This article proposes a complex inhibitory network of nucleus tractus solitarius (NTS) interneurons for the control of cough. This hypothesis has important implications in disease states, such as cough hyperresponsiveness. This may be largely a function of reduced inhibitory NTS control; thus reduced cough excitability may in part be due to enhanced inhibition. This hypothesis emphasizes that the levels of excitation and inhibition in a network determine sensory gating of afferent input.
Keywords: airway protection, computational modeling, cough, in vivo, inhibition
Abstract
We investigated the hypothesis, motivated in part by a coordinated computational cough network model, that second-order neurons in the nucleus tractus solitarius (NTS) act as a filter and shape afferent input to the respiratory network during the production of cough. In vivo experiments were conducted on anesthetized spontaneously breathing cats. Cough was elicited by mechanical stimulation of the intrathoracic airways. Electromyograms of the parasternal (inspiratory) and rectus abdominis (expiratory) muscles and esophageal pressure were recorded. In vivo data revealed that expiratory motor drive during bouts of repetitive coughs is variable: peak expulsive amplitude increases from the first cough, peaks about the eighth or ninth cough, and then decreases through the remainder of the bout. Model simulations indicated that feed-forward inhibition of a single second-order neuron population is not sufficient to account for this dynamic feature of a repetitive cough bout. When a single second-order population was split into two subpopulations (inspiratory and expiratory), the resultant model produced simulated expiratory motor bursts that were comparable to in vivo data. However, expiratory phase durations during these simulations of repetitive coughing had less variance than those in vivo. Simulations in which reciprocal inhibitory processes between inspiratory-decrementing and expiratory-augmenting-late neurons were introduced exhibited increased variance in the expiratory phase durations. These results support the prediction that serial and parallel processing of airway afferent signals in the NTS play a role in generation of the motor pattern for cough.
NEW & NOTEWORTHY
This article proposes a complex inhibitory network of nucleus tractus solitarius (NTS) interneurons for the control of cough. This hypothesis has important implications in disease states, such as cough hyperresponsiveness. This may be largely a function of reduced inhibitory NTS control; thus reduced cough excitability may in part be due to enhanced inhibition. This hypothesis emphasizes that the levels of excitation and inhibition in a network determine sensory gating of afferent input.
chronic cough is a debilitating condition, with broad social and health consequences (1, 17, 19, 21, 22, 26). A subset of patients with acute or chronic cough can experience paroxysmal coughing, defined as intense bouts of uncontrollable repetitive cough (27, 29, 31, 32, 37). Paroxysmal coughing is commonly associated with significant morbidity such as vomiting, headache (17, 19, 21), ruptured abdominal muscles, and broken ribs (17, 19, 21, 26). The success of therapeutic strategies aimed at reducing the morbidity of cough will depend on a greater understanding of the mechanisms of altered excitability across cough bouts.
These bouts or epochs of coughing are evoked by mechanical or chemical stimulation of the larynx (6, 14), pharynx (14, 28, 63), or tracheobronchial tree (14, 61) and are associated with esophageal pressures that can exceed 100 mmHg. Of the most commonly employed animal models of cough, the cat routinely produces intense bouts of repetitive coughing. Spatiotemporal features of coughing in the cat have been reported for large numbers of coughs across a population or within a single animal (45–48, 53, 54, 59, 61). The results of these and other studies (2, 4, 10–12, 49) support the existence of a central control system for cough that regulates the intensities of inspiratory and expiratory motor drive separately. Retrospective inspection of our own data suggested that, within individual intense cough epochs in the cat, the sequential pattern of expiratory motor drive is qualitatively different from inspiratory motor activity. The duration of the passive portion of the expiration phase (CTE2) is the primary determinant of the total cough cycle time (CTTOT) (61) and can be significantly prolonged to allow the execution of other protective behaviors, such as swallow, that have an airway clearance function. Additionally, our recent work (45) indicated that the duration of the E2 phase has a higher degree of variance during cough than during breathing and has a reflexive control system property appropriate for the “interleaving” of different behaviors between successive coughs and enhanced protection of the airway from intrusion of foreign material. This mechanism allows the brain stem control system to initiate and complete multiple airway protective behaviors in an orderly sequence while preserving the mechanical effectiveness of each. We have termed the coordinated execution of multiple airway protective behaviors a meta-behavioral control system (45).
The reflexive cough pathway is initiated by mechanoreceptors and/or C fibers in the laryngeal and tracheal mucosa (especially the area of the carina) which terminate on second-order neurons in the NTS (35, 62). These second-order neurons are hypothesized to project to pontine and medullary respiratory groups (24, 43, 53, 54). Mifflin (38) identified neurons with monosynaptic (second-order neurons) and polysynaptic responses to electrical stimulation of the superior laryngeal nerve (SLN); 50% of the second-order and 100% of the polysynaptic response neurons exhibited significant frequency-dependent reductions in excitatory postsynaptic potential amplitude in response to SLN stimulation. Neurons with polysynaptic responses often exhibited an excitatory-inhibitory postsynaptic potential sequence (38). These findings support the presence of feed-forward inhibitory mechanisms among the NTS second-order interneuronal population that processes afferent feedback from the larynx. More recently, Canning et al. (15) proposed that the tracheal sensory receptors that induce coughing differ from the classically defined rapidly adapting receptors (RAR) (57, 62–64). Specific information on the behavior of intrapulmonary RAR second-order neurons suggests that this population can undergo altered excitability as a compensatory response to heightened afferent input (52). Haji et al. (24) have provided information in support of the concept that second-order neurons in the NTS that respond to input from RARs act as filters that shape afferent input to downstream locations. However, the role of NTS second-order neurons in the processing of tracheobronchial cough receptor feedback and the production of cough is not well understood.
This prior work and gaps in knowledge motivated a computational modeling study of the breathing-cough neural network and in vivo experiments to investigate the mechanisms responsible for timing and modulation of motor drive to spinal respiratory muscle pathways during cough bouts. Our current neuromechanical model of the cough-respiratory system has only a single NTS second-order neuronal population that receives tracheobronchial afferent feedback (41, 48). We hypothesized that an enhanced model with additional NTS second-order neuron populations and additional recurrent inhibitory connections among extant ventral respiratory column (VRC) populations would confer sensory filtering functions for specific shaping and regulation of the cough motor pattern.
METHODS
Computational modeling methods.
Neural circuit components of the cough computational model were derived from previously described network models of discrete “integrate and fire” populations after MacGregor (30). These models were developed from in vivo experiments of fictive coughing in the cat as previously described by Rybak et al. (50), Poliaček et al. (48), and O'Connor et al. (41). The network model used herein is further described in results. The model published by Poliaček et al. (48) was used as the base model for comparison in this manuscript.
Computational models were implemented using a program package written in C language for the UNIX environment. Simulations were run on a 64-bit Intel multiprocessor-based computer under the Linux operating system. Figure 1 is a visual representation of the final network model designated version 2 (see results for version 1 and 2 modifications; details in Tables 3 and 4). The model is scaled in time with 1 s equivalent to 2,000 integration steps. In versions 1 and 2 the simulation coughs are approximately twice the rate of in vivo.
