The novel finding in this study is that high-intensity endurance training turned the heart failure phenotype in CaMKIIδC-overexpressing mice toward a more healthy phenotype. We report improved cardiac and cardiomyocyte function and Ca2+ handling by reducing diastolic Ca2+ leak and restoring sarcoplasmic reticulum (SR) Ca2+ content through compensatory mechanisms of restored SR Ca2+-ATPase (SERCA2a) function and Na+/Ca2+-exchanger (NCX) function and increased L-type Ca2+ currents. The present data extend the basis for further understanding of cardiac adaptations to exercise training.
Keywords: calcium handling, CaM kinase, exercise training, heart disease
Abstract
Several conditions of heart disease, including heart failure and diabetic cardiomyopathy, are associated with upregulation of cytosolic Ca2+/calmodulin-dependent protein kinase II (CaMKIIδC) activity. In the heart, CaMKIIδC isoform targets several proteins involved in intracellular Ca2+ homeostasis. We hypothesized that high-intensity endurance training activates mechanisms that enable a rescue of dysfunctional cardiomyocyte Ca2+ handling and thereby ameliorate cardiac dysfunction despite continuous and chronic elevated levels of CaMKIIδC. CaMKIIδC transgenic (TG) and wild-type (WT) mice performed aerobic interval exercise training over 6 wk. Cardiac function was measured by echocardiography in vivo, and cardiomyocyte shortening and intracellular Ca2+ handling were measured in vitro. TG mice had reduced global cardiac function, cardiomyocyte shortening (47% reduced compared with WT, P < 0.01), and impaired Ca2+ homeostasis. Despite no change in the chronic elevated levels of CaMKIIδC, exercise improved global cardiac function, restored cardiomyocyte shortening, and reestablished Ca2+ homeostasis to values not different from WT. The key features to explain restored Ca2+ homeostasis after exercise training were increased L-type Ca2+ current density and flux by 79 and 85%, respectively (P < 0.01), increased sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a) function by 50% (P < 0.01), and reduced diastolic SR Ca2+ leak by 73% (P < 0.01), compared with sedentary TG mice. In conclusion, exercise training improves global cardiac function as well as cardiomyocyte function in the presence of a maintained high CaMKII activity. The main mechanisms of exercise-induced improvements in TG CaMKIIδC mice are mediated via increased L-type Ca2+ channel currents and improved SR Ca2+ handling by restoration of SERCA2a function in addition to reduced diastolic SR Ca2+ leak.
NEW & NOTEWORTHY
The novel finding in this study is that high-intensity endurance training turned the heart failure phenotype in CaMKIIδC-overexpressing mice toward a more healthy phenotype. We report improved cardiac and cardiomyocyte function and Ca2+ handling by reducing diastolic Ca2+ leak and restoring sarcoplasmic reticulum (SR) Ca2+ content through compensatory mechanisms of restored SR Ca2+-ATPase (SERCA2a) function and Na+/Ca2+-exchanger (NCX) function and increased L-type Ca2+ currents. The present data extend the basis for further understanding of cardiac adaptations to exercise training.
in recent years, exercise training has arisen as an important clinical treatment strategy for cardiovascular disease. Not only does exercise training reduce cardiovascular risk factors, but also several studies show beneficial effects on cardiac function along with reversal of cellular abnormalities such as hypertrophy and remodeling, and aberrant Ca2+ handling and contractile function (7, 15, 20). Furthermore, improvements in maximal oxygen uptake (V̇o2 max) as well as cardiac function are reported more pronounced with high-intensity endurance training both in experimental animal models (12) as well as in patients with cardiovascular disease (34, 37). Regulation of the protein kinase Ca2+/calmodulin-dependent protein kinase II (CaMKII), which occurs in cardiac muscle after exercise training (11, 29) could contribute to these effects since CaMKII regulates several aspects of cardiomyocyte function.
In the heart the predominant isoform of CaMKII is the cytosolic δ isoform CaMKIIδC (6, 30), which targets several proteins involved in intracellular Ca2+ homeostasis, including the sarcoplasmic reticulum (SR) Ca2+ release channel (ryanodine receptor, RyR2), the L-type Ca2+ channel (LTCC), and phospholamban (PLN), which regulates SR Ca2+-ATPase (SERCA2a) activity. Several models of heart disease, including heart failure (9, 16) and diabetic cardiomyopathy (29), are associated with upregulation of CaMKII activity. In line with this, overexpression of the deltaC isoform CaMKIIδ (CaMKIIδc) has been shown to detrimentally alter Ca2+ handling and contractility (19, 25). Especially, increased RyR2 Ca2+ sensitivity that causes leaky RyR2s has received great attention in the phenotypic changes observed in cardiomyocytes with increased activity of CaMKIIδC (1, 5, 22).
We hypothesized that high-intensity endurance training could enable restoration of dysbalanced cardiomyocyte Ca2+ homeostasis and thereby ameliorate cardiac dysfunction even in the face of continuous and chronic elevated levels of CaMKIIδC.
MATERIALS AND METHODS
Animals.
Transgenic CaMKIIδC mice (TG) with increased CaMKII activity were generated as previously described (40). Briefly, hemagglutinin (HA)-tagged rat wild-type CaMKIIδC cDNA were subcloned into the SalI site of pBluescript-based TG vector between the 5.5-kb murine α-MHC promoter and a human growth hormone (HGH) polyadenylation sequences. Purified linear transgene fragments were injected into pronuclei of fertilized mouse oocytes. The resultant pups were screened for the presence of the transgene by PCR, using a CaMKII-specific primer (5′-TTGAAGGGTGCCATCTTGACA-3′) and a TG vector-specific primer (5′-GGTCATGCATGCCTGGAATC-3′). To determine the transgene copy number, Southern blot analysis was performed with EcoRI-digested genomic DNA and a P-labeled 1.7-kb EcoRI-SalI α-MHC fragment as a probe. Founder mice were bred with C57BL/6 or Black Swiss wild-type (WT) mice to generate TG and WT offspring. Three-month-old TG mice underwent aerobic interval endurance training (N = 12) or remained sedentary (N = 12) and were compared with age-matched sedentary WT littermate controls (N = 12) and aerobic interval endurance-trained WT littermate control mice (N = 12). Twenty-four hours after the last training session, the mice were euthanized, and cardiomyocytes were isolated to examine contractile function, Ca2+ cycling, and diastolic SR Ca2+ leak. The Norwegian Council for Animal Research approved the study, which was in accordance with the Guide for the Care and Use of Laboratory Animals published by the European Commission, Directive 86/609/EEC.
