Abstract
Adipose tissue PKA has roles in adipogenesis, lipolysis, and mitochondrial function. PKA transduces the cAMP signal downstream of G protein-coupled receptors, which are being explored for therapeutic manipulation to reduce obesity and improve metabolic health. This study aimed to determine the overall physiological consequences of PKA activation in adipose tissue. Mice expressing an activated PKA catalytic subunit in adipose tissue (Adipoq-caPKA mice) showed increased PKA activity in subcutaneous, epididymal, and mesenteric white adipose tissue (WAT) depots and brown adipose tissue (BAT) compared with controls. Adipoq-caPKA mice weaned onto a high-fat diet (HFD) or switched to the HFD at 26 wk of age were protected from diet-induced weight gain. Metabolic health was improved, with enhanced insulin sensitivity, glucose tolerance, and β-cell function. Adipose tissue health was improved, with smaller adipocyte size and reduced macrophage engulfment of adipocytes. Using metabolic cages, we found that Adipoq-caPKA mice were shown to have increased energy expenditure, but no difference to littermate controls in physical activity or food consumption. Immunoblotting of adipose tissue showed increased expression of uncoupling protein-1 (UCP1) in BAT and dramatic UCP1 induction in subcutaneous WAT, but no induction in the visceral depots. Feeding a HFD increased PKA activity in epididymal WAT of wild-type mice compared with chow, but did not change PKA activity in subcutaneous WAT or BAT. This was associated with changes in PKA regulatory subunit expression. This study shows that adipose tissue PKA activity is sufficient to increase energy expenditure and indicates that PKA is a beneficial target in metabolic health.
Keywords: protein kinase A, adipose, energy expenditure, uncoupling protein 1
adipose tissue is distributed throughout the body and performs specific functions to regulate whole body nutrient status. Following feeding, white adipose tissue (WAT) stores energy as triglyceride in a single, cytoplasmic lipid droplet and releases these lipids into the circulation during fasting. Hormonal signals play key roles in the control of lipid handling in adipose tissue. Insulin promotes lipid uptake and storage, while catecholamines are major regulators of lipolysis. Catecholamines bind Gs-coupled adrenergic receptors on the plasma membrane of adipocytes and stimulate a rise in cAMP, which activates the cAMP-dependent protein kinase, PKA. PKA phosphorylates target proteins, leading to the induction of lipolysis. WAT is distributed in visceral and subcutaneous depots, with these being further subdivided into regional depots. These WAT depots have distinct characteristics that play specific roles in obesity and metabolic diseases. Visceral WAT is strongly linked to metabolic syndrome (39), whereas the association between subcutaneous WAT and metabolic disease is less pronounced (15, 20, 41). Brown adipose tissue (BAT), although also a site of lipid storage, consumes rather than releases lipids upon catecholamine stimulation (4, 6, 23). BAT expresses uncoupling protein 1 (UCP1) that dissipates the mitochondrial membrane proton gradient to uncouple mitochondrial metabolism from ATP generation, resulting in the release of heat. In humans BAT activity reduces with age, but can still be found in depots in the neck and upper torso (11, 37). Moreover, obesity and insulin resistance are associated with diminished BAT function and/or mass (11, 37). The activation of energy consumption by BAT is a potential therapeutic target for reducing obesity and improving metabolic health.
PKA is a heterotetrameric serine/threonine kinase, composed of a regulatory dimer and two catalytic subunits (40). In the absence of bound cAMP, the regulatory dimer inhibits the kinase activity of the catalytic subunits. Adenylyl cyclase activation leads to a rise in cAMP, which binds two sites on each PKA regulatory subunit, thereby releasing the inhibition of the catalytic subunits. This activates the kinase function and leads to the phosphorylation of target proteins. PKA regulatory subunits are classified in two subtypes, RI and RII, with the RI regulatory subunits (RIα and RIβ) having a higher affinity for cAMP than RII regulatory subunits (RIIα and RIIβ). Thus, PKA-RI holoenzymes are more readily activated at low cAMP concentrations. Adipose tissue primarily expresses PKA-RIIβ, which is also expressed in the brain (30). This contrasts with the majority of other tissues, which express PKA-RIIα. In adipose tissue, PKA activation leads to lipolysis by promoting the hydrolysis of the esterified triglycerides in the lipid droplet. This is a sequential process: adipose triglyceride lipase (ATGL) converts triglyceride to diacylglyceride, which hormone-sensitive lipase (HSL) hydrolyzes to monoacylglyceride, and this is further hydrolyzed to glycerol and a nonesterified fatty acid. The activity of ATGL and HSL are tightly regulated by PKA, and access to the lipid droplet is controlled by perilipin, which is highly phosphorylated by PKA. In addition to controlling lipolysis, cAMP and PKA also regulate adipogenesis (22, 32, 34, 45, 47).
