Abstract
An increasing number of studies have linked high dietary phosphate (Pi) intake to hypertension. It is well established that the rise in sympathetic nerve activity (SNA) and blood pressure (BP) during physical exertion is exaggerated in many forms of hypertension, which are primarily mediated by an overactive skeletal muscle exercise pressor reflex (EPR). However, it remains unknown whether high dietary Pi intake potentiates the EPR-mediated SNA and BP response to exercise. Accordingly, we measured renal SNA (RSNA) and mean BP (MBP) in normotensive Sprague-Dawley rats fed a normal Pi diet (0.6%, n = 13) or high Pi diet (1.2%, n = 13) for 3 mo. As previously reported, we found that resting BP was significantly increased by 1.2% Pi diet in both conscious and anesthetized animals. Activation of the EPR by electrically induced hindlimb contraction triggered greater increases in ΔRSNA and ΔMBP in the 1.2% compared with 0.6% Pi group (126 ± 25 vs. 42 ± 9%; 44 ± 5 vs. 14 ± 2 mmHg, respectively, P < 0.01). Activation of the muscle mechanoreflex, a component of the EPR, by passively stretching hindlimb muscle also evoked greater increases in ΔRSNA and ΔMBP in the 1.2% compared with 0.6% Pi group (109 ± 27 vs. 24 ± 7%, 38 ± 7 vs. 8 ± 2 mmHg, respectively, P < 0.01). A similar response was produced by hindlimb intra-arterial capsaicin administration to stimulate the metaboreflex arm of the EPR. Thus, our data demonstrate a novel action of dietary Pi loading in augmenting EPR function through overactivation of both the muscle mechanoreflex and metaboreflex.
Keywords: phosphate, exercise pressor reflex, blood pressure, sympathetic nerve activity, hypertension, diet, Western diet
inorganic phosphates are widely used in the food industry as preservatives, flavor enhancers, and color stabilizers (1, 46). As a result, dietary phosphate (Pi) intake in the United States far exceeds the recommended daily allowance (1). High Pi intake is proposed to contribute to vascular calcification and cardiovascular mortality in patients with chronic kidney disease (18). However, little attention has been paid to the potential role of Pi excess on the cardiovascular system in the general population with normal kidney function. A high-Pi diet was recently shown to trigger blood pressure (BP) elevation in both normotensive rats (2) and spontaneously hypertensive rats (48), both with normal kidney function, in the resting condition. However, the influence of dietary Pi on BP regulation during physical exertion has not been determined.
Studies have demonstrated a correlation between dietary Pi intake and Pi concentration in the central nervous system, which may influence brain development (14, 15) and feeding behavior (5, 49). However, the influence of dietary Pi intake on the central nervous system control of the circulation has not been determined. This is a salient point as the sympathetic nervous system, an important autonomic component of the central nervous system, is known to play a major role in the pathogenesis of hypertension (8). Moreover, patients with primary hypertension not only display elevated levels of sympathetic nerve activity (SNA) at rest, but also augmented increases in SNA and BP during exercise (6, 50). In primary hypertension, the heightened sympathetic and pressor responses are mediated, in part, by an overactive reflex originating from skeletal muscle, known as the exercise pressor reflex (EPR). The augmentation in EPR function is evoked both by thin-fiber muscle afferents associated with metaboreceptors, which are activated slowly during ischemic muscle contraction and mechanoreceptors, which respond quickly to muscle deformation (30, 32). Sensory information from these afferents is processed in central nervous system centers within the brain stem that regulate sympathetic outflow. Given the known correlation between dietary Pi intake and Pi concentration in the brain combined with the central nervous system's role in regulating EPR-mediated sympathetic outflow, it is logical to suggest that dietary Pi loading could potentially induce sympathetic overactivity during stimulation of the EPR, possibly contributing to the pathogenesis of Pi-induced hypertension. However, to date, the influence of dietary Pi on EPR function has not been elucidated.
Accordingly, we conducted studies to determine the cardiovascular and sympathetic responses to high dietary Pi loading in the normotensive Sprague-Dawley (SD) rats with normal kidney function at rest and in response to muscle contraction (i.e., EPR activation), as well as during isolated mechanoreceptor and metaboreceptor stimulation.
METHODS
Animal Models
All experiments were performed in male SD rats. The animals were housed in standard rodent cages on 12:12-h light-dark cycles and were randomly divided into either a group fed a control diet (0.6% total Pi, including 0.3% inorganic Pi, LabDiet/TestDiet 5002; St. Louis, MO, n = 13) or a group fed a high-phosphorus diet (1.2% total Pi, including 0.9% inorganic Pi, LabDiet/TestDiet modified 5002 no. 1812250, n = 13) for 3 mo (beginning at 11–12 wk of age). Both diets contained 0.8% calcium (Ca), 0.3% sodium (Na), 0.2% magnesium (Mg), and 1% potassium (K). We chose a total Pi of 0.6% to be the control diet because this proportion has been shown to be optimal for normal rodents (45). Thus, to mimic the excess Pi consumed daily by Americans, which is approximately twice the recommended daily allowance, we chose a 1.2% Pi diet for the experimental group in our study. The animals were allowed to eat ad libitum and were given free access to water. All studies were conducted in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals.” The outlined procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Plasma and Urine Biochemistry
Rats were acclimatized for 2 days in metabolic cages before 24-h urine collection, and measurement of daily food intake (difference in the weight of food provided and food recovered). Plasma and urinary Pi, Ca, -K, and Na were determined 12 wk after consumption of either a high- (1.2%) or normal- (0.6%) Pi diet, using methods previously described (12, 34). Plasma creatinine concentrations were measured using a P/ACE MDQ Capillary Electrophoresis System and photodiode detector (Beckman-Coulter, Fullerton, CA) at 214 nm (52).
Measurement of Blood Pressure in Conscious Animals
Two to three days prior to surgical procedures below, systolic arterial pressure was assessed in the conscious state by tail cuff in a subset of animals in each group (n = 10), using a CODA blood pressure system (Kent Scientific). Animals were acclimated to the measurement procedure and trained for 2 wk by being placed in a restraining chamber and inflating the blood pressure cuff several times.
