Abstract
Neuronal circuits in the hypothalamus and hindbrain are of importance for control of food intake, energy expenditure, and fat mass. We have recently shown that treatment with exendin-4 (Ex-4), an analog of the proglucagon-derived molecule glucagon-like peptide 1 (GLP-1), markedly increases mRNA expression of the cytokine interleukin-6 (IL-6) in the hypothalamus and hindbrain and that this increase partly mediates the suppression of food intake and body weight by Ex-4. Endogenous GLP-1 in the central nervous system (CNS) is produced by preproglucagon (PPG) neurons of the nucleus of the solitary tract (NTS) in the hindbrain. These neurons project to various parts of the brain, including the hypothalamus. Outside the brain, IL-6 stimulates GLP-1 secretion from the gut and pancreas. In this study, we aim to investigate whether IL-6 can affect GLP-1-producing PPG neurons in the nucleus of the solitary tract (NTS) in mouse hindbrain via the ligand binding part of the IL-6 receptor, IL-6 receptor-α (IL-6Rα). Using immunohistochemistry, we found that IL-6Rα was localized on PPG neurons of the NTS. Recordings of these neurons in GCaMP3/GLP-1 reporter mice showed that IL-6 enhances cytosolic Ca2+ concentration in neurons capable of expressing PPG. We also show that the Ca2+ increase originates from the extracellular space. Furthermore, we found that IL-6Rα was localized on cells in the caudal hindbrain expressing immunoreactive NeuN (a neuronal marker) or CNP:ase (an oligodendrocyte marker). In summary, IL-6Rα is present on PPG neurons in the NTS, and IL-6 can stimulate these cells by increasing influx of Ca2+ to the cytosol from the extracellular space.
Keywords: IL-6, IL-6Rα, preproglucagon, glucagon-like peptide-1, hindbrain
it is well established that interleukin-6 (IL-6) contributes toward the stimulation of the innate immune system and induction of inflammation (1). In addition, IL-6 is important for the decreased appetite, enhanced energy metabolism, and increased body temperature often observed during disease (6, 36). During inflammation and disease, IL-6, along with other cytokines, acts via nervous afferents to induce a disease response in the brain (33). During more severe disease, circulating cytokines in the bloodstream are thought to act on the circumventricular organs to induce a more pronounced disease response (9).
In healthy mice, IL-6 acts in lower concentrations than those observed during disease to cause a tonic decrease in body fat mass and an increase in energy expenditure (24, 38, 39). We, as well as others, have shown that IL-6−/− mice develop mature-onset obesity (24, 39). The antiobesity effect of IL-6 in rodents is partly exerted at the level of the brain, presumably, the hypothalamus and the hindbrain (22, 38, 39). Deficiency of IL-6 decreases leptin sensitivity, while overexpression of IL-6 in the brain enhances this parameter (12, 30, 39). Taken together, these findings suggest that IL-6 levels below those found in normal healthy individuals promote obesity (12, 24, 29, 39), while levels above normal may reduce appetite (7, 30). Therefore, it is important to identify which cells in the brain express functional interleukin-6 receptor-α (IL-6Rα), as well as to determine whether these cells are localized in nuclei that regulate appetite and/or energy balance. Such a mapping of IL-6Rα has been done for body fat-regulating centers in the hypothalamus (3, 31, 32, 40), although there are fewer studies that focus on the hindbrain, another part of the brain that regulates metabolic functions (14).
The proglucagon system includes proglucagon-derived posttranslational cleavage products, such as glucagon-like peptide 1 (GLP-1) (2). GLP-1 is an incretin with important effects on both blood glucose levels and fat mass. Outside the central nervous system (CNS), GLP-1 is mainly synthesized in the entero-endocrine L-cells in the distal gut and to some extent in the proximal gut (37). GLP-1 may also be produced by pancreatic α-cells (11). Within the CNS, GLP-1 is expressed in preproglucagon neurons in the nucleus of the solitary tract (NTS) in the brain stem. The antiobesity effect of peripheral GLP-1 is assumed to be exerted via activation of vagal afferent fibers (10, 18). Whether these vagal afferents, in turn, activate the preproglucagon (PPG) neurons in the NTS to initiate central release of GLP-1 is currently under debate (17). Leptin and CCK are known to stimulate PPG neurons in the NTS, while several other appetite and/or peptides regulating body fat and food intake, including GLP-1 itself, have no effect (16, 17). These data are in line with the assumption that GLP-1 in NTS regulates body fat mass and that this effect is exerted via interaction with other peptides regulating body fat and food intake, such as leptin and CCK.
In recent studies, we reported that treatment with Exendin-4 (Ex4), a long-lasting GLP-1 analog, enhances the expression of IL-6 in both the hypothalamus and different parts of the hindbrain. We provided evidence that increased IL-6 expression mediates the antiobesity effect of GLP-1 (28, 34). Similarly, it has recently been shown that amylin, an antiobesity and blood glucose-regulating hormone, induces central IL-6 production which, in turn, leads to increased leptin sensitivity (21).
As discussed above, GLP-1 inhibits fat mass, in part, by stimulating IL-6 expression (34). To further investigate possible interactions between GLP-1 and IL-6, we sought to determine whether PPG neurons express functional IL-6Rα. Because GLP-1 in the CNS is almost exclusively produced in the NTS, we stained for IL-6Rα in brain slices from this area. Furthermore, we performed optical recordings using Glu-Cre/Rosa26-GCaMP3 transgenic mice to investigate whether IL-6 can affect these neurons by modulation of Ca2+ influx and cytosolic Ca2+ concentrations.
We have previously found that IL-6Rα is expressed in several different types of neurons in hypothalamic nuclei (3, 31, 32). Besides neurons, the parenchymal brain also consists of three different types of glial cells; astrocytes, microglia, and oligodendrocytes. In the present study, we aimed to systematically elucidate whether IL-6Rα in the NTS is expressed on neurons and/or different types of glial cells.
