Abstract
Preeclampsia (PE) is a pregnancy-associated disorder that affects 5–8% of pregnancies and is a major cause of maternal, fetal, and neonatal morbidity and mortality. Hallmark characteristics of PE are new onset hypertension after 20 wk gestation with or without proteinuria, chronic immune activation, fetal growth restriction, and maternal endothelial dysfunction. However, the pathophysiological mechanisms that lead to the development of PE are poorly understood. Recent data from studies of both clinical and animal models demonstrate an imbalance in the subpopulations of CD4+ T cells and a role for these cells as mediators of inflammation and hypertension during pregnancy. Specifically, it has been proposed that the imbalance between two CD4+ T cell subtypes, regulatory T cells (Tregs) and T-helper 17 cells (Th17s), is involved in the pathophysiology of PE. Studies from our laboratory highlighting how this imbalance contributes to vasoactive factors, endothelial dysfunction, and hypertension during pregnancy will be discussed in this review. Therefore, the purpose of this review is to highlight hypertensive mechanisms stimulated by inflammatory factors in response to placental ischemia, thereby elucidating a role
Keywords: cytokines, hypertension, inflammation, pregnancy
in the united state alone, preeclampsia (PE) is a common disorder, affecting 5–7% of all pregnancies and accounts for 18% of maternal deaths and is the number one reason for premature births, annually (41). According to the National High Blood Pressure Education Program (73a), a blood pressure of 140/90 mmHg during pregnancy in women who were not previously hypertensive before pregnancy, constitutes a PE pregnancy (75). PE is typically diagnosed past the 20th wk of gestation and worsens with progression of the pregnancy until delivery. While PE remains a significant contributor to maternal deaths, it is also a great cause of perinatal morbidity due to premature births. Although the manifestations of PE do not appear until 20 wk of gestation, the disease process is hypothesized to begin at implantation. Immune cells such as macrophages, uterine natural killer (NK) cells, dendritic cells (DCs), and T regulatory cells (Tregs) are present in the decidua and are required for the normal invasion of trophoblast cells during placentation. These immune cells communicate with the invading trophoblasts to allow for the remodeling of the uterine spiral arteries creating a low resistance system, leading to increased blood volume and flow to the uteroplacental unit. DCs present in the decidua are thought to function to promote a CD4+ T helper 2 (Th2) dominate state in the uterus and placenta to induce immunotolerance of the mother to the fetus. This communication is modulated by the release of cytokines and angiogenic factors. Importantly, inflammatory cells, such as DCs and cytolytic NK cells are decreased in the periphery during a normal pregnancy (91, 102).
Although the immune system is altered during a normal pregnancy compared with the nonpregnant state, women with PE and those suffering from unsuccessful pregnancies have an even greater alteration, with an increase in innate immune activation in the periphery as well as the uteroplacental unit (18). It is thought that factors secreted from immune cells such as CD4+ T helper 1 (Th1) cells, cytolytic NK cells, and B cells may play a role in the shallow trophoblast invasion and endothelial dysfunction, as well as contribute to decreased maternal vascular remodeling of the placental unit beginning early in the first trimester of a PE pregnancy. As the pregnancy progresses the disease worsens and such factors may also contribute to the maternal vascular and renal dysfunction and hypertension observed in the later trimesters of PE pregnancy. The shallow trophoblast invasion and lack of vascular remodeling contributes to an increase in uterine artery resistance, reduced placental blood flow, low oxygen and nutrient delivery, and the development of placental ischemia and intrauterine growth restriction of the fetus. The reduction in the supply of oxygenated blood to the uteroplacental unit causes a robust increase in oxidative stress and hypoxia-stimulated factors, such as soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), which also play a role in the development of hypertension (86). Historically, PE has not been classified as an inflammatory disorder; however, new findings suggests that the major shift in immune responses during PE versus normal pregnancies certainly suggests that PE shares many of the unique disease features displayed in autoimmune disorders.
Several studies have shown that changes in the CD4+ T helper population of cells (Th1 vs. Th2) are instrumental in maternal tolerance and overall health of a pregnancy. In fact, previous studies from the Zenclussen lab show that adoptive transfer of Th1-like splenocytes increases mean arterial pressure (MAP), fetal rejection, and inflammatory cytokines when transferred to normal pregnant mice (118). However, more recent analysis of circulating blood collected from PE women has demonstrated a much greater shift in the immunotolerance paradigm than what was previously suggested. Important clinical studies demonstrated a decrease in the proportion of circulating Treg cells and an increase in Th17 cells (27, 60, 81, 85–87). Th17 cells and cytokines such as interleukin (IL)-17, which have been known to be upregulated in autoimmune disorders such as lupus, psoriasis, and multiple sclerosis, play an important role in the host-defense mechanisms against extracellular bacteria by recruiting neutrophils to the site of infection (30). Th17 and neutrophils generate oxidative stress molecules for bactericidal action. Thus it is hypothesized that the increase in proinflammatory T helper cells and Th17 cells will increase the release of cytokines, recruitment of neutrophils, and production of oxidative stress in the placenta of PE patients (30, 85). Tregs are responsible for suppression of responses in the adaptive and innate immune system and control unwanted immune responses through various mechanisms (40, 46). Loss of Treg function has been shown to lead to autoimmune diseases and other immunopathology, including maternal loss of tolerance for the fetus during pregnancy (31, 39, 40, 46). Anti-inflammatory cytokines associated with Tregs and Th2 cells help regulate the immune response. Therefore, Tregs and Th2 and their respective cytokines IL-10 and IL-4 play important roles in a normal, successful pregnancy by providing a balance to the immune system (14, 108). The loss of Treg cells and their function could allow for an uncontrolled proinflammatory nature during PE by allowing for the observed increase in Th1 and Th17 cells.
