Abstract
A single bout of exhaustive exercise signals expression of vascular endothelial growth factor (VEGF) in the exercising muscle. Previous studies have reported that mice with life-long deletion of skeletal myofiber VEGF have fewer capillaries and a severe reduction in endurance exercise. However, in adult mice, VEGF gene deletion conditionally targeted to skeletal myofibers limits exercise capacity without evidence of capillary regression. To explain this, we hypothesized that adult skeletal myofiber VEGF acutely regulates skeletal muscle perfusion during muscle contraction. A tamoxifen-inducible skeletal myofiber-specific VEGF gene deletion mouse (skmVEGF−/−) was used to reduce skeletal muscle VEGF protein by 90% in adult mice. Three weeks after inducing deletion of the skeletal myofiber VEGF gene, skmVEGF−/− mice exhibited diminished maximum running speed (−10%, P < 0.05) and endurance capacity (−47%; P < 0.05), which did not persist after 8 wk. In skmVEGF−/− mice, gastrocnemius complex time to fatigue measured in situ was 71% lower than control mice. Contraction-induced perfusion measured by optical imaging during a period of electrically stimulated muscle contraction was 85% lower in skmVEGF−/− than control mice. No evidence of capillary rarefication was detected in the soleus, gastrocnemius, and extensor digitorum longus (EDL) up to 8 wk after tamoxifen-induced VEGF ablation, and contractility and fatigue resistance of the soleus measured ex vivo were also unchanged. The force-frequency of the EDL showed a small right shift, but fatigue resistance did not differ between EDL from control and skmVEGF−/− mice. These data suggest myofiber VEGF is required for regulating perfusion during periods of contraction and may in this manner affect endurance capacity.
Keywords: vascular endothelial growth factor, perfusion, fatigue, exercise, skeletal myofiber
many patients suffering from chronic disease, such as chronic obstructive pulmonary disease (COPD), peripheral artery disease, and chronic heart failure, exhibit poorly explained muscle weakness and exercise intolerance. This may stem from pathological changes in the muscle contractile apparatus or metabolism, a restriction in O2 available to the mitochondria within the myofiber, or a combination of vascular and myofiber changes. A key element for providing adequate O2 to mitochondria within myofibers is the number of surrounding capillaries. In addition, during exercise, oxygen delivery is increased to active skeletal muscles due to a rapid increase in perfusion in a process referred to as exercise-induced hyperemia (8, 26). Vascular endothelial growth factor (VEGF) has the potential to contribute to the regulation of these steps in the oxygen transport system that take place within locomotor skeletal muscle. First, low VEGF levels in skeletal muscle have been associated with capillary rarefaction in some, but not all, patients with COPD (23, 31). Second, VEGF has been reported to regulate vasodilatation in several vascular beds through a nitric-oxide dependent mechanism (2, 35, 41). Thus, these vascular mechanisms could potentially be regulated by VEGF signaling and contribute to the exercise intolerance observed in chronic disease states (14, 23).
Although several studies have shown that skeletal muscle VEGF expression increases in response to exercise (5, 19), and some studies have shown a link between VEGF expression and increased capillary number in exercise-trained muscle (29, 40), little attention has been given to the requirement of VEGF signaling for maintaining the microvascular network in mature skeletal muscle. Tang et al. (37) employed an adeno-associated virus Cre-LoxP strategy to successfully delete VEGF in all cell types within a small region of adult mouse gastrocnemius muscle (37). This led to capillary regression and apoptosis that persisted for at least 8 wk in the affected area. In a similar study by Kamba et al. (24), VEGF signaling was inhibited with either a small-molecule VEGF receptor (VEGFR) tyrosine kinase inhibitor, VEGF trap, or VEGF decoy receptors and was found to lead to capillary regression in some, but not all, adult organs. In this study, the tongue—the skeletal muscle studied—failed to show a decrease in capillary density. Recently, our laboratory reported that skeletal myofiber VEGF plays a key role in the angiogenic adaptation to exercise training, but capillaries in nonexercised, VEGF-deficient muscle did not regress (13).
In skeletal muscle, the myofiber is a major source of VEGF expression (21). Lifelong inhibition of myofiber-expressed VEGF, initiated during late gestation in a skeletal myofiber-targeted VEGF knockout mouse (Myo-Cre × VEGFLoxP mice), results in a dramatic decrease in skeletal muscle capillarity along with a severe limitation in exercise endurance capacity (38). This finding suggests that VEGF expressed by myofibers is necessary during development of the muscle microvasculature and is important for achieving a normal exercise capacity into adulthood. However, it is unknown whether VEGF has a role in acutely regulating blood perfusion to active muscle in response to contraction in a normally developed adult mouse.
In the present study, we hypothesized that adult skeletal myofiber-derived VEGF is necessary to regulate muscle perfusion, contractile function, and exercise capacity. To test this hypothesis, we made use of a skeletal myofiber-specific VEGF knockout mouse [skeletal actin-driven Cre recombinase (Cre)-tamoxifen-dependent mutant estrogen receptor (ERT2) recombinase mouse cross-bred with VEGFLoxP mouse] that allowed for almost complete (>90%) reduction of VEGF protein in the skeletal muscles analyzed. Using this model, we asked whether the inhibition of VEGF expression exclusively in adult skeletal myofibers would 1) alter contraction-induced muscle perfusion, 2) impair in vitro muscle contractile function and fatigue resistance, and 3) decrease exercise performance.
MATERIALS AND METHODS
Ethical approval.
This study was approved by the University of California, San Diego, Animal Care and Use Committee and was conducted in accordance to guidelines outlined by the Guide for the Care and Use of Laboratory Animals (National Research Council). Mice were housed 3 or 4 per cage in a pathogen-free vivarium, maintained on a 12:12-h day-night cycle and provided standard chow (Harlan Teklad 8604, Madison, WI) and tap water ad libitum. After all functional assessments were completed, mice were euthanized by surgical removal of the heart while under anesthesia (pentobarbital sodium, 60 mg/kg ip).
Generation of inducible, skeletal myofiber-specific VEGF gene-ablated mice.