METHODS
Animals.
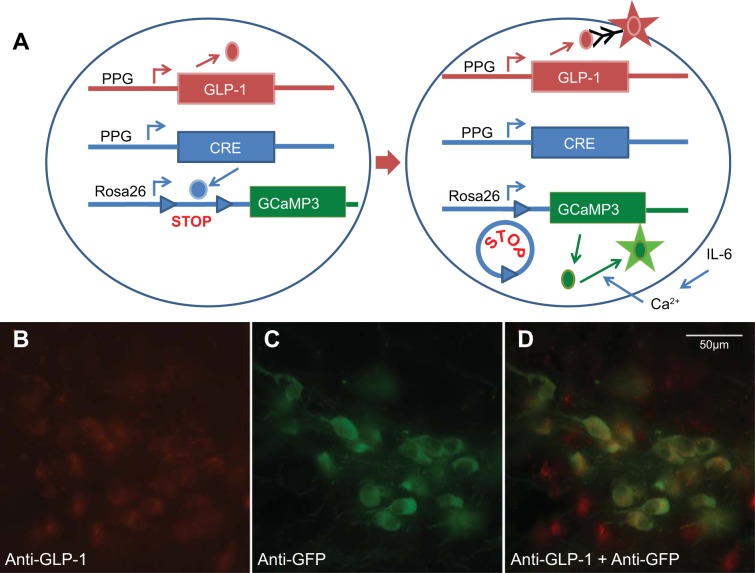
Two transgenic C57BL6 mouse models were used in this study. First, PPG neurons were visualized using glucagon promoter (Glu)-YFP mice, in which yellow fluorescent protein (YFP) is expressed under the control of the glucagon promoter (23, 27). These mice were used for double staining of YFP and immunoreactive IL-6Rα. GCaMP3 mice were used for immuohistochemical validation of the model. Standard wild-type C57BL6 mice were used for the rest of the immunohistochemistry. Glu-Cre/Rosa26-GCaMP3 transgenic mice expressing the genetically encoded calcium indicator GCaMP3 in a Cre-dependent manner were obtained by crossing Glu-Cre mice (26) with commercially available Rosa26-GCaMP3 reporter mice (Jax strain 014538) (41), resulting in the expression of the genetically encoded Ca2+ sensor GCAMP3 in PPG neurons (Fig. 1A).
Fig. 1.
A: PPG promoter (Glu)-Cre mice were mated with Rosa26-promoter-STOP-GCaMP3 mice. The Rosa26 promoter is active in most cell types. In cells with active PPG promoter, the cAMP response element (CRE) protein cleaved out the STOP sequence upstream of the GCaMP3 gene. This resulted in expression of the GCaMP3 protein and emission of green fluorescence after stimulation of Ca2+ influx into the cytosol, e.g., after stimulation by IL-6. B: immunofluorescence showing anti-glucagon-like peptide-1 (GLP-1) immunoreactivity in red in cells in the nucleus of the solitary tract (NTS) of Glu-Cre-GCaMP3 mice. C: immunofluorescence showing anti-GFP immunoreactivity in green in cells in the NTS of Glu-Cre-GCaMP3 mice. D: cells in the NTS of mice with anti-GLP-1 immunoreactivity in red, anti-GFP immunoreactivity in green, demonstrating colocalization of the two markers (yellow).
Animals had free access to water and standard chow pellets (Teklad Global, Harlan, The Netherlands) and were kept under standardized conditions on 12:12-h light-dark cycle with ad libitum food. The local ethics committees for animal care at the University of Gothenburg and University College London (UCL) approved all animal procedures, and studies at UCL were conducted in accordance with the U.K. Animals (Scientific Procedures) Act, 1986.
Tissue preparation for immunohistochemistry.
Mice were deeply anesthetized and perfused transcardially with heparinized saline (50 IU/ml) followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brains were removed and postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer containing 15% sucrose overnight at 4°C. They were then transferred to a 30% sucrose solution in 0.1 M phosphate buffer until sectioning. Coronal 20-μm-thick serial sections of the hypothalamus and hindbrain were cut using a Leica CM3050S cryostat (Leica Microsystem, Wetzlar, Germany) and were stored in cryoprotectant solution (25% ethylene glycol; 25% glycerol; 0.05 M phosphate buffer). For GFP/GLP-1 costaining, we instead used 30-μm sections that were not postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer containing 15% sucrose overnight. Coronal sections corresponding to bregma −7.32 to −7.64 (interneural −3.52 to −3.84) were selected for staining.
Immunohistochemistry.
Briefly, sections were rinsed in wash buffer (0.1 M Tris·HCl, pH 7.5, 0.15 M NaCl, 0.2% Triton-X-100) and were blocked for 1 h with Tris-NaCl-blocking buffer (TNB) (Perkin Elmer, Waltham, MA). Sections were incubated with primary antibodies (see Supplemental Table S1) for 2 days at 4°C. After rinsing, sections were incubated for 1 h with secondary antibodies (see Supplemental Table S1) diluted in TNB blocking reagent. Sections were rinsed, and the IL-6Rα signal was developed by incubating the sections in streptavidin-horseradish peroxidase in TNB-blocking reagent (1:100; TSA System; Perkin Elmer) for 30 min and then with biotinyl-tyramide in amplification diluent (1:50, TSA System; Perkin Elmer). Following signal amplification, sections were stained with streptavidin Alexa Fluor 568-conjugate (1:250, S11226; Molecular Probes, Carlsbad, CA). After a further wash, cell nuclei were stained with DAPI (1:5,000, D1306; Molecular Probes) for 15 min, rinsed, and mounted in mounting medium containing prolong gold antifade (P36930; Molecular Probes). As a control for the secondary antibodies, some sections were incubated with mismatching primary and secondary antibodies, resulting in negative staining (as a control for unwanted cross-reactivity). The rat anti-IL-6α antibody used in the present study has been validated by several earlier studies (31, 32). Primary antibodies, their dilutions, and catalog numbers, as well as the manufacturers that provided them, are listed in Table 1.
