Increases in total capillary length of the hippocampus occur linearly in response to increasing cyclical intermittent hypoxia (CIH) severity, but an increase in GLUT1 transporter along endothelial cells occurs only at very severe CIH. This highlights that there may be multiple mechanisms when exposed to different levels of CIH, providing a strong rationale for future CIH experiments to include multiple levels of CIH.
Keywords: obstructive sleep apnea, cyclical intermittent hypoxia, blood-brain barrier, angiogenesis, GLUT1 transporter, vascular endothelium, VEGF
Abstract
Recent studies have shown an association between obstructive sleep apnea (OSA) and cognitive impairment. This study was done to investigate whether varied levels of cyclical intermittent hypoxia (CIH) differentially affect the microvasculature in the hippocampus, operating as a mechanistic link between OSA and cognitive impairment. We exposed C57BL/6 mice to sham [continuous air, arterial O2 saturation (SaO2) 97%], severe CIH to inspired O2 fraction (FiO2) = 0.10 (CIH10; SaO2 nadir of 61%), or very severe CIH to FiO2 = 0.05 (CIH5; SaO2 nadir of 37%) for 12 h/day for 2 wk. We quantified capillary length using neurostereology techniques in the dorsal hippocampus and utilized quantitative PCR methods to measure changes in sets of genes related to angiogenesis and to metabolism. Next, we employed immunohistochemistry semiquantification algorithms to quantitate GLUT1 protein on endothelial cells within hippocampal capillaries. Capillary length differed among CIH severity groups (P = 0.013) and demonstrated a linear relationship with CIH severity (P = 0.002). There was a strong association between CIH severity and changes in mRNA for VEGFA (P < 0.0001). Less strong, but nominally significant associations with CIH severity were also observed for ANGPT2 (PANOVA = 0.065, PTREND = 0.040), VEGFR2 (PANOVA = 0.032, PTREND = 0.429), and TIE-2 (PANOVA = 0.006, PTREND = 0.010). We found that the CIH5 group had increased GLUT1 protein relative to sham (P = 0.006) and CIH10 (P = 0.001). There was variation in GLUT1 protein along the microvasculature in different hippocampal subregions. An effect of CIH5 on GLUT1 mRNA was seen (PANOVA = 0.042, PTREND = 0.012). Thus CIH affects the microvasculature in the hippocampus, but consequences depend on CIH severity.
NEW & NOTEWORTHY
Increases in total capillary length of the hippocampus occur linearly in response to increasing cyclical intermittent hypoxia (CIH) severity, but an increase in GLUT1 transporter along endothelial cells occurs only at very severe CIH. This highlights that there may be multiple mechanisms when exposed to different levels of CIH, providing a strong rationale for future CIH experiments to include multiple levels of CIH.
an estimated 70–80% of patients with Alzheimer's dementia meet the criteria for obstructive sleep apnea (OSA) [defined as an apnea hypopnea index (AHI) >5], with 38–48% of this patient group having an AHI > 20 (1–3, 16). In elderly women, the presence of OSA (AHI > 15) is associated with an increased risk of future development of dementia or mild cognitive impairment (43). This association is related to measures of hypoxia, but not to arousal index (43). Therefore, a likely underlying pathophysiological mechanism is chronic cyclical intermittent hypoxia (CIH). One role CIH may have on brain pathology may be its effects on the hippocampus, an area critical to memory retention.
The brain accounts for ∼2% of the body's weight, but 20% of the total oxygen consumption and 25% of the total glucose utilization (10). Oxygen is critical to generating ATP, and glucose is the main energy substrate for neurons, since 99% of circulating free fatty acids are bound to albumin and cannot cross the blood-brain barrier (BBB) (31). It is estimated that mean oxygen diffusion is ∼100 μm using a Krogh model (19); thus the proximity of cells to capillaries is important. Glucose, however, largely moves across the BBB via facilitated diffusion using the glucose transporter, GLUT1, transporting glucose at a rate up to 50,000 times greater than uncatalyzed transmembrane diffusion of glucose (29). The BBB maintains a strict homeostatic environment within the brain that is significantly different than that of blood, while dynamically responding to increased demands for oxygen and glucose (11, 44). Therefore, when the BBB is exposed to stressors, it strives to maintain homeostasis, although persistent stress may gradually lead to maladaptation (14). Therefore, one way chronic CIH can affect the hippocampus is via the BBB (for review of role of BBB in cognitive impairment, see Ref. 24).
Angiogenesis of skeletal muscle capillaries has been shown to increase in patients with OSA (40, 41). Angiogenesis has also been measured in hippocampal capillaries of neonatal rats exposed to one level of CIH (18). There are, however, no studies comparing the effects of different levels of CIH on angiogenesis, or potential mechanisms of CIH-induced angiogenesis. Another possible response to chronic CIH at the BBB may be changes in expression of various BBB transporters, for example, GLUT1. Although GLUT1 has been reported to not change significantly in response to CIH, only one level of hypoxia [inspired O2 fraction (FiO2) = 0.10] was tested (17). For other outcomes associated with CIH, a “dose-dependent” response to specific levels of CIH severity has been demonstrated. For example, Li et al. (22) demonstrated hypertriglyceridemia and hypercholesterolemia in mice exposed to very severe CIH (FiO2 0.21-0.05), but not at severe CIH (FiO2 0.21–0.10). Given the severity of CIH needed to observe this effect, it is possible that other pathways are involved at less severe CIH levels.
Epidemiological studies indicate the majority of OSA patients do not have severe or very severe OSA (26, 46), and thus do not experience hypoxia to the extent studied in rodent models of CIH (23). Further complicating the matter is one of scalar differences between mice and men in response to CIH consequences. Thus it is unknown whether the consequences of CIH seen in mice exposed to an FiO2 of 0.05 are relevant to the majority of patients with OSA. Our global hypothesis is that CIH leads to changes in the BBB, including increases in capillary length and expression of the BBB transporter, GLUT1. To address this hypothesis, this paper examines differences in these BBB responses to different levels of CIH.
One approach to resolve these issues is to design experiments in mice that look at multiple doses of CIH. To examine CIH “dose responses” in the hippocampus requires techniques that are sensitive enough to quantitate functional changes, such as angiogenesis and GLUT1 transporter protein expression within capillaries. Toward this end, we utilized neurostereology to quantitate capillary length (28), and, when measurements made in mice exposed to CIH compared with sham controls, this represents angiogenesis, the formation of new blood vessels from preexisting vessels. We also utilized a new semiquantitative method based on immunohistochemistry to quantify expression of GLUT1 protein on capillary endothelial cells in mice (38). In addition, we measured the mRNA expression of sets of ligands and their receptors known to be involved in angiogenesis, as well as genes involved in metabolism. We report a linear increase in the degree of angiogenesis with increasing CIH severity; expression of some, but not all, genes involved in angiogenesis also increases linearly in response to CIH. In addition, GLUT1 protein shows a strong increase only at the very severe CIH, but demonstrates a potential linear increase at the mRNA level. Altogether, results demonstrate that different severities of CIH modify different genes and proteins within the microvasculature of the hippocampus. It remains to be determined whether these changes are protective or have adverse consequences.