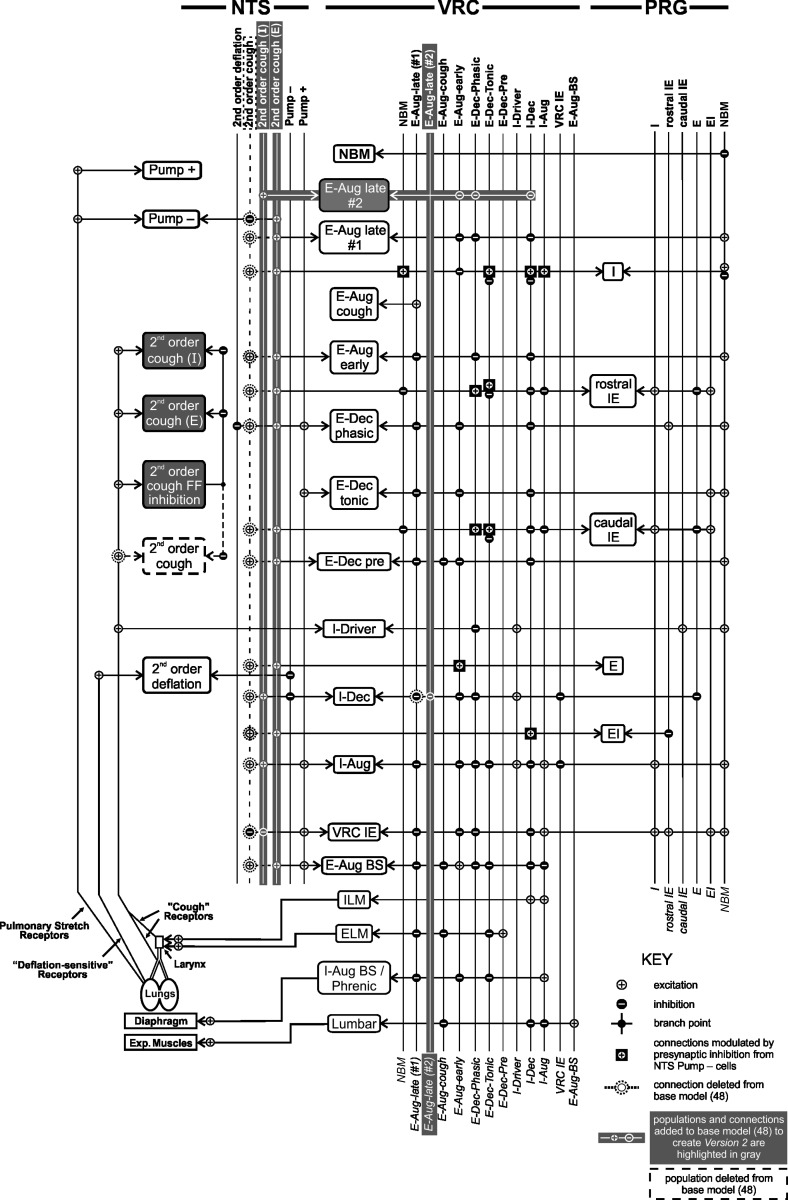
Fig. 1.
Schematic representation of version 2 of the pontomedullary respiratory network model. Cell populations are represented as rectangles and are labeled with the cells' respiratory discharge pattern; populations are vertically grouped according to anatomic location. Vertical lines to the right of each group represent the output(s) of each cell population; note that the lines and cell populations have the same names. Cell input is delineated at line intersections marked with circles containing a symbol to indicate the synapse type (excitatory or inhibitory; see Key); the horizontal lines point to the cell population receiving the input. The synapse type (excitatory or inhibitory) is indicated by small circles on cell population (see Key). For example, VRC IE neurons inhibit the VRC I-Dec and I-Aug cells and are excited by, among others, NTS Pump + cells. Nerve outputs are represented by the bottom four populations in the VRC column. Sensory receptors are shown at bottom left; they influence the cell populations via second-order populations (left column). Populations and connections present in the base model [as published by Poliaček et al. (48)] that were removed or transformed in version 2 are indicated by dashed lines. Added connections and populations are shown in white and highlighted in gray. NTS, nucleus tractus solitarius; VRC, ventral respiratory column; PRG, pontine respiratory group. Aug and Dec, neurons with augmenting or decrementing activity patterns, respectively, during the indicated phase (I, inspiratory; E, expiratory) of maximum firing rate. ELM, expiratory laryngeal motoneurons; EI and IE, neurons with a peak firing rate during the E-I and I-E phase transitions, respectively; ILM, inspiratory laryngeal motoneurons; Lumbar, lumbar nerve; NBM, nonbreathing modulated neurons; Phrenic, phrenic nerve; Pump cells, excitatory or inhibitory neurons excited by pulmonary stretch receptors and most active with lung inflation; BS, bulbospinal; FF, feed-forward; Exp., expiratory.
Table 3.
Population parameters for the three computational network models discussed
| Population Name | Size (N) | Resting Threshold (THO), mV | THO Variability, mV | Membrane Time Constant (TMEM) | Postspike Increase in GK+ (B) | Postspike GK+ Time Constant (TGK), ms | Adaptation Threshold Increase (C) | Adaptation (TTH), ms | Noise Amplitude | DC, mV |
|---|---|---|---|---|---|---|---|---|---|---|
| Second-order cough*§ | 100 | 10.0 | 1.0 | 9.0 | 20.0 | 7.0 | 0.3 | 500.0 | 0.1 | 0.0 |
| Second-order cough (insp)ठ| 100 | 10.0 | 1.0 | 9.0 | 20.0 | 7.0 | 0.9 | 600.0 | 0.1 | 0.0 |
| Second-order cough (exp)ठ| 250 | 10 | 1.0 | 9.0 | 20.0 | 7.0 | 0.9 | 600.0 | 0.1 | 0.0 |
| E-Aug late (#2)‡ | 600 | 10.0 | 1.0 | 9.0 | 27 | 2.5 | 0 | 250 | 0.1 | 27.0 |
| Second-order cough feed-forward inhibition†‡§ | 100 | 10.0 | 1.0 | 9.0 | 20.0 | 7.0 | 0.9 | 600.0 | 0.1 | 0.0 |
Symbols indicate neuronal populations added to the base model from Poliaček et al. (48) to create version 1 (†) and version 2 (‡) of the model and populations present in the base model (48) and version 1 that were removed from version 2 (
). Variable names used by MacGregor (30) are in parentheses. All values representing voltages are relative to the resting potential, which is considered equal to zero. N is the number of neurons simulated in each population. THO, the resting threshold, is normally distributed in the population around the value of THO with a SD equal to the “THO variability” value. TMEM is the membrane time constant. B is the amplitude of the postspike increase in potassium conductance. TGK is the time constant of the potassium conductance decay following an action potential. C and TTH define the change in threshold associated with spike adaptation. C is the ratio of the threshold increase to the membrane potential increase; its value is between 0 and 1. TTH is the time constant of the rise in threshold with spike adaptation. Noise amplitude: Each cell has an internal noise generator that acts like two synapses, one with an equilibrium potential of 70 mV above resting and the other with −70 mV. Each acts as though it has an incoming firing probability of 0.05 per time step and a synapse time constant of 1.5 ms. This parameter is the conductance that gets added to the synapse conductance on each (virtual) spike. DC: An injected current will raise the membrane potential by an amount that is inversely proportional to the membrane conductance. Instead of being specified directly as a current, this parameter is specified in mV, and it is interpreted as the current that is required to raise the membrane potential by the specified number of mV when the membrane conductance has its resting value. The effect on the membrane potential at other membrane conductances will be inversely proportional to the conductance. Note also that as in other types of integrate-and-fire (IF) neuron models, our neuron models do not actually generate action potential-like spikes but only identified moments of spikes, so “spiking” shown in all neuron simulations is represented graphically by assigning vertical spikelike lines at computed times of threshold crossing.
Neuron populations that relay perturbations of the network model. A fiber population consisting of 100 fibers, each with 100 excitatory synaptic terminals and a firing probability of 0.07 at each simulation time step, was used to represent cough receptor excitation. This fiber population excited the second-order cough and feed-forward inhibitory neuron populations (synaptic strengths, 0.12 and 0.04, respectively) and the I-Driver/Plateau population (synaptic strength, 0.02).
Table 4.