Although WAT and BAT have distinct functions, it is clear that WAT can gain characteristics of BAT, at least in mice. In WAT and BAT, catecholamines raise cAMP and activate PKA to mobilize lipid reserves by promoting the hydrolysis of triglycerides. Lipids mobilized in WAT are released during nutritional deprivation to maintain energy supply. In BAT, lipids are consumed in mitochondria during cold exposure to generate heat. However, PKA activity in WAT increases mitochondrial activity (46), similar to the role of PKA in driving mitochondrial activity in BAT. BAT is characterized by numerous small lipid droplets and a high density of mitochondria that give the tissue its brown appearance. PCG1α is the master regulator of mitochondrial biogenesis and oxidative metabolism, and its expression in BAT contributes to the high mitochondrial content and activity of BAT (35). Catecholamines have been shown to induce the acquisition of a brown phenotype by WAT in mice (17, 42), and it has been shown that cAMP acting via PKA and PCG1α induces UCP1 and promotes mitochondrial biogenesis (5, 16, 35). This acquisition of a brown adipose phenotype by WAT reduces body weight and improves metabolic health. It has recently been shown that sympathetic regulation of this process can be regulated by leptin and insulin acting synergistically in the hypothalamus (13), which may underlie the observation of diet-induced thermogenesis (36).
Two mouse models have been generated that alter adipose tissue PKA expression. Mice transgenically engineered to overexpress the transcription factor FOXC2 had a lean phenotype (7). This may, in part, be attributable to increased expression of PKA-RIα, which is more readily activated by cAMP, however, as a transcription factor, FoxC2 regulates the expression of many genes besides PKA-RIα. Thus, FoxC2 overexpression is likely to have effects beyond the observed increase in PKA-RIα expression and PKA activity (18). Similarly, a whole body knockout of PKA-RIIβ, which is the major PKA-R subunit expressed in BAT and WAT, drove a compensatory increase in BAT PKA-RIα and a lean phenotype (10). PKA-RIIβ is also widely expressed in the central nervous system (CNS) (30), and its ablation has been shown to be through central effects rather than at the adipose tissue (48). Although both models exhibit a subtype switch from PKA-RIIβ to PKA-RIα in adipose tissue, they do not establish whether increased adipose tissue PKA activity is sufficient to drive a lean phenotype.
The aim of this study was to determine the physiological consequences of activating PKA specifically in adipose tissue. As discussed above, PKA regulates many important processes in adipose tissue, including lipolysis, adipogenesis, and the induction of mitochondrial activity. It has been proposed that therapeutically targeting G protein-coupled receptors (GPCRs) expressed in adipose tissue to raise cAMP, such as the β3-adrenergic receptor (β3-AR), could reduce body weight and improve metabolic health (12, 29, 42). However, because of the wide-ranging effects of adipose PKA activation, the physiological consequences of specifically activating PKA throughout the adipose tissue are unknown. Here, we use the adiponectin:Cre-recombinase mouse line (Adipoq-Cre mice) (14) to induce expression of an activated PKA-Cα allele (PKA-CαR) (31) specifically in adipose tissue (Adipoq-caPKA mice). Using these mice, we show that activation of PKA specifically in adipose tissue is sufficient to protect against diet-induced obesity and improve metabolic health.
METHODS
Animals.
Adiponectin-Cre homozygous mice (Adipoq-Cre+/+) (14) on a C57BL/6J background were crossed to PKA-CαR (PKA-Cα+/R) (31) heterozygous mice on a C57BL/6J background. Adipoq-Cre mice use adiponectin gene promoter fragments to provide highly specific Cre expression in adipose tissue (14, 43). The activating mutation of PKA-Cα was targeted to the endogenous PKA-Cα allele, so it is referred to as the PKA-CαR allele. Only male offspring of this cross, Adipoq-caPKA mice (Adipoq-Cre+/−; PKA-Cα+/R) and littermate controls (Adipoq-Cre+/−; PKA-Cα+/+) were used in this study. Mice were maintained in the University of Chicago animal facility under the day-to-day care of the facility staff, according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Chicago. Mice were fed either a standard chow diet (24% protein, 18% fat, and 58% carbohydrate; Harlan Teklad, cat. no. 2918) or a high-fat diet (20% protein, 20% carbohydrate, 60% fat, Research Diets, cat. no. D12492), and housed at ∼21°C on a 12:12-h light-dark cycle.
PKA activity assays.
PKA activity was measured in lysates prepared from adipose tissue snap frozen in liquid nitrogen upon dissection. The method used was similar to that which we have previously described (25, 26) and is adapted from Clegg et al. (9). This assay measures PKA activity in response to cAMP added exogenously to the in vitro assay and so reflects the PKA capacity of the sample as a function of the combined expression of the PKA catalytic and regulatory subunits. The assay is based upon the phosphorylation of the added PKA substrate, kemptide, in response to known concentrations of added cAMP. Values were corrected to PKI-inhibited samples that identified the PKA-independent kinase activity.
Physiological and metabolic analyses.
Insulin sensitivity was assayed by insulin tolerance test (ITT), in which insulin at 0.75 U/kg of body wt was given by intraperitoneal administration to 4-h fasted mice. Blood glucose was measured prior to insulin injection and at the indicated time points thereafter. Glucose tolerance was measured with 1 g/kg body wt glucose by intraperitoneal glucose tolerance test in 4-h fasted mice. The arginine response of insulin secretion was measured by sampling tail vein blood prior to, and at 2 min following, an intraperitoneal bolus of l-arginine dissolved in saline at 1 g/kg body wt. Blood glucose was measured using a Freestyle Lite glucose meter (Abbott Laboratories). Insulin was measured in plasma using the Ultrasensitive Mouse Insulin ELISA (Alpco, cat. no. 80-INSMSU-E01).
