Abstract
Hindbrain glucagon-like peptide 1 (GLP-1) neurons project to numerous forebrain areas, including the lateral septum (LS). Using a fluorescently labeled GLP-1 receptor (GLP-1R) agonist, Exendin 4 (Ex4), we demonstrated GLP-1 receptor binding throughout the rat LS. We examined the feeding effects of Ex4 and the GLP-1R antagonist Exendin (9–39) (Ex9) at doses subthreshold for effect when delivered to the lateral ventricle. Intra-LS Ex4 suppressed overnight chow and high-fat diet (HFD) intake, and Ex9 increased chow and HFD intake relative to vehicle. During 2-h tests, intra-LS Ex9 significantly increased 0.25 M sucrose and 4% corn oil. Ex4 can cause nausea, but intra-LS administration of Ex4 did not induce pica. Furthermore, intra-LS Ex4 had no effect on anxiety-like behavior in the elevated plus maze. We investigated the role of LS GLP-1R in motivation for food by examining operant responding for sucrose on a progressive ratio (PR) schedule, with and without a nutrient preload to maximize GLP-1 neuron activation. The preload strongly suppressed PR responding, but blockade of GLP-1R in the intermediate subdivision of the LS did not affect motivation for sucrose under either load condition. The ability of the nutrient load to suppress subsequent chow intake was significantly attenuated by intermediate LS Ex9 treatment. By contrast, blockade of GLP-1R in the dorsal subdivision of the LS increased both PR responding and overnight chow intake. Together, these studies suggest that endogenous activity of GLP-1R in the LS influence feeding, and dLS GLP-1Rs, in particular, play a role in motivation.
Keywords: glucagon-like peptide-1, lateral septum, food intake
in the brain, glucagon-like peptide 1 (GLP-1) is primarily produced by neurons in the nucleus of the solitary tract (NTS) (18). These cells are activated by nutrient ingestion, gastric distention, cholecystokinin, and vagal afferent stimulation, and they have been implicated in the control of food intake (36). Intracerebroventricular GLP-1 suppresses food intake, and feeding is increased by intracerebroventricular administration of the GLP-1 receptor (GLP-1R) antagonist, Exendin (9–39) (Ex9) or by NTS GLP-1 mRNA knockdown (5, 37). The loss-of-function studies support the idea that central GLP-1 is essential to the physiological control of energy balance. NTS GLP-1 neurons project throughout the brain (29); however, research on GLP-1R signaling has largely focused on hypothalamic nuclei and the NTS (22, 33). Recent studies have shown that GLP-1R in the ventral tegmental area (VTA) and the nucleus accumbens influence food intake and reward (2, 7, 8), suggesting that GLP-1 plays a physiological role in the control of food intake in brain areas known to play a role in reward and motivation.
The lateral septum (LS) has not traditionally been considered key for control of feeding behavior, but the LS contains GLP-1 fibers and receptors (10, 23, 29). The LS has bidirectional connections with the lateral hypothalamus (LH), an area known to influence feeding (30). Olds and Milner's classic self-stimulation studies suggested that the LS plays a role in reward (25), and recent findings suggest that LS neurons projecting to the LH and the VTA play a role in cocaine reward (19, 32). Furthermore, septal GLP-1R have been shown to suppress behavioral responses to cocaine (13). LS neurons also play a role in anxiety and stress (3). Activation of corticotropin-releasing factor 2 (CRF2) receptors in the LS increases anxiety-like behaviors and decreases feeding in food-restricted rats (4). Other studies suggest that inhibition of LS neurons can reduce the anorexic effects of stress (24). LS α1-adrenoceptors have also been shown to influence feeding in food-deprived rats (34). These findings suggest that the LS is involved in coordinating feeding behavior during stress; however, little attention has been drawn to the potential role of the LS in the physiological control of feeding. Here, we focused on the LS as a site of action for GLP-1 effects on feeding in nondeprived rats. Our data suggest that GLP-1R signaling in the LS plays a physiological role in the control of food intake and highlight the LS as a contributor to the central control of feeding.
METHODS
Subjects.
Naïve male Wistar rats (Harlan, Indianapolis, IN) were maintained individually in temperature-controlled vivariums on a 12:12-h light-dark cycle in plastic cages. Rats had ad libitum access to distilled water and rat chow (Purina 5001) (3.02 kcal/g), except where otherwise noted. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Surgery.
Under 2–4% isoflurane delivered at a rate of 1 l/min, rats were implanted with unilateral 26 G guide cannulas (Plastics One, Roanoke, VA), targeting the lateral ventricle (LV) or the LS. Coordinates for the LV were 1.5 mm lateral to midline, 0.9 mm posterior to bregma, and 2.7 mm ventral to skull surface. LS coordinates were 0.6 mm lateral to midline, 1.0 mm anterior to bregma, and 4.0 mm ventral to skull surface. Coordinates for the dorsal LS (dLS) were 0.6 mm lateral to midline, 1.0 mm anterior to bregma, and 3.0 mm ventral to skull surface. Caudate putamen (CPu) coordinates were 3.0 mm lateral to midline, 1.0 mm anterior to bregma, and 4.0 mm ventral to skull surface. Injectors (33G) extending 2.0 mm below the end of the guide cannulas were used. LV cannula placements were verified before the start of experiments through observation of water intake induced by ANG II (Sigma-Aldrich, St. Louis, MO). Rats were given 1 wk to recover from surgery prior to the start of experimentation. LS placements were verified histologically at the conclusion of behavioral testing. Injection sites within the boundaries of the LS, as drawn in the atlas of Paxinos and Watson (26), were considered correct, and data from rats with correct placements were included in the analysis (80% hit rate). For the operant responding studies, one experiment included only intermediate lower septum (iLS) placements, and the other included only dLS placements.
Study 1: histological analysis of GLP-1R binding in LS.
Rats (n = 3) with cannulas targeting the LV received intracerebroventricular injection of 0.2 μg of Ex4, FAM-labeled (AnaSpec, Fremont, CA) in 4 μl of saline, administered over 5 min. Three hours after intracerebroventricular injection, rats were deeply anesthetized and transcardially perfused with 10 mM PBS followed by 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). This timing was based on the recent paper from Reiner et al. (27) using this technique. Brains were removed, sunk in 30% sucrose, and then frozen in isopentane on dry ice. Coronal cryostat sections (20 μm) through the LS were slide-mounted and stored at 4°C. Staining for NeuN was conducted as follows. Sections were blocked for 1 h at room temperature in 5% normal donkey serum and 1% BSA in PBS. The rabbit anti-NeuN primary by Millipore was diluted at 1:500 in 1% BSA in PBS. The secondary was diluted at 1:500, Cy5-conjugated AffiniPure donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA) 1% BSA in PBS. Sections were coverslipped with Fluoro-Gel (Electron Microscopy Sciences). Slides were examined and digital images were acquired with a Zeiss LSM 880 inverted confocal microscope. Adobe Photoshop CC was used to adjust contrast, add color, and merge images of FAM labeling and NeuN immunoreactivity.