Connectivity for the network model
| Conduction Times |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source Population | Target Population | Synaptic Type | Min | Max | Number of Terminals | Synaptic Strength | Source Population, n | Target Population, n | Divergence | Mean Number of Terminals | Convergence |
| E-Dec P‡ | E-Aug-late (#2) | Inh_1 | 2 | 4 | 150 | 0.02 | 300 | 600 | 132.51 ± 3.36 | 1.13 | 66.26 ± 7.63 |
| I-Dec‡ | E-Aug late (#2) | Inh_2 | 0 | 5 | 115 | 1.0 | 300 | 600 | 104.77 ± 2.71 | 1.10 | 52.39 ± 6.40 |
| E-Aug early‡ | E-Aug late (#2) | Inh_1 | 0 | 2 | 50 | 0.001 | 300 | 600 | 48.01 ± 1.32 | 1.04 | 24.00 ± 4.83 |
| E-Aug late (#1)* | I-Dec | Inh_1 | 0 | 4 | 55 | 0.01 | 300 | 300 | 50.33 ± 1.87 | 1.09 | 50.33 ± 6.68 |
| E-Aug late (#2)‡ | I-Dec | Inh_1 | 0 | 4 | 100 | 0.05 | 600 | 300 | 85.21 ± 3.03 | 1.17 | 170.43 ± 10.33 |
| Second-order cough*§ | I-Dec | Ex_1 | 0 | 3 | 100 | 0.038 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough*§ | I-Aug | Ex_1 | 0 | 3 | 100 | 0.02 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough*§ | VRC IE | Inh_1 | 0 | 3 | 100 | 0.2 | 100 | 99 | 63.13 ± 3.05 | 1.58 | 63.77 ± 5.20 |
| Second-order cough*§ | E-Dec P | Ex_1 | 0 | 3 | 100 | 0.015 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough*§ | E-Aug early | Ex_1 | 0 | 3 | 100 | 0.1 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough*§ | E-Aug late (#1) | Ex_1 | 0 | 3 | 100 | 0.06 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough*§ | E-Aug BS | Ex_1 | 0 | 4 | 125 | 0.001 | 100 | 300 | 102.81 ± 3.59 | 1.22 | 34.27 ± 4.61 |
| Second-order cough*§ | Pump- | Inh_1 | 0 | 4 | 250 | 0.4 | 100 | 300 | 170.45 ± 4.98 | 1.47 | 56.82 ± 4.58 |
| Second-order cough*§ | PRG cIE | Ex_1 | 0 | 3 | 100 | 0.001 | 100 | 100 | 63.59 ± 3.21 | 1.57 | 63.59 ± 5.84 |
| Second-order cough*§ | PRG rIE | Ex_1 | 0 | 3 | 100 | 0.001 | 100 | 100 | 63.59 ± 3.21 | 1.57 | 63.59 ± 5.84 |
| Second-order cough*§ | PRG I | Ex_1 | 0 | 3 | 100 | 0.001 | 100 | 100 | 63.59 ± 3.21 | 1.57 | 63.59 ± 5.84 |
| Second-order cough*§ | PRG E | Ex_1 | 0 | 3 | 100 | 0.001 | 100 | 100 | 63.59 ± 3.21 | 1.57 | 63.59 ± 5.84 |
| Second-order cough*§ | PRG EI | Ex_1 | 0 | 3 | 100 | 0.001 | 100 | 100 | 63.59 ± 3.21 | 1.57 | 63.59 ± 5.84 |
| Second-order cough*§ | E-Dec Pre | Ex_1 | 0 | 3 | 100 | 0.0025 | 100 | 300 | 85.14 ± 3.15 | 1.11 | 28.38 ± 3.97 |
| Second-order cough (insp)‡§ | I-Dec | Ex_1 | 0 | 3 | 100 | 0.038 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough (insp)‡§ | I-Aug | Ex_1 | 0 | 3 | 100 | 0.02 | 100 | 300 | 85.25 ± 2.83 | 1.17 | 28.42 ± 5.15 |
| Second-order cough (insp)‡§ | VRC IE | Inh_1 | 0 | 3 | 100 | 0.2 | 100 | 99 | 63.13 ± 3.05 | 1.58 | 63.77 ± 5.20 |
| Second-order cough (insp)‡§ | E-Aug late (#2) | Ex_1 | 0 | 3 | 100 | 0.07 | 100 | 600 | 92.11 ± 2.37 | 1.09 | 15.35 ± 4.27 |
| Second-order cough (exp)‡§ | E-Dec P | Ex_1 | 0 | 3 | 100 | 0.015 | 250 | 300 | 85.23 ± 2.93 | 1.17 | 71.02 ± 9.02 |
| Second-order cough (exp)‡§ | E-Aug early | Ex_1 | 0 | 3 | 100 | 0.1 | 250 | 300 | 85.23 ± 2.93 | 1.17 | 71.02 ± 9.02 |
| Second-order cough (exp)‡§ | E-Aug late (#1) | Ex_1 | 0 | 3 | 100 | 0.06 | 250 | 300 | 85.23 ± 2.93 | 1.17 | 71.02 ± 9.02 |
| Second-order cough (exp)‡§ | E-Aug BS | Ex_1 | 0 | 4 | 125 | 0.001 | 250 | 300 | 102.33 ± 3.45 | 1.22 | 85.28 ± 6.85 |
| Second-order cough (exp)‡§ | Pump- | Inh_1 | 0 | 4 | 250 | 0.4 | 250 | 300 | 170.67 ± 4.79 | 1.46 | 142.23 ± 7.14 |
| Second-order cough (exp)‡§ | PRG cIE | Ex_1 | 0 | 3 | 100 | 0.001 | 250 | 100 | 63.42 ± 3.05 | 1.58 | 158.54 ± 10.15 |
| Second-order cough (exp)‡§ | PRG rIE | Ex_1 | 0 | 3 | 100 | 0.001 | 250 | 100 | 63.42 ± 3.05 | 1.58 | 158.54 ± 10.15 |
| Second-order cough (exp)‡§ | PRG I | Ex_1 | 0 | 3 | 100 | 0.001 | 250 | 100 | 63.42 ± 3.05 | 1.58 | 158.54 ± 10.15 |
| Second-order cough (exp)‡§ | PRG E | Ex_1 | 0 | 3 | 100 | 0.001 | 250 | 100 | 63.42 ± 3.05 | 1.58 | 158.54 ± 10.15 |
| Second-order cough (exp)‡§ | PRG EI | Ex_1 | 0 | 3 | 100 | 0.001 | 250 | 100 | 63.42 ± 3.05 | 1.58 | 158.54 ± 10.15 |
| Second-order cough (exp)‡§ | E-Dec Pre | Ex_1 | 0 | 3 | 100 | 0.0025 | 250 | 300 | 85.08 ± 3.08 | 1.18 | 70.90 ± 6.53 |
| Second-order cough feed-forward inhibition† | Second-order cough | Inh_1 | 0 | 2 | 500 | 0.02 | 100 | 250 | 215.94 ± 3.74 | 2.32 | 86.38 ± 3.09 |
| Second-order cough feed-forward inhibition‡ | Second-order cough (exp) | Inh_1 | 0 | 2 | 500 | 0.02 | 100 | 250 | 215.94 ± 3.74 | 2.32 | 86.38 ± 3.09 |
| Second-order cough feed-forward inhibition‡ | Second-order cough (insp) | Inh_1 | 0 | 2 | 500 | 0.02 | 100 | 100 | 99.33 ± 0.91 | 5.03 | 99.33 ± 0.85 |
Symbols indicate connections present in the base model from Poliaček et al. (48) and version 1 that were removed from version 2 (
) and connections added to the base model (48) to create version 1 (
) and version 2 (
) of the computational model.
Connection relaying a perturbation to the network model. Connections between individual neurons were made according to a sequence of pseudorandom numbers calculated from a unique seed number for each source-to-target connection. Targets were chosen with replacement. This table includes the mean ± SD of the number of neurons in each target population innervated by each source neuron in each population. Corresponding values are also shown for source neurons that innervated each target neuron in each population. These data indicate the extent of divergence and convergence, respectively. Most neurons in each source population made a single terminal connection with each target neuron. Mean number of terminals, the mean number of terminals from each source neuron innervating each target neuron. The efficacy of connections between populations of neurons was influenced by the change in conductance associated with each action potential at a synapse (synaptic strength) and the number of terminals for each axon. Synaptic types were distinguished by their equilibrium potentials and time constants. The time constant of some synapses was slightly longer than others because troughs in cross-correlograms from which the particular synaptic connections were inferred tended to have longer durations. Six types of synapses were used in the simulation: type 1 excitatory (Ex_1, equilibrium potential of 115.0 mV; time constant, 1.5 ms), type 3 excitatory (Ex_3, equilibrium potential, 115.0 mV; time constant, 5.0 ms), type 1 inhibitory (Inh_1, equilibrium potential, −25.0 mV; time constant, 1.5 ms), type 2 inhibitory (Inh_2, equilibrium potential, −25.0 mV; time constant, 2.0 ms), type 4 inhibitory (Inh_4, equilibrium potential, −25.0 mV; time constant, 5.0 ms), and presynaptic modulation (Pre, time constant, 1.5 ms). If the value of the presynaptic modulatory strength parameter (synaptic strength) was <1.0, the strength of the connection it modulates was reduced to the product of the presynaptic synaptic strength parameter and target synapse conductance. If the presynaptic synaptic strength parameter was >1.0, the amount by which it was >1 is added to its target synapse's conductance. Minimum and maximum conduction times are expressed in 0.5-ms simulation clock ticks for each source-to-target axon population. Number of terminals, number of terminals from source neuron; cIE, caudal IE; rIE, rostral IE.