METHODS
Animals and Administration of CIH
The Institutional Animal Care and Use Committee at the University of Pennsylvania approved experiments in accordance to National Institutes of Health policy. We subjected C57BL/6 male mice, age 4–5 mo old (National Institute on Aging, Bethesda, MD) to CIH for 12 h/day during their lights on period (0600–1800) for 2 wk. Mice were fed a regular chow diet. We utilized the Hycon system to produce CIH as validated previously (23). The HyCon (S. Savransky, West Des Moines, IA) uses a closed-loop control mechanism to regulate air/N2 delivery by making direct comparisons between the oxygenation points set by the investigator to an oxygen sensor sampling the gas inside the home cage. A MouseOx was used to validate the Hycon in C57BL/6 (23) at multiple arterial O2 saturation (SaO2) nadirs (24). Mice were subject to one of three conditions: 1) sham (continuous air, SaO2 95%); 2) severe intermittent hypoxia from FiO2 0.21-0.10 (CIH10; SaO2 nadir 61%, 30 s for desaturation and 30 s for resaturation); or 3) very severe intermittent hypoxia from FiO2 0.21-0.05 (CIH5; SaO2 nadir 37%, 30 s for desaturation and 30 s for resaturation). Mice were killed at 10 AM, after 4 h of sham or intermittent hypoxia, and brains were harvested as previously described (38).
On the day of death, mice were taken out one at a time from their home cage (i.e., no recovery time out of the system). For neurostereology and immunohistochemistry semiquantification, the mouse was weighed, and then anesthetized with ketamine, 100–200 mg/kg body wt by intraperitoneal injection. After the mouse was under deep anesthesia, we performed an intracardiac perfusion at 120 mmHg of pressure, first with normal saline for 5 min and then with a 4% formaldehyde solution for 5 min. Brains were carefully harvested and placed in 4% formaldehyde at 4°C for 24 h and then cryopreserved with 30% sucrose at 4°C for 2 days and then a fresh 30% sucrose solution for another 2 days at 4°C. Brains were then quickly rinsed with tap water, dried with a kimwipe, frozen using dry ice that was pounded into a fine powder, and then stored at −80°C. For quantitative PCR (qPCR), the mice were not anesthetized and quickly decapitated. These brains were carefully harvested and immediately frozen using dry ice and then stored at −80°C until hippocampi were dissected out and RNA obtained.
Immunohistochemistry
Frozen brains were sliced on a cryostat in the coronal plane at 40 μm and stored in 0.1% sodium azide in PBS. Slices were initially quenched in 60% methanol and then blocked [4% normal donkey serum (Jackson ImmunoLab) and 1% BSA (Rockland) in Tris-buffered saline] for 90 min. We then used primary antibodies for 16 h at room temperature on an orbital shaker: CD31, a protein expressed in capillary endothelial cells, at 1:500 (goat, R&D Systems, cat. no. AF3628) and GLUT1 (rabbit, Abcam, cat. no. ab652) at 1:500. Slices were then incubated in biotinylated secondary antibody (Jackson ImmunoLab) at 1:5,000 for 2 h at room temperature and then placed in Vectastain Elite ABC (Vector Laboratories) diluted at 1:500 for 90 min. Lastly, we incubated the slices in a solution with diaminobenzidine (final concentration 0.2 mg/ml, Sigma) and nickel (final concentration 0.3%, Sigma-Aldrich) for exactly 20 min.
Assessment of Angiogenesis within the Hippocampus using Neurostereology
CD31, also known as platelet endothelial cell adhesion molecule-1, is found in the intercellular junctions between endothelial cells of the BBB and is commonly used to outline blood vessels. Slices stained with CD31 were subjected to neurostereology techniques using Stereo Investigator (mbf Bioscience) to acquire three-dimensional measurement of microvessel length, as previously described (28). A pilot study was conducted using one of every three slices of the entire dorsal hippocampus sections [landmarks described by Mouton et al. (28)], for a total of 18 sections. The pilot study determined 10 slices, counting both left and right hippocampi were needed to maintain a coefficient of error of <10% (calculated coefficient of error was between 4 and 6%). Therefore, 10 slices from each of five mice in each of the three conditions (150 total slices) were stained for CD31 and assessed for capillary length. Final settings using the hemisphere program to measure vessel length at ×100 are as follows: 1) setting an 18-μm radius hemisphere with 2) a guard zone of 1 μm on the top and 1 μm on bottom, resulting in 3) an average tissue height that ranged from 19 to 21 μm 4) in each of the two regions of interest (left and right hippocampus), whereby a 700 × 550 μm counting frame (sampling grid area of 385,000 μm2) was employed, resulting in 5) a range of 2–10 grids, with an average of 5–7 grids counted per region of interest; 6) we counted intersections with the hemisphere (that were in focus) at 1-μm steps, which were counted to determine capillary length in a three-dimensional space.
Assessment of GLUT1 Expression using Immunohistochemistry Semiquantification
Two hippocampal slices between bregma −1.82 mm to −2.06 mm were selected from each of five mice in each of the three conditions and stained for GLUT1 protein, which is known to be on the endothelial cells of the brain (15). We utilized immunohistochemistry semiquantification (ISQ), an algorithm we developed to automate the measurement of protein expression intensity on hippocampal capillaries (38).
Images were taken at ×20 magnification using a 5-megapixel CCD Bayer Array RGB filter camera (DFC-425, Leica, Germany) mounted on a digital light microscope (DM5500B, Leica). Digital images were acquired as 16-bit gray-scale 2,592 × 1,944 pixel frames and saved in TIFF format. The pixel-to-pixel distance at the specimen level is 0.5 μm. After a Kohler alignment was done at ×20 magnification, the same settings (exposure, gamma, gain) were applied to every image for GLUT1. Images of both the left and right hippocampus were taken, with each hippocampus resulting in ∼9–13 images/side of hippocampus. Each image consisted of a 5-μm z-stack range stepped at 1 μm, resulting in a set of six z-stack images. All sets of images were then run through ISQ to identify blood vessels exhibiting GLUT1 stain uptake, which virtually extracted and parameterized every vessel using a custom MATLAB workflow. Then, using feature recognition to identify vessels of all diameters, we quantitated protein expression by calculating the average intensity of the stain, equal to the total intensity normalized by the area of the blood vessel. When interpreting ISQ data, the lower the intensity, the blacker the stain; thus the lower the number, the higher the protein level of GLUT1 on the microvessel. For ease of interpretation, we inverted the intensity values by subtracting the calculated average intensity value from the maximum possible intensity (equal to 65,535); thus higher values mean more protein. We note that the relative direction of effect and differences among conditions is of particular interest in these experiments, not the absolute intensity values. The false positive rate of detecting a capillary was found to be 5.04% and correlated with the degree of background complexity (38). The false negative rate as a function of background complexity ranged from 6.25 to 8.25%, with the average being 7.49% (38).
Yoshihara et al. (45) showed that cortical capillaries of live mice started with a mean (±SD) diameter of 5.0 ± 1.0 and 4.9 ± 0.9 μm at day 0 before exposure was started to 10 and 8% O2 hypoxia conditions, and reached 6.7 ± 1.8 and 8.5 ± 1.9 μm, respectively, by day 21 of exposure. We may, therefore, expect the diameter of a capillary to range from 2 μm (equal to 5.0 − 3 SD) to 14.2 μm (equal to 8.5 + 3 SD). Using the minimum and maximum values that result from applying a threshold of 3 SD to the mean estimates is expected to cover >99% of the given distribution. Converting from micrometers to pixels results in an expected diameter range of 4–28.4 pixels; thus analyses comparing expression levels on capillaries among the conditions are restricted to vessels falling within this diameter range. Therefore, data that are presented are from microvessels with a diameter of 2–14.2 μm. A pilot study on 20 micrographs selected at random underwent random forest learning to identify microvessels from all of the segmented candidates in the image, resulting in a false positive rate of 5.04% for the identification of capillaries (38).
RNA Isolation and Reverse Transcription
Hippocampus regions were carefully dissected while the tissue was on dry ice. In brief, a coronal cut was first made using a no. 11 surgical blade at approximately bregma −1.5 mm using landmarks. Two sequential coronal slices from ventral to dorsal, each ∼0.5 mm thick, were made, and the entire hippocampus regions from these two slices were isolated and removed. The isolated hippocampi were placed into an Eppendorf tube and kept frozen until tissue was homogenized in Trizol reagent (Invitrogen). Total RNA was purified by phase separation with chloroform, and the top clear phase was mixed with equal volume of 70% ethanol and further purified with RNeasy Mini kit (Qiagen). Reverse transcription reactions were performed using High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems), following the manufacturer's instructions.
