Abstract
Teleosts living in seawater continually absorb water across the intestine to compensate for branchial water loss to the environment. The present study reveals that the Gulf toadfish (Opsanus beta) rectum plays a comparable role to the posterior intestine in ion and water absorption. However, the posterior intestine appears to rely more on SLC26a6 (a HCO3−/Cl− antiporter) and the rectum appears to rely on NKCC2 (SLC12a1) for the purposes of solute-coupled water absorption. The present study also demonstrates that the rectum responds to renoguanylin (RGN), a member of the guanylin family of peptides that alters the normal osmoregulatory processes of the distal intestine, by inhibited water absorption. RGN decreases rectal water absorption more greatly than in the posterior intestine and leads to net Na+ and Cl− secretion, and a reversal of the absorptive short-circuit current (ISC). It is hypothesized that maintaining a larger fluid volume within the distal segments of intestinal tract facilitates the removal of CaCO3 precipitates and other solids from the intestine. Indeed, the expression of the components of the Cl−-secretory response, apical CFTR, and basolateral NKCC1 (SLC12a2), are upregulated in the rectum of the Gulf toadfish after 96 h in 60 ppt, an exposure that increases CaCO3 precipitate formation relative to 35 ppt. Moreover, the downstream intracellular effects of RGN appear to directly inhibit ion absorption by NKCC2 and anion exchange by SLC26a6. Overall, the present findings elucidate key electrophysiological differences between the posterior intestine and rectum of Gulf toadfish and the potent regulatory role renoguanylin plays in osmoregulation.
Keywords: water secretion, Cl− secretion, HCO3− secretion, intestine, marine teleost, CFTR
seawater teleost fish live in an environment that is hypertonic to their blood plasma, resulting in continual water loss through the gills (34). To compensate for this, seawater teleosts drink copious volumes of water that are continually desalinated by the esophagus and gastrointestinal tract to produce a fluid that is roughly isotonic to blood plasma (17, 34). The constant absorption of ions, by the intestine, is critical as it lowers luminal osmolality and allows for solute-coupled water absorption, and, thereby, compensating for branchial water loss (45, 46). The decrease in luminal osmolality is achieved by ion transport mechanisms that not only favor salt absorption, but also result in the precipitation of osmolytes in the intestinal lumen that further reduce osmolality. Enterocytes express the apical electrogenic HCO3−/Cl−-antiporter (SLC26a6) that secretes multiple molecules of HCO3− in exchange for a single Cl−, although the exact ratio is unknown (20, 31). Secreted HCO3− binds to luminal Ca2+ (and to a lesser extent with Mg2+) to form CaCO3 (and MgCO3) precipitates, resulting in a 70–100 mosmol/kg H2O reduction in luminal osmolality (52). In addition, the titration of HCO3− by H+, secreted by apical V-type H+-ATPase (VHA), can further decrease luminal osmolality by up to 30 mosmol/kg H2O (16, 17, 19, 21, 22, 51, 52). Na+ is primarily taken up by the apical Na+/K+/2Cl−-cotransporter, NKCC2 (SLC12a1), and the apical Na+/Cl− cotransporter, NCC (SLC12a3) (25). Water absorption occurs through aquaporins (53), a process coupled to the ion transport mechanisms described above.
The seawater teleost rectum also plays a role in water absorption. For instance, in the sea bream (Sparus aurata) and Japanese eel (Anguilla japonica), fluid absorption by the rectum also occurs via aquaporins, but the rate of absorption is less than in the intestine (8, 15, 28, 36). The rectum of the Gulf toadfish (Opsanus beta) also expresses intestinal ion transporters and enzymes involved in solute-coupled water absorption, such as SLC26a6, the basolateral electrogenic Na+/HCO3−-cotransporter [NBCe1 (SLC4a4)], apical VHA, and cytosolic carbonic anhydrase (CAC) (20, 22, 42, 49). These cellular components are essential for fluid absorption by the marine teleost intestine, but there is a large gap in knowledge concerning their osmoregulatory role in the rectum.
The hydration mechanisms described above are vital to a seawater teleost's survival. However, recent studies have demonstrated the potential for inhibited fluid absorption by the intestine, initiated by the guanylin family of intestinal peptides [guanylin, uroguanylin, and renoguanylin (RGN)] (3, 4, 40, 41). These short-length hormones are expressed in the intestine and rectum of the Japanese eel, European eel (A. anguilla), and Gulf toadfish (41, 47, 48), and they are upstream regulators of apical guanylyl cyclase-C1 and -C2 (GC-C) receptors in the intestine (56). GC-C activation leads to increases in intracellular cGMP formation, whose downstream effects stimulate Cl− secretion by activation of the apical cystic fibrosis transmembrane conductance regulator (CFTR) and greatly inhibit ion and water absorption (3, 4, 6, 40). In the Gulf toadfish and Japanese eel, the guanylin peptide effect appears restricted to the mid- and posterior intestine, by reversing the absorptive short-circuit current (ISC) (from mucosa-to-serosa to serosa-to-mucosa), mediated primarily by a switch from net Cl− absorption to net secretion (4, 40, 41, 55). This reversal leads to either fluid secretion or inhibited fluid absorption (3, 4, 40, 41, 55). Moreover, the guanylin peptides, GC-C1, and GC-C2, are transcriptionally upregulated when freshwater-acclimated European and Japanese eels are transferred from freshwater to seawater (SW) (9, 10, 27, 54, 56), which suggests that the guanylin peptide system is important for seawater acclimatization. There is also immunohistochemical and molecular evidence for a putative Cl−-secretory pathway comprising an apical CFTR and basolateral NKCC1 (SLC12a2) in the posterior intestine of Gulf toadfish, killifish (Fundulus heteroclitus), and sea bream (15, 35, 40, 44).
It is hypothesized that the guanylin-stimulated Cl− secretory response is essential for removing solids (e.g., CaCO3 precipitates or undigested food) from the intestine (40, 41). The mechanical activity (e.g., peristalsis) along with presence of fluids in the intestinal lumen facilitates the movement of solids along the intestine (43). The presence of fluid in the intestinal lumen likely facilitates clearance of CaCO3 precipitates from the intestine. In support of this hypothesis, transcription of CFTR and NKCC1 is upregulated in the posterior intestine of Gulf toadfish (41) and Mozambique tilapia (Oreochromis mossambicus) (33) exposed to hypersalinity. Further support for the above hypothesis is the observation that RGN causes a larger reversal of the absorptive ISC and inhibits water absorption more greatly in the posterior intestine of Gulf toadfish exposed to hypersalinity (41). Although the above hypothesis remains to be fully validated, an important consideration is the fate of the fluid that is either secreted or not absorbed by the distal intestine of seawater teleosts, due to guanylin peptide stimulation.
The retention of water is vital for the survival of a seawater teleost, yet the inhibition of absorptive mechanisms in the intestine that occurs through guanylin peptide stimulation still exists. Either this water is lost by expulsion into the environment or reabsorbed by the rectum. To our knowledge, the duration of the in vivo guanylin peptide response in seawater teleosts is unknown. It is also unknown whether the duration of the guanylin peptide stimulation is long enough that it prevents the distal intestine and rectum from being able to absorb water secreted by the more proximal segments.
The purpose of this study was twofold. First, we sought to rectify the gap of knowledge with respect to transport physiology of the rectum by characterizing the normal osmoregulatory mechanisms of the Gulf toadfish rectum. Second, we wished to determine whether the rectum responded to RGN and compared this response to that of the posterior intestine. A third objective was to determine the mechanisms that decrease HCO3− secretion in the intestinal tract by RGN.
MATERIALS AND METHODS
Experimental Animals
Gulf toadfish (Opsanus beta) were caught as bycatch from Biscayne Bay, FL, by shrimp fishermen, and placed in a 3-min freshwater bath and treated with malachite green for ectoparasite removal, upon arrival to the laboratory. Individuals were separated by size and placed into 62-liter aerated tanks (33–36 ppt, 20–26°C), with continuous flow-through of sand-filtered SW from Key Biscayne. Gulf toadfish were fed squid to satiation once per week, but food was withheld for 3 days prior to experimentation. For the hypersalinity (60 ppt) studies, Gulf toadfish were held in experimental tanks in groups of 6–10, with recirculating biofilters containing charcoal. The tanks sat in a temperature-controlled water bath at 26°C. Individuals were acclimated to 35 ppt and 60 ppt (adjusted with Instant Ocean; SpectrumBrands, Madison, WI) for up to 4 days for the real-time, quantitative PCR (qPCR) study and at least 7 days for the physiological studies. Tanks were siphoned regularly and topped up with SW adjusted to the proper salinity. Water chemistry was monitored daily (temperature and salinity) and twice weekly (pH). Fish husbandry and experimental procedures followed an approved University of Miami Animal Care Protocol (Insitutional Animal Care and Use Committee no. 13–225, renewal 02). Gulf toadfish were killed using 0.2 g/l MS-222 (Argent, Redmond, WA) solution buffered with 0.3 g/1 NaHCO3− (Sigma-Aldrich, St. Louis, MO), followed by severing the spinal cord at the cervical vertebra and pithing of the brain.
Composition of Salines, Hormones, and Inhibitors
Mucosal (pH = 7.8) and serosal (pH = 7.8) salines were prepared as described in Table 1 and were used to bathe intestinal and rectal tissues of Gulf toadfish acclimated to both 35 and 60 ppt. The osmolalities of plasma, posterior intestinal, and rectal fluids from Gulf toadfish at these two salinities are not significantly different (13, 37) and, accordingly, would influence neither cell volume nor osmotic transepithelial water movement. Eel RGN (Peptide Institute, Osaka, Osaka Prefecture, Japan) was used in the present study for two reasons. First, the sequence for eel RGN (ADLCEICAFAACTGCL, accession no.: BAC76010.1) is nearly identical to that of Gulf toadfish (tf) guanylin (MDVCEICAFAACTGC, accession no.: AIA09902.1). Second, because of its close homology with tf guanylin, eel RGN has the greatest physiological effects on Gulf toadfish intestinal tissues (40). Ethoxzolamide (Sigma-Aldrich) was dissolved in DMSO (Sigma-Aldrich) and administered at a concentration of 100 μmol/l in saline containing 0.1% DMSO. Vehicle controls for 0.1% DMSO have been shown not to affect the physiological parameters tested in the present study (40). Bumetanide (Sigma-Aldrich) was dissolved in ethanol (Sigma-Aldrich) and administered at 10 μmol/l in saline containing 0.05% ethanol. Control experiments revealed that at this concentration, ethanol does not affect the physiological parameters tested in the present study (data not shown).
