Abstract
THE EARLIEST DESCRIPTIONS OF LUNG DISEASE IN PEOPLE WITH CYSTIC FIBROSIS (CF) DEMONSTRATED THE INVOLVEMENT OF THREE INTERACTING PATHOPHYSIOLOGICAL ELEMENTS IN CF AIRWAYS: MUCUS OBSTRUCTION, INFLAMMATION, AND INFECTION. OVER THE PAST 7 DECADES, OUR UNDERSTANDING OF CF RESPIRATORY MICROBIOLOGY AND INFLAMMATION HAS EVOLVED WITH THE INTRODUCTION OF NEW TREATMENTS, WITH INCREASED LONGEVITY, AND WITH INCREASINGLY SOPHISTICATED LABORATORY TECHNIQUES. IN THIS CHAPTER, WE WILL REVIEW THE CURRENT STATE OF UNDERSTANDING OF THE ROLES OF INFECTION AND INFLAMMATION AND THEIR ROLES IN DRIVING LUNG DISEASE. WE WILL ALSO DISCUSS HOW THIS CONSTANTLY EVOLVING INFORMATION IS USED TO INFORM CURRENT THERAPEUTIC STRATEGIES, MEASURES AND PREDICTORS OF DISEASE SEVERITY, AND RESEARCH PRIORITIES.
Keywords: CYSTIC FIBROSIS, INFLAMMATION, INFECTION, MICROBIOLOGY, NEUTROPHIL, BIOMARKER
Introduction
The earliest published descriptions of cystic fibrosis (CF) lung disease described the following triad of pathophysiologic elements that still form the basis of most current models:
airway obstruction
infection
inflammation
In her landmark 1938 publication describing the pathologic and clinical features of children who died from this disorder, Dorothy Andersen identified the common features of “bronchitis, bronchiectasis, pulmonary abscesses arising in the bronchi,” which were plugged with “tenacious, greenish gray mucopurulent material”, and that “Staphylococcus aureus was the usual bacteriologic agent”1,2. On the basis of these findings, she and others began treating children with CF with antibiotics targeting S. aureus, specifically sulfonamides and penicillin2. This approach was followed by several important developments: First, the children generally improved clinically. Second, cultures showed that they were increasingly infected with penicillin-resistant S. aureus, as well as pathogens not seen prior to antibiotics, including Pseudomonas aeruginosa3.
These early observations are instructive on many levels. The primary defect that causes CF, mutational dysfunction of the CF transmembrane conductance regulator (CFTR), leads to a cycle in the airways of defective mucus clearance, obstruction, infection and inflammation. Improvements in nutrition, mucus clearance, and treatment of inflammation and infection have led to dramatic improvements in CF respiratory morbidity and mortality4; however, current treatments have not been able to halt disease progression. As patients live increasingly longer, their respiratory microbiology evolves4, perhaps driven by elements of care intended to mitigate their lung disease (such as antibiotics and attending clinics at CF care centers). Put differently, the treatments that have so greatly helped people with CF are often followed by microbiological changes, which in turn may alter their clinical course. The causal relationships between specific pathogens, clinical changes, and our constantly evolving therapies are difficult to sort out.
Andersen and her colleagues made special mention of the apparent link between infection and inflammation2. While these are omnipresent features of CF lung disease, the causal relationships between them are controversial. Observations from a variety of studies of patients5 and, most recently, animals engineered with CFTR mutations6 have led some authors to postulate that infection is required for CF airway inflammation, while others have suggested that inflammation can precede infection7. While these disputes continue, great progress has been made in defining the mediators and mechanisms driving the intense inflammation within CF airways, identifying not only new candidate therapeutic targets, but also promising biomarkers of early disease.
In this chapter, we will review the current understanding of the roles of infection and inflammation in CF lung disease pathogenesis. While these two features of the CF airway are mechanistically linked by data from observational studies and, as a result, in our current pathophysiological models, for simplicity we consider them separately. While we will touch on therapeutic approaches to both infection and inflammation, we are currently experiencing a period of particularly rapid evolution in CF treatment strategies and protocols. Therefore, where appropriate, we will refer the reader to recent, in-depth reviews of CF therapeutic strategies8,9 for more information. Similarly, there have been a number of excellent reviews of CF respiratory microbiology and inflammation in general that the reader may find useful10–16.
I. CF MICROBIOLOGY
The microbiology of CF lung disease: The evolving role of methodology
Dorothy Andersen and her contemporaries identified a number of important characteristics of CF lung disease. For example, she and others who later built upon her work17–20 noted that the microbes infecting CF lungs were nearly always confined to airway luminal mucus, rather than invading tissue. The role of S. aureus was highlighted; however, other bacteria were quickly recognized as important CF pathogens. Blending observations from culture and microscopy, two of the most sophisticated methods available at the time, provided higher sensitivity and yielded a richer depiction of infection pathogenesis than either method alone. As both treatments and laboratory methods have evolved, so has our understanding of the pathogenesis of CF respiratory infections and the spectrum of likely pathogens. Before discussing the most common pathogens, it is instructive to consider how improvements in treatment, innovations in clinical microbiology, and increases in patient longevity combine to create constant evolution in the field of CF microbiology.
CF clinical microbiology generally relies on conventional laboratory cultivation of respiratory samples (e.g. sputum, bronchoalveolar lavage fluid, oropharyngeal swabs, or sinus samples) to identify and study causative organisms. Using these techniques, bacteria and fungi can be identified, enumerated, isolated, and characterized; their growth characteristics and in vitro antibiotic susceptibilities are defined. These methods are invaluable for diagnosing infections in individual patients, directing antibiotic treatment, and generating epidemiological information that informs our models of CF infection pathogenesis. Despite the enormous utility and power of these conventional methods, recent work with advanced laboratory techniques has added to the ever-growing list of CF-associated microbes. To show how newer methods might complement culture, we will briefly review how current CF respiratory cultures are performed, and how information is generated and used to direct treatment.
Current methods rely on synthetic laboratory growth media that, together with incubation conditions (generally aerobic and at body-temperature for CF microbiology), have been chosen to select for the microbes customarily associated with lung disease pathogenesis. In addition, current protocols21 usually involve selecting a small number of isolates of those “traditional pathogens” from those cultures to test susceptibilities, and to inform treatment.
However, conventional culture methods can introduce bias or have other shortcomings. For example, it is now known that CF airway mucus has anaerobic niches that alter microbial metabolism18 and that are not accurately modeled by these laboratory methods. In line with this finding, both advanced culture-based and culture-independent (sequencing) techniques have identified diverse microbes, including anaerobes and other otherwise undetected bacteria together with traditionally-cultured pathogens, in respiratory samples from many CF patients22. Microscopic and biochemical investigations18,23 suggest that microbes infecting CF airways may commonly exist in biofilms, which are gel-encased communities of cells that are resistant to killing compared with the liquid-suspended, dispersed microbial cells usually studied in the laboratory. In-depth studies of traditional CF pathogens (such as P. aeruginosa and S. aureus)24 have also revealed that they evolve and diversify over time, meaning that one or a few isolates will not accurately reflect the behaviors of an entire, chronically-infecting population of those bacteria. And studies of the microbes in different specimen types (such as swabs, sputum, and lavages) collected concurrently from individual CF patients indicates that each may sample different anatomic sites, raising the question of which sample is best for informing treatment or prognosis.
These new findings highlight the constantly evolving and controversial field of CF microbiology, which has largely been, and continues to be, informed by the results of standard cultures. Accordingly, the rest of this section will focus on what is known from conventional CF microbiology, with a concluding section on how recent developments may change clinical practice.
