Abstract
Diabetic polyneuropathy (DPN) refers to peripheral nerve dysfunction as a complication of diabetes mellitus. This condition is relatively common and is likely a result of vascular and/or metabolic disturbances related to diabetes. In the early or less severe stages of DPN it typically results in sensory impairments but can eventually lead to major dysfunction of the neuromuscular system. Some of these impairments may include muscle atrophy and weakness, slowing of muscle contraction, and loss of power and endurance. Combined with sensory deficits these changes in the motor system can contribute to decreased functional capacity, impaired mobility, altered gait, and increased fall risk. There is no pharmacological disease-modifying therapy available for DPN and the mainstay of treatment is linked to treating the diabetes itself and revolves around strict glycemic control. Exercise therapy (including aerobic, strength, or balance training-based exercise) appears to be a promising preventative and treatment strategy for patients with DPN and those at risk. The goal of this Physiology in Medicine article is to highlight important and overlooked dysfunction of the neuromuscular system as a result of DPN with an emphasis on the physiologic basis for that dysfunction. Additionally, we sought to provide information that clinicians can use when following patients with diabetes or DPN including support for the inclusion of exercise-based therapy as an effective, accessible, and inexpensive form of treatment.
diabetic polyneuropathy (DPN) refers generally to peripheral neural dysfunction as a complication of diabetes mellitus (DM). The Centers for Disease Control report that 29.1 million people or 9.3% of the population of the United States has been diagnosed with diabetes (21). Of this group, almost 95% are individuals with type 2 DM (21). Neuropathy is a common and costly complication of both type 1 and type 2 diabetes and DPN is estimated to occur in at least 20% of diagnosed patients (18).
In its broadest context, DPN can refer to any of a group of neuropathic conditions with relatively heterogeneous patterns of neurological involvement. These diverse patterns may include any combination of dysfunction in the sensory, motor, or autonomic nervous systems. More specifically, typical DPN is described as a chronic, symmetrical, length-dependent sensorimotor polyneuropathy, which is the condition referred to throughout this review (25, 26). Typical DPN features sensory (i.e., numbness and paresthesia) and motor (i.e., atrophy and weakness) dysfunction that progresses in a distal to proximal, or length-dependent, manner (25, 26). Academic and clinical discussion of DPN is often centered on its sensory component whereas important DPN-related impacts to the motor and neuromuscular systems, associated with more severe forms of the disease, often are underappreciated and overlooked.
DPN PATHOGENESIS
The precise mechanisms underlying the development of DPN are complex and not entirely clear. DPN is likely caused by DM-related metabolic or vascular disturbances that are not mutually exclusive and may be interrelated or synergistic. These mechanisms lead to axonal loss via retrograde degradation, as well as peripheral nerve segmental demyelination (62). Moreover, the etiologies of DPN caused by type 1 DM (T1DM) vs. type 2 DM (T2DM) may feature subtle distinctions (19). Notwithstanding, the general pathophysiology of DPN is subsequently outlined in brief and reviewed in detail elsewhere (64, 69).
Microangiopathy has been shown to develop early in DPN, and these abnormalities may relate directly to subsequent nerve dysfunction. Additionally, DM has been associated with increases in blood viscosity, impaired oxygen release from blood to tissue, and dysfunctional structural alterations of red blood cells (50, 53). Thus these combined vascular or hemodynamic changes may contribute to chronic hypoxia and ischemia resulting in nerve damage (56, 69). Metabolic disturbances related to DM also play a causal role in the development of DPN. Neuronal dysfunction or death may be triggered by chronically elevated intraneuronal glucose concentrations, which upregulate several damaging pathways (64, 69). Additionally, neuronal damage may be imparted through the formation of advanced glycated end-products (AGEs) (69); dyslipidemia (65) and impairments in neuronal interaction with various neurotrophic factors (36, 61, 74).
Key Points
DPN is a common complication of diabetes mellitus.
The precise etiology of DPN has not been completely elucidated; however, it appears a variety of dysfunctional vascular and intraneuronal metabolic processes likely underlie its development.
Despite greatly improved understanding of mechanisms underlying DPN, no treatments have been found that stop or slow its progression.