Another group of rats (n = 3) received unilateral, 0.5-μl intra-LS microinjections of 0.025 μg HiLyte Fluor 647-labeled Ex4 (AnaSpec, Fremont, CA) under 2–4% isoflurane in 1 liter of oxygen/min inhaled throughout surgery. Each rat was injected with a 28 G Hamilton microsyringe (VWR International, Radnor, PA) and pump (World Precision Instruments, Sarasota, FL) at a rate of 300 nl/min. Stereotaxic coordinates for the injection were 0.6 mm lateral to midline, 1.0 mm anterior to bregma, and 6.0 mm ventral to skull surface. Three hours after intra-LS injection, rats were transcardially perfused, and brains were removed as described above. Coronal cryostat sections (30 μm) through the LS were slide-mounted and stored at 4°C. Sections were then coverslipped with Fluoro-Gel. Slides were examined with an Olympus BX41 fluorescence microscope, and monochromatic digital images were acquired with a Retiga EXI Aqua camera and Q-Capture software (Hunt Optics, Pittsburgh, PA). Adobe Photoshop CC was used to adjust brightness and contrast.
General methods for behavioral experiments.
Rats were habituated to all experimental procedures before the start of testing. For habituation to intra-LV and intra-LS injection procedures, rats received a 2-μl or 0.5-μl injection of sterile 0.9% saline at a rate of 0.5 μl/min. Injectors were then left in place for an additional minute before removal. Body weights were recorded daily, and all drug treatments were separated by at least 48 h.
Study 2: effects of LS GLP-1R stimulation or blockade on chow intake.
First, we conducted a within-subjects dose response for LV delivery of Ex4 (American Peptides, Vista, CA) to determine a subthreshold dose. On experimental days, food was removed 4 h before the onset of the dark cycle. Thirty minutes before dark, rats (n = 10) received a LV injection of 0, 0.025, 0.05, or 0.1 μg of Ex4 in 2 μl of saline. Chow was returned immediately before dark, and intake was measured 1, 2, 4, and 20 h later.
A within-subjects counterbalanced design was used to determine the feeding response to intra-LS Ex4 (0.01 or 0.025 μg; n = 9), and in a separate group of rats, the response to Ex9 (5.0 or 10 μg; n = 7) was determined. Doses of Ex4 and Ex9 (American Peptides, Vista, CA) used here were subthreshold for effect when delivered to the LV in our previous studies (8). On experimental days, food was removed 4 h before the onset of the dark cycle. Thirty minutes before dark, rats received intra-LS injection of Ex9 or Ex4 in 0.5 μl of saline. Chow was returned immediately before dark, and intake was measured 1, 2, 4, and 20 h later.
In another group of rats (n = 5) with cannulas targeting the CPu, the same design, as described above, was used to determine the effect of intra-CPu Ex4 on chow intake.
Study 3: effect of LS GLP-1R stimulation on kaolin and chow intake.
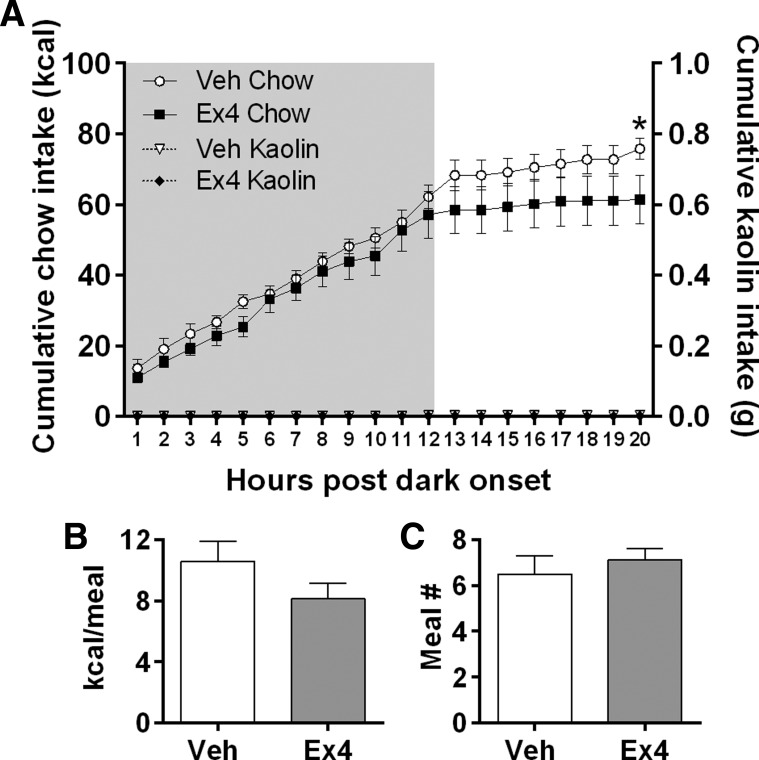
To determine whether intra-LS Ex4 causes malaise or nausea, we assessed pica (ingestion of nonnutritive materials) using a within-subjects design in rats (n = 8) housed in the BioDAQ (Research Diets, New Brunswick, NJ) continuous food intake monitoring system. Rats had ad libitum access to chow and kaolin pellets (aluminum silicate; Research Diets) for 7 days before testing. As a positive control, on the 6th day, rats were injected with 3 mEq/kg ip of 0.15 M LiCl. On experiment days, the gates allowing access to food hoppers were closed 4 h before dark onset. Rats received intra-LS injection of saline or Ex4 (0.025 μg) 30 min before dark onset. Gates were opened to resume access immediately before onset of the dark cycle. Chow and kaolin intake was continuously recorded for 20 h. Meal pattern analyses were conducted with the Data Viewer software from Research Diets. A single meal was defined as the consumption of at least 0.25 g of chow separated by ≥15 min from subsequent intake.
Study 4: effects of LS GLP-1R stimulation or blockade on high-fat diet (HFD) intake.
LS-cannulated rats (n = 8) housed in the BioDAQ system had ad libitum access to one hopper of HFD (60% fat; Research Diets) (5.24 kcal/g) and another of chow (13.5% fat). Rats were adapted to the availability of HFD and chow for 10 days before the start of the experiment. This experiment was conducted, as described above, with rats receiving intra-LS injections of either saline, Ex4 (0.025 μg), or Ex9 (10 μg) 45 min before dark-cycle onset.
Study 5: effects of LS GLP-1R blockade on sucrose and corn oil intake.
LS-cannulated naïve male rats (n = 20) were habituated to receive daily 2-h access to a preweighed bottle filled with 0.25 M sucrose (0.34 kcal/g) in their home cages during the mid-light phase. Bottles were weighed every 30 min for the 2-h test session, during which water and chow were not present in the cage. After the test period, water and chow were returned. Experimentation began after sucrose intake during these test sessions showed less than 10% variation over three consecutive days, which occurred after 20 days. On experiment days, rats received intra-LS injections of either saline vehicle or Ex9 (10 μg) 30 min before the 2-h sucrose intake test, in counterbalanced order with test days separated by at least 48 h. Overnight chow intake was measured, and daily 2-h sucrose sessions continued between treatment days.
The same design was used with another group (n = 20) that received daily 2-h access to an emulsion of 4% corn oil and 0.6% emulsifier (Emplex, Corbion Caravan, Lenexa, KS) (0.36 kcal/g).
Study 6: effect of LS GLP-1R stimulation on behavior in the elevated plus maze.