Traditional CF respiratory pathogens: Epidemiology
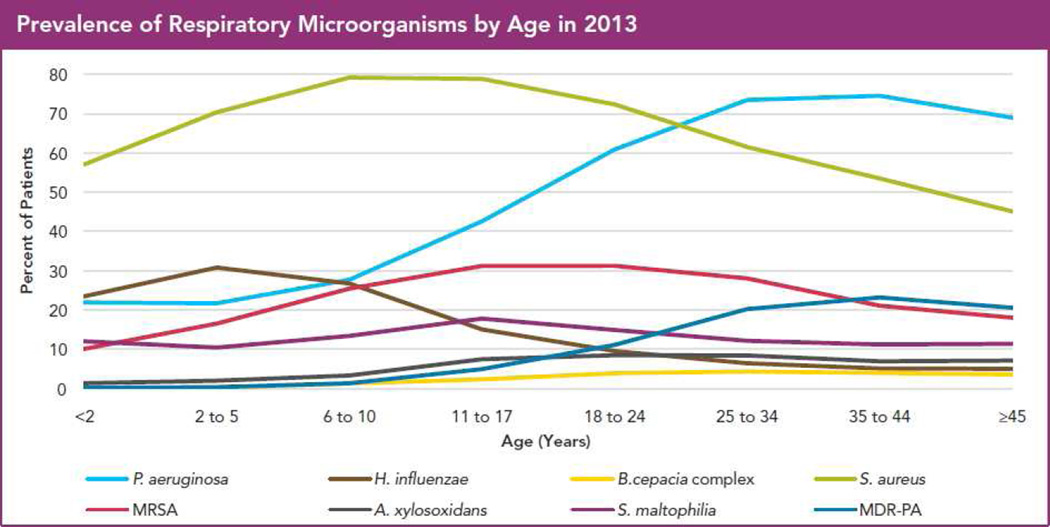
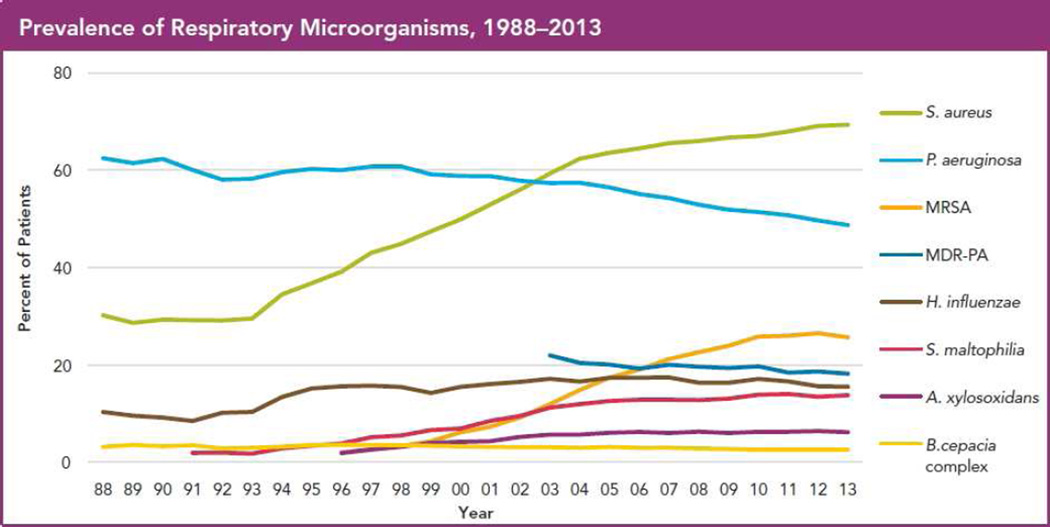
The two graphs in Figure 1 demonstrate that CF airway infections exhibit three key features:
They are diverse.
They are frequently polymicrobial.
They are constantly evolving.
Figure 1.
Epidemiology of traditional CF pathogens in 2013, from the US CF Patient Registry. (a) Percent of patients reported to be culture-positive for the indicated bacteria in 2013, by age group. (b) Overall prevalence in CF patients per year of these bacteria.
Cystic fibrosis patients under care at CF Foundation-accredited care centers in the United States, who consented to have their data entered. Courtesy of CF Foundation, Broomall, PA.
Figure 1a shows the US prevalence in 2013 by patient age of the bacteria cultured most commonly from respiratory cultures (as recorded in the Cystic Fibrosis Foundation Patient Registry4). This graph shows that S. aureus was particularly common among young children, while P. aeruginosa was most common in adults. The S. aureus subtype methicillin-resistant S. aureus (MRSA) had peak CF prevalence in the mid-teens to early 20’s. P. aeruginosa resistant to multiple antibiotics (MDR-PA) was relatively uncommon but followed the same age distribution as P. aeruginosa in general. A brief consideration of these data reveals that, for each age group, many patients must have had more than one of these bacteria during the year.
Figure 1b provides a slightly different perspective of the 2013 data. Consider, for example, how the prevalences of S. aureus and P. aeruginosa have changed in the US since 1988. S. aureus prevalence has dramatically increased, while that of P. aeruginosa has decreased somewhat. Therefore, even considering just these 6 commonly-cultured bacterial species, one can appreciate the diverse, polymicrobial, and constantly evolving nature of CF lung infections. Further, some culturable pathogens (such as nontuberculous mycobacteria, NTM) are not depicted on these graphs. In the following sections, we will briefly discuss each of these species, paying particular attention to S. aureus and P. aeruginosa, given their high prevalence and clinical associations. For brevity, we will provide key points for less common CF respiratory microbes. We will also discuss clinically-relevant variants of common pathogens, (such as MRSA and MDR-PA), as well as emerging pathogens such as NTM, with attention to their epidemiology, associations with disease, and treatment.
Staphylococcus aureus
Epidemiology and clinical associations
As described above, this gram-positive organism was the bacterium first identified as an important CF respiratory pathogen. The earliest CF antibiotic treatments targeted S. aureus, and early proponents of these treatments described the substantial clinical improvements that resulted. However, the role of S. aureus in driving CF lung disease, and the appropriate clinical response to its detection, are matters of ongoing debate. A review of published observations regarding the association between S. aureus and lung disease illustrates the basis for this controversy.
In addition to the high prevalence of S. aureus among children, pediatric CF studies have provided further reason to worry: S. aureus has been associated with higher airway inflammation25,26, lower lung function27,28, and even higher subsequent mortality when detected together with P. aeruginosa29. Further, these associations were for all S. aureus; as described below, specific subtypes of S. aureus (such as MRSA and small-colony variants of S. aureus (SCVs)) may be associated with even worse outcomes than other S. aureus.
However, when considering all S. aureus in adults with CF, the picture becomes less clear. Studies of adults found S. aureus to be associated with a lower risk of mortality30, better lung function31, and a lower risk of exacerbations32. Therefore, S. aureus pathogenesis may either be context-dependent (that is, perhaps it is either more pathogenic either in children or in the absence of P. aeruginosa, or it adapts over time to become less pathogenic). Alternatively, pathogenesis may be higher for specific subtypes of S. aureus (such as MRSA or SCVs), or S. aureus may not be universally pathogenic itself but rather serves as a marker of early (as with children) or mild (as with adults) disease.
Answering these questions is particularly pressing because of the recent increase in S. aureus prevalence shown in Figure 1b. Recent studies demonstrated similar trends in parts of Europe and Australia33 by the mid-2000s. However, S. aureus prevalence has remained comparatively low in the United Kingdom33, perhaps related to differences in antibiotic use (discussed below). Importantly, both longevity and lung function have continued to improve in the general CF population4, including in the age groups with the highest S. aureus prevalences. These combined observations make it difficult to predict the best approach to infection with S. aureus.