NEUROMUSCULAR PATHOPHYSIOLOGY OF DPN
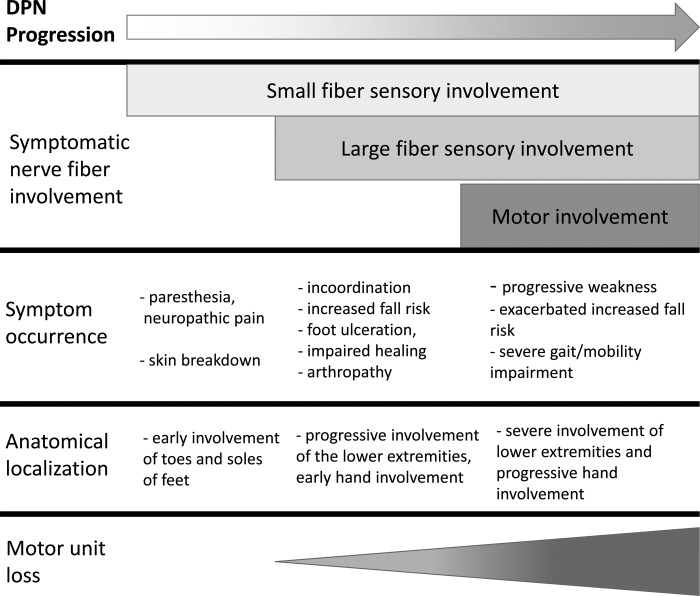
Perhaps the most impactful consequence of DPN on the neuromuscular system is the accelerated loss of motor axons or motor units (3, 4, 29, 48), which is associated with neurogenic muscle atrophy (9-13, 32) and likely occurs later in the disease process, following sensory deficits (Fig. 1). DPN-related motor unit loss appears to follow a length-dependent progression (52) and is linked with disease duration and severity (29). Reduced motor unit number estimates (MUNEs), a technique performed using electromyography (EMG), have been documented in the intrinsic muscles of the feet (29), tibialis anterior (3), and first dorsal interosseous muscles (3). The progressive loss of motor units is associated with the development of muscle weakness, muscle atrophy, and intramuscular fatty infiltration (4, 32) (Fig. 2). It is reasonable to conclude from these studies that during the course of DPN, motor axons are progressively lost and collateral reinnervation is outpaced by further denervation, ultimately leading to the death of orphaned muscle fibers. Over years in DPN patients, this process leads to a clinically detectable loss of muscle mass and muscle weakness (4, 13, 32). In addition to neurogenic muscle atrophy, several investigations have demonstrated that patients with DPN have reduced muscle quality, as assessed by strength per unit of muscle area or volume, compared with matched controls (4, 32). This exacerbates the strength loss experienced by DPN patients beyond muscle atrophy alone.
Fig. 1.
A simplified depiction of the progression of diabetic polyneuropathy in humans.
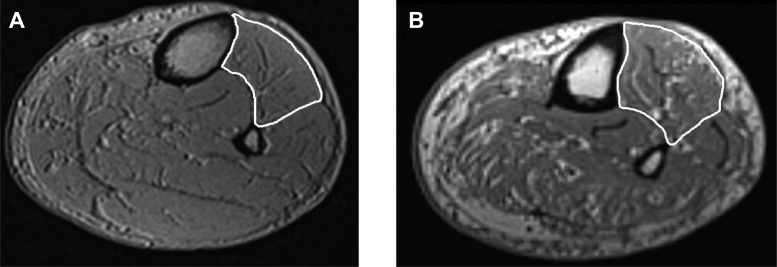
Fig. 2.
Sample MRI images of the leg in an age-matched (∼65 year old) male control (A) and diabetic polyneuropathy (DPN) patient (B; Ref 5). The anterior compartment of the leg is outlined in white. Note: the greater amounts of intramuscular fatty infiltration and noncontractile tissue found in the DPN patient leg compared with the control leg.
Additionally, there is a concomitant slowing of skeletal muscle contractile properties associated with DPN (4, 32). This has been demonstrated by reduced rates of torque development and prolonged muscle relaxation rates (4, 32). These findings may be related to a preferential loss of type 2 (fast) motor units and muscle fibers. This is supported by reduced motor unit firing rates and an impaired ability to elicit postactivation potentiation (4, 28, 67). Muscle slowing could also be caused by impairments in actomyosin cross-bridge kinetics or alterations in whole muscle morphology, including fascicular disruption, which could reduce the number of sarcomeres in series, thereby reducing contractile velocity.
Key Points
In more severe or longer duration cases of DPN, functionally relevant neuromuscular deficits manifest.