Table 1.
Basic information about the primary and secondary antibodies used in this study
| Antibody | Dilution | Cat. No | Manufacturer |
|---|---|---|---|
| Rat anti-IL-6Rα | 1:20 | BAM18301 | R&D Systems, Minneapolis, MN, USA |
| Rabbit anti-GFAP | 1:200 | Z 0334 | DakoCytomation, Glostrup, Denmark |
| Rabbit anti-Iba1 | 1:200 | 019–19741 | Wako Chemicals, Richmond, VA, USA |
| Mouse anti-CNP:ase | 1:100 | MAB326 | Millipore, Billerica, MA, USA |
| Rabbit anti-NeuN | 1:100 | ABN78 | Millipore, Billerica, MA, USA |
| Rabbit anti-GLP-1 (7–36) amide | 1:200 | T-4057 | Peninsula Laboratories/Bachem, Torrance, CA, USA |
| Goat anti-rat biotin | 1:1000 | ab7096 | Abcam, Cambridge, UK |
| Donkey Alexa Fluor 488-conjugated anti-rabbit IgG | 1:250 | A21206 | Molecular Probes, Carlsbad, CA, USA |
| Rabbit Alexa Fluor 488-conjugated anti-mouse IgG | 1:250 | A11059 | Molecular Probes, Carlsbad, CA, USA |
| Donkey Alexa Fluor 568-conjugated anti-rabbit IgG | 1:250 | A10042 | Molecular Probes, Carlsbad, CA, USA |
| Donkey Alexa Fluor 568-conjugated anti-mouse IgG | 1:250 | A10037 | Molecular Probes, Carlsbad, CA, USA |
Confocal microscopy and cell counting.
Images of the stained sections were obtained using either a confocal microscope system (LSM 700; ZEISS, Oberkochen, Germany), together with a Plan APO 63× aperture/1.40 oil lens (for close-up pictures) or a Plan Fluor 20/0.75× lens (for anatomical overview pictures) and a solid-state laser. For colocalization between IL-6rα and GLP-1, focus stacking was used to achieve a greater depth of field and, as such, make it possible to more accurately detect possible colocalization.
NTS YFP-labeled cells and IL6-rα-positive cells were quantified from at least four 20-μm sections per brain. Triple-channel confocal images (to cover the entire NTS) were generated with a Plan Fluor 20/0.75× lens and a solid-state laser. A tile scan of 3 × 3 tiles was obtained from the center of the NTS, covering the entirety of the nucleus. Neurons were considered IL-6Rα-labeled when the staining was clearly above background. The emission spectrum of the secondary fluorescent antibody is well known. By adjusting the splits of the confocal microscope, the signal of the fluorophore was maximized while minimizing background fluorescence. The evaluation of IL-6Rα-labeling was done with the cell nucleus in the plane of focus.
Calcium imaging.
Glu-Cre/Rosa26-GCaMP3 transgenic mice express the calcium indicator GCaMP3 in PPG neurons selectively. To confirm that GCaMP3-positive cells in the NTS produce GLP-1, double immunofluorescence was carried out with an anti-GFP antibody (Abcam) to detect GCaMP3 (25) and an anti-GLP-1 antibody (Bachem) to detect GLP-1 (15). As shown in Fig. 1, B–D, the anti-GLP-1 antibody detected essentially all GCaMP3/GFP-positive cells, but also cells that were not GCaMP3/GFP-positive. This could indicate that visualization of GCaMP3/GFP-expressing cells has incomplete penetrance, e.g., due to lack of IL-Rα in about 60% of GLP-1-expressing cells (Fig. 2G). Alternatively, the GLP-1 antibody may bind nonspecifically to additional cells that do not have GLP-1 and GCaMP3 expression and, thereby, are not stained. All cells expressing GCaMP3 were positive for GLP-1.