In vivo methods.
Experiments were performed on six spontaneously breathing adult male cats. The protocol was approved by the University of Florida Institutional Animal Care and Use Committee (IACUC). The animals were initially anesthetized with sodium pentobarbital (35–40 mg/kg iv), and supplementary doses were administered as needed (1–3 mg/kg iv). A dose of atropine sulfate (0.1–0.2 mg/kg iv) was given at the beginning of the experiment to reduce secretions from repeated tracheal stimulation. Cannulas were placed in the femoral artery, femoral vein, and trachea. An esophageal balloon was placed via an oral approach to measure pressure in the midthoracic esophagus. Arterial blood pressure and end-tidal CO2 were continuously monitored. Po2 was maintained using air mixtures with enriched oxygen (25–60%) to maintain values above 100 mmHg. Body temperature was monitored and maintained at 37.5 using a heating pad. Arterial blood samples were periodically removed for blood gas analysis.
Electromyograms (EMG) were recorded using bipolar insulated fine wire electrodes. The activities of the parasternal (inspiratory) and the rectus abdominis (expiratory) muscles were used to determine cough. Electrodes were placed in the parasternal muscle at T3. For the rectus abdominis muscle, the electrodes were placed through a small incision in the skin, 7 cm caudal to the xiphoid process and 1 cm lateral to the midline. All electrodes were placed 2–3 mm apart, and their positions were confirmed by visual inspection and EMG activity patterns during breathing and cough. Cough was induced by mechanical stimulation of the extrathoracic and intrathoracic trachea using a thin polyethylene catheter (diameter 0.5–1.0 mm). The catheter was continuously rotated along the length of the intrathoracic trachea for 20 s. All EMG signals were amplified, filtered (200–5,000 Hz), rectified, and integrated (time constant = 50 ms). EMG amplitude measures were normalized to the peak of the largest signal from each muscle recorded during the stimulus-evoked coughs. Parameters from the first 13 coughs that occurred during the 20-s stimulus were measured.
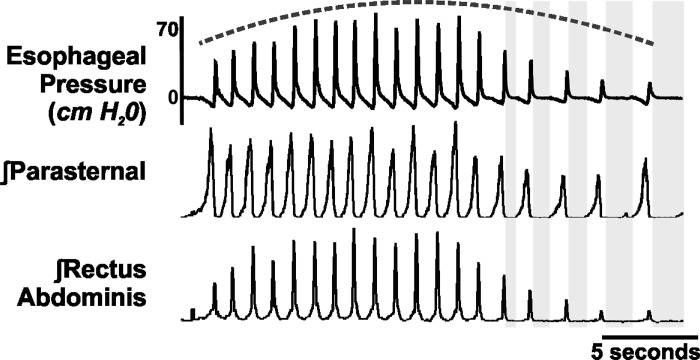
Cough was operationally defined as a burst of activity in the parasternal EMG and rectus abdominis EMG, along with a negative to positive change in esophageal pressure (Fig. 2), or a burst in the phrenic followed by lumbar (Fig. 3). Cough phase durations were measured using the definitions from Wang et al. (61). The inspiratory phase duration (CTI) was defined as the period of time from the onset of inspiratory activity (integrated activity of the parasternal in vivo, phrenic in computer simulations) until the maximum inspiratory burst, duration of the first expiratory phase (CTE1) was defined as the maximum inspiratory burst to the end of the expiratory activity (integrated activity of the rectus abdominis in vivo, lumbar in computer simulations), and the duration of the second expiratory phase (CTE2) was defined as the end of the expiratory motor burst to the onset of the inspiratory activity for the next cough in the epoch (Fig. 2; gray boxes). Total cough cycle duration (CTTOT) was defined as the sum of CTI, CTE1, and CTE2.
Fig. 2.
In vivo representative example demonstrating the dynamic expiratory drive over a cough epoch (dashed line) and the change in CTE2 (gray boxes). Note the change in the CTE2, with longer E2 durations occurring at the end of the cough bout.
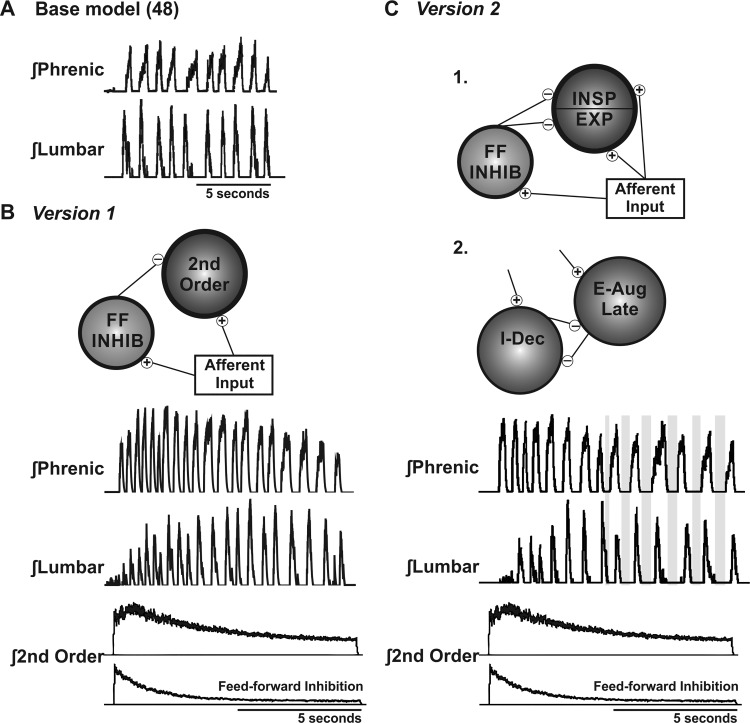
Fig. 3.
Evolution of the computational model. A: base model of Poliaček et al. (48). Note the limited lumbar (expiratory) amplitude variance and the stable CTE2 during coughing. B: version 1 model changes. A feed-forward inhibitory population was added, which accommodated over the cough epoch. C: version 2 model changes. 1. The second-order population was split into inspiratory (INSP) and expiratory (EXP) populations, which received identical excitatory and feed-forward inhibitory (FF INHIB) inputs. 2. A second E-Aug late population was added, which increased the expiratory control over the initiation of inspiration, increasing the CTE2 at the end of the cough epoch (gray boxes). This figure does not indicate anatomical locations for the proposed neuronal populations, although E-Aug late neurons are subsets of E-Aug neurons located in the Botzinger complex in vivo (53, 54). Second-order neurons are located in the NTS (14, 15), and feed-forward populations are also proposed to be located in this area of the dorsal medulla.
Results are expressed as means ± SE. A pairwise two-sided t-test with nonpooled SE was used to assess differences in amplitude of the inspiratory and expiratory activity, and the P values were adjusted for multiple testing (25). A pairwise one-sided t-test with nonpooled SE was used to assess whether CTE2 was longer at the end of a cough bout. Each series was divided into equal halves, unless the epoch was an odd number (the odd cough was included in the second half of the cough epoch). A difference was considered significant if the adjusted P value ≤ 0.05. Relationships between durations and normalized inspiratory and expiratory burst amplitudes during cough to total cough duration were evaluated by linear regression analysis (r2) (Table 2).
Table 2.
Correlation coefficients (r2) from each measure with CTTOT for in vivo and computational model simulations
| r2 | In Vivo (61) | In Vivo (Present) | Base Model (48) | Version 1 | Version 2 |
|---|---|---|---|---|---|
| I amplitude | 0.07 | <0.01 | 0.50* | 0.05 | 0.11 |
| E amplitude | 0.11 | 0.01 | 0.50* | <0.01 | 0.15 |
| CTI | 0.24 | 0.10 | 0.70* | 0.55* | 0.14 |
| CTE1 | 0.09 | <0.01 | 0.60* | 0.52* | 0.05 |
| CTE2 | 0.89* | 0.79* | 0.85* | 0.68* | 0.93* |
In vivo (61), in vivo data from Wang et al. (61); the base model is from Poliaček et al. (48). Note the strong association between CTTOT and the duration of the second expiratory phase (CTE2) in the in vivo conditions. Version 2 is the only model that predicted this important in vivo interaction and did not predict association of other measures with CTTOT.