DPN may result in the progressive loss of motor units (more slowly than in other neuromuscular diseases such as amyotrophic lateral sclerosis, but more quickly than in natural adult aging), which likely occurs in an axonal, length-dependent process.
DPN patients have slowed muscle contractile properties and may experience unstable or intermittently failed neuromuscular signal transmission.
FUNCTIONAL CONSEQUENCES OF DPN-RELATED NEUROMUSCULAR ALTERATIONS
Muscle weakness.
The various insults and alterations occurring within the neuromuscular system associated with DPN have important functional consequences. A well-documented impact of DPN is muscle weakness (9–13, 13, 63) or muscle wasting that is commonly observed in the intrinsic foot muscles (13, 63) and dorsiflexors (i.e., tibialis anterior; 4, 10). However, significant weakness has also been reported in the plantar flexors and knee extensors of DPN patients (9, 13). DPN-related weakness is associated with the severity of neuropathy (10, 63), emphasizing the role of neurogenic factors. Also, not surprisingly weakness has been identified as having a close association with reduced mobility and gait speed (66). Importantly, in the early stages of DPN-related muscle weakness, the loss of strength may not be detectable using manual muscle testing alone (10).
Loss of muscle power.
In addition to weakness, there is a DPN-related loss of muscle power (work per unit time; Refs. 32, 49). Muscle power is defined as the product of muscle strength and muscle contractile velocity. The decrement in muscle power is greater than the loss of strength alone (32); thus the slowing of muscle contractile properties mentioned previously likely plays a role in power-related decrements reported in DPN patients (4, 32). Because most activities of daily living involve dynamic action (i.e., movement), loss of muscle power may have more impactful functional consequences than the loss of muscle strength alone (49, 51). Indeed, loss of muscle power in the lower limbs has been reported as a key factor underlying impairments in both balance and gait in DM patients (47) and in older adults with impaired mobility (23, 43, 51). However, standard measures of strength, including manual muscle testing and isometric dynamometry, do not provide comprehensive information regarding muscle power. Advanced dynamometers, available commercially (e.g., Biodex System 4 or CSMI Humac Norm), with isokinetic- and isotonic-modes are useful in assessing muscle power as well as examining muscle force generating capabilities across the entire range of motion in most major muscles groups (17, 32, 43). Previous studies have demonstrated dorsiflexion power loss is associated with the loss of tibialis anterior motor units in adult aging (43) and deficits in knee extensor power are a stronger predictor of functional ability than knee extensor strength in knee osteoarthritis (17). However, unfortunately these advanced dynamometers are costly, time consuming, and often inaccessible to many clinicians. Performance in clinical tests of functional capacity such as the timed up and go test or the shuttle walk test may be relatively preserved in DPN patients who feature early motor dysfunction only in distal muscles. Thus, at this time, clinically feasible and useful assessments of muscle power, particularly in distal muscle groups such as the dorsiflexors and plantar flexors, remain limited.
Changes in muscle fatigability.
DPN may also cause changes in neuromuscular endurance or fatigability, although those changes may depend on the severity of neuropathy and the specific fatigue task (6, 7, 9). One fatigue study examining muscle endurance in insulin-dependent DM without any clinical signs of DPN found a substantial decrease (approximately −50%) in knee extensor muscle time to fatigue during a sustained isometric task compared with controls (7). Reduced endurance of the DM group in that study was related to lower motor unit firing rates and reduced neuromuscular transmission velocity. Furthermore, a more recent study using a sustained, maximal, isometric contraction to induce fatigue, found DPN patients had significantly reduced neuromuscular endurance despite equally high levels of voluntary activation (6). Interestingly, endurance capacity under sustained high-intensity contractions may be linked to potential neuromuscular transmission failure in patients with relatively severe DPN (5, 39). Additionally, changes in muscle metabolism and blood flow associated with DM may play a role in altered fatigability. For example, DM has been associated with slowed oxygen kinetics and faster: PCr loss, pH decrease, and muscle deoxygenation relative to controls (50). Furthermore, DM is closely linked with dysfunction in microvasculature as well as slowed oxygen kinetics during exercise (16, 40), both of which could reduce skeletal muscle oxidative metabolism and adversely affect endurance.
Increased fall risk.