For the metabolic analyses and measurement of body composition, mice were weaned on to a high-fat diet (HFD) at 4 wk of age and maintained on the HFD to 25 wk of age. Male mice at 25 wk of age were individually housed in metabolic cages (LabMaster System, TSE Systems), and maintained on the HFD in the metabolic cages for 7 days: a 3-day acclimatization period, followed by 4 days during which data were collected on oxygen consumption, carbon dioxide production, energy expenditure, activity, and food and water consumption. Food consumption was also measured in mice housed under standard facility conditions with 2 or 3 male mice of the same genotype per cage. The respiratory exchange ratio was measured as the ratio of O2 inhalation to CO2 production. Following the 7 days in the metabolic cages, body composition was analyzed by DEXA (dual-energy X-ray absorptiometry; Lunar PIXImus densitometer system; GE Healthcare) using PIXImus 2 software after system calibration, according to the manufacturer's instructions. This analysis was performed with the assistance of the Metabolic Testing Facility of the Diabetes Research and Training Center at the University of Chicago.
Histology.
Adipose tissue depots were removed from killed mice and fixed overnight at 4°C in paraformaldehyde diluted to 4% in PBS. The tissue samples were washed in PBS and embedded in paraffin; 5-μm sections were cut in the Human Tissue Resource Center at The University of Chicago. Sections were deparaffinized and either stained with hematoxylin or processed for immunostaining. Immunostained samples were incubated with primary antibodies for Mac2 (Cederlane, cat no. CL8942AP; 1:1,000), UCP1 (Abcam, cat no. ab10983; 1:1,000) and perilipin (Cell Signaling, cat. no. 9349; 1:500).
Immunoblotting.
Harvested adipose tissue was immediately frozen in liquid nitrogen. Ground tissue samples of ∼50 mg were sonicated at 1 mg/10 μl in 240 mmol/l Tris-acetate, 1.0% SDS, 0.5% glycerol, 5 mmol/l dithiothreitol, protease inhibitors (Sigma-Aldrich, cat. no. P8340), phosphatase inhibitor (Santa Cruz Biotechnology, catalog no. sc-45044), 1 mmol/l Na3VO4, 10 mmol/l β-glycerophosphate; boiled for 10 min; and sonicated again. Lysates were resolved by gel electrophoresis and transferred to nitrocellulose. Immunoblotting used the following antibodies: anti-UCP-1 (Abcam, cat. no. ab10983, diluted 1:1,000); anti-tubulin (Cell Signaling Technologies, cat. no. 5346, diluted 1:2,000). Horseradish peroxidase-linked secondary antibodies were obtained from Jackson Laboratories, and images were developed using a chemiluminescent kit (Perkin Elmer, cat. no. NEL103001EA). Images for quantification were acquired using a Bio-Rad ChemiDoc MP system and are expressed in arbitrary units relative to the loading control.
Statistical analyses.
Data, expressed as means ± SD, were analyzed by unpaired Student's t-tests, one-way ANOVA, and two-way ANOVA using Prism software (GraphPad). P < 0.05 was considered significant.
RESULTS
Adipoq-caPKA mice have increased adipose PKA activity and reduced adiposity.
Male Adipoq-caPKA and littermate control mice were weaned at 4 wk of age onto a HFD or were weaned onto chow before being switched to a HFD at 25 wk of age. Mice weaned onto the HFD were killed at 25 wk of age for tissue analysis. In lysates prepared from WAT depots (subcutaneous, epididymal, and mesenteric) and from BAT, basal PKA activity was measured without added exogenous cAMP. In all four depots, basal PKA activity was approximately five-fold higher in tissue from the Adipoq-caPKA mice, compared with tissue from littermate controls (Fig. 1). These activated PKA values in adipose tissue from Adipoq-caPKA mice are approximately one-third of the activity seen in the presence of 0.1 μM cAMP (a typical basal cellular cAMP level; see Ref. 3) and 2% of the maximal activity (with 10 μM cAMP). Adipoq-caPKA mice accumulated body weight at a slower rate than the littermate controls (Fig. 2). In mice weaned to a HFD, body weight accumulated at a lower rate than in controls (Fig. 2A). At 25 wk of age DEXA revealed that the lean body mass was similar in Adipoq-caPKA mice and littermate controls, but the fat mass was significantly reduced in Adipoq-caPKA mice (Fig. 2B). Similarly, in mice weaned onto chow and then switched to the HFD at 25 wk of age, the increase in body weight was significantly reduced in Adipoq-caPKA mice, compared with littermates, although the Adipoq-caPKA mice did gain weight on HFD at a greater rate than when they were fed chow (Fig. 2, C and D). Therefore, the Adipoq-caPKA mice have a reduced body weight accumulation compared with control littermates, which is attributable to a reduced accumulation of adipose tissue mass. Adipoq-caPKA mice maintained to 35 wk of age on a chow diet did not differ in body weight gain compared with littermates (Fig. 2, E and F), showing that PKA activity only protects against diet-induced obesity and does not cause weight reduction under normal feeding conditions.