Subtypes
As hinted above, two subtypes of S. aureus are of particular significance for CF lung disease and treatment: MRSA and SCVs.
MRSA (or ORSA for oxacillin-resistant S. aureus) are usually identified either by their resistance to these β-lactams or by carriage of the mecA gene, which encodes this resistance. MRSA prevalence in CF has increased in the past 2 decades (Figure 1b) in parallel with all S. aureus and methicillin-susceptible S. aureus (MSSA). MRSA acquisition in CF is strongly associated with exposure to hospitals and to antibiotics34–36, as it is in the general population, which also experienced a parallel increase in MRSA prevalence in the US. Interestingly, the increase seen in the US CF population has not occurred as dramatically elsewhere33, suggesting that environmental, treatment, or other factors unique to specific locations are responsible for this epidemiologic change.
The relationship between MRSA and CF lung disease outcomes is complex. While a number of studies have demonstrated an association between MRSA infection and lower lung function in CF patients34,35,37, some studies indicated that patients with MRSA had faster lung function decline prior to MRSA detection, suggesting MRSA may be a marker for severe disease rather than the cause35. Other studies, however, found MRSA to be an independent risk factor for failure to recover lung function during treatment for respiratory exacerbations38 and for increased mortality39.
S. aureus SCVs are slow-growing, antibiotic-resistant variants that are difficult to detect with conventional cultures. Small studies of both European40–42 and US CF43 populations have identified SCVs among 8–30% of patients using special laboratory methods. These studies have also found SCVs to be associated with lower lung function, faster lung function decline, and higher rates of preceding treatment with antibiotics known to select for SCVs, including aminoglycosides and sulfonamides, in addition to higher rates of co-infection with P. aeruginosa, which may also select for SCVs44.
The similarities between MRSA and S. aureus SCVs, particularly their relationships with clinical outcomes, antibiotic treatment and resistance, are instructive. Each of these bacterial types is associated with:
Preceding antibiotic treatment
Subsequent antibiotic resistance
Higher lung disease severity
However, these findings are largely from observational studies, and whether MRSA and SCVs cause worse outcomes, whether they reflect patients with worse preexisting disease and resulting higher antibiotic treatment burdens, or both, remains to be seen. Additional observational and interventional studies may resolve these questions of causality. Until then, the best therapeutic approaches to each subtype remains to be defined.
Treatment
Determining the best approach to S. aureus infection in CF is a controversial subject. Some countries provide antistaphylococcal prophylaxis during childhood; this practice has been associated in some trials with earlier detection of P. aeruginosa which, given its association with worse lung disease, led many countries to not adopt this approach45. Some centers provide antistaphylococcal antibiotics aimed at eradicating S. aureus on first detection46, and strategies and attitudes differ for MRSA versus MSSA; evidence supports the microbiological efficacy of this strategy, but clinical benefit for both MRSA and MSSA remain to be determined46,47. By comparison, for patients with S. aureus and no other detected pathogens who have an exacerbation, there is consensus that antistaphylococcal antibiotics should be used. The drugs used most often in this case have been reviewed8.
Pseudomonas aeruginosa
Epidemiology and clinical associations
With the advent of effective antistaphylococcal therapy, the gram-negative bacterium P. aeruginosa emerged as a common and important pathogen in CF lung disease (reviewed in12). P. aeruginosa infection has been associated with48:
Worse lung function outcomes
Higher respiratory tract inflammation
A greater risk of respiratory exacerbations
Higher risk of mortality
While chronic P. aeruginosa infection usually cannot be eradicated, current evidence indicates that initial P. aeruginosa detection presents a “window of opportunity” for eradication with antibiotics. There is also good evidence that suppressing P. aeruginosa can improve measures of lung disease. For CF exacerbations among people with P. aeruginosa, antipseudomonal antibiotics have been shown to improve outcomes49. However, whether early eradication provides similar clinical benefits is still under investigation50.
As shown in Figure 1a, P. aeruginosa prevalence tends to be higher in adults than in children; its overall prevalence has decreased slightly in the past few decades (Figure 1b), perhaps related to new eradication approaches upon initial detection. Longitudinal analyses indicate that P. aeruginosa tends to “exclude” other microbes that were previously detected in the respiratory tract, and that it is frequently the predominant, and often the only, bacterium identified in CF lungs during end-stage disease51. These aggregate findings have earned P. aeruginosa the reputation as the most important CF respiratory pathogen.
P. aeruginosa has a very large genome, encoding numerous potential toxins controlled by a complex array of regulatory elements. During chronic CF infections, P. aeruginosa undergoes diverse adaptive changes, including inactivation of many of these “virulence factors” and their regulators52. One of the most common phenotypic changes observed for P. aeruginosa is the exuberant production of alginate, resulting in a mucoid colony appearance in vitro53. Mucoidy has been associated with both persistence and duration of infection; however, it was not associated with failure of early eradication in recent studies. Interestingly, those studies also identified mucoid isolates upon initial detection of P. aeruginosa52. As mucoidy has traditionally been thought to represent adaptation to the airway, these results may indicate that current detection methods are not perfectly sensitive for earliest infection.
Epidemiologic evidence has identified genetically-related isolates of P. aeruginosa (referred to as epidemic strains) among CF populations in several countries (reviewed in reference 54). In some cases, these isolates were associated with worse outcomes than non-epidemic P. aeruginosa, including higher risks of death and need for lung transplantation. Similarly, P. aeruginosa resistant to multiple antibiotics (MDR) have been found to be associated with worse lung disease by several measures, but as with MRSA and S. aureus SCVs, whether these resistant P. aeruginosa identify patients with higher antibiotic exposures, or whether they are more pathogenic than other P. aeruginosa, remains to be determined55.
Treatment
Prophylaxis for P. aeruginosa is not recommended. However, treatment with antibiotics upon initial detection, with the goal of eradication, has proven to be microbiologically effective, and to decrease the risk of subsequent exacerbations. Inhaled antibiotics, such as tobramycin and more recently aztreonam, form the basis of the current guidelines56 for eradication, with no benefit found by adding an oral drug of another class (ciprofloxacin). Similarly, inhaled antibiotics are recommended as chronic maintenance medications for CF patients with persistent P. aeruginosa. For exacerbation treatment in patients chronically infected with P. aeruginosa, limited evidence indicates that treatment with two classes of antipseudomonal antibiotics leads to longer periods of clinical stability than does a single class. Treatments for P. aeruginosa in CF have been the subject of recent reviews and guidelines9,56.
Burkholderia cepacia complex (BCC)
Epidemiology and clinical associations
The BCC group of gram-negative bacteria is comprised of at least 18 distinct species (a related species, B. gladioli, is often discussed with BCC because it can cause similar infections). Of these, two species are among the most common and most associated with CF lung infections and disease:
B. cenocepacia
B. multivorans
By comparison, other BCC species are less common and their clinical associations less well-defined (as is the case for B. gladioli)57. As shown in Figure 1, Burkholderia species have infected between 3–4% of CF patients in the US for many years4,14, primarily affecting adolescents and adults. B. multivorans prevalence was highest, followed by B. cenocepacia and B. gladioli.
Burkholderia CF infections are notorious for several characteristics:
They are associated with more severe lung disease. These associations are more pronounced for some Burkholderia species than others; some small studies have shown B. cenocepacia to be associated with more rapid lung function decline than B. multivorans, and a recent Canadian study found the strongest association with subsequent mortality to be with B. cenocepacia, followed by B. multivorans57.