Maximal oxygen uptake (V̇o2max).
The mice warmed up for 20 min at 50–60% of the maximal oxygen uptake (V̇o2max), whereupon treadmill velocity was increased by 0.03 m/s every 2 min until V̇o2 reached a plateau despite increased workload. V̇o2max recordings were obtained by treadmill placed in a closed metabolic chamber according to previous validated methods (10, 35).
Endurance training.
The aerobic interval endurance-training program was performed as previously described (13, 35). During training, the mice ran uphill (25°) on a treadmill for 80 min: following 20 min of warm-up at a speed corresponding to 50–60% V̇o2 max, the mice performed intervals during a period of 60 min, alternating between 4 min at an exercise intensity corresponding to 85–90% of V̇o2 max and 2-min active recovery at 50–60%, giving a total of 40 min (10 intervals) at high intensity and a total of 20 min of recovery between intervals. Exercise was performed 5 days/wk over 6 wk; controls were age-matched CaMKIIδC TG or WT mice that remained sedentary or exercised. The time frame of the intervention period was chosen on background of previous publications showing a robust change in V̇o2 max, as well as in cardiomyocyte function and calcium handling in experimental animal models (10, 13, 35). In exercising animals, V̇o2 max was measured every second week to adjust band speed in order to maintain the intended intensity throughout the experimental period, whereas in the sedentary group V̇o2 max was measured before and after the experimental period.
Cardiomyocyte shortening and Ca2+ cycling.
At the end of the exercise-training period the heart was removed during 3% isoflurane anesthesia and immediately transferred for cardiomyocyte cell isolation by retrograde Langendorff perfusion and collagenase type II (Worthington, UK) as described earlier (40). Isolated cardiomyocytes were loaded with Fura-2/AM for detection of Ca2+-handling properties (2 μmol/l, Molecular Probes, Eugene, OR). To ensure similar loading of the cardiomyocytes, we incubated the cells for exactly 30 min, and all cells were allowed at least 10 min in normal HEPES solution before any recordings. Cardiomyocytes were stimulated by bipolar electrical pulses with increasing frequencies 1–3 Hz on an inverted epifluorescence microscope (Nikon TE-2000E; Tokyo, Japan), whereupon cell shortening was recorded by video-based myocyte sarcomere spacing (SarcLen; IonOptix, Milton, MA) and intracellular Ca2+ concentration ([Ca2+]i) was measured by fluorescence after excitation by alternating 340- and 380-nm wavelengths (F340/380 ratio) (Optoscan; Cairn Research, Kent, UK). During the stimulation protocol, cells were continuously perfused with normal physiological HEPES-based solution (1.8 mmol/l Ca2+, 37°C). In a subset of experiments, H-89 (3 μmol/l for 1 h; Sigma, St. Louis, MO), to block protein kinase A (PKA), or autocamtide-2-related inhibitory peptide (AIP, 1 μmol/l for 1 h; Sigma), to block CaMKIIδC, were added to the solutions. Cell size was measured in cardiomyocytes not introduced to FURA2-AM with a graticule on the microscope, and volume was calculated with the following formula: cell area (length × cell midpoint width) μm2 × 0.00759 ρL/μm2, as previously established by two-dimensional (2-D) light and 3-D confocal microscopy (26).
Diastolic Ca2+ leak.
A method similar to that established by Shannon et al. (27) was used to determine diastolic Ca2+ leak from the SR. To bring the cellular Ca2+ content to a steady state, we stimulated the cardiomyocytes electrically at 1 Hz in normal HEPES-based 1.8 mmol/l Ca2+ solution for 30–60 s. After the last electric stimulus, we rapidly switched the perfusion to a 0 Na+/0 Ca2+-containing solution and measured diastolic Ca2+ concentration in quiescent nonstimulated cardiomyocytes (1 min) ± tetracaine (1 mmol/l). The 0 Na+/0 Ca2+ solution prevents the Na+-Ca2+ exchange, which is the primary Ca2+ influx and efflux mechanism at rest. Tetracaine blocks the Ca2+ leak over the RyR (21, 27). The quantitative difference between diastolic Ca2+ concentration with and without tetracaine determines leak. After the 1-min period in 0 Na+/0 Ca2+ ± tetracaine solution, we added caffeine (10 mmol/l) to assess SR Ca2+ content. Diastolic Ca2+ leak is presented as diastolic [Ca2+]i in relation to total SR Ca2+ content. In a subset of experiments, H-89 (3 μmol/l for 1 h), to block PKA, or AIP (1 μmol/l for 1 h), to block CaMKII, were added to the solutions.
Ca2+ waves.
Cardiomyocytes loaded with Fluo-3/AM (10 μmol/l, Molecular Probes) were used to determine frequency of Ca2+ waves by confocal line scan (Pascal; Carl Zeiss, Jena, Germany).
Voltage clamp.