Fig. 1.

PKA activity is increased in adipose depots of Adipoq-caPKA mice. Adipose tissue was harvested from WAT depots: epididymal (Epi), subcutaneous (Sub), mesenteric (Mes), and from brown adipose tissue (BAT) of control (open bars) and Adipoq-caPKA (solid bars) mice. Basal PKA activity was measured in lysates prepared from these tissue samples and assayed in the absence of added cAMP. Basal PKA activity was significantly increased in each depot of Adipoq-caPKA mice examined: ***P < 0.001 by one-way ANOVA.
Fig. 2.
Adipoq-caPKA mice gain less weight on a high-fat diet. A: Adipoq-caPKA (■, n = 7) and littermate controls (○, n = 12) were weaned onto a 60% high-fat diet at 28 days of age, and their weight was monitored. Adipoq-caPKA mice gained significantly less weight than littermate controls: ***P < 0.001 by two-way ANOVA. B: At 25 wk of age, Adipoq-caPKA mice (black bar; n = 3) and littermate controls (white bar; n = 5) weaned onto the high-fat diet (HFD) were euthanized, and the body composition was analyzed by DEXA. Although lean mass of the mice was similar, the fat mass was significantly less in Adipoq-caPKA mice: ***P < 0.001; ns, not significant by Student's t-test. Adipoq-caPKA mice (■ and black bars) and littermate controls (○ and white bars) were weaned onto a chow diet and were either switched to the HFD at 25 wk of age (C and D; n = 5–8) or remained on the chow diet (E and F; n = 3/group). Body weight was measured (C and E), and the change in body weight was calculated, either after 4 wk of HFD (D) or over the 10–35-wk period on the chow diet (F). Body weight was compared in C and E between Adipoq-caPKA mice and controls using two-way ANOVA and Bonferroni post hoc test analyses. Changes in body weight (D and F) were compared by Student's t-test. ***P < 0.001; ns, not significant.
Adipoq-caPKA mice have improved metabolic health.
Obesity is associated with insulin resistance and impaired glucose clearance. Insulin sensitivity and glucose tolerance were studied to determine whether the reduced adiposity improved the metabolic health of Adipoq-caPKA mice. Glucose tolerance was measured by intraperitoneal glucose tolerance test at 25 wk of age in mice weaned onto the HFD (Fig. 3A). In mice weaned on to chow and later switched to a HFD, glucose tolerance was measured after 10 wk of the HFD (Fig. 3B). In both groups, Adipoq-caPKA mice exhibited significantly better glucose tolerance than littermate controls. Insulin sensitivity was measured by ITT both in mice weaned on to the HFD at 25 wk of age (Fig. 3C) and in mice weaned on to chow and then transferred to the HFD at 25 wk of age (Fig. 3D). In these mice, Adipoq-caPKA mice retained significantly better insulin sensitivity than control mice. β-cell function was examined using the arginine tolerance test. Plasma insulin levels were measured after a 4-h fast and again 2 min after an intraperitoneal injection of 1 g/kg body wt l-arginine. The 2-min postarginine insulin value was expressed relative to the baseline fasting value (Fig. 3E). In control mice, this ratio was 1.1, indicating very little response of the β-cells to the arginine. In contrast, the ratio in Adipoq-caPKA mice was 1.9, indicating that the β-cells retained a functional response to the arginine. In agreement with these findings of better insulin sensitivity and β-cell function, ad libitum-fed Adipoq-caPKA mice had lower circulating glucose levels, and 5-h fasted mice had lower insulin levels than controls, consistent with more effective insulin action (Fig. 3, F and G). These data show that the reduced body weight due to the activation of PKA in adipose tissue is associated with better metabolic health. In Adipoq-caPKA mice, both β-cell function and insulin sensitivity were improved relative to littermates.
Fig. 3.
Adipoq-caKA mice are metabolically healthier than littermates on a HFD. Glucose tolerance in Adipoq-caPKA mice (■) and littermate controls (○) was measured at 26 wk of age in mice weaned on a HFD (A) or after 10 wk of a HFD in mice weaned onto chow and switched to the HFD at 25 wk of age (B). Insulin tolerance was measured in Adipoq-caPKA (■) and littermate controls (○) was measured at 26 wk of age in mice weaned on a HFD (C) or after 10 wk of a HFD in mice weaned onto chow and switched to the HFD at 25 wk of age (D). After 10 wk of the HFD, insulin sensitivity and glucose tolerance were significantly better in Adipoq-caPKA mice than littermate controls. For A-D: **P < 0.01; ***P < 0.001 by two-way ANOVA. E: insulin response to a 1 g/kg l-arginine challenge (The ratio of 2 min plasma insulin/0 min plasma insulin) showed better β-cell function in Adipoq-caPKA mice (solid bars) compared with littermate controls (open bars). Plasma insulin in 5-h fasted mice (F) and blood glucose in ad libitum-fed mice (G), were significantly lower in Adipoq-caPKA mice (solid bars) compared with littermate controls (open bars): **P < 0.01, *P < 0.05 by Student's t-test.
Adipose depots of Adipoq-caPKA show smaller adipocytes and less macrophage infiltration.