While both innate and adaptive immune cells populate blood vessels under normal conditions, low-grade inflammation has most recently been established as a mechanism contributing to the development of cardiovascular disease (76, 88). During inflammation, leukocyte numbers in the vascular wall greatly increases due to increased adhesion molecule and cytokine expression stimulating migration and proliferation of white blood cells in the vasculature (76). This leads to oxidative stress and increased inflammation, causing impaired vascular function (30). The inflammatory state leads to endothelial cell barrier dysfunction that results in increased vascular permeability (96). Proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, and IL-17 promote cytotoxic and inflammatory responses permeability (26, 29, 49, 59, 62, 120). In the vasculature, increased TNF-α and IL-6 both contribute to endothelial dysfunction, a hallmark feature of PE, and is characterized by increased adhesion molecules and endothelial cell permeability, whereas IL-17 promotes oxidative stress from neutrophils and Th17 cells (20, 50). During PE, increased TNF-α, IL-17, and IL-6 are present in the circulation and in the trophoblast cells of the placenta, whereas IL-10 and IL-4 are decreased (7, 39, 49). Studies have demonstrated that the increased inflammation observed during PE antepartum persists postpartum as well. Vitoratos et al. (103) demonstrated that women with PE remained under inflammatory stress up to 12–14 wk postpartum. A later study by Kvehaugen et al. (56) showed evidence of chronic systemic inflammation and persistent endothelial dysfunction 5–8 yr postpartum in women and their offspring after PE (56). Furthermore, a preclinical study by Pruthi et al. (81) demonstrated that exposure to experimental PE led to increased vascular damage after injury compared with normal pregnancy in mice. It is possible that the vascular dysfunction observed in PE women postpartum is due to the persistent inflammation after delivery. Nevertheless, such studies suggest that persistent inflammation, postpartum, may play a role in future cardiovascular disease risk in women with PE.
Several studies have reported that in addition to the hypertension and inflammation that occurs during PE, there are also changes in the central nervous system. During pregnancy there is diminished vascular resistance and hyperpermeability in the maternal brain, which increases the risk of neurological complications and cerebral edema; both of which are exacerbated during acute hypertension (13, 24). Women with PE have been reported to have an increased risk of developing stroke, white matter damage, and impaired cerebral autoregulation (97, 121, 122). Cerebral complications such as stroke, structural changes in the brain, cerebrovascular alterations, and tight junction impairments are common in inflammatory-based pathological diseases such as multiple sclerosis, rheumatoid arthritis, and HIV, suggesting that immune mediators in the circulation may have a role in impairing the blood-brain barrier (BBB) (6, 38, 52). Studies by Amburgey et al. (4) demonstrated that circulating factors in plasma from women with PE significantly increases BBB permeability. Furthermore, other studies have reported that inflammatory cytokines such as TNF-α or immune cells like CD4+ T cells are also capable of damaging the BBB (8, 54, 65). It is possible that the inflammatory factors and acute hypertension that is experienced by women with PE during pregnancy damages the vascular endothelium of the BBB, thereby increasing the permeability and entry of inflammatory mediators into the brain, which in turn contribute to neuroinflammation and neurovascular hypertension (68, 113).
Various animal models of placental ischemia exhibit an increase in the production of antiangiogenic factors (sFlt-1), inflammatory cytokines, and immune cells (such as CD4+ T cells), which is associated with the development of hypertension during pregnancy (7, 27, 59, 71, 72, 99, 101, 103, 104, 118, 119). Furthermore, work over recent years has demonstrated a role for these factors to lead to increased vasoconstrictor endothelin-1 (ET-1) and decreased vasodilator, nitric oxide (NO), oxidative stress, and autoantibodies activating the angiotensin II (ANG II) type I receptor (AT1-AA) and hypertension, all of which are associated with clinical presentation of PE (10, 42, 72, 79, 99, 104–106, 119). However, studies to determine whether inflammatory CD4+ helper T-cell populations and other such factors remain elevated postpartum in preeclamptic women should be performed to determine their role in mediating cardiovascular disease in this patient population later in life.
Reduced Uterine Perfusion Pressure Model
The reduced uterine perfusion pressure (RUPP) rat model is the most reproducible animal model of PE. RUPP is used to create placental ischemia and thereby examine mechanisms involved in the pathophysiology resulting in hypertension, which are associated with PE (66, 106). In the RUPP model, a 0.203-mm ID silver clip is placed around the aorta above the iliac bifurcation and two 0.100-mm ID silver clips are placed on the right and left uterine artery arcades on day 14 of gestation. These clips placed on the rats' blood vessels allows for reduction in uterine flow in the pregnant rat by ∼40% causing placental ischemia. Placental ischemia in these rats is associated with an increase in oxidative stress, decreased antioxidant activity similar to what is observed in PE women (89). Placental ischemia in RUPP rats also causes the release of several antiangiogenic factors such as sEng and sFlt-1, an antiangiogenic factor that antagonizes vascular endothelial growth factor (VEGF), and placental growth factor (PlGF) (33, 34). In addition, placenta ischemia stimulates chronic inflammation, plasma IL-6, TNF-α, and IL-17, and AT1-AA (22, 60, 64). RUPP rats display an 13% increase in circulating CD4+ T cells compared with normal pregnant rats and a 47% decrease in Treg cells and increase in Th17 cells in the peripheral circulation compared with normal pregnant rats (106).
Effect of RUPP CD4+ T Cells to Cause Hypertension During Pregnancy
Similar to what is seen in women with PE, CD4+ T cells are increased in placental ischemic RUPP rats compared with normal pregnant rats (106). In recapitulation of the idea from the Zenclussen lab, we examined the role of CD4+ T cells from RUPP rats to induce hypertension in normal pregnant rats. Through a series of studies, CD4+ T cells from RUPP rats were adoptively transferred into normal pregnant rats, which not only repeatedly resulted in a significant increase in MAP, but also resulted in an increase in both TNF-α and sFlt-1, both of which have also been demonstrated to contribute to hypertension during pregnancy (59, 69, 106). Even though the mechanisms associated with hypertension during pregnancy have not been fully elucidated, several studies have indicated that increased TNF-α and sFlt-1 during pregnancy mediate hypertension via activation of the AT1-AA, endothelin, and/or reactive oxygen species (ROS) pathways, in addition to the other antiangiogenic or inflammatory effects stimulated by sFlt-1 or TNF-α (59, 73, 99).