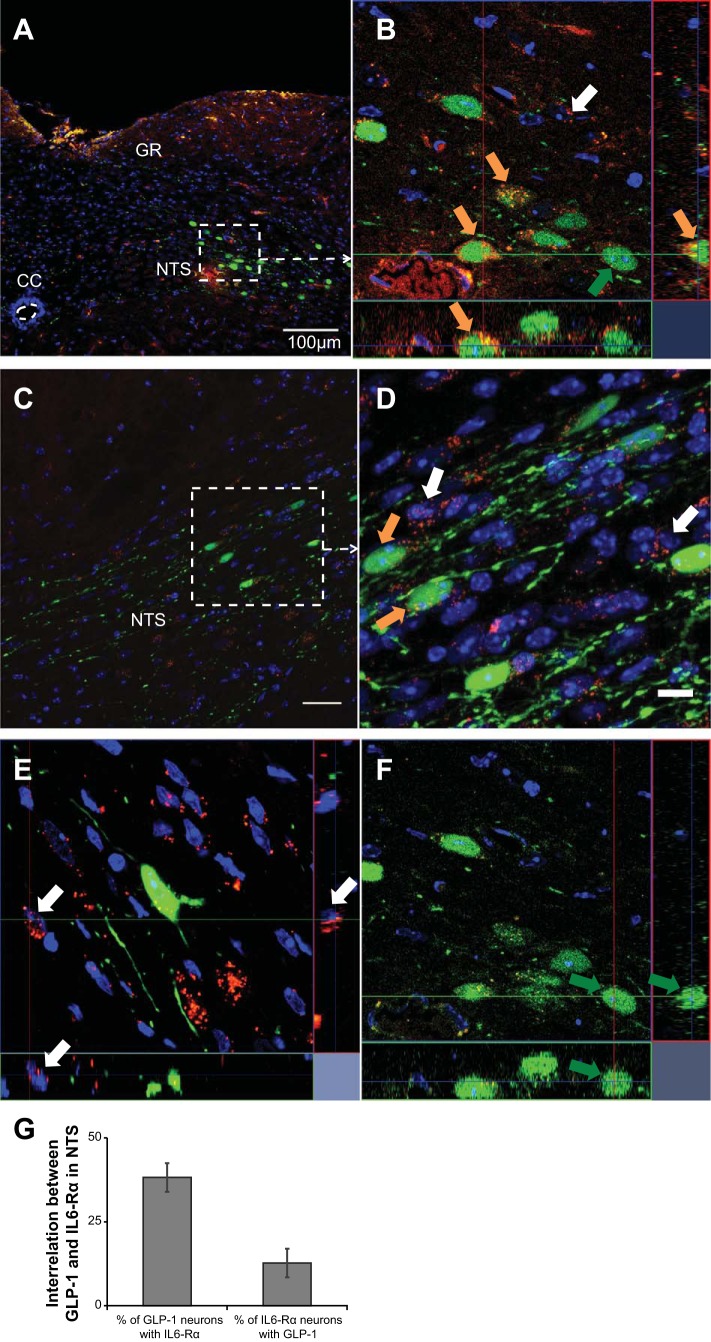
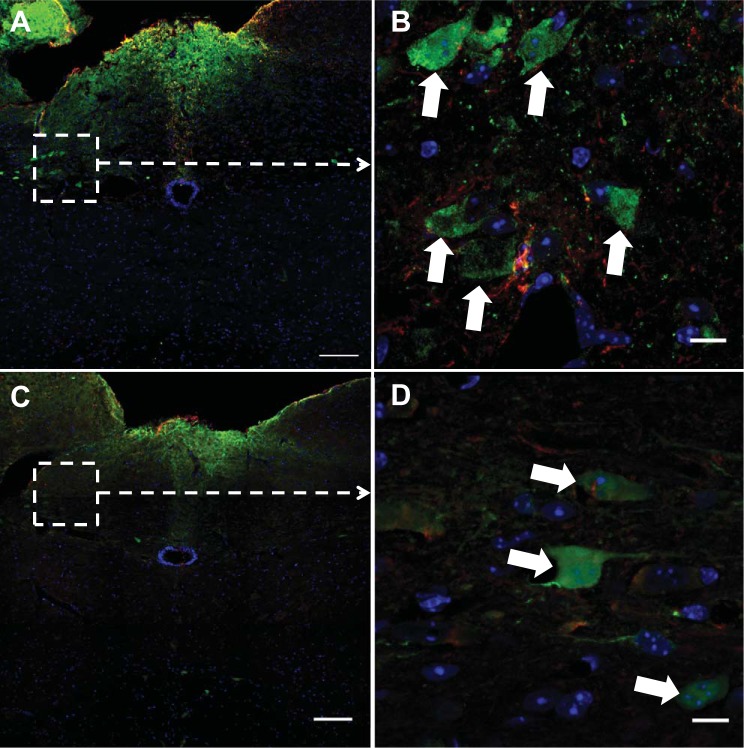
Fig. 2.
Interleukin-6 receptor α (IL-6Rα) is colocalized with glucagon-like peptide-1 (GLP-1) in the nucleus of the solitary tract (NTS) of the hindbrain. Immunohistochemistry showing IL-6Rα-immunoreactivity in red, GLP-1-immunoreactivity in green, and nuclear staining (DAPI) in blue in a coronal section of the mouse hindbrain. A and C: overviews of the NTS. B and D: magnifications of the areas indicated in A and C, respectively. One cell each in B, E, and F is subject to orthogonal analysis using z-stacks. D was made by focal stacking. Orange arrows indicate examples of colocalization between IL-6Rα and GLP-1. White and green arrows indicate examples of cells with only IL-6Rα (E) and only GLP-1 (F), respectively (D). Results of cell counting concerning the interrelation between GLP-1 and IL-6Rα are shown in 2G. Roughly 38% of all GLP-1-positive cells also stain positively for IL-6Rα and roughly 16% of all IL-6Rα-positive cells stain positively for GLP-1. Images were obtained using the confocal microscope system described in materials and methods. CC, central canal; NTS, nucleus of the solitary tract; GR, gracile nucleus. Scale bars = 100 μm (overview), 10 μm (magnification).
On the day of recording, mice underwent deep terminal anesthesia using isoflurane. The brain stem was removed and placed in ice-cold high-magnesium/low-calcium artificial cerebrospinal fluid (ACSF) (composition in mM: 2.5 KCl, 200 sucrose, 28 NaHCO3, 1.25 NaH2PO4, 7 glucose, 7 MgCl2, 0.5 CaCl2; pH 7.4). Two-hundred-micrometer-thick coronal sections were cut on a vibratome (Campden Instruments) and left to incubate in high-magnesium/low-calcium recovery solution (in mM: 3 KCl, 118 NaCl, 25 NaHCO3, 1.2 NaH2PO4, 2.5 glucose, 7 MgCl2, 0.5 CaCl2; pH 7.4) at 34°C for 45 min. Subsequently, the sections were transferred to standard ACSF (in mM: 3 KCl, 118 NaCl, 25 NaHCO3, 10 glucose, 1 MgCl2, 2 CaCl2; pH 7.4) and left to incubate at 34°C for a minimum of 30 min before imaging. All solutions were constantly bubbled with 95% O2-5% CO2. Imaging was performed on a Zeiss Axioskop wide-field microscope using a 40× water immersion lens, charge-coupled device camera (Q-click; QImaging), and a LED light source (CoolLED pE300white). Sections were continuously superfused with standard ACSF at 32°C at a flow rate of 3 or 4 ml/min. GCaMP3 was excited every 5 s for 250 ms at 460 ± 25 nm. Regions of interest (ROIs) and an area representing background fluorescence were outlined, and the mean pixel intensity was calculated for each ROI. Background fluorescence was subtracted from each ROI, and recordings are presented as ΔF/F0, with F0 being the average intensity over 5 min prior to the stimulus and ΔF being the fluorescence intensity minus F0.