They can be transmissible between persons with CF. Epidemic strains of B. cenocepacia have been shown to infect CF patients after interpatient contact at camps and clinics58 and have even been shown to replace B. multivorans after transmission. This transmissibility was one important driving force behind the institution of stringent infection control measures, which evidence suggests have been highly infective for controlling spread of these strains.
Clinical outcomes of BCC infections are unpredictable. Associated outcomes often range from clinical quiescence to rapidly progressive, necrotizing pneumonia and fatal septic disease (“cepacia syndrome”).
Treatment
Currently, the practice of antibiotic treatment for eradication on early isolation is controversial, and the utility of this practice is not yet clear59,60. Burkholderia species are also notorious for their antibiotic resistance—both intrinsic and acquired—and therapy is usually limited to specific antibiotics as needed. Systematic reviews have recently been published61.
Considerations for the following microbes in CF infections will be presented briefly:
Stenotrophomonas maltophilia13,14
Gram-negative
Increased US CF prevalence in recent years.
Particularly common among adolescents and young adults.
Conflicting data regarding clinical associations.
Both intrinsically and adaptively resistant to many antibiotics
Inadequate evidence for the clinical impact of therapy; a Cochrane Review of current approaches recently became available.
Haemophilus influenzae14
Gram-negative
Often the first organism detected in CF respiratory cultures; prevalent in children, less common in adults.
Association with CF clinical outcomes is controversial, but associated with adverse outcomes in other chronic respiratory diseases: non-CF bronchiectasis and chronic obstructive pulmonary disease.
Difficult to culture, requiring specific conditions for detection.
Since the introduction in the US of the H. influenzae type B (HIB) vaccine, most isolates are “nontypeable” and unencapsulated.
Can produce β-lactamase; therefore, treatments usually include a β-lactamase inhibitor (e.g., amoxicillin-clavulanate).
Achromobacter xylosoxidans10,13
Gram-negative bacterium similar to P. aeruginosa.
Increased recent US prevalence, but remains low (<10% of CF patients, Figure 1).
Associated with worse radiographic and spirometric measures of lung disease.
Similar to P. aeruginosa and BCC, A. xylosoxidans can be the dominant, and sometimes only, bacterium isolated from CF patients at end-stage.
Notorious for resistance to many antibiotics, limiting treatment options.
Nontuberculous Mycobacteria (NTM)11,13,14
Estimated CF prevalence 6—30%.
- Two groups of Mycobacteria, accounting for 6 species, are currently considered important CF pathogens:
-
○Mycobacterium-avium complex (MAC)
-
○Mycobacterium abscessus complex
-
○
- NTM treatment approaches usually involve two phases:
-
○Multiple intravenous (IV) antibiotics for weeks to months
-
○Multiple inhaled and oral antibiotics for months to years
-
○Side-effects and toxicities are common and can be troublesome.
-
○
Guidelines for NTM infection diagnosis and treatment from a joint committee based in both the US and Europe expected to be published in 2015-6.
Fungi and viruses11,14
- Numerous fungi are frequently isolated from CF patients, including:
- Yeasts (particularly Candida spp.)
- Filamentous fungi (including Aspergillus spp).
Whether detection of fungi requires treatment is a matter of current debate.
Allergic Bronchopulmonary Aspergillosis (ABPA): Patients with CF and other chronic airway diseases can develop an IgE-mediated allergic airway disease known as ABPA, the treatment for which primarily involves steroids, although the addition of an antifungal (such as itraconazole) may allow for lower doses of steroids.
Human respiratory viruses are not thought to chronically infect the CF airway, but they have been shown both to be important and common triggers of CF respiratory exacerbations.
The CF airway microbiome and future directions
The application of DNA-based microbiological techniques to CF respiratory samples has afforded a very different view of their microbiota. To date, dozens of such studies using a variety of methods have been performed. This collective work provides evidence for dynamic, diverse, and highly resilient CF respiratory microbial communities (reviewed in reference 22). In general, these respiratory microbiota tend to be more diverse, with more microbial species and not dominated by any individual microbe, in CF patients who are younger, have better lung function, and/or have had fewer courses of antibiotics. Conversely, this diversity tends to be lower in patients who are older, who have more severe lung disease, and who have had been treated with more antibiotics. The microbiota in lungs from patients with end-stage CF disease tend to be dominated by one or very few species, most often P. aeruginosa, BCC, or A. xylosoxidans. However, these associations between age, disease severity, microbial diversity, and antibiotic burden make it difficult to sort out cause and effect; it is not clear yet whether the decrease in diversity “drives” disease severity, or whether patients with worse preexisting disease receive more antibiotics that “prune” the microbiota to select for the most adaptable and resistant microbes. Ongoing and future studies aim to better define the causal relationships between infection dynamics, therapy, and disease progression.
CF microbiology: Summary
There have been remarkable changes and developments since the first descriptions of CF lung infections in the 1930s-40s. Antibiotics have played a central role in improving lung disease outcomes and overall longevity. However, findings over the past 70 years from both culture-based, classical microbiological methods, and those from newer culture-independent techniques, repeatedly demonstrate the resilient and dynamic nature of chronic CF lung infections. These findings warrant ongoing vigilance and innovation, and they highlight the many questions that have arisen in this rapidly-moving field. In the years to come, there are certain to be new revelations regarding the mechanisms of infection and the microbial determinants of both CF lung disease and response to treatment, which will lead to new recommendations for therapy and infection control. However, the many controversies discussed above regarding the relationships between airway microbes and clinical outcomes underscore the importance of host response in driving CF lung disease. The next section will discuss these inflammatory mechanisms.
II. HOST- RESPONSE IN CF LUNG DISEASE
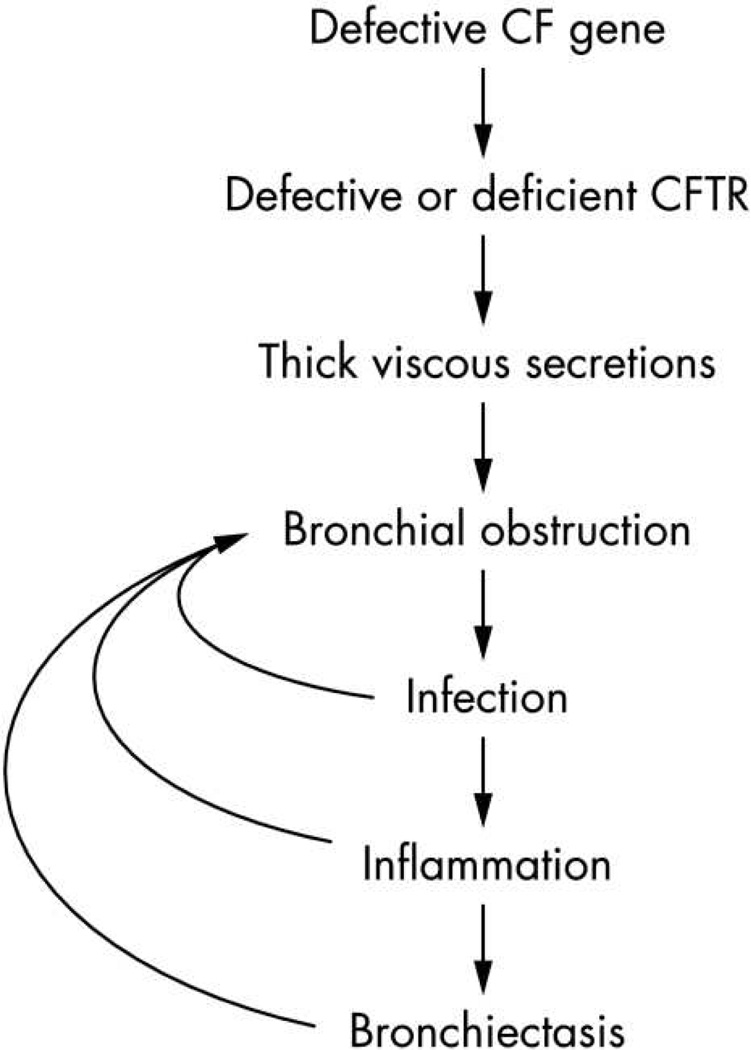
As emphasized above, chronic airway infections are a major driver of progressive airways destruction in CF. Much of the damage seen with chronic infection is mediated by a vigorous and abnormal host-response to airway infections, resulting in a vicious cycle that leads to irreversible bronchiectasis and lung function decline8,62 (Figure 2a). Over the past several decades, numerous studies have documented a heightened, primarily neutrophilic inflammatory response in CF airways. Much of the inflammatory response is driven by the presence of bacterial pathogens, but the reaction is exaggerated even when accounting for the presence of infection63,64. Debate continues over how much of the inflammatory response is related to underlying CFTR dysfunction and whether airway inflammation precedes the onset of infection.