Real-Time Quantitative PCR
Gene expression was measured in an ABI Prism 7900HT Real Time PCR system using TaqMan FAM-labeled specific probes (Applied Biosystems). The following TaqMan probes were used: for housekeeping genes to normalize data, TATA box binding protein (TBP; Mm00446971_m1) and hypoxanthine-guanine phosphoribosyl transferase (HPRT; Mm01545399_m1); for genes of ligands involved in the angiogenesis process, vascular endothelial growth factor A (VEGFA; Mm01281449_m1), angiopoietin-1 (ANGPT1; Mm00456503_m1), angiopoietin-2 (ANGPT2; Mm00545822_m1), brain-derived neurotrophic factor (BDNF; Mm04230607_s1), fibroblast growth factor 2 (FGF-2; Mm00433287_m1), and delta-like ligand 4 (DLL4; Mm00444619_m1), a negative regulator of angiogenesis; for genes of receptors of the above ligands, FMS-like tyrosine kinase 1 (FLT-1, alias VEGFR1; Mm00438980_m1), kinase insert domain protein receptor (KDR, alias VEGFR2; Mm01222421_m1), tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE-1; Mm00441786_m1), which acts as a cell-surface receptor for ANGPT1, endothelial-specific receptor tyrosine kinase (TEK, alias TIE-2; Mm00443243_m1), which acts as a cell-surface receptor for ANGPT2, neurotrophic tyrosine kinase receptor type 2 (NTRK2, alias TRK2; Mm00435422_m1), which acts as a cell-surface receptor for BDNF, FGF receptor 1 (FGFR-1; Mm00438930_m1), FGF receptor 2 (FGFR-2; Mm01269930_m1), and Notch1 (NOTCH1; Mm00435249_m1), which acts as a cell-surface receptor for DLL4; for genes of transcription factors, hypoxia inducible factor 1, α-subunit (HIF-1α; Mm00468869_m1), peroxisome proliferative activated receptor-γ coactivator 1α (PPARGC-1α, alias PGC-1α; Mm00447181_m1), peroxisome proliferative activated receptor-γ coactivator 1β (PPARGC-1β, alias PGC-1β; Mm00504720_m1); for genes of glucose metabolism, solute carrier family 2 (facilitated glucose transporter) member 1 (SLC2A1, alias GLUT1; Mm00441480_m1), mannoside acetylglucosaminyltransferase 5 (MGAT5; Mm00455036_m1), insulin-like growth factor I (IGF-I; Mm00439560_m1), insulin receptor (INSR; Mm01211875_m1). Gene expression levels were normalized to the geometric mean of TBP and HPRT; expression of these housekeeping genes did not change with CIH (data not shown).
Statistical Analysis
Descriptive statistics.
Continuous variables are summarized using means, SDs, SEs, and/or 95% confidence intervals, where appropriate. Continuous variables were assessed for normality and transformed, if necessary, to meet model assumptions. Categorical variables are summarized using frequencies and percentages.
Neurostereology: power, reliability, and reproducibility.
Within our sample, we observed a pooled standard deviation of 2,360,361, and between CIH condition differences of 5,267,825 (effect size = 2.2 SDs) between sham and CIH5, 3,252,148 (effect size = 1.4 SD) between CIH10 and CIH5, and 2,015,677 (effect size = 0.85 SD) between CIH10 and sham. These estimates correspond to an overall linear contrast effect size of 1.6. Using this effect size estimate and an α = 0.05, our total of five mice per group results in 76% power to detect the observed effect and more than 85% power for the observed between group difference of 2.2 SDs between CIH5 and sham. Importantly, in the current analyses, we observed a statistically significant difference in total length among CIH conditions (PANOVA = 0.013), with a significant difference between CIH5 and sham (P = 0.004) and a borderline difference between CIH10 and CIH5 (P = 0.050). Thus, independent of a priori power, we can conclude that CIH significantly affects vessel length.
To assess the reliability and reproducibility of the neurostereology methodology, we had two independent raters both score one set of five slices, randomly selected, to assess interscorer reliability, and one of the scorers scored the same set of five slices twice to assess intrascorer reliability. The two raters were well-trained laboratory technologists. We then used a linear mixed model to calculate intraclass correlation coefficients estimating the proportion of variability due to mouse (true biological variability), rater within mouse (i.e., differences between the 2 raters in a given mouse), and residual error (due to a combination of technical and replication errors).
Comparisons of different CIH severities within mice.
We compared the outcome measures obtained via neurostereology, ISQ, and qPCR among the three CIH conditions using two complementary methodologies. 1) A P value from the analysis of variance (ANOVA) assessing the null hypothesis of no differences between groups tested whether there are any differences among the groups (e.g., CIH conditions). This was obtained by including the condition as a categorical variable and used to test whether there was any relationship between CIH group and outcome (using a 2 degree-of-freedom test of significance), regardless of the shape of this relationship. 2) modeling condition as an ordinal variable (0 = sham, 1 = CIH10, and 2 = CIH5) to assess whether there was a linear trend (i.e., dose effect) across the conditions. The P value for trend assesses whether there is a linear relationship between increasing CIH severity and changes in outcomes, such that the difference between the sham and CIH10 groups is similar to the difference between CIH10 and CIH5 groups. This analysis is performed by including CIH group as an ordinal/continuous variable in analyses and using a 1 degree-of-freedom test of significance. Combined with observed values, differences in these two P values help clarify the nature of the relationship between CIH and outcome. If a significant difference among conditions was observed (P < 0.05), we performed pairwise comparisons between conditions.
For outcome measures that contained only one measure per mouse (neurostereology and qPCR), a standard linear regression model was used. To satisfy assumptions of normality, expression levels obtained through qPCR were analyzed using −ΔΔCT values, which is defined as negative 1 times the difference between (the ΔCT in a given animal) and (the average ΔCT in the sham animals for each gene). For ease of interpretation, results are shown as fold change (equal to 2−ΔΔCT). For GLUT1 data from ISQ, which quantifies GLUT1 intensity on a large number of vessels per mouse, we used a linear mixed-model accounting for correlations within mouse and subregion, and adjusting for vessel diameter. As previously mentioned, we restricted our ISQ analyses to vessels determined likely to be capillaries, based on data of Yoshihara et al. (45).
qPCR gene subsets.
We designed the qPCR experiment to evaluate specific groups of genes thought to be involved in the angiogenesis and glucose metabolism. Given this, genes were separated into four distinct domains/subgroups: 1) ligands of angiogenesis: VEGFA, ANGPT1, ANGPT2, BDNF, FGF, and DLL4; 2) ligand receptors of angiogenesis: VEGFR1, VEGFR2, TIE-1, TIE-2, TRK2, FGFR-1, FGFR-2, NOTCH1; 3) transcription factors involved in angiogenesis: HIF-1α, PGC-1α, PGC-1β; and 4) glucose metabolism: GLUT1, MGAT5, IGF-I, and INSR. Given the independent hypothesized pathways for each of these gene subsets, we used a domain-specific Bonferroni correction for the number of genes within each subgroup to determine statistical significance. Thus we had the following significance levels: 1) ligands, P < 0.0083 (equals 0.05/6); 2) ligand receptors, P < 0.00625 (equals 0.05/8); 3) signaling pathway, P < 0.0167 (equals 0.05/3); and 4) glucose metabolism, P < 0.0125 (equals 0.05/4). Any result with a P < 0.05 was described as nominally significant.
