We show that deliberate elevations in operating lung volumes during interval exercise do not prevent bronchoconstriction when workrate is reduced in asthmatic adults. In our subjects, exercise airway caliber fluctuated with changing workrate both when subjects ventilated spontaneously and when tidal volume and end-inspiratory lung volume were voluntarily and substantially increased at the lower of two exercise workloads. Thus exercise airway function behaved independently from lung stretch and oscillations in lung stretch.
Keywords: airway caliber, asthma, bronchodilation, exercise, lung stretch
Abstract
In asthmatic adults, airway caliber fluctuates during variable intensity exercise such that bronchodilation (BD) occurs with increased workrate whereas bronchoconstriction (BC) occurs with decreased workrate. We hypothesized that increased lung mechanical stretch would prevent BC during such variable workrate exercise. Ten asthmatic and ten nonasthmatic subjects completed two exercise trials on a cycle ergometer. Both trials included a 28-min exercise bout consisting of alternating four min periods at workloads equal to 40 % (Low) and 70% (High) peak power output. During one trial, subjects breathed spontaneously throughout exercise (SVT), such that tidal volume (VT) and end-inspiratory lung volume (EILV) were increased by 0.5 and 0.6 liters during the high compared with the low workload in nonasthmatic and asthmatic subjects, respectively. During the second trial (MVT), VT and EILV were maintained constant when transitioning from the high to the low workload. Forced exhalations from total lung capacity were performed during each exercise workload. In asthmatic subjects, forced expiratory volume 1.0 s (FEV1.0) increased and decreased with the increases and decreases in workrate during both SVT (Low, 3.3 ± 0.3 liters; High, 3.6 ± 0.2 liters; P < 0.05) and MVT (Low, 3.3 ± 0.3 liters; High, 3.5 ± 0.2 liters; P < 0.05). Thus increased lung stretch during MVT did not prevent decreases in airway caliber when workload was reduced. We conclude that neural factors controlling airway smooth muscle (ASM) contractile activity during whole body exercise are more robust determinants of airway caliber than the ability of lung stretch to alter ASM actin-myosin binding and contraction.
NEW & NOTEWORTHY
We show that deliberate elevations in operating lung volumes during interval exercise do not prevent bronchoconstriction when workrate is reduced in asthmatic adults. In our subjects, exercise airway caliber fluctuated with changing workrate both when subjects ventilated spontaneously and when tidal volume and end-inspiratory lung volume were voluntarily and substantially increased at the lower of two exercise workloads. Thus exercise airway function behaved independently from lung stretch and oscillations in lung stretch.
bronchodilation (BD) occurs during whole body exercise in both nonasthmatic and asthmatic persons, although the magnitude of this BD is considerably greater in the asthmatic with baseline airway obstruction (10, 16, 20, 22, 30, 46). In the asthmatic with baseline airway narrowing, the exercise BD is a particularly critical response that allows for increased airflow during the exercise (10, 16, 37). Following the initial exercise-induced BD in the asthmatic, bronchoconstriction (BC) has been shown to occur during exercise when workrate is reduced (5, 20). Furthermore, subsequent increases in workrate once again cause BD. Thus BD is the initial default response during exercise in the asthmatic, but the airways may narrow or dilate thereafter depending on exercise intensity. The physiological mechanisms accounting for these complicated airway responses to exercise in the asthmatic are not known.
Based on the known coupling between lung volume (VL) and airway diameter (8, 26), it is reasonable to suggest that changes in operating VL might regulate, at least in part, airway caliber during exercise in the asthmatic. The airways are embedded within the lung parenchymal tissues, which impart an outward acting force, referred to as radial traction or tethering, on the airways. This tethering is a key component of the sum of forces that determine airway caliber at any given instant (29). Indeed, recent imaging studies and application of the forced oscillation technique have shown a clear influence of VL on airway lumen diameter in both nonasthmatic and asthmatic adults (6, 8, 25). Furthermore, in nonasthmatic and asthmatic subjects with pharmacologically induced airway narrowing (e.g., methacholine), both a single deep inhalation to total lung capacity or several such inhalations dilate the airways in both subject groups (18, 38).
During exercise, tidal volume (VT) and end-inspiratory lung volume (EILV) increase in proportion to exercise workload. These increases in operating VL and the corresponding increase in lung stretch should dilate the airways due to the augmented radial traction. Likewise, reductions in exercise intensity are met with decreases in both VT and EILV, which should theoretically decrease airway caliber due to a reduced mechanical load applied to the airways. Therefore, changes in operating VL, and the consequent changes in lung stretch, during variable intensity exercise might contribute to both the BD and BC that occur during such exercise in the asthmatic.
The purpose of this study was to determine whether changes in VT and EILV (i.e., variations in lung stretch) are responsible for variations in airway caliber during interval exercise in the mild-to-moderate asthmatic adult. We hypothesized that, due to the forces of interdependence between the airways and parenchyma, increased VT and EILV will prevent BC during variable intensity exercise when workrate is reduced. To test this hypothesis, asthmatic and nonasthmatic subjects completed two variable workrate exercise studies. During one study, subjects breathed spontaneously throughout the interval exercise. During the second study, breathing was deliberately controlled such that VT and EILV were increased at the low-intensity workloads.
METHODS
Subjects
Ten adult male and female asthmatic subjects and ten nonasthmatic subjects were recruited through advertisements in local newspapers and by flyers posted on the Johnson State College campus and in the community. Before participation, subjects were informed of the procedures, risks, and benefits of the study. Written informed consent was subsequently obtained. The experiments described in this manuscript were approved by the Johnson State College Institutional Review Board for research involving human subjects.
All participants were nonsmokers between the ages of 18 and 45 yr with a negative history for cardiovascular, metabolic, and pulmonary disease, other than asthma. All subjects had an absence of respiratory infection during the 4 wk before participation. All asthmatic subjects had a previous clinician diagnosis of asthma. Based on a clinical history, responses to Juniper's Asthma Control Questionnaire (21), and pulmonary function values via spirometry, disease severity in the asthmatic subjects represented a continuum between “intermittent” and “moderate-persistent” disease that was either “well-controlled” or “not well-controlled” at the time of study (45). Several asthmatic subjects had a prescriptive history for antihistamines, inhaled steroids, or nasal steroids; however, none were using these medications at the time of their participation in the studies. A total of 9/10 asthmatic subjects used a short-acting β2-agonist on an as-needed basis; these subjects were instructed to refrain from their use for 12 h before each study. All nonasthmatic and asthmatic subjects were instructed to refrain from ingesting any caffeinated food or beverages for 8 h before each study.
Screening Studies
All subjects completed three separate screening studies on different days to determine eligibility for participation as an asthmatic or nonasthmatic subject.
Bronchodilator reversibility.
Following baseline pulmonary function tests (PFT), subjects inhaled four actuations of a fast-acting β2-agonist. For each actuation, subjects exhaled to residual volume, depressed the actuator, inhaled slowly through a holding chamber (Aerochamber) to total lung capacity, and held their breath for 5 s before exhaling. Pulmonary function was assessed at several time points following β2-agonist inhalation.
Eucapnic voluntary hyperpnea challenge.
Following baseline PFTs, subjects completed a eucapnic voluntary hyperpnea (EVH) challenge according to previously published methods (1). Briefly, subjects ventilated dry gas from a compressed gas tank (21% O2, 5% CO2, balance N) for 6 min at a target ventilation rate equal to forced expiratory volume 1.0 s × 30. Pulmonary function was assessed serially following hyperpnea, and the lowest value was used to determine the maximal airway narrowing response to the hyperpnea.
Maximal exercise test.
Subjects completed a maximal incremental exercise test on a cycle ergometer. The test consisted of 2-min stages with the initial workload set at 60 watts for females and 65 watts for males; workload was increased by 30 and 35 watts per stage for females and males, respectively. The purposes of this study were twofold: 1) provide familiarization with exercise on the cycle ergometer and with the other apparati used during the experimental trials; and 2) determine each subject's exercise workloads for the two experimental trials.
To qualify as an asthmatic, subjects were required to meet one or both of the following two criteria: 1) >12% increase in forced expiratory volume 1.0 s (FEV1.0) after inhalation of the fast-acting β2-agonist; and 2) >25% decrease in FEV1.0 after the EVH challenge. Those not meeting these criteria completed the studies as nonasthmatic subjects.