Table 1.
Composition of salines for short-circuit current, pH-stat titration, and sac preparation experiments
| Compound | Mucosal* | Serosal† | HCO3−/CO2-free serosal |
|---|---|---|---|
| NaCl, mmol/l | 169.0 | 151.0 | 151.0 |
| KCl, mmol/l | 5.0 | 3.0 | 3.0 |
| MgSO4, mmol/l | 77.5 | 0.88 | 0.88 |
| MgCl2, mmol/l | 22.5 | ||
| Na2HPO4, mmol/l | 0.5 | 0.5 | |
| KH2PO4, mmol/l | 0.5 | 0.5 | |
| CaCl2, mmol/l | 5.0 | 1.0 | 1.0 |
| NaHCO3, mmol/l | 5.0 | ||
| HEPES, free acid, mmol/l | 11.0 | 11.0 | |
| HEPES, Na+ salt, mmol/l | 11.0 | 11.0 | |
| Urea, mmol/l | 4.5 | 4.5 | |
| Glucose, mmol/l | 5.0 | 5.0 | |
| Osmolality§, mosmol/kg H2O | 330 | 330 | 330 |
| pH | 7.8† | 7.8 | 7.8 |
| Gas‡ | 100% O2 | 0.3% CO2 in O2 | 100% O2 |
Mucosal application of blockers and pharmaceuticals: Renoguanylin was added for a final concentration of 10−7 and 5 × 10−7 mol/l in Ussing chamber and sac preparation experiments, respectively. Bumetanide and ethoxzolamide were added to Ussing chambers for final concentrations of 10 and 100 μmol/l, respectively. Saline solutions were adjusted for osmolality with mannitol (§). When measuring HCO3− secretion, mucosal pH in Ussing chambers was maintained at 7.8 by pH-stat titration (†). Salines were gased for at least 1 h prior to experiments (‡).
RNA Isolation and Molecular Cloning
Tissues were collected from Gulf toadfish kept in 33–35 ppt seawater for the tissue distribution study and from Gulf toadfish acclimated to 0 (control), 6, 12, 24, and 96 h in 60 ppt for the hypersalinity study. Collected tissues were homogenized with an Ultra-Turrax T8 homogenizer (Ika-Werke, Breisgau, Baden-Württemberg, Germany) and 50–100 mg/ml of total RNA was extracted with RNA STAT-60 (Tel-Test, Friendswood, TX). Traces of genomic DNA were removed with DNase I (Turbo DNA-free kit, Ambion, Foster City, CA) from each isolate (10 μg/sample). A spectrophotometer (NanoDrop 1000 Spectrophotometer, Thermo Scientific, Grand Island, NY) was used to quantify total RNA at 260 nm. A subsample of isolates was spot-checked using gel electrophoresis to confirm RNA integrity. Isolated RNA (1 μg) was reversed transcribed into cDNA using SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Genes coding for tf guanylin, tf uroguanylin, tf guanylyl cyclase-C (GC-C), tfNKCC1, and tfCFTR were previously cloned (40, 41) by PCR.
Real-Time, Quantitative PCR
Gene expression levels of tf guanylin, tf uroguanylin, tfGC-C, tfNKCC1, tfNKCC2, and tfCFTR were detected using qPCR. Gulf toadfish tissues were dissected and immediately snap-frozen in liquid nitrogen, after which RNA was isolated and cDNA produced, as described above. Gene expression was measured using gene-specific primers (Table 2) and Power SYBR Green (Applied BioSystems, Foster City, CA) as the reporter dye, for qPCR in an Mx4000 cycler (Stratagene, San Diego, CA). Cycling parameters were as follows: 95°C for 10 min, 40–50 cycles of 95°C for 30 s, 55–60°C for 30 s, and 72°C for 30 s. The specificity for all PCR products was confirmed by observing a distinct, corresponding melting peak for each product following each qPCR run using a melting curve analysis (guanylin: 79–85°C, uroguanylin: 81–86°C, GC-C: 80–86°C, NKCC1: 81–86°C, NKCC2: 77–83°C, and CFTR: 80–85°C), as reported previously (41). Gene expression was normalized to EF-1α and was scaled relative to the lowest expressing tissue (tissue distribution study) and the 0-h control (hypersalinity study), which were given a value of 1.0, as described by Pfaffl (39). Primers for tfNKCC1, tfNKCC2, and tfCFTR were designed from an annotated Gulf toadfish intestinal transcriptome (LeMoine CMR, Corradi N, Walshun PJ, unpublished data).
Table 2.
Primers used for qPCR experiments
| Name | Sequence |
|---|---|
| tfGN-F | 5′-AGCAAAGGGCAGCATCTGCA-3′ |
| tfGN-R | 5′-TGGCAAGATGTTTGTGGCTTTGC-3′ |
| tfUGN-F | 5′-CCGACCCTCTCATGCCGCAGG-3′ |
| tfUGN-R | 5′-TGCACGGAGGCATCGAGCTG-3′ |
| tfGC-C-F | 5′-CAGAGGCCACCATGCGGCGTCACCTA-3′ |
| tfGC-C-R | 5′-TTCACCAAGCGCTGCTCCGACCAA-3′ |
| tfNKCC1-F | 5′-TCCTGCAGCAGCTCGTTGAG-3′ |
| tfNKCC1-R | 5′-GAGCACGTGGCGGCCTTCGAGGAT-3′ |
| tfNKCC2-F | 5′-AAGGCACCATAGACGTGTGG-3′ |
| tfNKCC2-R | 5′-CCTCAACCGACAGTCCTTCC-3′ |
| tfCFTR-F | 5′-GTTTCATCACCGGCATGAACG-3′ |
| tfCFTR-R | 5′-GTGCCTTTCTGTAGATGGCTCCAA-3′ |
| EF-1α-F | 5′-AGGTCATCATCCTGAACCAC-3′ |
| EF-1α-R | 5′-GTTGTCCTCAAGCTTCTTGC-3′ |
| Universal Primer* | 5′-CTAATACGACTCACTATAGGGCAAGCGTGGTATCAACGCAGAGT-3′ |
| 5′-CTAATACGACTCACTATAGGGC-3′ |
Universal primer (Clontech SMARTer RACE cDNA amplification kit) consists of both a long and short sequence. tf, Gulf toadfish; F, forward; R, reverse; GN, guanylin, UGN, uroguanylin; GC-C, (guanylyl cyclase-C), NKCC1 (Na+/K+/2Cl−-cotransporter type 1, SLC12a2), NKCC2 Na+/K+/2Cl−-cotransporter type 2, SLC12a1; CFTR, cystic fibrosis transmembrane conductance regulator; EF-1α, elongation factor 1-α.
General Experimental Protocol: Short-Circuit Current and HCO3− Secretion
Short-circuit current (ISC), transepithelial potential (TEP), and transepithelial conductance (GTE) of Gulf toadfish tissues were measured by Ussing chambers (model 2400; Physiologic Instruments, San Diego, CA). These experiments were paired with the posterior intestine and the rectum of an individual Gulf toadfish (15–30 g) measured in parallel. The posterior intestine and rectum were excised, cut open along the midline, and mounted onto P2413 tissue holders (Physiologic Instruments), which exposed 0.71 cm2 of tissue and placed between the two half-chambers of the Ussing apparatus. ISC and TEP were measured by silver (Ag) current and Ag/AgCl voltage electrodes, respectively, and were connected to an amplifier (model VCC600, Physiologic Instruments).
ISC studies were performed under symmetrical conditions, in which 2 ml of serosal saline (Table 1) was added to both the mucosal (apical membrane/luminal side) and serosal (basolateral membrane/blood side) half-chambers. The salines were continually mixed by airlift gassing (0.3% CO2 in O2) and maintained at 25°C by a recirculating bath (model no. 1160S, VWR, Radnor, PA), as described in Grosell and Genz (18). ISC was measured by current electrodes under voltage-clamp conditions (0.0 mV), with 3 s of 2-mV pulses (mucosal-to-serosal) every 60 s. TEP studies were performed under asymmetrical conditions, in which 2 ml each of mucosal and serosal saline (Table 1) were added to the mucosal and serosal half-chamber, respectively. The mucosal saline was continually mixed by airlift gassing (100% O2), and the serosal saline, as described above, was maintained at 25°C. TEP was measured by voltage electrodes under current-clamp conditions (0.0 μA), with 3 s of 30-μA pulses (mucosal-to-serosal) every 60 s. GTE was calculated by Ohm's law and was determined from the deflections in ISC and TEP during pulsing. Acqknowledge software (v. 3.8.1, BIOPAC Systems, Goleta, CA) recorded ISC and TEP onto a computer.
A pH-stat titrator (model TIM 854 or 856; Radiometer, Brea, CA) was set up in tandem with an Ussing chamber to measure HCO3− secretion along with TEP (described above), respectively, on the isolated tissues, following the protocol in Grosell and Genz (18). A pH electrode (Radiometer, model no. PHC4000.8) and a microburette tip (from which acid is delivered) were immersed in the mucosal saline bath and maintained a pH of 7.8, allowing for symmetrical pH conditions on either side of the epithelium. Titramaster software (Radiometer, v. 5.1.0), installed on a computer, recorded pH and the volume of acid titrant (0.005 mol/l HCl) injected into the mucosal bath. The HCO3− secretion rate from a tissue preparation was calculated from the rate of titrant secreted and its concentration, as described in Grosell and Genz (18).
Experimental set 1.
To determine baseline ISC and HCO3− secretion rates, as well as the effects of RGN, on posterior intestinal and rectal tissues of Gulf toadfish acclimated to 35 and 60 ppt. RGN (10−7 mol/l) was added to mucosal half-chamber once baseline values stabilized.
Experimental set 2.
To determine the source of decreased HCO3− secretion rates by RGN, bicarbonate was removed from the serosal saline, leaving CAC as the sole source of intracellular HCO3−. As before, RGN was added to the mucosal half-chamber once the HCO3− secretion rates of the posterior intestine and rectum stabilized (of fish acclimated to 35 ppt). A subsequent experiment tested the potential influence of RGN on CAC with ethoxzolamide (10−4 mol/l) [a known inhibitor of CAC (18)]. This experimental set served to elucidate the role of RGN on the transport rates of HCO3− by apical SLC26a6 and basolateral NBCe1, and on HCO3− formation by CAC.
Experimental set 3.