Reduced adiposity could be a consequence of either reduced adipocyte size or a reduction in the number of adipocytes. Adipose tissue from three depots was examined by histological staining: inguinal subcutaneous WAT, epididymal visceral WAT, and supraclavicular BAT (Fig. 4A). Hematoxylin staining of BAT showed a significant reduction in the lipid content of the tissue. Similarly, staining showed smaller adipocytes in subcutaneous and epididymal depots (Fig. 4A). Smaller adipocytes are associated with healthier adipose tissue (24, 27, 38). Consistent with healthier adipose tissue, macrophage engulfment of adipocytes was observed in epididymal adipose tissue from control mice but not Adipoq-caPKA mice (Fig. 4B). At low magnification, crown-like structures (indicative of macrophage recruitment to dead adipocytes) were seen across the tissue sample in control mice (Fig. 4, B, a and B, c) but not in Adipoq-caPKA mice (Fig. 4, B, b and B, d). To confirm that these structures were accumulations of macrophages, sections were immunostained for Mac2 to identify macrophages. This revealed numerous adipocytes surrounded by macrophages in epididymal adipose tissue from control mice (Fig. 4B, e) but not in tissue from Adipoq-caPKA mice (Fig. 4B, f). Thus, the activation of PKA in the adipose tissue of Adipoq-caPKA mice results in smaller adipocytes and healthier adipose tissue.
Fig. 4.
PKA activation improves adipose tissue health. Adipose depots were harvested from Adipoq-caPKA and littermate control mice fed a HFD for 26 wk from weaning. Adipose tissue was fixed, embedded in paraffin, and 5-μm sections were cut, which were either stained with hematoxylin (A and B, a–d), or immunostained for perilipin and the macrophage marker, Mac2 (B, e and f). A: adipocytes from inguinal subcutaneous (scWAT), epididymal visceral (vWAT), and BAT were smaller in Adipoq-caPKA mice compared with controls (bar = 100 μm). B: macrophage engulfment of adipocytes was evident in control epididymal adipose tissue but not in Adipoq-caPKA epididymal adipose tissue. Left panels (a, b) show a low magnification (scale bar = 200 μm) with arrowheads indicating adipocytes surrounded by macrophages. Middle panels (c, d) show a higher magnification (scale bar = 50 μm), with the arrowheads indicating nuclei of engulfing macrophages. Right panels (e, f) show immunofluorescent staining of adipose tissue macrophages with Mac2 (green) surrounding individual adipocytes (perilipin, purple; scale bar = 100 μm).
Adipoq-caPKA mice have increased energy expenditure.
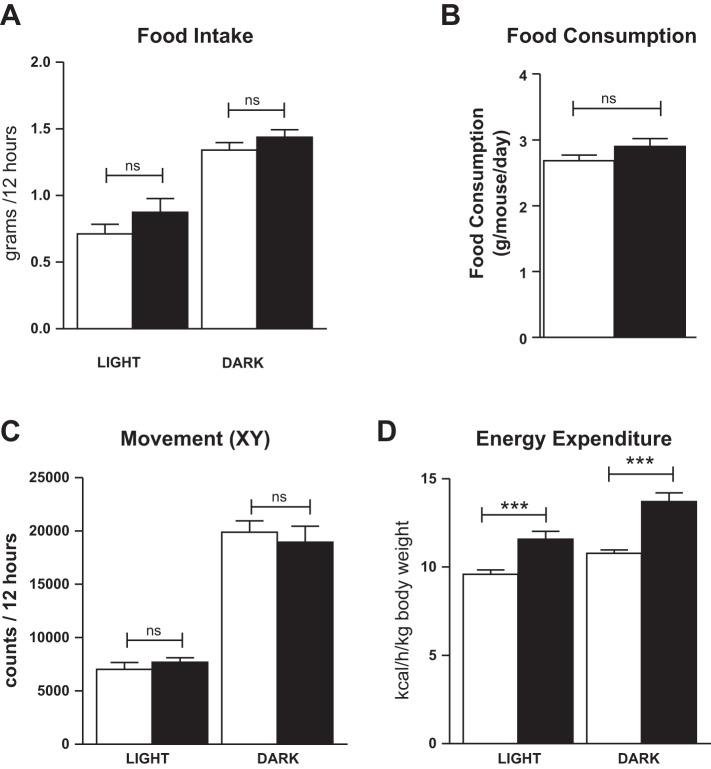
To determine the reason for the reduced adiposity with PKA activation, Adipoq-caPKA and littermate control mice were placed in metabolic cages. After a 3-day acclimatization period, data were collected for 4 days. Food intake in the metabolic cages did not differ between Adipoq-caPKA mice and control littermates (Fig. 5A), and this was confirmed by measuring food consumption in group-housed mice under standard animal facility conditions (Fig. 5B). Activity, measured on the x-y axes and on the y-axis, was not different between Adipoq-caPKA mice and controls (Fig. 5C and data not shown). Measurement of energy expenditure revealed that Adipoq-caPKA mice have a higher metabolic rate than littermate controls, under both the light and dark cycles (Fig. 5D). These data show that the resistance of the Adipoq-caPKA mice to gaining weight on a HFD is solely due to increased energy expenditure and cannot be attributed to increased activity or reduced food intake.