RESULTS
Linear Increase in Capillary Length with Increasing Severity of CIH
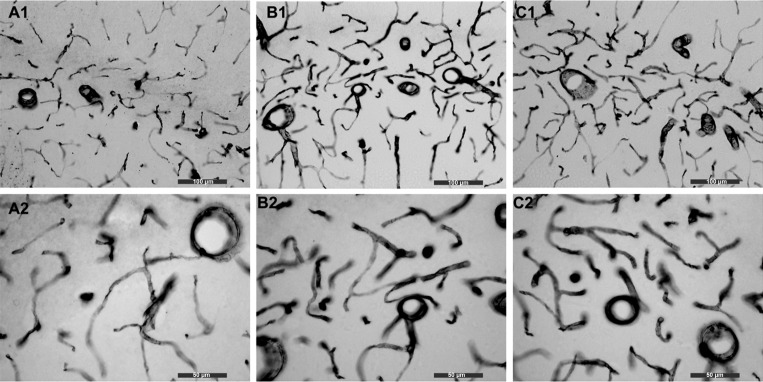
Performing unbiased estimates of total length for linear objects (e.g., capillaries or neuronal fibers) within a tissue region has allowed us to have some confidence in measuring increases and decreases in length of linear objects in response to stressors, aging, and disease (8). Figure 1 presents representative images of CD31 staining of hippocampal capillaries from each of the three conditions (sham, CIH10, and CIH5) at ×20 (Fig. 1, A1, B1, and C1) and ×40 magnification (Fig. 1, A2, B2, and C2). Although there is visual appreciation that angiogenesis (defined as growth of new blood vessels that would translate to an increase in capillary length) is different between the conditions, to directly quantify total capillary length, we used the neurostereology technique, as described in methods.
Fig. 1.
Immunohistochemistry for CD31 to quantitate angiogenesis. We used immunohistochemistry for CD31 followed by neurostereology to count the total length of microvessels in our three conditions. A: sham (A1 is at ×20 and A2 is at ×40 magnification). B: CIH10 (B1 is at ×20 and B2 is at ×40 magnification). C: CIH5 (C1 is at ×20 and C2 is at ×40 magnification). It is appreciated by visual inspection that there are more blood vessels in the CIH10 group compared with sham, and more blood vessels in the CIH5 group compared with sham and CIH10. ×20 bar = 100 μm; ×40 bar = 50 μm.
First, to quantify reproducibility and reliability, we had two separate raters score five randomly chosen mice twice. We observed that 64% of the total variability was attributable to differences between mice, i.e., to real biological variability, indicating that the technique provides a reliable measure that captures the differences in capillary length. There was also good concordance between the two raters: <4% of the total variability was due to differences in the two rater's scores for a given mouse. There was a moderate level of residual variability not explained by the two factors above, which is likely explained by technical variability in the neurostereology analysis caused by randomly selecting regions to count length.
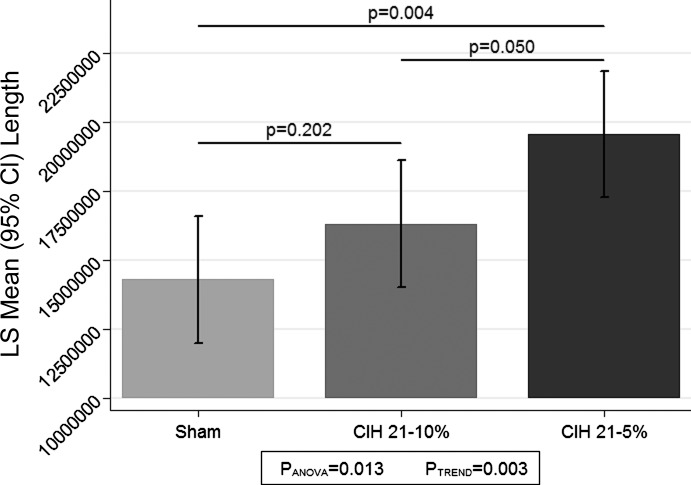
Comparisons of total capillary length among the three CIH conditions are shown in Fig. 2. We observed a statistically significant difference in average total capillary length among the three conditions (P = 0.013). When examining the pairwise differences between conditions, we note that the very severe CIH group (CIH5) had a significantly greater total length than sham (P = 0.004), and borderline had a greater length compared with CIH10 (P = 0.050). While there was no significant difference between the sham and CIH10 conditions (P = 0.202), we observed strong evidence for a linear trend across the three CIH conditions (P = 0.003), indicating that there is a dose-response relationship between increased CIH severity and total vessel length.
Fig. 2.
Angiogenesis (total vessel length) as measured by neurostereology. The figure illustrates the estimated least squares (LS) mean and 95% confidence interval (CI) of total vessel length among the 3 conditions: sham, CIH10, and CIH5. For each condition, n = 5 mice, 2 slices per mouse, for both the left and right hippocampus. The average probed volume are as follows: sham = 7,282,841 μm3, CIH10 = 7,194,943 μm3, and CIH5 = 7,519,552 μm3. There was a significant difference among the three conditions (P = 0.013) and strong evidence for a linear trend across conditions (P = 0.003). In pairwise comparisons between groups, CIH5 had significantly longer vessel length compared with sham (P = 0.004) and borderline significantly longer length compared with CIH10 (P = 0.050); there was no statistically significant difference between sham and CIH10 (P = 0.202).
CIH Alters Gene Expression of VEGFA and Other Genes Related to Angiogenesis
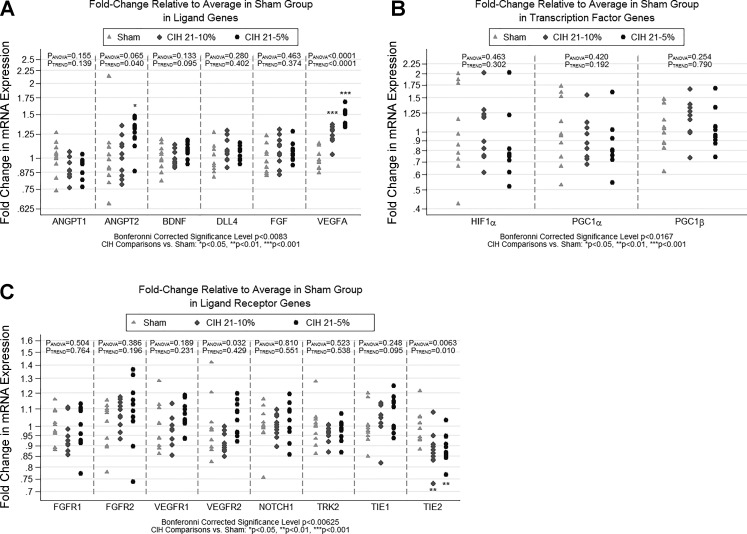
Figure 3 shows the relationship between the three CIH conditions and mRNA expression in ligand, ligand receptor, and transcription factor genes known to be related to angiogenesis. We observed strong differences among the CIH conditions for expression of VEGFA (P < 0.0001), which met our Bonferroni-corrected level of significance. Specifically, the CIH5 condition had significantly increased VEGFA expression compared with both sham (P < 0.0001) and CIH10 (P = 0.0002), and CIH10 had increased VEGFA expression compared with sham (P < 0.0001); given this relationship, there was clear evidence of a linear dose response (P < 0.0001). We observed nominally or borderline significant associations between CIH conditions and ANGPT2 (P = 0.065), VEGFR2 (P = 0.032), and TIE-2 (P = 0.006). For ANGPT2, we observed significantly increased expression in the CIH5 group compared with both sham (P = 0.039) and CIH10 (P = 0.046). As shown in Fig. 3, there was one sham animal with a large fold-increase in ANGPT2, which may be considered an outlier. Removing this point resulted in a Bonferroni-corrected significant difference among CIH conditions (P = 0.002). For VEGFR2, we did not see the expected linear dose response across conditions. Rather, we saw a significant difference between CIH10 and CIH5 (P = 0.011), with CIH10 showing reduced expression and CIH5 increased expression. Neither condition was significantly different from sham, as reflected in the nonsignificant linear trend (P = 0.429). For TIE-2, the overall P value observed (P = 0.0063) fell just slightly above our Bonferroni-corrected significance level of P < 0.00625; given the conservative nature of Bonferroni corrections, it is likely that this may prove to be a real effect. When comparing between groups, there was significantly decreased expression of TIE-2 for both CIH5 (P = 0.008) and CIH10 (P = 0.004) compared with sham. All other genes related to angiogenesis showed no changes in expression.