To examine whether LS GLP-1R stimulation increases anxiety-like behavior, naïve LS-cannulated rats were tested on the elevated plus maze (EPM), a validated approach for assessing anxiety-like behavior. On the test day, one cohort of rats received intra-LS saline (n = 6) or Ex4 (0.025 μg) (n = 6) 45 min before placement into the center of the EPM. Another cohort received saline (n = 11) or intra-LS Ex4 (n = 16) 8 h before the test, to match testing to the time at which Ex4 began to significantly suppress HFD intake in experiment 4. The rat EPM comprised two open arms (50 × 10 cm) and two closed arms (50 × 10 × 60 cm) extending from the center platform (10 × 10 cm) elevated 60 cm above the floor. The testing room was dimly lit with a red light. Behavior in the EPM was video recorded for 5 min, and then rats were returned to their home cage. Videos were scored using NDL-EXBP-0001, Ethovision XT Base software.
Study 7: effects of iLS GLP-1R blockade on operant responding.
LS cannulated rats (n = 8) were trained to lever press for 45-mg sucrose pellets (TestDiet, Richmond, IN) on fixed ratio (FR and FR3) schedules with a 5-s timeout after each reinforcement in operant-conditioning chambers (Coulbourn Instruments, Allentown, PA), as described by Kay et al. (15). Two levers were present in each chamber; presses on the active lever were reinforced, whereas inactive lever presses were not reinforced (rats rarely pressed the inactive lever). Following 16 training days of FR, rats showed less than 10% day-to-day variation in responding and were switched to a progressive ratio (PR) schedule that followed the algorithm of Richardson and Roberts (28): 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, etc., lever presses for reinforcement. PR sessions ended when the rat failed to press the active lever for 30 min, with a maximum duration of 2 h. Rats were then returned to home cages with ad libitum food and water access. Experimentation began after 14 days of PR training, at which point rats showed stable responding on the PR schedule. On experiment days, rats received an intra-LS injection of saline vehicle or 10 μg Ex9 45 min before the start of the session. Chow intake was measured 24 h after injections. Rats received both treatment conditions in counter-balanced order. Between treatment days, rats continued to receive PR sessions.
Next, we tested PR responding following a nutrient preload intended to increase activation of hindbrain GLP-1 neurons and, potentially, release GLP-1 in the LS. The same cohort of rats were trained to drink original chocolate Ensure as a nutrient-dense (0.93 kcal/ml) preload during the 10 min before the 2-h PR sessions. Experimental days were as described above, with the addition of 10-min access to Ensure beginning at 15 min prior to start of the PR session.
Our LS cannula coordinates preferentially targeted the iLS, and for this study, we included only subjects whose injection placements were histologically revealed to be in the iLS.
Study 8: effects of dLS GLP-1R blockade on operant responding.
In another group of rats (n = 13) with cannulas targeting the dLS, we used the same basic design as described above to determine whether GLP-1R activity in this area may influence motivation for food reward. Unlike the previous study, we did not do a preload manipulation here; rats were only tested “without” a nutrient load prior to the operant responding session. Rats were trained to lever press for sucrose pellets over 6 days on a FR1 schedule of reinforcement, followed by 4 days on a FR3 schedule of reinforcement. Rats were then placed on a PR schedule of reinforcement and trained for 16 days before the start of the experiment. On experimental days, rats received an intra-dLS injection of saline vehicle or 10 μg Ex9 45 min before the start of the session. Chow intake was measured 24 h after injections. Rats received both treatment conditions in counter-balanced order. Between treatment days, rats continued to receive PR sessions.
Statistical analysis.
Data are reported as means ± SE. Effects were evaluated by within- or between-subjects Student's t-test or within- or between-subjects one- or two-way ANOVA, as appropriate. Post hoc comparisons were made with Holm-Bonferroni tests. P values of <0.05 were taken as significant.
RESULTS
Study 1: histological analysis of GLP-1R binding in LS.
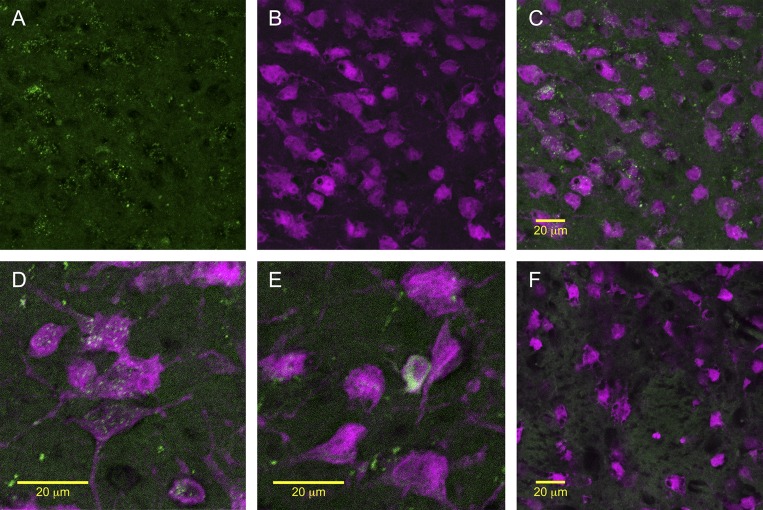
In rats that received intra-LV injection of FAM-labeled Ex4, we observed GLP-1R binding throughout the LS. Many LS neurons, identified by NeuN staining, appeared to internalize the fluoro-Ex4 (Fig. 1). The nearby caudate putamen (CPu) contained no fluoro-Ex4 labeling (Fig. 1F). Throughout the brain, we observed clear fluoro-Ex4 present in other areas where GLP-1R expression and binding have been reported previously, including the NTS and PVN (not shown).
Fig. 1.
A: representative images of fluorescent immunostaining and fluorescently labeled GLP-1R agonist, Exendin-4 (Ex4) binding throughout the lateral septum (LS). A–E: many LS neurons, identified by NeuN staining (magenta) appeared to internalize the fluoro-Ex4 (green). A: LS neurons that appeared to internalize the fluoro-Ex4. B: neurons in the same area identified by NeuN staining. C: merged images of fluoro-Ex4 and NeuN. D and E: higher-magnification merged images of fluoro-Ex4 and NeuN in the LS. F: nearby caudate putamen (CPu) contained no Ex4 labeling, consistent with reports that GLP-1Rs are not expressed there.