Therefore, we performed additional studies to determine the mediator of the hypertension observed in recipients of RUPP CD4+ T cells. We found that there was indeed increased placental and renal production of ET-1. Furthermore, serum from recipient rats significantly increased vascular endothelial cell secretion of ET-1 from human umbilical vein endothelial cells (HUVECs) in culture. Blockade of the ETA receptor by administration of an ETA receptor antagonist significantly decreased the hypertension in normal pregnant recipient rats of RUPP CD4+ T cells (105), suggesting that activation of the endothelin pathway by CD4+ T cells is one mechanism causing hypertension during pregnancy. Additionally, oxidative stress was found to be stimulated in normal pregnant recipients of RUPP CD4+ T cells (104). Reduction of oxidative stress with the antioxidant apocynin, significantly reduced MAP, CD4+ T cells, and placental and cortical ROS in recipients of RUPP CD4+ T cells (104). Subsequent studies demonstrated that adoptive transfer of RUPP CD4+ T cells into NP rats induced AT1-AA and impaired renal function in recipient rats (77). Blockade of the AT1 receptor with losartan attenuated the hypertension in recipients of RUPP CD4+ T cells (77), suggesting that B cell secretion of AT1-AA can be stimulated by placental ischemic CD4+ T cells. Therefore, in a follow-up study we incubated RUPP CD4+ T cells with an antibody to the CD40 ligand (CD40L) before adoptive transfer into normal pregnant rats. One interaction that is essential for long-term B cell activation is between CD40 on B cells and CD40L on T cells. We hypothesized that communication of activated T cells with endogenous immune and/or vascular cells via CD40-CD40L interactions during PE mediate pathophysiology in response to placental ischemia-stimulated CD4+ T cells and is crucial to stimulating production of the AT1-AA. We found that blockade of CD40L on RUPP T cells before adoptive transfer attenuated the AT1-AA from being produced and resulted in less ET-1, placental ROS, and hypertension in normal pregnant recipient rats (15). These data suggest the AT1-AA is produced by a T cell-dependent response dependent on CD40L. Finally, endogenous T cell costimulation was inhibited in normal pregnant rats before the RUPP procedure to determine the effects of CD4+ T cells on placental ischemia (78). In this study, administration of abatacept (Orencia), a fusion molecule of CTLA4 that inhibits T cell costimulation, significantly decreased CD4+ T cells and hypertension, circulating sFlt-1 and TNF-α, and AT1-AA compared with untreated control RUPP rats. Furthermore, when the RUPP procedure was performed in pregnant nude/athymic rats, rats naturally deficient in T cells, there was no change in blood pressure or fetal weights (78). These studies suggested that CD4+ T cells have a direct role in not only contributing to hypertension in pregnancy but also inflammation and the antiangiogenic imbalance that is present during placental ischemia.
Angiotensin II Type 1 Receptors Autoantibodies Mediate Hypertension During Pregnancy
In the 1960s, human studies performed by Gant et al. (32) demonstrated that women that went on to develop PE exhibited increased vasoconstriction and blood pressure sensitivity to intravenously infused ANG II than those that went on to have normal pregnancies. However, biochemical assessment indicates PE women have normal plasma renin activity and ANG II levels (32). Several years later in the 1990s Wallukat et al. (107) reported that PE women produce AT1-AA. This AT1-AA binds to and activates the AT1R, thereby increasing chronotropic events in cultured cardiomyocytes, very similarly to ANG II, and are suggested to be an important link between placental ischemia and the development of hypertension. AT1-AAs bind with a high affinity to the seven amino acid sequence on the second extracellular loop of the ANG II type 1 receptor (AT1R) and increase AT1 receptor activity, intracellular calcium levels, and activation of intracellular mitogen-activated kinase/ERK kinase pathways (19, 100, 116). AT1-AAs activity on the AT1 receptor was verified by antibody isolation and affinity column antibody purification. Bioactivity was determined analyzing chronotropic events stimulated in cultured neonatal rat cardiomyocytes, which was blocked with losartan, the AT1R antagonist (107). AT1-AA increases tissue factor, ROS production, NADPH oxidase components, and activation of nuclear factor κ-β (NFκ-β) from vascular smooth muscle cells (VSMC) and trophoblast cells (21). AT1-AAs cultured with rat neonatal cardiomyocytes not only increase chronotropic events in cardiomyocytes but later cause apoptosis of cardiomyocytes via a TNF-α-mediated pathway (11, 47), suggesting their role in cellular or tissue necrosis and death.
Although the antigen for the generation of AT1-AA is unknown, we have shown the AT1-AA to be generated in pregnant rats with placental ischemia (RUPP rats) or by infusion of TNF-α, IL-6, IL-17, or by adoptive transfer of RUPP CD4+ T cells into normal pregnant rats (22, 64). We can hypothesize that AT1-AAs are derived from an immunological loss of self-tolerance toward the AT1R, which results in the accumulation of antibodies against the AT1R. However, antibodies can also be generated by molecular mimicry, a process in which a different molecule from a different gene or their protein product can generate autoantibodies due to similarities in structure to the epitope binding region of the antigen of interest (79, 98). For instance the human parvovirus B19 (PVB19) capsid proteins shares a six amino acid homology with the seven amino acid sequence on the second extracellular loop of the AT1R (42, 98). PVB19 is a single-stranded DNA virus that codes for nonstructural proteins and two capsid proteins (VP1 and VP 2) (42, 98). The PVB19 has a seroprevalance of ∼80% of the population and associated with several autoimmune diseases and PE (42, 98). Although this is a proposed mechanism of AT1-AA generation, more research is warranted to determine whether the PVB19 capsid proteins may serve as an antigen for AT1-AA production.