To determine the effects of mucosally applied bumetanide on ISC and HCO3− secretion. In the Japanese eel and Gulf toadfish, Na+ absorption is inhibited by guanylin peptide stimulation, suggesting altered NKCC2 activity (3, 4, 41, 55). To test for this in the Gulf toadfish, bumetanide (10 μmol/l), which specifically binds to and inhibits NKCC isoforms, was added to the mucosal half-chamber, and either ISC or HCO3− and TEP were measured.
Intestinal and Rectal Sac Preparations
Sac preparations were used to determine the effects of RGN on water, Cl−, and Na+ fluxes. The posterior intestine (1.5–3 cm) and rectum (1.5–2 cm) from Gulf toadfish (50–80 g) were excised as described above. The flared end of a PE-50 catheter was inserted into either the posterior intestine or rectum and tied off with a silk suture. Mucosal saline (Table 1) was then injected into the catheter to rinse the tissue of any debris. The tissue was then tied off at the open end with a silk suture to form either an intestinal or rectal sac. The sac preparations were then injected with mucosal saline (control or containing 5 × 10−7 mol/l RGN). A subsample of injected mucosal saline was collected from which initial Na+ and Cl− concentrations were measured. The catheter was sealed, the sac preparations were blot-dried, and initial masses were weighed. The sac preparations were then placed in a scintillation vial containing serosal saline and continually gassed with 0.3% CO2 (Table 1) and underwent a 2-h flux period. At the end of the flux period, the sac preparations were removed from the serosal saline, blot-dried once again, and final masses were weighed. The sealed catheter was opened, and the fluid was collected. The silk sutures of an empty sac preparation were cut, the tissue was cut down its midline, thoroughly dried, weighed, and its surface area was outlined onto tracing paper. Water, Na+, and Cl− flux rates were calculated by the differences in the product between final and initial volumes and concentrations (volume × concentration), divided by the flux period and surface area. Na+ and Cl− concentrations were measured using flame spectrometry (Varian 220FS, Palo Alto, CA) and anion chromatography (DIONEX 120, Sunnyvale, CA), respectively.
Statistical Analyses
The data are presented as means ± SE and are evaluated using parametric and nonparametric statistical tests, as described in the figure legends. The 50% effective concentration (EC50) was calculated with a nonlinear regression using a global curve fitting. All data were analyzed and plotted onto graphs with SigmaPlot 13.0. Means were considered significantly different with P ≤ 0.05. Control measurements for ISC, TEP, and HCO3− secretion were calculated from the final 30 min of the control fluxes, and treatment values were calculated from the final 30 min of a 70-min treatment exposure. One-tailed tests were used to analyze the qPCR results; a previous study in the Gulf toadfish (41) revealed that tfGC-C, tfCFTR, and tfNKCC1 levels of expression increased in 60 ppt. One-tailed tests were also used to analyze changes in Na+, Cl−, and fluid absorption, which have been shown to be inhibited after RGN stimulation in the Gulf toadfish (40, 41).
RESULTS
Tissue Density
Tissue masses and surface areas collected for the sac preparations revealed that the rectum of the Gulf toadfish is significantly denser than the posterior intestine (16.16 ± 1.29 vs. 20.59 ± 2.36 mg/cm2). There were no differences in tissue densities of fish acclimated to 35 and 60 ppt. Significant differences were revealed by Mann-Whitney rank sum tests.
Dose Response of the Rectum to Renoguanylin
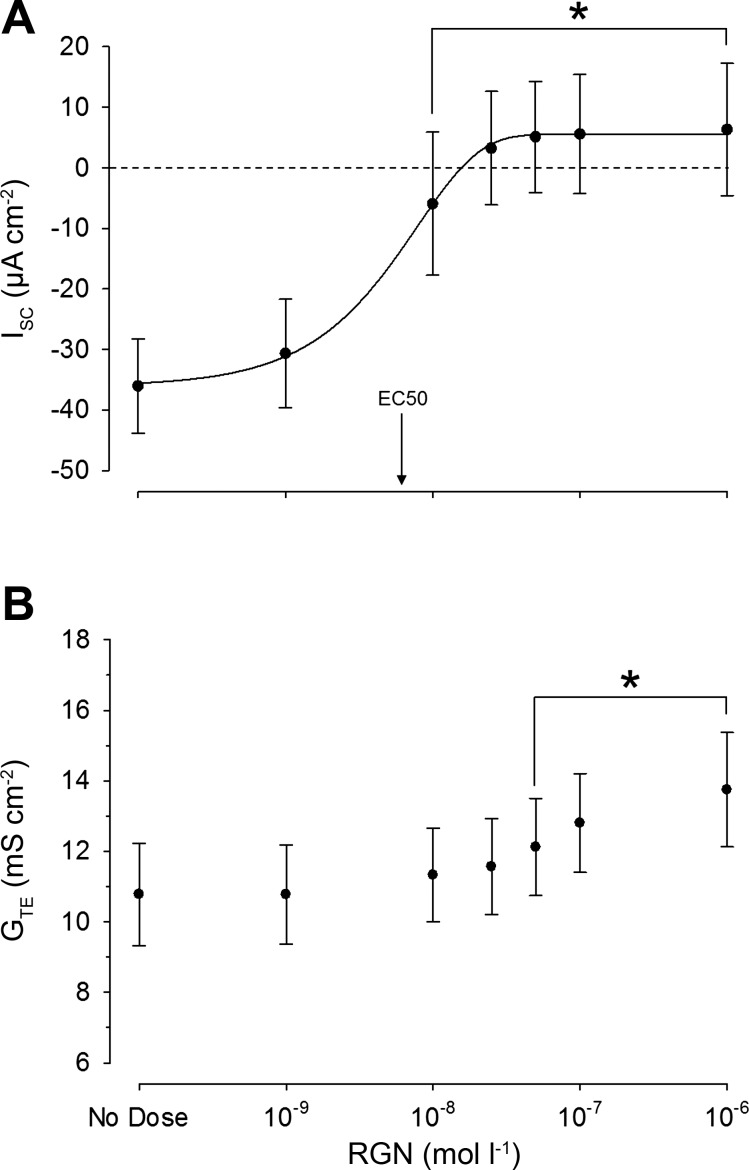
Stimulation of rectal tissues with increasing RGN concentrations (10−9→ 10−6 mol/l) resulted in pronounced decreases in the absorptive ISC in a sigmoidal, dose-dependent manner. On average, the absorptive ISC was reversed (mucosa-to-serosa to serosa-to-mucosa) at a concentration of 2.5 × 10−8 mol/l RGN, with an EC50 of 6.12 × 10−9 mol/l RGN (Fig. 1A). GTE also increased significantly at RGN concentrations ≥5.0 × 10−8 mol/l (Fig. 1B)
Fig. 1.
Changes in short-circuit current (ISC) (A) and transepithelial conductance (GTE) (B) in rectal tissues as a function of renoguanylin (RGN) dose after mucosal application (log scale). The concentration of RGN was increased every 30 min when ISC values became stable. Values are expressed as means ± SE (n = 6). Significant differences from the control (no dose) were revealed by one-way, repeated-measures ANOVAs, followed by Holm-Sidak tests (*P ≤ 0.05). The 50% effective concentration (EC50) of RGN was calculated using a nonlinear regression, global curve fitting (n = 6; P ≤ 0.05). Positive and negative ISC values indicate secretory and absorptive currents, respectively.
Quantitative PCR Analysis
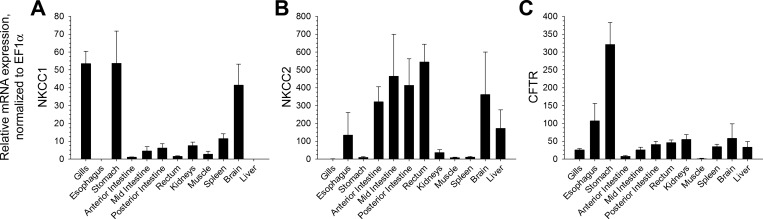
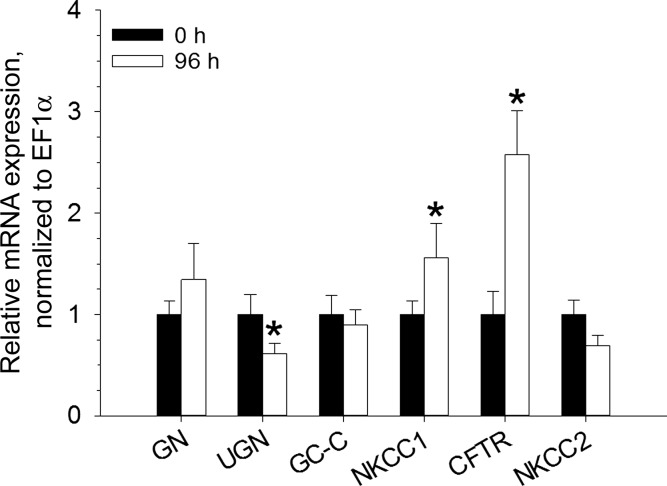
Gene expression levels were normalized to elongation factor (EF)-1α expression. NKCC1, NKCC2, and CFTR gene expression was present in all intestinal segments and the rectum (Fig. 2). Increased levels of rectal NKCC1 and CFTR gene expression occurred after a 96-h exposure to 60 ppt; conversely, a decreased level of rectal uroguanylin gene expression occurred after 96 h in 60 ppt (Fig. 3).
Fig. 2.
Relative gene expression levels of NKCC1 (A), NKCC2 (B), and CFTR (C) in various tissues of the Gulf toadfish. Gene expression is normalized to elongation factor (EF)-1α and scaled relative to the tissue with the lowest expression value (NKCC1: anterior intestine, NKCC2: gills, and CFTR: muscle), which was given a value of 1.0. NKCC1 gene expression was not detected in the esophagus and liver. Values are expressed as means ± SE (n = 8).
Fig. 3.
Relative gene expression levels of guanylin (GN), uroguanylin (UGN), guanylyl cyclase-C (GC-C), NKCC1, CFTR, and NKCC2 in the rectum of Gulf toadfish acclimated to 0 (control) and 96 h in 60 ppt seawater. The level of expression for each gene is normalized to elongation factor (EF)-1α and scaled relative to the 0-h control, which was given a value of 1.0. Values are means ± SE (n = 8). Significant differences between the 0 and 96-h groups were revealed by one-tailed Student's t-tests (*P ≤ 0.05).