Experimental Design
All nonasthmatic and asthmatic subjects completed two experimental exercise studies separated by at least 48 h. Both experimental studies included the same 28-min exercise protocol on a cycle ergometer. The protocol consisted of alternating four min exercise periods at 40 (Low) and 70% (Hi) of the peak workload obtained during the maximal exercise test. The entire protocol consisted of four stages at the low workload interspersed with three stages at the high workload (i.e., Low-1, Hi-1, Low-2, Hi-2, Low-3, Hi-3, and Low-4). As described below, the two trials differed only in how subjects ventilated during the exercise.
Experimental Exercise Studies
All subjects completed two experimental exercise studies. During the first study, subjects breathed spontaneously throughout the exercise protocol [spontaneous ventilation trial (SVT)]. During the second study, subjects maintained VT and breathing frequency (fb) constant while exercising at the low exercise workloads following each high workload period [maintained ventilation trial (MVT)]. To control VT, a computer monitor with a real-time volume trace was placed in front of the subject with the goal VT clearly marked by a piece of tape; subjects voluntarily controlled inspiration to achieve the required volume. Breathing frequency was controlled by a computerized metronome with the duty cycle set at 0.5. To prevent hypocapnia during the MVT trial, 5 or 10% CO2 (21% O2, balance N) was added to the inspiratory tubing at a rate that maintained end-tidal CO2 constant (33). To prevent airway drying and its potential for provoking BC, the compressed gas was saturated with water by first passing through a water bath before entering the inspiratory tubing.
Following the first and third minutes of each 4-min exercise workload, subjects performed a maximal inhalation to total lung capacity (TLC) followed immediately by a maximal forced exhalation. Subjects were instructed to exhale as forcefully as possible for at least 3 s and to resume spontaneous or controlled breathing immediately thereafter. Subjects were regularly reminded to fill their lungs completely and to exhale as forcefully as possible during each maneuver.
Exercise Apparatus and Measurements
Exercise was completed on a magnetically braked cycle ergometer (Velotron). Subjects breathed through a two-way, nonrebreathing valve (Hans-Rudolph) with nose clips in place. Separate Fleisch pneumotachographs (Hans Rudolph) were used to determine inspiratory and expiratory flow-rates. Separate oxygen and carbon dioxide gas analyzers (AEI Technologies) were used to measure expired gases. An additional CO2 analyzer (VacuMed) was used to continuously monitor end-tidal CO2 (ETCO2) during the MVT trial. A Powerlab 16-channel data acquisition system (ADinstruments) interfaced with a laptop computer was used to collect exercise data.
Inspired and expired airflow rates and expired gases were used to calculate metabolic oxygen consumption (V̇o2) and carbon dioxide production (V̇co2), minute ventilation (V̇e), VT, fb, total breathing cycle time (Ttot), and inspiratory duty cycle (TI/TTot). Inspiratory capacity (IC), FEV1.0, and peak expiratory flow (PEF) were determined from each forced exhalation maneuver completed after the first and third minutes of each exercise workload. EILV was calculated as [vital capacity-(IC-VT)]. A Polar heart rate monitor (POLAR) was used to record heart rate during exercise.
Pulmonary Function
Resting pulmonary function (Medgraphics) was completed in the seated, upright position according to recommendations by the American Thoracic Society and European Respiratory Society (31). During each measurement, subjects completed maximal volitional flow-volume maneuvers for determination of forced vital capacity (FVC), FEV1.0, PEF, and forced expiratory flow at 50% of FVC (FEF50%). Predicted values are from Quanjer (36).
Statistical Analysis
A three-factor [GROUP (asthmatic vs. nonasthmatic) × TRIAL (SVT vs. MVT) × WORKLOAD (Low-1, Hi-1, Low-2, Hi-2, etc.)] repeated-measures ANOVA was used to analyze the exercise responses. When significant main effects were found, Bonferroni-corrected multiple pairwise comparisons were employed for post hoc analysis. Least squares linear regression was used to analyze relationships between selected variables. Tabular data are presented as means ± SD. Data in figures are presented as means ± SE. Significance was set at α < 0.05. The statistical software program SYSTAT (version 12) was used to analyze all data.
RESULTS
Descriptive characteristics, selected results from the maximal exercise test, and results from the two inclusion criteria studies assessing airways responsiveness are shown in Table 1. The groups were similarly matched for age, height, and exercise capacity. Baseline pulmonary function was lower in asthmatic compared with nonasthmatic subjects (FEV1.0/FVC P = 0.003; FEF50% P = 0.015). The increase in FEV1.0 following inhalation of β2-agonist (P = 0.004) and the decrease in FEV1.0 following the EVH challenge were greater (P = 0.004) in asthmatic compared with nonasthmatic subjects. Thus baseline pulmonary function was compromised in the asthmatic subjects and they exhibited significantly greater airways responsiveness than the nonasthmatic subjects.
Table 1.
Descriptive characteristics and inclusion criteria results for nonasthmatic and asthmatic subjects
| Nonasthmatic Group | Asthmatic Group | |
|---|---|---|
| Male/female | 7/3 | 9/1 |
| Age, yr | 24.8 ± 7.4 | 28.7 ± 7.3 |
| Height, m | 1.72 ± 0.11 | 1.75 ± 0.07 |
| Weight, kg | 70.3 ± 14.2 | 84.0 ± 19.1 |
| V̇o2peak, ml·kg−1·min−1 | 46.9 ± 8.2 (116 ± 17%) | 47.9 ± 13.2 (106 ± 28%) |
| Peak power, watts | 217 ± 43 | 255 ± 33* |
| Peak power, watts/kg | 3.1 ± 0.5 | 3.2 ± 0.8 |
| FVC, liters | 4.8 ± 1.1 (104 ± 11) | 5.3 ± 1.0 (109 ± 14) |
| FEV1.0, liters | 3.9 ± 0.7 (100 ± 11) | 3.7 ± 0.7 (90 ± 12) |
| FEV1.0/FVC | 82.3 ± 7.5 | 70.1 ± 8.6* |
| FEF50%, l/s | 4.4 ± 1.3 (87 ± 28) | 3.1 ± 1.0 (58 ± 16)* |
| PEF, l/s | 9.1 ± 2.0 (103 ± 21) | 8.9 ± 1.5 (94 ± 10) |
| Asthma inclusion criteria | ||
| β2-Agonist reversibility, FEV1.0 % | +5.8 ± 4.6 | +16.6 ± 8.9* |
| EVH, FEV1.0 %change | −9.0 ± 6.9 | −32.1 ± 19.1* |
Values are means ± SD. EVH, eucapnic voluntary hyperpnea challenge; FEF50%, forced expiratory flow at 50% FVC; FEV1.0, forced expiratory volume 1.0 s; FVC, forced vital capacity; PEF, peak expiratory flow. Percent predicted values in parentheses.
P < 0.05, significant difference between nonasthmatic and asthmatic subjects.
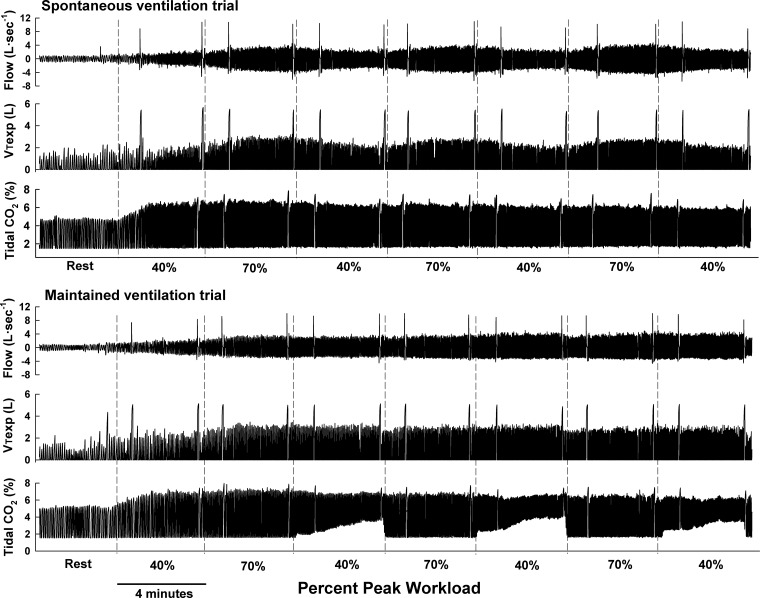
Figure 1 depicts airflow, expiratory VT, and tidal CO2 at rest and throughout the exercise protocol for the SVT and MVT in one asthmatic subject. Note the expected fluctuations in airflow and VT with changing workrate during SVT. Conversely, during MVT, airflow and VT remained constant at the low workloads following each high-intensity workload, as per the study design. Also note the maintained ETCO2 while exercise was performed at the low workload during MVT. Finally, the spikes in airflow and VT indicate the forced exhalation maneuvers used to determine FEV1.0 and PEF.