Studies with chronic AT1-AA infusion into pregnant rats increased blood pressure which was associated with increased ROS, ET-1, and sFlt-1 production (80). Furthermore, AT1-AA have been shown in an animal model to result in enhanced sensitivity to ANG II (112) as was observed in PE women by Gant et al. (31). When ANG II along with AT1-AA is administered acutely to rats during pregnancy, there is a 40-mmHg increase in blood pressure above what is observed with ANG II administered alone (10, 112). In addition, chronic ANG II and AT1-AAs administered together not only further increases MAP but also heightens oxidative stress, ET-1 secretion, and renal artery resistive index above ANG II or AT1-AA administered alone (Fig. 1) (10, 112). Studies from our lab have shown that HUVECs incubated 6 h with ANG II (10−7 M) and AT1-AAs exhibited a 100-fold increase in ET-1 (112), which was not observed in HUVECs incubated with ANG II or AT1-AAs alone. These data suggest a synergistic effect among ANG II, AT1-AA, and activation of AT1R (112). One could also extrapolate from these data that there is an enhancement of ANG II signaling when AT1-AAs are present (112).
Fig. 1.
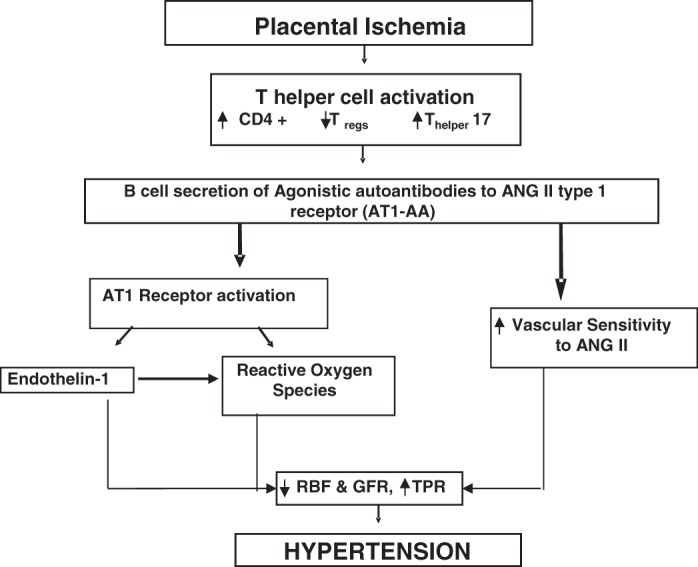
Schematic illustrates our hypothesis that T cell profile altered in response to placental ischemia can lead to hypertension by stimulating B cell secretion of angiotensin II (ANG II) type I receptor (AT1-AA), thus leading to endothelin 1, reactive oxygen species, and enhanced vascular sensitivity to ANG II during pregnancy.
Although the mechanisms of increased ANG II sensitivity in PE is unknown, we hypothesize that AT1-AAs increase ANG II sensitivity in PE by increasing the AT1R affinity for ANG II when the AT1-AA are present in circulation. One mechanism by which the AT1-AA can increase ANG II sensitivity is by increasing the dimerization of the AT1R (1, 83, 115). When the AT1R heterodimerizes with the vasodepressor bradykinin receptor (B2), an increase in AT1R responsiveness to ANG II occurs (115). AbdAlla et al. showed that women with PE have increased B2 protein amounts and that AT1/B2 heterodimerzation is present in the platelets and the omental vessels of PE patients (1, 83, 115). Thus the mechanism by which AT1-AAs increase ANG II sensitivity in PE could be due to AT1R receptor dimerization, however, more studies are needed to verify this hypothesis.
AT1-AAs are not only elevated in women with PE but are also elevated in pregnancies with uterine growth-restricted fetuses, kidney transplant recipients, patients with systemic sclerosis, vasculopathy, tissue fibrosis, hypertension, renovascular disease, and pregnant women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome (23, 25, 28, 67, 119). AT1-AAs appear to be elevated as early as the second trimester of pregnancy (108) and exist up to 1 year postpartum in ∼18% of PE patients (44). In addition, AT1-AA are associated with greater severity of the disease and could be an indicator in midgestation of worsening progression of the pregnancy toward PE (94). Most recently, we have shown that AT1-AA may be a causal factor for many of the neurological symptoms associated with PE. Warrington et al. (109) recently demonstrated that hypertension in response to chronic AT1-AA infusion compromised cerebral blood flow. The increase in the AT1-AA during the second trimester coincide with the development of headaches, blurred vision, and other neurological difficulties. With this most recent evidence one can infer that the AT1-AAs play a causal role not only in the hypertension but neurological symptoms during PE. Furthermore, one can suggest that the persistence of AT1-AAs after delivery maybe a risk factor for cardiovascular and renal disease and neurological complications that women with PE are likely to suffer from later in life.
Alterations in Th17 and Tregs Cause Hypertension During Pregnancy
As described above, PE is associated with a chronic immune activation that leads to an increased production of inflammatory cytokines by proinflammatory T cells, and a decrease in regulatory and anti-inflammatory cytokines, which further promotes an inflammatory state during pregnancy (9, 26, 36, 110). This imbalance between proinflammatory and regulatory cytokines could be in response to the placental ischemia that occurs during a PE pregnancy. This imbalance worsens as the pregnancy continues, which coincides with the worsening of PE symptoms (58).