Quantifications were made by calculating the relative change in fluorescence between the fluorescent intensity in the absence of drug (Fno-drug) and the peak fluorescent intensity (Fpeak): (Fpeak − Fno-drug)/Fno-drug. Fno-drug was defined as the average of 1 min before drug response onset and 1 min after recovery from the response. IL-6 was dissolved in water and added directly to the ACSF. For recordings in the nominal absence of Ca2+, Ca2+ was replaced with Mg2+. All sections were exposed to 2 nM IL-6, followed by a second exposure to IL-6 in 0 mM Ca2+, and finally exposed again to 2 nM IL-6 in standard ACSF. Data are reported as means ± SE. Statistics were analyzed using one-way ANOVA, and post hoc comparisons were made using the Bonferroni method.
RESULTS
IL-6Rα immunoreactivity is present in the NTS and is colocalized with GLP-1.
Immunofluorescence staining of hindbrain sections from Glu-YFP mice revealed YFP-positive neurons activated by the Glu promoter (in green) colocalized with IL-6Rα immunoreactivity (in red) (Fig. 2, A–D). Examples of cells expressing both IL-6 Rα and YFP, are shown in Fig. 2, B and D (orange arrows). Orthogonal images made from z-stacks focused on single cells clearly identified cells with both IL-6Rα and GLP-1, as shown in in Fig. 2B. Orthogonal images made from z-stacks, focused on single cells with only IL-6Rα (white arrow) or only GLP-1 (green arrow), are shown in Fig. 2, E and F, respectively. Cell counting showed that 38.5% of the GLP-1-positive cells of the NTS also stained positively for IL-6Rα. Conversely, 13% of the IL-6Rα-positive cells of the NTS also stained positively for GLP-1 (Fig. 2G). Use of separate filters on the same area of the NTS, as seen in Fig. 2B, verified the colocalization results described above (not shown). In the intermediate reticular nucleus, another known site of GLP-1-positive neurons in the hindbrain, ∼10% of the GLP-1-positive neurons also stained positively for IL-6Rα.
PPG neurons in the NTS respond to IL-6 with an increase in intracellular Ca2+.
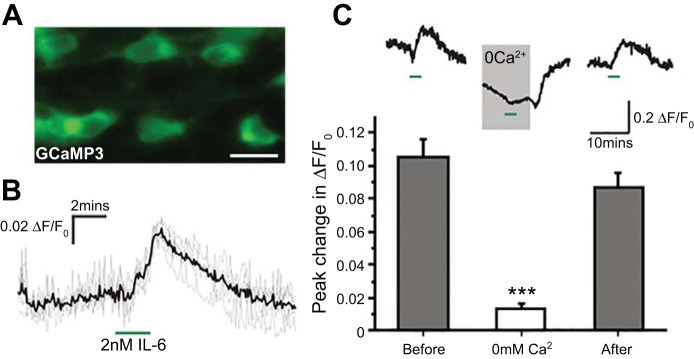
In vitro calcium imaging was performed using adult Glu-Cre/Rosa26-GCaMP3 mice to characterize the functional response of PPG neurons to IL-6 (Fig. 3). Bath application of 2 nM IL-6 led to an 8% ± 1.5% increase in GCaMP3 fluorescence in PPG neurons (Fig. 3B). To determine the source of the increase in intracellular Ca2+ experiments, we repeated the exposures to IL-6, but in the absence of extracellular Ca2+. Removal of extracellular calcium strongly reduced the intracellular Ca2+ (Fig. 3C, top) an effect that was reversed upon reintroduction of extracellular Ca2+. The fact that the effect of IL-6 was reduced by about 90% in the absence of extracellular Ca2+ suggests that Ca2+ influx is necessary for the response to IL-6 (Fig. 3C, bottom).
Fig. 3.
Preproglucagon neurons in the NTS respond to IL-6 with a rise in intracellular Ca2+. A: representative image of GCaMP3 fluorescence in PPG neurons in the NTS. Scale bar = 20 μm. B: traces showing the increase in intracellular Ca2+ in response to 2 nM IL-6. Gray trace shows individual cells, and the black trace shows the average. n = 6. C: rise in intracellular Ca2+ in response to IL-6 is dependent on influx of extracellular Ca2+. Top: representative trace showing the response to IL-6 (green bars) before, during, and after exposure to Ca2+-free solution. Note that removal of extracellular Ca2+ (0 Ca2+) also reduces the basal intracellular Ca2+ concentration. Bottom: mean peak change in intracellular Ca2+ in the presence (gray bars: n = 19 and 18, respectively) and absence (white bar, n = 28) of extracellular Ca2+. ***P < 0.001.
IL-6Rα immunoreactivity in the NTS is present on neurons and oligodendrocytes, but not on astrocytes or microglia.