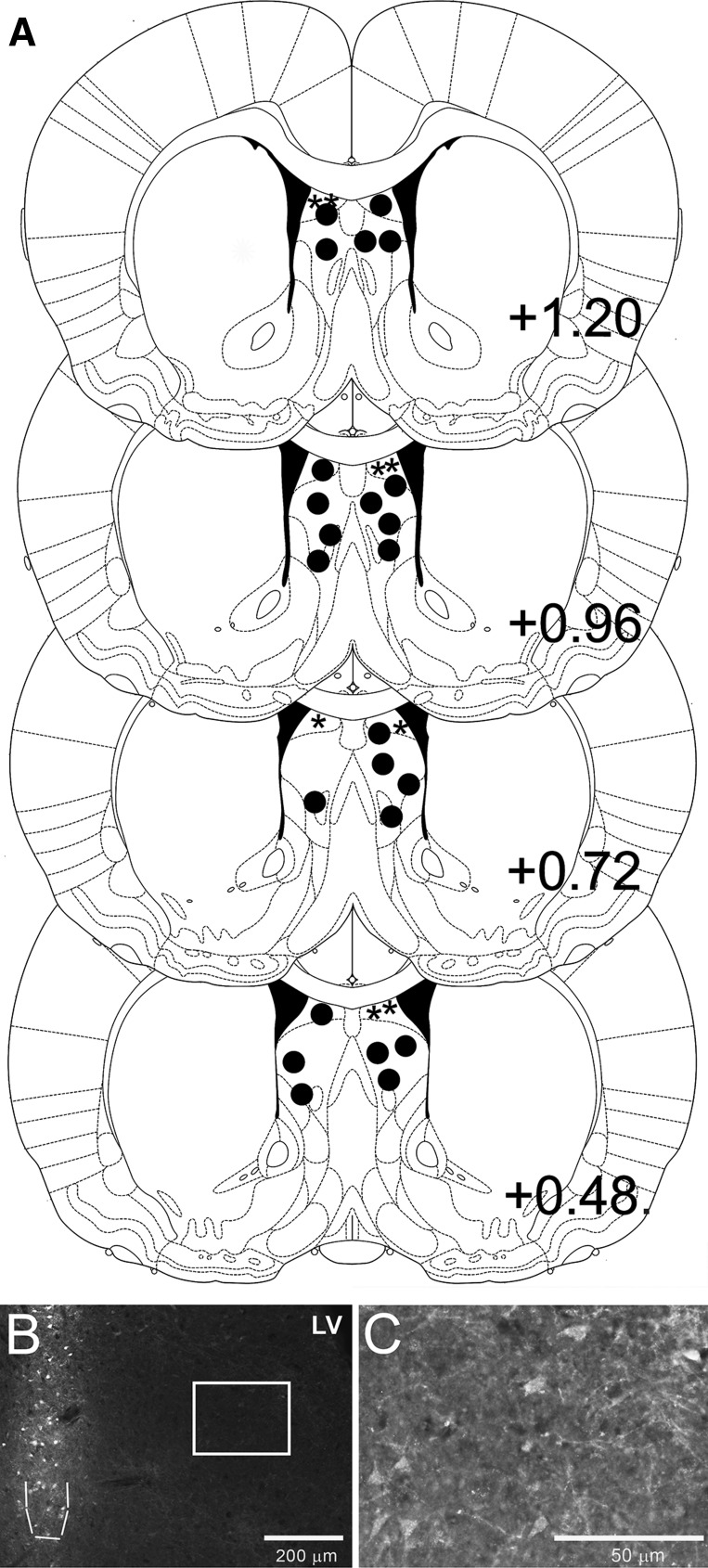
Rats that received intra-LS injection of HiLyte Fluor 647-labeled Ex4 showed ∼0.6-mm spread of the labeled Ex4 around the site of injection (medial-lateral and anterior-posterior). Fluorescence intensity was highest at the site of injection (Fig. 2B), but faint labeling was visible for about 0.6 mm around the site of injection in both the medial-lateral and anterior-posterior directions (Fig. 2C). Beyond ∼0.6 mm from the injection site, no labeling could be observed or photographed. The labeled Ex4 was visible along the injection track within the LS, but only below the corpus callosum (CC). There was clear mechanical damage above the CC from the syringe, and the lack of fluoro-Ex4 labeling there supports the previous reports that there are no GLP-1R above the CC in this anterior-posterior location (10). Depending on the exact location of the injection within the LS, faint labeling could sometimes be observed in the medial septum. No labeling was observed in other adjacent nuclei.
Fig. 2.
A: diagram of representative LS injection placements based on the atlas of Paxinos and Watson (26). Additional subjects' injection sites were identified in similar locations at points between the anterior-posterior levels displayed here. Solid circles (●) represent LS placements from studies 2–7, while asterisks (*) represent dorsal lateral septum (dLS) placements from study 8. B: representative image of intra-LS HiLyte Fluor 647-labeled Ex4 injection. The site of injection is outlined with a dashed line. Faint fluoro-Ex4 labeling to ∼0.6 mm around the site of injection can be observed. C: higher-magnification image taken from the area inside the white box in B shows the lower-density fluoro-Ex4 labeling.
Study 2: effects of LS GLP-1R stimulation or blockade on chow intake.
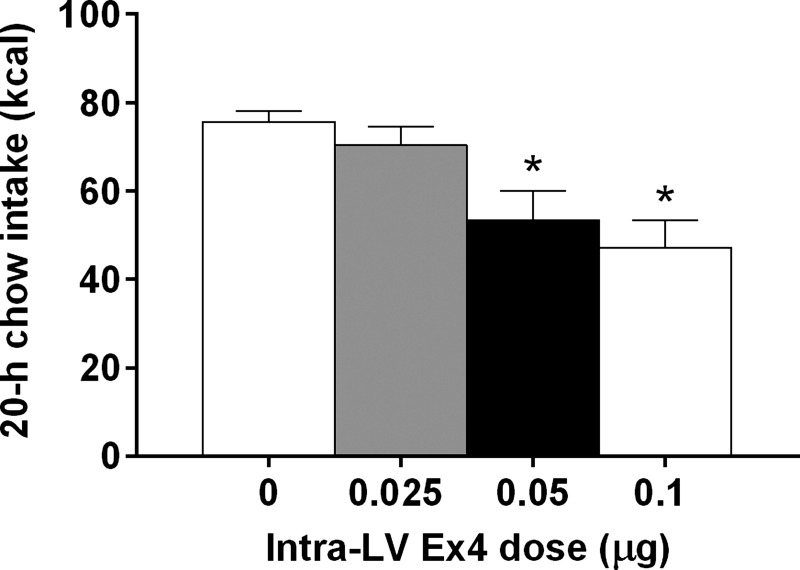
Intra-LV injection of 0.025, 0.05, and 0.1 μg of Ex4 had no effects on feeding at 1, 2, or 4 h after dark onset. At 20 h after dark onset, there was a significant main effect of LV Ex4 [F(3,30) = 9.8; P < 0.01] (Fig. 3). Post hoc comparisons showed that the 0.05 and 0.1 μg dose of Ex4 reduced chow intake relative to saline vehicle at 20 h after dark onset (P < 0.05), while the lower dose, 0.025 μg of Ex4, did not differ from saline. These results informed our choices of doses for subsequent studies.
Fig. 3.
Dose-response for the 20-h chow intake effects of left ventricle (LV)-injected Ex4, *P < 0.05 relative to saline vehicle.