Fig. 3.
Fold change in qPCR gene expression for angiogenesis-related genes. The figure illustrates the results of qPCR analyses examining expression of three subsets of genes hypothesized to be related to angiogenesis. Results are shown as fold change in expression relative to the sham condition. Fold change is measured as 2−ΔΔCT for each gene, where the first Δ is the average CT of the housekeeping genes, and the second Δ is the average ΔCT in the sham condition. Analyses were based on −ΔΔCT values, which are measured on a linear scale. Based on a priori hypothesized pathways, genes were separated into 3 distinct domains/subgroups. A: ligands: VEGFA, ANGPT1, ANGPT2, BDNF, FGF, and DLL4. B: ligand receptors: VEGFR1, VEGFR2, TIE-1, TIE-2, TRK2, FGFR-1, FGFR-2, NOTCH1. C: transcription factors: HIF-1α, PGC-1α, and PGC-1β. Statistical significance was based on a domain-specific Bonferroni corrected α-level (equal to 0.05 divided by the number of genes in the domain). Significant or nominally significant differences were observed for VEGFA, ANGPT2, VEGFR2, and TIE-2. The strongest differences among conditions were seen for VEGFA (P < 0.0001; significant after correction for multiple ligand genes), with both CIH5 and CIH10 showing significantly increased expression compared with sham. We also observed nominal or borderline significant differences among the conditions for the ligand gene ANGPT2 and ligand receptors VEGFR2 and TIE-2. We note that the TIE-2 result was just slightly above our Bonferroni-corrected threshold for significance (P < 0.00625). Significant difference vs. sham: *P < 0.05, **P < 0.01, ***P < 0.001.
CIH Alters Gene Expression of GLUT1, But Not Other Genes Related to Metabolism
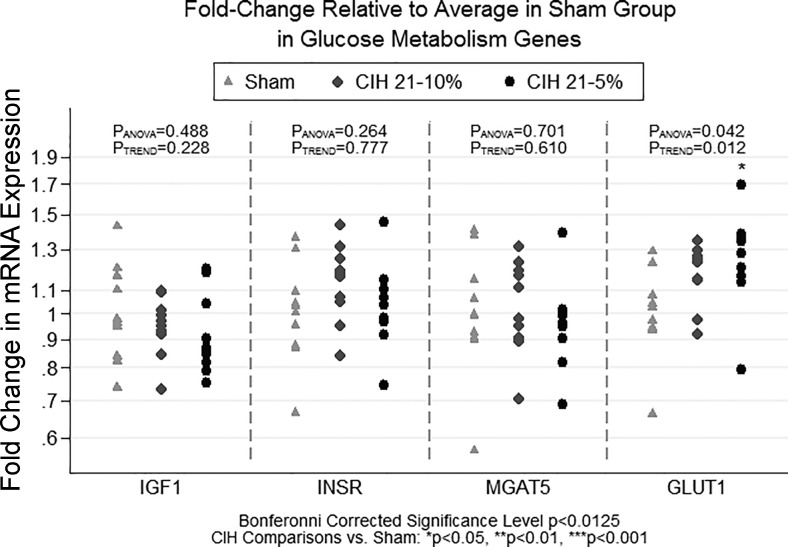
Figure 4 shows the relationship between the three CIH conditions and mRNA expression of glucose metabolism genes. We observed a borderline significant association among CIH conditions for GLUT1 (P = 0.042). GLUT1 expression was significantly increased in CIH5 compared with sham (P = 0.014); we observed no significant difference in mRNA expression of GLUT1 between the CIH5 and CIH10 conditions (P = 0.353) and no difference between CIH10 and sham (P = 0.102). We note that there was evidence of a linear trend across the conditions (PTREND = 0.012).
Fig. 4.
Fold change in qPCR gene expression for glucose metabolism genes. The figure illustrates the results of qPCR analyses examining expression of IGF-I, INSR, MGAT5, and GLUT1, genes hypothesized to be related to metabolism within the hippocampus across CIH severities. Results are shown as fold change in expression relative to the sham condition. Fold change is measured as 2−ΔΔCT for each gene, where the first Δ is the average of the housekeeping genes, and the second Δ is the average ΔCT in the sham condition. Analyses were based on −ΔΔCT values, which are measured on a linear scale. We observed a significant difference in GLUT1 expression among CIH severity groups, with the CIH5 group showing significantly higher expression compared with sham. There is also evidence of a linear dose response in mRNA expression across CIH conditions. Significant difference vs. sham: *P < 0.05.
CIH Alters Protein Expression of GLUT1 at Only the Very Severe Level of CIH
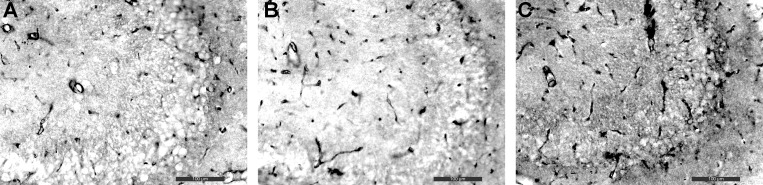
We used an ISQ algorithm to assess the quantity of GLUT1 protein along the luminal and abluminal surface of the endothelial cell. A clear benefit of immunohistochemistry over Western blots is that it can identify the specific location of proteins such as GLUT1 within the hippocampus, specifically the endothelial cells of the BBB. Representative images of the hippocampus are presented from each of the three conditions at ×20 magnification (Fig. 5). Visual inspection of microvessel darkness appears to be similar for sham and CIH10, with CIH5 noticeably darker (e.g., more protein) compared with other conditions, indicating increased expression of GLUT1.
Fig. 5.
Immunohistochemistry staining for GLUT1 proteins. Representative images of GLUT1 staining in the hippocampus are shown for the three CIH conditions: sham (A), CIH10 (B), and CIH5 (C). It is appreciated by visual inspection that there is more GLUT1 protein in the CIH5 group, as evidenced by the darker staining, compared with sham and CIH10. ×20 bar = 100 μm.
We quantified the average intensity of GLUT1 expression using ISQ, as described previously (38). A total of 1,073,264 vessels were identified and quantified for GLUT1 expression (measured as the intensity of stain/area of vessel). Of these, 740,614 (69.0%) were considered to be capillaries based on the expected diameter range described above (2–14.2 μm), and carried forward in the analyses comparing GLUT1 expression among CIH conditions. The total number of identified capillaries across the three conditions was 233,278 in mice exposed to sham; 239,944 in the CIH10 condition; and 267,392 in the CIH5 condition.