Fig. 5.
Metabolic characterization of Adipoq-caPKA mice. Adipoq-caPKA (black bars; n = 3) and littermate control (white bars; n = 5) mice weaned onto the HFD were placed in metabolic cages at 25 wk of age, remaining on the HFD. Food intake (A), movement in the x-y axes (C), and energy expenditure (D) were measured over 4 days after an initial acclimatization period of 3 days: one-way ANOVA, ***P < 0.001; ns, not significant. B: food consumption was also measured in mice housed at two or three per standard cage; Student's t-test, ns, not significant.
Adipoq-caPKA mice express UCP1 in subcutaneous WAT and increased UCP1 in BAT.
To determine the reason for the increased energy expenditure of Adipoq-caPKA mice, WAT and BAT depots were harvested and analyzed by immunoblotting for UCP1 expression. UCP1 expression is associated with energy expenditure in BAT leading to heat generation, but its expression is usually not associated with WAT. Consistent with this, BAT from both Adipoq-caPKA and control mice showed strong UCP1 expression, whereas epididymal and mesenteric WAT depots showed no UCP1 expression (Fig. 6A). However, UCP1 expression was strongly upregulated in the inguinal subcutaneous adipose of Adipoq-caPKA mice but was undetectable in subcutaneous adipose from control mice (Fig. 6A). To assess the UCP1 expression in BAT more fully, less protein was loaded for the immunoblotting analysis. This clearly revealed a modest but consistent increase of 17% in UCP1 expression in the BAT tissue from Adipoq-caPKA mice over the level observed in littermate control mice (Fig. 6, B and C). Consistent with the immunoblotting, immunostaining of subcutaneous WAT tissue showed no UCP1 in control mice, but widespread UCP1 expression throughout the subcutaneous depot. Thus, the increased energy expenditure of Adipoq-caPKA mice is most likely due to the induction of UCP1 expression in both BAT and subcutaneous WAT. These data show that PKA activation is sufficient to induce changes to a brown-like phenotype in the subcutaneous WAT and to increase UCP1 expression in BAT.
Fig. 6.
Adipose PKA activation induces UCP1 expression in adipose tissue. Adipose depots were harvested from control and Adipoq-caPKA mice, which had been weaned onto a high-fat diet and killed at 25 wk of age. Lysates prepared from subcutaneous, epididymal, mesenteric, and brown adipose depots were probed for uncoupling protein 1 (UCP1), with tubulin as a loading control (A and B). Less protein was loaded in B (compared with A) to allow observation of differences in UCP1 expression. The data in B were quantified (C), and expression compared between Adipoq-caPKA and control BAT samples (Students t-test: *P < 0.05). UCP1 staining of inguinal subcutaneous adipose tissue, harvested at 25 wk of age from male control (D) and Adipoq-caPKA (E) mice that had been maintained on a HFD since weaning at 4 wk of age.
DISCUSSION
PKA regulates many important processes in the establishment and function of adipose tissue. The aim of this study was to determine the physiological consequences of activating PKA throughout the adipose tissue depots. We used a genetic approach to activate PKA specifically in white and brown adipocytes, through the use of the aponectin/Cre-recombinase mouse model. This model has been thought to only increase PKA activity in adipocytes already sufficiently differentiated to activate the adiponectin promoter, and so we did not expect to affect adipogenesis, but rather affect the function of mature adipocytes. However, recent data indicate that the adiponectin promoter may be active in preadipocytes (21). We had considered it possible that activation of adipose PKA might reduce metabolic health by driving unrestricted lipolysis. However, this was not the case. In our mouse model, we observed improved metabolic health and resistance to diet-induced obesity. We attributed this effect to increased energy expenditure, the basis of which was most likely the increase in the already high level of uncoupling protein 1 (UCP1) expression in BAT, as well as a potential contribution from the dramatic induction of UCP1 expression in subcutaneous WAT. However, it should be noted that even upon this dramatic induction of UCP1 in subcutaneous WAT, UCP1 levels in BAT are much higher than those observed in the subcutaneous WAT. Presumably mitochondrial uncoupling in both these depots contributes to the increased energy expenditure, although these experiments did not determine the relative contribution of the BAT and subcutaneous WAT to the total increase in energy expenditure.
Previously, it has been reported that increasing adipose tissue PKA activity leads to a lean phenotype (7, 10). In the first of these studies (7), PKA activity was increased by transgenic overexpression of FoxC2. The second study knocked out expression of PKA-RIIβ (10), which is the predominant PKA regulatory subunit in adipose tissue, but which is also expressed in the CNS (10). In both studies, expression of PKA-RIα was increased relative to PKA-RIIβ, which led to an increased sensitivity of PKA holoenzymes to cAMP due to the higher affinity of PKA-RI subunits for cAMP, compared with PKA-RII subunits. However, overexpression of the transcription factor FoxC2 affects many adipocyte genes (7) and potentially nonadipose genes through the use of the aP2 promoter (28). Ablation of PKA-RIIβ increases adipose PKA-RIα expression that may be expected to increase adipose tissue PKA activity, similar to FOXC2 overexpression. However, its effects have been found to be largely in the CNS (48). The lack of an adipose tissue phenotype in mice lacking PKA-RIIβ may be due to a significant reduction in the expression of PKA-Cα that negates the change in regulatory subtype (1). Activation of adrenergic and other GPCRs that act via cAMP have been shown to increase energy expenditure in mouse models (8). However, many signaling pathways are activated downstream of GPCRs. Overall, although these studies alter adipose PKA activity, they do not establish the functional effects of PKA activity. The importance of our data is that it shows that activation of PKA alone in adipose tissue is sufficient to induce resistance to diet-induced obesity.