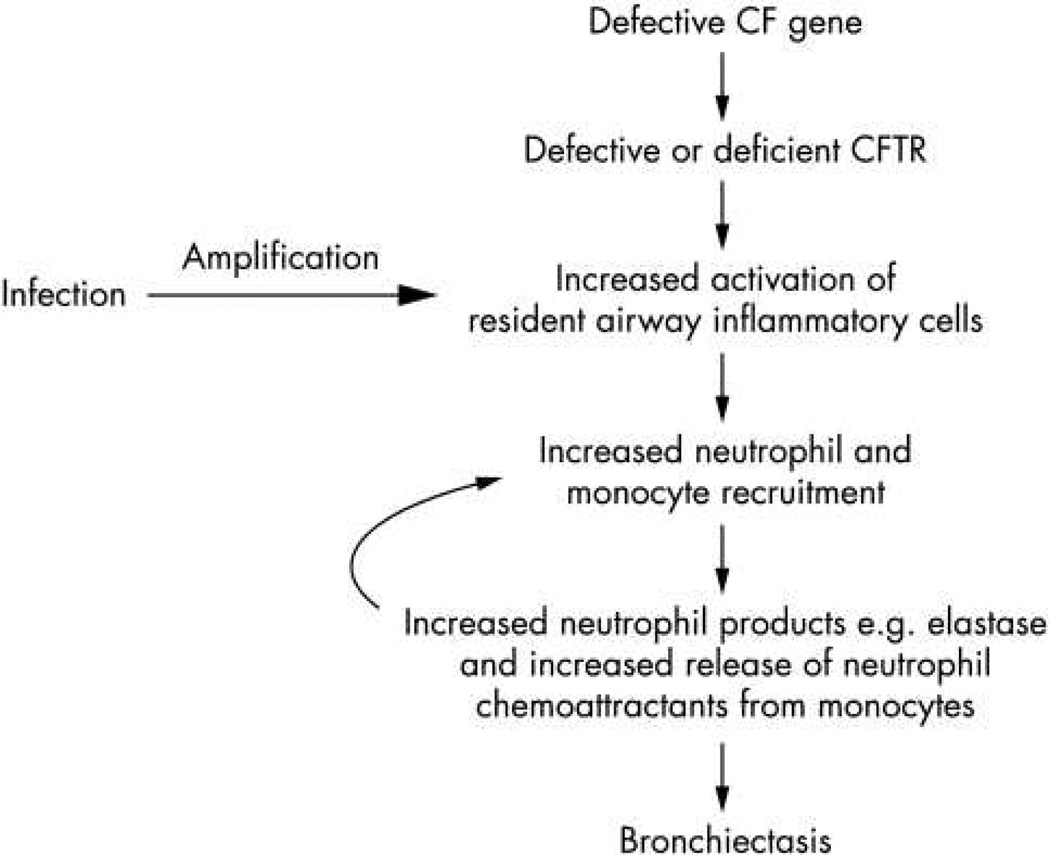
Figure 2.
Pathophysiology of lung disease in CF. (a) Traditional view of pathology of lung disease in CF. Inflammation is triggered by chronic bacterial infection. (b) Potential alternative mechanism for airway inflammation in CF. Underlying CFTR dysfunction contributes to altered host-response.
From: Rao S and Grigg J, New insights into pulmonary inflammation in cystic fibrosis Arch Dis Child. 2006 Sep; 91(9): 786–788; with permission.
In this section, we will consider evidence for the role of CFTR dysfunction in altered host-defense; how imbalances in the airway environment contribute to disease pathophysiology and may lead to new therapies; and how specific measures of inflammation, particularly neutrophil elastase (NE), may be useful biomarkers of disease. Finally, we will consider current and future approaches to anti-inflammatory therapies and highlight challenges in moving these therapies from the bench to bedside.
CFTR, impairments in host-defense and inflammation
The role of CFTR in altered host-defense has been examined extensively using in vitro cell culture systems, in vivo animal models and human subject studies. Two recent review articles explore this issue in depth16,15. Briefly, several lines of evidence now link CFTR dysfunction to altered host-defense through primary and secondary mechanisms (Figure 2b). Airway surface liquid dehydration and acidification results from lack of CFTR function in the epithelial cells lining the airways. Loss of the airway surface liquid layer results in abnormal mucociliary clearance, a first layer of defense against microbes. Acidification, due to lack of bicarbonate secretion by CFTR, likely alters periciliary mucins, resulting in a dense and overly adherent mucus layer lining the cilia65,66. Neutrophils and macrophages also express CFTR, and lack of functional CFTR channels likely reduce bacterial killing and clearance by these cells15.
In many ways, CF airways disease can be best understood as imbalances initiated by CFTR dysfunction and perpetuated by chronic infection, a necessary but overly exuberant inflammatory response, and airway structural injury that progressively worsens mucociliary clearance. Bacterial burden is typically high in CF airways; however, the neutrophilic inflammatory response is elevated for a given bacterial load in CF compared to non-CF patients63,64. The neutrophilic response is worsened by defects in neutrophil apoptosis and impaired clearance of debris. Defects in mucociliary clearance and macrophage dysfunction lead to accumulation of neutrophils and their products15,67. Proteases, primarily NE, are released by neutrophils and overwhelm the normally counteracting anti-proteases (e.g. alpha-1-antitrypsin, a serine protease inhibitor, and secretory leukocyte protease inhibitor), leading to airways damage68,69. Antioxidants in the CF airways are also overwhelmed by oxidative stress from persistent infection and inflammatory responses. CFTR dysfunction may also lead to worsening oxidative imbalance through impairment of Nrf2, a transcription factor crucial in maintaining oxidative reducing capacity. Finally, lipid mediators that regulate antiinflammatory responses to insults are likely abnormal in CF, further limiting the ability of the host to mitigate an overly vigorous inflammatory reaction. Therefore, therapies aimed at restoring airway equilibrium may help reduce airway injury.