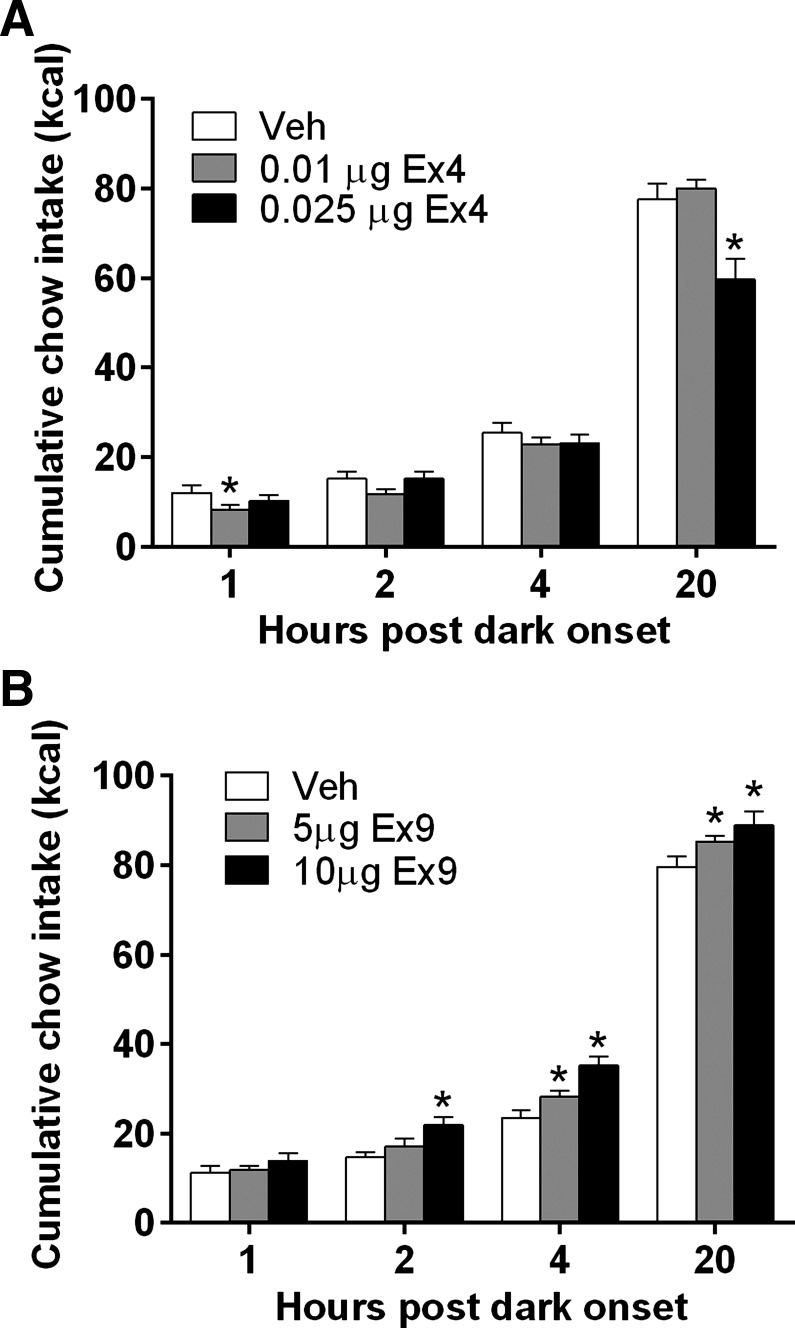
LS Ex4, at doses subthreshold for effect when delivered to the ventricle, significantly reduced chow intake. At 1 h after dark onset [F(2,16) = 6.5, P < 0.05], the 0.01 μg of Ex4 suppressed chow intake relative to saline (P < 0.05). There were no effects at 2 or 4 h after dark onset. The significant effect of intra-LS Ex4 at 20 h [F(2,16) = 22.5, P < 0.01] was driven by intake suppression at the 0.025 μg Ex4 dose (P < 0.01) (Fig. 4A). LS Ex4 reduced body weight overnight [F(2,16) = 25.7, P < 0.01] (weight change: vehicle, −3.7 ± 1.7 g; 0.01 μg Ex4, 0.7 ± 1.3 g; 0.025 μg Ex4, −11.6 ± 1.4 g).
Fig. 4.
A: intra-LS injection of Ex4 reduced chow intake at 1 and 20 h after dark onset. *P < 0.05. B: LS Ex9 injection increased chow intake. *P < 0.05.
As shown in Fig. 4B, intra-LS injection of Ex9 significantly increased chow intake at 2, 4, and 20 h. At 2 h [F(2,12) = 7.688, P < 0.01], 10.0 μg Ex9 effectively increased chow intake (P < 0.01), and at 4 h [F(2,12) = 43.87, P < 0.0001] and 20 h after dark onset [F(2,12) = 7.494, P < 0.05], both the 5.0 and 10.0 μg doses of Ex9 significantly increased chow intake (P < 0.05). There was no effect on overnight body weight change.
Intra-CPu injection of 0.025 μg Ex4 had no effects on chow intake at 1, 2, or 4 h after dark onset. At 20 h after dark onset, there was still no effect on chow intake (vehicle, 82.26 ± 5.4 kcal; Ex4, 80.1 ± 2.63 kcal; P = 0.33). Intra-CPu injection of Ex4 also had no effect on overnight body weight change relative to vehicle injection.
Study 3: effects of LS GLP-1R stimulation on kaolin and chow intake.
Throughout the experiment, rats consumed little to no kaolin (mean = <0.5 g/day), and intra-LS GLP-1R stimulation did not increase kaolin intake. Repeated-measures ANOVA showed a significant Ex4 × time interaction [F(19,133) = 2.394; P < 0.05], where Ex4 suppressed cumulative chow intake at 20 h [P < 0.05] (Fig. 5A). There was no effect on body weight in this experiment. During the dark phase after LS Ex4 injection, there was a trend toward a decrease in average dark-phase meal size (P = 0.09) (Fig. 5B) and no effect on meal number (Fig. 5C). Likewise, during the light phase after LS Ex4 injection, there was a trend toward a decrease in average light-phase meal size (P = 0.051), and no effect on meal number. The LiCl-positive control strongly promoted consumption of kaolin in the same subjects (mean 20-h kaolin intake = 4.1 ± 1.9 g).
Fig. 5.
A: intra-LS Ex4 suppressed overnight chow intake but did not induce kaolin intake. *P < 0.05. LS Ex4 did not significantly affect average dark cycle meal size (B) or meal number (C).
Study 4: effects of LS GLP-1R stimulation or blockade on HFD intake.
Throughout this experiment, rats consumed little low-fat food (mean = <1 g/day), and this was not affected by drug treatment. LS Ex4 significantly suppressed cumulative HFD intake over the 12-h dark cycle relative to vehicle treatment, and this effect was maintained throughout the next 12-h light cycle, during which rats ate nearly no food regardless of treatment condition. On the basis of this pattern, we analyzed intake during the dark phase. There was a significant Ex4 × time interaction [F(11,77) = 9.261, P < 0.001], with significant Ex4-induced suppression of intake beginning at 9 h into the dark phase and continuing for the remainder of the dark cycle (P < 0.05) (Fig. 6A). At 20 h postinjection, LS Ex4 suppressed body weight relative to vehicle treatment [t(7) = 3.89, P < 0.05] (20-h body weight change: vehicle: 3.4 ± 0.8 g vs. 0.025 μg; Ex4: −5.7 ± 2.2 g). For Ex9, there was also a drug × time interaction [F(11,77) = 3.078; P < 0.01], with significant increases in intake at 2, 4, 6, and 7 h after dark onset (P < 0.05) (Fig. 6A). There was no drug effect on body weight change overnight after injections.
Fig. 6.
A: Ex4 injection into the LS decreased HFD intake and LS Ex9 increased HFD intake over the dark cycle. *P < 0.05. B: intra-LS Ex4 decreased average meal size over the dark cycle. C: intra-LS Ex4 also reduced the number of meals consumed over the dark phase. *P < 0.05.
LS Ex4 suppressed average meal size over the 12-h dark cycle [t(7) = 2.839; P < 0.05] relative to vehicle (Fig. 6B). Intra-LS Ex4 also reduced the number of meals taken during the dark phase [t(7) = 2.65; P < 0.05] (Fig. 6C). Blockade of GLP-1R with Ex9 had no significant effects on meal pattern during the dark cycle (Fig. 6, B and C), and there was only a trend toward an increase in average meal size during the first 7 h of dark, when cumulative intake was increased (vehicle, 13.91 ± 1.12 kcal; Ex9, 15.92 ± 1.65 kcal, P = 0.18).