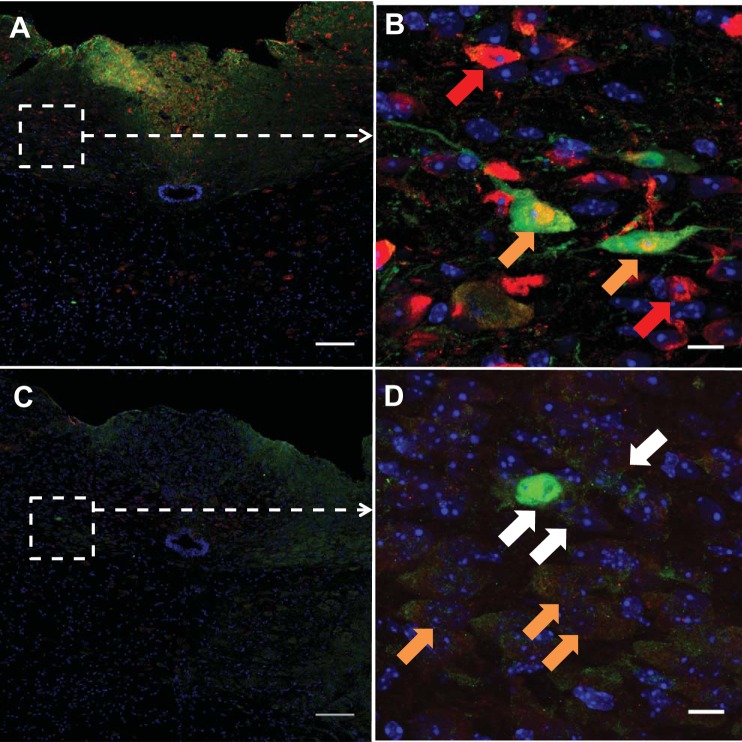
In the hindbrain, immunofluorescent costaining revealed that IL-6Rα immunoreactivity (in green) in many cells was colocalized with a marker for neuronal nuclei (NeuN, in red) (Fig. 4, A and B, orange arrows). There were numerous NeuN-positive cells without IL-6Rα (Fig. 4B; red arrows show examples). Additionally, IL-6Rα immunoreactivity (in green) colocalized in many cells with a marker for oligodendrocytes (CNP:ase, in red). (Fig. 4, C and D; orange arrows show examples). There were IL-6Rα-positive cells without CNP:ase (white arrows show examples). In contrast, IL-6Rα immunoreactivity was not coexpressed with markers for microglia (Iba-1; Fig. 5, A and B) or astrocytes (GFAP; Fig. 5, C and D). The colocalization between IL-6Rα and the four cellular markers was verified in a focus-stacking projection (Figs. 4, B and D, 5, B and D).
Fig. 4.
Interleukin-6 receptor α (IL-6Rα) is localized on neurons and oligodendrocytes. Immunohistochemistry showing IL-6Rα-immunoreactivity in green, NeuN immunoreactivity in red (A and B), CNP:ase immunoreactivity in red (C and D), and nuclear staining (DAPI) in blue in a coronal section of the mouse hindbrain. A and C: overview of the NTS. B and D: magnification of the areas indicated in A and C. Examples of cells with both IL-6Rα and NeuN (B, orange arrows), cells with both IL-6Rα and CNP:ase (D, orange arrows), as well as a cell only positive for NeuN (B, red arrows) or IL-6Rα (D, white arrows) are shown. Images were obtained using the confocal microscope system described in materials and methods. Scale bars = 100 μm (overview), 10 μm (magnification).
Fig. 5.
IL-6Rα is not localized on astrocytes or microglia. Immunohistochemistry showing IL-6Rα immunoreactivity in green and nuclear staining (DAPI) in blue (A–D) in a coronal section of the hindbrain. Iba-1-immunoreactivity (A and B) and GFAP-immunoreactivity (C and D) are shown in red. A and C: overviews of the mouse NTS, and a magnification (D) of the area indicated in C. B show examples of IL-6-Rα-positive and -negative neurons. White arrows indicate cells containing IL-6-Rα. There was no colocalization between glial cell markers, GFAP, and Iba-1 on one hand, and IL-6Rα on the other. Images were obtained using the confocal microscope system described in materials and methods. Scale bars = 100 μm (overview), 10 μm (magnification).
Cell counting showed that 30% of the cells expressing NeuN and CNP:ase were also IL-6Ra-positive. Conversely, 60% and 30% of the IL-6Rα-positive cells, were also positive for NeuN and CNP:ase, respectively. There was no colocalization between GFAP and Iba-1 on one hand and IL-6Rα on the other (Table 2).
Table 2.
Overlap between IL-6Rα-positive cells and neuronal/glial cell markers
| % | % | ||
|---|---|---|---|
| 100x NeuN/IL-6Rα | 58 | 100x IL-6Rα/NeuN | 32 |
| 100x CNP:ase/IL-6Rα | 28 | 100x IL-6Rα/CNP:ase | 30 |
| 100x GFAP/IL-6Rα | 0–2 | 100x IL-6Rα/GFAP | 0–2 |
| 100x Iba-1/IL-6Rα | 0–2 | 100x IL-6Rα/Iba-1 | 0–2 |
Results of cell counting suggest that cells with markers for neurons (NeuN) and oligodendrocytes (CNP:ase), but not astrocytes (GFAP) or microglia (Iba-1), are responsive to IL-6 in the nucleus of the solitary tract of healthy mice.
DISCUSSION
It has previously been shown that IL-6 in CNS is of importance in regulation of energy metabolism and that IL-6 acts at the CNS level to decrease fat mass (20, 31, 34). IL-6−/− mice develop mature-onset obesity, as well as insulin and leptin resistance (24, 38). GLP-1 analogs, like exendin-4 (Ex4), have also been shown to decrease fat mass by acting at the CNS level (15). Ex4 is widely used as a treatment for Type 2 diabetes, and beneficial side effects of its use include mild weight loss (10). Recently, liraglutide, another GLP-1 analog, has been used for the treatment of obesity per se (19). We have previously shown that intracerebroventricular injections of Ex4 increases IL-6 mRNA levels both in several parts of the hindbrain and in the hypothalamus and that IL-6 is mediating the antiobesity effect exerted by this GLP-1 analog (28, 34). These findings led us to further study the possible interactions between these two neuromodulators, to elucidate how they may interact to decrease fat mass.
Our present finding that IL-6Rα is expressed in PPG neurons in the NTS of the hindbrain indicates that IL-6 has the capacity to influence GLP-1-expressing neurons in the CNS via direct effects on IL-6Rα located on these neurons, in a similar way as shown for leptin (16). As noted above, treatment with a GLP-1 analog increases the levels of IL-6 mRNA in the hypothalamus and the hindbrain (34). Taken together, these findings are in line with a bidirectional interaction between PPG neurons and IL-6 neurons. Such interactions are common in biological context, and there are both general (4) and specific (13) examples of this. However, further studies are needed to investigate whether there is a bidirectional interaction between GLP-1- and IL-6-producing cells, and if so, the nature of this interaction.