Homozygous VEGFLoxP mice (provided by Dr. N. Ferrara, Genentech, San Francisco, CA) (15) were crossed with a heterozygous human skeletal actin (HSA)-Cre-ERT2 mice (34). In HSA-Cre-ERT2 mice, Cre-ERT2 is selectively expressed in skeletal myofibers (controlled by human skeletal muscle α-actin sequence) and Cre recombinase is fused to a mutant form of the estrogen receptor that is selective for tamoxifen. Upon administration of tamoxifen, the Cre-ERT2 fusion protein translocates to the nucleus and initiates a recombination event with the loxP sites surrounding a region of VEGF exon 3 (34). All mice were homozygous for the VEGFLoxP gene and either heterozygous for HSA-Cre-ERT2 (premutant skmVEGF−/−) or null for HSA-Cre-ERT2 (VEGF+/+). Post-tamoxifen VEGF gene-deleted mice are designated skmVEGF−/−. Subsequent breeding pairs were sibling-sibling matings. Tail DNA was used to genotype mice, as previously described (30). Tibialis anterior muscle DNA from D21 post-tamoxifen mice (n = 5–7) was used to confirm VEGF gene deletion by real-time RT-PCR using primers previously described that amplify the 3′ LoxP site (28).
Study design.
In this study, skeletal myofiber-specific VEGF gene-deleted, male mice (skmVEGF−/−) were compared with male, control (VEGF+/+) littermates. Female mice were not used to eliminate potential effects of endogenous estrogen on the tamoxifen-inducible Cre-ERT2 used in this study. Premutant skmVEGF−/− and VEGF+/+ mice (4–5 mo of age) were weighed and tested for maximal running speed, and the following day, endurance was tested. After 2 days, all mice were treated with tamoxifen (1 mg/day ip) for five consecutive days (D0 to D4). On days D21 (a sufficient time for VEGF expression to be decreased) and D56 (to allow for sufficient time for capillary regression and test for potential compensatory effects), separate groups of mice were retested for maximal running speed. On D22 and D57, mice were retested for endurance. On D23 and D58, mice were weighed and anesthetized with pentobarbital sodium; then, the soleus (45% type I and 55% type IIa fibers), extensor digitorum longus (EDL) (14% type IIa, 84% type IIb fibers), and gastrocnemius [which contains a deep region of oxidative fiber types, the majority (90%) of which are type IIb fibers (18, 39)], heart, lung, brain, and kidney were collected for VEGF-specific ELISA. SkmVEGF−/− mice that did not have >90% reduction in VEGF levels were excluded. Contralateral gastrocnemius, soleus, and EDL were used for histochemical assays. Additional groups of mice were used for ex vivo and in situ muscle stimulation protocols.
VEGF protein levels.
VEGF protein levels were measured using an ELISA kit for mouse (VEGF mouse ELISA, R&D Systems, La Jolla, CA) and were normalized to total protein measured by Bio-Rad DC protein assay.
Exercise tests.
All mice were familiarized on a treadmill (model no. CL-4, Omnitech, Columbus, OH) for 10 min on each of several days prior to performing the exercise tests. The maximal running speed protocol began at 20 m/min [above the critical speed for C57BL/6J mice (3)], 10° incline for 1 min, after which running speed was increased by 2 m/min every minute, until exhaustion. Endurance capacity was determined by running mice at 20 m/min, on a 10° incline until exhaustion. For each running test, exhaustion was defined as the point at which the mouse was no longer able to maintain normal running position on the treadmill and/or sitting on a shock grid (≤0.2 milliamps) at the rear of the treadmill for 10 consecutive seconds.
Skeletal muscle capillary parameters.
Capillaries and fibers were counted in 10-μm gastrocnemius complex frozen sections, as previously described (30). Morphometric measurements were made from 700-2,000 randomly selected fibers from each lateral section of gastrocnemius (including both deep and superficial regions), from >300 fibers in the soleus and >400 fibers in the EDL.
Isolated muscle contraction and fatigue measurements.
Mice were anesthetized with pentobarbital sodium, and the soleus and EDL muscles were dissected with both tendons intact. For each test, the muscle was mounted in a chamber that was continuously perfused with oxygenated (95% O2-5% CO2) Tyrode's solution (121 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 0.4 mM NaH2PO4, 24 mM NaHCO3, 5.5 mM glucose, and 0.1 mM EGTA) at pH 7.4 at room temperature. One tendon was tied with silk thread to a force transducer, and the other tendon was tied to an adjustable tube at the opposite end of the chamber, allowing muscle length to be changed incrementally to set the optimal muscle length (Lo). Muscles were stimulated with an S48 stimulator (Grass Technologies, Quincy, MA) at supramaximal voltage using platinum electrodes placed on either side of the muscle. Following 15 min of equilibration, the muscles were tested for contractile function and fatigue resistance. To assess contractile function, the force frequency relationship was established for each muscle. Maximum isometric tetanic force was measured by recording force output at stimulation frequencies of 1, 15, 30, 50, 80, 100, and 120 Hz (500-ms train duration, 0.2-ms pulse duration) for soleus and 1, 10, 15, 30, 45, 60, 75, 90, 120, and 150 Hz (300-ms train duration, 0.2 ms pulse duration) for EDL. The muscles were stimulated to contract once every 2 min to prevent fatigue. Stability of the muscle was checked by periodically stimulating the muscle at maximal frequency (100 Hz for soleus and 150 Hz for EDL) throughout the course of the experiment. To measure fatigue, isometric tetanic contractions were elicited with 500-ms (soleus) or 300-ms (EDL) train duration and 0.2-ms pulse duration at 50 Hz (soleus) or 70 Hz (EDL). Stimulation frequency was increased every 2 min (soleus) or 1 min (EDL) in a progressive manner (from a contraction every 8 s to 4 to 3 to 2 to 1 s). Time to fatigue was measured at the time it took each muscle to reach 60% of the maximal developed force for that muscle. When all testing was finished, Lo was measured using a reticle with a surgical microscope (Zeiss OPMI, Thornwood, NY), tendons were removed, and the muscles were blotted and weighed. Cross-sectional area was calculated, as previously described (9), and the maximal isometric tetanic force (Po) was expressed in Newtons per square centimeter.
In situ skeletal muscle fatigue.
Mice were anesthetized with ketamine: xylazine (10 mg:1 mg/kg), and a saline-filled PEO8 catheter was inserted into the left carotid artery and advanced into the aortic arch to allow measurement of blood pressure (VEGF+/+ 64.7 ± 8.0 mmHg, skmVEGF−/− 63 ± 2.3 mmHg). The sciatic nerve was exposed and connected to electrodes. The gastrocnemius complex was then separated from the bone, and the tendons connected by a suture to a force transducer. The gastrocnemius complex was contracted with a single pulse (10 volts, 200-ms duration electrical stimulation) via the sciatic nerve. The force generated by the contraction was recorded, and then the resting tension of the muscle was slightly increased. This was repeated until the contraction generated a maximum amount of force. The gastrocnemius complex was then stimulated to contract with 10 volts at a rate of 0.25 trains per second (tps) until the force generated fell to 50% of the initial force output, and the time was recorded.