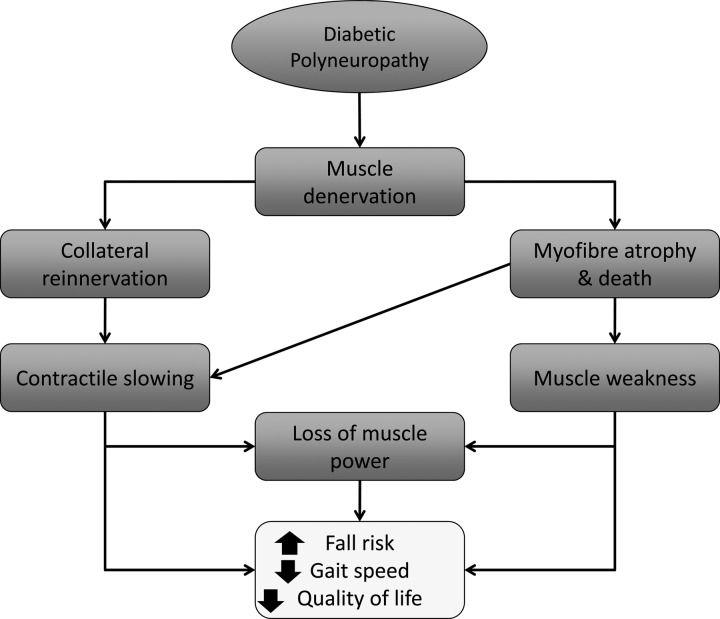
Loss of muscle strength, speed, power, and endurance, particularly in lower limb muscles, may synergistically reduce functional capacity and contribute to altered gait, increased fall risk, and impaired balance in patients with DPN (Fig. 3). Additionally, neuromuscular control may be impaired or altered via the loss of motor units and increased average motor unit size, disturbing normal, orderly motor unit recruitment. When motor units are lost, and others enlarged, there may be a reduced ability to finely grade force output compared with healthy controls. This impairment, in conjunction with DPN-related sensory and proprioceptive deficits (62), may further contribute to altered gait, which is associated with an increased fall risk (44). Moreover, an increased fall risk is an especially important concern for DPN patients as their falls are substantially more likely to be injurious, and lead to bone fractures, poorly healing wounds, and chronic infections (33, 54, 55). Therefore, patient education and regular proper foot care is critical to reduce the risk of these debilitating and costly complications.
Fig. 3.
The neuromuscular and functional motor consequences of diabetic polyneuropathy.
Key Points
In some cases of DPN, the progressive loss of motor units may lead to muscle atrophy and functionally significant weakness.
Loss of muscle power (force per unit time) and endurance in DPN may have more important functional consequences than the development of weakness alone.
Weakness and loss of power, in combination with sensory deficits, synergistically lead to altered gait, impaired balance, and increased fall risk in DPN, which can have extremely debilitating consequences such as fractures, ulceration, and amputation.
NEUROMUSCULAR DYSFUNCTION IN DPN: PATHOPHYSIOLOGICAL BASIS FOR PREVENTION AND TREATMENT
Preventative strategies and early interventions are the mainstay of treatment for both DM and DPN (8, 60). Such strategies include aggressive glycemic control, as well as management of blood pressure and serum lipid levels using standard pharmacological therapy and lifestyle modifications (18, 19). Many patients with DM may have early, undiagnosed, or yet to be diagnosed neuropathy (19). These patients may be at greater risk for skin breakdown, ulceration, and other early complications of diabetic foot dysfunction, due to sensation loss or alteration (1). However, those in the early stages of DPN are unlikely to incur dysfunction related to large fiber sensory afferent or motor fibers such as significant proprioceptive issues including falls, muscle weakness, or gait impairment. Although quantitative EMG and MUNE, in addition to quantitative dynamometry, may not be applicable to early sensory changes in diabetic neuropathy, they may be useful measures of progressive disability in diabetes, particularly those individuals with severe disability (3). Another potentially useful assessment for identifying and following patients with DPN may include electromyographic measures of peripheral nerve excitability (14, 38, 39). These measures provide assessment of the degree of excitability of a nerve and are described in detail elsewhere (14, 38, 39). Excitability studies have shown DPN is associated with shortened nerve refractory periods and decreased excitability based on the minimal current needed to depolarize a motor axon (34, 38, 39, 42). These measures have shown subtle differences in early DPN and thus may warrant further investigation as a possible tool in the early detection of progressive motor dysfunction.