Moderate and strong associations.
RESULTS
In vivo.
We conducted 18 tracheobronchial stimulation trials in 6 cats (3 per animal). Mechanical stimulation of the tracheobronchial airway elicited 16.7 ± 1 coughs per trial with a range of 13–21 coughs. Moving averages of electromyography (EMG) traces for parasternal and abdominal muscles during cough are represented in Fig. 2. Means and SE for percent of maximum of the parasternal and abdominal EMGs are presented in Table 1.
Table 1.
In vivo percent of maximum parasternal and abdominal EMG amplitude, and computational model simulations percent of maximum phrenic (inspiratory) and lumbar (expiratory) activities
| In Vivo |
Version 1 |
Version 2 |
||||
|---|---|---|---|---|---|---|
| Cough | Parasternal | Abdominal | Phrenic | Lumbar | Phrenic | Lumbar |
| C1 | 72 ± 7 | 41 ± 11 | 58 ± 9 | 42 ± 6 | 94 ± 2 | 59 ± 2 |
| C2 | 71 ± 7 | 46 ± 7 | 76 ± 7 | 42 ± 6 | 89 ± 4 | 55 ± 7 |
| C3 | 62 ± 5 | 44 ± 7 | 91 ± 4† | 60 ± 6 | 91 ± 3 | 56 ± 10 |
| C4 | 64 ± 4 | 55 ± 10 | 92 ± 3† | 64 ± 5 | 88 ± 2 | 66 ± 7 |
| C5 | 74 ± 5 | 61 ± 9 | 94 ± 2† | 64 ± 7 | 86 ± 3 | 70 ± 9 |
| C6 | 76 ± 6 | 62 ± 5 | 81 ± 5† | 51 ± 10 | 79 ± 2 | 75 ± 7 |
| C7 | 85 ± 2 | 69 ± 7† | 85 ± 4 | 69 ± 4 | 78 ± 3† | 81 ± 8 |
| C8 | 84 ± 3 | 69 ± 4† | 81 ± 4 | 81 ± 8 | 70 ± 2† | 72 ± 10 |
| C9 | 84 ± 5 | 72 ± 6† | 74 ± 3 | 87 ± 8* | 71 ± 2† | 83 ± 4† |
| C10 | 77 ± 4 | 68 ± 5† | 74 ± 4 | 82 ± 4† | 68 ± 3† | 75 ± 5† |
| C11 | 82 ± 2 | 69 ± 4 | 68 ± 5 | 81 ± 8 | 66 ± 4† | 76 ± 2† |
| C12 | 76 ± 4 | 67 ± 5 | 67 ± 5 | 88 ± 1† | 60 ± 7† | 68 ± 5 |
| C13 | 82 ± 5 | 70 ± 9 | 59 ± 4 | 83 ± 3† | 71 ± 3† | 66 ± 2† |
| C14 | 62 ± 8 | 87 ± 5 | ||||
| C15 | 67 ± 8 | 85 ± 5 | ||||
| C16 | 58 ± 13 | 80 ± 4 | ||||
| C17 | 53 ± 6 | 77 ± 4 | ||||
| C18 | 58 ± 9 | 74 ± 5 | ||||
| C19 | 50 ± 3 | 61 ± 6 | ||||
Values are means ± SE for each cough in an epoch. The cough number is expressed as coughs C1 to Cn. Symbols indicate a significant difference from the corresponding value during cough C1 (
P < 0.01; †P < 0.05).
The percent of maximum parasternal EMG activity during in vivo cough C1 was not significantly different comparing cough C1 in the epoch to each subsequent cough (coughs C2–C13) (Table 1). There was a significant change in the peak amplitude of the abdominal EMG burst, comparing cough C1 to subsequent coughs in the epoch beginning at cough C7 to cough C10 in the series (Table 1).
The average CTI for cough C1 was 692 ± 84 ms, ∼200 ms longer than any subsequent cough. Coughs C2–C13 demonstrated very little variation in CTI, averaging between 416 ± 42 ms for cough C8 and 472 ± 46 for cough C12. CTE1 was stable throughout the cough epoch; average values ranged from 230 ± 26 ms for cough C3 to 268 ± 26 ms for cough C12. Average CTE2 values, however, ranged from 383 ± 71 ms for cough C7 to 618 ± 155 ms for cough C12 (a difference of ∼62%). Taken with the high correlation between CTE2 and CTTOT (Table 2), this suggests, in agreement with previous research (61), that the primary determinant of CTTOT was CTE2.
The CTE2 over the first half of the cough epoch (464 ± 72 ms) was significantly different from the second half (652 ± 99 ms; P = 0.03) and is demonstrated in Fig. 2.
Computational model and simulations.
Five simulated trials were run using each model version. Values (means ± SE) for the percent maximum of the phrenic (inspiratory) and lumbar (expiratory) outputs are presented in Table 1. Simulations from version 1 resulted in 19 coughs, and simulations from version 2 resulted in 13 coughs.
Figure 3A shows a simulated motor output from the preliminary version of the model, published by Poliaček et al. (48). Version 1 of the model (Fig. 3B) incorporated an additional second-order circuit for NTS processing of the afferent signal, expanding the number of second-order populations involved with cough from one to two. The added population consisted of a feed-forward inhibitory circuit that received the same excitation as the first second-order cough population and inhibited it. The feed-forward inhibitory circuit accommodated over time, decreasing its suppression of cough excitability over the cough trial (Tables 3 and 4). Early iterations of the model resulted in simulations with irregular and nonphysiologic expiratory drive, so the number of terminals from the feed-forward population to the original second-order cough relay population was increased from 100 to 250 to increase spatial and temporal dispersion of the inhibition (Tables 3 and 4).
The NTS circuit was further expanded in version 2 by dividing the original second-order population into second-order inspiratory and expiratory populations (Fig. 3C). The discharge identity of these populations was determined by their activities during the inspiratory and expiratory portions of the cough rather than the breathing cycle. The second-order inspiratory population was connected to inspiratory portions of the network through primarily excitatory synapses (I-Aug, I-Dec, and Late-I), and the second-order expiratory population was connected to expiratory portions of the network (E-Aug early, E-Aug late, E-Dec P, and E-Aug BS in the VRC, and E, EI, rostral IE, and caudal IE populations in the pons; see Fig. 1 for model population abbreviations), also via excitatory synapses (Tables 3 and 4). Both the inspiratory and expiratory second-order populations received identical inputs from the cough receptor fiber populations and the feed-forward inhibition population. To limit inspiratory amplitude variance, the cough receptor fiber populations were connected to the I-Driver/Plateau population.
The excitability of the I-Dec population is proposed to be an important determinant of the onset of the cough I phase; this group of neurons was inhibited in the base model by the E-Aug late population. To better account for increased variance of the expiratory phase, relative to that during breathing, we added a second E-Aug late population that received many of the same inputs as the extant E-Aug late population and an inhibitory synapse to I-Dec neurons; the I-Dec inhibition by the first E-Aug late population was removed. The number of cells in the added E-Aug late population was doubled, and the number of terminals onto the I-Dec population was nearly doubled (from 55 to 100) with the intent of enhancing temporal dispersion of inhibitory postsynaptic potentials (Tables 3 and 4). In addition, increased inhibition produced a greater postinhibitory rebound, further affecting timing in the model. The resulting increase in early I-Dec activity would further inhibit the beginning of inspiratory motor activity and contribute to the prolongation of the E2 phase. To achieve modulation of motor drive to inspiratory pathways during the E2 phase, inhibitory synapses from E-Aug late neurons to I-Dec were added. The resultant simulations of coughing exhibited an inverse relationship between the peak amplitude of cough expiratory drive and the I-Dec population discharge rate. This connection ultimately resulted in prolongation of the CTE2 when cough expiratory drive declined, similar to observations in vivo.