Real-time imaging of contraction-induced muscle perfusion.
Contraction-induced muscle perfusion was measured in situ at the 21-day post-tamoxifen time point. Mice were anesthetized with ketamine: xylazine. A catheter (MTV-03; Braintree Scientific, Braintree, MA) connected to a 1-ml syringe was filled with 40 μl of 20-nm near-infrared spheres (Qtracker 800 nontargeted quantum dots, Q21071MP; Invitrogen, Molecular Probes, Carlsbad, CA) in 200 μl of saline. The dead space in the syringe was filled with perfluorocarbon (FC-75 perfluoro-compound; Acros Organics, Geel, Belgium). The nanosphere-loaded catheter was then inserted into the tail vein and glued into place. The sciatic nerve of one hind limb was connected to an electrode to allow the gastrocnemius complex to be electrically stimulated to contract (ES). The contralateral sciatic nerve was severed and a ground was connected to the mouse. The mouse was then placed into an optical imaging chamber (IVIS Spectrum, Caliper Life Sciences, Hanover, MD) prewarmed to 37°C, and anesthesia was switched over to isoflurane (1.5%) to maintain a constant level during the ES period. Nanospheres were delivered at a constant rate over 2 min. Thirty seconds into the delivery, one gastrocnemius complex was electrically stimulated to contract (6 V, 0.25 tps, 200-ms duration) using a Grass S88X Stimulator (Astro-Med, West Warwick, RI). Images were collected every 5 s (0.5-s image capture time, excitation 710, emission 800) for 30 s pre-ES period, 3-min ES period and 3-min post-ES period. Images were background corrected by subtracting the autofluorescence from a preimage (before Qdot delivery) from each of the subsequent 80 images captured using Live Image 3.2 software (Caliper Life Sciences). Fluorescent signals, simultaneously collected from the ES and Rest gastrocnemius complexes in the same mouse, were measured and graphed as the average ES/Rest ratio over time. The use of a nanosized fluorescent spheres allowed the tracer to pass through the lung and allowed the accumulation of signal in the muscle to be detected by optical imaging and reflect active perfusion of the microvascular system.
Statistical analyses.
All data are presented as means ± SE. Separate repeated-measures ANOVA 2 (group: VEGF+/+ or skmVEGF−/−) × 2 (time: pre- and post-tamoxifen) were conducted for the dependent variables (maximum running speed and time to exhaustion). The muscle perfusion data were averaged over 30-s intervals. A two-way ANOVA was used to detect a difference between the experimental groups and time. For force generated by the EDL muscle, a 2 (group: VEGF+/+ or skmVEGF−/−) × 10 (frequency: 1, 10, 15, 30, 45, 60, 75, 90, 120, and 150 Hz) mixed-factor ANOVA was performed. Similarly, for soleus muscle force, a 2 (group: VEGF +/+ or skmVEGF −/−) × 7 (frequency: 1, 15, 30, 50, 80, 100, and 120 Hz) mixed-factor ANOVA was performed. Specific differences were identified with Bonferroni's post hoc tests. Pairwise comparisons for body and muscle weights, VEGF protein levels, capillary-to-fiber ratios, maximal isometric tetanic force, and time to fatigue were made with the use of Student's t-test. P ≤ 0.05 was considered significant.
RESULTS
VEGF protein levels.
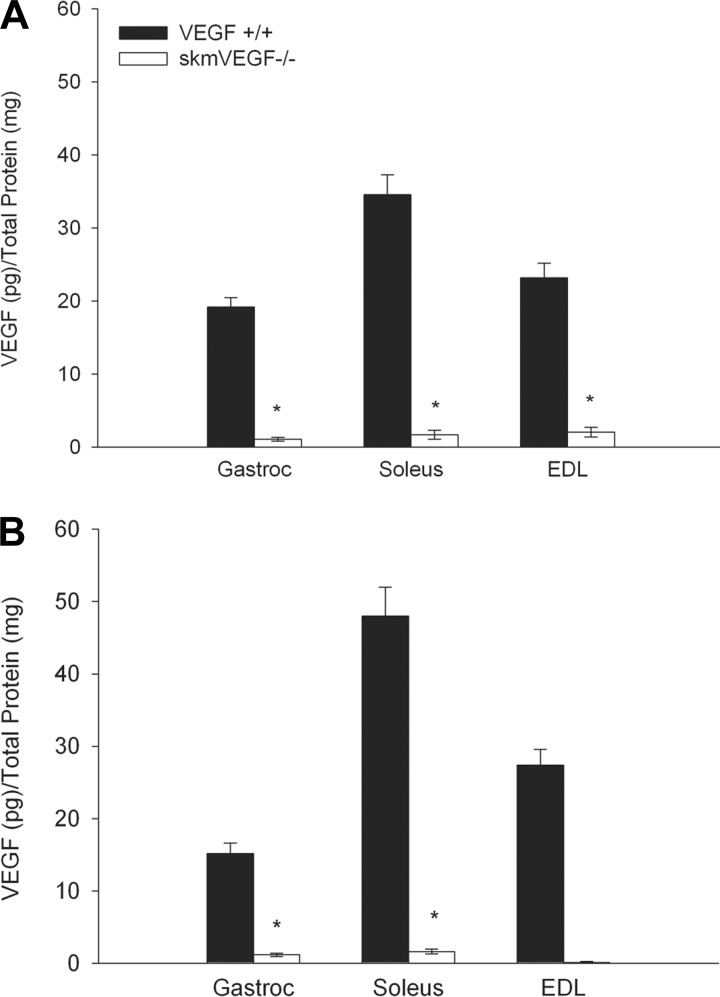
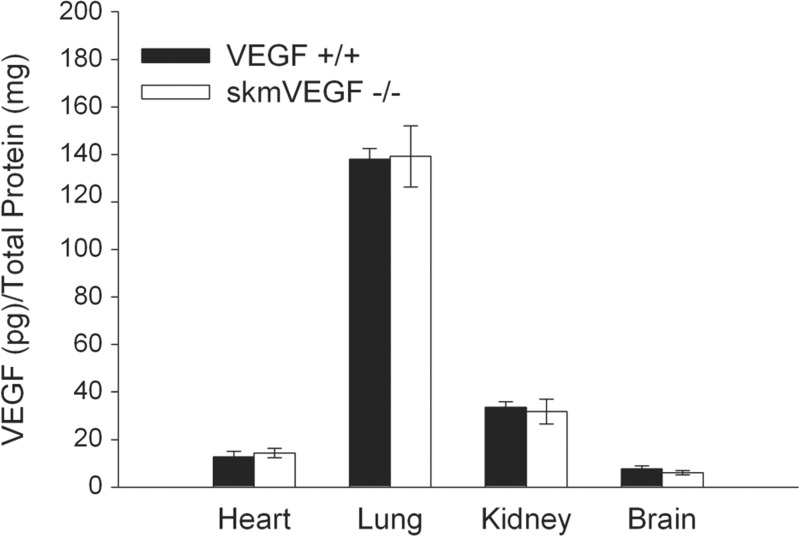
On day 21 post-tamoxifen administration, >40% gene deletion efficiency was confirmed by real-time PCR using DNA isolated from the tibialis anterior from skmVEGF−/− mice (n = 5–7, P < 0.05). On day 23 post-tamoxifen, VEGF protein in the gastrocnemius, soleus, and EDL muscles were 94, 97, and 91%, respectively, lower in skmVEGF−/− mice than the control, VEGF+/+ group (Fig. 1A). Similarly, on day 58 post-tamoxifen, VEGF levels were reduced by 92 and 97% in the gastrocnemius and soleus, respectively (Fig. 1B). VEGF protein levels in skmVEGF−/− EDL were below detection levels in some samples (Fig. 1B). VEGF+/+ and premutant skmVEGF−/− mice that were not given tamoxifen showed no reduction in skeletal muscle VEGF protein levels (data not shown). VEGF levels in the heart, lung, kidney, and brain from skmVEGF−/− mice were normal (Fig. 2).