Experimental Protocols
General surgical procedures.
After consumption of either a high- (1.2%) or normal- (0.6%) Pi diet for 12 wk, rats were anesthetized with 1–4% isoflurane in oxygen and intubated for mechanical ventilation, as described previously (29). Arterial blood pressure was continuously measured by a pressure transducer connected to a left carotid arterial catheter. To obtain electrocardiograph recordings, needle electrodes were placed on the back of the animal. Heart rate (HR) was calculated from the time between successive R waves. To measure RSNA, a branch of the renal nerve was exposed and attached to a pair of stainless-steel wire electrodes. The nerve and electrodes were covered with silicone glue for insulation and fixation. The preamplified nerve signal was band-pass filtered at 150–1,000 Hz then full-wave rectified and low-pass filtered with a cutoff frequency of 30 Hz. Animals were held in a stereotaxic head unit and then, for rendering the animals insentient, a precollicular decerebrate procedure was performed. Immediately after the decerebration, isoflurane anesthesia was discontinued.
Procedures to activate the EPR.
A laminectomy exposing the lower lumbar portions of the spinal cord (L2–L6) was performed, as described previously (29, 40). The L4 and L5 ventral roots were carefully isolated and sectioned. The cut peripheral ends of the roots were placed on bipolar electrodes. The gastrocnemius and soleus muscles of the right hindlimb were isolated. The calcaneal bone of the right hindlimb was cut and the Achilles tendon connected to a force transducer for the measurement of muscle tension. To activate the EPR, the gastrocnemius and soleus muscles were contracted by electrically stimulating the L4 and L5 ventral roots for 30 s using constant current stimulation at 3 times motor threshold with a pulse duration of 0.1 ms at 40 Hz.
Procedures to activate the mechanically sensitive component of the EPR.
To evoke a mechanical stimulus similar to that elicited during muscle contraction, the gastrocnemius and soleus muscles of the right hindlimb were passively stretched for 30 s using a rack-and-pinion system (30, 32). Care was taken to manually generate the same pattern, as well as the magnitude of muscle tension developed during static muscle contractions. This maneuver was used to selectively activate the mechanoreceptors associated with the mechanically sensitive afferent fibers of the skeletal muscle mechanoreflex.
Procedures to activate the chemically sensitive component of the EPR.
Selective activation of chemically sensitive metaboreceptors and associated skeletal muscle afferent fibers was achieved by injecting capsaicin into the arterial supply of the right hindlimb (0.3 μg/100 μl). Capsaicin was administered into the right common iliac artery, while a reversible ligature placed around the right common iliac vein was occluded for 2 min. This maneuver was used to selectively activate the afferent fibers associated with the skeletal muscle metaboreflex (30, 32).
End experiment procedures.
To validate that RSNA signals were recorded from postganglionic renal sympathetic fibers, an intravenous infusion of hexamethonium bromide (60 mg/kg) was given to abolish SNA at the end of experiments. RSNA background noise was determined over a 30-min period after the insentient decerebrated animal was humanely killed by intravenous injection of saturated potassium chloride (4 M, 2 ml/kg). The heart and lungs were excised and weighed. Further, tibial length was measured.
Data Acquisition
Mean arterial pressure (MAP), HR, RSNA, and contractile tension data were acquired, recorded, and analyzed using data acquisition software (LabChart, ADInstruments) for the PowerLab analog-to-digital convertor (PowerLab8/30, ADInstruments) at a 1-kHz sampling rate. To analyze RSNA, full-wave rectified signals of RSNA, as well as background noise signals, were obtained. The noise signal component, which was defined as the signal recorded post mortem, was subtracted from rectified RSNA. To quantify RSNA responses to EPR stimulation, baseline values were calculated by averaging 30 s of data immediately prior to the onset of stimulation and were considered 100% of basal RSNA. Subsequently, changes in RSNA were expressed as a percentage of baseline, and the relative changes in RSNA (ΔRSNA, %) from baseline were evaluated. Data sets of 1-s averages for MAP, HR, RSNA, and hindlimb tension were analyzed. Baseline values for all variables were determined by evaluating 30 s of recorded data before a muscle contraction. The peak response of each variable was defined as the greatest change from baseline elicited by contraction.
Statistical Analyses
Data were analyzed using unpaired t-tests to identify differences between specific group means. The significance level was set at P < 0.05. Results are presented as means ± SE.
RESULTS
Morphometric characteristics, biochemical changes, as well as baseline hemodynamics, are presented in Table 1. The heart weight-to-body weight ratio tended to be higher in the 1.2% Pi than the 0.6% Pi diet group, but the difference did not reach statistical significance (2.89 ± 0.10 vs. 2.67 ± 0.04 mg/g, respectively, P = 0.051). There were no significant differences in the heart weight-to-tibial length ratio or the lung weight-to-body weight ratio between the two groups. Twenty-four hour urinary excretion of calcium (Ca), sodium (Na), and potassium (K) were not significantly different between the two groups. Twenty-four-hour urinary Pi excretion in the 1.2% Pi group was markedly higher compared with the 0.6% Pi group (77 ± 5 vs. 12 ± 1 mg/day, respectively, P < 0.01). There was no significant difference in serum creatinine or creatinine clearance between the two groups (0.35 ± 0.01 vs. 0.37 ± 0.01 mg/dl, respectively, P = 0.3 and 2.83 ± 0.17 vs. 2.64 ± 0.30 ml/min, respectively, P = 0.6). Additionally, there were no differences in urine volume, food intake, serum Pi, Ca, Na, and K between the two groups (all P values > 0.1).
Table 1.