Single isolated mouse cardiomyocytes were superfused with a HEPES-buffered Krebs-Henseleit solution containing (in mM) 140 NaCl, 4 KCl, 5 HEPES, 1 MgCl2, 1.8 CaCl2, 11.1 glucose, 5 4-aminopyridine (to block K+ currents), 0.1 niflumic acid (to block Ca2+-activated Cl− currents), and 5 μM tetrodotoxin (to block INa), pH 7.4 with NaOH (37°C) in a chamber mounted on the stage of an inverted microscope. Microelectrode pipettes were filled with an intracellular solution of composition (in mM): 20 KCl, 100 K aspartate, 20 tetraethylammonium chloride (TEA-Cl), 10 HEPES, 4.5 MgCl2, 4 disodium ATP, 1 disodium creatine phosphate, and 0.01 EGTA, pH 7.25 with KOH. L-type Ca2+ current (ICaL) protocol was as follows: voltage clamp was achieved via whole cell ruptured patch technique using an Axoclamp 2B amplifier (Axon Instruments) in discontinuous (switch clamp) mode. Pipette resistance was ∼6 MΩ. Whole cell patch clamp was performed on single isolated mouse cardiomyocytes. The cell was clamped at −80 mV, and the voltage stepped to −40 mV for 50 ms, before stepping to 0 mV for 150 ms. The protocol was repeated at 2 Hz for 90 s. The last 10 L-type Ca2+ current recordings were averaged and analyzed.
Western blot analyses.
Cardiac tissue was homogenized in Tris buffer containing (in mmol/l) 20 Tris-HCl, 200 NaCl, 20 NaF, 1 Na3VO4, 1 dithiothreipol, 1% Triton X-100 (pH 7.4), PhosSTOP (Roche Diagnostics, Grenzach-Wyhlen, Germany), and complete protease inhibitor cocktail (Roche Diagnostics, Grenzach-Wyhlen, Germany). Protein concentration was determined by bicinchoninic acid assay (Thermo Fisher Scientific, Rockford, IL). Denatured tissue homogenates (30 min at 37°C or 5 min at 95°C, 2% beta-mercaptoethanol) were used for Western blotting (8–15% sodium dodecylsulfate-polyacrylamide gel) using anti-CaMKIIδ (1:15,000; gift from D. M. Bers, University of California, Davis), anti-phospho-CaMKII (1:1,000; Thermo Fisher Scientific), anti-RYR2 (1:10,000; Sigma), anti-RYR2 phospho serine-2814 (1:5,000; Badrilla, Leeds, UK), anti-glyceraldehyde-3-phosphate dehydrogenase (1:20,000; Biotrend Chemikalien, Köln, Germany) as primary, and horseradish peroxidase conjugated donkey anti-rabbit and sheep anti-mouse immunoglobulin G (1:10,000; Amersham Biosciences, Freiburg, Germany) as secondary antibodies. Chemiluminescent detection was performed with Millipore Immobilion Western (Millipore, Billerica, MA). For SERCA2a and L-type Ca2+ channel determination, primary antibodies were anti-SERCA2a (1:2,000; Badrilla), and for L-type Ca2+ channel the primary antibody was anti-CACNA1C (1:350; Abcam, Cambridge, UK) and anti-GAPDH (1:2,000, MA5-15738; Thermo Fisher Scientific). Fifty micrograms of protein were separated on Bis-Tris SDS-PAGE ready gels and transferred to polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific). Secondary antibodies used were IRDye 800CW goat anti-mouse (1:10,000; LI-COR Biotechnology, Lincoln, NE) and IRDye 680LT donkey anti-rabbit (1:30,000; LI-Cor Biotechnology). Protein bands were visualized using an Odyssey fluorescence imaging system, and band intensities were quantified using LI-COR Image Studio 3.1 (LI-COR Biotechnology).
Statistical analysis.
Data are shown as means ± SD, except where indicated. One-way ANOVA with Bonferroni post hoc test adjusted for multiple comparisons was used to identify the statistical differences between the groups, and Mann-Whitney U-test was used when appropriate. P < 0.05 was considered statistically significant.
RESULTS
Total CaMKIIδ protein expression was increased sevenfold in TG mice compared with WT, whereas CaMKII phosphorylation at the autoactivation site threonine-286 increased twofold. Exercise did not modify either of these parameters (Fig. 1, A–C). However, despite no effect of exercise training on regulation of these proteins, we observed that the TG mice adapted to high-intensity exercise training such that parameters of several aspects of in vivo cardiac and ex vivo cardiomyocyte function improved or were restored to levels comparable to basal levels (WT untrained). Moreover, the training response with regard to aerobic capacity and cardiac and cardiomyocyte function followed the same pattern as seen after exercise training in the WT group. Exercise was well tolerated in all groups, and we did not observe any adverse effects in any of the animals. No mortality was observed during the experimental period.
Fig. 1.
A: CaMKII total protein levels. B: phosphorylated CaMKII at threonine-286. Protein measurements are presented as means ± SE (number of animals in each group N = 4). C: examples of Western blots of protein regulation. D: maximal oxygen uptake was measured in all animals included in the study. V̇o2 max was reduced in transgenic (TG) CaMKIIδC-overexpressing mice (N = 12) compared with WT sedentary (N = 12); exercise increased V̇o2 max in both TG (N = 12) and WT (N = 12). E: cardiomyocyte volume was significantly larger in TG mice (N = 5) compared with WT (N = 5); exercise reduced cell volume in TG (N = 5) but increased cell volume in WT (N = 5). Data in D and E are presented as means ± SD. †P < 0.01 vs. sedentary WT, *P < 0.05 vs. sedentary WT, #P < 0.05 vs. sedentary TG.
Aerobic capacity, cardiac function, and response to exercise training.
The increased expression of CaMKIIδC led to a significant reduction in aerobic capacity as maximal oxygen uptake (V̇o2 max) in sedentary TG mice was 75% to that of WT mice. However, 6 wk of exercise training restored V̇o2 max in TG mice to levels similar to WT mice (Fig. 1D). As aerobic capacity is closely related to cardiac pump function, we measured cardiac parameters by echocardiography. Cardiac output, stroke volume, and ejection fraction were significantly reduced in sedentary TG mice, suggesting cardiac dysfunction, whereas parameters of left ventricle (LV) lumen dimensions indicated dilation (Table 1). Exercise training improved cardiac output, stroke volume, and ejection fraction significantly (P < 0.01, Table 1). Hence deficits in both aerobic capacity and global cardiac function were improved by exercise training in TG mice. Similar effects were seen after exercise in WT mice.