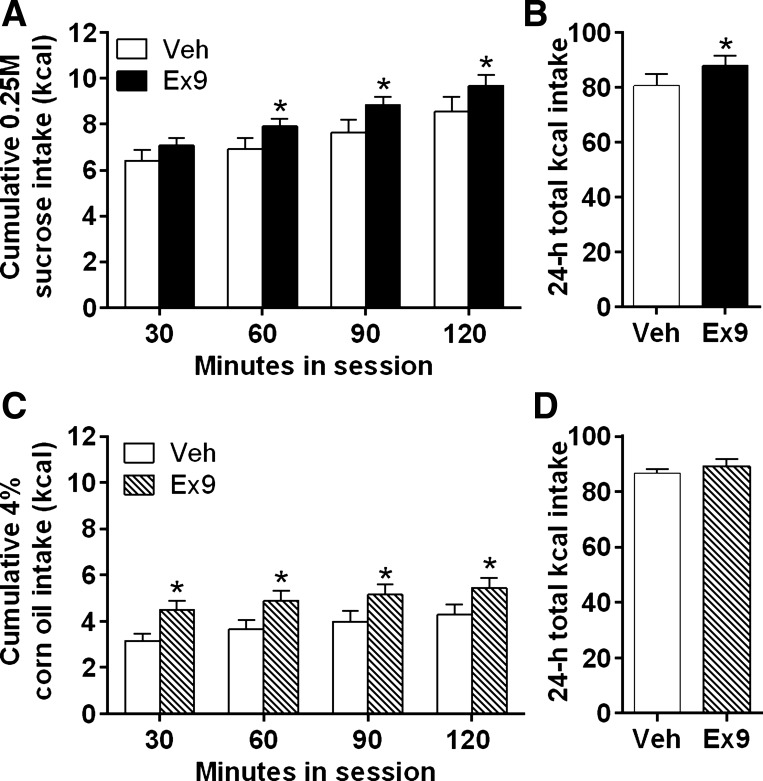
Study 5: effects of LS GLP-1R blockade on sucrose and corn oil intake.
Intra-LS Ex9 injection elevated sucrose intake during the 2-h test session [F(1,19) = 5.862, P < 0.05], with significant effects at 60, 90, and 120 min postinjection (P < 0.05) (Fig. 7A). Intra-LS Ex9 injection did not affect chow intake at 24 h; however, total kilocalories consumed (from sucrose plus chow) was significantly increased by Ex9 relative to vehicle [t(19) = 1.73, P < 0.05] (Fig. 7B). Ex9 did not affect body weight in this experiment. In rats with incorrect cannula placements, such that injections were made into the LV (n = 6), there were no effects of Ex9 (2-h sucrose intake: vehicle, 10.66 ± 1.83 kcal; Ex9, 10.56 ± 1.81 kcal; P = 0.46), confirming that the 10-μg dose of Ex9 is subthreshold for effect when delivered to the ventricle.
Fig. 7.
A: blockade of LS GLP-1R by Ex9 increased cumulative 0.25 M sucrose intake over a 2-h session. *P < 0.05. B: intra-LS Ex9 injection significantly increased total overnight kilocalorie intake (kilocalories from chow plus kilocalories from sucrose intake during 2-h session). *P < 0.05. C: LS Ex9 increased 4% corn oil intake over a 2-h session. *P < 0.05. D: intra-LS Ex9 injection did not affect 24-h total kilocalorie intake following the 4% corn oil session.
Intra-LS injection of Ex9 increased 4% corn oil emulsion intake during the 2-h test [F(1,19) = 30.8; P < 0.01], with significant effects at 30, 60, 90, and 120 min postinjection (P < 0.05) (Fig. 7C), but no effects on subsequent chow and kcal intake (Fig. 7D). Ex9 did not affect body weight in this experiment.
Study 6: effects of LS GLP-1R stimulation on behavior in the elevated plus maze.
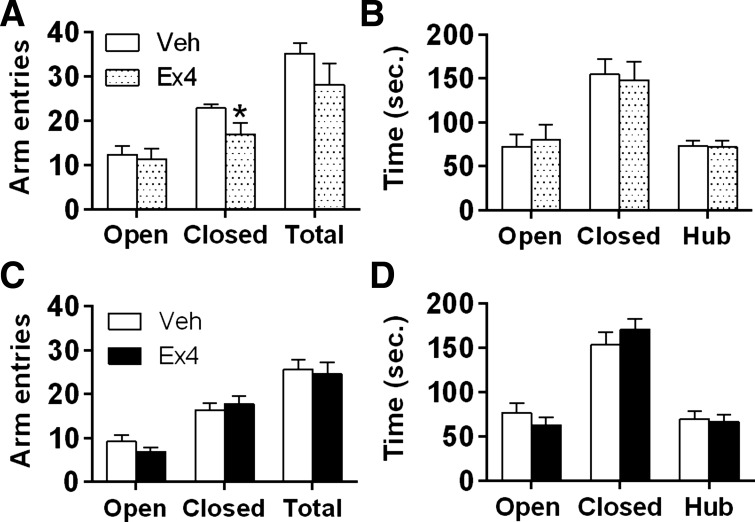
Ex4 given 45 min before testing had no effect on total arm entries or entries into open arms, but did significantly decrease the number of entries into closed arms [t(10) = 2.197; P < 0.05] (Fig. 8A). Intra-LS Ex4 delivered 45 min before EPM had no effect on the time spent in the EPM center zone (hub), open arms, or closed arms (Fig. 8B). When Ex4 was injected into the LS 8 h before EPM, there were no effects on any behavior measured in the test (Fig. 8, C and D). Regardless of injection time, LS Ex4 significantly suppressed overnight chow intake and body weight (EPM test 45 min postinjection: 20-h chow intake after vehicle, 72.5 ± 4.8 g vs. Ex4, 50.4 ± 11.5 kcal; 20 h body weight change after vehicle, 1.4 ± 1.6 g vs. Ex4, −11.2 ± 7.3 g) (EPM test 8 h postinjection: 20-h chow intake after vehicle, 74.6 ± 3.9 kcal vs. Ex4, 57.1 ± 5.1 kcal; 20-h body weight change after vehicle, −2.7 ± 2.3 g vs. Ex4, −7.6 ± 2.9 g) (P < 0.05).
Fig. 8.
Activation of LS GLP-1R with Ex4 did not affect anxiety-like behavior in the elevated plus maze (EPM). A: LS Ex4 suppressed arm entries into the closed arm 45 min postinjection. *P < 0.05. B: intra-LS Ex4 had no effect on time spent in either arm when assessed 45 min postinjection. LS Ex4 did not affect arm entries (C) or time spent in either arm (D) when measured 8 h postinjection.
Study 7: effects of iLS GLP-1R blockade on operant responding.
To focus on the role of GLP-1R in the intermediate subdivision of the LS specifically, because most of our LS placements in the food intake studies above were located there, we excluded one rat that was injected into the dLS from this PR study. We excluded a second rat that was injected into the iLS but was an outlier (more than two SDs above the mean) for active lever presses and breakpoint (e.g., active lever presses after vehicle, 196; Ex9, 522) under all conditions. Exclusion of this outlier did not change the outcome of the experiment, but it reduces variability.