Fig. 1.
Vascular endothelial growth factor (VEGF) protein levels in hind limb skeletal muscles. VEGF levels were measured by ELISA in muscles collected from skmVEGF−/− and VEGF+/+ mice. A: 23 days post-tamoxifen. B: 58 days post-tamoxifen. Values are expressed as means ± SE; n = 11–16 for gastrocnemius, n = 5–6 for soleus, and n = 3–6 for extensor digitorum longus (EDL). *Significant difference compared with VEGF+/+ mice (P < 0.001).
Fig. 2.
Post-tamoxifen VEGF protein levels in heart, lung, kidney, and brain. As expected, there were no differences in VEGF levels between the two groups in heart, lung, kidney, or brain. Values are expressed as means ± SE; n = 6.
Exercise limitation.
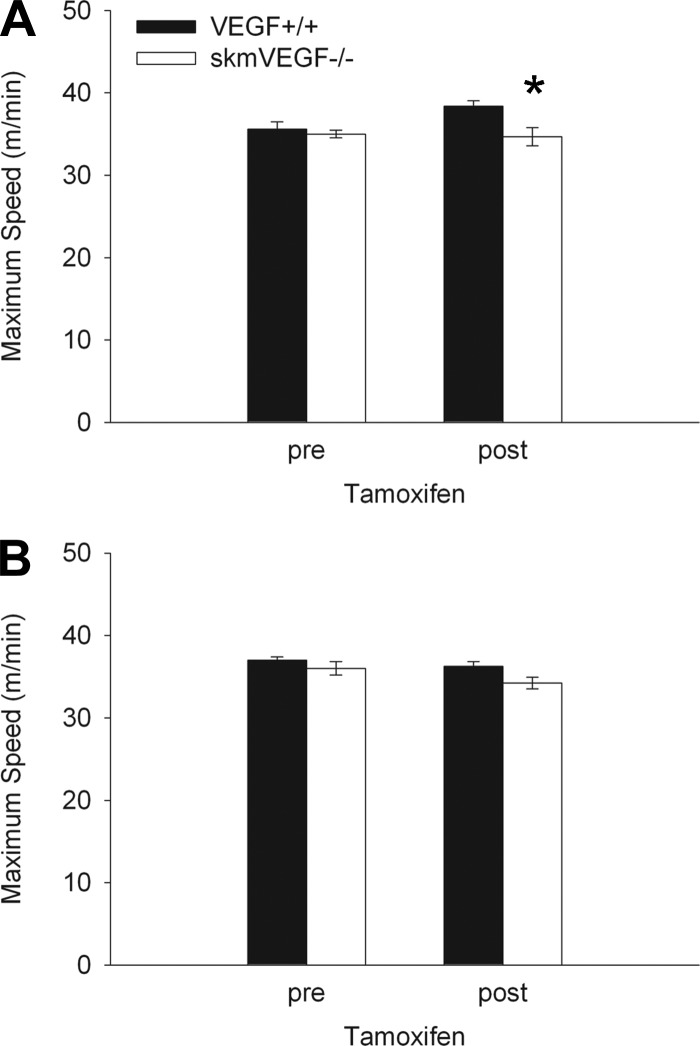
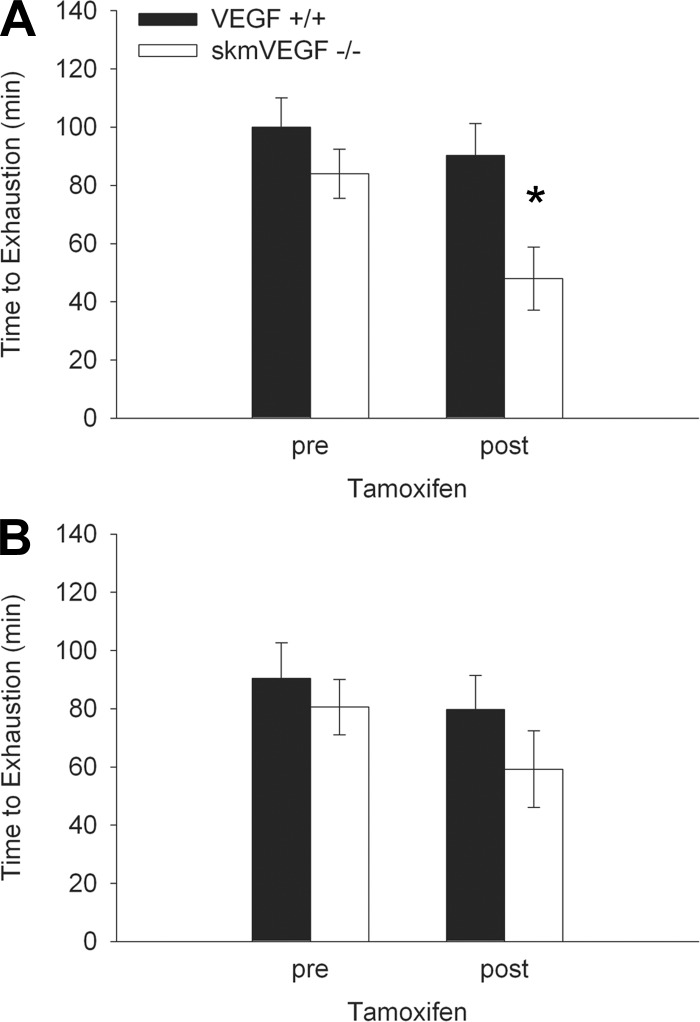
On day 21 post-tamoxifen administration, the maximum running speed of skmVEGF−/− mice was 10% (P < 0.05) lower than the control group (Fig. 3A). Maximum running speed was not significantly different between the groups 56 days post-tamoxifen treatment. On day 22 following tamoxifen delivery, skmVEGF−/− mice exhibited a 47% decrease (P < 0.05) in time to exhaustion (Fig. 4A). At 57 days following tamoxifen treatment, endurance capacity was no longer different between the genotypes (Fig. 4B).