Table 1.
Global cardiac left ventricle function (echocardiography)
| CaMKIIδC TG |
Wild Type |
|||
|---|---|---|---|---|
| Sedentary | Exercise | Sedentary | Exercise | |
| LV cardiac output, ml/min | 12.3 ± 2.8† | 17.6 ± 1.1* | 19.0 ± 1.2* | 23.0 ± 3.0*† |
| LV stroke volume, μl | 25.2 ± 4.6† | 35.2 ± 1.8* | 35.5 ± 2.6* | 42.2 ± 4.9*§ |
| LV ejection fraction, % | 19.4 ± 3.0† | 29.7 ± 5.8*† | 50.7 ± 3.7* | 64.5 ± 4.5*§ |
| LV fractional shortening, % | 8.9 ± 1.4† | 14.0 ± 3.0*† | 25.5 ± 2.2* | 34.8 ± 3.4*§ |
| LV diameter, end systole, mm | 4.7 ± 0.2† | 4.3 ± 0.4† | 3.0 ± 0.2* | 2.5 ± 0.2*§ |
| LV diameter, end diastole, mm | 5.2 ± 0.2† | 5.0 ± 0.3† | 4.0 ± 0.2* | 3.9 ± 0.2* |
| LV volume, end systole, μl | 105 ± 12.4† | 86.1 ± 17.7† | 35.1 ± 5.8* | 23.5 ± 4.5*§ |
| LV volume, end diastole, μl | 130.2 ± 14.0† | 121.3 ± 16.1† | 70.6 ± 7.6* | 65.6 ± 8.0* |
Data are means ± SD. CaMKII, Ca2+/calmodulin-dependent kinase II; LV, left ventricle. Difference from sedentary CaMKIIδC TG:
P < 0.01. Difference from sedentary WT:
P < 0.01,
P < 0.05.
Cardiomyocyte size and contractility.
We found significantly larger cardiomyocyte size in TG mice compared with WT mice; exercise training reduced the volume significantly (Fig. 1E), indicating a reversal of the pathologic hypertrophy. In the WT exercise group, we observed the opposite scenario with increased cardiomyocyte size, indicating a physiologic hypertrophy that commonly is observed after exercise in healthy individuals. Cardiomyocyte contractility, measured as fractional shortening, was reduced by ∼47% in TG mice compared with WT mice, whereas exercise training fully restored cardiomyocyte fractional shortening (Fig. 2, A and B). Also, time to 50% relengthening was prolonged in isotonically contracting cardiomyocytes from TG mice, but exercise training normalized this (Fig. 2C).
Fig. 2.
A: representative sample tracings of cardiomyocyte fractional shortening from sedentary and exercise-trained transgenic (TG) CaMKIIδC-overexpressing mice and sedentary and exercised WT mice. B: fractional shortening was significantly reduced in TG, whereas exercise training in TG restored this to WT levels. C: time to 50% relengthening was longer in TG and restored after exercise training, with a comparable response to that of exercise training in WT. **P < 0.01 vs. other groups. There were no significant differences between exercise-trained TG and WT mice. Cells in each group n = 25–30.
L-type Ca2+ current (ICaL).
Since transmembrane Ca2+ flux initiates cardiomyocyte excitation-contraction coupling and contractility, we examined ICaL. Exercise training in TG mice increased the ICaL density and flux significantly by 79 and 85%, respectively (P < 0.01, Fig. 3). Similar alterations were observed in exercised WT mice. The increased L-type Ca2+ channel current after exercise training was at least partly explained by the significantly increased protein expression in exercised TG mice compared with TG sedentary (P < 0.05, Fig. 3).
Fig. 3.
A: Ca2+ flux through ICaL was reduced in sedentary TG compared with trained TG. B: representative L-type Ca2+ current (ICaL) recordings from sedentary transgenic (TG) CaMKIIδC-overexpressing mice (red), trained TG (blue), sedentary WT (green), and trained WT (black). C: ICaL density was reduced in sedentary TG compared with trained TG. In C, sedentary WT, n = 14 cells; exercise WT, n = 14 cells; sedentary TG, n = 19 cells; exercise TG, n = 14 cells. D: protein expression on L-type Ca2+ channel was significantly increased after exercise training in TG mice (number of mice in each group N = 4). Data are presented as means ± SE. *P < 0.05 vs. trained TG. #P < 0.5 between exercise-trained WT vs. sedentary WT.
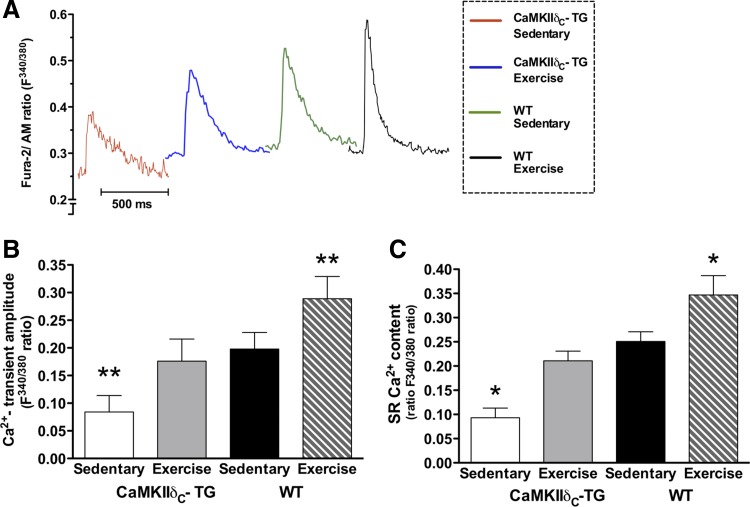
Ca2+ transients and SR Ca2+ content.