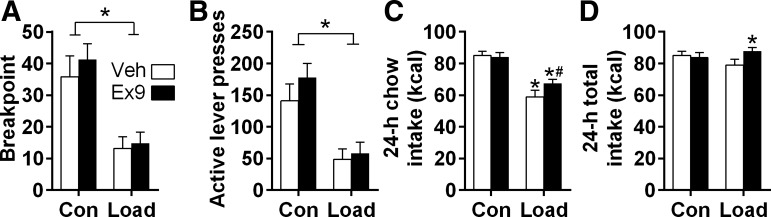
There were no significant main effects of LS Ex9 nor interactions between drug and preload condition for breakpoint (the last completed ratio of the session), active lever presses (Fig. 9, A and B), or number of reinforcers earned (control vehicle, 9.67 ± 0.67 pellets vs. control Ex9, 10.33 ± 0.46 pellets, P = 0.12). The Ensure preload strongly suppressed break point [F(1,5) = 16.5; P < 0.01], active lever presses [F(1,5) = 22.59, P < 0.01] (Fig. 9, A and B), and number of reinforcers earned [F(1,5) = 24.59, P < 0.01] (load vehicle, 5.83 ± 1.03 pellets vs. load Ex9, 6.33 ± 0.96 pellets, P = 0.36) (post hoc P < 0.01).
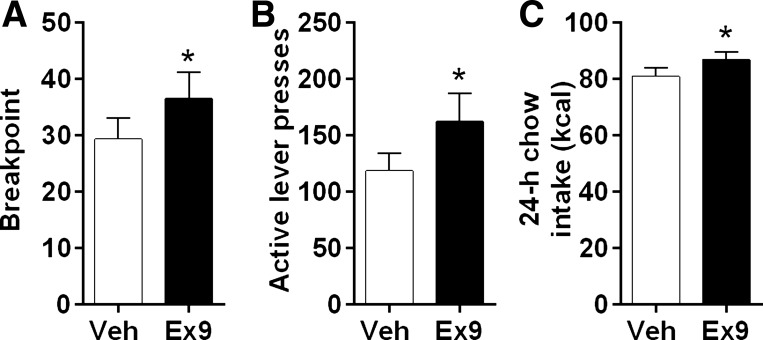
Fig. 9.
A–D: in rats responding on a progressive ratio (PR) schedule of reinforcement, intra-LS Ex9 did not affect the number of sucrose reinforcers earned or total active lever presses in both the nonpreload (Con) and Ensure (load) condition. The Ensure load significantly reduced the breakpoint and total active lever presses relative to the control condition. *P < 0.05 relative to Con. C: Ensure load reduced chow 24-h chow intake relative to the control no-preload condition. *P < 0.01 relative to Con. Intra-LS Ex9 injection attenuated this effect on 24-h chow intake. #P < 0.01 load Veh vs. load Ex9. D: after Ensure load, LS blockade of GLP-1R by Ex9 injection increased 24-h total kilocalorie intake.
Overnight chow intake was suppressed by the preload [F(1,5) = 39.9; P < 0.01], and there was a significant interaction with Ex9 [F(1,5) = 7.95; P < 0.01], such that intra-LS Ex9 injection attenuated the preload-induced intake suppression (P < 0.05) (Fig. 9C). We also assessed effects on total kilocalories consumed, which was exclusively from chow in the no-load condition but includes both Ensure and chow in the preload condition. Here, we observed an interaction between Ex9 and preload [F(1,5) = 6.92; P < 0.05], in which rats failed to compensate for the preload after intra-LS Ex9 and, thus, consumed significantly more kilocalories on that day (P < 0.05) (Fig. 9D). There was no effect on overnight body weight change in this experiment.
Study 8: effects of dLS GLP-1R blockade on operant responding.
Intra-dLS injection of Ex9 significantly increased breakpoint [t(12) = 2.33; P < 0.05], total active lever presses [t(12) = 2.52; P < 0.05] (Fig. 10, A and B), and number of reinforcers earned [t(12) = 2.54, P < 0.05] (vehicle, 8.84 ± 0.54 pellets vs. Ex9, 9.61 ± 0.55 pellets) during the PR session, as well as overnight chow intake [t(12) = 1.98, P < 0.05] following the PR session (Fig. 10C). There was no effect on overnight body weight change in this experiment.
Fig. 10.
In rats responding on a PR schedule of reinforcement, intra-dLS Ex9 significantly increased breakpoint and total active lever presses (A and B), as well as overnight chow intake (C). *P < 0.05.
DISCUSSION
Here, we demonstrated GLP-1R binding throughout the rat LS using a fluorescently labeled GLP-1R agonist, Ex4. Our behavioral data demonstrate that these LS GLP-1Rs play a role in feeding behavior. Pharmacological activation of LS GLP-1R, at doses that were ineffective when delivered to the LV, suppressed both overnight chow and HFD intake. This decrease in HFD intake after intra-LS Ex4 was a result of reductions in meal size and meal number, suggesting that GLP-1R in the LS may influence satiation and satiety. Intra-LS injection of ventricle-subthreshold doses of the GLP-1R antagonist Ex9 significantly increased chow, HFD, 0.25 M sucrose, and 4% corn oil emulsion intake. The effect on HFD may be mediated by an increase in meal size, although our analysis was limited by the relatively short duration of the Ex9 effect. Together, these findings suggest that endogenous GLP-1R stimulation in the LS plays a physiological role in limiting chow intake under normal feeding conditions. Although the presence of GLP-1 fibers and receptors in the LS was previously documented, this is the first demonstration of a role for this pathway in feeding behavior (10, 23, 29).
Signs of visceral illness and conditioned taste aversion have been exhibited in rodents after Ex4 or GLP-1 treatment (14, 35), and in humans, nausea is the most common side effect in patients receiving exenatide or liraglutide treatment (6). However, in rats, GLP-1 and Ex4 can suppress feeding without evidence of nausea when delivered to the VTA, nucleus accumbens, and lateral parabrachial nucleus (1, 2, 7, 8). Here, we found that intra-LS injection of Ex4, at a dose that significantly suppressed chow and HFD intake, did not induce kaolin intake, suggesting that the intake-suppressive effects of LS GLP-1R activation are not due to nausea.
Although the LS is known to play a role in anxiety-related behaviors (3), and central GLP-1 signaling has been shown to activate HPA axis stress responses (9), we found that LS GLP-1R activation does not increase anxiety-like behavior measured on the EPM. This is consistent with another recent report examining effects of LS GLP-1R in mice (13). Our finding supports the idea that an effect on anxiety or stress does not underlie the intake suppression observed after intra-LS GLP-1R stimulation. However, GLP-1 neurons do seem to play a role in the response to stress (20, 21), and LS GLP-1R may contribute to stress-induced anorexia. Further studies are necessary to evaluate this possibility.
Because the LS plays a role in drug reward (13, 32), we hypothesized that LS GLP-1R may be involved in food reward. We used the PR-operant responding task to assess motivation for sucrose and found that Ex9 administered to the iLS, at a dose that reliably increased food intake, failed to affect responding. We speculated that because the PR sessions occurred during the mid-light phase when rats would not have been eating large amounts of food, GLP-1 neurons may not be strongly stimulated at this time, leaving little endogenous GLP-1 in the LS for Ex9 to block. To increase the likelihood of GLP-1 neuron activation and GLP-1 release in the LS, we performed the same PR test after rats consumed a large Ensure preload (17, 38). This preload strongly suppressed PR responding, but LS Ex9 still had no effect. Although there is a trend toward an increase in PR responding after Ex9 treatment in the control (nonpreload) condition, it failed to reach significance. Thus, these results suggest that endogenous GLP-1 in the iLS does not affect motivation to obtain sucrose, at least under our test conditions.