Morphometric characteristics and 24-hour urine and baseline hemodynamics
| 0.6% Pi | 1.2% Pi | |
|---|---|---|
| Number of animals | 13 | 13 |
| Body weight, g | 492 ± 9 | 496 ± 11 |
| Heart weight/body weight, mg/g | 2.67 ± 0.04 | 2.89 ± 0.10 |
| Heart weight/tibial length, mg/mm | 30.9 ± 0.7 | 33.7 ± 1.5 |
| Lung weight/body weight, mg/g | 4.86 ± 0.19 | 4.87 ± 0.23 |
| Food intake, g/day | 19.8 ± 0.7 | 19.0 ± 1.7 |
| Serum Levels | ||
| Creatinine, mg/dl | 0.37 ± 0.01 | 0.35 ± 0.01 |
| Calcium, mg/dl | 9.31 ± 0.10 | 9.46 ± 0.08 |
| Sodium, mmol/l | 132 ± 0.46 | 132 ± 0.27 |
| Potassium, mmol/l | 3.98 ± 0.06 | 4.03 ± 0.06 |
| Phosphate, mg/dl | 3.70 ± 0.10 | 3.76 ± 0.08 |
| 24-hour urinary excretion | ||
| Urine volume, ml/day | 16.5 ± 1.4 | 13.6 ± 1.3 |
| Creatinine, mg/day | 14.9 ± 0.8 | 13.0 ± 1.2 |
| Calcium, mg/day | 1.1 ± 0.1 | 0.8 ± 0.1 |
| Sodium, mmol/day | 3.1 ± 0.2 | 2.3 ± 0.2 |
| Potassium, mmol/day | 4.6 ± 0.2 | 5.1 ± 0.2 |
| Phosphate, mg/day | 12 ± 1 | 77 ± 5* |
| Creatinine clearance, ml/min | 2.83 ± 0.17 | 2.64 ± 0.30 |
| Hemodynamic variables | ||
| Tail cuff systolic BP under anesthesia, mmHg | 95 ± 2 | 109 ± 3* |
| Mean BP, mmHg | 93 ± 4 | 120 ± 5* |
| HR, beats/min | 333 ± 9 | 355 ± 5† |
| Baseline RSNA to noise ratio | 5.5 ± 1.1 | 4.0 ± 0.6 |
| Tail cuff systolic BP after decerebration | ||
| Mean BP, mmHg | 96 ± 6 | 100 ± 7 |
| HR, beats/min | 410 ± 21 | 400 ± 12 |
| Baseline RSNA to noise ratio | 5.2 ± 1.4 | 6.4 ± 1.3 |
Values are expressed as means ± SE BP, blood pressure; HR, heart rate; RSNA, renal sympathetic nerve activity.
P < 0.01,
P < 0.05 compared with 0.6% Pi.