Table 1 includes amplitude measures from versions 1 and 2 of the computational models for the percent of maximum phrenic and lumbar activity during cough. Similar to in vivo CTE2, an analysis was performed by splitting the cough epochs into two halves. In version 1, CTE2 in the first half of the cough epoch (53 ± 22) was not significantly different from the second half (78 ± 28; P = 0.09), but in version 2, CTE2 in the first half of the cough epoch (14 ± 6) was significantly different from that in the second half (40 ± 9; P = 0.01). Version 2 demonstrated the prolongation of CTE2 observed in the in vivo results (Fig. 3, gray boxes).
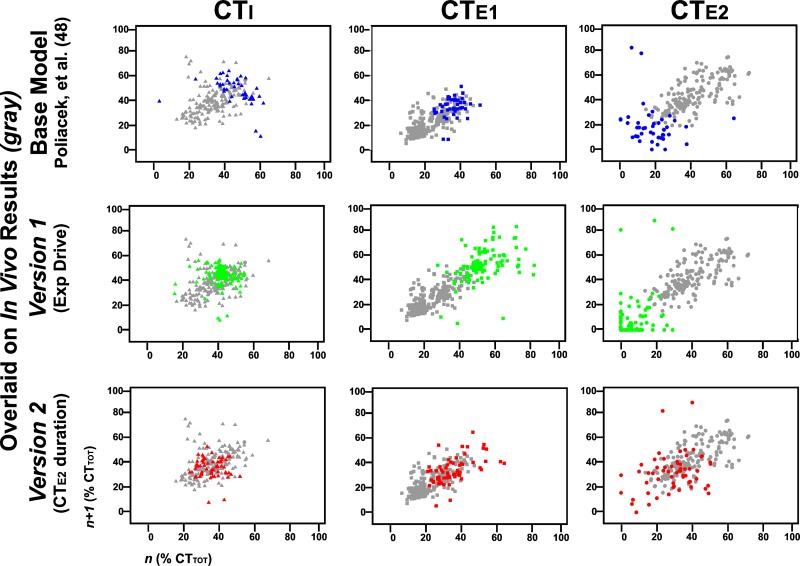
Poincaré plots of cough phase durations.
For the purpose of visually displaying the variability of cough, Poincaré plots (Fig. 4) were used to compare cough phase durations obtained from the in vivo data, from the base model simulations, and from simulations created using versions 1 and 2. The x-axis is the phase duration during cycle n, and the y-axis is the duration of the same phase during cycle n + 1, repeated for each pair of cycles within each cough bout. To normalize the comparisons, in vivo and simulated phase durations were calculated as a percent of CTTOT. Results of model simulations exhibited alterations in the variability of cough cycle durations. The model from Poliaček et al. (48) had limited variability, with each phase in nonphysiologic clusters. Version 1 simulations had increased variability in CTE1; however, there were many cycles with a CTE2 of zero, which is not a feature of cough in vivo. Simulations using version 2 of the computational model exhibited variability more closely resembling in vivo data in each of the three cough phases.
Fig. 4.
Poincaré plots (n vs. n + 1; see methods) of cough phase duration over model evolutions and overlaid on in vivo experiments represented by gray symbols. Data for CTI are represented as triangles, CTE1 as squares, and CTE2 as circles. The base model from Poliaček et al. (48) is in blue, version 1 is in green, and version 2 is in red. Version 1 had instances of CTE2 equal to zero, which is not seen during in vivo repetitive coughing. Note the greater similarity of points generated from in vivo data and version 2 of the computational model. This and the absence of zero-duration E2 phases suggest an improved model.
DISCUSSION
Model simulations of repetitive cough bouts predicted that feed-forward and reciprocal inhibition would produce increased variance in expiratory motor drive. In vivo data revealed that expiratory drive during repetitive coughs is not uniform, but increases from the first cough, peaking about the eighth to ninth cough, and then decreases through the remainder of the bout. The base computational model for cough was first presented by Shannon et al. (53) with the following core hypotheses: 1) the NTS is activated by cough receptors, and this information is distributed to the core network; and 2) medullary neuron drive for breathing and cough arise from, in part, the same neurons. We further hypothesized that behavior excitability is controlled by neurons in the NTS that filter incoming afferent information and initiate an appropriate behavioral response.
The neuronal network for the central pattern of cough has been described by a number of studies (6, 41, 42, 51, 53, 54). This network model has been predictive of the results of in vivo studies for modulation of cough by blood pressure (48) and central chemoreceptor effects (42). However, the computational model of repetitive cough presented by Poliaček et al. (48) had limited expiratory drive variability (Fig. 3A), with all parameters having a moderate to strong relationship with CTTOT (Table 2). The second-order population received the “fiber” population input, which was subject to limited perturbation and is represented as an essentially square wave input. However, the current in vivo data and results given by Wang et al. (61) demonstrate that there is variability in expiratory drive within a cough bout.
To begin to control gain via expiratory excitability, another second-order circuit for NTS processing of the afferent signal was added to the base model to produce version 1 (Fig. 3B). This new feed-forward inhibitory population and all the second-order cough neurons received the same level of excitability. The inhibitory circuit directly inhibited other NTS neurons, but accommodated at a faster rate. The added circuit acted as a sensorimotor gate locally controlling afferent excitability to regulate the motor pattern (13, 16, 38, 40, 44). This process allowed for state-dependent gain control by filtering the afferent signal which resulted in stronger coughs and longer bouts, which is a common feature of repetitive cough (8, 18, 48, 61). The unique advantage conferred by our modeling efforts allowed us to directly investigate the effects of a feed-forward circuit on motor drive in a computational model that has potential predictive value.
Early iterations of version 1 had irregular and nonphysiologic changes in expiratory drive across the cough epoch and even fractionation of the motor burst within a single cough. On the basis of the work of McGovern et al. (35), there is a large population of NTS interneurons rostral to obex that is labeled subsequent to injection of pseudorabies virus into the trachea in the rat. Even though there is evidence that the rat does not cough, this large population of NTS interneurons may represent a neural substrate for “smoothing” temporal variations in synaptic drive to the downstream elements of the respiratory-related network, as seen in the visual cortex (23), eye movement in the cerebellum (36), and arm movement (33). To simulate this process and minimize variance in motor bursts during the cough-bout model simulations, we increased the convergence to second-order cough population neurons from the feed-forward inhibitory population fivefold. We hypothesized that a large number of neurons and/or connections would be needed within a neural circuit that is responding to rapid shifts in excitability from multiple afferent populations, such as the process of transition from eupnea to coughing, to affect temporal and spatial dispersion of afferent feedback to stabilize phase transitions.
In version 2, the second-order population of neurons was split into two discrete populations: one controlling the excitability of the inspiratory core of the network and a second for regulating the expiratory core. This change allowed “parallel” pathways to independently control excitability of each portion of the cough motor pattern. This circuit arrangement represents a plausible explanation for dynamic changes in expiratory motor drive and relatively little change in inspiratory drive over a cough epoch in vivo. Through work on cough suppression induced by pharmacologic agents, Bolser and colleagues (3–6, 9, 11, 12, 45–49, 61) and Mutolo and colleagues (39, 43) have hypothesized that motor drive to the inspiratory and expiratory portions of the network is supplied by distinct interneuronal pathways. More specifically, Bolser and DeGennaro (8) demonstrated differential effects of codeine on inspiratory and expiratory motor drive in the cat: administration of codeine resulted in a dose-dependent decline in expiratory motor drive during coughs, whereas inspiratory drive was less affected [Fig. 2 of Bolser and DeGennaro (8)]. These effects also have been seen with nociceptin (17-amino acid neuropeptide and endogenous ligand for the nociceptin receptor) (12), a substance P receptor antagonist (11), and more recently with baclofen administration in cats (18). A common feature of each of these studies was the stability of the inspiratory motor drive during the coughing following administration of a cough suppressant drug.