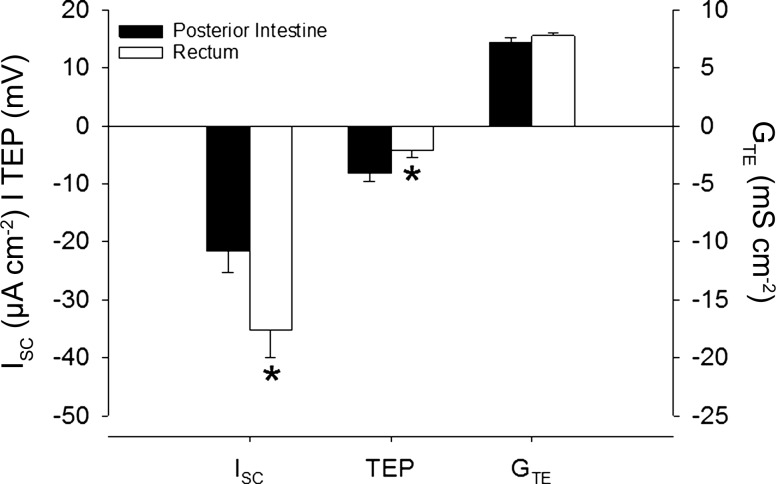
Baseline Parameters: Posterior Intestine Versus Rectum
The posterior intestine and rectum of the Gulf toadfish both displayed an absorptive ISC, which was significantly greater in the rectum (Fig. 4). The basal TEP of both the posterior intestine and rectum was negative, and significantly more so in the posterior intestine (Fig. 4).
Fig. 4.
Baseline (control) values of the short-circuit current (ISC), transepithelial potential (TEP), and transepithelial conductance (GTE) displayed by the posterior intestine and rectum of Gulf toadfish. Values are expressed as means ± SE and were pooled from various experiments in the present study (ISC: n = 11 and 13, TEP: n = 13 and 13, and GTE: n = 25 and 27, posterior intestine and rectum, respectively). Significant differences were revealed by Student's t-tests (*P ≤ 0.05). Positive and negative ISC values indicate secretory and absorptive currents, respectively. Positive and negative TEP values indicate net anion secretion and absorption, respectively.
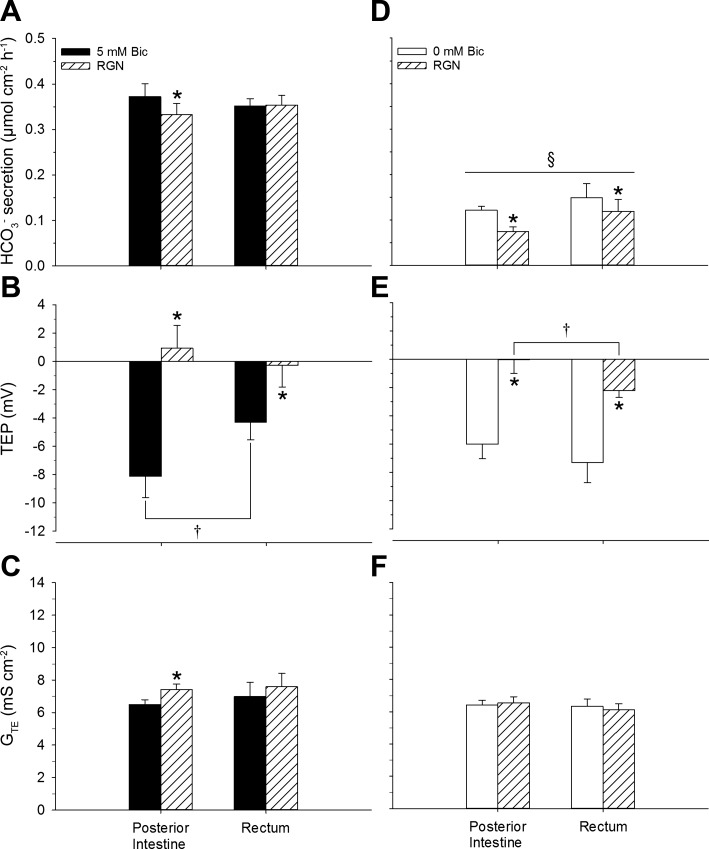
Effects of Renoguanylin on Bicarbonate Secretion, Transepithelial Potential, and Transepithelial Conductance
Tissues bathed in a serosal saline containing 5 mmol/l HCO3−.
In the posterior intestine, RGN significantly reduced HCO3− secretion, reversed the TEP (on average), and significantly increased the GTE (Fig. 5, A–C). Conversely, RGN had no effect on HCO3− secretion in the rectum, but did significantly reduce the magnitude of the TEP (Fig. 5B). The control TEP of the posterior intestine was significantly more negative than that of the rectum (Fig. 5B).
Fig. 5.
HCO3− (Bic) secretion (A and D), transepithelial potential (TEP) (B, E), and transepithelial conductance (GTE) (C and F) displayed by the posterior intestine and rectum of Gulf toadfish before and after treatment with renoguanylin (RGN). Pretreatment values were taken from the final 30 min of the control flux, and post-treatment values were taken from the final 30 min of a 70-min treatment flux. Values are expressed as means ± SE (n = 7 and 8, 5 and 0 mM Bic groups, respectively). Significant differences: control vs. RGN revealed by paired t-tests (*P ≤ 0.05), posterior intestine vs. rectum revealed by Student's t-tests (†P ≤ 0.05), and 5 mM Bic vs. 0 mM (within tissue and within treatment) revealed by Student's t-tests (§P ≤ 0.05). The presence (5 mM Bic) and absence (0 mM Bic) of serosal HCO3− is indicated by solid and open bars, respectively. Mucosally administered RGN treatment is indicated by hatched bars.
Tissues bathed in a serosal saline devoid of serosal HCO3−.
In both the posterior intestine and rectum, RGN significantly reduced both HCO3− secretion and the TEP (Fig. 5, D and E). All control and RGN-treated tissues bathed in the serosal saline devoid of HCO3− displayed significantly lower HCO3− secretion rates than their counterparts bathed with 5 mmol/l HCO3− (Fig. 5, A and D). The TEP of the RGN-treated rectum was significantly more negative than that of the RGN-treated posterior intestine (Fig. 5E).
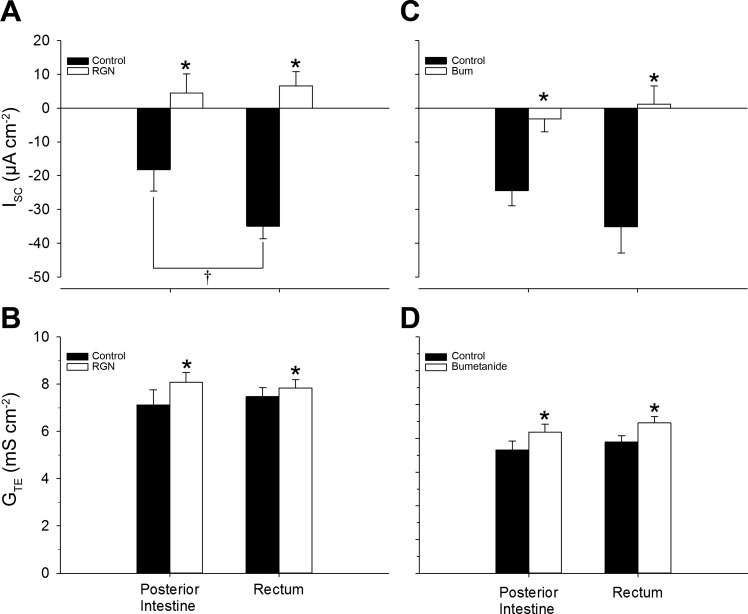
Effects of Renoguanylin and Bumetanide on Short-Circuit Current and Transepithelial Conductance
On average, RGN reversed the absorptive ISC (from mucosa-to-serosa to serosa-to-mucosa) and significantly increased the GTE of both the posterior intestine and rectum of the Gulf toadfish (Fig. 6, A and B); these results are in line with previous reports (40, 41). Bumetanide had similar effects as RGN; in both tissues, it significantly increased GTE and reduced the magnitude of the absorptive ISC of the posterior intestine and, on average, reversed it in the rectum (Fig. 6, C and D).
Fig. 6.
Short-circuit current (ISC) (A, C) and transepithelial conductance (GTE) (B, D) displayed by the posterior intestine and rectum of Gulf toadfish before and after treatment with renoguanylin (RGN) or bumetanide (Bum). Pretreatment values were taken from the final 30 min of the control flux and post-treatment values were taken from the final 30-min of a 70-min treatment flux. Values are expressed as means ± SE (n = 6 and 6 or 7, RGN and Bum experiments, respectively). Significant differences: control vs. treatment revealed by paired t-tests (*P ≤ 0.05) and posterior intestine vs. rectum revealed by Student's t-tests (†P ≤ 0.05). Control and treatment values are indicated by solid and open bars, respectively. Positive and negative ISC values indicate secretory and absorptive currents, respectively.