Fig. 1.
Breath-by-breath airflow, expiratory tidal volume (VTexp), and tidal CO2 during spontaneous (SVT) and maintained (MVT) ventilation trial in 1 asthmatic subject. Vertical dashed lines indicate changes in exercise workrate. Spikes in airflow and VTexp indicate the forced exhalations at the beginning and end of each 4-min exercise workload. During SVT, airflow and VT increased and decreased (as expected) with increases and decreases in workrate. During MVT, airflow and VT were stable despite the changing workrate. End-tidal CO2 was maintained constant at the low-intensity exercise periods during MVT by adding 5% CO2 to the inspirate; note the concomitant increases in inspiratory CO2 at the low-intensity workloads during the MVT trial.
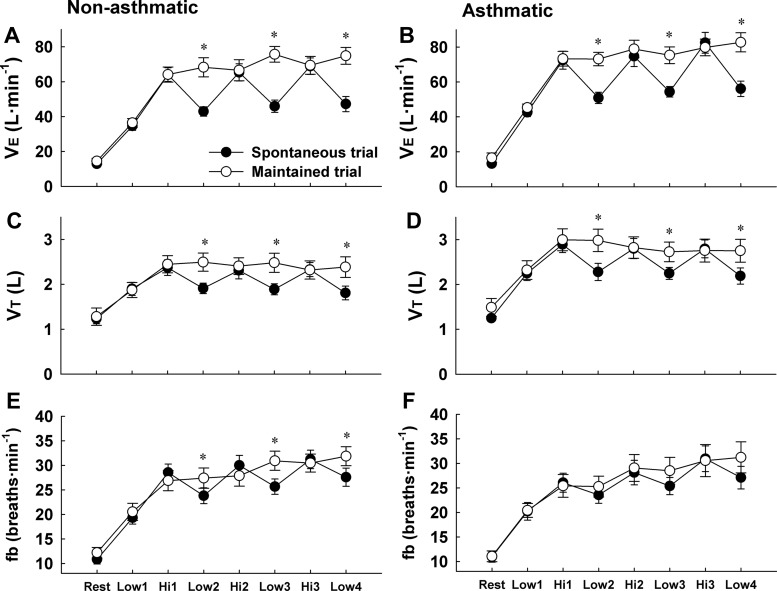
Ventilation and Breathing Pattern
During SVT, V̇e, VT and fb fluctuated similarly with exercise workload in both subject groups (Fig. 2). The average difference in V̇e between the low and high workload was 24 ± 7 (SD) and 27 ± 9 l/min in nonasthmatic and asthmatic subjects, respectively (P < 0.05 for both groups). Similarly, the average difference in VT between the workloads was 0.46 ± 0.24 (SD) and 0.58 ± 0.27 liters in nonasthmatic and asthmatic subjects (P < 0.05 for both groups). During MVT, all ventilatory variables were maintained nearly constant during the transitions from the high to the low workload in both subject groups. During low-intensity exercise, VT was 131 and 126% higher during MVT relative to SVT in nonasthmatic and asthmatic subjects [+0.6 ± 0.3 (SD) and 0.6 ± 0.4 liters], respectively (P < 0.05).
Fig. 2.
Minute ventilation (V̇e), tidal volume (VT), and breathing frequency (fb) in nonasthmatic (A, C, and E) and asthmatic subjects (B, D, and F) during spontaneous (SVT) and maintained (MVT) ventilation trials. During SVT, V̇e, VT, and fb fluctuated with the changing exercise workload in both subject groups. During MVT, VT and fb, and thus V̇e, were maintained constant during the final 3 low-intensity workloads (i.e., Low-2, Low-3, Low-4). There were no differences between nonasthmatic and asthmatic subjects. *P < 0.05 vs. SVT trial.
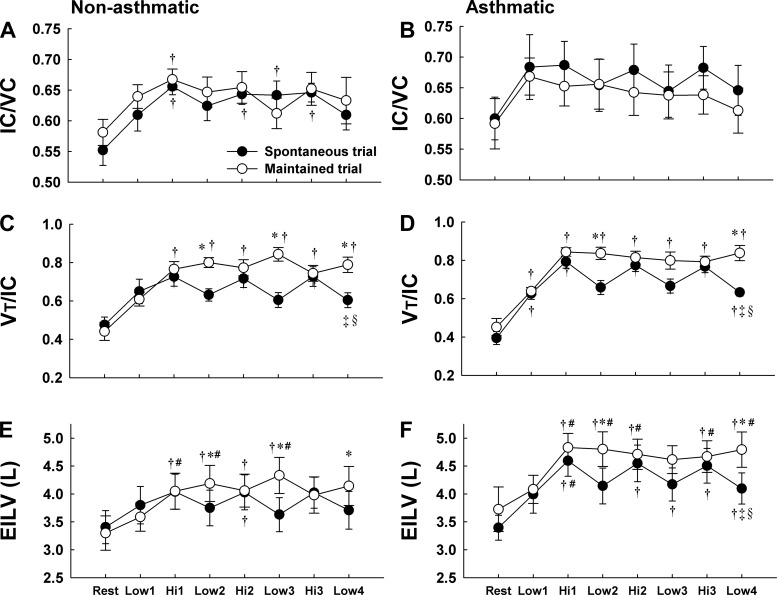
Operating Lung Volumes
In nonasthmatic subjects, IC increased with exercise during SVT and MVT (P < 0.05 vs. rest), demonstrating similar variation with changes in workload during both trials (Fig. 3A and Table 2). In asthmatic subjects, there was a significant time main effect for the increase in IC during SVT and MVT (P < 0.05); however, post hoc pairwise comparisons were not significant (Fig. 3B and Table 3). During SVT, IC fluctuated with exercise workload in asthmatic subjects (not significant). During MVT, however, IC did not vary with workload; rather, it remained at a nearly constant value throughout the exercise protocol.
Fig. 3.
Ratio of inspiratory capacity to vital capacity (IC/VC), ratio of tidal volume to inspiratory capacity (VT/IC), and end-inspiratory lung volume (EILV) in nonasthmatic (A, C, and E) and asthmatic (B, D, and F) subjects during spontaneous (SVT) and maintained (MVT) ventilation trials. In nonasthmatic subjects, IC/VC responded similarly during SVT and MVT. In asthmatic subjects, IC/VC fluctuated with exercise workload during SVT whereas it remained constant throughout exercise during MVT. In both subject groups, the higher VT/IC and EILV at the low-intensity workloads during MVT compared with SVT indicates increased operating lung volumes and greater lung stretch at the low workload during MVT. *P < 0.05 vs. SVT; †P < 0.05 vs. rest; #P < 0.05 vs. Low-1; ‡P < 0.05 vs. Hi-1; §P < 0.05 vs. Hi-2.
Table 2.