IL-17 and Th17 Cells Mediate Hypertension, Oxidative Stress, and AT1-AA During Pregnancy
Clinical studies show an association between PE and increased Th17 cells and IL-17. The IL-17 family is made up of six cytokines: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F. IL-17A is the prototype member, and IL-17A and IL-17F have similar functions, are genetically linked, and bind to the same heterodimeric receptor, consisting of IL-17 receptor A and C (55, 70). Binding of the IL-17 receptor activates the NFκB and mitogen-activate protein kinase pathways (55) acting in a feedback loop for expansion of Th17s and are therefore key cytokines responsible for proliferation, recruitment, activation, and migration of neutrophils (55, 70, 71, 117). IL-17 stimulates the production of other cytokines by neutrophils for cell-to-cell communication, and more importantly, it stimulates these cells to release antimicrobial substances, such as ROS as a host-defense mechanism used by neutrophils and macrophages against bacterial infection (70, 117). However, a role for Th17 and IL-17 in mediating the pathophysiology of PE had not previously been established. To determine whether increased IL-17 is relevant in the pathology of PE, Dhillon et al. (22) examined the effect of IL-17 infusion on blood pressure. In this study, IL-17 infusion into normal pregnant rats resulted in a significant increase in MAP and oxidative stress as measured by urinary isoprostane and NADPH-stimulated placental ROS, increased circulating Th17 cells, and AT1-AA. Blockade of the AT1 receptor by administration of losartan attenuated the blood pressure response and decreased placental production of ROS in response to chronic IL-17. Additionally, depletion of B cells with rituximab blunted the blood pressure response and decreased circulating Th17 cells in IL-17-infused rats. Finally, administration of the superoxide dismutase mimetic tempol attenuated the hypertension placental production of ROS and circulating AT1-AAs produced in response to IL-17 infusion (22). The effect of tempol on IL-17-induced pathophysiology suggests that the ROS stimulated by Th17s, via IL-17, may be an important signaling molecule for the activation of B cells to produce AT1-AAs.
To support a role for IL-17 in the pathophysiology of PE, we examined the effect of IL-17 blockade in the RUPP rat model. A soluble form of the IL-17 receptor C (IL-17RC) was infused from gestational day 14–19 in RUPP rats to block the IL-17 signaling pathway. This resulted in significant decreases in blood pressure, intrauterine growth restriction, oxidative stress, and AT1-AA and lowered circulating Th17 cells. Importantly, infusion of IL-17RC increased pup weight, which was accompanied by increased placental weight and decreased uterine artery resistance index (17). Thus inhibition of this immune pathway could normalize placental inflammation and improve fetal demise as a direct result of decreased placental stress thus serving as a fetal protective mechanism during PE. Therefore, this form of immunosuppression may have an important role to preserve the health in both mother and fetus.
Effect of Decreased Tregs and IL-10 in Preeclampsia
Anti-inflammatory cytokines that help regulate the immune response, like IL-10 and IL-4, play important roles in a normal, successful pregnancy by providing a balance to the immune system (111). Along with increased levels of inflammatory cytokines and T cells, regulatory cytokines and T cells are decreased during PE and in response to placental ischemia (RUPP). These cells are identified by the expression of cell surface markers CD4+CD25+ and their specific internal transcription factor forkhead box protein 3 (Foxp3+) (53). Tregs are responsible for suppression of responses in the adaptive and innate immune system and control unwanted immune responses through various mechanisms. Loss of Treg function has been shown to lead to autoimmune diseases and other immunopathology, including maternal loss of tolerance for the fetus during pregnancy (53). Tregs are increased very early in normal pregnancy and reach their highest levels during the second trimester before decreasing back to normal levels (82). In diseases such as PE where Tregs and their secretion of IL-10 and TGF-β are reduced (45), stimulation and proliferation of inflammatory T cells remains unchecked, leading to excess secretion of inflammatory cytokines such as TNF-α, IL-6, and IL-17, which contribute to endothelial dysfunction, oxidative stress, AT1-AA, and the increase in blood pressure during PE.
A number of studies have provided evidence for reduced Tregs to contribute to PE. Circulating and decidual Tregs have been shown to be decreased in women with PE, and the decrease in Tregs is directly proportional to the severity of the disease (87). IL-10 is an important anti-inflammatory cytokine that is secreted by Tregs and also stimulates Tregs from naïve T cells (46, 74, 90). IL-10 inhibits Th1 inflammatory cytokines secretion and inflammation at the fetal-maternal interface. Proinflammatory cytokines downregulated by IL-10 include IFN-y, IL-2, and TNF-α (35). Levels of IL-10 are increased throughout normal pregnancy and first begin to drop when labor begins (95). In addition to Tregs, villous cytotrophoblasts secrete IL-10 during pregnancy. Moreover, the expression of IL-10 (12) and its receptor have been reported on a number of cell types in the decidua, including trophoblasts, stromal cells, macrophages, and uterine natural killer cells. It is not clear how IL-10 may influence trophoblast invasion; however, the presence of IL-10 at the fetal-maternal interface and its strong anti-inflammatory properties are suspected to contribute to allogeneic tolerance of the fetus during a normal pregnancy (39, 48, 57, 71, 84, 101).
Most recent data indicate that Treg cells can be further classified into Helios natural Treg (nTreg) cells and Helios-adaptive Treg (iTreg) cells (43). nTreg cells are generated in the thymus, and iTreg cells are derived from naive CD4+ T cells in the periphery. Newer evidence indicates that iTreg cell expansion occurs in healthy pregnancy but not in PE, suggesting that this may originate from the decidua, where distinct dendritic antigen presenting cells reside that are especially proficient to cause iTreg cell induction, essential for healthy pregnancy. Therefore, both iTreg cell induction and expansion are impaired in PE thereby allowing for chronic peripheral and placental inflammation, further compromising the pregnancy (43). Induction of iTreg cells may be important for immune tolerance toward the fetus, and if this is missing it becomes instrumental in causing the exacerbated inflammation observed during PE.