Host response and inflammation as biomarkers of disease
In addition to contributing to the pathophysiology of CF airway disease, mediators of host-response may serve as important biomarkers of disease progression. NE has been the most well-documented inflammatory biomarker to date. NE is more abundant in induced sputum in CF compared to control children70; in school-aged children with CF, elevated NE in induced sputum has been associated with subsequent lung function decline71. Elevated NE in BALF is associated with bronchiectasis in infants and preschool aged children72 and with lung function measurements in infants with CF27. Sputum measures of airway inflammation have also been shown to decrease after treatment of a pulmonary exacerbation with IV antibiotics73. In a study of IV antibiotics, higher sputum NE on day 14 of therapy for a pulmonary exacerbation was associated with increased risk of subsequent exacerbation74.
Circulating inflammatory proteins have also been examined as potential biomarkers of disease in CF. In a multicenter study measuring a panel of plasma proteins at onset of a pulmonary exacerbation and following treatment with IV antibiotics, significant reductions in 10 of 15 proteins were observed75. Baseline measurements of IL-8, NE-protease complexes and alpha-1-antitrypsin, when combined with changes in protein markers and lung function, predicted a better treatment response, suggesting that a panel of circulating markers may be useful clinically and for future therapeutic trials. A clinical trial in CF demonstrated reduction in systemic inflammation following six months of treatment with chronic azithromycin76, although treatment with several agents, including ibuprofen and ivacaftor in patients with the G551D mutation, that were expected to decrease inflammation did not demonstrate this anticipated effect77,78.
Host-response to Pseudomonas aeruginosa
A number of studies have examined the host response to P. aeruginosa in an effort to understand the clinical associations with this pathogen79–81. The interaction between P. aeruginosa and the CF host is complex and beyond the scope of this review. However, it is likely that CFTR dysfunction predisposes the host to infection with P. aeruginosa; subsequent adaptation by the bacteria then allows for chronic infection and reduced opportunity for eradication. It is also likely that P. aeruginosa interacts with other bacterial pathogens including Staphylococcus aureus and Burkholderia cepacia complex in a way to further alter the inflammatory response25,82.
Anti-inflammatory therapies in CF
As described above, an overly exuberant inflammatory response in CF leads to airways damage and worsening bronchiectasis. However, the CF immune response also plays a critical role in containing microbes within the airways and preventing invasive disease. While therapies that blunt the inflammatory response theoretically may limit airway damage, they could potentially lead to worsening infections. Several anti-inflammatory therapies have been studied with variable results, likely due to difficulties in maintaining this balance.
Ibuprofen
In cell and animal models, ibuprofen has been shown to inhibit neutrophil migration and aggregation, including in CF animal models83. The effect of chronic high-dose ibuprofen was evaluated in a two-center, randomized, double-blind, placebo controlled trial84. Patients in the treatment group had a slower rate of lung function decline overall, although in a subgroup analysis by age, the difference was significant only in patients younger than 13 years. There was no reduction in hospitalization rate seen with treatment. Several follow-up studies confirmed these findings, and showed reasonable safety data, although gastrointestinal bleeding was more common in those on chronic therapy85. A recent Cochrane review concluded that high-dose ibuprofen could slow the progression of lung disease in CF, particularly in children with mild disease86. Despite these findings, clinical use of ibuprofen is relatively uncommon particularly compared to other CF therapies87. Reasons are likely related to persistent clinician concerns about adverse effects (e.g. kidney impairment, gastric bleeding), and need for careful pharmacokinetic monitoring to achieve and maintain therapeutic dosing. Based on pulmonary clinical guidelines published in 2013, the CF Foundation recommends chronic use of ibuprofen in children ages 6–17 years with FEV1 > 60% (Grade B, moderate net benefit), but does not recommend treatment in adults with CF88.
Chronic azithromycin
Azithromycin is a macrolide antibiotic with immunomodulatory effects, which are likely the basis for its effectiveness in CF and other chronic inflammatory conditions89. Chronic azithromycin has been studied in two CF populations: patients with and those without chronic P. aeruginosa infection90,91. Patients with chronic P. aeruginosa infection treated with thrice weekly azithromycin for 6 months had improved FEV1, decreased risk of pulmonary exacerbation and improved weight gain compared to placebo90. In a second study, patients without P. aeruginosa treated with azithromycin had a 50% reduction in pulmonary exacerbations and a modest improvement in weight91. However, lung function did not improve compared to placebo. CF clinical guidelines recommend azithromycin for people with CF ages 6 years and older with chronic P. aeruginosa (Grade B, moderate net benefit), and consideration of treatment for those without P. aeruginosa (Grade C, moderate net benefit)88. The durability of effect with azithromycin remains unclear however, and there are concerns about emergence of bacterial resistance92,93; thus, treatment should be reassessed every 6–12 months. In addition, patients with NTM should not receive azithromycin unless it is prescribed in combination with other anti-mycobacterial medications as part of NTM therapy.
Corticosteroids and leukotriene receptor antagonists
Systemic and inhaled corticosteroids have been studied in patients with CF. Although systemic corticosteroids have been shown to improve lung function, the adverse effects, including poor somatic growth, outweigh any benefit94. Inhaled corticosteroids have also not been shown to be effective in CF95. Thus, the CF Foundation recommends against the use of chronic systemic or inhaled corticosteroids88. Montelukast, a leukotriene receptor antagonist, showed modest effects on lung function and inflammatory markers in a small pediatric study (n=28), however more robust data are lacking96. A phase 2 clinical trial of a leukotriene B4 receptor antagonist, BIIL 284 BS, was terminated after an interim analysis revealed increased pulmonary-related, serious adverse events and incidence of pulmonary exacerbations in those on treatment, highlighting the potential risks of suppressing the immune response in patients with chronic infections97.
Clinical trials
Several compounds are currently in the CF drug development pipeline. Alpha-one-proteinase inhibitor (Alpha-1 HC) is an aerosolized protease inhibitor with anti-inflammatory properties. A phase 2 randomized, double-blind, placebo-controlled study of Alpha-1 HC given for three weeks to adults with CF showed safety and tolerability at two dose levels98. CTX-4430 is a LTB4 receptor antagonist that is currently in phase 2 testing; risk of pulmonary adverse events and exacerbations will be closely monitored particularly given the previous above results with BIIL 284 BS (Trial number NCT02443688, clinicaltrials.gov). Ajulemic acid (JBT-101) is an oral synthetic cannabinoid agonist that binds to the CB2 receptor expressed on activated immune cells and fibroblasts, and thought to help resolve inflammation and limit fibrosis99. A 12-week, phase 2 study in adults with CF is currently recruiting (Trial number NCT02465450, clinicaltrials.gov).
III. CONCLUSIONS
Airway obstruction, infection and inflammation lead to much of the morbidity and mortality associated with CF. P. aeruginosa, S. aureus and BCC are the bacteria most frequently associated with CF lung disease; improved and aggressive antimicrobial treatment of these pathogens has contributed greatly to improvements in life expectancy. However, airway microbial communities are complex and likely influenced by environment, host characteristics, disease progression, and antibiotic treatment. Host-response to microbes in the CF airways is robust, likely due in part of underlying CFTR protein dysfunction. The overly exuberant host-inflammatory response and decreased host ability to clear microbes and inflammatory debris from the airways leads to further damage. Therapies targeting airway infections and host-inflammatory response are effective in reducing disease activity, but do not fully stop progression. Fortunately, a number of anti-inflammatory and antimicrobial therapies are in the therapeutic development pipeline. In addition, CFTR modulator therapy, newly available for ~50% of people with CF, and with the hope of expanded treatments for all with CF, will undoubtedly influence both airway microbiology and inflammatory responses.
Key points.