Intake of a high Pi diet for 3 mo significantly increased tail cuff systolic BP compared with consumption of a normal Pi diet (109 ± 3 vs. 95 ± 2 mmHg, respectively, P < 0.001). Similarly, resting mean BP (120 ± 5 vs. 93 ± 4 mmHg, P < 0.01), as well as HR (355 ± 5 vs. 333 ± 9 beats/min, P < 0.05) under 1% isoflurane anesthesia was significantly higher in the 1.2% Pi group compared with the 0.6% Pi group. After decerebration, however, there were no significant differences in baseline hemodynamics between groups. The baseline SNA-to-noise ratio was also not different between the two groups.
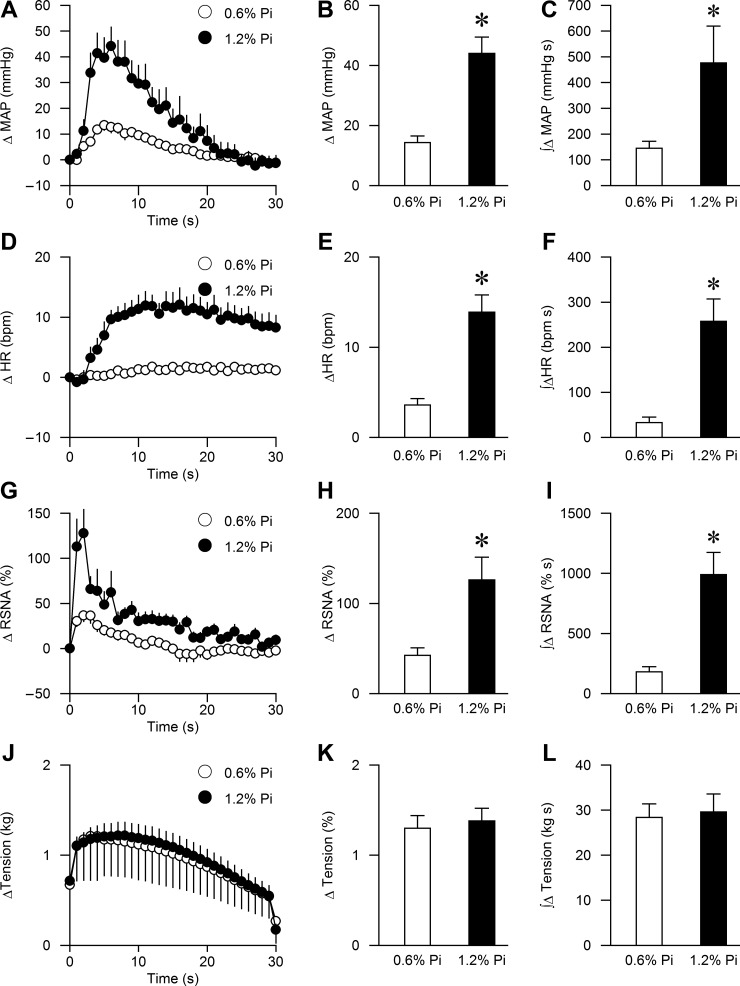
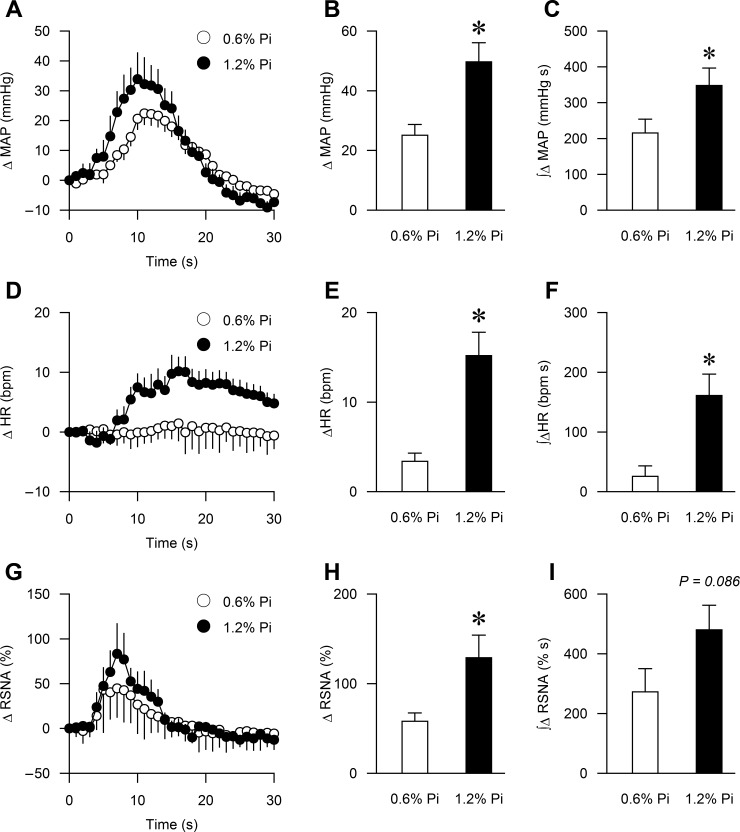
The peak BP, HR, and RSNA responses to activation of the EPR during static muscle contraction were significantly augmented in the high Pi group compared with the control group (44 ± 5 vs. 14 ± 2 mmHg, P < 0.01; 14 ± 2 vs. 4 ± 1 bpm, P < 0.01; 126 ± 25 vs. 42 ± 9%, P < 0.01, respectively, Fig. 1 and Fig. 2, B, E, H, and K). Importantly, muscle tension developed during ventral root stimulation was similar between the two groups (1.3 ± 0.1 vs. 1.4 ± 0.1 kg, respectively, P > 0.1, Figs. 1 and 2). The integrated changes in MAP, HR, RSNA, and tension presented as area under the curve (AUC) over 30 s demonstrated similar results (Fig. 2 C, F, I, and L).
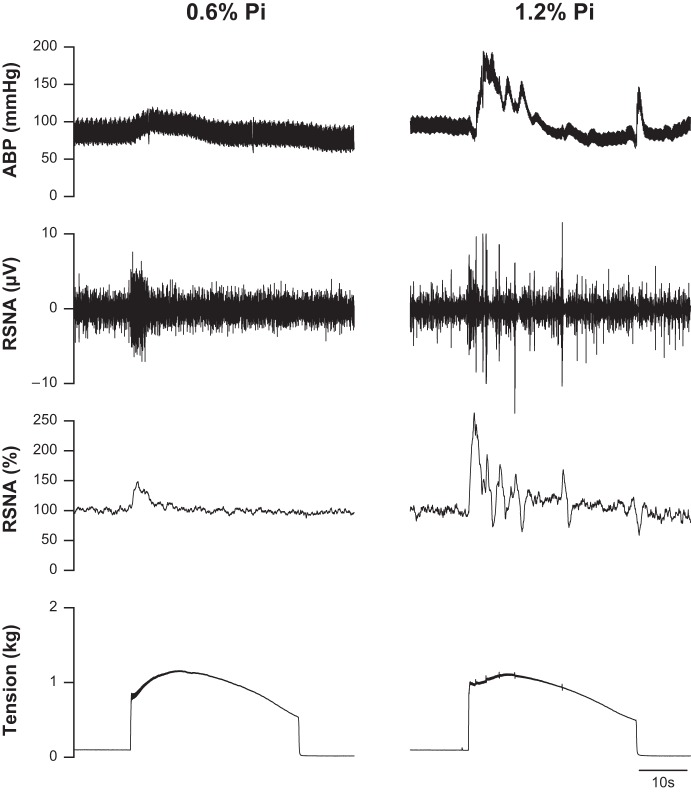
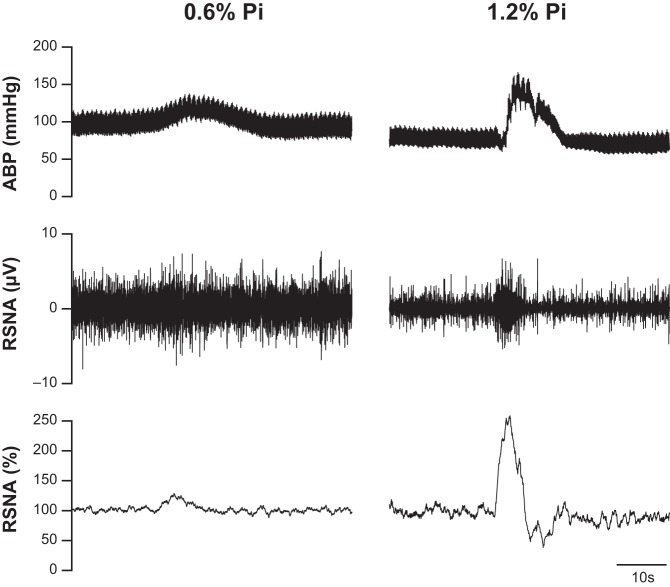
Fig. 1.
Characteristic cardiovascular and sympathetic responses during static muscle contraction in representative Sprague-Dawley (SD) rats fed with 0.6% dietary phosphate (Pi) (left) and 1.2% Pi (right) for 12 wk. The contraction-induced increases in arterial blood pressure (ABP), as well as raw and normalized renal sympathetic nerve activity (RSNA, %) were larger in the SD rat treated with 1.2% Pi compared with 0.6% Pi diet.