The Ca2+-transient amplitude was ∼58% lower in TG mice compared with WT mice, but this difference was absent after exercise training, indicating that the Ca2+-transient amplitude was corrected by exercise training (Fig. 4, A and B). This increase in Ca2+-transient amplitude in response to exercise training was comparable with the effect observed in WT mice. Reduced Ca2+-transient amplitude in TG has been suggested to result from reduced SR Ca2+ content compared with that observed in cardiomyocytes from WT mice (19, 25). We confirmed that caffeine-evoked SR Ca2+ content was reduced in TG compared with WT; exercise training restored the SR Ca2+ content to sedentary WT levels (Fig. 4C).
Fig. 4.
A: representative traces of Ca2+ transients by Fura-2/AM ratio (F340/380) recordings. B: twitch-stimulated Ca2+-transient amplitude (Fura-2/AM ratio F340/380) was reduced in transgenic (TG) CaMKIIδC-overexpressing mice compared with WT. Exercise training increased the Ca2+-transient amplitude in both TG and WT, in TG to levels comparable with WT mice. C: caffeine-evoked Ca2+-transient amplitude (SR Ca2+ content) was reduced in TG mice compared with WT. Exercise training increased the SR Ca2+ content in both TG and WT, in TG to levels comparable with sedentary WT. **P < 0.01 vs. other groups, *P < 0.05 vs. other groups. There were no significant differences between exercise-trained TG and sedentary WT mice. Cells in each group n = 25–30.
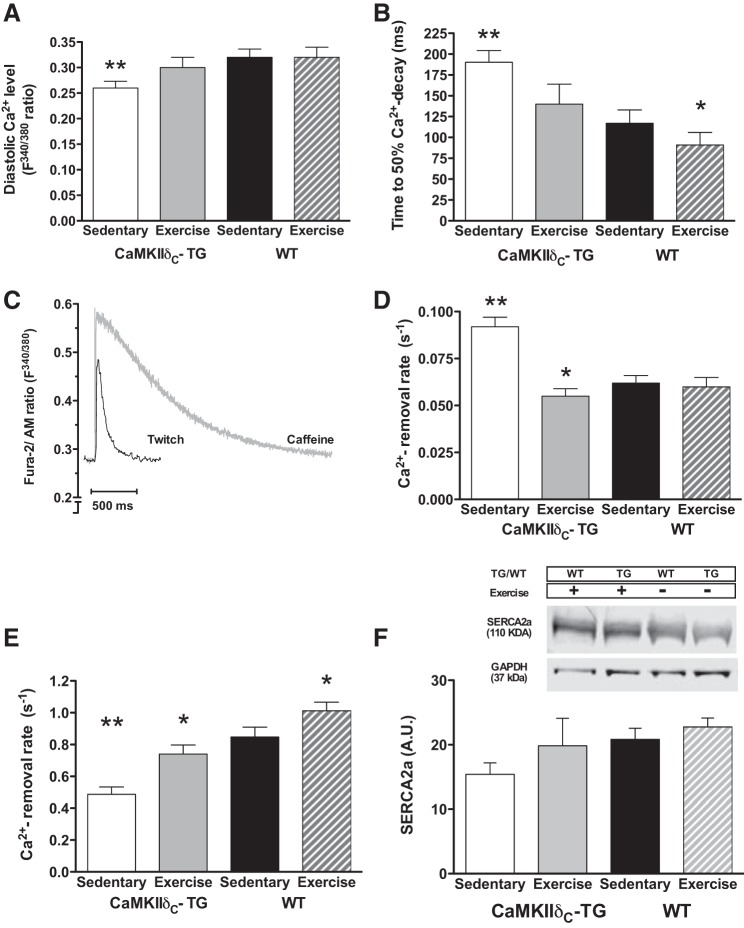
Diastolic Ca2+ control.
Diastolic Ca2+ levels during twitch contractions were lower in TG mice compared with WT mice, whereas exercise training restored diastolic Ca2+ to levels comparable with WT mice (Fig. 5A).
Fig. 5.
A: diastolic Ca2+ levels were lower in sedentary transgenic (TG) CaMKIIδC-overexpressing mice, but this was raised to sedentary WT levels by exercise training; exercise training had, however, no effect in WT. B: time to 50% Ca2+ decay was prolonged in TG mice compared with WT but reduced by exercise training to WT levels; exercise training also reduced time to 50% Ca2+ decay in WT. C: example traces of Ca2+ transients evoked by twitch stimulations and caffeine stimulations. D: calculated NCX rate constant of Ca2+ removal; the NCX rate was increased in TG, whereas exercise training normalized the rate. Exercise training had no effect in WT. E: calculated SERCA2a rate constant of Ca2+ removal; SERCA2a rate was reduced in TG mice compared with WT, whereas exercise training increased the rate in both TG and WT. **P < 0.01 vs. other groups, *P < 0.05 vs. WT. Cells in each group n = 25–30. F: protein expression of SERCA2a (protein expressions are presented as means ± SE, number of mice in each group N = 4, no significant differences were observed between groups).