Fig. 3.
Pre- and post-tamoxifen maximum running speeds, showing a small but significant reduction after VEGF gene deletion. A: 21 days post-tamoxifen. B: 56 days post-tamoxifen. Values are expressed as means ± SE; n = 6–10. *Significant difference between VEGF+/+ and the skeletal myofiber-specific VEGF gene deletion mouse (skmVEGF−/−) at the post-tamoxifen time point (P < 0.01).
Fig. 4.
Impaired endurance exercise capacity in skeletal myofiber VEGF gene-deleted mice. A: 22 days following tamoxifen. B: 57 days following tamoxifen. Values are expressed as means ± SE; n = 6–10. *Significant difference between VEGF+/+ and skmVEGF−/− at the post-tamoxifen time point (P < 0.05).
Body mass and muscle size.
Body weights were not different between VEGF+/+ and skmVEGF−/− groups before tamoxifen (data not shown) or at 21 and 56 days post-tamoxifen (Table 1). Muscle mass and cross-sectional areas of the gastrocnemius, soleus, and EDL in the skmVEGF−/− mice were also not different than the control group (Table 1).
Table 1.
Body mass, muscle weight, and muscle cross-sectional area
| VEGF+/+ | skmVEGF−/− | VEGF+/+ | skmVEGF−/− | |
|---|---|---|---|---|
| Days post-tamoxifen | 21 | 21 | 56 | 56 |
| Body mass, g | 24.8 ± .3 | 25.0 ± 0.3 | 26.4 ± .3 | 25.7 ± 0.4 |
| Gastrocnemius weight, mg | 116 ± 3 | 116 ± 4 | 123 ± 3 | 117 ± 4 |
| Soleus weight, mg | 8.8 ± 0.2 | 8.7 ± 0.2 | 9.1 ± 0.4 | 9.0 ± 0.4 |
| Soleus CSA, mm2 | 0.78±.01 | 0.76 ± .02 | 0.76±.04 | 0.79 ± 0.05 |
| EDL weight, mg | 10.5 ± 0.6 | 9.2 ± 0.4 | 10.4 ± 0.7 | 10.1 ± 0.3 |
| EDL CSA, mm2 | 0.87±.05 | 0.73 ± .03 | 0.87±.08 | 0.83 ± 0.03 |
Values are expressed as means ± SE.
VEGF, vascular endothelial growth factor; EDL, extensor digitorum longus; CSA, cross-sectional area; skmVEGF, skeletal myofiber-specific VEGF gene deletion mouse.
No significant differences between groups were found for any variable at either time.
Capillary-to-fiber ratio.
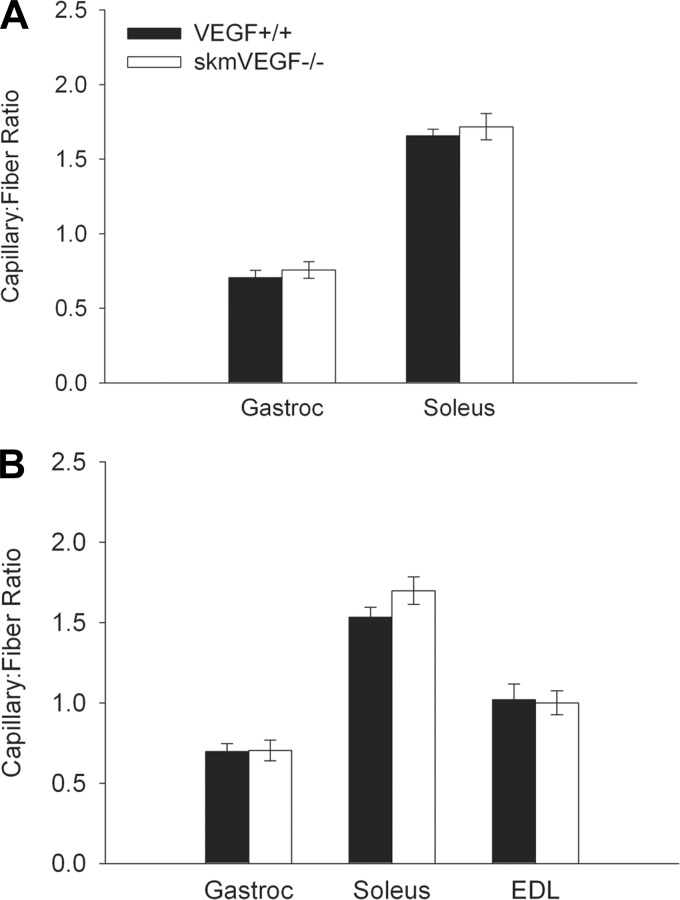
SkmVEGF−/− mice did not reveal a change in the capillary-to-fiber ratio in the gastrocnemius or soleus 23 days post-tamoxifen or in the gastrocnemius, soleus, or EDL 58 days post-tamoxifen (Fig. 5). EDL sections at the 23-day time point were not analyzed due to freezing artifacts. Neither increased apoptosis (caspase 3-positive cells) nor histological evidence of muscle damage (necrotic fibers and regenerating fibers with central nuclei) were observed in gastrocnemius and soleus muscle sections from skmVEGF−/− 24 h following an endurance run to exhaustion (data not shown).
Fig. 5.
Loss of myofiber VEGF does not alter the skeletal muscle capillary-to-fiber ratio. A: 23 days post-tamoxifen. B: 58 days post-tamoxifen. Values are expressed as means ± SE; n = 4–6. No differences were found in any muscle at either time.
Skeletal muscle function in vitro and in situ.