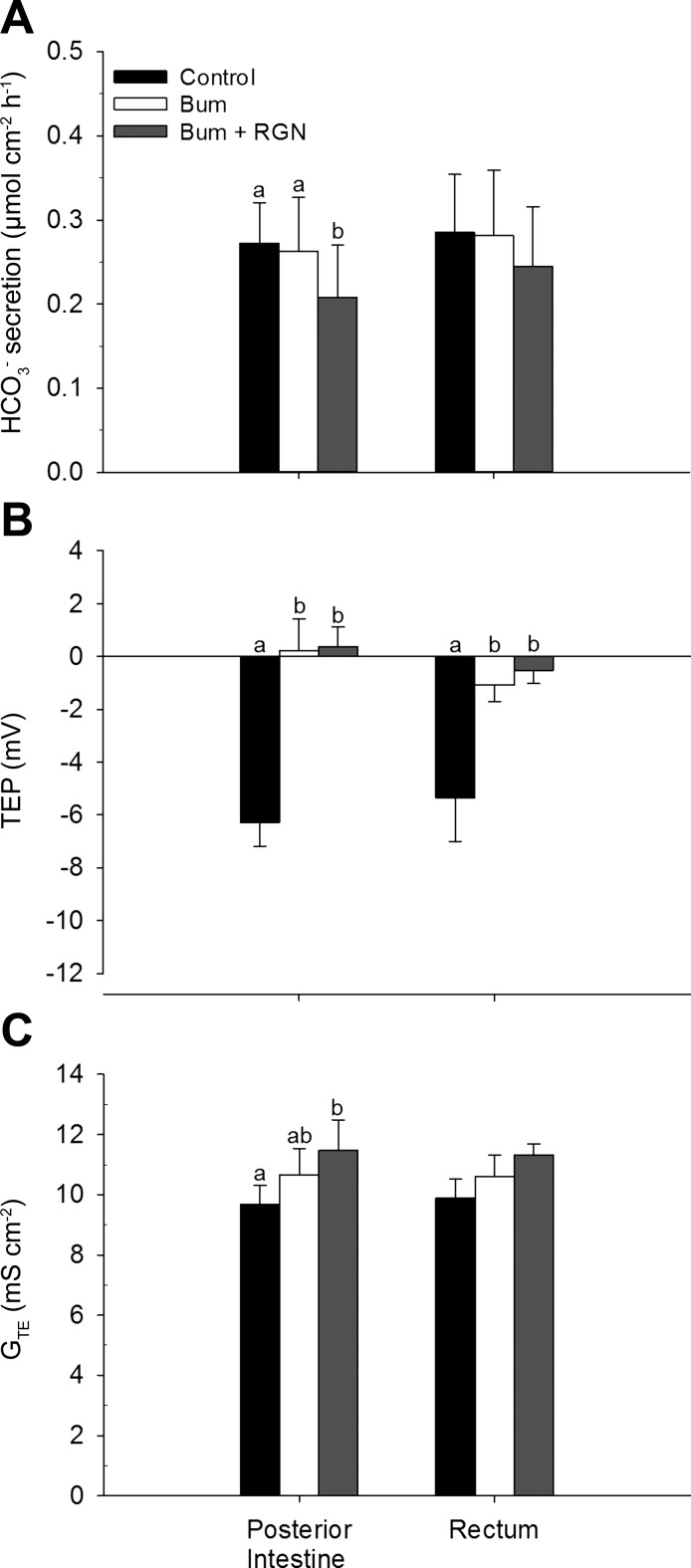
Effects of Bumetanide and Renoguanylin on Bicarbonate Secretion, Transepithelial Potential, and Transepithelial Conductance
In the posterior intestine, HCO3− secretion was significantly decreased in the presence of combined bumetanide and RGN treatment, but not with bumetanide alone (Fig. 7A). Bumetanide reversed the TEP (on average), and this effect was not enhanced by RGN (Fig. 7B). GTE significantly increased in the presence of combined bumetanide and RGN treatment, but not with bumetanide alone (Fig. 7C). In the rectum, only the TEP was affected by bumetanide, with a significant decrease, and this effect was not enhanced by RGN (Fig. 7B).
Fig. 7.
HCO3− secretion (A), transepithelial potential (TEP) (B), and transepithelial conductance (GTE) (C) displayed by the posterior intestine and rectum of Gulf toadfish. Control values (solid bars) were taken from the final 30 min of the control flux. Bumetanide (Bum) (open bars) was then added, and its effects were measured from the final 30 min of a 70-min treatment flux. Finally, renoguanylin (RGN) (shaded bars) was added in combination with Bum, and its effects were measured from the final 30 min of a 70-min treatment flux (ttotal = 170 min). Values are expressed as means ± SE. Significant differences were revealed by one-way, repeated-measures ANOVAs, followed by Holm-Sidak tests (a,bP ≤ 0.05). Positive and negative TEP values indicate net anion secretion and absorption, respectively.
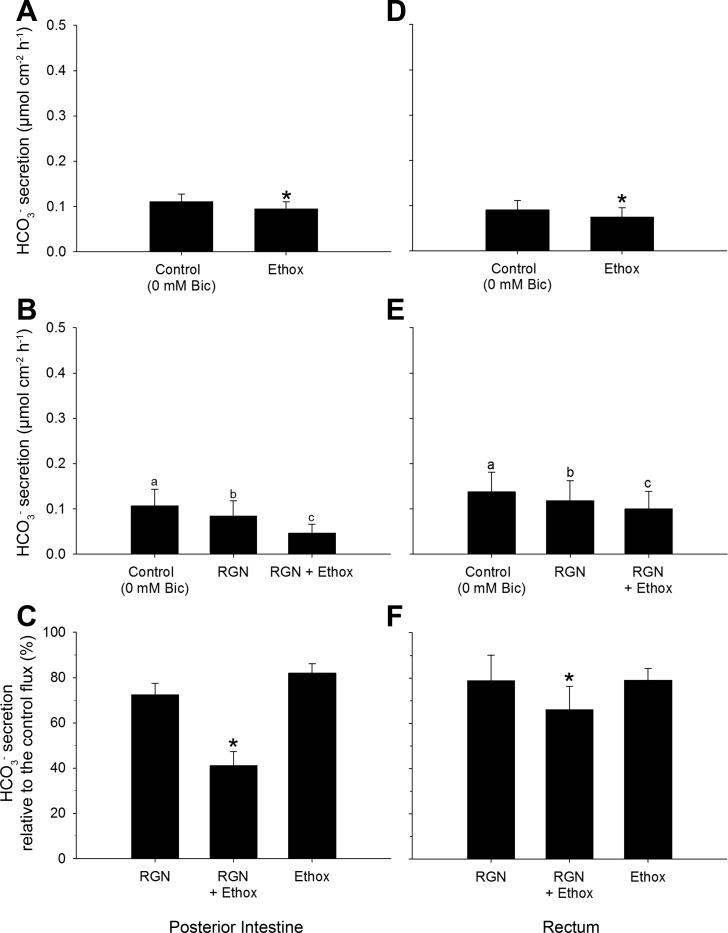
Effects of Ethoxzolamide on Bicarbonate Secretion in the Posterior Intestine and Rectum, When Bicarbonate/CO2 Is Absent from the Serosal Saline
Under bicarbonate-free conditions, ethoxzolamide decreased bicarbonate secretion in both the posterior intestine and rectum (Fig. 8, A and D). As expected, RGN decreased bicarbonate secretion in both the posterior intestine and rectum, and further by the administration of ethoxzolamide into the mucosal saline (Fig. 8, B and E). The effects of RGN and ethoxzolamide were equal when applied individually and additive when applied together (Fig. 8, C and F).
Fig. 8.
Absolute (A, B, D, E) and relative (C, F) HCO3− secretion rates displayed by the posterior intestine and rectum of Gulf toadfish when exposed in the absence (0 mmol/l) of serosal HCO3− (Bic). A and D: ethoxzolamide (Ethox) was added alone to the mucosal saline (n = 7 and 8, posterior intestine and rectum, respectively; ttotal = 100 min). Values are expressed as means ± SE. Significant differences were revealed by paired t-tests (*P ≤ 0.05). B and E: RGN was initially added to the mucosal saline, after which Ethox was also added to the mucosal saline (n = 6 and 7, posterior intestine and rectum, respectively; ttotal = 170 min). Significant differences were revealed by one-way, repeated-measures ANOVAs, followed by Holm-Sidak tests (a,b,cP ≤ 0.05). C and F: combined effects of RGN and Ethox were compared with their individual treatments. Significant differences: RGN vs. RGN + Ethox revealed by paired t-tests and Ethox vs. RGN + Ethox revealed by Student's t-tests (*P ≤ 0.05). Control values were taken from the final 30 min of the control flux. Treatment values were taken from the final 30 min of a 70-min treatment flux.
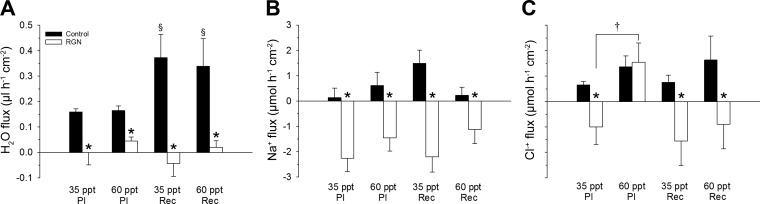
Effects of Renoguanylin on Water, Cl−, and Na+ Fluxes in Posterior Intestinal and Rectal Sac Preparations of Gulf Toadfish Exposed to 35 and 60 ppt
In the posterior intestine, RGN treatment led to net water secretion of fish exposed to 35 ppt, and decreased water absorption in fish exposed to 60 ppt (Fig. 9A). RGN caused net Na+ secretion in posterior sac preparations of fish exposed to both 35 and 60 ppt (Fig. 9B), and led to net Cl− secretion in posterior sac preparations of fish exposed to 35 ppt, but not from the 60 ppt group (Fig. 9C). In the rectum, RGN led to net water secretion in rectal sac preparations of fish exposed to 35 ppt and decreased water absorption in fish exposed to 60 ppt (Fig. 9A). Moreover, the response to RGN of the rectum was greater than in the posterior intestine of fish exposed to 60 ppt (Fig. 9A). RGN led to net Na+ and Cl− secretion in the rectal sac preparations of fish exposed to both 35 and 60 ppt (Fig. 9, B and C).
Fig. 9.
Water (A), Na+ (B), and Cl− (C) fluxes displayed by the posterior intestine (PI) and rectum (Rec) of Gulf toadfish acclimated to 35 or 60 ppt and treated with RGN. Values are expressed as means ± SE. Three-way ANOVAs, followed by one-tailed Holm-Sidak tests revealed significant differences in Na+, Cl−, and water fluxes (P ≤ 0.05; ntotal = 55). Control vs. RGN (*), PI: 35 vs. 60 ppt (†), and 35 or 60 ppt: PI vs. Rec (§). Positive and negative values indicate net absorption or net secretion, respectively.