The results from version 1 revealed an improved model; however, there were additional cycle-by-cycle timing variations in in vivo data that were not observed in model simulations. The Poincaré plots (Fig. 4) from version 1 display the abbreviated CTE2, and several coughs in the series are missing this phase. Relatively invariant CTE1 and significant variations in CTE2 were observed by Wang et al. (61) for tracheobronchial coughing. Computational models of repetitive cough must account for this temporal regulation of the motor pattern (i.e., a fixed CTE1 with a highly variable CTE2). Additionally, Wang et al. (61) demonstrated that CTE2 was the only temporal measurement to correlate with overall cough duration; this observation was confirmed with the present data set. This feature of the temporal control of coughing differs significantly from that of breathing, in which the inspiratory phase is a major determinant of the total breathing cycle time (20). Table 2 shows regression data including correlations presented by Wang et al. (61), along with in vivo data in the present paper and results from simulations using versions 1 and 2 of the computational model. An additional circuit was added in version 2 to produce an increase in CTE2 as the second-order afferent input decreased excitability. A similar reciprocal inhibitory circuit was used to inhibit the I-Dec population by increased activity in the additional E-Aug late group. We note that both of these populations of neurons are located in the ventral respiration column and not the NTS (53, 55). However, the afferent input to this E-Aug late population did not decrement over time and thus resulted in extended suppression of the I-Dec group to delay the onset of the next inspiratory phase of cough.
The impacts of these model revisions are evident in the correlation coefficients (Table 2, version 2) and the Poincaré plots (Fig. 4) and illustrate that simulations of version 2 produced the salient in vivo relationship between cough phase durations. The relationship between CTE2 and CTTOT is an important feature for the control system of cough and is integral for our understanding of how cough interacts with swallow (45). For example, when cough and swallow occur in sequence, swallow is only present in the E2 phase of cough. These model enhancements recognize the permissive nature of the E2 phase for appropriate integration of the behavior of the cough and swallow pattern generators. The balance of inspiratory and expiratory neuronal synaptic effects in shaping phase transitions from multiple stimuli is manifest by inspection of other parts of the repetitive cough model and more recently has been examined by Segers et al. (51), who demonstrated the action of expiratory neurons in shaping inspiratory drive in response to peripheral chemoreceptor stimulation. More specifically, reductions in the inhibitory synaptic drive from tonic expiratory neurons can enhance inspiratory drive during breathing and cough (51).
The hypothesis that E-Aug late neurons play an integral role in the timing of the transition from expiration to inspiration is limited by the fact that in vivo studies have not demonstrated a robust frequency of occurrence of this neuron type (53, 54). An alternative hypothesis is that other populations of neurons, with different (perhaps nonrespiratory) firing patterns during breathing or the early part of a cough series, change their activity patterns during later coughs in an epoch. Additionally, the extent to which our novel computational model predicts repetitive cough in humans is not known. Smith et al. (56) investigated airflow, volume, and driving pressures in peals (epochs) of coughs in humans. Human cough is characterized by an initial inspiratory effort followed by a cycling of compression and expiration with no intervening inspiratory phases. This laryngeal compression to expiration cycle can be repeated upward of 10 times in adults. Fig. 6 in the report by Smith et al. (56) shows changes in volume and pressure for the first cough in the peal, a middle cough, and the last cough. In humans, the expelled volume of air is greatest during the first cough and becomes progressively smaller as the coughing continues. Thoracoabdominal pressure is a measure of the intensity of cough motor drive, and these investigators showed that the first and middle coughs had similar pressures. This sustained expiratory motor drive over the epoch and the ramping of expiratory motor drive in the cat may be a species difference or a result of differences in the cough stimuli.
As noted above, temporal regulation of the breathing motor pattern differs from that of cough, in particular the importance of inspiratory (breathing) or expiratory (cough) phase duration for controlling total cycle time. The dominant role of CTE2 in total cycle time (61) is a critical determinant in how cough and swallow cooperate to maintain pharyngeal, laryngeal, and tracheobronchial airway patency (45). The fundamental temporal differences between cough and breathing appear difficult to reconcile in light of the accepted hypothesis that the core network for breathing is also responsible for phase timing during coughing. Shannon et al. (55) envisioned a core network that undergoes a process of “reconfiguration” to produce both behaviors, presenting evidence of functional interaction of multiple neuron populations. Our current modeling efforts suggest several caveats to the reconfiguration hypothesis.
First, the reconfiguration hypothesis does not fully account for the contribution of neurons that have no role in breathing but are important in the neurogenesis of coughing. By definition, second-order interneurons in cough receptor pathways are likely quiescent during breathing and are recruited during cough; these interneurons are incorporated in the present modeling efforts. VRC recruitment during cough was incorporated into previous models; however, we believe that the full extent of recruited and/or incorporated populations in the VRC, pons, etc., in cough genesis remains underestimated.
Second, there are profound differences in the sensory regulation of cough and breathing that are grounded in the function of the two behaviors. While the function of breathing is gas exchange, the sole function of cough is airway clearance. As such, the main sensory input that induces cough is located in the airways (larynx, trachea, and bronchi). However, breathing can be produced in the absence of vagal feedback and relies primarily on sensory feedback from the carotid sinus and ventral surface of the brain stem. The model incorporates vagal sensory feedback for the production of coughing but does not specifically account for the synaptic input from peripheral and central chemoreceptors. This input is simulated by an excitatory input current to the populations of neurons that participate in the core network. Cough can be produced during hyperventilation below apneic threshold (58). Preliminary simulations have shown that reduction of this input current consistent with hyperventilation will result in apnea, and coughing can still be produced by the model. Unlike breathing, there is no relationship between volume and phase timing during coughing (7). Our model retains excitatory pump cells, and these neurons excited E-Dec populations and therefore can have a role in cough phase timing. In the absence of specific information regarding the behavior of pump cells during coughing, we have not revised this aspect of the model. However, we acknowledge that neural processing of sensory information related to lung volume during cough is likely to differ significantly from that for breathing.
The proposed presence of a complex inhibitory network of NTS interneurons controlling coughing may have important implications in disease states in which cough excitability is altered. Cough hyperresponsiveness due to airway inflammation may be largely a function of reduced inhibitory control in NTS interneuronal populations. Further, reduced cough excitability could in part be due to enhanced inhibition by the same populations of neurons. The latter hypothesis is really a manifestation of the network scale of organization, in which the balance of excitatory and inhibitory inputs in a system determines function, rather than the presence or absence of highly excitable elements. This idea is effectively the hypothesis of “detailed balance” (60), in which the balance of excitatory and inhibitory activity in a network determines sensory gating of afferent input. In the medial NTS, GABAergic inhibition on vagal second-order interneurons is considerably less robust than excitatory glutaminergic neurotransmission (34), consistent with a distributed network of inhibitory axons that control second-order interneuron activity through temporal synchrony. If this mechanism is involved in the regulation of cough, it is likely that our proposed feed-forward inhibitory population consists of neurons with highly synchronized activities. As such, central processes that influence synchrony in this population may represent a primary mechanism responsible for the regulation of cough excitability.
Summary/conclusions.
This study demonstrates the variance in pattern of expiratory drive over a cough epoch in the cat. These modulations in timing and gain could not be modeled solely by inclusion of a second-order NTS population with a feed-forward function, but required the addition of separate inspiratory and expiratory second-order populations and a stronger inhibitory synaptic effect from neurons controlling phase transition from expiration to inspiration. These results support the hypothesis that afferent signals and their processing are a significant feature of the motor pattern for cough. Further, our results support the presence of multiple subtypes of NTS neurons that respond to tracheobronchial sensory feedback and have an important role in controlling cough motor bursts on a cycle-by-cycle basis.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-89104, HL-103415, HL-109025, and HL-111215, The Kentucky Spinal Cord and Head Injury Trust, The Commonwealth of Kentucky Challenge for Excellence, Rebecca F. Hammond Trust, and Veterans Affairs Rehabilitation, Research & Development B9249-S and B7165-R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.P., K.F.M., I.P., M.J.R., B.G.L., P.W.D., and D.C.B. conception and design of research; T.P., K.F.M., M.J.R., and D.C.B. performed experiments; T.P., K.F.M., L.S.S., I.P., M.J.R., and D.C.B. analyzed data; T.P., K.F.M., I.P., B.G.L., P.W.D., D.R.H., and D.C.B. interpreted results of experiments; T.P., K.F.M., L.S.S., D.R.H., and D.C.B. prepared figures; T.P., K.F.M., L.S.S., D.R.H., and D.C.B. drafted manuscript; T.P., K.F.M., L.S.S., I.P., M.J.R., B.G.L., P.W.D., D.R.H., and D.C.B. edited and revised manuscript; T.P., K.F.M., L.S.S., I.P., M.J.R., B.G.L., P.W.D., D.R.H., and D.C.B. approved final version of manuscript.