The induction of energy expenditure by adipose tissue is currently an area of intense research activity, where the activation of preexisting BAT or the acquisition of a brown-like phenotype by WAT appears to have considerable health benefits. Although the acquisition of characteristics of BAT by WAT is well established in rodents, it has not been established in humans. Therefore, our demonstration that PKA activity increases UCP1 expression in BAT may be more relevant to human therapy. The origin of the brown-like adipocytes in WAT tissue is keenly debated. Data have been presented to support both the gain of a brown adipose-like phenotype by preexisting WAT and the expansion of brown adipose-like precursor cells within WAT depots (2, 19, 33, 42, 44). Our use of the adiponectin promoter to induce PKA activity, indicates that the expression of PKA in cells already sufficiently differentiated to express adiponectin is able to induce these cells to acquire the brown adipose-like phenotype. Thus, preexisting WAT adipocytes are able to gain a brown adipose phenotype, but only in the subcutaneous and not the epididymal or mesenteric depots. However, this interpretation is complicated by the recent observation that lipid-lacking proliferating preadipocytes also express adiponectin (21). These new data require closer examination to determine whether preadipocytes do express adiponectin and when Cre-recombinase is expressed in mouse strains that employ adiponectin promoter sequences to drive Cre-expression. An alternative explanation of our data is that PKA activation in mature adipocytes in our mouse model releases factors (possibly lipids) that induce a change in local precursor cells. We do not think that this is likely, as staining of subcutaneous adipose for UCP1 shows a homogenous pattern of UCP1 expression, indicating that nearly all cells are expressing UCP1. We conclude from these data that the activation of PKA in the subcutaneous WAT of mice induces the acquisition of brown adipose-like characteristics in mature, preexisting adipocytes, but this conclusion is dependent upon the determination that Cre-recombinase in the Adipoq-Cre mice is only expressed in mature adipocytes. Our study does not exclude that possibility that a nonwhite adipocyte precursor in which the adiponectin promoter is activated is the precursor for the cells that express increased UCP1 in the Adipoq-caPKA mice.
Perspectives and Significance
In summary, our data show that activating PKA in adipose depots protects against diet-induced obesity and improves metabolic health through increased energy expenditure. Benefits to metabolic health of PKA activation lie in reduced adiposity, smaller adipocytes, reduced macrophage engulfment of adipocytes, increased insulin sensitivity, and retained β-cell function. As PKA is an important mediator of the cAMP signal downstream of GPCRs, which are important targets for the development of new therapies, targeting adipose GPCRs that activate PKA may provide opportunities to develop drugs to reduce obesity and improve metabolic health. Taken together, these findings support the interest in therapeutically targeting PKA in adipose tissue.
GRANTS
This work was supported by grants from the National Institutes of Health (NIH) to B. Wicksteed (DK-085129), by an American Diabetes Association grant (7-13-BS-033) to R. N. Cohen and by University of Chicago Diabetes Research and Training Center funding from the NIH (DK-020595) to B. Wicksteed and R. N. Cohen. B. T. Layden was supported by NIH Grant DK-104927 and Department of Veterans Affairs, Veterans Health Administration, Office of Research, and Development, Career Development Grant (1IK2BX001587-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.D., R.N.C., and B.W. conception and design of research; L.M.D., S.G., and B.W. performed experiments; L.M.D., S.G., and B.W. analyzed data; L.M.D., B.T.L., R.N.C., and B.W. interpreted results of experiments; L.M.D. and B.W. prepared figures; L.M.D., B.T.L., R.N.C., and B.W. edited and revised manuscript; L.M.D., S.G., B.T.L., R.N.C., and B.W. approved final version of manuscript; B.W. drafted manuscript.