Previous data suggest a selective role for the dLS subregion in motivation (19). Therefore, we hypothesized that while iLS GLP-1R activity may not affect motivation, dLS GLP-1 would do so. We tested this by examining the effect of dLS GLP-1R blockade with the antagonist Ex9 on operant responding for sucrose pellets on a PR schedule employing the same design that was used for the nonpreload condition of the experiment targeting the iLS. Here, we found that intra-dLS Ex9 significantly increased lever presses for sucrose pellets, total reinforcers earned, and breakpoint during the PR session, as well as overnight chow intake relative to vehicle. Together, these data support the idea that GLP-1R in both of these divisions of the LS influence feeding, and GLP-1Rs in the dLS, in particular, play a role in motivation.
Although intra-LS Ex9 treatment increased overnight food intake in most of our studies, it failed to do so when delivered to the iLS prior to the PR test sessions conducted with no nutrient load. We initially hypothesized that this could be due to the relatively low gastrointestinal nutrient stimulation during the first few hours after injection in this situation. In the other studies in which we saw a significant effect of Ex9 on feeding, rats had ad libitum access to food during the first few hours after treatment, and the bulk of the Ex9 effect occurred during that time. The results obtained after the Ensure preload support the idea that nutrient stimulation is required for the effect of iLS Ex9 on later intake. Unsurprisingly, we found that the Ensure preload suppressed 24-h chow intake relative to the no-preload condition. However, intra-iLS Ex9 treatment significantly attenuated this compensatory reduction in chow intake after the preload. We suggest that the LS GLP-1R population receives meal-related information through the GLP-1 projection from the NTS and that GLP-1 action in the LS during and after meals may play a role in limiting subsequent food intake. This is consistent with other reports that neurons in the LS are responsive to gastrointestinal signals, including gastric distention (11, 12).
It is already well established that central GLP-1 plays a role in the control of feeding behavior, but the specific populations of GLP-1R that contribute to feeding behavior and the behavioral mechanisms through which these receptors work, have not been fully elucidated. Previous work from our laboratory and others have demonstrated that GLP-1R populations in different brain regions, and even neighboring subregions of the same brain nuclei, can have distinct behavioral effects (2, 8, 33). In some brain areas, GLP-1Rs appear to affect glucose homeostasis (31) and produce a conditioned taste aversion (16) without affecting food intake. Here, we identify a new role for LS GLP-1R in the control of food intake, and we find subregion specificity for the effect on motivation for food. Together, the previous studies and our current data suggest that GLP-1R populations throughout the CNS contribute in the control of feeding behavior and energy balance though a variety of mechanisms.
Perspectives and Significance
Overall, these data substantiate a role for the LS in the physiological control of feeding behavior. The LS has not traditionally been considered to play an important role in the homeostatic control of food intake, but here, we show that GLP-1R activation in the LS influences feeding, and endogenous LS GLP-1R stimulation limits chow, HFD, sucrose solution, and corn oil intake, as well as motivation for sucrose pellets. The LS has reciprocal connections with a broad array of other brain regions, including feeding-relevant areas, such as the LH and VTA, and connections differ across subregions of the LS. Thus, there are a number of efferent pathways out of the LS that may mediate effects on feeding. LS cells are primarily GABAergic; therefore, we speculate that projections from the LS inhibit orexigenic neurons, which ultimately leads to suppression of feeding behavior. The diversity of neuronal projections to the LS and receptors expressed by LS neurons also raises the possibility that feeding behavior is influenced by interactions among a variety of neurotransmitters and neuropeptides at this site (e.g., GLP-1, CRH, ghrelin, and GABA). Additional studies will be necessary to elucidate the neural circuitry that mediates the effects of LS GLP-1R stimulation observed here, as well as the role of the LS in feeding behavior more generally.
GRANTS
This work was funded by the National Institutes of Health Grant R01-DK-095757 to D. L. Williams.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.T. and D.L.W. conception and design of research; S.J.T., C.M.J., H.E.G., N.L., C.B.M., and S.V. performed experiments; S.J.T. and D.L.W. analyzed data; S.J.T. and D.L.W. interpreted results of experiments; S.J.T. and D.L.W. prepared figures; S.J.T. drafted manuscript; S.J.T. and D.L.W. edited and revised manuscript; S.J.T., C.M.J., H.E.G., N.L., C.B.M., S.V., and D.L.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Charles Badland for his assistance with figure production and Ruth Didier for her assistance with acquiring images using the Zeiss LSM 880 at Florida State University College of Medicine. We also thank the reviewers for their helpful suggestions on the first submission of this manuscript.
REFERENCES
- 1.Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 39: 2233–2243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, Anderson DJ. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 156: 522–536, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakshi VP, Newman SM, Smith-Roe S, Jochman a K, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci 27: 10,568–10,577, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrera JG, Jones KR, Herman JP, D'Alessio a D, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calara F, Taylor K, Han J, Zabala E, Carr EM, Wintle M, Fineman M. A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic exendin-4). Clin Ther 27: 210–215, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14,453–14,457, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav 122: 201–207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Gong Y, Xu L, Guo F, Pang M, Shi Z, Gao S, Sun X. Effects of ghrelin on gastric distension sensitive neurons and gastric motility in the lateral septum and arcuate nucleus regulation. J Gastroenterol 49: 219–230, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Xu L, Wang H, Guo F, Sun X, Gao S. Involvements of the lateral hypothalamic area in gastric motility and its regulation by the lateral septum. Gen Comp Endocrinol 194: 275–285, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology 40: 1969–1978, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62: 1916–1927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay K, Parise EM, Lilly N, Williams DL. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl) 231: 419–427, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinzig KP, D'Alessio a D, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10,470–10,476, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreisler AD, Davis a E, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav 136: 47–54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen P, Tang-Christensen M, Holst J, Ørskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Luo AH, Tahsili-Fahadan P, Wise a R, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333: 353–357, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing peptide or glucagon-like peptide 1. Front Neurosci 6: 1–17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci 35: 10,701–10,714, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274: R23–R29, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Mitra A, Lenglos C, Timofeeva E. Inhibition in the lateral septum increases sucrose intake and decreases anorectic effects of stress. Eur J Neurosci 41: 420–433, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47: 419–427, 1954. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC, Hayes MR. Astrocytes regulate GLP-1 receptor-mediated effects on energy balance. J Neurosci 36: 3531–3540, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev 24: 115–195, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Sandoval a D, Bagnol D, Woods SC, Alessio a D D, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci 32: 4623–4631, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schick RR, Zimmermann JP, Walde vorm T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 284: R1427–R1435, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Scopinho a a Resstel LBM, Corrêa a FM. α1-Adrenoceptors in the lateral septal area modulate food intake behaviour in rats. Br J Pharmacol 155: 752–756, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, Alessio DD. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci 7: 181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285: R470–R478, 2003. [DOI] [PubMed] [Google Scholar]