Time to 50% Ca2+-transient decay was significantly prolonged in TG mice compared with WT, whereas exercise training abolished this difference (Fig. 5B). To further analyze the characteristics of diastolic Ca2+ handling, we examined the rate constants of cytoplasmic Ca2+ removal (Fig. 5C). During a normal twitch-induced Ca2+ transient, Ca2+ is removed by SERCA2a, Na+/Ca2+ exchanger (NCX), and the plasma membrane Ca2+-ATPase (PMCA), and the rate constant of Ca2+ decline in this situation (Ktw) can be described as the sum of the rate constants associated with each efflux mechanism. During caffeine-induced Ca2+ transients, the contribution from SERCA2a is abolished, and the decay rate constant thus depends only on NCX and PMCA. To derive the rate constant of NCX (KNCX), the rate constant of Ca2+ removal during caffeine-induced Ca2+ transients in a solution containing 0 Na+ and 0 Ca2+ was measured and subtracted from the rate constant in the presence of these ions (3). First, the rate constant attributed to PMCA was negligibly small, and there were no differences between groups. The rate constant of Ca2+ removal during a caffeine-induced Ca2+ transient (SERCA2a contribution thus abolished) was significantly higher in TG mice, indicating increased NCX function (Fig. 5D). To quantify the contribution from SERCA2a, a simple model was used based on the following assumptions: SERCA2a transport rate is KSERCA2a = KTW - KNCX, and the relative contribution by SERCA2a is KSERCA2a/KTW. Thus, for WT mice, Ktw = 0.91 s−1, KNCX = 0.06 s−1, and KSERCA2a = 0.85 s−1, and 93% of the total Ca2+ removal was attributed to SERCA2a (Fig. 5E). In TG mice, Ktw (0.58 s−1) was reduced, and KNCX (0.09 s−1) was increased, resulting in a KSERCA2a of 0.49 s−1.
This implies that SERCA2a was responsible for 84% of the total Ca2+ removal, which was reduced by 42% compared with WT mice (from 0.85 to 0.49 s−1). In contrast, NCX function was increased by ∼50% (from 0.06 to 0.09 s−1) in the TG group. After exercise training in TG mice, Ktw = 0.8 s−1, KNCX = 0.06 s−1, and KSERCA = 0.74 s−1, which indicates that both SERCA2a and NCX functions were restored to normal levels (Fig. 5, C–E). At the protein level, SERCA2a was 26% lower in TG mice compared with WT. SERCA2a protein expression was 28% higher in exercised TG mice (Fig. 5F, not significant) compared with sedentary TG, which is in agreement with functional SERCA2a data from isolated cardiomyocytes.
Diastolic SR Ca2+ leak.
In TG mice the diastolic SR Ca2+ leak was higher (19 ± 3% of total SR Ca2+ in TG vs. 3 ± 2% in WT, P < 0.01, Fig. 6A), which was associated with a significant reduction in the total SR Ca2+ content compared with WT mice. Exercise training normalized SR Ca2+ leak to levels comparable with WT mice. The increased Ca2+ leak in TG mice was related to the overexpression of CaMKIIδC, since inhibition of CaMKIIδC by autocamtide 2-inhibitory peptide (AIP) reduced the leak to levels of WT mice (Fig. 6B). To control for a PKA-related effect on Ca2+ leak, separate cells were incubated with H-89, but under these conditions no effect on SR Ca2+ leak was observed (Fig. 6B). None of the CaMKII or PKA inhibitors had any effect on Ca2+ leak in sedentary WT mice, exercise-trained WT mice, or exercise-trained TG mice; however, in these groups the baseline Ca2+ leak was already minimal (Fig. 6A). In line with this, Ca2+ wave frequency was increased in TG mice compared with WT mice, but exercise training reduced the wave generation to WT levels (Fig. 6C).
Fig. 6.
A: diastolic SR RyR Ca2+ leak in normal HEPES 1.8 mmol/l Ca2+ solution in sedentary and exercise-trained transgenic (TG) CaMKIIδC-overexpressing mice and WT mice. B: RyR Ca2+ leak after incubation by AIP (to inhibit CaMKII) and H-89 (to inhibit PKA) in sedentary TG mice. Note that exercise training reduced the Ca2+ leak to levels found in WT mice and inhibiting CaMKII with AIP abolished Ca2+ leak. PKA inhibition by H-89 had no significant effect (NS) on reducing Ca2+ leak. No significant effects of H-89 or AIP were seen in any of the other groups. C: frequency of spontaneous Ca2+ waves was higher in sedentary TG compared with WT; exercise training reduced Ca2+ wave frequency to WT levels. Number of animals in each group for cardiomyocyte data N = 5, number of cells in each group n = 25–30. D: phosphorylation of serine-2814 residues at RyR2; example blots in inset (protein expressions are presented as means ± SE, number of rats in each group N = 4). **P < 0.01 vs. other groups, *P < 0.05 vs. other groups, #P < 0.05 between sedentary TG and sedentary WT.
Finally, we examined the mechanism of reduced diastolic SR Ca2+ leak by analyzing protein phosphorylation of RyR2 at the CaMKII-specific residue serine-2814. We found that the phosphorylation was increased by over 100% in sedentary TG mice compared with WT mice (P < 0.05) (Fig. 6D) and that this increase remained despite normalization of the SR Ca2+ leak. The serine-2814 phosphorylation status was not changed by exercise training in WT mice.
DISCUSSION
The present study demonstrates for the first time that exercise training suppresses the detrimental cardiac-based effects of transgenic CaMKIIδC overexpression in vivo and in vitro without significantly changing the CaMKIIδC expression level or its phosphorylation. After exercise training the following aspects of cardiac function were improved or restored to levels similar to those observed in the WT (untrained) animals: 1) global cardiac function in vivo and cardiomyocyte contractility, 2) ICaL, 3) diastolic Ca2+ levels and twitch Ca2+-transient amplitude, 4) propensity for spontaneous SR Ca2+ release, 5) SR Ca2+ content, 6) SERCA2a-mediated SR Ca2+ uptake, and 7) Ca2+ efflux by NCX.
Cardiomyocyte function and Ca2+ transients.