Exercise responses during spontaneous and controlled ventilation exercise in control subjects
| Rest | Low-1 | Hi-1 | Low-2 | Hi-2 | Low-3 | Hi-3 | Low-4 | |
|---|---|---|---|---|---|---|---|---|
| V̇o2, ml·kg−1·min−1 | ||||||||
| SVT | 5.5 ± 1.3 | 21.5 ± 4.2 | 33.2 ± 6.0 | 22.5 ± 3.9 | 32.0 ± 5.5 | 23.5 ± 2.9 | 33.6 ± 5.1 | 23.7 ± 3.5 |
| MVT | 6.3 ± 1.7 | 21.7 ± 3.1 | 32.0 ± 4.7 | — | 31.1 ± 4.7 | — | 31.4 ± 5.2 | — |
| V̇co2, ml·kg−1·min−1 | ||||||||
| SVT | 5.1 ± 1.5 | 19.1 ± 4.7 | 34.4 ± 6.5 | 21.2 ± 4.2 | 32.0 ± 5.8 | 21.5 ± 3.2 | 33.2 ± 5.6 | 22.1 ± 3.8 |
| MVT | 5.6 ± 1.5 | 18.9 ± 4.1 | 33.8 ± 6.3 | — | 31.6 ± 5.8 | — | 32.5 ± 4.5 | — |
| RER | ||||||||
| SVT | 0.95 ± 0.3 | 0.89 ± 0.1 | 1.04 ± 0.1 | 0.94 ± 0.1 | 1.00 ± 0.1 | 0.92 ± 0.1 | 0.99 ± 0.1 | 0.93 ± 0.04 |
| MVT | 0.91 ± 0.3 | 0.87 ± 0.1 | 1.05 ± 0.1 | — | 1.02 ± 0.1 | — | 1.05 ± 0.2 | — |
| V̇e/V̇o2 | ||||||||
| SVT | 35.5 ± 14.0 | 24.2 ± 3.2 | 29.2 ± 3.9 | 28.5 ± 4.5 | 30.3 ± 4.6 | 28.9 ± 4.7 | 30.4 ± 4.4 | 29.7 ± 3.9 |
| MVT | 33.8 ± 10.2 | 24.2 ± 2.9 | 29.0 ± 3.1 | — | 30.5 ± 4.2 | — | 32.0 ± 4.3 | — |
| V̇e/V̇co2 | ||||||||
| SVT | 36.7 ± 6.5 | 27.4 ± 3.2 | 28.2 ± 3.1 | 30.2 ± 3.6 | 30.1 ± 3.9 | 31.6 ± 4.3 | 30.7 ± 3.7 | 31.9 ± 4.4 |
| MVT | 36.8 ± 4.6 | 28.1 ± 2.4 | 27.6 ± 3.0 | — | 30.1 ± 3.8 | — | 30.8 ± 3.9 | — |
| PEF, l/s | ||||||||
| SVT | 8.6 ± 1.9 | 8.8 ± 2.1 | 9.4 ± 2.2* | 9.4 ± 2.4 | 9.3 ± 2.1 | 8.9 ± 2.1 | 9.6 ± 2.4 | 9.3 ± 2.1 |
| MVT | 8.8 ± 2.0 | 9.3 ± 2.1 | 9.3 ± 2.0 | 9.4 ± 2.2 | 9.1 ± 2.1 | 9.0 ± 2.0 | 9.3 ± 1.9 | 9.1 ± 1.9 |
| IC, liters | ||||||||
| SVT | 2.6 ± 0.6 | 2.9 ± 0.7 | 3.1 ± 0.6* | 3.0 ± 0.6 | 3.1 ± 0.7 | 3.1 ± 0.7 | 3.1 ± 0.7 | 2.9 ± 0.6 |
| MVT | 2.8 ± 0.7 | 3.1 ± 0.8 | 3.2 ± 0.7* | 3.1 ± 0.8 | 3.2 ± 0.9 | 3.0 ± 0.8 | 3.1 ± 0.9 | 3.0 ± 0.9 |
| ETCO2, % | ||||||||
| SVT | 4.9 ± 0.8 | 5.6 ± 0.7 | 5.2 ± 0.6 | 5.1 ± 0.6 | 5.0 ± 0.6 | 5.0 ± 0.6 | 4.9 ± 0.6 | 4.9 ± 0.6 |
| MVT | 4.9 ± 0.7 | 5.6 ± 0.7 | 5.2 ± 0.7 | 5.2 ± 0.8 | 5.0 ± 0.8 | 5.0 ± 0.7 | 4.8 ± 0.7 | 4.8 ± 0.7 |
| TTot, s | ||||||||
| SVT | 6.2 ± 1.8 | 3.2 ± 0.8 | 2.1 ± 0.4 | 2.6 ± 0.5 | 2.0 ± 0.5 | 2.4 ± 0.4 | 2.0 ± 0.4 | 2.3 ± 0.5 |
| MVT | 5.8 ± 1.6 | 3.0 ± 0.8 | 2.3 ± 0.5 | 2.3 ± 0.6 | 2.2 ± 0.6 | 2.0 ± 0.4 | 2.0 ± 0.4 | 1.9 ± 0.4 |
| TI/TTot | ||||||||
| SVT | 0.46 ± 0.07 | 0.46 ± 0.03 | 0.49 ± 0.03 | 0.47 ± 0.04 | 0.49 ± 0.02 | 0.47 ± 0.04 | 0.49 ± 0.03 | 0.46 ± 0.03 |
| MVT | 0.47 ± 0.05 | 0.46 ± 0.04 | 0.48 ± 0.03 | 0.47 ± 0.03 | 0.48 ± 0.02 | 0.48 ± 0.02 | 0.49 ± 0.02 | 0.48 ± 0.04 |
| HR, beats/min | ||||||||
| SVT | 76 ± 4.8 | 121 ± 9.3 | 159 ± 13.1 | 135 ± 16.6 | 167 ± 13.0 | 141 ± 15.9 | 170 ± 14.0 | 145 ± 17.3 |
| MVT | 75 ± 9.2 | 120 ± 15.0 | 157 ± 16.7 | 132 ± 17.9 | 165 ± 16.1 | 140 ± 17.4 | 168 ± 14.6 | 144 ± 17.7 |
Values are means ± SD. ETCO2, end-tidal CO2; HR, heart rate; IC, inspiratory capacity; MVT, maintained ventilation trial; PEF; peak expiratory flow; RER, respiratory exchange ratio; SVT, spontaneous ventilation trial; TI/TTot, inspiratory to total breath cycle time; TTot, total breath cycle time; V̇e/V̇o2, ventilatory equivalent for oxygen consumption; V̇e/V̇co2, ventilatory equivalent for carbon dioxide production; V̇co2, carbon dioxide production; V̇o2, oxygen uptake.
P < 0.05 vs. rest.
Table 3.
Exercise responses during spontaneous and controlled ventilation exercise in asthmatic subjects
| Rest | Low-1 | Hi-1 | Low-2 | Hi-2 | Low-3 | Hi-3 | Low-4 | |
|---|---|---|---|---|---|---|---|---|
| V̇o2, ml·kg−1·min−1 | ||||||||
| SVT | 5.9 ± 1.2 | 21.9 ± 4.2 | 32.8 ± 8.8 | 23.4 ± 5.9 | 32.7 ± 8.2 | 23.9 ± 6.6 | 34.2 ± 8.7 | 23.8 ± 5.8 |
| MVT | 5.9 ± 2.3 | 22.1 ± 4.2 | 30.8 ± 7.5 | — | 31.0 ± 7.2 | — | 32.1 ± 7.6 | — |
| V̇co2, ml·kg−1·min−1 | ||||||||
| SVT | 4.6 ± 1.1 | 19.9 ± 3.8 | 34.0 ± 9.1 | 22.0 ± 5.3 | 32.9 ± 8.7 | 22.3 ± 6.1 | 34.7 ± 8.8 | 22.2 ± 5.6 |
| MVT | 5.2 ± 1.7 | 19.1 ± 4.3 | 32.4 ± 7.2 | — | 32.2 ± 6.3 | — | 32.7 ± 6.7 | — |
| RER | ||||||||
| SVT | 0.79 ± 0.1 | 0.91 ± 0.1 | 1.04 ± 0.1 | 0.94 ± 0.05 | 1.01 ± 0.04 | 0.93 ± 0.05 | 1.02 ± 0.04 | 0.93 ± 0.04 |
| MVT | 0.86 ± 0.2 | 0.87 ± 0.1 | 1.07 ± 0.2 | — | 1.05 ± 0.1 | — | 1.03 ± 0.1 | — |
| V̇e/V̇o2 | ||||||||
| SVT | 26.4 ± 5.3 | 24.5 ± 1.8 | 28.0 ± 3.1 | 27.8 ± 2.5 | 28.5 ± 2.8 | 28.9 ± 2.4 | 30.2 ± 2.7 | 29.6 ± 2.7 |
| MVT | 31.8 ± 6.2 | 25.2 ± 2.3 | 28.9 ± 5.3 | — | 28.7 ± 2.4 | — | 29.0 ± 2.7 | — |
| V̇e/V̇co2 | ||||||||
| SVT | 33.6 ± 4.3 | 27.0 ± 1.9 | 27.0 ± 2.6 | 29.5 ± 2.6 | 28.4 ± 3.0 | 31.0 ± 2.8 | 29.8 ± 3.1 | 31.8 ± 2.9 |
| MVT | 36.9 ± 3.2 | 28.3 ± 2.2 | 26.7 ± 2.4 | — | 28.7 ± 2.4 | — | 29.0 ± 2.7 | — |
| PEF, l/s | ||||||||
| SVT | 8.2 ± 1.5 | 9.0 ± 1.3 | 9.1 ± 1.1 | 8.6 ± 1.9 | 9.0 ± 1.4 | 8.2 ± 1.6† | 9.1 ± 1.6 | 8.4 ± 1.8 |
| MVT | 8.2 ± 2.2 | 8.7 ± 1.8 | 9.0 ± 1.7 | 8.8 ± 1.6 | 8.6 ± 1.9 | 8.2 ± 1.9 | 8.8 ± 2.3 | 8.3 ± 1.8 |
| IC, liters | ||||||||
| SVT | 3.2 ± 0.7 | 3.6 ± 0.8 | 3.6 ± 0.7 | 3.5 ± 0.9 | 3.6 ± 0.8 | 3.4 ± 0.8 | 3.6 ± 0.8 | 3.4 ± 0.8 |
| MVT | 3.1 ± 0.6 | 3.6 ± 0.9 | 3.5 ± 0.9 | 3.5 ± 1.0 | 3.4 ± 1.0 | 3.4 ± 1.0 | 3.4 ± 0.9 | 3.3 ± 0.9 |