Our most recent studies demonstrated that adoptive transfer of Tregs from normal pregnant rats into RUPP rats lowered blood pressure, minimized intrauterine growth restriction, reduced the inflammatory response, and significantly decreased ET-1 and placental oxidative stress. Importantly, Tregs attenuated the production of AT1-AA in response to placental ischemia (16). These data further support a role for decreased Tregs in the pathophysiology of PE. In addition we have supplemented IL-10 in RUPP rats, via osmotic mini pumps, to restore the circulating levels IL-10 to that of normal pregnant rats (37). IL-10 supplementation lowered the total population of circulating CD4+ T cells while increasing the population of circulating Tregs to that of normal pregnant rats. In addition to normalizing Tregs, IL-10 supplementation also normalized proinflammatory cytokines TNF-α and IL-6 and, importantly, supplementation of IL-10 to RUPP rats significantly lowered AT1-AA, placental ROS and ET-1, which proved beneficial in decreasing blood pressure (37). It could be that the lower levels of IL-10 during PE would allow for increased T cell activation and differentiation into a proinflammatory phenotype, thus, leading to an increased number of inflammatory T helper cells, proinflammatory cytokines, and possibly B cell stimulation. Furthermore, it could be the decrease in Tregs that results in lower levels of IL-10 during PE. Although adoptive transfer studies may not be feasible for treatment of PE, supplementation with IL-10 could be a beneficial therapeutic to consider.
Challenges in Treating Preeclampsia
Women with PE are at high risk for eclamptic seizures and therefore are given magnesium sulfate for seizure prevention (114). In severe PE where the systolic blood pressure of the mother is 160–180 mmHg or above and diastolic blood pressure is 105–110 mmHg or higher, intravenous antihypertensive therapy such as hydralazine (vasodilator) or labetalol (β-blocker) is used to manage the blood pressure (73a, 92). Other classes of antihypertensive drugs like angiotensin-converting enzyme (ACE) inhibitors and ANG II receptor blockers cannot be used during pregnancy due to their potential teratogenic effects on the fetus (93). Since current treatments for PE are not always effective to improve maternal and fetal outcomes, a greater emphasis on drug discovery for treatment of PE is essential. Over the last few years, we have utilized several anti-inflammatory agents that decrease hypertension and circulating factors in RUPP rats that are known to be elevated in PE. Such agents include TNF-α inhibitor etanercept (61), B lymphocyte-depleting agent rituximab (63), and most recently, T cell suppressor abatecept (Orencia) (78), and IL-17RC, all of which decreased blood pressure and inflammation in the RUPP rat model of PE. However, these pharmacological inhibitors of the inflammatory cytokines or lymphocytes have not been considered obstetrically safe for use in PE women. Although, the benefits of immune suppression therapies for the mothers and fetuses are not well understood, administration of the IL-17 RC may have minimal effects on the innate response while improving fetal weight and could be considered further for use during pregnancy.
Interestingly, 17-hydroxyprogesterone caproate (17-OHPC), a progesterone derivate, is obstetrically safe and can be used for the attenuation of preterm labor and has been shown to exhibit anti-inflammatory actions in rat models of PE, its efficacy in the either treatment or management of PE is unclear. Our previous studies have demonstrated that 17-OHPC administered during late gestation to RUPP rats improved hypertension by suppressing immune CD4+ T cells and inflammatory cytokines, along with the vasoconstrictor ET-1. In addition, 17-OHPC also improves NO bioavailability and reduced sFlt-1 during pregnancy (2, 3, 51). These data suggest that an intervention such as 17-OHPC to enhance current management for affected patients could be positive for mother and child.
Perspectives and Significance
While PE is the primary global cause of prematurity and perinatal morbidity/mortality, there is a tremendous need for improved treatment modalities for this disorder, especially to arrest its development and progression when otherwise very preterm delivery would be necessary. Indeed, our recent findings could have profound implications on identifying possible immunotargets for the treatment of PE. However, further studies are necessary to better understand which therapies could be appropriate to add to the management of PE that will provide safe outcomes for both mothers and fetuses.
GRANTS
This work was supported by National Institutes of Health Grants HL105324, HL126301, HL124715, HL51971, HL78147, and HD067541.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.D.L. and D.C.C. conception and design of research; B.D.L., D.C.C., A.C.H., L.M.A., M.W.C., J.L.F., and K.W. performed experiments; B.D.L., D.C.C., A.C.H., L.M.A., M.W.C., and K.W. analyzed data; B.D.L., D.C.C., A.C.H., L.M.A., M.W.C., and K.W. interpreted results of experiments; B.D.L. prepared figures; B.D.L., D.C.C., A.C.H., L.M.A., M.W.C., J.L.F., and K.W. drafted manuscript; B.D.L., D.C.C., A.C.H., L.M.A., M.W.C., J.L.F., and K.W. edited and revised manuscript; B.D.L., D.C.C., A.C.H., L.M.A., M.W.C., J.L.F., and K.W. approved final version of manuscript.
