of Ang II in the hematopoietic SOCS3+/− model.
The low concentration of Ang II used in the present experiments did not alter endothelial function in lethally irradiated SOCS3+/+ mice reconstituted with CD45.1 WT bone marrow. Such results are consistent with effects of Ang II on vessels from nonirradiated SOCS3+/+ mice. Similarly, local effects of Ang II on endothelial function of lethally irradiated SOCS3+/− mice reconstituted with SOCS3+/− bone morrow were consistent with nonirradiated controls. Both experiments suggest that bone marrow transplantation per se did not alter vascular function. These results further suggest that circulating hematopoietic cells did not alter the impact of SOCS3 in relation to local (direct) effects of Ang II on the vessel wall. Collectively, these combined approaches suggest that the endothelial dysfunction produced by Ang II in arteries from SOCS3+/− mice can be attributed to the local effects of SOCS3 within the vessel wall (within resident vascular cells).
In conclusion, both genetic and pharmacological approaches provided strong evidence that SOCS3 plays a divergent role in relation to local vs. systemic effects of Ang II on the vasculature. Endothelial dysfunction produced by local Ang II was augmented in SOCS3+/− arteries, suggesting that, under normal conditions, SOCS3 protects vessels against detrimental effects of Ang II by suppressing IL-6/STAT3 signaling events. Systemic infusion of a pressor dose of Ang II, which induced endothelial dysfunction in arteries from WT mice, surprisingly caused minimal impairment in arteries from SOCS3+/− mice, suggesting that normal expression of SOCS3 facilitates detrimental effects of Ang II on vascular function. Last, results from bone marrow transplantation studies suggest that bone marrow-derived cells are responsible for protective effects of SOCS3 deficiency on vascular function. Understanding mechanisms by which SOCS3+/− cells protect against Ang II may eventually support the development of new therapeutic strategies for hypertension and associated end-organ damage.
Recent studies have begun to highlight the importance of bone marrow in hypertension, including the role of lymphocyte adapter protein (49, 55). The present findings extend this concept by providing new insight into functional effects of SOCS3 in vascular and bone marrow compartments. As noted by us, and others (17, 49), complete loss of gene expression or protein function is unusual. Thus is it noteworthy that only partial deficiency of SOCS3 (loss of one gene copy) was sufficient to substantially alter effects of Ang II on vascular function and the impact of bone marrow-derived cells.
GRANTS
This work was supported by the National Institutes of Health (HL-62984, HL-113863, and NS-096465), the Department of Veterans Affairs (BX001399), and a Foundation Leducq Transatlantic Network of Excellence (12CVD01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and F.M.F. conception and design of research; Y.L., D.A.K., M.L.M., and L.L.P. performed experiments; Y.L. and F.M.F. analyzed data; Y.L., L.L.P., and F.M.F. interpreted results of experiments; Y.L. prepared figures; Y.L. drafted manuscript; Y.L. and F.M.F. edited and revised manuscript; Y.L., D.A.K., M.L.M., L.L.P., and F.M.F. approved final version of manuscript.
REFERENCES
- 1.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension 51: 867–871, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasier AR. The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86: 211–218, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cascino T, Csanyi G, Al Ghouleh I, Montezano AC, Touyz RM, Haurani MJ, Pagano PJ. Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxid Redox Signal 15: 1507–1515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Wang S, Zhang J, Ren X, Zhang R, Shi W, Lv Y, Zhou Y, Yan X, Chen L, He L, Zhang B, Nan X, Yue W, Li Y, Pei X. A novel molecule Me6TREN promotes angiogenesis via enhancing endothelial progenitor cell mobilization and recruitment. Sci Rep 4: 6222, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrissobolis S, Banfi B, Sobey CG, Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol 113: 184–191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol 22: 611–616, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 4: 540–545, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid Redox Signal 20: 102–120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55: 99–108, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, Casares S. Expression of HLA class II molecules in humanized NOD. Rag1KO IL2RgcKO mice is critical for development and function of human T and B cells. PLoS One 6: e19826, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension 46: 1147–1153, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 54: 619–624, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 91: 938–944, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-γ. Circulation 105: 2296–2302, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzau VJ. Vascular renin-angiotensin system and vascular protection. J Cardiovasc Pharm 22, Suppl 5: S1–S9, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol 300: H1566–H1582, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: New insights from genetic models. J Cereb Blood Flow Metab 26: 449–455, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol 100: 2089–2093, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol Heart Circ Physiol 274: H564–H570, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension 55: 445–452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta 1231: 215–219, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F. NF-κB transcription factor: Role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications. Clin Ter 152: 249–253, 2001. [PubMed] [Google Scholar]
- 24.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells 22: 487–500, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κB transcription factors. Circ Res 84: 695–703, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Harty JT, Bevan MJ. CD8 T-cell recognition of macrophages and hepatocytes results in immunity to Listeria monocytogenes. Infect Immun 64: 3632–3640, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higashi Y, Maruhashi T, Noma K, Kihara Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc Med 24: 165–169, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Johnson AW, Kinzenbaw DA, Modrick ML, Faraci FM. Small-molecule inhibitors of signal transducer and activator of transcription 3 protect against angiotensin II-induced vascular dysfunction and hypertension. Hypertension 61: 437–442, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol Heart Circ Physiol 271: H1626–H1634, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli. Pharmacol Rev 67: 754–819, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: Role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β1. J Exp Med 203: 1021–1031, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: Characterization and clinical application. Crit Rev Oral Biol Med 10: 165–181, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 51: 127–133, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Ma DD, Da WM, Purvis-Smith S, Biggs JC. Chromosomal analysis of bone marrow stromal fibroblasts in allogeneic HLA compatible sibling bone marrow transplantations. Leuk Res 11: 661–663, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle JN. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell 98: 617–627, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112: 2677–2685, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Bruning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15: 423–430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289: 1550–1554, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Michie AM, Carlyle JR, Zuniga-Pflucker JC. Early intrathymic precursor cells acquire a CD4(low) phenotype. J Immunol 160: 1735–1741, 1998. [PubMed] [Google Scholar]
- 42.Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system—focusing on the vascular system. Peptides 32: 2141–2150, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 38: 655–659, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22: 377–384, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: Enhancement by angiotensin II. Proc Natl Acad Sci USA 94: 14483–14488, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Ortega M, Lorenzo O, Ruperez M, Suzuki Y, Egido J. Angiotensin II activates nuclear transcription factor-κB in aorta of normal rats and in vascular smooth muscle cells of AT1 knockout mice. Nephrol Dial Transplant 16, Suppl 1: 27–33, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol 22: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension 43: 661–666, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol 27: 2576–2581, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, Nagata K, Yoshimura A. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest 108: 1781–1788, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterstrat A, Liang Y, Swiderski CF, Shelton BJ, Van Zant G. Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may influence hematopoietic stem cell engraftment. Blood 115: 408–417, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol 27: 677–86, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang G, Hisha H, Cui Y, Fan T, Jin T, Li Q, Lian Z, Hosaka N, Li Y, Ikehara S. A new assay method for late CFU-S formation and long-term reconstituting activity using a small number of pluripotent hemopoietic stem cells. Stem Cells 20: 241–248, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 4: 551–556, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura A. Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J Med 58: 73–83, 2009. [DOI] [PubMed] [Google Scholar]