DISCUSSION
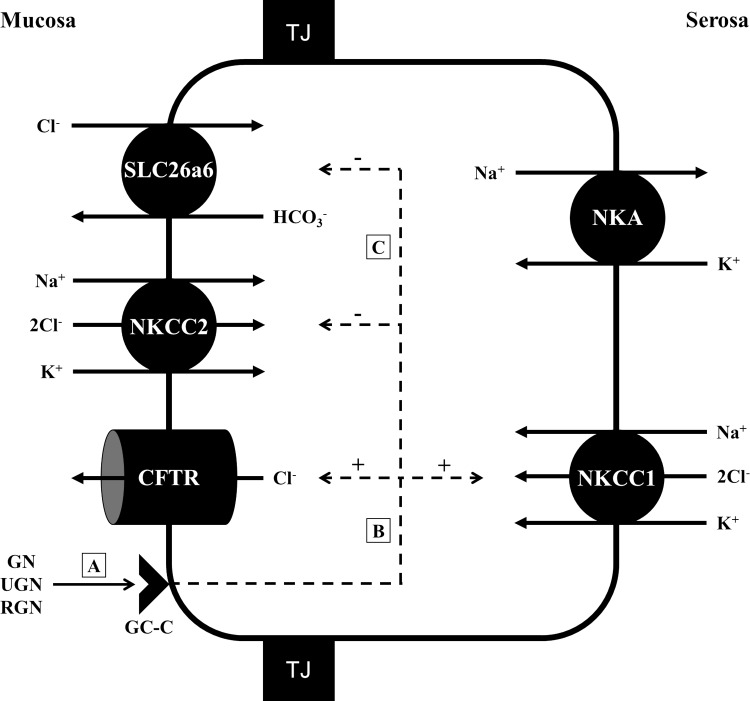
The present study reveals that the absorptive ISC of the rectum is greater than that of the posterior intestine, yet, the TEP of the posterior intestine is more negative than that of the rectum. Furthermore, it is demonstrated that RGN affects the rectum similarly to the posterior intestine. However, in the rectum, RGN inhibits water absorption more greatly than in the posterior intestine and, in contrast to the posterior intestine, does not reduce HCO3− secretion when bicarbonate is present in the serosal saline. The present study also shows that the downstream effects of RGN likely act on SLC26a6 and lead to the observed reduction of the HCO3− secretion from endogenous CO2, in addition to stimulating the Cl−-secretory pathway, made up of apical CFTR, and basolateral NKCC1 (Fig. 10).
Fig. 10.
Simplified proposed effects of the guanylin peptides in the posterior intestinal and rectal epithelia of Gulf toadfish (Opsanus beta) acclimated to 35 ppt seawater. Guanylin (GN), uroguanylin (UGN), and renoguanylin (RGN) bind to a guanylyl cyclase-C (GC-C) receptor on the apical membrane of an enterocyte (A). The stimulation of GC-C leads to enhanced formation of cGMP, whose downstream effects increase the phosphorylation of PKA and PKG, both of which can activate CFTR and, perhaps, NKCC1 (B). The downstream effects of cGMP also lead to inhibited ion transport activity by NKCC2 and decreased anion exchange activity by SLC26a6 (C). The combined effects of GC-C stimulation results in the reversal of ion flux, from net ion absorption (mucosa-to-serosa) to net ion secretion (serosa-to-mucosa), which result in either inhibition of water absorption or net water secretion. NKA, Na+/K+-ATPase (maintains electrochemical gradients that enables ion transport); TJ, tight junction, stimulatory (+) and inhibitory (−) effects. Cell diagram modified from Ruhr et al. (41).
Comparative Physiology of the Posterior Intestine and Rectum
The present study reinforces the current knowledge on the tissue distribution patterns of NKCC1, NKCC2, and CFTR gene expression in seawater teleosts. The high relative mRNA expression of CFTR and NKCC1 in gill tissues is not surprising. In the seawater teleost gill, CFTR and NKCC1 are located on the apical and basolateral membranes, respectively, and constitute a Cl−-secretory pathway that excretes excess Cl− for the purposes of maintaining homeostasis of plasma osmolality (for a review, see Ref. 34). Conversely, NKCC1 transcripts were not detected in the esophagus of the Gulf toadfish, a result that is unsurprising given the importance of esophageal desalination, rather than salt secretion, for the purposes of decreasing the osmolality of imbibed seawater (from ∼1,000 to ∼500 mosmol/kg H2O) (for a review, see Ref. 17). It is interesting to note relatively high CFTR gene expression in the esophagus, which could be expressed in the apical membrane as an absorptive isoform or on the basolateral membrane as a secretory transporter. In any case, the Gulf toadfish likely takes advantage of the large inward electrochemical gradient for Cl− that permits its passive absorption from the esophageal lumen into the interstitial fluid. The high mRNA expression of CFTR and NKCC1 in the stomach is also unsurprising given the role of HCl plays as a component of gastric juices used to break down food. All of the intestinal segments and the rectum displayed relatively lower CFTR and NKCC1 gene expression, although they appear to be expressed at the protein level, as evidenced by their immunofluorescent-like reactivity [CFTR in the posterior intestine and NKCC1 in the anterior and posterior intestine (40)]. The anterior intestine is the site of the greatest ion absorption in the seawater teleost intestine, while the middle and posterior intestine are the sites of high water absorption (17, 34). Accordingly, it is not surprising that the entire intestine displays rather low relative mRNA expression of CFTR and NKCC1, since its primary osmoregulatory role is ion and fluid absorption for the purposes of maintaining whole body hydration. Indeed, the seawater teleost intestine expresses absorptive-type ion transporters important for fluid absorption (17), including NKCC2 shown in the present and previous studies (12, 23, 25, 38). Moreover, in the Gulf toadfish rectum, absorptive-type ion transporters and proteins necessary for ion absorption, including NKCC2, as well as CFTR and NKCC1, are also expressed at the mRNA level. These consist of SLC26a6 (20), VHA (22), NBCe1 (49), and CAC (42). Consequently, the Gulf toadfish rectum has absorbing and secretory ion transporters that enable it to absorb and secrete water, respectively, much like in the posterior intestine.
Indeed, the present study shows that the posterior intestine and rectum of Gulf toadfish acclimated to 35 ppt absorbed Na+ and Cl− at roughly the same rates. Interestingly, the rectum displayed a greater absorptive ISC (under symmetrical conditions) and absorbed more water (under asymmetrical, in vivo-like conditions) than the posterior intestine, while the magnitude of the TEP of the posterior intestine was roughly twice the magnitude of the rectum. These data suggest that the posterior intestine relies more on an HCO3−/Cl− exchange by SLC26a6 and the rectum on Na+/K+/2Cl−-cotransport by NKCC2. This is due to the electrogenic nature of SLC26a6 (20), which exchanges multiple bicarbonate anions for a single Cl−, as opposed to the electroneutral transport of NKCC2. The differences in the electrophysiological and osmoregulatory properties between the posterior intestine and rectum are likely not due to transcriptional differences of proteins involved in ion absorption [i.e., SLC26a6, VHA, NBCe1, CAC, and NKCC2 (present study)], which are expressed equally (20, 22, 42, 49).
An objective of the present study was to determine whether the rectum would respond to RGN stimulation. The present study confirms the presence of the components of the Cl−-secretory pathway, CFTR and NKCC1, by qPCR, which seem necessary for responding to RGN, at least for the parameters measured in previous studies (3, 4, 40, 41). In prior studies on the Gulf toadfish and Japanese eel, inhibited water absorption and, in some instances, water secretion have been observed in the middle and posterior intestine, but not in the anterior intestine, after the mucosal administration of guanylin, uroguanylin, or RGN (3, 4, 55). It should first be noted that the rectum seems to be more sensitive to RGN than the posterior intestine, with the EC50 of the rectum (6.12 × 10−9 mol/l RGN) being significantly lower than that of the posterior intestine [11.6 × 10−9 mol/l RGN (40)]. Moreover, RGN causes a significant decrease and a reversal of the absorptive ISC in the rectum at 10−8 and 2.5 × 10−8 mol/l (present study), respectively, and at 2.5 × 10−8 mol/l (for both measurements) in the posterior intestine (40). These differences might be related to a combination of the greater tissue density of the rectum (which could express greater GC-C on the apical membrane) and a greater affinity for RGN by rectal GC-C than in the posterior intestine.
The present study shows that the posterior intestine and rectum of Gulf toadfish acclimated to 35 ppt respond to RGN by inhibiting water absorption and displaying net secretion of Cl− and Na+. However, their electrophysiological responses to RGN are distinct: RGN decreases the absorptive ISC of the rectum more greatly than that of the posterior intestine; conversely, RGN decreases the TEP of the posterior intestine more greatly than that of the rectum. These differences might be due to the proposed reliance on SLC26a6 by the posterior intestine and NKCC2 by the rectum for ion absorption (see above). The larger decrease in the TEP of the posterior intestine is likely the result of SLC26a6 being inhibited more greatly than in the rectum, in contrast to the more tissue-dense rectum, where the effects of RGN might be more pronounced on the overall ion-absorptive capacity of the tissue. In both tissues, however, GTE was significantly increased, despite inhibition of ion absorption. It has been suggested that anion secretion by the opening of CFTR (40, 41) and, possibly, increased paracellular cation secretion (3) might be responsible for the increases in conductance when intestinal tissues are treated with the guanylin peptides. Inhibition of water absorption partially occurs as a result of Cl− secretion, likely by CFTR activation, which is fueled, in part, by NKCC1, which takes up Cl− from the blood (3, 4, 40, 41, 55), and is necessary to inhibit water absorption. Although counterintuitive, decreased water absorption is a strategy that would benefit the removal of CaCO3 precipitates that naturally form along the intestine (as part of the normal osmoregulatory processes). It has been demonstrated that the retention of water in the guinea pig ileum can make digested food more aqueous and allow it to be moved out of the intestinal tract more easily by muscle contractions (43). A similar mechanism would allow the removal of CaCO3 precipitates from the intestine and reduce the occurrence of intestinal blockages. Consequently, the trade-off between decreasing water absorption in the posterior intestine, and the risk of dehydration associated with this behavior, is made by decreasing the risk of harmful obstructions in the narrow intestinal passage. Nevertheless, the osmoregulatory mechanisms necessary for water absorption are maintained in the anterior intestine of the seawater teleost, as it does not seem to be affected by the guanylin peptides (40, 55).
Effects of Renoguanylin on NKCC2 and SLC26a6 Transport Activity
Another objective of the present study was to determine whether the downstream effects of RGN affected the transport activity of NKCC2 and SLC26a6. To test the effects of RGN on NKCC2, bumetanide (a specific blocker of NKCC isoforms) was used to determine whether its effects on electrophysiological characteristics were similar to that of RGN. Indeed, the reductions to the absorptive ISC and TEP of the posterior intestine and rectum by bumetanide were similar to those of RGN. Of particular interest, however, is the failure of bumetanide to change tissue conductance in the posterior intestine and rectum under in vivo-like conditions (asymmetrical saline exposures), as opposed to RGN. This suggests that RGN does not inhibit NKCC2 as greatly as bumetanide, and the reductions to the absorptive ISC and TEP, as well as the increases in GTE, are the sum effects of NKCC2 inhibition, apical CFTR activation, and possible paracellular Na+ secretion, which leads to net Na+ and Cl− secretion observed in tissues of fish acclimated to 35 ppt. Evidence from the Japanese eel supports this suggestion, because the magnitude of the decreases to the absorptive ISC and TEP of the middle and posterior intestine were similar for both guanylin and bumetanide (4).