| ETCO2, % | ||||||||
| SVT | 5.1 ± 0.3 | 5.7 ± 0.6 | 5.5 ± 0.6 | 5.4 ± 0.5 | 5.2 ± 0.5 | 5.2 ± 0.5 | 5.0 ± 0.6 | 5.0 ± 0.5 |
| MVT | 5.0 ± 0.2 | 5.7 ± 0.4 | 5.6 ± 0.5 | 5.6 ± 0.5 | 5.3 ± 0.5 | 5.3 ± 0.5 | 5.2 ± 0.5 | 5.1 ± 0.5 |
| TTot, s | ||||||||
| SVT | 6.4 ± 2.2 | 3.1 ± 0.8 | 2.4 ± 0.4 | 2.6 ± 0.5 | 2.3 ± 0.5 | 2.4 ± 0.5 | 2.0 ± 0.4 | 2.3 ± 0.6 |
| MVT | 6.4 ± 3.5 | 3.1 ± 0.5 | 2.5 ± 0.5 | 2.6 ± 0.6 | 2.3 ± 0.5 | 2.2 ± 0.5 | 2.1 ± 0.5 | 2.1 ± 0.4 |
| Ti/TTot | ||||||||
| SVT | 0.44 ± 0.05 | 0.45 ± 0.02 | 0.45 ± 0.03 | 0.44 ± 0.02 | 0.45 ± 0.02 | 0.45 ± 0.02 | 0.47 ± 0.02 | 0.45 ± 0.01 |
| MVT | 0.41 ± 0.05 | 0.45 ± 0.02 | 0.47 ± 0.03 | 0.48 ± 0.03* | 0.47 ± 0.02 | 0.48 ± 0.03 | 0.48 ± 0.03 | 0.49 ± 0.03* |
| HR, beats/min | ||||||||
| SVT | 81 ± 13.9 | 118 ± 7.2 | 152 ± 8.1 | 134 ± 8.3 | 161 ± 9.8 | 142 ± 11.6 | 168 ± 10.5 | 148 ± 10.7 |
| MVT | 82 ± 17.7 | 124 ± 12.4 | 149 ± 9.6 | 140 ± 14.0 | 159 ± 11.0 | 145 ± 14.6 | 164 ± 10.0 | 146 ± 13.2 |
Values are means ± SD. ETCO2, end-tidal CO2; HR, heart rate; IC, inspiratory capacity; MVT, maintained ventilation trial; PEF; peak expiratory flow; RER, respiratory exchange ratio; SVT, spontaneous ventilation trial; TI/TTot, inspiratory to total breath cycle time; TTot, total breath cycle time; V̇e/V̇o2, ventilatory equivalent for oxygen consumption; V̇e/V̇co2, ventilatory equivalent for carbon dioxide production; V̇co2, carbon dioxide production; V̇o2, oxygen uptake.
P < 0.05 vs. spontaneous; †P < 0.05 vs. Hi-1.
In nonasthmatic and asthmatic subjects, VT/IC and EILV increased with exercise during SVT and varied regularly with workload throughout the trial (Fig. 3, C–F). During MVT, VT/IC and EILV did not fluctuate with exercise workload in either subject group; both variables were higher at the low workload during MVT compared with SVT in both subject groups (P < 0.05). Thus EILV was +0.53 ± 0.14 (SD) and +0.6 ± 0.37 liters higher at low-intensity exercise during MVT compared with SVT in nonasthmatic and asthmatic subjects, respectively.
Additional Exercise Responses
All variables responded to exercise as expected (Tables 2 and Table 3). Due to the variable addition of CO2 to the inspirate while exercise was performed at Low-2, Low-3, and Low-4 during MVT, several of the parameters requiring known inhaled gas fractions were not obtainable at these workloads during MVT. There were no significant differences between nonasthmatic and asthmatic subjects for any of the variables.
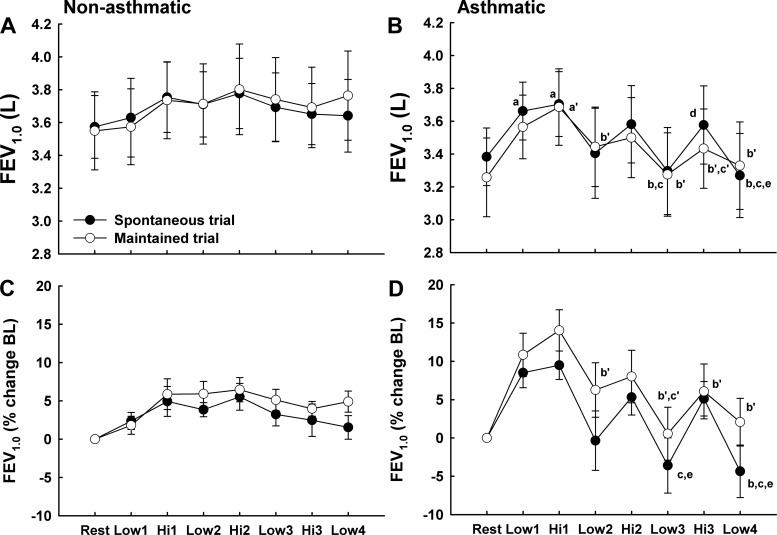
Exercise Airway Function
The results for FEV1.0 shown in Fig. 4 are from the forced exhalation maneuver performed during the fourth minute of exercise at each workload. In both subject groups, FEV1.0 increased during exercise; however, the magnitude of the increase and fluctuations due to changing workload were different between asthmatic and nonasthmatic subjects. In nonasthmatic subjects, FEV1.0 increased by a maximum of 8.4 and 8.5% during SVT and MVT [0.31 ± 0.17 (SD) and 0.30 ± 0.16 liters], respectively; there was a significant main effect for time during both trials, but Bonferroni-corrected comparisons were not significant. Following the initial increases in FEV1.0 during Low-1 and Hi-1, it remained relatively constant throughout exercise during both trials. In asthmatic subjects, FEV1.0 initially increased by 12.2 and 16.0% throughout Low-1 and Hi-1 during SVT and MVT [0.41 ± 0.15 (SD) and 0.49 ± 0.19 liters, P < 0.05]. Following Hi-1, FEV1.0 fluctuated with exercise workload during both SVT and MVT, such that there was an average difference of 0.25 ± 0.2 (SD) and 0.19 ± 0.1 liters between low- and high-intensity exercise during SVT and MVT, respectively (P < 0.05). Additionally, FEV1.0 decreased progressively throughout the 28-min exercise protocol during both trials. There were no significant differences between SVT and MVT in either the nonasthmatic or asthmatic group. In general, PEF responded similar to FEV1.0 during the exercise trials in both subject groups (Tables 2 and 3).
Fig. 4.
Forced expiratory volume 1.0 s (FEV1.0) in liters and as a percent change from baseline (BL) in nonasthmatic (A and C) and asthmatic (B and D) subjects during spontaneous (SVT) and maintained (MVT) ventilation trials. The FEV1.0 is from the forced exhalation maneuver completed during the fourth min of exercise at each workload. In both subject groups, a progressive increase in FEV1.0, signifying bronchodilation (BD), occurred during the 1st 2 exercise workloads during SVT and MVT; however, the magnitude of BD was greater in asthmatic subjects. Following Hi-1, airway caliber fluctuated with exercise workload in the asthmatic subjects whereas it remained constant in the nonasthmatic subjects. Note that FEV1.0 fluctuated with exercise workrate similarly during SVT and MVT in asthmatic subjects. SVT trial: aP < 0.05 vs. rest; bP < 0.05 vs. Hi-1; cP < 0.05 vs. Hi-2; dP < 0.05 vs. Low-3; eP < 0.05 vs. Hi-3. MVT trial: a′P < 0.05 vs. rest; b′P < 0.05 vs. Hi-1; c′P < 0.05 vs. Hi-2.