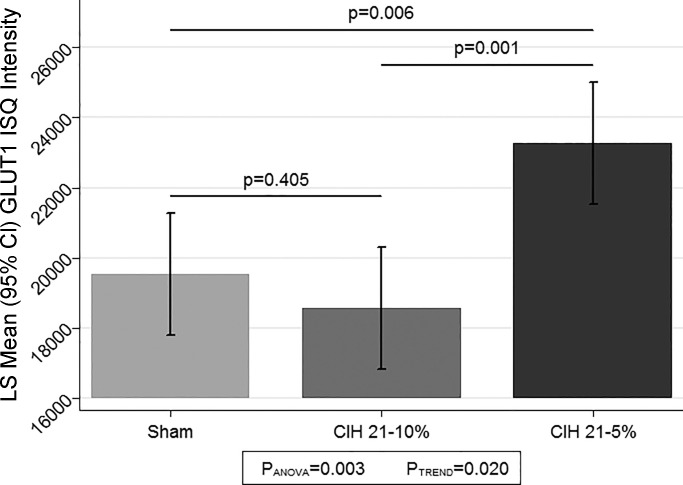
The comparison of average GLUT1 intensity across the three CIH conditions is illustrated in Fig. 6. As with the total capillary length, e.g., angiogenesis data, we observed a significant difference in GLUT1 among the three conditions (P = 0.003). In pairwise comparisons between the CIH groups, the very severe CIH5 group had significantly more GLUT1 than both the sham (P = 0.006) and CIH10 (P = 0.001) conditions; there was no significant difference between the sham and CIH10 groups (P = 0.405). When examining the linear trend, we see significant evidence for a linear dose response across the CIH conditions (P = 0.020); however, this result is not as strong as the overall ANOVA and is likely driven by the strong difference between sham and CIH5. This nonlinearity is illustrated in the graph, where we see that the CIH10 condition has no significant increase in GLUT1 intensity values compared with sham, while the CIH5 condition has a larger intensity value on average (Fig. 6). We note that results were similar when considering the GLUT1 intensity in all vessels measured, rather than restricting to those that were likely capillaries based on diameter (data not shown).
Fig. 6.
Immunohistochemistry semiquantification (ISQ) for GLUT1. We used immunohistochemistry to stain for GLUT1 and then applied our ISQ technique to determine the intensity of the GLUT1 protein on the microvessels of the 3 CIH conditions. We observed a significant difference among the groups (PANOVA = 0.003), with the CIH5 having significantly greater GLUT1 intensity compared with both sham (P = 0.006) and CIH10 (P = 0.001); there was no difference between CIH10 and sham (P = 0.405), with CIH10 actually showing less GLUT1, on average. While there was evidence for a linear trend (P = 0.020), this result is solely driven by the strong difference between sham and CIH5. LS, least squares; CI, confidence interval; IQ, ??.
There Are Differences in GLUT1 Protein Expression within Hippocampal Capillaries between Different Regions within the Hippocampus
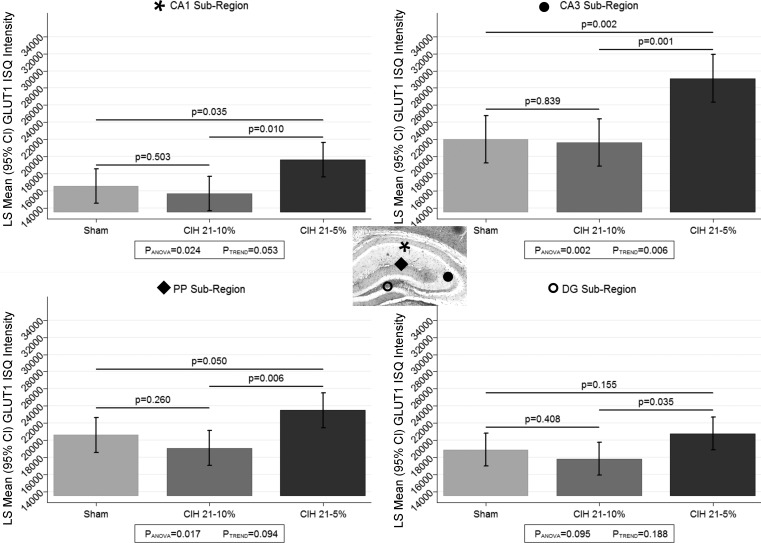
One of the strengths of the ISQ technique is the ability to generate GLUT1 intensity estimates specific to different subregions of the hippocampus [i.e., CA1, CA3, dentate gyrus (DG) and perforant pathway (PP)]. Thus we examined whether the relationship between the conditions differed across these four subregions (Fig. 7). We observed a significant interaction between hippocampal subregion and CIH condition (P = 0.025). When looking within each region separately, we note that a significant difference among the CIH groups was seen in the CA1 (P = 0.024), CA3 (P = 0.002), and PP (P = 0.017) regions, but not among conditions in the DG (P = 0.095). When examining between condition comparisons, we again observed that the CIH5 group had the highest GLUT1 expression compared with sham and CIH10 in the CA1, CA3, and PP; the difference between CIH5 and sham was borderline (P = 0.050) in the PP region. There were no differences between CIH10 and sham conditions in any of the subregions. Interestingly, when collapsing across all CIH conditions, we observed significant (P < 0.0001) differences in GLUT1 expression among hippocampal subregions. All pairwise comparisons among the subregions were significant, with the CA3 region showing more GLUT1 expression than the other three subregions (P < 0.0001) and the CA1 subregion showing significantly less GLUT1 protein expression than the other subregions (P < 0.0001), followed by the DG region, and then the PP regions.
Fig. 7.
ISQ for GLUT1 within brain subregions. We used the ISQ technique to assess the intensity of the GLUT1 protein on the microvessels in our 3 CIH conditions and present results stratified by brain subregion [CA1 (denoted by *), CA3 (denoted by ●), PP (denoted by ⧫), and DG (denoted by ○); see inset]. We observed significant differences among the conditions in all but the DG subregion. In general, as with the overall analysis, CIH5 showed significantly higher GLUT1 intensity compared with either the CIH10 or sham conditions. There were significant differences in the overall GLUT1 intensity across subregions, with the CA3 showing the most GLUT1 and CA1 the least. IQ, ??.
DISCUSSION
We have demonstrated two consequences of CIH on hippocampus microvasculature: increases in total capillary length, and changes in the GLUT1 transporter. Our data reveal that the quantitative nature of these effects depends on the severity of CIH. Total capillary length shows a linear change in response to increased CIH severity, with clear differences in gene expression for VEGFA at each CIH severity, as well as nominal differences in expression for other genes associated with angiogenesis. On the other hand, while there was some evidence of a linear change in mRNA expression of GLUT1, changes in the GLUT1 protein appeared to only occur at the very severe CIH5 level. Future CIH experiments that include different severity levels may demonstrate different molecular mechanisms that are activated at different levels of CIH.
Angiogenesis Response to CIH
One important response to CIH is angiogenesis. Kanaan et al. (18) reported increased capillary density within the cortex and the hippocampus in response to both chronic sustained hypoxia at FiO2 0.11 and chronic intermittent hypoxia to a nadir of FiO2 0.11 in CD-1 pups from postnatal day 2 for both 2 and 4 wk. We employed neurostereology to quantitate the entire dorsal hippocampal region to obtain total length (μm) of capillaries within each mouse and then averaged within each condition. Using this method, we observe a significant linear trend of increasing capillary length across increasing levels of CIH, gaining statistical significance only at CIH5 in pairwise comparisons. By utilizing this sensitive and objective technique, we were able to demonstrate with some confidence that increasing severity of CIH resulted in a linear increase in total capillary length. This reveals that, even at milder degrees of CIH, the brain may be sensitive to CIH and responds through angiogenesis.
To understand which genes are involved in CIH-induced angiogenesis, we used qPCR analysis to examine mRNA expression. We found that VEGFA expression increased robustly and in a linear fashion with increasing severity of CIH. Interestingly, VEGFR2 was slightly decreased at CIH10, but slightly increased at CIH5; this may contribute to more angiogenesis at CIH5 than CIH10, but requires further study. A decrease in TIE-2 at both CIH10 and CIH5 and an increase in ANGPT2 at CIH5 were also observed, again supporting our angiogenesis findings. Lobov et al. (25) demonstrated in rat capillary basal lamina of the eye that ANGPT2 becomes proangiogenic when it is in an environment that has high levels of VEGFA. Interestingly, Felcht et al. (12) demonstrated that ANGPT2 regulates proangiogenesis based on the context of the endothelial cell, to be specific, a context where TIE-2 expression is downregulated in angiogenic endothelial cells; this allows ANGPT2 to bind to several integrins that then activate angiogenesis. Thus the observed changes in ANGPT2 and TIE-2 will also contribute to more angiogenesis at CIH5 than CIH10.