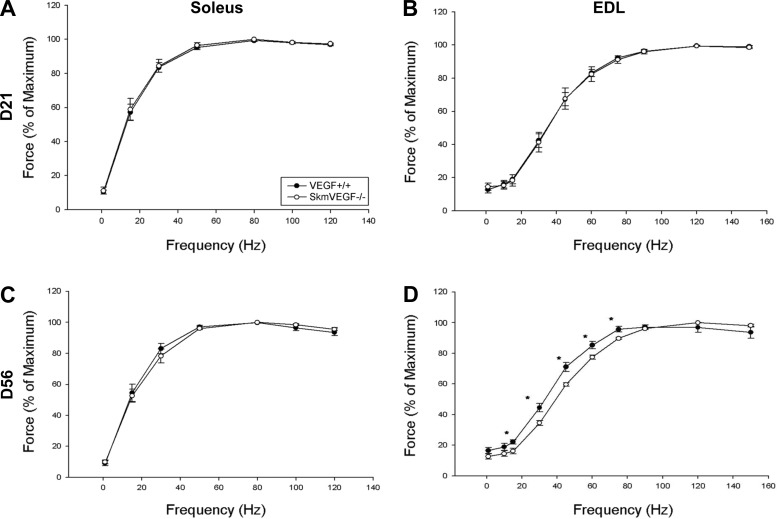
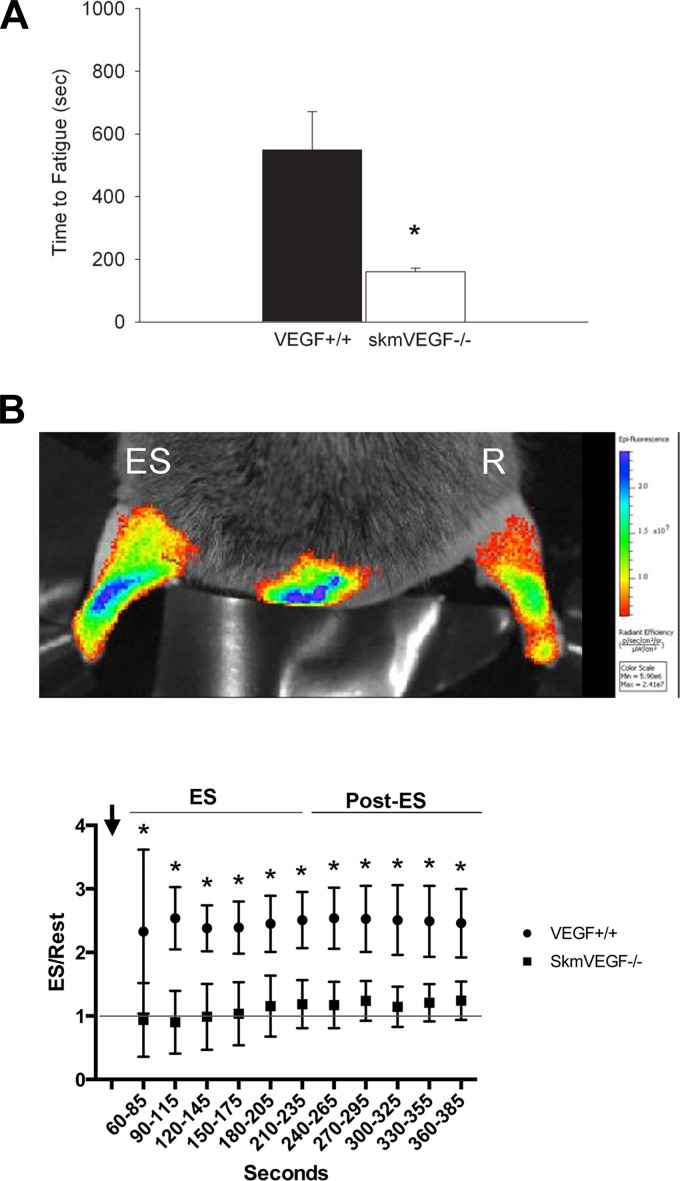
At 21 and 56 days after the initial tamoxifen administration, there were no differences in maximal isometric tetanic force or time to fatigue in the soleus or EDL muscles between skmVEGF−/− and VEGF+/+ mice (Table 2). At 21 days post-tamoxifen, soleus and EDL force-frequency curves also showed no difference between groups. At 56 days post-tamoxifen, soleus force frequency curves were normal in skmVEGF−/− mice; however, the EDL force-frequency relationship in skmVEGF−/− mice revealed a small but significantly right-shifted curve (P < 0.05) (Fig. 6). In situ time to fatigue of the gastrocnemius complex was 71% lower (P = 0.02) in the skmVEGF−/− group than the VEGF+/+ group at the 21-day post-tamoxifen time point (Fig. 7A).
Table 2.
Isolated soleus and EDL maximal isometric tetanic force and time to fatigue
| VEGF+/+ | skmVEGF−/− | VEGF+/+ | skmVEGF−/− | |
|---|---|---|---|---|
| Days posttamoxifen | 21 | 21 | 56 | 56 |
| Soleus | ||||
| Po, N/cm2 | 10.9 ± 2.0 | 9.3 ± 2.9 | 14.4 ± 2.5 | 12.1 ± 1.9 |
| Time to fatigue, s | 444 ± 21 | 441 ± 28 | 435 ± 35 | 415 ± 16 |
| EDL | ||||
| Po, N/cm2 | 18.4 ± 3.4 | 23.5 ± 2.5 | 18.0 ± 2.8 | 20.7 ± 2.4 |
| Time to fatigue, s | 165 ± 8 | 151 ± 10 | 148 ± 11 | 152 ± 5 |
Values are expressed as means ± SE.
Po, maximal isometric tetanic force.
No significant differences between groups were found for any variable at either time.
Fig. 6.
Contractile properties in isolated EDL and soleus muscles from skeletal myofiber-specific gene-ablated mice. Force was measured as a function of stimulation frequency to assess the contractile function of each muscle. A: soleus 21 days following tamoxifen (n = 4–6 muscles per group). B: EDL 21 days following tamoxifen (n = 5 or 6 muscles per group). C: soleus 56 days following tamoxifen (n = 5–7 muscles per group). D: EDL 56 days following tamoxifen (n = 6–7 muscles per group). The EDL muscle, 56 days after tamoxifen delivery, showed a significant right shift in the force-frequency curve. There were no significant differences in any other muscle between skmVEGF−/− and VEGF+/+. Values are expressed as means ± SE. *Significant difference (P < 0.05) between skmVEGF−/− and VEGF +/+ muscles.
Fig. 7.