While results obtained by immunohistochemistry should always be interpreted with caution, there is good reason to believe the colocalization between GLP-1 and IL-6Rα observed herein is valid. First, we have previously identified IL-6Rα by use of two independent antibodies (31, 32). Second, the Glu-YFP transgenic mouse used here has been thoroughly validated (16, 23, 27, 28). Third, the costaining was verified using focal stacking, as well as three-dimensional analysis seen in Fig. 2, B, and D–F. Finally, the fact that PPG/GLP-1-expressing cells (identified by both glucagon promoter activity and GLP-1 immunoreactivity) in the NTS responded to the addition of IL-6 strongly support that these cells express functional IL-6Rα. Taken together, these methods make it reasonable to believe that the costaining shown is reliable.
GCaMP3 recordings revealed that adding IL-6 to mouse acute hindbrain sections resulted in an influx of Ca2+ in PPG neurons, similar to what has previously been shown in hippocampal neurons (25). Our finding that IL-6 seems to stimulate PPG neurons adds further information concerning the interaction between these substances. We have previously shown that intracerebroventriculular injections of Ex4 in rat causes an increase in IL-6 mRNA (34), and we show here that IL-6, in turn, can stimulate PPG neurons. One possible interpretation is that a GLP-1 analog like Ex4 can stimulate endogenous GLP-1 secretion, acting on NTS PPG neurons via IL-6. It has been shown that GLP-1 itself cannot activate these PPG neurons (16). This would constitute a feed-forward effect, a type of effect seen, for instance, in connection to immune stimulation. Further studies are needed to investigate this issue.
It should be noted that IL-6 outside the brain has been found by Donath and colleagues (11) to stimulate the secretion of GLP-1 from L-cells of the gut and α-cells of the pancreas (11). Our current data suggest that IL-6 also stimulates a third GLP-1-expressing cell type, the PPG neurons in the NTS. Currently, it is unclear whether circulating IL-6 or IL-6 produced locally in the brain influences PPG neurons in the NTS. Circulating IL-6 may not cross the blood-brain barrier, and, therefore, may not reach the NTS. Unlike, for example, the area postrema, the NTS is not a circumventricular organ and, thus, cannot be affected directly by peripherally produced peptides like IL-6 (5). The fact that IL-6 levels in the cerebrospinal fluid often are higher than in the blood circulation also argues that IL-6 found in the brain is produced locally rather than in the periphery (35).
We have previously reported that IL-6Rα is expressed in important energy-regulatory neurons in the paraventricular nucleus (3), in the arcuate nucleus (31), and in the lateral hypothalamic area of the hypothalamus (32). Neurons expressing IL-6Rα have been reported to express several neuropeptides with energy balance regulating potential, such as TRH, corticotropin-releasing hormone, oxytocin, melanin-concentrating hormone, orexin, neuropeptide Y, and α-MSH (3, 31, 32). Here, we show that IL-6Rα is expressed in parts of the brain stem, as well as in the hypothalamus.
There was no obvious colocalization between IL-6Rα and markers for astrocytes and microglia, although it cannot be completely ruled out that IL-6Rα and the markers are localized in different compartments within the same cell. In contrast, we found CNP:ase immunoreactivity in about 30% of the IL-6Rα-positive cells of the NTS. Therefore, IL-6Rα seems to be present on oligodendrocytes in this nucleus. This is in accordance with previous findings that IL-Rα can be found on oligodendrocytes in humans with multiple sclerosis, as well as healthy controls (8). Moreover, we found that a large part (about 60%) of the cells that expressed IL-6Rα in the NTS also expressed the neuronal marker NeuN. These findings from the caudal hindbrain are in line with results obtained from different parts of the hypothalamus (3, 31, 32). Taken together, these results indicate that IL-6 exerts its effects in caudal parts of the hindbrain and in the hypothalamus of healthy mice primarily on neurons and oligodendrocytes.
In conclusion, our present findings show stimulation by IL-6 of PPG neurons and localization of IL-6Rα on these cells. In the caudal hindbrain of healthy mice, IL-6Rα is expressed on neurons and oligodendrocytes, while we found no evidence of expression on astrocytes and microglia.
Perspectives and Significance
In a previous study, we found evidence that GLP-1 stimulates IL-6 expression in the CNS and that IL-6 may mediate the antiobesity effects of GLP-1. The present findings demonstrate that IL-6, in turn, activates PPG neurons in the NTS. Given that GLP-1 stimulates IL-6 expression, there is a possibility for bidirectional interaction between cells producing GLP-1 and IL-6 in the CNS. Alternatively, IL-6 that is produced in the periphery may affect GLP-1-producing neurons in the CNS, as it has been shown to activate L-cells in the gut and α-cells in the pancreas. The present findings are of clinical interest, given that both GLP-1 analogs and IL-6 receptor blockers are used for treatment in humans.