Mechanisms of angiogenesis within the brain in response to CIH are not well understood. Genes known to regulate angiogenesis, such as VEGF, have been previously shown to increase in response to severe hypoxia through a combination of both transcriptional and posttranscriptional mechanisms (5). Furthermore, transcription factors that are oxygen sensors, for example HIF-1α and HIF-2α (6, 36), as well as nutrient and oxidative stress sensors, for example PGC-1α (4, 9, 34) and PGC-1β (42), have a significant role in the regulation of angiogenesis. HIF-1α transcription factor responds posttranscriptionally to hypoxia (36), although there is evidence of transcriptional regulation in response to other stimuli (20). We, however, did not observe changes in HIF-1α, PGC-1α, and PGC-1β genes in response to CIH10 or CIH5; therefore, angiogenesis pathways in response to CIH are most likely regulated at a posttranscriptional level.
GLUT1 Response to CIH
In addition to angiogenesis, blood-brain endothelial cells can respond to stressors such as CIH by inducing changes in transporters, which are not dependent on mechanical disruption of the BBB for unwanted solutes to enter the brain. Thus dysfunction or subtle changes in the concentration of over 20 BBB transporters could alter the microenvironment of the brain over time. Kalaria et al. (17) examined the effects of CIH on GLUT1 expression along the BBB by exposing rats to intermittent hypoxia to 10% O2 for 4 h/day, 6 days/wk, followed by room air for the remaining 20 h, for a total of 56 days. A pairwise comparison between normoxia and CIH showed no statistically significant increases in GLUT1 protein expression in multiple regions of the brain. This negative result with respect to CIH likely reflects that the CIH was not sufficiently severe and/or was not daily. Our GLUT1 protein semiquantification using ISQ is consistent with Kalaria's findings, as we observed no significant increase in GLUT1 protein for the CIH10 compared with sham. However, we did find increased GLUT1 at the more severe CIH5 compared with both sham and CIH10. Because ISQ is not predicated on selecting regions of interest, we were able to observe that the CA3 region has the highest GLUT1 intensity across conditions. This raises the interesting question of whether CA3 has different metabolic requirements than CA1, perhaps based on different functions (27), and deserves further study. Our data highlight the advantage of quantifying an entire region to allow analysis of subregions, rather than selecting regions of interest that may skew results unintentionally. When examining the differences in mRNA expression of GLUT1, we again observed an increased expression within the CIH5 group. However, the qPCR data also suggested a linear trend across the conditions, with the CIH10 group showing intermediate levels of expression (although not significantly different from sham or the CIH5 groups).
What could explain an increase in GLUT1 protein at very severe CIH5, but not at severe CIH10? One explanation may be the activation of HIF-1α at very severe CIH5 levels, but not at CIH10 levels. The question then becomes, under what CIH conditions are HIF-1α proteins stabilized and/or activated to move from cytoplasm to nucleus? HIF-1α transcription factor responds posttranscriptionally to hypoxia and increases GLUT1 transcription (13, 30, 35, 47). However, the effect of sustained hypoxia on HIF-1α protein depends on not only the degree of hypoxia, but also the cell type (39). Shao et al. (37) used in vitro studies to demonstrate that the degree of sustained hypoxia (2, 5, and 10%) affected GLUT1 mRNA and protein levels. At a hypoxia level of 2% for 12 h, GLUT1 mRNA increased 11-fold in primary epithelial cells, but increased to a lesser degree when exposed to 5% oxygen, and did not change when exposed to 10%. Next, when using Mac-T cells, they found hypoxia of 2% for 12 h did not change HIF-1α mRNA, but there was an increase in HIF-1α protein movement from cytoplasm to nucleus. Lastly, when small interfering RNA was used to knock down HIF-1α in cells exposed to 2% oxygen, the cells no longer had an increase in GLUT1. Thus they concluded HIF-1α is partially responsible for the increase in GLUT1 when exposed to very severe hypoxic conditions. Therefore, one potential explanation of increased GLUT1 only at CIH5 is that HIF-1α activation (movement to the nucleus) may only occur at this level of CIH severity. Li et al. used a mouse model to demonstrate HIF-1α activity is associated with hypertriglyceridemia at very severe CIH (FiO2 0.21-0.05) (21), but not at severe CIH (FiO2 0.21-0.10) (22). Collectively, results by Shao et al. (37), Li et al. (22), and the GLUT1 data presented in this paper suggest that it is possible that the HIF-1α mechanism may explain changes in GLUT1 and lipids, but only at very severe levels of CIH. Definitive studies need to assess whether HIF-1α is stabilized/activated at less severe levels of CIH and identify other pathways that may be operative at less severe CIH.
Integration of Angiogenesis and GLUT1 Responses: Oxygen/Glucose Index
Both angiogenesis and changes in BBB transporters like GLUT1 will alter the brain's metabolic response to increased demand via the oxygen-to-glucose consumption ratio [oxygen/glucose index (OGI)] (32, 33). Conceptually, OGI is when brain activation [either in normal physiological circumstances or pathological activation such as seizures (7)] increases cerebral blood flow to deal with the increase in oxygen and metabolic demand to maintain brain function, as the brain has little reserve and demand/consumption is dynamic. However, chronic perturbations such as CIH may result in varying fold changes and pathways being activated in response to OGI. Combining capillary length and glucose transporter data at each level of CIH allows us to make several interesting observations about the oxygen-to-glucose consumption ratio. First, at severe CIH10, it may be that oxygen demands are increased to the point that new capillary growth is needed, since diffusion is the major mode of oxygen transport through the BBB. Meanwhile, the number of GLUT1 transporters is adequate (or another mechanism is activated) until at very severe CIH5, when more GLUT1 transporters are needed to maintain energy demand because facilitative diffusion is no longer efficient enough. Thus the combination of angiogenesis and GLUT1 response to different CIH severity is a potentially interesting mechanism that reveals how the brain maintains homeostasis and how this response may vary. However, it is conceivable that long-term dysregulation of this homeostasis results in cognitive impairment.
Technological Considerations
CIH.
The strength of using the Hycon to deliver intermittent hypoxia is in delivering precise FiO2 according to a preset schedule within each cage. However, due to mice normally sleeping on top of each other, this behavior may create variability in the actual FiO2 delivered to each mouse. This biological variability may account at least in part for the range in the results.
Neurostereology.
The strength of using neurostereological techniques to quantitate angiogenesis is that a large region such as the dorsal hippocampus can be objectively measured using a computer-generated algorithm that eliminates selection bias and sampling errors. One down side is that current software does not allow automatic analysis of subregions within the hippocampus to assess whether angiogenesis is differentially affected. There is a need for further enhancement of computerized techniques. In addition, investigations linking increases in capillary length to function need to be performed in the future.
ISQ.
Although Western blots of protein have long been the standard for protein semiquantification, Western blots require pooling 6–10 total brains to isolate blood vessels, thereby losing the ability to detect regional differences of protein semiquantification. In light of this, we consider the use of ISQ to provide additional information (38). ISQ is similar to Image Pro Plus in that it measures pixel intensity of immunohistochemistry stains, with the difference being ISQ is 1) automated, which eliminates human error in hand-drawing each blood vessel; 2) high-throughput, which eliminates selection bias because regions of interest no longer need to be selected; 3) objective, which eliminates focus bias because we quantitate the protein at multiple focus planes of a torturous blood vessel; and 4) able to be analyzed both by overall region and subregions. Although the algorithm is publically available, improving the user interface for use is needed and underway.
qPCR.