Fig. 2.
Summary data showing cardiovascular and sympathetic responses to activation of the exercise pressor reflex (EPR) during static muscle contraction in the 0.6% Pi (n = 13) and 1.2% Pi (n = 13) rats. The time course of changes in mean arterial pressure (MAP), heart rate (HR), renal sympathetic nerve activity (RSNA), and muscle tension are shown in A, D, G, and J. The peak MAP, HR, RSNA, and muscle tension responses to muscle contraction are shown in B, E, H, and K. The integrated changes in MAP, HR, RSNA, and muscle tension presented as area under the curve (AUC) over 30 s are shown in C, F, I, and L. *P < 0.01 compared with 0.6% Pi.
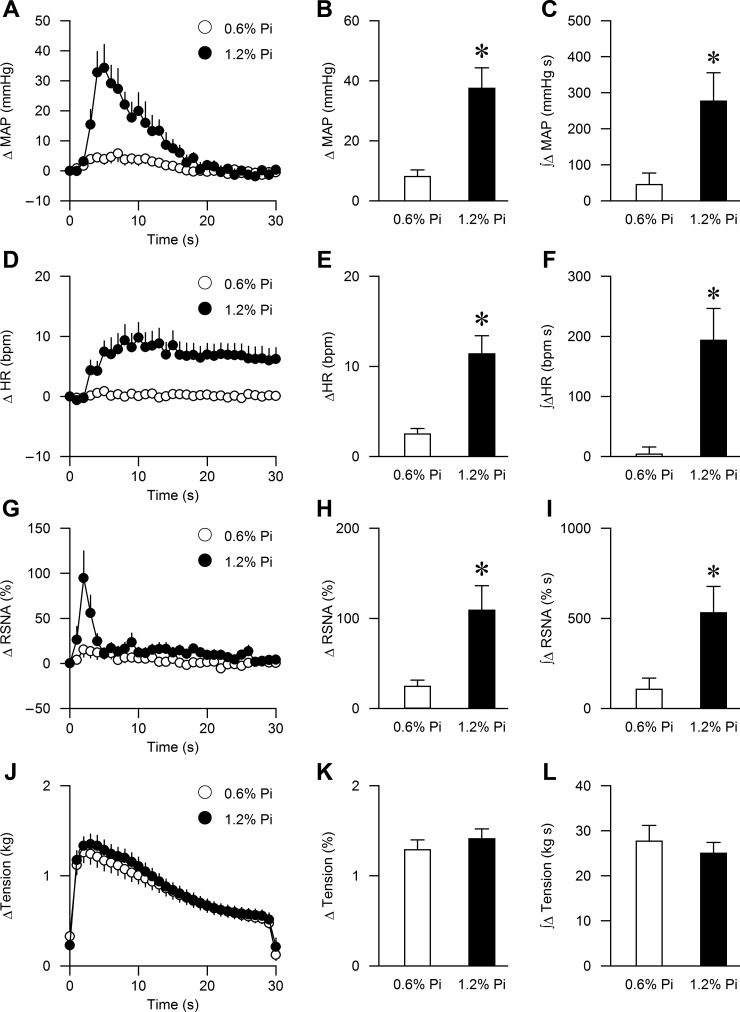
Dietary Pi loading significantly exacerbated the rise in peak BP, HR, and RSNA in response to stimulation of the muscle mechanoreflex during passive muscle stretch compared with rats fed a normal diet (38 ± 7 vs. 8 ± 2 mmHg, P < 0.01; 11 ± 2 vs. 3 ± 1 bpm, P < 0.01; 109 ± 27 vs. 24 ± 7%, P < 0.01, respectively, Figs. 3 and 4, B, E, H, and K). The integrated changes in MAP, HR, RSNA, and muscle tension showed identical results (Fig. 4, C, F, I, and L). Dietary Pi loading also enhanced the BP, HR, and RSNA responses to stimulation of the muscle metaboreflex during capsaicin administration compared with the 0.6% Pi group (50 ± 6 vs. 25 ± 4 mmHg, P < 0.01; 15 ± 2 vs. 3 ± 1 bpm, P < 0.01; 129 ± 25 vs. 58 ± 9%, P < 0.05, respectively; P < 0.05, Figs. 5 and 6). The integrated changes in MAP and HR were significantly higher in the 1.2% Pi compared with the 0.6% Pi group (Fig. 6, C and F; P < 0.01). The AUC of RSNA during capsaicin administration tended to be higher in in the 1.2% Pi compared with the 0.6% Pi group, but the difference did not reach statistical significance (P = 0.086, Fig. 6I).
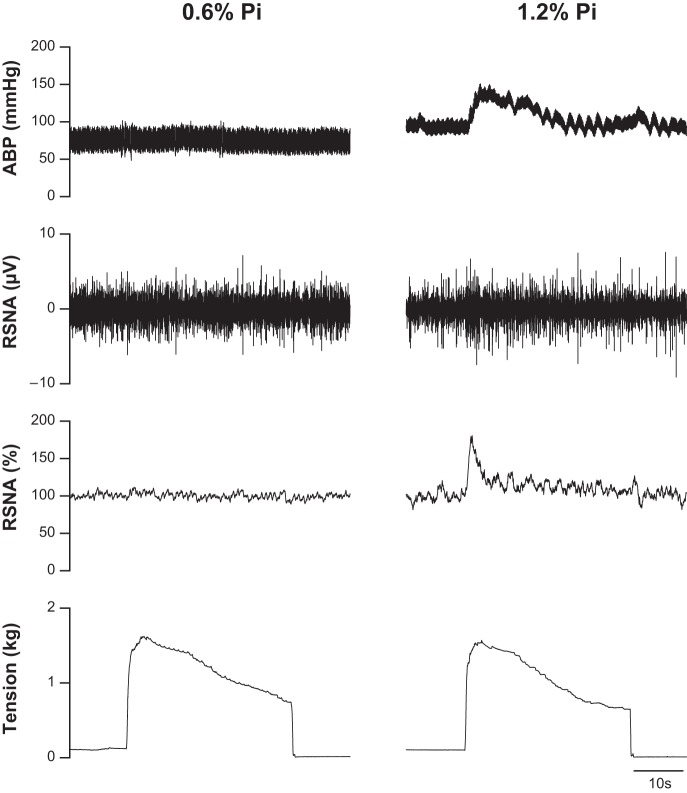
Fig. 3.
