Abstract
Background and purpose
Combining components from different manufacturers in total hip arthroplasty (THA) is common practice worldwide. We determined the proportion of THAs used in the Netherlands that consist of components from different manufacturers, and compared the revision rates of these mixed THAs with those of non-mixed THAs.
Patients and methods
Data on primary and revision hip arthroplasty are recorded in the LROI, the nationwide population-based arthroplasty register in the Netherlands. We selected all 163,360 primary THAs that were performed in the period 2007–2014. Based on the manufacturers of the components, 4 groups were discerned: non-mixed THAs with components from the same manufacturer (n = 142,964); mixed stem-head THAs with different manufacturers for the femoral stem and head (n = 3,663); mixed head-cup THAs with different head and cup manufacturers (n = 12,960), and mixed stem-head-cup THAs with different femoral stem, head, and cup manufacturers (n = 1,773). Mixed prostheses were defined as THAs (stem, head, and cup) composed of components made by different manufacturers.
Results
11% of THAs had mixed components (n = 18,396). The 6-year revision rates were similar for mixed and non-mixed THAs: 3.4% (95% CI: 3.1w–3.7) for mixed THAs and 3.5% (95% CI: 3.4–3.7) for non-mixed THAs. Revision of primary THAs due to loosening of the acetabulum was more common in mixed THAs (16% vs. 12%).
Interpretation
Over an 8-year period in the Netherlands, 11% of THAs had mixed components—with similar medium-term revision rates to those of non-mixed THAs.
There is a discrepancy between what guidelines recommend and the practice of mixing implant components (stem, head, or cup) from different manufacturers in assembling a total hip replacement. National arthroplasty register data show that components from different manufacturers are often combined, contrary to the advice in the product guidelines for these components. Mixed prostheses are defined as total hip arthroplasties (THAs) (stem, head, and cup) composed of components made by different manufactures. Non-mixed prostheses are defined as prostheses made up from components produced by one and the same manufacturer. With very little published in the literature on the consequences of implanting mixed prostheses, there is a need for evaluation of large numbers of mixed and non-mixed prostheses. Such data can be obtained from national arthroplasty registers (Graves 2010).
Mixing and matching of THA component brands is common worldwide. Surgeons may use various combinations of cups, heads, and stems made by different manufacturers. Using the National Joint Registry of England and Wales (NJR), Tucker et al. (2015) identified over 90,000 cases in which mixing of components was recorded between 2003 and 2013. However, the manufacturers emphasize that their implants were not designed, tested, or validated to be combined. In addition, there is a liability issue. Legally, the advice is not ever to implant a mixed arthroplasty unless you have familiarized yourself with the manufacturer’s product compatibility information (Michel 2009).
It has been hypothesized that mixing and matching of components from different manufacturers can lead to adverse effects (Ljung et al. 1989, Barrack et al. 1993, Morlock et al. 2001, Andrew et al. 2008, Higgs et al. 2013, Kurtz et al. 2013). However, recent research from the NJR of England and Wales revealed that combining a cemented stem with a polyethylene cup from a different manufacturer did not result in higher revision rates (Tucker et al. 2015).
We determined the proportion of THAs used in the Netherlands that consist of mixed components and examined the revision rate for mixed THAs. We compared this with revision rates for non-mixed THAs. We hypothesized that mismatch between stem, head, and cup would result in higher revision rates for mixed THAs than for non-mixed THAs.
Patients and methods
The Dutch Arthroplasty Register
The Dutch Arthroplasty Register (LROI) is a nationwide population-based registry that has information on joint arthroplasties in the Netherlands since 2007. The LROI was initiated by the Netherlands Orthopedic Association (NOV), and almost all Dutch orthopedic surgeons are members of this society. The LROI is well-supported by these members, resulting in an inter-institutional database with a completeness of more than 95% for primary THAs and 88% for hip revision arthroplasty (van Steenbergen et al. 2015).
Data collection
The LROI contains information on patient characteristics such as age, sex, and general health (ASA score), hospital of surgery, type of surgery, date of surgery, fixation, and prosthesis characteristics. The acetabular cup, femoral stem, femoral head, and inlay component of the hip prostheses can be registered in the LROI. Stickers supplied by the manufacturer, containing information on the implanted component, are attached to the registration form. Prosthesis characteristics are derived from an implant library within the LROI, which contains several core characteristics of all the prostheses used in the Netherlands since 2007, including the name and type of the prosthesis, the manufacturer, the material, and the head size of the hip prosthesis. The characteristics are supplied by all the implant manufacturers or distributors in the Netherlands.
A primary THA is defined as the first implantation of a hip prosthesis, to replace a hip joint. Hip revision arthroplasty is defined as any exchange (placement, replacement, or removal) of one or more components of the hip prosthesis, including head exchange (van Steenbergen et al. 2015).
The vital status of all patients was obtained from Vektis (2015), the national insurance database on healthcare in the Netherlands. For the present study, we included all the patients who underwent a primary THA in a Dutch hospital, from the start of the registry in 2007 until 2014 (n = 171,255). Patients with unknown prosthesis components were excluded (n = 4,711; 2.8%), as were cases with missing components (n = 5,184; 3.0%). These excluded patients generally had similar patient and treatment characteristics, although a slightly higher proportion underwent THA for reasons other than osteoarthritis than in the study population. The median length of follow-up was 3.0 years, with a maximum of 8.0 years.