GRANTS
This work was supported by grants from Swedish Research Council (K2016-54X-09894-19-3, 2011–3054, and 325–2008-7534), Johan and Jakob Söderbergs Foundation, Marcus Borgströms Foundation, Nilsson-Ehle Foundation, NovoNordisk Foundation, Novo Nordisk Foundation Excellence project grant (to K. P. Skibicka), Inga-Britt and Arne Lundbergs Foundation, Swedish Medical Society, Swedish Society for Medical Research, Kungl and Hvitfeldtska Foundation, EC FP7 funding (Health-F2-2010-259772, FP7/2007–2013 Grant Agreement 245009, Full4Health FP7-KBBE-2010-4- 266408, TORNADO [Grant 222720]). M. K. Holt holds a University College London Graduate Research Fellowship, and S. Trapp is supported by a grant from the Medical Research Council (MRC) UK (MR/J013293/2). F. M. Gribble and F. Reimann are supported by a grant from the Wellcome Trust (106262/Z/14/Z, 106263/Z/14/Z), and the MRC Metabolic Diseases Unit (MRC_MC_UU_12012/3).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.B.A. and J.-O.J. conception and design of research; F.B.A., M.K.H., and C.S. performed experiments; F.B.A., M.K.H., E.S., V.P., K.P.S., S.T., and J.-O.J. analyzed data; F.B.A., M.K.H., E.S., V.P., K.P.S., S.T., and J.-O.J. interpreted results of experiments; F.B.A., M.K.H., and S.T. prepared figures; F.B.A. and J.-O.J. drafted manuscript; F.B.A., E.S., V.P., F.R., F.M.G., C.S., K.P.S., S.T., and J.-O.J. edited and revised manuscript; F.B.A., E.S., V.P., F.R., F.M.G., C.S., K.P.S., S.T., and J.-O.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Centre for Cellular Imaging at the University of Gothenburg for the use of imaging equipment, as well as support received from Julia Fernandez-Rodriguez, Maria Smedh, and Carolina Tängemo. We also thank Edwin Gidestrand for assistance with immunohistochemistry.
REFERENCES
- 1.Arora S, Anubhuti. Role of neuropeptides in appetite regulation and obesity—a review. Neuropeptides 40: 375–401, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2125, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Benrick A, Schéle E, Pinnock SB, Wernstedt-Asterholm I, Dickson SL, Karlsson-Lindahl L, Jansson JO. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol 21: 620–628, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Blalock JE. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev 69: 1989. [DOI] [PubMed] [Google Scholar]
- 5.Borison HL. Area postrema: Chemoreceptor circumventricular organ of the medulla oblongata. Progr Neurobiol 32: 351–390, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Cahlin C, Körner A, Axelsson H, Wang W, Lundholm K, Svanberg E. Experimental cancer cachexia: the role of host-derived cytokines interleukin (IL)-6, IL-12, interferon-gamma, and tumor necrosis factor α evaluated in gene knockout, tumor-bearing mice on C57/BL background and eicosanoid-dependent cachexia. Cancer Res 60: 5488–5493, 2000. [PubMed] [Google Scholar]
- 7.Campbell IL, Erta M, Lim SL, Frausto R, May U, Rose-John S, Scheller J, Hidalgo J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci 34: 2503–2513, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannella B, Raine CS. Multiple sclerosis: Cytokine receptors on oligodendrocytes predict innate regulation. Ann Neurol 55: 46–57, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol 526: 653–661, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24–32, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17: 1481–1489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes 55: 2554–2561, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nat Med 395: 763–770, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 2: 320–330, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes 59: 1890–1898, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves α1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes 60: 2701–2709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Iepsen EW, Torekov SS, Holst JJ. Therapies for inter-relating diabetes and obesity—GLP-1 and obesity. Expert Opin Pharmacother 15: 2487–2500, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Jansson JO, Wallenius K, Wernstedt I, Ohlsson C, Dickson SL, Wallenius V. On the site and mechanism of action of the anti-obesity effects of interleukin-6. Growth Horm IGF Res 13: S28–S32, 2003. [DOI] [PubMed] [Google Scholar]
- 21.LeFoll C, Johnson MD, Dunn-Meynell AA, Boyle CN, Lutz TA, Levin BE. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64: 1621–1631, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Klein RL, Matheny M, King MA, Meyer EM, Scarpace PJ. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience 115: 879–889, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 28: 111–121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, Jowett JB, Watt MJ, Jansson JO, Bruce CR, Febbraio MA. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53: 2431–2441, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Orellana DI, Quintanilla RA, Gonzalez-Billault C, Maccioni RB. Role of the JAKs/STATs pathway in the intracellular calcium changes induced by interleukin-6 in hippocampal neurons. Neurotox Res 8: 295–304, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55: 2445–2455, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532–539, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 155: 4356–4367, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Klarlund Pedersen B, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55: S48–S52, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Sadagurski M, Norquay L, Farhang J, D'Aquino K, Copps K, White MF. Human IL6 enhances leptin action in mice. Diabetologia 53: 525–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schéle E, Benrick A, Grahnemo L, Egecioglu E, Anesten F, Pálsdóttir V, Jansson JO. Inter-relation between interleukin (IL)-1, IL-6 and body fat regulating circuits of the hypothalamic arcuate nucleus. J Neuroendocrinol 25: 580–589, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Schéle E, Fekete C, Egri P, Füzesi T, Palkovits M, Keller É, Liposits Z, Gereben B, Karlsson-Lindahl L, Shao R, Jansson JO. Interleukin-6 receptor α is co-localised with melanin-concentrating hormone in human and mouse hypothalamus. J Neuroendocrinol 24: 930–943, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Schettini G, Grimaldi M, Landolfi E, Meucci O, Ventra C, Florio T, Scorziello A, Marino A. Role of interleukin-6 in the neuroendocrine system. Acta Neurol (Napoli) 13: 361–367, 1991. [PubMed] [Google Scholar]
- 34.Shirazi R, Palsdottir V, Collander J, Anesten F, Vogel H, Langlet F, Jaschke A, Schürmann A, Prévot V, Shao R, Jansson JO, Skibicka KP. Glucagon-like peptide 1 receptor induced suppression of food intake and body weight is mediated by central IL-1 and IL-6. Proc Natl Acad Sci USA 110: 16,199–16,204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenlöf K, Wernstedt I, Fjällman T, Wallenius V, Wallenius K, Jansson JO. Interleukin-6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J Clin Endocrinol Metab 88: 4379–4383, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 89: 1681–1684, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Toräng S, Rehfeld JF, Poulsen SS, Holst JJ. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 156: 847–857, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun 293: 560–565, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Vallières L, Rivest S. Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology 140: 3890–3903, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci 32: 3131–3141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]