Characteristic cardiovascular and sympathetic responses during passive muscle stretch in representative SD rats fed with 0.6% Pi (left) and 1.2% Pi (right) for 12 wk. The stretch-induced increases in arterial blood pressure (ABP), as well as raw and normalized RSNA (%), were larger in the SD rat treated with 1.2% Pi compared with 0.6% Pi diet.
Fig. 4.
Summary data showing cardiovascular and sympathetic responses to activation of the mechanically sensitive component of the EPR in the 0.6% Pi (n = 13) and 1.2% Pi (n = 13) rats. The time course of changes in MAP, HR, RSNA, and muscle tension are shown in A, D, G, and J. The peak MAP, HR, RSNA, and muscle tension responses to muscle stretch are shown in B, E, H, and K. The integrated changes in MAP, HR, RSNA, and muscle tension, which are presented as area under the curve (AUC) over 30 s, are shown in C, F, I, and L. *P < 0.01 compared with 0.6% Pi.
Fig. 5.
Characteristic cardiovascular and sympathetic responses during selective activation of chemically sensitive metaboreceptors by injecting capsaicin into the right iliac artery in representative SD rats fed with 0.6% Pi (left) and 1.2% Pi (right) for 12 wk. The capsaicin-induced increases in ABP, as well as raw and normalized RSNA (%), were larger in the SD rat treated with 1.2% Pi compared with 0.6% Pi diet.
Fig. 6.
Summary data showing cardiovascular and sympathetic responses to activation of the metabolically sensitive component of the EPR in the 0.6% Pi (n = 13) and 1.2% Pi (n = 13) rats. The time course of changes in MAP, HR, and RSNA are shown in A, D, and G. The peak MAP, HR, RSNA, and tension responses to intrafemoral capsaicin are shown in B, E, and H. The integrated changes in MAP, HR, and RSNA, which are presented as AUC over 30 s, are shown in C, F, and I. *P < 0.05 compared with 0.6% Pi.
DISCUSSION
The major findings from this investigation are threefold. First, consumption of a high-Pi diet augments cardiovascular and sympathetic responses to EPR stimulation during muscle contraction. Second, high dietary Pi intake potentiates BP, HR, and RSNA responses to both passive muscle stretch and intra-arterial capsaicin administration in the hindlimb, suggesting enhanced mechanoreflex and metaboreflex function. Third, this detrimental effect of a high-Pi diet occurs in the absence of renal failure, a disease condition known to be associated with hypertension and augmented EPR function (35, 36). Collectively, our present study provides the first direct evidence that chronic exposure to a high-Pi diet in otherwise healthy animals induces sympathetic overactivation during EPR stimulation, resembling the phenotype observed in rodent models of non-Pi-induced hypertension (28, 30).
The high Pi diet used in our study had the content that mimics the excess Pi consumed by the general American population as it contained twice the amount of total Pi compared with the control diet (1.2% vs. 0.6% total Pi) and was enriched with inorganic Pi (0.9% vs. 0.3% inorganic Pi). The extreme elevation in 24-h urinary Pi excretion in the high Pi group is reflective of the exceedingly absorbable nature of inorganic Pi. Our study in rodents has revealed a novel action of Pi on the neural control of BP during physical exertion as the high-Pi diet unequivocally potentiated the increase in SNA, BP, and heart rate to muscle contraction compared with the resting condition. The sympathoexcitation induced by the high-Pi diet was mediated by both functional components of the exercise pressor reflex: the muscle mechanoreflex and metaboreflex. Although previous studies indicated that high-salt intake enhanced EPR function in normotensive SD rats (28, 51), our current data indicated a similar property of dietary Pi, which is independent of Na intake, as evidenced by equivalent 24-h urinary Na excretion rates.
The mechanisms underlying Pi-induced enhancements in EPR function remain unknown. Ingestion of an excess amount of inorganic Pi has been shown to promote renal injury in rodents (10, 25). Furthermore, previous studies in patients with chronic kidney disease have demonstrated augmented EPR function, possibly via enhanced mechanoreflex function (35, 36). However, impaired renal function is not likely to explain our study results for two reasons. First, serum creatinine and creatinine clearance in the high-Pi group were identical to the control group. Second, the decline in renal function associated with high dietary Pi was not typically observed in rodents with intact kidneys until total Pi content exceeded 2% (10, 33). A high Pi diet has also been shown to induce cardiomyopathy in mice with normal renal function (12). Previous studies in cardiomyopathic rats and patients with congestive heart failure have demonstrated enhanced EPR activity via selective mechanoreflex sensitization (27, 41, 43). In our study, rats subjected to chronic exposure of a high-Pi diet tended to have increased heart weight-to-body weight ratios, suggesting development of left ventricular hypertrophy. However, the lung weight-to-body weight ratio in the high Pi group was not increased, indicating that congestive heart failure had not developed. Thus, it is unlikely that cardiomyopathy induced by a high-Pi diet is the primary cause of EPR dysfunction.
Aside from indirect effects of Pi on renal or cardiac function, another possibility includes direct stimulatory effects of Pi on the autonomic or preautonomic neurons in the central nervous system. Sodium-phosphate transporters (NaPi-2c) have been identified in many diencephalic regions, including the amygdala and third ventricle (11, 31). Expression of these transporters is altered by dietary Pi intake and concentration of Pi in the cerebrospinal fluid (CSF) (31). Another Pi transporter, PiT2, has also been identified in the choroid plexus of sharks and implicated in the removal of Pi from the CSF (9). Dietary Pi restriction has been shown to reduce Pi levels in the serum and CSF, which was accompanied by increased Pi appetite and Pi-seeking behavior within 48 h (5, 49). In our study, serum Pi was not altered by the high-Pi diet, although the 24-h urinary Pi excretion was significantly increased. Whether consumption of the high-Pi diet induced an elevated Pi milieu in the brain, causing direct stimulation of brain stem centers involved in the generation of muscle reflex-induced central sympathetic outflow remains to be determined.