REFERENCES
- 1.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med 7: 1003–1009, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JNJ Jr, LaMarca B. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 65: 225–231, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J, Wallukat G, Martin JN Jr, Dechend R, LaMarca B. Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin II type I receptor in response to elevated interleukin-6 during pregnancy. Am J Obstet Gynecol 211: 158.e1–158.e6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension 56: 1003–1008, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res 74: 255–265, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J Soc Gynecol Investig 12: 335–342, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71: 667–675, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bennett WA, Lagoo-Deenadayalan S, Stopple JA, Barber WH, Hale E, Brackin MN, Cowan BD. Cytokine expression by first-trimester human chorionic villi. Am J Reprod Immunol 40: 309–318, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN Jr, Lamarca B. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 62: 886–892, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai W, Zhang W, Jin Z, Feng Y, Kuang Y, Zhi J. Angiotensin II type I receptor agonistic autoantibody-induced apoptosis in neonatal rat cardiomyocytes is dependent on the generation of tumor necrosis factor-α. Acta Biochim Biophys Sin (Shanghai) 44: 984–990, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Chaouat G, Cayol V, Mairovitz V, Dubanchet S. Localization of the Th2 cytokines IL-3, IL-4, IL-10 at the fetomaternal interface during human and murine pregnancy and lack of requirement for Fas/Fas ligand interaction for a successful allogeneic pregnancy. Am J Reprod Immunol 42: 1–13, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Cipolla MJ. The adaptation of the cerebral circulation to pregnancy: mechanisms and consequences. J Cereb Blood Flow Metab 33: 465–478, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, Scott J, Herse F, Haase N, Moseley J, Wallukat G, Dechend R, LaMarca B. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R884–R891, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelius DC, Castillo J, Porter J, Amaral LM, Campbell N, Paige A, Thomas AJ, Harmon A, Cunningham MW Jr, Wallace K, Herse F, Wallukat G, Dechend R, LaMarca B. Blockade of CD40 ligand for intercellular communication reduces hypertension, placental oxidative stress, and AT1-AA in response to adoptive transfer of CD4+ T lymphocytes from RUPP rats. Am J Physiol Regul Integr Comp Physiol 309: R1243–R1250, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, Scott J, Herse F, Haase N, Moseley J, Wallukat G, Dechend R, LaMarca B. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R884–R891, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis D, Kaufmann R, Moticka EJ. Nonspecific immunity in pregnancy: monocyte surface Fcgamma receptor expression and function. J Reprod Immunol 40: 119–128, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 101: 2382–2387, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Dechend R, Homuth V, Wallukat G, Müller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig 13: 79–86, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Dechend R, Viedt C, Müller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 107: 1632–1639, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN Jr, Dechend R, LaMarca B. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol 303: R353–R358, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Duvekot JJ, Peeters LL. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv 49, Suppl: S1–S14, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Fischer T, Wallukat G, Schneider MP, Schlembach D, Munz W, Homuth V. HELLP syndrome in the 18th week of gestation in association with elevated angiotensin AT(1)-receptor autoantibodies. Eur J Obstet Gynecol Reprod Biol 97: 255–257, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Formby B. Immunologic response in pregnancy. Its role in endocrine disorders of pregnancy and influence on the course of maternal autoimmune diseases. Endocrinol Metab Clin North Am 24: 187–205, 1995. [PubMed] [Google Scholar]
- 27.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 44: 708–714, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Fu ML, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, Sjögren KG, Hjalmarson A, Müller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens 18: 945–953, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Gaffen SL. An overview of IL-17 function and signaling. Cytokine 43: 402–407, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol 38: 1807–1813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol 164: 5721–5728, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Harmon A, Cornelius D, Amaral L, Paige A, Herse F, Ibrahim T, Wallukat G, Faulkner J, Moseley J, Dechend R, LaMarca B. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy 34: 291–306, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol 163: 3491–3495, 1999. [PubMed] [Google Scholar]
- 40.Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM, Cho ML, Kwok SK, Ju JH, Park KS, Cho SG, Park SH, Kim HY, Min JK. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett 127: 150–156, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Herse F, LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am J Reprod Immunol 69: 413–418, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modrow S, Wallukat G, Luft FC, Redman CW, Dechend R. Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension 53: 393–398, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, Nanan R. Altered decidual DC-Sign+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol 181: 2149–2160, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49: 612–617, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28: 870–880, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204: 273–283, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Z, Wang J, Zhang W, Zhang G, Jiao X, Zhi J. Changes in cardiac structure and function in rats immunized by angiotensin type 1 receptor peptides. Acta Biochim Biophys Sin (Shanghai) 43: 970–976, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Kalkunte S, Lai Z, Tewari N, Chichester C, Romero R, Padbury J, Sharma S. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta 29: 871–878, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, Granger JP, Martin JN Jr, Lamarca B. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am J Hypertens 22: 1120–1125, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharfi A, Giguère Y, Sapin V, Massé J, Dastugue B, Forest JC. Trophoblastic remodeling in normal and preeclamptic pregnancies: implication of cytokines. Clin Biochem 36: 323–331, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Kiprono LV, Wallace K, Moseley J, Martin J Jr, Lamarca B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. Am J Obstet Gynecol 209: 44. e1–44. e6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol 201: 319–327, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev 259: 231–244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kooij G, van Horssen J, de Lange EC, Reijerkerk A, van der Pol SM, van Het Hof B, Drexhage J, Vennegoor A, Killestein J, Scheffer G, Oerlemans R, Scheper R, van der Valk P, Dijkstra CD, de Vries HE. T lymphocytes impair P-glycoprotein function during neuroinflammation. J Autoimmun 34: 416–425, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Korn T, Bettelli E, Oukka M, Kuchroo VK. Il-17 and Th17 cells. Annu Rev Immunol 27: 485–517, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension 58: 63–69, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension 57: 505–514, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol 62: 105–120, 2010. [PMC free article] [PubMed] [Google Scholar]
- 59.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005. [DOI] [PubMed] [Google Scholar]