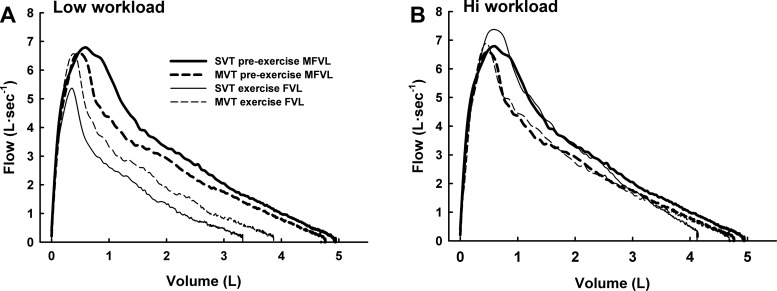
Figure 5 shows preexercise maximal flow-volume loops and exercise maximal flow-volume loops at both exercise intensities during SVT and MVT for one asthmatic subject. In this subject, preexercise expiratory airflow was higher during the SVT; this reveals the typical daily variation in airway function in asthmatic patients. During both SVT and MVT, the exercise maximal flow-volume loops were markedly larger during the high-intensity exercise than the low-intensity exercise.
Fig. 5.
Preexercise maximal volitional flow-volume loops (MFVL) and exercise maximal flow-volume loops (FVL) during spontaneous (SVT) and maintained (MVT) ventilation trials in one asthmatic subject. A: low workload; B: high workload. During exercise at the low workload, maximal airflow was decreased, indicating bronchoconstriction. During exercise at the high workload, maximal airflow was increased to preexercise levels, indicating bronchodilation. Note that the bronchoconstriction occurred at the low workload during both exercise trials.
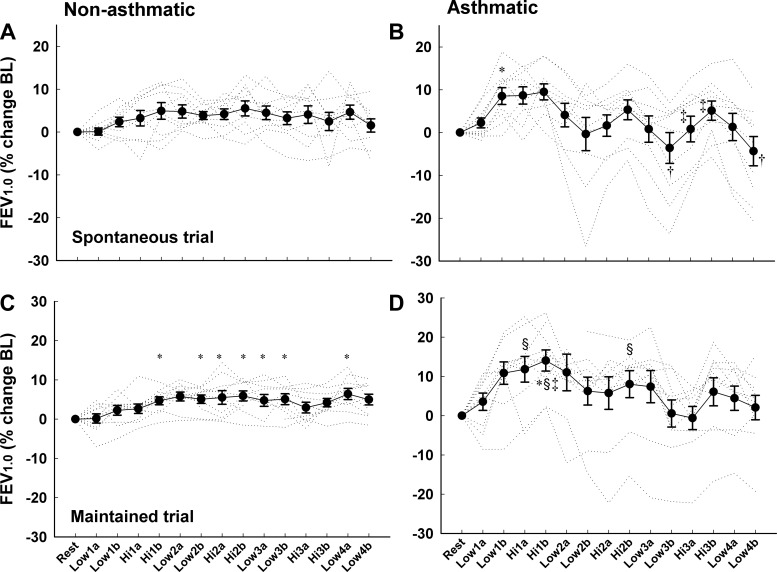
In Fig. 6, individual subject and group mean FEV1.0 values are shown for both of the forced exhalation maneuvers completed during each 4-min exercise stage. Between- and within-subject variability was more pronounced in the asthmatic subjects. In both subject groups, FEV1.0 increased progressively over time throughout the first low- and high-intensity stages. Thereafter, FEV1.0 remained stable in nonasthmatic subjects. In asthmatic subjects, FEV1.0 changed gradually over time during each 4-min workload during SVT and MVT. At the three low workloads following Hi-1 (i.e., Low-2, Low-3, Low-4), FEV1.0 decreased by an average of 0.15 ± 0.1 and 0.13 ± 0.1 liters [4.9 ± 3.7 (SD) and 4.0 ± 3.7%] between the first and fourth minutes of exercise during SVT and MVT, respectively. Conversely, at the high workloads, FEV1.0 increased by an average of 0.13 ± 0.1 and 0.14 ± 0.1 liters [4.1 ± 3.2 (SD) and 4.6 ± 3.6%] between the first and fourth minutes of exercise during SVT and MVT, respectively. Finally, the pattern of significant differences in asthmatic subjects demonstrates a progressive BC over time during the exercise bouts. There were no significant differences between SVT and MVT for nonasthmatic or asthmatic subjects.
Fig. 6.
Individual subject and group mean forced expiratory volume 1.0 s (FEV1.0) expressed as a percent change from baseline in nonasthmatic (A and C) and asthmatic (B and D) subjects during the spontaneous and maintained ventilation trials. The FEV1.0 values from the beginning and end of each 4-min workload are shown. The fluctuations in FEV1.0 during exercise and the between-subject variability were greater in the asthmatic compared with nonasthmatic subjects. These figures also show that FEV1.0 changed gradually over time at each workload, particularly in asthmatic subjects. There were no significant differences between SVT and MVT in nonasthmatic or asthmatic subjects. *P < 0.05 vs. rest and Low-1A; †P < 0.05 vs. Hi-2B; §P < 0.05 vs. Hi-3A; ‡P < 0.05 vs. Low-4B.
DISCUSSION
In asthmatic adults, airway caliber fluctuates during variable workrate exercise; BD occurs with increased workrate whereas BC occurs with decreased workrate. Due to the forces of interdependence between the airways and parenchyma, we hypothesized that increased VT and EILV would prevent BC during variable workrate exercise in asthmatic adults. The main finding of this study is that increased VT and EILV do not prevent a decrease in airway caliber during exercise when workload is reduced. Indeed, we found that FEV1.0 fluctuated similarly with changing exercise workload when subjects breathed spontaneously and when VT and EILV were increased at the low workload. These findings suggest that changes in operating VL, and accompanying changes in lung stretch, during variable intensity exercise do not contribute to the fluctuating airway caliber seen during such exercise. Rather, physiological inputs other than lung stretch and oscillations in lung stretch must be more potent regulators of airway function during exercise in the mild-to-moderate asthmatic.
Exercise Model
There are several reasons we chose interval exercise to investigate the influence of lung mechanical stretch on airway function during exercise. Firstly, as shown previously (5, 20) and as replicated in this study, interval exercise causes alternating periods of BC and BD during a single exercise session. This fluctuating airway caliber provides a robust physiological signal for assessing the effects of our exercise intervention, namely, altered operating VL. Secondly, the protocol consisted of multiple repeats of each low and high workload, which allowed us to analyze the effects of exercise duration on the relationships between lung mechanical strain and airway caliber. Finally, forced exhalations performed at the beginning and at the end of each 4-min workload allowed us to examine the rate of change in airway caliber when intensity was changed.
Rationale for Hypothesis
Our hypothesis that increased operating VL would prevent decreases in airway caliber during exercise was founded on basic principles of lung mechanics and informed by several findings in the literature. Fundamental pulmonary mechanics dictate that airway diameter increases as a function of VL (26). This coupling derives from the increased elastic recoil and consequent increase in tethering forces between the lung parenchyma and the intraparenchymal airways at larger VL. Recent studies using high-resolution computed tomography and the forced oscillation technique show that increased VL distends the airways in asthmatic humans (6–8, 25), although the degree of airway dilation may be less than in healthy persons.
In addition to the in vivo demonstrations of in-phase interdependence between VL and airway diameter, a burgeoning number of studies at reduced biological scales support the postulate that increased VL will attenuate airway narrowing during exercise. In vitro studies of airway smooth muscle (ASM) strips and investigations of airway sections from healthy animals have demonstrated a powerful capacity of increased mechanical stress and strain to reduce ASM force (3, 11, 15, 27, 34). Recent investigations of airway sections from both asthmatic mice and humans provide evidence that increased lung mechanical strain inhibits force generation in the asthmatic, as well (19, 32). Moreover, the reduction in ASM force is, in general, proportional to the magnitude of oscillatory strain (3, 15, 27, 34). These strain-induced declines in ASM force are thought to reflect perturbed myosin-actin cross-bridge binding due to stretching of the airway wall and ASM. The principle limitation of these reduced preparations is that they do not replicate the functional interdependence between the airways and surrounding parenchyma in the whole organism.
Accordingly, our hypothesis linking VL with exercise airway caliber was based on the following reasoning. During exercise, increased VT and EILV will distend the airways in proportion to the increased VL. The distended airways should stretch the ASM, and if the degree of stretch is sufficient, myosin-actin binding will be perturbed. If the reapplication of force by ASM activation is slow relative to the rate of airway distention dictated by fb, airway caliber should remain increased (13). To the best of our knowledge, the current study represents the first in vivo attempt at manipulating lung stretch as a method to uncover the lung mechanical mechanisms accounting for variable airway caliber during exercise in the asthmatic.