Diabetic Neuropathy, the Neuromuscular System, and Exercise
Treatment options directed towards improvement of DPN are limited and have focused on pharmacological and dietary strategies linked to strict glycemic control as mentioned above (8). However, there is evidence of the benefits of exercise training-based interventions in treating and preventing the development or progression of DPN (46, 58). These may be related to exercise-related improvements in glycemic control, as well as other more direct effects on the neuromuscular system. Indeed, several reports suggest aerobic, strength, and balance training regimens all can prove effective in management of DPN (see Ref. 58 for brief review). Integrating these disparate investigations into specific and actionable clinical knowledge remains challenging due to the variety of patient populations, exercise modalities, length of interventions, and outcome measures used. Even so, it does appear that overall, increased physical activity levels and exercise training regimens have positive impacts on DPN.
One longitudinal study conducted over 4 yr found DM patients who engaged in a low-intensity aerobic exercise program (i.e., brisk treadmill walking) were less likely to develop clinical signs of DPN than DM patients who did not partake in the exercise program (15). The exercise group maintained normal motor nerve conduction velocity and vibration sensation, whereas the control group showed relative deterioration in those same measures. Interestingly, there were no differences between the exercise and control group in body mass index, waist circumference, or metabolic profile perhaps due to the low intensity. Thus the authors speculated the effect of exercise on DPN is more complex than simply related to improved glycemic control (15).
Another report showed DPN patients in a combined strength and endurance exercise regimen of moderate intensity had improved clinical symptoms of neuropathy and improved cutaneous nerve branching as assessed by biopsy after only 10 wk of moderate-intensity training (37). No changes were detected in the motor nervous system, although the patients included in the study did not feature severe motor involvement at recruitment. Some studies investigating the effect of more intense strength training regimens based on models of high-intensity progressive resistance training reported large (30–60%) increases in strength, as well as increases in muscle quality in patients with DM (see Ref. 46 for review). How such training programs affect individuals with severe DPN remains unclear. Several investigations have also assessed the effects of balance training (e.g., tai chi) on patients with DPN (2, 35, 41, 57). These studies showed improvements in balance and gait, as well as improved motor, sensory, and metabolic symptoms of DPN (2, 35, 41, 57). It is important to note that all of the studies discussed above concluded exercise of different modalities and intensities is safe for patients with DPN, with only minor and infrequent adverse effects reported (i.e., strained muscle). However, caution should be taken, especially for patients with DPN who may have other complications (e.g., ulcers) or co-morbidities (e.g., cardiac disease). For example, the patient with foot ulceration or major lower limb sensory loss should likely minimize chronic, repetitive, weight-bearing exercise to avoid exacerbating that condition.
Exercise training can indirectly treat or prevent DPN by contributing to the maintenance of healthy glycemic control (see Ref. 22 for review). This is the most discussed and best understood mechanism by which aerobic or strength training can impact DM, and by extension, DPN. Exercise can improve glycemic control primarily via increased insulin sensitivity, increased muscle mass, and increased substrate utilization (8, 20). However, exercise may be beneficial to DPN patients, and those at risk of developing DPN, through more direct mechanisms related to neuromuscular health. This could include upregulation of various neurotrophic factors (e.g., BDNF, IGF; Refs. 24, 27, 31), improved nerve blood flow (45), and improved neuronal resiliency related to intracellular metabolic stressors such as inflammation (30, 68). Although further research is necessary to uncover the precise physiological mechanisms underlying exercise-related benefits to neuromuscular health in DPN, at this time it appears that chronic exercise represents an accessible and inexpensive intervention for individuals with DPN.
Key Points
Strict glycemic control remains the primary goal in the treatment of DPN.
More detailed assessment of DPN-related neuromuscular progression via quantitative strength testing, motor unit number estimates, or nerve excitability may be helpful in tracking and managing the disease.
Cardiovascular and strength training regimens are both likely to impart meaningful beneficial effects to DPN patients through improvements in glycemic control and direct effects on the neuromuscular system.