Bumetanide also had no effect on HCO3− secretion in the posterior intestine and rectum of fish acclimated to 35 ppt (under in vivo-like, asymmetrical conditions). Upon the administration of RGN, however, the posterior intestine displayed reduced HCO3− secretion and increased GTE, without further reductions to the TEP. This suggests that, at least in the Gulf toadfish intestine, Cl− does not recirculate across the apical membrane through NKCC2 and SLC26a6 for the purposes of preserving ion and fluid absorption, which is hypothesized to occur in the Japanese eel (4, 55). Interestingly, the present study shows that RGN does not reduce HCO3− secretion in the Gulf toadfish rectum as it does in the posterior intestine (when HCO3− is present in the serosal saline). A previous study demonstrated that the downstream effects of RGN inhibited the exchange activity of SLC26a6, when CO2 was the only source of HCO3− (via CO2 hydration and CAC) with which to fuel SLC26a6 (41). By removing bicarbonate from the serosal saline, the basolateral HCO3− transporter, NBCe1, was rendered functionless and, as before, CO2 was the sole source of HCO3− fueling SLC26a6. Under these conditions, RGN reduced HCO3− secretion in both the posterior intestine and rectum. These observations support the previous study's conclusions (41) that the transport activity of SLC26a6 is being directly affected by the downstream effects of RGN.
We also tested a possible mechanism in mammals that is proposed to decrease HCO3− secretion, via SLC26a6, when PKC phosphorylates SLC26a6 at a site that decreases its ability to bind CAC, and, thereby, disrupts the SLC26a6-CAC metabolon (1). It is possible that the effects of RGN could lead to PKG or PKA [which are both phosphorylated downstream of GC-C activation (5)] binding to Gulf toadfish intestinal SLC26a6 at a location on or near the CAC binding site and limit the exchange capacity of SLC26a6 as well. To test this in the Gulf toadfish intestine, the CAC-specific inhibitor, ethoxzolamide [tested in Gulf toadfish previously (18)], was used along with RGN in tissues bathed with a serosal saline absent of HCO3−, to determine their individual and combined effects on HCO3− secretion. With this approach, as with the previous experiment (see above), CO2 was the only source of HCO3− to fuel SLC26a6. The results of the present study reveal that in both the posterior intestine and rectum, the individual doses of RGN and ethoxzolamide statistically decreased HCO3− secretion equally, and the combined doses were additive and different than the individual doses. Consequently, these data do not suggest that the downstream effects of RGN stimulation affects SLC26a6 by interfering with the binding of CAC and disrupting the SLC26a6-CAC metabolon. Instead, the data support the idea that the downstream effects of RGN have a direct effect on the transport capacity of SLC26a6 for HCO3− that leads to decreased exchange activity in the posterior intestine (in both the presence and absence of serosal HCO3−) and in the rectum (in the absence of HCO3−). Moreover, three previous studies (including one on the Gulf toadfish) also demonstrate that CFTR does not seem to transport HCO3− (30, 40, 50), and that Gulf toadfish SLC26a6 transport activity is limited by HCO3− and not Cl− (40). With respect to the guanylin peptide system in the Gulf toadfish intestine observed in the present and previous studies (40, 41), it is possible that inhibition of SLC26a6 (and HCO3− secretion) and activation of CFTR due to RGN stimulation leads to a maximal Cl− current that manifests itself, physiologically, as a reversal of the absorptive ISC.
Increased Response to Renoguanylin in Hypersalinity
The present study reveals changes in gene expression for NKCC1 and CFTR in the rectum after Gulf toadfish are transferred from 35 to 60 ppt, and these results are comparable to what is seen in the posterior intestine (41). The data from both tissues show that gene expression of NKCC1 and CFTR is upregulated in 60 ppt, but differ concerning the expression of GC-C, which is upregulated in the posterior intestine (40), but not in the rectum. The previous study suggested that the response to RGN in the posterior intestine of Gulf toadfish acclimated to 60 ppt was limited by the level of GC-C, NKCC1, and/or CFTR gene expression. At least in the rectum of the Gulf toadfish, the response to RGN is likely limited to NKCC1 and/or CFTR. Despite acclimation to 60 ppt, the current and previous studies (41) demonstrate that water absorption by the posterior intestine and rectum of Gulf toadfish does not increase as expected, as opposed to the sea bream intestine when acclimated to 55 ppt (15). Moreover, both the posterior intestine and rectum of fish exposed to 35 and 60 ppt displayed decreased water absorption in response to RGN that correlates with net Na+ and Cl− secretory fluxes (a switch from mucosa-to-serosa to serosa-to-mucosa), with the exception of the posterior intestine from the 60 ppt. When considering our hypothesis that the guanylin peptide-induced inhibition of water absorption is necessary to facilitate the removal of solids (such as CaCO3 precipitates from the intestine), the occurrence of water absorption being inhibited more greatly in 60 ppt (in response to RGN) would be more important. In 50 ppt, intestinal CaCO3 precipitation in Gulf toadfish increases 2.3-fold (14). Thus, by upregulating gene expression of the components of the Cl−-secretory pathway (apical CFTR and basolateral NKCC1) and inhibiting NKCC2 transport activity by RGN (which seems to occur at 35 ppt as well), more fluid can remain in the intestine to facilitate the removal of increased CaCO3 precipitates formed as a result of hypersalinity.
A notable difference in Cl− flux between the posterior intestine and rectum of Gulf toadfish exists, as mentioned above, in response to RGN. In the present and previous study, net Cl− secretion does not occur in the posterior intestine of fish acclimated to 60 ppt when treated with RGN, and this is observed in parallel with increased HCO3− secretion (41). It has been suggested that this occurs to maintain the drinking rate of the fish (2). In hypersalinity, there is even greater branchial fluid loss relative to normal seawater. Consequently, to stave off dehydration, a seawater teleost must increase its rate of drinking concurrently with the increases in branchial water loss. In 35 ppt, decreases in HCO3− secretion by the posterior intestine is beneficial to promote net Cl− secretion and inhibit both water absorption and CaCO3 precipitation (40, 41). Conversely, in 60 ppt, stimulating net Cl− secretion and limiting the posterior intestine's ability to absorb water and secrete HCO3− could be detrimental. The consequence of limiting HCO3− secretion in 60 ppt is the reduction of CaCO3 precipitation that is essential for the absorption of water in seawater teleosts. Likewise, increases in luminal Cl− concentration in fish decreases their drinking rate in parallel (2). Accordingly, the RGN response with respect to HCO3− secretion (41) and Cl− in the posterior intestine of Gulf toadfish acclimated to 60 ppt is a possible adaptation for maintaining a high rate of drinking to compensate for increases in branchial fluid loss. In this fascinating process, Cl− secretion likely still occurs in response to RGN (as evidenced by inhibited water absorption), but is completely reabsorbed by SLC26a6 in exchange for HCO3−. Yet, these mechanisms are not observed in the rectum of fish acclimated to 60 ppt, with Cl− secretion occurring equally to what is seen in both the posterior intestine and rectum of fish acclimated to 35 ppt. The adaptive benefit of this response might be to ensure that enough water is present in the rectum (via inhibited water absorption/stimulation of water secretion) to removed CaCO3 precipitates (or other solids) from the intestine.
Significance and Perspectives
The posterior intestine and rectum of Gulf toadfish display comparable osmoregulatory physiologies with respect to Na+, and Cl− fluxes, yet they possess key distinct mechanisms by which they regulate ion and fluid movement. The posterior intestine appears to rely more on electrogenic anion exchange by SLC26a6 than the rectum (as evidence by the larger magnitude of its TEP), while the rectum appears to rely more on ion cotransport by NKCC2 than the posterior intestine (as evidenced by the large magnitude of its absorptive ISC and the greater absolute change in ISC when bumetanide is applied). In addition, the rectum absorbs more water than the posterior intestine, and this observation correlates with the greater absorptive ISC and tissue density of the intestine. The rectum responds more strongly to RGN than the posterior intestine in reversing the absorptive ISC and inhibiting water absorption. In support of the hypothesis that the tissue response of the intestine to the guanylin peptides is necessary to facilitate the removal of solids from the intestine, limiting water absorption would permit more water to remain in the rectum, which would enable solids to be removed from the intestinal tract (at least in response to RGN from the Japanese eel).
The downstream effects of cGMP formation, due to RGN binding to GC-C, might act to inhibit both NKCC2 and SLC26a6, in addition to stimulating the opening of apical CFTR. If these intracellular effects do occur, it elevates the guanylin peptides as potent modulators of intestinal physiology due to their modification of the osmoregulatory processes. The intracellular mechanisms that regulate these changes should be investigated further and be focused on the roles that cGMP/PKG and cAMP/PKA might play.
The present study also reveals that uroguanylin gene expression significantly decreases in the rectum of fish exposed to 60 ppt and compares well to what is seen in the posterior intestine in 60 ppt (41). This suggests that Gulf toadfish guanylin and uroguanylin might have different functions. Indeed, in the rat intestine and in T84 cells expressing rat GC-C, uroguanylin was most effective in a pH of 5.5, while guanylin was most effective in a pH of 7.4 and 8.0 (24, 26). In mammals, guanylin and uroguanylin cause increased HCO3− secretion into the intestinal lumen; accordingly, it has been suggested that uroguanylin might play a role in defending the pH of the intestine, as acidic chyme enters from the stomach (11). Conversely, guanylin transcription and secretion are increased in rat intestinal tissues when perfused with high salt concentrations and decreased with low salt intake (7, 29, 32). Conceivably, in seawater teleosts, uroguanylin plays a more prominent role in digestion, and guanylin takes a more active part in osmoregulatory processes.
It should be emphasized that the guanylin peptide system in Gulf toadfish has, so far, been studied on fasted fish. The potential interactions between osmoregulation and digestion in the context of guanylin peptide function are an interesting avenue of further study.
GRANTS
M. Grosell is Maytag Professor of Ichthyology and is supported by National Science Foundation (Grant IOS 1146695).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.R. and M.G. conception and design of research; I.M.R. performed experiments; I.M.R. and M.G. analyzed data; I.M.R., Y.T., and M.G. interpreted results of experiments; I.M.R. prepared figures; I.M.R. drafted manuscript; I.M.R., Y.T., and M.G. edited and revised manuscript; M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. M. Danielle McDonald from the Rosenstiel School of Marine and Atmospheric Science at the University of Miami for the generous use of her equipment. We also thank Drs. Patrick J. Walsh, Nicolas Corradi, and Christophe M. R. LeMoine from the University of Ottawa for providing us with the annotated intestinal transcriptome of the Gulf toadfish.