CYSTIC FIBROSIS LUNG DISEASE INVOLVES A CYCLE OF MUCUS OBSTRUCTION, INFLAMMATION, AND INFECTION IN THE AIRWAYS, AND EACH OF THESE ELEMENTS IMPACTS THE OTHERS AS DISEASE PROGRESSES.
OBSERVATIONS FROM THE EARLIEST STUDIES OF CF DISEASE IN THE 1930S-40S DEMONSTRATED THESE ELEMENTS, BUT OUR UNDERSTANDING AND TREATMENT OF EACH HAS EVOLVED OVER TIME.
CF RESPIRATORY MICROBIOLOGY HAS ALSO EVOLVED AS NEW TREATMENTS HAVE BEEN INTRODUCED, AS PEOPLE HAVE LIVED LONGER WITH THIS DISEASE, AND AS DETECTION METHODS HAVE BECOME MORE SOPHISTICATED.
THE CF AIRWAY ALSO DEMONSTRATES ALTERED HOST-DEFENSE, AND IMBALANCES IN THE AIRWAY ENVIRONMENT ARE IMPORTANT FOR PATHOPHYSIOLOGY AND MAY INDICATE BOTH NEW TREATMENT DIRECTIONS AND USEFUL DISEASE BIOMARKERS.
BECAUSE MOST INFORMATION REGARDING THE RELATIONSHIPS BETWEEN INFECTION, INFLAMMATION, AND DISEASE SEVERITY COME FROM OBSERVATIONAL STUDIES, THEIR CAUSAL RELATIONSHIPS ARE NOT ALWAYS CLEAR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclose any relationship with a commercial company that has a direct financial interest in subject matter or materials discussed in article or with a company making a competing product. If nothing to disclose, please state “The Authors have nothing to disclose.”
Contributor Information
Edith T. Zemanick, ASSOCIATE PROFESSOR OF PEDIATRICS, UNIVERSITY OF COLORADO SCHOOL OF MEDICINE, AURORA, COLORADO.
Lucas R. Hoffman, Email: LHOFFM@UW.EDU, ASSOCIATE PROFESSOR OF PEDIATRICS AND ADJUNCT ASSOCIATE PROFESSOR OF MICROBIOLOGY, UNIVERSITY OF WASHINGTON, SEATTLE, WASHINGTON.
Bibliography
- 1.Andersen D. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Child. 1938;56:344–399. [Google Scholar]
- 2.Di Sant’agnese PEA, Andersen DH. Celiac syndrome; chemotherapy in infections of the respiratory tract associated with cystic fibrosis of the pancreas; observations with penicillin and drugs of the sulfonamide group, with special reference to penicillin aerosol. Am. J. Dis. Child. 1911. 1946;72:17–61. [PubMed] [Google Scholar]
- 3.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2013 Annual Data Report to the Center Directors. 2014 [Google Scholar]
- 5.Armstrong DS, et al. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr. Pulmonol. 2005;40:500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 6.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan TZ, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 8.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 9.Waters V, Smyth A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015;14:551–560. doi: 10.1016/j.jcf.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Chmiel JF, et al. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann. Am. Thorac. Soc. 2014;11:1120–1129. doi: 10.1513/AnnalsATS.201402-050AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chmiel JF, et al. Antibiotic Management of Lung Infections in Cystic Fibrosis. II. Nontuberculous Mycobacteria, Anaerobic Bacteria, and Fungi. Ann. Am. Thorac. Soc. 2014;11:1298–1306. doi: 10.1513/AnnalsATS.201405-203AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilligan PH. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkins MD, Floto RA. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015;14:293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015;50(Suppl 40):S39–S56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 16.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015;14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich M, et al. Localization of Staphylococcus aureus in infected airways of patients with cystic fibrosis and in a cell culture model of S. aureus adherence. Am. J. Respir. Cell Mol. Biol. 1998;19:83–91. doi: 10.1165/ajrcmb.19.1.3137. [DOI] [PubMed] [Google Scholar]
- 18.Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baltimore RS, Christie CD, Smith GJ. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am. Rev. Respir. Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- 20.Schwab U, et al. Localization of Burkholderia cepacia Complex Bacteria in Cystic Fibrosis Lungs and Interactions with Pseudomonas aeruginosa in Hypoxic Mucus. Infect. Immun. 2014;82:4729–4745. doi: 10.1128/IAI.01876-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saiman L, et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect. Control Hosp. Epidemiol. 2014;35(Suppl 1):S1–S67. doi: 10.1086/676882. [DOI] [PubMed] [Google Scholar]
- 22.Caverly LJ, Zhao J, LiPuma JJ. Cystic fibrosis lung microbiome: Opportunities to reconsider management of airway infection: Cystic Fibrosis Lung Microbiome. Pediatr. Pulmonol. 2015;50:S31–S38. doi: 10.1002/ppul.23243. [DOI] [PubMed] [Google Scholar]
- 23.Singh PK, et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 24.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin. Microbiol. Rev. 2011;24:29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagel SD, et al. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 2009;154:183–188. doi: 10.1016/j.jpeds.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangell C, et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011;53:425–432. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 27.Pillarisetti N, et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 28.Cogen J, et al. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr. Pulmonol. 2015;50:763–770. doi: 10.1002/ppul.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson VL, Wielinski CL, Regelmann WE. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. J. Pediatr. 1993;122:854–860. doi: 10.1016/s0022-3476(09)90007-5. [DOI] [PubMed] [Google Scholar]
- 30.Liou TG, et al. Predictive 5-year survivorship model of cystic fibrosis. Am. J. Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer-Hamblett N, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007;175:822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlgren HG, et al. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm. Med. 2015;15:67. doi: 10.1186/s12890-015-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2011;10:298–306. doi: 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Ren CL, et al. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr. Pulmonol. 2007;42:513–518. doi: 10.1002/ppul.20604. [DOI] [PubMed] [Google Scholar]
- 35.Sawicki GS, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 2008;43:1117–1123. doi: 10.1002/ppul.20914. [DOI] [PubMed] [Google Scholar]
- 36.Muhlebach MS, et al. Multicenter Observational Study on Factors and Outcomes Associated with Different MRSA Types in Children with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2015 doi: 10.1513/AnnalsATS.201412-596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent Methicillin-resistant Staphylococcus aureus and Rate of FEV1 Decline in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2008;178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 38.Sanders DB, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am. J. Respir. Crit. Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasenbrook EC, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 40.Besier S, et al. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 2007;45:168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahl B, et al. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 1998;177:1023–1029. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 42.Schneider M, et al. Clinical characteristics associated with isolation of small-colony variants of Staphylococcus aureus and Pseudomonas aeruginosa from respiratory secretions of patients with cystic fibrosis. J. Clin. Microbiol. 2008;46:1832–1834. doi: 10.1128/JCM.00361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolter D, et al. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in children with CF: A cohort study. Pediatr. Pulmonol. 2012;S35:276. [Google Scholar]
- 44.Hoffman LR, et al. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19890–19895. doi: 10.1073/pnas.0606756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth AR, Walters S. Prophylactic anti-staphylococcal antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. 2014;11:CD001912. doi: 10.1002/14651858.CD001912.pub3. [DOI] [PubMed] [Google Scholar]
- 46.Dalbøge CS, Pressler T, Høiby N, Nielsen KG, Johansen HK. A cohort study of the Copenhagen CF Centre eradication strategy against Staphylococcus aureus in patients with CF. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2013;12:42–48. doi: 10.1016/j.jcf.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Lo DKH, Hurley MN, Muhlebach MS, Smyth AR. Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst. Rev. 2015;2:CD009650. doi: 10.1002/14651858.CD009650.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Langton Hewer SC, Smyth AR. In: Cochrane Database of Systematic Reviews. The Cochrane Collaboration; Langton Hewer SC, editor. John Wiley & Sons, Ltd; 2009. at < http://doi.wiley.com/10.1002/14651858.CD004197.pub3>. [Google Scholar]