REFERENCES
- 1.Birkebæk NH, Kristiansen M, Seefeldt T, Degn J, Møller A, Heron I, Andersen PL, Møller JK, Østergård L. Bordetella pertussis and chronic cough in adults. Clin Infect Dis 29: 1239–1242, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bolser DC. Cough suppressant and pharmacologic protussive therapy. Chest 129: 238S–249S, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolser DC. Pharmacologic management of cough. Otolaryngol Clin North Am 43: 147–155, xi, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolser DC, Aziz SM, DeGennaro FC, Kreutner W, Egan RW, Siegel MI, Chapman RW. Antitussive effects of GABAB agonists in the cat and guinea-pig. Br J Pharmacol 110: 491–495, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Curr Opin Allergy Clin Immunol 7: 32–36, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther 15: 221–225, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bolser DC, Davenport PW. Volume-timing relationships during cough and resistive loading in the cat. J Appl Physiol 89: 785–790, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bolser DC, DeGennaro FC. Effect of codeine on the inspiratory and expiratory burst pattern during fictive cough in cats. Brain Res 662: 25–30, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Bolser DC, DeGennaro FC, O'Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol 113: 1344–1348, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolser DC, DeGennaro FC, O'Reilly S, McLeod RL, Hey JA. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968, in the guinea-pig and cat. Br J Pharmacol 121: 165–170, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol 86: 1017–1024, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Bolser DC, McLeod RL, Tulshian DB, Hey JA. Antitussive action of nociceptin in the cat. Eur J Pharmacol 430: 107–111, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Bonham AC, Sekizawa S, Chen CY, Joad JP. Plasticity of brainstem mechanisms of cough. Respir Physiol Neurobiol 152: 312–319, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Canning BJ. Anatomy and neurophysiology of the cough reflex: Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest 129: 33S–47S, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 24: 3916–3926, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney IK, Gibson PG, Murree-Allen K, Saltos N, Olson LG, Hensley MJ. A systematic evaluation of mechanisms in chronic cough. Am J Respir Crit Care Med 156: 211–216, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Castillo D, Pitts T. Influence of baclofen on laryngeal and spinal motor drive during cough in the anesthetized cat. Laryngoscope 123: 3088–3092, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 371: 1364–1374, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Clark F, von Euler CV. On the regulation of depth and rate of breathing. J Physiol 222: 267–295, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis CL. ABC of palliative care: breathlessness, cough, and other respiratory problems. BMJ 315: 931–934, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jongste J, Shields M. Cough. 2: chronic cough in children. Thorax 58: 998–1003, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deneve S, Latham PE, Pouget A. Reading population codes: a neural implementation of ideal observers. Nat Neurosci 2: 740–745, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Haji A, Ohi Y, Kimura S. Cough-related neurons in the nucleus tractus solitarius of decerebrate cats. Neuroscience 218: 100–109, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 65–70, 1979. [Google Scholar]
- 26.Irwin RS. Complications of cough. Chest 129: 54S–58S, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med 343: 1715–1721, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Irwin RS, Rosen MJ, Braman SS. Cough: a comprehensive review. Arch Intern Med 137: 1186–1191, 1977. [DOI] [PubMed] [Google Scholar]
- 29.Linnemann CC Jr, Nasenbeny J. Pertussis in the adult. Annu Rev Med 28: 179–185, 1977. [DOI] [PubMed] [Google Scholar]
- 30.MacGregor R. Neural and Brain Modeling. San Diego, CA: Academic, 1987. [Google Scholar]
- 31.Marchant JM, Masters IB, Taylor SM, Chang AB. Utility of signs and symptoms of chronic cough in predicting specific cause in children. Thorax 61: 694–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mateo I, Pascual J. Coexistence of chronic paroxysmal hemicrania and benign cough headache. Headache 39: 437–438, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Maynard E, Hatsopoulos N, Ojakangas C, Acuna B, Sanes J, Normann R, Donoghue J. Neuronal interactions improve cortical population coding of movement direction. J Neurosci 19: 8083–8093, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDougall SJ, Andresen MC. Low-fidelity GABA transmission within a dense excitatory network of the solitary tract nucleus. J Physiol 590: 5677–5689, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGovern A, Davis-Poynter N, Farrell M, Mazzone S. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience 207: 148–166, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci 27: 6832–6842, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mello CJ, Irwin RS, Curley FJ. Predictive values of the character, timing, and complications of chronic cough in diagnosing its cause. Arch Intern Med 156: 997–1003, 1996. [PubMed] [Google Scholar]
- 38.Mifflin SW. Laryngeal afferent inputs to the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 265: R269–R276, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol 295: R243–R251, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Mutolo D, Cinelli E, Bongianni F, Pantaleo T. Inhibitory control of the cough reflex by galanin receptors in the caudal nucleus tractus solitarii of the rabbit. Am J Physiol Regul Integr Comp Physiol 307: R1358–R1367, 2014. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor R, Segers LS, Morris KF, Nuding SC, Pitts T, Bolser DC, Davenport PW, Lindsey BG. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Front Physiol 3: 264, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott MM, Nuding SC, Segers LS, Lindsey BG, Morris KF. Ventrolateral medullary functional connectivity and the respiratory and central chemoreceptor-evoked modulation of retrotrapezoid-parafacial neurons. J Neurophysiol 105: 2960–2975, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pantaleo T, Bongianni F, Mutolo D. Central nervous mechanisms of cough. Pulm Pharmacol Ther 15: 227–233, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Pearson K. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16: 265–297, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Pitts T, Rose MJ, Mortensen AN, Poliaček I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol 189: 543–551, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poliaček I, Corrie LW, Rose MJ, Wang C, Bolser DC. Influence of microinjections of d,l-homocysteic acid into the Botzinger complex area on the cough reflex in the cat. J Physiol Pharmacol 59, Suppl 6: 585–596, 2008. [PMC free article] [PubMed] [Google Scholar]
- 47.Poliaček I, Corrie LWC, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol 102: 1014–1021, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poliaček I, Morris KF, Lindsey BG, Segers LS, Rose MJ, Corrie LWC, Wang C, Pitts TE, Davenport PW, Bolser DC. Blood pressure changes alter tracheobronchial cough: computational model of the respiratory-cough network and in vivo experiments in anesthetized cats. J Appl Physiol 111: 861–873, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poliaček I, Wang C, Corrie LWC, Rose MJ, Bolser DC. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J Appl Physiol 108: 858–865, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O'Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol 113: 352–368, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekizawa S, Joad JP, Pinkerton KE, Bonham AC. Secondhand smoke exposure alters K+ channel function and intrinsic cell excitability in a subset of second−order airway neurons in the nucleus tractus solitarius of young guinea pigs. Eur J Neurosci 31: 673–684, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Shannon R, Baekey D, Morris K, Lindsey B. Brainstem respiratory networks and cough. Pulm Pharmacol 9: 343–347, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Shannon R, Baekey D, Morris K, Nuding S, Segers L, Lindsey B. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther 17: 369–376, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol 84: 2020–2035, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Smith JA, Aliverti A, Quaranta M, McGuinness K, Kelsall A, Earis J, Calverley PM. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J Physiol 590: 563–574, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 402: 411–420, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomori Z, Sedíková V, Javorka K. Changes in blood gas tensions and acid-base balance during respiratory reflexes. Physiol Bohemoslov 22: 305–314, 1973. [PubMed] [Google Scholar]
- 59.Tomori Z, Widdicombe J. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J Physiol 200: 25–49, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogels TP, Abbott L. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci 12: 483–491, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Saha S, Rose MJ, Davenport PW, Bolser DC. Spatiotemporal regulation of the cough motor pattern. Cough 5: 12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widdicombe J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A Discov Mol Cell Evol Biol 270: 2–10, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Widdicombe J. Neurophysiology of the cough reflex. Eur Respir J 8: 1193–1202, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Widdicombe J. Reflexes from the lungs and airways: historical perspective. J Appl Physiol 101: 628–634, 2006. [DOI] [PubMed] [Google Scholar]