In addition to the potential direct effect of Pi on the sympathetic nervous system, consumption of a diet high in phosphate may alter baroreflex sensitivity constituting another mechanism by which EPR function could be augmented. It has been demonstrated that the baroreflex normally acts to restrain EPR-induced increases in BP and RSNA (42). In our study, the 1.2% Pi group displayed both elevated resting heart rate and blood pressure, suggesting impaired baroreflex control of heart rate at rest. Reductions in sensitivity could compromise the buffering capacity of the baroreflex contributing to the EPR overactivity observed. Future studies are needed to assess the effects of high dietary Pi on baroreflex control of blood pressure, heart rate, and SNA during exercise. Exposure to a high-Pi environment may induce alterations in the levels of many hormones involved in Pi homeostasis, which could affect EPR function. Previous studies demonstrated that acute Pi loading by either enteral or intravenous routes induced release of phosphaturic hormones, including fibroblast growth factor 23 (FGF-23) and parathyroid hormone (PTH) (3, 7, 38, 39). FGF23 is a major hormone released from osteocytes in bone to augment renal phosphate excretion, thereby minimizing phosphate overload (19, 37). This action of FGF23 requires klotho, which functions as a coreceptor for transduction of FGF23 signaling (13). A high-Pi diet has also been shown to reduce soluble klotho levels in the serum of mice (12). Soluble klotho is a multifunctional protein present in biological fluids, including blood, urine, and CSF. Whether a high-Pi diet induces hypertension and sympathetic overactivation via increased expression of FGF23 and/or PTH or downregulation of klotho remains to be determined.
Pi is an essential component of many biologic molecules and is crucial for a large number of cellular functions (21). The recommended daily allowance (RDA) of phosphorus in adults is 700 mg/day, according to the Institute of Medicine (44). Pi derived from vegetables or plants, which contain mainly organic phosphate, is poorly absorbed because it is present in the nonhydrolyzable phytate form (16, 17). Unfortunately, the Western diet is well known to contain enormous amounts of inorganic Pi, which is readily absorbed in the gastrointestinal tract (17). Inorganic Pi-containing food additives were found to be present in more than 40% of top-selling grocery items, including frozen foods, dry food mixes, packaged meat, and soup (22). It is estimated that an average U.S. adult consumes Pi of ∼1,200 mg/day, which is almost twice the recommended daily allowance (4). Emerging evidence suggests that excessive phosphate intake can potentially instigate a number of pathological sequelae, including atherosclerosis, myocardial infarction, vascular calcification, and stroke in patients with and without renal disease (4, 18, 26). Adding to this list, our study demonstrated that dietary Pi excess detrimentally affects the sympathetic regulation of BP during exercise by potentiating the function of both the muscle mechanoreflex and metaboreflex, the two major components of the EPR and, in doing so, may contribute to the pathogenesis of Pi-induced hypertension. Just as important, numerous epidemiological studies have demonstrated that exercise BP predicts subsequent development of stroke, myocardial infarction, and death (20, 23, 24, 47), independent of resting BP.
Our study is limited by several factors, including a lack of molecular insight into mechanisms linking high dietary Pi to the pathogenesis of EPR dysfunction. Moreover, measurement of BP yielded lower levels of conscious BP than those obtained during isoflurane anesthesia, which was unexpected. However, the tail cuff measurement was limited to a single time point, which may not reflect fluctuations in BP over the longer time period analyzed under anesthesia. Despite the limitations of this technique, both tail cuff BP and BP under anesthesia were higher in the 1.2% Pi group compared with 0.6% Pi group. Thus, our study demonstrated a consistent BP-raising effect of dietary Pi loading in otherwise normal rodents. Our study may provide an explanation for the elevation in SNA and augmented EPR function in patients with chronic kidney disease (35, 36), who are prone to develop Pi retention and hypertension. If our findings are confirmed in humans, our study results would call for a revision in the food-labeling process to include inorganic phosphate. As with Na, such a revision would not only allow the American public to monitor their phosphate intake but also serve as a warning that their health could be compromised by excess consumption of Pi.
GRANTS
This research was supported by a grant from the National Institutes of Health Heart, Lung and Blood Institute (HL-113738 to W. Vongpatanasin), the Kaplan Chair in Hypertension Research (to W. Vongpatanasin), the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to J. H. Mitchell), the National Institutes of Diabetes and Digestive and Kidney Diseases (DK-092461 to M. C. Hu, DK-091392 to O. W. Moe, and RO1-DK100605 to C.-L. Huang), the UT Southwestern O'Brien Kidney Research Center (National Institutes of Health Grant P30DK-079328 to O. W. Moe and Clinical and Translational Core Grant to W. Vongpatanasin), and the University of Texas Southwestern O'Brien Kidney Research Pilot and Feasibility Program (to M. Maalouf).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M., O.W.M., S.A.S., and W.V. conception and design of research; M.M. and S.C. performed experiments; M.M., S.C., M.-C.H., and W.V. analyzed data; M.M., J.H.M., S.C., C.-L.H., N.M.M., O.W.M., S.A.S., and W.V. interpreted results of experiments; M.M. prepared figures; M.M., J.H.M., C.-L.H., N.M.M., M.-C.H., O.W.M., S.A.S., and W.V. drafted manuscript; M.M., J.H.M., S.C., C.-L.H., N.M.M., M.-C.H., O.W.M., S.A.S., and W.V. edited and revised manuscript; M.M., S.C., C.-L.H., N.M.M., M.-C.H., O.W.M., S.A.S., and W.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Martha Romero and Julius Lamar, Jr., for their expert technical assistance. In addition, we thank the O'Brien Kidney Research Core Center and the Mineral Metabolism Clinical Laboratories at the Pak Center for providing materials and support for the measurement of urine.