Exercise Airway Function
In contrast to our hypothesis, we found that increased lung stretch did not prevent decreases in airway caliber during exercise when workrate was reduced in mild-to-moderate asthmatic adults. At the onset of exercise, however, airway caliber increased in nearly all of our asthmatic subjects (see Figs. 4 and 6). Airway caliber also increased during exercise in the nonasthmatic subjects, although the magnitude of dilation was less than in the asthmatics. Others have also shown that BD occurs during exercise in asthmatic and nonasthmatic adults (10, 16, 30, 46). In fact, Crimi et al. (10) showed that the bronchodilatory stimulus of exercise is powerful enough to increase airway caliber markedly in asthmatics that are in the midst of a spontaneous asthma attack. Similarly, another study reported a rapid BD in asthmatics that began exercise when their airways were significantly narrowed from a previous exercise bout (16). Both the rapidity and the magnitude of BD with exercise support the notion that this BD is at least partly due to increased VL and airway tethering. Indeed, a modest BD also occurs during seated isocapnic hyperpnea in asthmatic subjects (41, 43).
Following the initial BD at exercise onset, airway caliber fluctuated with changing workrate throughout both exercise trials. Thus, during the MVT trial, exercise airway caliber behaved independently from the nearly constant VT and EILV. Presumably, ASM contractile force varied in concert with workload throughout exercise whether or not the degree of lung stretch was maintained. Our findings thus imply that the influence of lung stretch (at least at the levels seen in this study) on ASM contractile activity is overwhelmed by coexisting influences operating during exercise that oppose the ability of lung stretch to maintain airway caliber.
One possible explanation for not only the initial exercise BD but also for the workload related fluctuations in airway caliber is altered cholinergic tone. Studies have shown that the initial exercise BD is likely due, at least in part, to the immediate withdrawal of cholinergic tone that accompanies whole body exercise (22, 28, 46). Also, parasympathetic activity decreases progressively with increases in workrate (28); presumably, its activity increases similarly when workrate is reduced. Studies in anesthetized dogs demonstrate that a neurogenic reflex originating in contracting skeletal muscle is probably responsible for the exercise-induced withdrawal of cholinergic tone (24). An additional input that might modulate cholinergic tone is lung inflation. In situ experiments in dogs have shown reflex withdrawal of cholinergic tone with increases in both fb and VT, an effect likely mediated by pulmonary stretch receptors (40, 47). However, our findings of decreased airway caliber (i.e., increased cholinergic tone) at the low workload despite maintained VT and VL suggest that cholinergic tone is more sensitive to other reflexes active during exercise. Given all of this, we speculate that increased cholinergic tone was at least partly responsible for the airway narrowing that occurred when exercise workload was reduced in this study. If this is the case, it implies that the skeletal muscle reflex control of cholinergic tone and hence, ASM contractile activity, exerts a more potent influence on ASM cross-bridge behavior than the combative effect of increased lung stretch to disrupt cross-bridge binding and reduce ASM contraction.
Another novel finding in this study is that the workload-induced changes in airway caliber occurred gradually over the 4-min exercise stages (Fig. 6). The immediate increase in heart rate at the onset of exercise is due to decreased cholinergic tone, which responds within seconds of altering workrate (28). Similarly, the altered airway caliber seen during the first minute of exercise at each stage in this study may be due to altered cholinergic activity. The mechanism(s) for the further changes in airway caliber seen over time within each exercise stage are not known. Based on the findings from SVT, one could reasonably postulate that the time-dependent changes in airway caliber were due to cumulative effects of repetitive oscillations in lung stretch acting on ASM contraction. Similarly, gradual release of bronchoactive or bronchoprotective biological mediators during each exercise stage might also be expected to result in progressive changes in airway caliber. However, the finding that increased VL during MVT failed to prevent reduced airway caliber at the lower exercise workload makes these possibilities unlikely. Sympathetic nervous system activity and circulating epinephrine increase progressively with both workload and exercise time (12). It is tempting to speculate that the progressive increases in airway caliber over time at the high workload were due to increased epinephrine binding to ASM β2-receptors. However, β2-receptor blockade does not prevent BD during exercise (46). Furthermore, BD occurs during isocapnic hyperpnea in asthmatic subjects (41, 43); a condition in which sympathetic activation is minimal. Collectively, we can only state that the balance between bronchodilatory and bronchoconstricting influences changes not only with altered exercise intensity but also over time within a given intensity. There is much room for future work directed at determining the control of airway function during exercise in asthma.
A final observation in this study providing insight into the control of airway function during exercise in the asthmatic is the gradual reduction in FEV1.0 over time during both of the 28-min exercise bouts. Suman et al. (43) compared pulmonary function during a 20-min exercise bout with a 20-min period of isocapnic hyperpnea in asthmatic subjects. Following an initial BD, pulmonary function declined slowly throughout both trials; however, the airway narrowing during the 20-min exercise bout was of a greater magnitude than during the 20-min hyperpnea bout. In combination with these findings, our current results suggest that exercise-induced alterations in neuromuscular reflexes, circulating chemicals, or airway-derived mediators have a powerful influence on ASM tone; physiological inputs that supersede the interdependence between lung stretch and airway caliber.
The gradual airway narrowing is intuitively compatible with the slow release of one or more BC mediators during the exercise. A variety of chemical mediators released from resident or nonresident lung cells have been proposed as regulators of airway function during exercise in the asthmatic (4). Indeed, the airway narrowing that occurs after exercise in the asthmatic (i.e., exercise-induced bronchospasm) is principally due to the effects of proinflammatory mediators released from mast cells and potentially other cells during or after exercise (2, 14). However, due to the logistical difficulties inherent in obtaining biological samples during an exercise bout (as opposed to before and after exercise), we are not aware of any data addressing interactions between exercise time and inflammatory mediators in health or in the asthmatic. Any biological mediators released during exercise would also necessarily interact with concurrent neural and lung mechanical factors that control airway function. Finally, nitric oxide and epithelial cell-derived prostaglandin E2 are both known to relax ASM, but their roles in modulating exercise airway function are not clear (42, 44). An improved understanding of airway inflammatory mediator release during exercise and the influences of such molecules on airway function will be necessary to understand the complicated control of exercise airway caliber in asthma.
Relationship to Deep Inspiration Literature
How do our findings relate to studies investigating the influence of deep inhalations (DI) on airway function? In nonasthmatic subjects, airway responsiveness is significantly increased when DIs are prohibited before inhalation of a direct-acting spasmogen (9, 23, 38, 39). In the asthmatic, however, this bronchoprotective effect of DI is variably impaired, exposing a defect in the relationship between VL and airway behavior (23, 38). In contrast, in both nonasthmatic and asthmatic subjects with methacholine-induced airway narrowing, a single DI causes an immediate increase in airway caliber that returns to pre-DI levels within ∼60 s (18, 35, 38). The design and question in the current study are actually an amalgam of the two types of DI literature: 1) does increased lung strain prevent BC during exercise in the asthmatic (i.e., bronchoprotective effect of DI); or alternatively, 2) does increased lung strain maintain BD during exercise in the asthmatic (i.e., bronchdilatory effect of DI)?
Collectively, the DI literature limits the stimulus of increased lung mechanical stress and strain to between one and five perturbations to TLC. In the current study, exercise VT and EILV were increased continuously by ∼500–600 ml for 4 min at a time. Given the differences in both the amplitude and number of perturbations in lung strain, we suggest that the nature of the stimulus in the current study does not compare well with that applied in the DI literature. However, our finding that repetitive increases in VL did not prevent decreases in airway caliber when exercise intensity was reduced complements previous findings demonstrating a reduced ability for lung stretch to prevent ASM contraction in response to inhaled agonists in asthmatic subjects (23, 38). Creative manipulations of airway function, VL, and exercise workload will be necessary to further clarify the role of lung stretch in determining exercise airway caliber in the asthmatic.
Conclusions
The airway responses to exercise in the asthmatic are dynamic and complicated. We investigated the effect of increased lung mechanical stretch on airway caliber during variable workrate exercise in nonasthmatic and mild-to-moderate asthmatic adults. Following an initial BD with exercise in asthmatic subjects, airway caliber subsequently fluctuated with decreased and increased workload throughout the exercise. In contrast to our hypothesis, increased lung mechanical stretch did not prevent reduced airway caliber when workload was decreased. We conclude that workrate-induced changes in skeletal muscle metabolic or mechanical reflexes are more robust determinants of ASM contractile activity than the influence of lung stretch on ASM myosin-actin binding and its subsequent force generation.