SUMMARY AND CONCLUSIONS
Severe DPN has debilitating impacts on the neuromuscular system that can be underappreciated clinically. The assessment of DPN would benefit from more precise methods for diagnosis and prognostication. Advances in electrophysiological techniques may have the potential to provide greater information regarding DPN and its motor system impacts as well as the effects of different interventions. Finally, patients with DPN appear to benefit from exercise regimens both via a decreased severity of diabetes but also potentially through direct effects on peripheral nerves and skeletal muscle. Both cardiovascular training and strength training should be recommended. Further research should aim to determine which exercise interventions (i.e., duration, frequency, intensity, and strength vs. aerobic regimens) are most effective in attenuating the progression of DPN given a patient's status and risk factors.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.D.A., T.J.D., C.L.R., and K.K. conception and design of research; M.D.A. and K.K. prepared figures; M.D.A., T.J.D., C.L.R., and K.K. drafted manuscript; M.D.A., T.J.D., C.L.R., and K.K. edited and revised manuscript; M.D.A., T.J.D., C.L.R., and K.K. approved final version of manuscript.
REFERENCES
- 1.Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 21: 1071–1075, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev 49: 333–338, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Allen MD, Kimpinski K, Doherty TJ, Rice CL. Length dependent loss of motor axons and altered motor unit properties in human diabetic polyneuropathy. Clin Neurophysiol 125: 836–843, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Allen MD, Major B, Kimpinski K, Doherty TJ, Rice CL. Skeletal muscle morphology and contractile function in relation to muscle denervation in diabetic neuropathy. J Appl Physiol 116: 545–555, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen MD, Stashuk D, Kimpinski K, Doherty TJ, Hourigan ML, Rice CL. Increased neuromuscular transmission instability and motor unit remodeling with diabetic neuropathy as assessed using novel near fibre motor unit potential parameters. Clin Neurophysiol 126: 7947–80, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Allen MD, Kimpinski K, Doherty TJ, Rice CL. Decreased muscle endurance associated with diabetic neuropathy may be attributed partially to neuromuscular transmission failure. J Appl Physiol 118: 1014–1022, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida S, Riddell MC, Cafarelli E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve 37: 231–240, 2008. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 37, Suppl 1: S14–80, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Andersen H. Muscular endurance in long-term IDDM patients. Diabetes Care 21: 604–609, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Andersen H, Stålberg E, Gjerstad MD, Jakobsen J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve 21: 1647–1654, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia 40: 1062–1069, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care 27: 2382–2385, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Andreassen C, Jakobsen J, Andersen H. Muscle weakness a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 55: 806–812, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Bae JS, Kim OK, Kim JM. Altered nerve excitability in subclinical/early diabetic neuropathy: evidence for early neurovascular process in diabetes mellitus? Diabetes Res Clin Pract 91: 183–189, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Balducci Iacobellis SG, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J. Diabetes Complications 20: 216–223, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Bauer T, Reusch J. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes 30: 2880–288, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Berger MJ, McKenzie CA, Chess DG, Goela A, Doherty TJ. Quadriceps neuromuscular function and self-reported functional ability in knee osteoarthritis. J Appl Physiol 113: 255–262, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D; American Diabetes Association . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28: 956–962, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 13: CD007543, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 25: 2335–2341, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014 (Online). http://www.cdc.gov/diabetes [2014]. [Google Scholar]
- 22.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B; American College of Sports Medicine; American Diabetes Association . Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 33: 2692–2696, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled men and women. J Gerontol 59: 1200–1206, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140: 823–833, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Dyck PJ, Albers JW, Andersen H; Toronto Expert Panel on Diabetic Neuropathy . Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 37: 620–628, 2011. [DOI] [PubMed] [Google Scholar]