This study shows that overexpression of CaMKIIδC leads to cardiac dysfunction reminiscent of heart failure, with depressed Ca2+ cycling, cardiomyocyte malfunction, and increased diastolic SR Ca2+ leak. The data confirm as such previous findings in this model (19, 25, 40), with a functionally detrimental effect of chronically increased CaMKII signaling. The prolonged time to Ca2+ removal was mainly due to the ∼42% reduction in SERCA2a function in TG mice. NCX function was increased by ∼48%, which would favor Ca2+ extrusion across the sarcolemma and a reduction of diastolic Ca2+ concentration (19). This is not unexpected since commonly reduced SERCA2 activity is accompanied by increased NCX activity in models of cardiac pathology (8, 18, 23). Increased activity of CaMKIIδC would normally be expected to chronically enhance SERCA2a function by augmenting phosphorylation of threonine-17 PLN (40), but as previously reported, SERCA2a expression is reduced in the TG model (19, 40), an effect that dominates over the stimulation of SERCA2a activity from enhanced CaMK phosphorylation. As previously reported in CaMKIIδC TG mice (39), reduced SR Ca2+ content can be linked to reduced SERCA2a activity and the NCX-linked reduction of diastolic Ca2+ levels, both of which will reduce SERCA2a activity and subsequent SR Ca2+ content. Therefore the exercise-training effect in TG mice, with reduced extrusion of Ca2+ across the plasma membrane via the NCX combined with increased L-type Ca2+ currents, would in combination with the increased SERCA2a activity enable more SR Ca2+ loading and explain the restored Ca2+ homeostasis observed after exercise training.
SR Ca2+ leak.
Increased diastolic SR Ca2+ leak via RyR2 and increased spontaneous Ca2+ wave generation observed in TG mice have previously been linked to reduced Ca2+-transient amplitude and reduced SR Ca2+ content, i.e., changes that would limit contractility (2, 33). A recent study of the same TG mice found a higher frequency of delayed afterdepolarizations and increased propensity to arrhythmias as a result of increased SR Ca2+ leak (25). The increased SR Ca2+ leak is believed to result from the increased activity of CaMKII leading to hyperphosphorylation of the RyR2 at serine-2814. This would increase the RyR2 sensitivity to Ca2+ and thereby increase the open probability of RyR2 (1, 19, 25). The data from the present study showing AIP to abolish the high SR Ca2+ leak observed in sedentary TG mice support this concept. However, despite compelling evidence considering RyR serine-2814 phosphorylation to be causal in SR Ca2+ leak, the exercise training-induced reduction in SR Ca2+ leak was not due to a reduction in overall CaMKII activity or phosphorylation status of the RyR at serine-2814. Changes in antioxidant enzyme activity and oxidative stress following the exercise-training period could possibly alter the activation state of CaMKII, as oxidation of CaMKII increases its activity and consequently causes more leaky RyR channels (32). Our data identifying no exercise-induced changes in the phosphorylation status of either the threonine-286 site of CaMKII or the serine-2814 site of RyR2 does, however, indicate that it is unlikely that oxidation of CaMKII could be a central player in modulating the exercise-induced reduction in RyR2-associated SR Ca2+ leak, at least in this model of continuous TG overexpression of CaMKIIδC. Further analyses are therefore needed to determine the compensatory mechanisms by exercise that counteract the chronic high levels of CaMKII and serine-2814 phosphorylation upon SR Ca2+ leak in these TG mice.
A link between increased RyR2-mediated SR Ca2+ leak and increased propensity for arrhythmias has received attention lately, especially in heart failure (4, 23, 28, 31, 38), and novel Ca2+ release channel-stabilizing drugs have been proposed on this basis (17). The finding that exercise training reduces diastolic SR Ca2+ leak is interesting since it ameliorates a deleterious defect in failing hearts through a physiological adaptation mechanism and may therefore provide an alternative route to the same outcome. This mechanism has also been suggested to be activated by exercise training in the postmyocardial infarction heart failure model (14). It is also important to note that exercise training reverses the increased NCX activity. Thus these effects suggest that exercise training may have the potential to reduce delayed afterdepolarizations that potentially trigger ventricular arrhythmias, by synergistically improving diastolic intracellular Ca2+ homeostasis via reduced spontaneous SR Ca2+ release and reduced NCX activity. The data on reduced frequency of spontaneous Ca2+ waves after exercise training in TG CaMKIIδC mice do indeed support reduced potential for triggering of ventricular arrhythmias.
Functional cardiac and cardiomyocyte properties.
V̇o2 max is regarded as the best indicator of cardiorespiratory endurance, where cardiac output is a key determinant of V̇o2 max as it sets the upper limit for O2 supply to working muscles (24). Chronic overexpression of CaMKIIδC has previously been shown to cause a significant depression of cardiac function and remodeling of the heart, similar to observations in heart failure (19, 40); our finding of significantly reduced V̇o2 max in TG mice was therefore in agreement with our hypothesis. Reduced cardiac function in the TG CaMKIIδC overexpression model has previously been explained by pathological remodeling of the heart and breakdown of normal Ca2+ handling via phosphorylation of Ca2+ regulatory proteins (19, 40), which was confirmed in the present study. The improvements observed in V̇o2 max after exercise training are furthermore in line with improvements in cardiomyocyte functional properties as well as improvements observed in stroke volume and cardiac output. In addition to restoring cardiomyocyte contractility, exercise training also reduced the pathological cellular hypertrophy in TG mice, although it did not completely normalize cell size. Improvements in cardiomyocyte function followed the same pattern as changes in Ca2+ cycling and are consistent with previous studies using the same exercise-training model in animals with postmyocardial infarction heart failure (36) and diabetic cardiomyopathy (29). LV ejection fraction increased from ∼20 to 30%, which has important clinical value. However, the improvements of in vivo cardiac function measured by echocardiography are less pronounced compared with findings in isolated cardiomyocytes. This may suggest that structural remodeling in the TG mice with continuously activated CaMKII mice cannot be completely normalized by exercise training under the current conditions. The comparisons between single-cell contractility and that of the whole heart are made complex because of the additional factors that apply to the intact myocardium including 1) isometric and isotonic components to the contractile event in whole heart (only isotonic in single cell), 2) interstitial fibrosis in whole hearts, and 3) changes in system peripheral resistance. Our data reflect the physiological relevance of in vivo measurements in addition to in vitro assessments of isolated cardiomyocytes contracting in nonisometric conditions. Further work is required to investigate the basis of the differences between whole heart and single-cell contractility parameters.