Using the entire hippocampus led to assessment of changes in gene expression in a mixed population of cells. Thus the magnitude of changes in specific cell types may have been underestimated. Future studies need to assess changes in gene expression in different specific cell populations. Our studies show that the hippocampal microvasculature has multiple ways to respond to the challenges represented by CIH.
Conclusion
Our studies show that the hippocampal microvasculature has different responses to the challenges represented by CIH. We demonstrate that the degree of CIH severity is important when studying its effects on the hippocampus in a quantitative way. This is likely to be an important consideration when studying the effect of CIH on other systems. We should be cautious to assert that consequences occurring at one level of CIH occur at all other levels of CIH, and we should be cautious in extrapolating effects seen at one CIH level to the majority of patients with OSA.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants T32-HL-07713 and K12-HL-090021 and the American Sleep Medicine Foundation [Physician Scientist Training Award (ASMF 56-PA-10)] (D. C. Lim); NHLBI Grant HL-094307 (A. I. Pack); Science Without Borders Program-Coordination for the Improvement of Higher Education Personnel, Ministry of Education, Brazil (L. Valverde); and the Han Eol Program (W. Y. Kim and M. J. Park).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.C.L. and A.I.P. conception and design of research; D.C.L., D.C.B., R.S., E.Y.K., L.V., X.G., W.Y.K., M.J.P., R.J.G., and J.A.S. performed experiments; D.C.L., D.C.B., R.S., E.Y.K., L.V., B.T.K., X.G., W.Y.K., J.A.S., and A.I.P. analyzed data; D.C.L., B.T.K., X.G., W.Y.K., J.A.S., and A.I.P. interpreted results of experiments; D.C.L., B.T.K., and X.G. prepared figures; D.C.L., B.T.K., X.G., and A.I.P. drafted manuscript; D.C.L., D.C.B., R.S., E.Y.K., L.V., B.T.K., X.G., W.Y.K., M.J.P., R.J.G., J.A.S., and A.I.P. edited and revised manuscript; D.C.L., D.C.B., R.S., E.Y.K., L.V., B.T.K., X.G., W.Y.K., M.J.P., R.J.G., J.A.S., and A.I.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Stephen Robinson (Royal Melbourne Institute of Technology) for suggesting that studies of the BBB in OSA are needed.
REFERENCES
- 1.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep 17: 77–83, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Klauber MR, Butters N, Parker L, Kripke DF. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc 39: 258–263, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc 56: 2076–2081, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Arcondeguy T, Lacazette E, Millevoi S, Prats H, Touriol C. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res 41: 7997–8010, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer 8: 967–975, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodersen P, Paulson OB, Bolwig TG, Rogon ZE, Rafaelsen OJ, Lassen NA. Cerebral hyperemia in electrically induced epileptic seizures. Arch Neurol 28: 334–338, 1973. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun ME, Mouton PR. Length measurement: new developments in neurostereology and 3D imagery. J Chem Neuroanat 21: 257–265, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A 106: 21401–21406, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Basica Neurochemistry: Molecular, Cellular and Medical Aspects, edited by Siegel GJ, Agranoff BW, Albers RW, Fisher L, and Uhler MD. Philadelphia, PA: Lippincott, 1998, p. 637–670. [Google Scholar]
- 11.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal 9: 1363–1371, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu JH, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122: 1991–2005, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 235: 1492–1495, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Gaohua L, Kimura H. A mathematical model of brain glucose homeostasis. Theor Biol Med Model 6: 26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmila JM, Drewes LR. Glucose transporter (GLUT1) expression by canine brain microvessel endothelial cells in culture: an immunocytochemical study. Adv Exp Med Biol 331: 13–18, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Hoch CC, Reynolds CF 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry 47: 499–503, 1986. [PubMed] [Google Scholar]
- 17.Kalaria RN, Spoors L, Laude EA, Emery CJ, Thwaites-Bee D, Fairlie J, Oakley AE, Barer DH, Barer GR. Hypoxia of sleep apnoea: cardiopulmonary and cerebral changes after intermittent hypoxia in rats. Respir Physiol Neurobiol 140: 53–62, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol 290: R1105–R1114, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kreuzer F. Oxygen-supply to tissues–the Krogh model and its assumptions. Experientia 38: 1415–1426, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Kuschel A, Simon P, Tug S. Functional regulation of HIF-1alpha under normoxia–is there more than post-translational regulation? J Cell Physiol 227: 514–524, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics 25: 450–457, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol (1985) 102: 557–563, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lim DC, Brady DC, Po P, Chuang LP, Marcondes L, Kim EY, Keenan BT, Guo X, Maislin G, Galante RJ, Pack AI. Simulating obstructive sleep apnea patients' oxygenation characteristics into a mouse model of cyclical intermittent hypoxia. J Appl Physiol (1985) 118: 544–557, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: Addressing the blood-brain barrier. Sleep Med Rev 18: 35–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A 99: 11205–11210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med 10: 355–362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuseki K, Royer S, Diba K, Buzsaki G. Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons. Hippocampus 22: 1659–1680, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc 206: 54–64, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nelson DL, Cox MM. Lehninger, Principles of Biochemistry. New York: Freeman, 2008. [Google Scholar]
- 30.Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 275: 21797–21800, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Pardridge WM, Connor JD, Crawford IL. Permeability changes in the blood-brain barrier: causes and consequences. CRC Crit Rev Toxicol 3: 159–199, 1975. [DOI] [PubMed] [Google Scholar]
- 32.Paulson OB. Blood-brain barrier, brain metabolism and cerebral blood flow. Eur Neuropsychopharmacol 12: 495–501, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cereb Blood Flow Metab 30: 2–14, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saint-Geniez M, Jiang A, Abend S, Liu L, Sweigard H, Connor KM, Arany Z. PGC-1alpha regulates normal and pathological angiogenesis in the retina. Am J Pathol 182: 255–265, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 64: 2627–2633, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Shao Y, Wellman TL, Lounsbury KM, Zhao FQ. Differential regulation of GLUT1 and GLUT8 expression by hypoxia in mammary epithelial cells. Am J Physiol Regul Integr Comp Physiol 307: R237–R247, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Soans R, Lim DC, Keenan BT, Pack AI, Shackleford JA. A high-throughput method for immunohistochemistry quantification of proteins in blood vessels at the blood-brain barrier. PLoS One 11: e0148411, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DAH, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15: 2445–2453, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Wahlin Larsson B, Kadi F, Ulfberg J, Piehl Aulin K. Skeletal muscle morphology and aerobic capacity in patients with obstructive sleep apnoea syndrome. Respiration 76: 21–27, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Wahlin-Larsson B, Ulfberg J, Aulin KP, Kadi F. The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve 40: 556–561, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Yadav V, Matsakas A, Lorca S, Narkar VA. PGC1beta activates an antiangiogenic program to repress neoangiogenesis in muscle ischemia. Cell Rep 8: 783–797, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306: 613–619, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto YL, Phillips KM, Hodge CP, Feindel W. Microregional blood flow changes in experimental cerebral ischemia. Effects of arterial carbon dioxide studied by fluorescein angiography and xenon 133 clearance. J Neurosurg 35: 155–166, 1971. [DOI] [PubMed] [Google Scholar]
- 45.Yoshihara K, Takuwa H, Kanno I, Okawa S, Yamada Y, Masamoto K. 3D analysis of intracortical microvasculature during chronic hypoxia in mouse brains. Adv Exp Med Biol 765: 357–363, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31: 1071–1078, 2008. [PMC free article] [PubMed] [Google Scholar]
- 47.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA Jr, Velculescu VE, Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325: 1555–1559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]