- 26.England JD, Bronseth GS, Franklin G, Miller RG, Ashbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella MD, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetric polyneuropathy: a definition for clinical research. Neurology 64: 199–207, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125: 129–139, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiol Scand 178: 165–173, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Hansen S, Ballantyne JP. Axonal dysfunction in the neuropathy of diabetes mellitus: a quantitative electrophysiological study. J Neurol Neurosurg Psychiatry 40: 555–564, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Sumpter R, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 8: 1548–1551, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellweg R, Raivich G, Hartung H, Christoph H, Kreutzberg GW. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol 130: 24–30, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 88: 1336–1344, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horlings CG, van Engelen BG, Allum JH, Bloem BR. A weak balance: the contribution of muscle weakness to postural instability and falls. Nat Clin Pract Neurol 4: 504–515, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Horn S, Quasthoff S, Grafe P, Bostock H, Renner R, Schrank B. Abnormal axonal inward rectification in diabetic neuropathy. Muscle Nerve 19: 1268–1275, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Hung JW, Liou CW, Wang PW, Yeh SH, Lin LW, Lo SK, Tsai FM. Effect of a 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med 41: 924–929, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab 23: 133–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma NK, Wright DE. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications 26: 424–229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan AV, Kiernan MC. Altered nerve excitability properties in established diabetic neuropathy. Brain 128, 1178–1187, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan AV, Lin CS, Kiernan MC. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain 131: 1209–1216, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med 231: 305–314, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Mackel R, Brink E. Conduction of neural impulses in diabetic neuropathy. Clin Neurophysiol 114: 248–255, 2003. [DOI] [PubMed] [Google Scholar]
- 43.McKinnon NB, Montero-Odasso M, Doherty TJ. Motor unit loss is accompanied by decreased peak muscle power in the lower limb of older adults. Exp Gerontol 70: 111–118, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Meier MR, Desrosiers J, Bourassa P, Blaszczyk J. Effect of type II diabetic peripheral neuropathy on gait termination in the elderly. Diabetologia 44: 585–592, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Olver TD, Grisé KN, McDonald MM, Dey A, Allen MD, Medeiros PJ, Lacefield JC, Jackson DN, Rice CL, Melling CW, Noble EB, Shoemaker JK. Exercise training preserves insulin-stimulated nerve vasodilation in rats with insulin-treated experimental diabetes. Am J Physiol Regul Integr Comp Physiol 306: R941–R950, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Orlando G, Balducci S, Bazzucchi I, Pugliese G, Sacchetti M. Neuromuscular dysfunction in type 2 diabetes: underlying mechanisms and effect of resistance training. Diabetes Metab Res Rev 32: 40–50, 2016. [DOI] [PubMed] [Google Scholar]
- 47.Orr R, Tsang T, Lam P, Comino E, Sing MF. Mobility impairment in type 2 diabetes: association with muscle power and effect of Tai Chi intervention. Diabetes Care 29: 2120–2122, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Ramji N, Toth C, Kennedy J, Zochodne DW. Does diabetes mellitus target motor neurons? Neurobiol Dis 26: 301–311, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus skeletal muscle contracting at moderate intensity. J Appl Physiol 85: 310–317, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Ringsberg K, Gerdhem P, Johansson J, Obrant KJ. Is there a relationship between balance, gait perforamnce and muscular strength in 75-year-old women? Age Ageing 28: 289–293, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Said G, Slama G, Selva J. Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy: a clinical and pathological study. Brain 106: 791–807, 1983. [DOI] [PubMed] [Google Scholar]
- 53.Schmid-Schonbein H, Volger E. Red-cell aggregation and red-cell deformability in diabetes. Diabetes 25: 897–902, 1976. [PubMed] [Google Scholar]
- 54.Schwartz A, Vittinghoff E. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes 31: 1147–1152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz AV. Diabetes mellitus: does it affect bone? Calcified Tissue Int 73: 515–519, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Sone H, Mizuno S, Yamada N. Vascular risk factors and diabetic neuropathy. N Engl J Med Med. 352: 1925–1927, 2005. [PubMed] [Google Scholar]
- 57.Song CH, Petrofsky JS, Lee SW, Lee KJ, Yim JE. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther 13: 803–811, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Streckmann F, Zopf EM, Lehmann HC, Kathrin M, Rizza J, Zimmer P, Gollhofer A, Bloch W, Baumann FT. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med 44: 1289–1304, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki T, Bean J, Fielding R. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc 41: 1161–1167, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Tesfaye S, Boulton AJ, Dyck PJ; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33: 2285–2293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes 46, Suppl 2: S43–49, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther 29: 718–726, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Van Schie CH, Vermigli C, Carrington AL, Boulton A. Muscle weakness and foot deformities in diabetes: relationship to neuropathy and foot ulceration in caucasian diabetic men. Diabetes Care 27: 1668–1673, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 7: 573–583, 2011. [DOI] [PubMed] [Google Scholar]
- 65.Vincent AM, Hinder LM, Pop-busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 14: 257–267, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volpato S, Bianchi L, Lauretani F. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes 35: 1672–1679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe K, Gazzoni M, Holobar A, Miyamoto T, Fukuda K, Merletti R, Moritani T. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve 48: 806–813, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Yoon H, Thakur V, Isham D, Fayad M, Chattopadhyay M. Moderate exercise training attenuates inflammatory mediators in DRG of type 1 diabetic rats. Exp Neurol 267: 107–114, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve 36: 144–166, 2007. [DOI] [PubMed] [Google Scholar]