- 60.LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology (Bethesda) 28: 225–233, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 52: 1161–1167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaMarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Int J Interferon Cytokine Mediat Res 2011: 65–70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 865–871, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee NY, Kang YS. The decrease of paclitaxel efflux by pretreatment of interferon-γ and tumor necrosis factor-α after intracerebral microinjection. Brain Res 1499: 158–162, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao YH, Wei YM, Wang M, Wang ZH, Yuan HT, Cheng LX. Autoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertens Res 25: 641–646, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Liu KK, Dorovini-Zis K. Differential regulation of CD4+ T cell adhesion to cerebral microvascular endothelium by the β-chemokines CCL2 and CCL3. Int J Mol Sci 13: 16119–16140, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu F, Bytautiene E, Maner W, Makhlouf M, Al-Hendy A, Longo M, Saade G. Role of sFlt-1 in hypertension of pregnancy. In: 26th Annual Meeting of the Society for Maternal-Fetal Medicine Elsevier, 2005, vol. 193, p. S13. [Google Scholar]
- 70.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 181: 8–18, 2012. [DOI] [PubMed] [Google Scholar]
- 71.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta 27: 445–451, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy 89: 49–61, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73a.National Heart, Lung, and Blood Institute National High Blood Pressure Education Program. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 183: S1–S22, 2000. [PubMed] [Google Scholar]
- 74.Nevers T, Kalkunte S, Sharma S. Uterine Regulatory T cells, IL-10 and hypertension. Am J Reprod Immunol 66, Suppl 1: 88–92, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol 1: 98–114, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol 1: 98–114, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novotny S, Wallace K, Herse F, Moseley J, Darby M, Heath J, Gill J, Wallukat G, Martin JN Jr, Dechend R, LaMarca B. CD4+ T cells play a critical role in mediating hypertension in response to placental ischemia. J Hypertens (Los Angel) 2: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J 12: 1255–1265, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens 24: 835–840, 2011. [DOI] [PubMed] [Google Scholar]
- 81.Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy 28: 300–311, 2009. [DOI] [PubMed] [Google Scholar]
- 82.Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol 91: 76–82, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Quitterer U, Lother H, Abdalla S. AT1 receptor heterodimers and angiotensin II responsiveness in preeclampsia. Semin Nephrol 24: 115–119, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Rein DT, Breidenbach M, Hönscheid B, Friebe-Hoffmann U, Engel H, Göhring UJ, Uekermann L, Kurbacher CM, Schöndorf T. Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine 23: 119–125, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183: 7023–7030, 2009. [DOI] [PubMed] [Google Scholar]
- 86.Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online 13: 680–686, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol 149: 139–145, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol 29: 543–548, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaw MH, Freeman GJ, Scott MF, Fox BA, Bzik DJ, Belkaid Y, Yap GS. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J Immunol 176: 7263–7271, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Shin S, Jang JY, Roh EY, Yoon JH, Kim JS, Han KS, Kim S, Yun Y, Choi YS, Choi JD, Kim SH, Kim SJ, Song EY. Differences in circulating dendritic cell subtypes in pregnant women, cord blood and healthy adult women. J Korean Med Sci 24: 853–859, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 102: 181–192, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol 100: 369–377, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 55: 386–393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J Clin Endocrinol Metab 83: 4332–4337, 1998. [DOI] [PubMed] [Google Scholar]
- 96.Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 299: L639–L651, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stennett AK, Khalil RA. Neurovascular mechanisms of hypertension in pregnancy. Curr Neurovasc Res 3: 131–148, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Stepan H, Wallukat G, Schultheiss HP, Faber R, Walther T. Is parvovirus B19 the cause for autoimmunity against the angiotensin II type receptor? J Reprod Immunol 73: 130–134, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Tam Tam KB, Lamarca B, Arany M, Cockrell K, Fournier L, Murphy S, Martin JN Jr, Granger JP. Role of reactive oxygen species during hypertension in response to chronic antiangiogenic factor (sFlt-1) excess in pregnant rats. Am J Hypertens 24: 110–113, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC III, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation 110: 1612–1619, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens 22: 1314–1319, 2009. [DOI] [PubMed] [Google Scholar]
- 102.Ueda Y, Hagihara M, Okamoto A, Higuchi A, Tanabe A, Hirabayashi K, Izumi S, Makino T, Kato S, Hotta T. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum Immunol 64: 1144–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 103.Vitoratos N, Economou E, Iavazzo C, Panoulis K, Creatsas G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators Inflamm 2010: 908649, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wallace K, Cornelius DC, Scott J, Heath J, Moseley J, Chatman K, LaMarca B. CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension 64: 1151–1158, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallace K, Novotny S, Heath J, Moseley J, Martin JN Jr, Owens MY, LaMarca B. Hypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol 303: R144–R149, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension 46: 1275–1279, 2005. [DOI] [PubMed] [Google Scholar]
- 109.Warrington J, Fan F, LaMarca B, Dechend R, Wallukat G, Roman R, Drummond H, Granger J, Ryan M. Agonistic autoantibodies to angiotensin II Type 1 receptor contributes partly to placental ischemia-induced cerebrovascular abnormalities. Hypertension 66: A127, 2015. [Google Scholar]
- 110.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14: 353–356, 1993. [DOI] [PubMed] [Google Scholar]
- 111.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14: 353–356, 1993. [DOI] [PubMed] [Google Scholar]
- 112.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58: 77–84, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wheway J, Obeid S, Couraud PO, Combes V, Grau GE. The brain microvascular endothelium supports T cell proliferation and has potential for alloantigen presentation. PLoS One 8: e52586, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Witlin AG, Sibai BM. Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet Gynecol 92: 883–889, 1998. [DOI] [PubMed] [Google Scholar]
- 115.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res 113: 78–87, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang S, Zhong Q, Qiu Z, Chen X, Chen F, Mustafa K, Ding D, Zhou Y, Lin J, Yan S, Deng Y, Wang M, Zhou Y, Liao Y, Zhou Z. Angiotensin II receptor type 1 autoantibodies promote endothelial microparticles formation through activating p38 MAPK pathway. J Hypertens 32: 762–770, 2014. [DOI] [PubMed] [Google Scholar]
- 117.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3: 811–821, 1995. [DOI] [PubMed] [Google Scholar]
- 118.Zenclussen AC, Fest S, Joachim R, Klapp BF, Arck PC. Introducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol 34: 377–387, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L, Cui L, Miao GB, Zhao WS, Wang SY, Liu XL. [Study of autoantibodies against the G-protein-coupled beta 2- and alpha 1-adrenergic and AT1 receptors in patients with primary hypertension]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 24: 367–369, 2002. [PubMed] [Google Scholar]
- 120.Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol 186: 6024–6034, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zunker P, Happe S, Georgiadis AL, Louwen F, Georgiadis D, Ringelstein EB, Holzgreve W. Maternal cerebral hemodynamics in pregnancy-related hypertension. A prospective transcranial Doppler study. Ultrasound Obstet Gynecol 16: 179–187, 2000. [DOI] [PubMed] [Google Scholar]
- 122.Zunker P, Ley-Pozo J, Louwen F, Schuierer G, Holzgreve W, Ringelstein EB. Cerebral hemodynamics in pre-eclampsia/eclampsia syndrome. Ultrasound Obstet Gynecol 6: 411–415, 1995. [DOI] [PubMed] [Google Scholar]