REFERENCES
- 1.Amieux PS, Cummings DE, Motamed K, Brandon EP, Brandon EP, Wailes LA, K Le, Idzerda RL, McKnight GS. Compensatory regulation of RIalpha protein levels in protein kinase A mutant mice. J Biol Chem 272: 3993–3998, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Borner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ, Nikolaev VO. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat Protoc 6: 427–438, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cao W, Medvedev AV, Daniel KW, Collins S. β-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem 276: 27,077–27,082, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Carneheim C, Nedergaard J, Cannon B. β-adrenergic stimulation of lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am J Physiol Endocrinol Metab 246: E327–E333, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106: 563–573, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry A, Granneman JG. Adrenergic regulation of neonatal brown fat adenylyl cyclase and Gs α-activity. Am J Physiol Regul Integr Comp Physiol 263: R34–R38, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Clegg CH, Correll LA, Cadd GG, McKnight GS. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem 262: 13,111–13,119, 1987. [PubMed] [Google Scholar]
- 10.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII β-subunit of protein kinase A. Nature 382: 622–626, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo F, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21: 33–38, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160: 88–104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13: 249–259, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox C. S., Massaro J. M., Hoffmann U., Pou K. M., Maurovich-Horvat P., Liu C. Y., Vasan R. S., Murabito J. M., Meigs J. B., Cupples L. A., D'Agostino R. B Sr., O'Donnell C. J. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Fredriksson J. M., Thonberg H. Ohlson K. B. Ohba K. Cannon B. Nedergaard J. Analysis of inhibition by H89 of UCP1 gene expression and thermogenesis indicates protein kinase A mediation of β3-adrenergic signalling rather than β3-adrenoceptor antagonism by H89. Biochim Biophys Acta 1538: 206–217, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Ghorbani M. and Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord 21: 465–475, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Gronning LM, Baillie GS, Cederberg A, Lynch MJ, Houslay MD, Enerback S, Tasken K. Reduced PDE4 expression and activity contributes to enhanced catecholamine-induced cAMP accumulation in adipocytes from FOXC2 transgenic mice. FEBS Lett 580: 4126–4130, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia 51: 900–902, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, Koh GY. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 142: 2623–2632, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Jia B, Madsen L, Petersen RK, Techer N, Kopperud R, Ma T, Doskeland SO, Ailhaud G, Wang J, Amri EZ, Kristiansen K. Activation of protein kinase A and exchange protein directly activated by cAMP promotes adipocyte differentiation of human mesenchymal stem cells. PLoS One 7: e34114, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joel CD. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J Biol Chem 241: 814–821, 1966. [PubMed] [Google Scholar]
- 24.Julius U, Leonhardt W, Schneider H, Schollberg K, Hanefeld M, Schulze J, Haller H. Basal and stimulated hyperinsulinemia in obesity: relationship to adipose-cell size. Endokrinologie 73: 214–220, 1979. [PubMed] [Google Scholar]
- 25.Kaihara KA, Dickson LM, Ellenbroek JH, Orr CM, Layden BT, Wicksteed B. PKA enhances the acute insulin response leading to the restoration of glucose control. Diabetes 64: 1688–1697, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaihara KA, Dickson LM, Jacobson DA, Tamarina N, Roe MW, Philipson LH, Wicksteed B. β-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes 62: 1527–1536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurencikiene J, Skurk T, Kulyte A, Heden P, Astrom G, Sjolin E, Ryden M, Hauner H, Arner P. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab 96: E2045–E2049, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Lee K. Y., Russell S. J. Ussar S. Boucher J. Vernochet C. Mori M. A. Smyth G. Rourk M. Cederquist C. Rosen E. D. Kahn B. B. and Kahn C. R. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62: 864–874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowell BB, Flier JS. Brown adipose tissue, β3-adrenergic receptors, and obesity. Annu Rev Med 48: 307–316, 1997. [DOI] [PubMed] [Google Scholar]
- 30.McKnight GS, Cummings DE, Amieux PS, Sikorski MA, Brandon EP, Planas JV, Motamed K, Idzerda RL. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog Horm Res 53: 139–159; discussion 160-131, 1998. [PubMed] [Google Scholar]
- 31.Niswender CM, Willis BS, Wallen A, Sweet IR, Jetton TL, Thompson BR, Wu C, Lange AJ, McKnight GS. Cre recombinase-dependent expression of a constitutively active mutant allele of the catalytic subunit of protein kinase A. Genesis 43: 109–119, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RK, Madsen L, Pedersen LM, Hallenborg P, Hagland H, Viste K, Doskeland SO, Kristiansen K. Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol Cell Biol 28: 3804–3816, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peverelli E, Ermetici F, Corbetta S, Gozzini E, Avagliano L, Zappa MA, Bulfamante G, Beck-Peccoz P, Spada A, Mantovani G. PKA regulatory subunit R2B is required for murine and human adipocyte differentiation. Endocr Connect 2: 196–207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979. [DOI] [PubMed] [Google Scholar]
- 37.Saito M., Okamatsu-Ogura Y. Matsushita M. Watanabe K. Yoneshiro T. Nio-Kobayashi J. Iwanaga T. Miyagawa M. Kameya T. Nakada K. Kawai Y. and Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salans LB, Bray GA, Cushman SW, Danforth E Jr, Glennon JA, Horton ES, Sims EA. Glucose metabolism and the response to insulin by human adipose tissue in spontaneous and experimental obesity. Effects of dietary composition and adipose cell size. J Clin Invest 53: 848–856, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 85: 1–10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta 1834: 1271–1278, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res 53: 619–629, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 151: 2933–2939, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang DC, Tsay HJ, Lin SY, Chiou SH, Li MJ, Chang TJ, Hung SC. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One 3: e1540, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yehuda-Shnaidman E, Buehrer B, Pi J, Kumar E, Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes 59: 2474–2483, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev 27: 1223–1232, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng R, Yang L, Sikorski MA, Enns LC, Czyzyk TA, Ladiges WC, McKnight GS. Deficiency of the RIIβ subunit of PKA affects locomotor activity and energy homeostasis in distinct neuronal populations. Proc Natl Acad Sci USA 110: E1631–E1640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]