- 49.Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am. Rev. Respir. Dis. 1990;141:914–921. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- 50.Mayer-Hamblett N, et al. Impact of Sustained Eradication of New Pseudomonas aeruginosa Infection on Long-term Outcomes in Cystic Fibrosis. Clin. Infect. Dis. 2015;61:707–715. doi: 10.1093/cid/civ377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer-Hamblett N, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am. J. Respir. Crit. Care Med. 2014;190:289–297. doi: 10.1164/rccm.201404-0681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 54.Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updat. 2015;21–22:41–59. doi: 10.1016/j.drup.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Merlo CA, et al. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest. 2007;132:562–568. doi: 10.1378/chest.06-2888. [DOI] [PubMed] [Google Scholar]
- 56.Mogayzel PJ, et al. Cystic Fibrosis Foundation Pulmonary Guideline*. Pharmacologic Approaches to Prevention and Eradication of Initial Pseudomonas aeruginosa Infection. Ann. Am. Thorac. Soc. 2014;11:1640–1650. doi: 10.1513/AnnalsATS.201404-166OC. [DOI] [PubMed] [Google Scholar]
- 57.Zlosnik JEA, et al. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada. 30 years’ experience. Ann. Am. Thorac. Soc. 2015;12:70–78. doi: 10.1513/AnnalsATS.201408-395OC. [DOI] [PubMed] [Google Scholar]
- 58.Lipuma JJ. Update on the Burkholderia cepacia complex. Curr. Opin. Pulm. Med. 2005;11:528–533. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- 59.Horsley A, Webb K, Bright-Thomas R, Govan J, Jones A. Can Early Burkholderia cepacia Complex Infection in Cystic Fibrosis be Eradicated with Antibiotic Therapy? Front. Cell. Infect. Microbiol. 2011;1 doi: 10.3389/fcimb.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regan KH, Bhatt J. In: Cochrane Database of Systematic Reviews. The Cochrane Collaboration, editor. John Wiley & Sons, Ltd; 2014. at < http://doi.wiley.com/10.1002/14651858.CD009876.pub2>. [Google Scholar]
- 61.Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int. J. Antimicrob. Agents. 2009;33:394–404. doi: 10.1016/j.ijantimicag.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Ratjen F, Döring G. Cystic fibrosis. Lancet Lond. Engl. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 63.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am. J. Respir. Crit. Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 64.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J. Infect. Dis. 1997;175:638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 65.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 66.Pezzulo AA, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alexis NE, Muhlebach MS, Peden DB, Noah TL. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2006;5:17–25. doi: 10.1016/j.jcf.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birrer P, et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1994;150:207–213. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 69.Weldon S, et al. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. Baltim. Md 1950. 2009;183:8148–8156. doi: 10.4049/jimmunol.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sagel SD, Kapsner R, Osberg I, Sontag MK, Accurso FJ. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am. J. Respir. Crit. Care Med. 2001;164:1425–1431. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 71.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sly PD, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 73.Ordonez CL, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 74.Waters VJ, et al. Factors associated with response to treatment of pulmonary exacerbations in cystic fibrosis patients. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015;14:755–762. doi: 10.1016/j.jcf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Sagel SD, et al. Effect of treatment of cystic fibrosis pulmonary exacerbations on systemic inflammation. Ann. Am. Thorac. Soc. 2015;12:708–717. doi: 10.1513/AnnalsATS.201410-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ratjen F, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest. 2012;142:1259–1266. doi: 10.1378/chest.12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowe SM, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chmiel JF, et al. Use of ibuprofen to assess inflammatory biomarkers in induced sputum: Implications for clinical trials in cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015 doi: 10.1016/j.jcf.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Aldallal N, et al. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;166:1248–1256. doi: 10.1164/rccm.200206-627OC. [DOI] [PubMed] [Google Scholar]
- 80.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buchanan PJ, Ernst RK, Elborn JS, Schock B. Role of CFTR, Pseudomonas aeruginosa and Toll-like receptors in cystic fibrosis lung inflammation. Biochem. Soc. Trans. 2009;37:863–867. doi: 10.1042/BST0370863. [DOI] [PubMed] [Google Scholar]
- 82.Chattoraj SS, et al. Pseudomonas aeruginosa alginate promotes Burkholderia cenocepacia persistence in cystic fibrosis transmembrane conductance regulator knockout mice. Infect. Immun. 2010;78:984–993. doi: 10.1128/IAI.01192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konstan MW, Vargo KM, Davis PB. Ibuprofen attenuates the inflammatory response to Pseudomonas aeruginosa in a rat model of chronic pulmonary infection. Implications for antiinflammatory therapy in cystic fibrosis. Am. Rev. Respir. Dis. 1990;141:186–192. doi: 10.1164/ajrccm/141.1.186. [DOI] [PubMed] [Google Scholar]
- 84.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N. Engl. J. Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 85.Konstan MW. Ibuprofen therapy for cystic fibrosis lung disease: revisited. Curr. Opin. Pulm. Med. 2008;14:567–573. doi: 10.1097/MCP.0b013e32831311e8. [DOI] [PubMed] [Google Scholar]
- 86.Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst. Rev. 2013;6:CD001505. doi: 10.1002/14651858.CD001505.pub3. [DOI] [PubMed] [Google Scholar]
- 87.Konstan MW, et al. Trends in the use of routine therapies in cystic fibrosis: 1995–2005. Pediatr. Pulmonol. 2010;45:1167–1172. doi: 10.1002/ppul.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mogayzel PJ, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 89.Zarogoulidis P, et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur. J. Clin. Pharmacol. 2012;68:479–503. doi: 10.1007/s00228-011-1161-x. [DOI] [PubMed] [Google Scholar]
- 90.Saiman L, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 91.Saiman L, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 92.Saiman L, et al. Open-label, follow-on study of azithromycin in pediatric patients with CF uninfected with Pseudomonas aeruginosa. Pediatr. Pulmonol. 2012;47:641–648. doi: 10.1002/ppul.21601. [DOI] [PubMed] [Google Scholar]
- 93.Tramper-Stranders GA, Wolfs TFW, Fleer A, Kimpen JLL, van der Ent CK. Maintenance azithromycin treatment in pediatric patients with cystic fibrosis: long-term outcomes related to macrolide resistance and pulmonary function. Pediatr. Infect. Dis. J. 2007;26:8–12. doi: 10.1097/01.inf.0000247109.44249.ac. [DOI] [PubMed] [Google Scholar]
- 94.Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J. Pediatr. 1995;126:515–523. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 95.Balfour-Lynn IM, Welch K. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst. Rev. 2014;10:CD001915. doi: 10.1002/14651858.CD001915.pub4. [DOI] [PubMed] [Google Scholar]
- 96.Stelmach I, et al. Effects of montelukast treatment on clinical and inflammatory variables in patients with cystic fibrosis. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2005;95:372–380. doi: 10.1016/S1081-1206(10)61156-8. [DOI] [PubMed] [Google Scholar]
- 97.Konstan MW, et al. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2014;13:148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaggar A, et al. Inhaled alpha1-proteinase inhibitor therapy in patients with cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015 doi: 10.1016/j.jcf.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burstein SH, Zurier RB. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009;11:109–119. doi: 10.1208/s12248-009-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]