REFERENCES
- 1.Benini O, D'Alessandro C, Gianfaldoni D, Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J Ren Nutr 21: 303–308, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Bozic M, Panizo S, Sevilla MA, Riera M, Soler MJ, Pascual J, Lopez I, Freixenet M, Fernandez E, Valdivielso JM. High-phosphate diet increases arterial blood pressure via a parathyroid hormone mediated increase of renin. J Hypertens 32: 1822–1832, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Burnett SAM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chang AR, Lazo M, Appel LJ, Gutierrez OM, Grams ME. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr 99: 320–327, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czarnogorski M, Woda CB, Schulkin J, Mulroney SE. Induction of a phosphate appetite in adult male and female rats. Exp Biol Med 229: 914–919, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 116: 976–990, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerreiro PM, Bataille AM, Parker SL, Renfro JL. Active removal of inorganic phosphate from cerebrospinal fluid by the choroid plexus. Am J Physiol Renal Physiol 306: F1275–F1284, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N. Renal toxicity of phosphate in rats. Kidney Int 17: 722–731, 1980. [DOI] [PubMed] [Google Scholar]
- 11.Hisano S, Haga H, Li Z, Tatsumi S, Miyamoto KI, Takeda E, Fukui Y. Immunohistochemical and RT-PCR detection of Na+-dependent inorganic phosphate cotransporter (NaPi-2) in rat brain. Brain Res 772: 149–155, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro OM, Hill JA, Moe OW. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 26: 1290–1302, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H, Hwang SK, Kwon JT, Lee YS, An GH, Lee KH, Prats AC, Morello D, Beck GR Jr, Cho MH. Low dietary inorganic phosphate affects the brain by controlling apoptosis, cell cycle and protein translation. J Nutr Biochem 19: 16–25, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Hwang SK, Yu K, Anderson HK, Lee YS, Lee KH, Prats AC, Morello D, Beck GR Jr, Cho MH. A high inorganic phosphate diet perturbs brain growth, alters Akt-ERK signaling, and results in changes in cap-dependent translation. Toxicol Sci 90: 221–229, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Karalis M. Food and Drug Administration petition on food labeling: an update from the American Dietetic Association and National Kidney Foundation. J Ren Nutr 17: 423–424, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Karp H, Ekholm P, Kemi V, Itkonen S, Hirvonen T, Narkki S, Lamberg-Allardt C. Differences among total and in vitro digestible phosphorus content of plant foods and beverages. J Renal Nutr 22: 416–422, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Ketteler M, Wolf M, Hahn K, Ritz E. Phosphate: a novel cardiovascular risk factor. Eur Heart J 34: 1099–1101, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens 19: 1343–1348, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9: 650–660, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Leon JB, Sullivan CM, Sehgal AR. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Renal Nutr 23: 265–270, e262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O'Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol 101: 1614–1620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim PO, Donnan PT, MacDonald TM. Blood pressure determinants of left ventricular wall thickness and mass index in hypertension: comparing office, ambulatory and exercise blood pressures. J Hum Hypertens 15: 627–633, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Mackay EM, Oliver J. Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J Exp Med 61: 319–334, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGovern AP, de Lusignan S, van Vlymen J, Liyanage H, Tomson CR, Gallagher H, Rafiq M, Jones S. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community-based cohort study. PloS One 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno M, Downey RM, Mitchell JH, Auchus RJ, Smith SA, Vongpatanasin W. Aldosterone and salt loading independently exacerbate the exercise pressor reflex in rats. Hypertension 66: 627–633, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulroney SE, Woda CB, Halaihel N, Louie B, McDonnell K, Schulkin J, Haramati A, Levi M. Central control of renal sodium-phosphate (NaPi-2) transporters. Am J Physiol Renal Physiol 286: F647–F652, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Murphy MN, Mizuno M, Mitchell JH, Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol 301: H1191–H1204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neves KR, Graciolli FG, dos Reis LM, Pasqualucci CA, Moyses RM, Jorgetti V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int 66: 2237–2244, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85: 855–870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Campese VM, Middlekauff HR. Exercise pressor reflex in humans with end-stage renal disease. Am J Physiol Regul Integr Comp Physiol 295: R1188–R1194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J, Quyyumi AA, Middlekauff HR. Exercise pressor response and arterial baroreflex unloading during exercise in chronic kidney disease. J Appl Physiol 114: 538–549, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prie D, Urena Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int 75: 882–889, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Scanni R, vonRotz M, Jehle S, Hulter HN, Krapf R. The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol 25: 2730–2739, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol London 577: 1009–1020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board, Institute of Medicine. DRI Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press, 1997. [Google Scholar]
- 45.Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academic Press, 1995. [Google Scholar]
- 46.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Renal Nutr 17: 350–354, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung J, Ouyang P, Silber HA, Bacher AC, Turner KL, DeRegis JR, Hees PS, Shapiro EP, Stewart KJ. Exercise blood pressure response is related to left ventricular mass. J Hum Hypertens 17: 333–338, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y, Mitsushima S, Kato A, Yamaguchi T, Ichihara S. High-phosphorus/zinc-free diet aggravates hypertension and cardiac dysfunction in a rat model of the metabolic syndrome. Cardiovasc Pathol 23: 43–49, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Sweeny JM, Seibert HE, Woda C, Schulkin J, Haramati A, Mulroney SE. Evidence for induction of a phosphate appetite in juvenile rats. Am J Physiol Regul Integr Comp Physiol 275: R1358–R1365, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol 589: 1209–1220, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol 306: H450–H454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zinellu A, Caria MA, Tavera C, Sotgia S, Chessa R, Deiana L, Carru C. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 342: 186–193, 2005. [DOI] [PubMed] [Google Scholar]