Gastrocnemius complex fatigue and perfusion monitored in situ is inhibited in mice with skeletal myofiber-targeted VEGF gene deletion. A: time to fatigue was measured in the gastrocnemius complex electrically stimulated to contract via the sciatic nerve. Values are expressed as means ± SE; n = 5 or 6. *P < 0.02. B: real-time measurements of muscle perfusion were collected simultaneously by optically imaging electrically stimulated (ES) and resting (R) gastrocnemius complexes in mice delivered near-infrared fluorescent nanospheres during (ES) and after (Post-ES) periods of muscle contraction. Nanospheres were delivered at a constant rate over the first 2 min. The muscle was stimulated to contract 30 s into the delivery period (arrow). Data are represented as the average over 30-s intervals of the ratio of fluorescence in the stimulated (ES) hind limb muscles to the nonstimulated (Rest) in the same mouse over time. Values are expressed as means ± SD; n = 5 VEGF+/+ mice, and n = 5 skmVEGF−/− mice. *P < 0.0001 between genotypes.
Contraction-induced muscle perfusion.
At 21 days following tamoxifen induction, perfusion to the gastrocnemius complex, measured both during (3 min) and after (3 min) the electrical stimulation (ES) period, was greater in the control, VEGF+/+ mice than the skmVEGF−/− mice (P < 0.0001). In skmVEGF−/− muscle, the average perfusion over the 3 min following the ES period was 23% higher in the ES gastrocnemius than the resting muscle. During this same period, the average perfusion to the control, VEGF+/+-stimulated muscle was 151% above resting values (Fig. 7B).
DISCUSSION
Major findings.
The major findings of this study are 1) myofiber-expressed VEGF is necessary for regulating the acute increase in perfusion to contracting muscle; 2) conformation that myofiber-expressed VEGF is not necessary to maintain the capillary bed (at least over the 8-wk time period studied in these mice); and 3) whole body exercise performance was acutely impaired in skeletal myofiber VEGF-deficient mice. Furthermore, inhibition of VEGF expression in the myofiber did not appear to exhibit an autocrine role in slow-twitch muscle as intrinsic myofiber contractile properties were unaffected in the soleus. The small right shift in the force-frequency relationship of EDL observed at the 56-day post-tamoxifen time point indicates a reduction in contractility in the skeletal myofiber VEGF-deficient fast-twitch muscle. However, this small change is not expected to impair whole body exercise performance. These data suggest that in mice VEGF-dependent vascular reactivity is required to provide adequate oxygen supply to mitochondria during treadmill running exercise.
Myofiber VEGF and capillary maintenance.
Similar to our recent study to evaluate the angiogenic response to exercise training (13), deletion of the VEGF gene, exclusively from skeletal myofibers in cage-confined adult mice, does not affect skeletal muscle capillarity. This finding is surprising given that greater than 90% of muscle VEGF comes from the myofiber. In a previous study, in which the VEGF gene was deleted from all cell types within a small region of adult gastrocnemius, we reported a substantial capillary loss (37). The contrasting results found between the study of Tang et al. (37) and the current model lead us to suggest that other cellular sources of VEGF in skeletal muscle are important for the stability of the adult capillary network. It is important to note that in the present study, we intentionally did not inhibit VEGF expression in all cell types present in skeletal muscle. The remaining ∼10–20% of VEGF produced could have been expressed by endothelial cells, satellite cells, muscle-derived stem cells, pericytes, macrophages, and fibroblasts (6, 7, 12, 17, 25). For instance, endothelial cell-targeted VEGF deletion has been reported to result in endothelial apoptosis and unstable vascular structures that eventually lead to hemorrhage and sudden death in greater than 50% of VE-cadherin-Cre/VEGFlox/lox mice (25). Interestingly, lifelong deletion of VEGF in endothelial cells alone does not reduce capillary density in the heart, but lifelong global myofiber VEGF gene deletion leads to a 50% reduction in cardiac capillarity (25, 30). In our skeletal myofiber-specific VEGF deletion model, we assume that VEGF production by cells other than the myofibers was not affected and, therefore, could support the survival of the endothelial cells in mature capillaries. While VEGF-dependent cell-cell interactions in vivo will require further investigation, it appears that once the skeletal muscle capillary bed has matured in an adult mouse, VEGF expression solely in the myofiber is not required for capillary maintenance in skeletal muscle.
VEGF signaling from the myofiber and regulation of muscle perfusion.
Aerobic exercise capacity is dependent on oxygen delivery being able to meet the metabolic demands of active muscle groups (32). In addition to maintaining the number of capillaries that interface with each myofiber (20), the ability to direct oxygenated blood to contracting muscles during periods of intense exercise is a key factor in achieving maximal exercise capacity (8, 26). In the present study, a more rapid time to fatigue using an in situ perfused preparation, with no reduction in capillarity and no evidence of muscle contractile or metabolic dysfunction studied in vitro, points to a VEGF-dependent change in vascular reactivity and/or perfusion of the capillaries. In vivo, real-time monitoring of the delivery of near-infrared fluorescent nanospheres to hind limb muscles, both during and after periods of electrically stimulated muscle contraction, allowed us to assess the level of muscle perfusion. The lower fluorescent signal in the VEGF-deficient gastrocnemius complex in response to repetitive contractions suggests VEGF may play a role in regulating O2 or nutrient delivery to the muscle.
Potential mediators of VEGF-dependent muscle perfusion.
A reduction in exercise-induced hyperemia or perfusion could be due to limited nitric oxide (NO) bioavailability secondary to the decrease in VEGF levels in the skeletal muscles. VEGF has been shown to upregulate the expression of endothelial nitric oxide synthase (eNOS) mRNA and protein and induce NO release from endothelial cells through Flk-1/VEGFR2 (1). Furthermore, mice or rats treated with Nω-nitro-l-arginine (l- NNA), a universal nitric oxide synthase inhibitor, or s-methyl-l-thiocitrulline, a more specific inhibitor of nNOS, experience a reduction in limb blood flow during intense exercise accompanied by decreased maximal oxygen consumption and aerobic work (11, 26). However, NO is not the only mediator of vasodilation and blood flow during exercise. Prostaglandins, endothelium-derived hyperpolarizing factor and AMPK are all examples of additional factors that may compensate for the loss of VEGF and NO to maintain perfusion during periods of aerobic exercise (4, 22, 36). Uncovering the mechanism of the impaired vascular perfusion response to exercise is well beyond the scope of the present study, and further work is needed to determine whether VEGF-dependent NO bioavailability and potential arterial remodeling contribute to skeletal muscle blood flow during exercise. Of note is the observation of a lower force-frequency relationship in the glycolytic, EDL muscle measured ex vivo than the more oxidative soleus, suggesting that the glycolytic muscles may be preferentially altered in the skeletal myofiber VEGF-deficient mice. Previous studies have demonstrated that blood flow is diverted to glycolytic fibers during exercise performed above critical speed (10). Thus, it is possible that muscle containing more glycolytic fibers may preferentially adapt to an impairment in contraction-induced perfusion. Further studies are required to directly address the possibility that VEGF-dependent microvascular perfusion occurs in glycolytic fibers during very strenuous exercise.