GRANTS
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Grant P20-GM-103449. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.K., A.M.-T., S.A., D.L., and H.C.H. performed experiments; A.K., A.M.-T., S.A., D.L., and H.C.H. analyzed data; A.K., C.G.I., and H.C.H. interpreted results of experiments; A.K. and H.C.H. drafted manuscript; A.K., C.G.I., A.M.-T., S.A., D.L., and H.C.H. edited and revised manuscript; A.K., C.G.I., A.M.-T., S.A., D.L., and H.C.H. approved final version of manuscript; C.G.I. and H.C.H. conception and design of research; H.C.H. prepared figures.
ACKNOWLEDGMENTS
We thank our subjects for generous donation of time and consistent efforts during the studies. Many thanks to Dr. Jerome Dempsey for critical review of this manuscript.
REFERENCES
- 1.Anderson SD, Brannan JD. Methods for “indirect” challenge tests including exercise, eucapnic voluntary hyperpnea, and hypertonic aerosols. Clin Rev Allergy Immunol 24: 27–54, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol 122: 225–235, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Ansell TK, McFawn PK, Mitchell HW, Noble PB. Bronchodilatory response to deep inspiration in bronchial segments: the effects of stress vs. strain. J Appl Physiol 115: 505–513, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Beck KC. Control of airway function during and after exercise in asthmatics. Med Sci Sports Exerc 31: S4–S11, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Beck KC, Offord KP, Scanlon PD. Bronchoconstriction occurring during exercise in asthmatic subjects. Am J Respir Crit Care Med 149: 352–357, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Benfante A, Bellia M, Scichilone N, Cannizzaro F, Midiri M, Brown R, Bellia V. Airway distensibility by HRCT in asthmatics and COPD with comparable airway obstruction. COPD 10: 560–566, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Brown NJ, Salome CM, Berend N, Thorpe CW, King GG. Airway distensibility in adults with asthma and healthy adults, measured by forced oscillation technique. Am J Respir Crit Care Med 176: 129–137, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 163: 994–1001, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chapman DG, Berend N, King GG, Salome CM. Effect of deep inspiration avoidance on ventilation heterogeneity and airway responsiveness in healthy adults. J Appl Physiol 110: 1400–1405, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Crimi E, Pellegrino R, Smeraldi A, Brusasco V. Exercise-induced bronchodilation in natural and induced asthma: effects on ventilatory response and performance. J Appl Physiol 92: 2353–2360, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol 38: 70–76, 1975. [DOI] [PubMed] [Google Scholar]
- 13.Gunst SJ. Contractile force of canine airway smooth muscle during cyclical length changes. J Appl Physiol Respir Environ Exercise Physiol 55: 759–769, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrand TS. New insights into pathogenesis of exercise-induced bronchoconstriction. Curr Opin Allergy Clin Immunol 12: 42–48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey BC, Parameswaran H, Lutchen KR. Can tidal breathing with deep inspirations of intact airways create sustained bronchoprotection or bronchodilation? J Appl Physiol 115: 436–445, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haverkamp HC, Dempsey JA, Miller JD, Romer LM, Pegelow DF, Lovering AT, Eldridge MW. Repeat exercise normalizes the gas-exchange impairment induced by a previous exercise bout in asthmatic subjects. J Appl Physiol 99: 1843–1852, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hayes D Jr, Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med 185: 1190–1196, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson AC, Murphy MM, Rassulo J, Celli BR, Ingram RH Jr. Deep breath reversal and exponential return of methacholine-induced obstruction in asthmatic and nonasthmatic subjects. J Appl Physiol 96: 137–142, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Jo-Avila M, Al-Jumaily AM, Lu J. Relaxant effect of superimposed length oscillation on sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L479–L484, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BD, Scanlon PD, Beck KC. Regulation of ventilatory capacity during exercise in asthmatics. J Appl Physiol 79: 892–901, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 14: 902–907, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kagawa J, Kerr HD. Effects of brief graded exercise on specific airway conductance in normal subjects. J Appl Physiol 28: 138–144, 1970. [DOI] [PubMed] [Google Scholar]
- 23.Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol 89: 711–720, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman MP, Rybicki KJ, Mitchell JH. Hindlimb muscular contraction reflexly decreases total pulmonary resistance in dogs. J Appl Physiol 59: 1521–1526, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Kelly VJ, Brown NJ, Sands SA, Borg BM, King GG, Thompson BR. Effect of airway smooth muscle tone on airway distensibility measured by the forced oscillation technique in adults with asthma. J Appl Physiol 112: 1494–1503, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Lai-Fook SJ, Hyatt RE, Rodarte JR. Effect of parenchymal shear modulus and lung volume on bronchial pressure-diameter behavior. J Appl Physiol Respir Environ Exercise Physiol 44: 859–868, 1978. [DOI] [PubMed] [Google Scholar]
- 27.Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, Dowell ML. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am J Respir Crit Care Med 186: 225–232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maciel BC, Gallo L Jr, Marin Neto JA, Lima Filho EC, Martins LE. Autonomic nervous control of the heart rate during dynamic exercise in normal man. Clin Sci (Lond) 71: 457–460, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Macklem PT. A theoretical analysis of the effect of airway smooth muscle load on airway narrowing. Am J Respir Crit Care Med 153: 83–89, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Milanese M, Saporiti R, Bartolini S, Pellegrino R, Baroffio M, Brusasco V, Crimi E. Bronchodilator effects of exercise hyperpnea and albuterol in mild-to-moderate asthma. J Appl Physiol 107: 494–499, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo RO, Enright P, Van der grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pederson OF, Pellegrino R, Viegi G, Wanger J. Standardization of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Noble PB, Jones RL, Cairncross A, Elliot JG, Mitchell HW, James AL, McFawn PK. Airway narrowing and bronchodilation to deep inspiration in bronchial segments from subjects with and without reported asthma. J Appl Physiol 114: 1460–1471, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Olin JT, Dimmen AC, Subudhi AW, Roach RC. A simple method to clamp end-tidal carbon dioxide during rest and exercise. Eur J Appl Physiol 112: 3439–3444, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Pascoe CD, Seow CY, Pare PD, Bosse Y. Decrease of airway smooth muscle contractility induced by simulated breathing maneuvers is not simply proportional to strain. J Appl Physiol 114: 335–343, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrino R, Wilson O, Jenouri G, Rodarte JR. Lung mechanics during induced bronchoconstriction. J Appl Physiol 81: 964–975, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal Official Statement of the European Respiratory Society. Eur Respir J Suppl 16: 5–40, 1993. [PubMed] [Google Scholar]
- 37.Rossman MJ, Nader S, Berry D, Orsini F, Klansky A, Haverkamp HC. Effects of altered airway function on exercise ventilation in asthmatic adults. Med Sci Sports Exerc 46: 1104–1113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med 163: 413–419, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorkness R, Vidruk E. Reflex effects of isocapnic changes in ventilation on tracheal tone in awake dogs. Respir Physiol 69: 161–172, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Stirling DR, Cotton DJ, Graham BL, Hodgson WC, Cockcroft DW, Dosman JA. Characteristics of airway tone during exercise in patients with asthma. J Appl Physiol 54: 934–942, 1983. [DOI] [PubMed] [Google Scholar]
- 42.Suman OE, Beck KC. Role of endogenous nitric oxide on lung function during and after exercise in mild asthma. J Appl Physiol 93: 1932–1938, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Suman OE, Beck KC, Babcock MA, pegelow D, Reddan AW. Airway obstruction during exercise and isocapnic hyperventilation in asthmatic subjects. J Appl Physiol 87: 1107–1113, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Suman OE, Morrow JD, O'Malley KA, Beck KC. Airway function after cyclooxygenase inhibition during hyperpnea-induced bronchoconstriction in guinea pigs. J Appl Physiol 89: 1971–1978, 2000. [DOI] [PubMed] [Google Scholar]
- 45.US Department of Health and Human Services, National Heart, Lung and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Washington, DC: DHHS/NIH, 2007. [Google Scholar]
- 46.Warren JB, Jennings SJ, Clark TJ. Effect of adrenergic and vagal blockade on the normal human airway response to exercise. Clin Sci (Lond) 66: 79–85, 1984. [DOI] [PubMed] [Google Scholar]
- 47.Widdicombe JG, Nadel JA. Reflex effects of lung inflation on tracheal volume. J Appl Physiol 18: 863–868, 1963. [DOI] [PubMed] [Google Scholar]