REFERENCES
- 1.Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J 24: 2499–2511, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando M, Nagashima K. Intestinal Na+ and Cl− levels control drinking behavior in the seawater-adapted eel Anguilla japonica. J Exp Biol 199: 711–716, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Ando M, Takei Y. Guanylin activates Cl−1 secretion into the lumen of seawater eel intestine via apical Cl− channel under simulated in vivo conditions. Am J Physiol Regul Integr Comp Physiol 308: R400–R410, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Ando M, Wong MK, Takei Y. Mechanisms of guanylin action on water and ion absorption at different regions of seawater eel intestine. Am J Physiol Regul Integr Comp Physiol 307: R653–R663, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Arshad N, Visweswariah SS. Cyclic nucleotide signaling in intestinal epithelia: getting to the gut of the matter. Wiley Interdiscip Rev Syst Biol Med 5: 409–424, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Arshad N, Visweswariah SS. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett 586: 2835–2840, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Carrithers SL, Jackson BA, Cai WY, Greenberg RN, Ott CE. Site-specific effects of dietary salt intake on guanylin and uroguanylin mRNA expression in rat intestine. Regul Pept 107: 87–95, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho ES, Gregorio SF, Power DM, Canario AV, Fuentes J. Water absorption and bicarbonate secretion in the intestine of the sea bream are regulated by transmembrane and soluble adenylyl cyclase stimulation. J Comp Physiol B 182: 1069–1080, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Comrie MM, Cutler CP, Cramb G. Cloning and expression of guanylin from the European eel (Anguilla anguilla). Biochem Biophys Res Commun 281: 1078–1085, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cramb G, Martinez AS, McWilliam IS, Wilson GD. Cloning and expression of guanylin-like peptides in teleost fish. Ann NY Acad Sci 1040: 277–280, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Forte LR, Hamra FK. Guanylin and uroguanylin: Intestinal peptide hormones that regulate epithelial transport. News Physiol Sci 11: 17–24, 1996. [Google Scholar]
- 12.Frizzell RA, Smith PL, Vosburgh E, Field M. Coupled sodium-chloride influx across brush border of flounder intestine. J Membr Biol 46: 27–39, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Genz J, McDonald MD, Grosell M. Concentration of MgSO4 in the intestinal lumen of Opsanus beta limits osmoregulation in response to acute hypersalinity stress. Am J Physiol Regul Integr Comp Physiol 300: R895–R909, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Genz J, Taylor JR, Grosell M. Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid-base balance in Opsanus beta. J Exp Biol 211: 2327–2335, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Gregorio SF, Carvalho ES, Encarnacao S, Wilson JM, Power DM, Canario AV, Fuentes J. Adaptation to different salinities exposes functional specialization in the intestine of the sea bream (Sparus aurata L.). J Exp Biol 216: 470–479, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Grosell M. Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209: 2813–2827, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Grosell M. The role of the gastrointestinal tract in salt and water balance. In: Fish Physiology, 2010, p. 135–164. [Google Scholar]
- 18.Grosell M, Genz J. Ouabain-sensitive bicarbonate secretion and acid absorption by the marine teleost fish intestine play a role in osmoregulation. Am J Physiol Regul Integr Comp Physiol 291: R1145–R1156, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Grosell M, Genz J, Taylor JR, Perry SF, Gilmour KM. The involvement of H+-ATPase and carbonic anhydrase in intestinal HCO3− secretion in seawater-acclimated rainbow trout. J Exp Biol 212: 1940–1948, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Grosell M, Mager E, Williams C, Taylor J. High rates of HCO3− secretion and Cl− absorption against adverse gradients in the marine teleost intestine: the involvement of an electrogenic anion exchanger and H+-pump metabolon? J Exp Biol 212: 1684–1696, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Grosell M, Wood CM, Wilson RW, Bury NR, Hogstrand C, Rankin C, Jensen FB. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am J Physiol Regul Integr Comp Physiol 288: R936–R946, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Guffey S, Esbaugh A, Grosell M. Regulation of apical H+-ATPase activity and intestinal HCO3− secretion in marine fish osmoregulation. Am J Physiol Regul Integr Comp Physiol 301: R1682–R1691, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Halm D, Krasny E, Frizzell R. Electrophysiology of flounder intestinal mucosa. II. Relation of the electrical potential profile to coupled NaCl absorption. J Gen Physiol 85: 865–883, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamra FK, Eber SL, Chin DT, Currie MG, Forte LR. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci USA 94: 2705–2710, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiroi J, Yasumasu S, McCormick SD, Hwang PP, Kaneko T. Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211: 2584–2599, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Joo NS, London RM, Kim HD, Forte LR, Clarke LL. Regulation of intestinal Cl− and secretion by uroguanylin. Am J Physiol Gastrointest Liver Physiol 274: G633–G644, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Kalujnaia S, Wilson GD, Feilen AL, Cramb G. Guanylin-like peptides, guanylate cyclase and osmoregulation in the European eel (Anguilla anguilla). Gen Comp Endocrinol 161: 103–114, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Kim YK, Ideuchi H, Watanabe S, Park SI, Huh M, Kaneko T. Rectal water absorption in seawater-adapted Japanese eel Anguilla japonica. Comp Biochem Physiol A 151: 533–541, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Kita T, Kitamura K, Sakata J, Eto T. Marked increase of guanylin secretion in response to salt loading in the rat small intestine. Am J Physiol Gastrointest Liver Physiol 277: G960–G966, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J 21: 5662–5672, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Knowles JW, Goyeau D, Prabhakar S, Short DB, Perkins AG, Goy MF. Low salt intake down-regulates the guanylin signaling pathway in rat distal colon. Gastroenterology 111: 1714–1721, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Lui EY, Wilson JM, Ip YK, Lin Q, Lam TJ, Lam SH. Expression of key ion transporters in the gill and esophageal-gastrointestinal tract of euryhaline Mozambique tilapia Oreochromis mossambicus acclimated to fresh water, seawater and hypersaline water. PLoS One 9: e87591, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall W, Grosell M. Ion transport, osmoregulation, and acid-base balance. In: The Physiology of Fishes, 2006, p. 177–230. [Google Scholar]
- 35.Marshall W, Howard J, Cozzi R, Lynch E. NaCl and fluid secretion by the intestine of the teleost Fundulus heteroclitus: involvement of CFTR. J Exp Biol 205: 745–758, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Martos-Sitcha JA, Campinho MA, Mancera JM, Martinez-Rodriguez G, Fuentes J. Vasotocin and isotocin regulate aquaporin 1 function in the sea bream. J Exp Biol 218: jeb 114546, 2015. [DOI] [PubMed] [Google Scholar]
- 37.McDonald MD, Grosell M. Maintaining osmotic balance with an aglomerular kidney. Comp Biochem Physiol A 143: 447–458, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Musch MW, Orellana SA, Kimberg LS, Field M, Halm DR, Krasny EJ Jr, Frizzell RA. Na+-K+-Cl− co-transport in the intestine of a marine teleost. Nature 300: 351–353, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruhr IM, Bodinier C, Mager EM, Esbaugh AJ, Williams C, Takei Y, Grosell M. Guanylin peptides regulate electrolyte and fluid transport in the Gulf toadfish (Opsanus beta) posterior intestine. Am J Physiol Regul Integr Comp Physiol 307: R1167–R1179, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Ruhr IM, Mager EM, Takei Y, Grosell M. The differential role of renoguanylin in osmoregulation and apical Cl−/HCO3− exchange activity in the posterior intestine of the Gulf toadfish (Opsanus beta). Am J Physiol Regul Integr Comp Physiol 309: R399–R409, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Sattin G, Mager EM, Beltramini M, Grosell M. Cytosolic carbonic anhydrase in the Gulf toadfish is important for tolerance to hypersalinity. Comp Biochem Physiol A 156: 169–175, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Schulze KS. The imaging and modelling of the physical processes involved in digestion and absorption. Acta Physiol (Oxf) 213: 394–405, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Singer TD, Tucker SJ, Marshall WS, Higgins CF. A divergent CFTR homologue: highly regulated salt transport in the euryhaline teleost F. heteroclitus. Am J Physiol Cell Physiol 274: C715–C723, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Skadhauge E. Coupling of transmural flows of NaCl and water in the intestine of the eel (Anguilla anguilla). J Exp Biol 60: 535–546, 1974. [DOI] [PubMed] [Google Scholar]
- 46.Skadhauge E. The mechanism of salt and water absorption in the intestine of the eel (Anguilla anguilla) adapted to waters of various salinities. J Physiol 204: 135–158, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takei Y. Exploring novel hormones essential for seawater adaptation in teleost fish. Gen Comp Endocrinol 157: 3–13, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Takei Y, Yuge S. The intestinal guanylin system and seawater adaptation in eels. Gen Comp Endocrinol 152: 339–351, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Taylor J, Mager E, Grosell M. Basolateral NBCe1 plays a rate-limiting role in transepithelial intestinal HCO3− secretion, contributing to marine fish osmoregulation. J Exp Biol 213: 459–468, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J 25: 5049–5057, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson RW, Grosell M. Intestinal bicarbonate secretion in marine teleost fish—source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim Biophys Acta 1618: 163–174, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Wilson RW, Wilson JM, Grosell M. Intestinal bicarbonate secretion by marine teleost fish—why and how? Biochim Biophys Acta 1566: 182–193, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Wood CM, Grosell M. Independence of net water flux from paracellular permeability in the intestine of Fundulus heteroclitus, a euryhaline teleost. J Exp Biol 215: 508–517, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Yuge S, Inoue K, Hyodo S, Takei Y. A novel guanylin family (guanylin, uroguanylin, and renoguanylin) in eels: possible osmoregulatory hormones in intestine and kidney. J Biol Chem 278: 22,726–22,733, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Yuge S, Takei Y. Regulation of ion transport in eel intestine by the homologous guanylin family of peptides. Zoolog Sci 24: 1222–1230, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Yuge S, Yamagami S, Inoue K, Suzuki N, Takei Y. Identification of two functional guanylin receptors in eel: multiple hormone-receptor system for osmoregulation in fish intestine and kidney. Gen Comp Endocrinol 149: 10–20, 2006. [DOI] [PubMed] [Google Scholar]