Conclusions.
Exercise training improved in vivo cardiac function, restored cardiomyocyte function, plasma membrane, and sarcolemmal and intracellular Ca2+ fluxes, and abolished the abnormally high diastolic SR Ca2+ leak in mice with TG overexpression of CaMKIIδC. Thus, despite a continuous background of abnormally high CaMKIIδC, exercise training triggers mechanisms such as improved L-type Ca2+ channels and SR Ca2+ handling by restoration of SERCA2a function in addition to reduced diastolic SR Ca2+ leak thereby restoring cardiomyocyte Ca2+ homeostasis.
GRANTS
This work was supported by grants from the Norwegian Council of Cardiovascular Disease to U. Wisløff and M. A. Høydal; the Norwegian Research Council to U. Wisløff; the K. G. Jebsen Foundation to U. Wisløff, M. A. Høydal, and T. O. Stølen; Funds for Cardiovascular and Medical Research at St. Olav's University Hospital, Trondheim; the British Heart Foundation to O. J. Kemi and G. L. Smith; the Deutsche Forschungsgemeinschaft (DFG) through a Heisenberg grant (MA1982/4-1) and the Klinische Forschergruppe (MA1982/2-2) to L. S. Maier; and in part by the Foundation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease and the National Heart, Lung, and Blood Institute (HL-080101) to J. H. Brown. The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A.H., T.O.S., S.K., L.S.M., J.H.B., O.J.K., G.L.S., and U.W. conception and design of research; M.A.H., T.O.S., S.K., L.S.M., J.H.B., T.S., D.C., G.C., and U.W. performed experiments; M.A.H., T.O.S., S.K., L.S.M., T.S., D.C., G.L.S., and U.W. analyzed data; M.A.H., T.O.S., S.K., L.S.M., J.H.B., T.S., D.C., G.C., O.J.K., G.L.S., and U.W. interpreted results of experiments; M.A.H., T.O.S., S.K., L.S.M., T.S., G.L.S., and U.W. prepared figures; M.A.H. and O.J.K. drafted manuscript; M.A.H., T.O.S., S.K., L.S.M., J.H.B., T.S., D.C., G.C., O.J.K., G.L.S., and U.W. edited and revised manuscript; M.A.H., T.O.S., S.K., L.S.M., J.H.B., T.S., D.C., G.C., O.J.K., G.L.S., and U.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the work of Ragnhild Elisabeth Nyhus Røsbjørgen for technical assistance and isolation of cardiomyocytes, Anne Marie Ormbostad Berre for sampling of echocardiography data, and Nathan Scrimgeour and Karin Solvang-Garten for Western blot analyses.
REFERENCES
- 1.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force (2nd ed). Dordrecht, The Netherlands: Kluwer Academic, 2001. [Google Scholar]
- 4.Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann N Y Acad Sci 1080: 165–177, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100: 391–398, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Edman CF, Schulman H. Identification and characterization of delta B-CaM kinase and delta C-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim Biophys Acta 1221: 89–101, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122: 1221–1238, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation 99: 641–648, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res 84: 713–721, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14: 753–760, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G, Wisløff U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol 43: 354–361, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisløff U, Ellingsen Ø. Moderate vs high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 67: 161–172, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Kemi OJ, Loennechen JP, Wisløff U, Ellingsen Ø. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol 93: 1301–1309, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kemi OJ, MacQuaide N, Hoydal MA, Ellingsen O, Smith GL, Wisloff U. Exercise training corrects control of spontaneous calcium waves in hearts from myocardial infarction heart failure rats. J Cell Physiol 227: 20–26, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Kemi OJ, Wisløff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol (Oxf) 199: 425–439, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res 42: 254–261, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Lehnart SE. Novel targets for treating heart and muscle disease: stabilizing ryanodine receptors and preventing intracellular calcium leak. Curr Opin Pharmacol 7: 225–232, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Litwin SE, Bridge JH. Enhanced Na(+)-Ca2+ exchange in the infarcted heart: implications for excitation-contraction coupling. Circ Res 81: 1083–1093, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 92: 904–911, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Mann N, Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation 126: 2625–2635, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overend CL, O'Neill SC, Eisner DA. The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes. J Physiol 507, Part 3: 759–769, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol 42: 196–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Richardson RS, Harms CA, Grassi B, Hepple RT. Skeletal muscle: master or slave of the cardiovascular system? Med Sci Sports Exerc 32: 89–93, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail 2: 664–675, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh H, Delbridge LM, Blatter LA, Bers DM. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J 70: 1494–1504, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res 107: 1150–1161, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Stølen TO, Høydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisløff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res 105: 527–536, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Uemura A, Okazaki K, Takesue H, Matsubara T, Hidaka H. A novel Ca2+/calmodulin-dependent protein kinase lacking autophosphorylation activity in the rabbit heart. Biochem Biophys Res Commun 211: 562–569, 1995. [DOI] [PubMed] [Google Scholar]
- 31.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation 122: 2669–2679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res 108: 555–565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48: 1227–1234, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Wisløff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H1301–H1310, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Wisløff U, Loennechen JP, Currie S, Smith GL, Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res 54: 162–174, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Kimbrough JT, Colbran RJ, Anderson ME. Calmodulin kinase is functionally targeted to the action potential plateau for regulation of L-type Ca2+ current in rabbit cardiomyocytes. J Physiol 554: 145–155, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH. Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res 106: 354–362, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92: 912–919, 2003. [DOI] [PubMed] [Google Scholar]