Limitations and future directions.
While the present study has focused on microvascular-myofiber interactions in limb muscle, several compensatory changes in the overall oxygen transport system could come into play to restore exercise tolerance. This is especially true if O2 available to the myofiber mitochondria reaches low enough levels to activate hypoxia-responsive genes. For example, an upregulation of HIF-1α and downstream glycolytic enzyme targets could contribute to a switch in substrate utilization.
This type of compensatory mechanism has been reported in myoglobin gene-ablated mice along with an increase in nitric oxide-dependent vasodilation, type I to type II fiber type transition, and increased expression of stress proteins (16, 27, 33). Future experiments to look at substrate utilization and glycogen stores, vasodilator mechanism and regulation of hemoglobin and myoglobin levels could provide insight into the potential compensatory mechanisms that allow mice with deficient skeletal myofiber VEGF expression to restore exercise tolerance.
Conclusion.
Using an inducible, skeletal myofiber VEGF knockout mouse, this study has revealed that, in the adult mouse, myofiber-expressed VEGF is important in acutely regulating muscle perfusion and exercise capacity. However, VEGF expressed by the myofiber alone is not critical for maintaining skeletal muscle capillarity or contractile function. The compensatory mechanisms that act to restore the transient decline in exercise endurance capacity remain to be elucidated. Taken together, with results from our previous studies, the present findings suggest that cells within skeletal muscle other than or in addition to the myofiber, are critical to adult skeletal muscle capillary maintenance. However, myofiber-expressed VEGF does play an important role in capillary perfusion and may, thus, contribute to the impaired exercise performance that occurs in older sedentary individuals or patients with chronic diseases accompanied by low skeletal muscle VEGF.
GRANTS
This article was funded by National Institutes of Health Grant1 PO1 HL-091830-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.C.B. and A.E.K. conception and design of research; E.C.B., A.E.K., D.G., H.D., B.M.T., and K.T. performed experiments; E.C.B., A.E.K., D.G., H.D., B.M.T., and K.T. analyzed data; E.C.B. and A.E.K. interpreted results of experiments; E.C.B. and A.E.K. prepared figures; E.C.B. and A.E.K. drafted manuscript; E.C.B., A.E.K., M.C.H., and P.D.W. edited and revised manuscript; E.C.B., A.E.K., M.C.H., and P.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to acknowledge Harrieth Wagner and Jeff Struthers for their technical support in completing this study. The H-CreERT2 cell line was generously provided by Dr. Metzeger and Dr. Chambon from the Institut de Génétique et de Biologie Moléculaire et Cellulaire.
Dr. Knapp's current affiliation is with the Department of Biology, Framingham State University, MA.
REFERENCES
- 1.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res 99: 715–722, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Ashrafpour H, Huang N, Neligan PC, Forrest CR, Addison PD, Moses MA, Levine RH, Pang CY. Vasodilator effect and mechanism of action of vascular endothelial growth factor in skin vasculature. Am J Physiol Heart Circ Physiol 286: H946–H954, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol (1985) 98: 1258–1263, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bradley EA, Eringa EC, Stehouwer CD, Korstjens I, van Nieuw Amerongen GP, Musters R, Sipkema P, Clark MG, Rattigan S. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol 30: 1137–1142, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol (1985) 97: 393–403, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972. [DOI] [PubMed] [Google Scholar]
- 10.Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588: 5077–5087, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol 591: 2885–2896, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther 17: 1788–1798, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D, Breen EC. Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol 306: R586–R595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol 33: 1956–1963, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Grange RW, Meeson A, Chin E, Lau KS, Stull JT, Shelton JM, Williams RS, Garry DJ. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am J Physiol Cell Physiol 281: C1487–C1494, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175–189, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hamalainen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41: 733–743, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Hang J, Kong L, Gu JW, Adair TH. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am J Physiol Heart Circ Physiol 269: H1827–H1831, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol 88: 560–566, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Hoier B, Prats C, Qvortrup K, Pilegaard H, Bangsbo J, Hellsten Y. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. FASEB J 27: 3496–3504, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, Kaley G. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol Heart Circ Physiol 278: H762–H768, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA, Simard C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil 18: 432–437, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560–H576, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130: 691–703, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation 98: 369–374, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Meeson AP, Radford N, Shelton JM, Mammen PP, DiMaio JM, Hutcheson K, Kong Y, Elterman J, Williams RS, Garry DJ. Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circ Res 88: 713–720, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Mirones I, Conti CJ, Martinez J, Garcia M, Larcher F. Complexity of VEGF responses in skin carcinogenesis revealed through ex vivo assays based on a VEGF-A null mouse keratinocyte cell line. J Invest Dermatol 129: 730–741, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD. Skeletal muscle capillarity and angiogenic mRNA levels after exercise training in normoxia and chronic hypoxia. J Appl Physiol 91: 1176–1184, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu- Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak V̇o2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD. Evidence for tissue diffusion limitation of V̇o2 max in normal humans. J Appl Physiol 67: 291–299, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Schlieper G, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flogel U, Godecke A, Schrader J. Adaptation of the myoglobin knockout mouse to hypoxic stress. Am J Physiol Regul Integr Comp Physiol 286: R786–R792, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Schuler M, Ali F, Metzger E, Chambon P, Metzger D. Temporally controlled targeted somatic mutagenesis in skeletal muscles of the mouse. Genesis 41: 165–170, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Sellke FW, Wang SY, Stamler A, Lopez JJ, Li J, Li J, Simons M. Enhanced microvascular relaxations to VEGF and bFGF in chronically ischemic porcine myocardium. Am J Physiol Heart Circ Physiol 271: H713–H720, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18: 63–69, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Tang K, Gu Y, Dalton ND, Wagner H, Peterson KL, Wagner PD, Breen EC. Selective life-long skeletal myofiber-targeted VEGF gene ablation impairs exercise capacity in adult mice. J Cell Physiol 231: 505–511, 2016. [DOI] [PubMed] [Google Scholar]
- 39.Tang K, Wagner PD, Breen EC. TNF-α-mediated reduction in PGC-1α may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol 222: 320–327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol 287: C1342–C1348, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Wei W, Jin H, Chen ZW, Zioncheck TF, Yim AP, He GW. Vascular endothelial growth factor-induced nitric oxide- and PGI2-dependent relaxation in human internal mammary arteries: a comparative study with KDR and Flt-1 selective mutants. J Cardiovasc Pharmacol 44: 615–621, 2004. [DOI] [PubMed] [Google Scholar]