Implant information
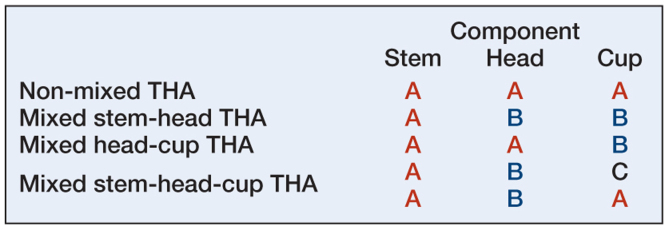
The category of mixed THAs was based on the manufacturer of the femur, the femoral head, and the acetabular component. Prostheses consisting of components from the same manufacturer were defined as non-mixed THAs (manufacturer A (femur) – manufacturer A (head) – manufacturer A (cup)). Mixed THAs were named after the manufacturer of the femoral component. Prostheses consisting of an acetabulum and a femoral head from the same manufacturer, but a femur from a different manufacturer were defined as mixed stem-head THAs (A-B-B). Similarly, mixed head-cup THAs were defined as a femur and a femoral head from the same manufacturer combined with an acetabulum from a different manufacturer (A-A-B). A fourth category, mixed stem-head-cup THAs, consisted of THAs with a different manufacturer for all components (femur, femoral head, and acetabulum) or THAs with the same manufacturer for femur and acetabulum, but a different manufacturer for the femoral head (A-B-C or A-B-A) (Figure 1).
Figure 1.
Combinations of components used in assembling a non-mixed or mixed THA, where A, B, and C represent different manufacturers.
In addition to the retrieved prosthesis information, we collected demographic data on all patients who received a THA in the period 2007–2014 in the Netherlands (Table 1). There were 3 age categories: < 60, 60–74, and ≥75 years. Overall physical condition of the patient was scored using the ASA score (I–IV). Diagnosis was categorized as osteoarthritis or non-osteoarthritis (consisting of mainly acute fracture, osteonecrosis, dysplasia, and late posttraumatic conditions). Previous operation of the same hip mainly involved osteosynthesis and osteotomy. Fixation of the hip was categorized as cementless, hybrid (where the acetabular component is implanted uncemented and the femoral component is implanted cemented), cemented, reversed hybrid (where the acetabular component is implanted cemented and the femoral component is implanted uncemented), or unknown. Head size was categorized as 22–28 mm, 32 mm, 36 mm, or ≥38 mm. Hip arthroplasty articulation was differentiated based on the bearing surface of the head and the bearing surface of the inlay or monoblock cup, and categorized as ceramic-on-polyethylene (PE), metal-on-PE, metal-on-metal, ceramic-on-ceramic, or other. Period of surgery was divided into 2007–2009, 2010–2011, and 2012–2014. Reasons for revision were infection, periprosthetic fracture, symptomatic metal-on-metal bearing, dislocation, loosening of the femoral or acetabular component, wear of the liner/cup, periarticular ossification, or establishment of a Girdlestone situation.
Table 1.
Demographic and clinical data on all patients who received a THA in the period 2007–2014 in the Netherlands (n = 161,360)
| Non-mixed THA (n = 142,964) |
Mixed stem- head THA (n = 3,663) |
Mixed head- cup THA (n = 12,960) |
Mixed stem- head-cup THA (n = 1,773) |
Total (n = 161,360) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Age, years | ||||||||||
| < 60 | 26,586 | 19 | 502 | 14 | 3,078 | 24 | 280 | 16 | 30,446 | 19a |
| 60–74 | 75,156 | 53 | 1,743 | 48 | 6,436 | 50 | 875 | 50 | 84,210 | 52 |
| ≥ 75 | 40,891 | 29 | 1,412 | 39 | 3,421 | 26 | 611 | 35 | 46,335 | 29 |
| Sex | ||||||||||
| Male | 46,656 | 33 | 1,093 | 30 | 4,147 | 32 | 583 | 33 | 52,479 | 33a |
| Female | 95,491 | 67 | 2,554 | 70 | 8,755 | 68 | 1,180 | 67 | 107,980 | 67 |
| ASA score | ||||||||||
| I | 33,483 | 25 | 947 | 26 | 3,508 | 29 | 383 | 25 | 38,321 | 25a |
| II | 85,068 | 63 | 1,973 | 55 | 6,846 | 57 | 977 | 64 | 94,864 | 62 |
| III–IV | 16,745 | 12 | ´686 | 19 | 1,772 | 15 | 161 | 11 | 19,364 | 13 |
| Diagnosis | ||||||||||
| Osteoarthritis | 124,464 | 87 | 3,191 | 87 | 10,568 | 82 | 1,545 | 87 | 139,768 | 87a |
| Non-osteoarthritis | 18,500 | 13 | 472 | 13 | 2,392 | 18 | 228 | 13 | 21,592 | 13 |
| Previous operation | ||||||||||
| No | 127,244 | 94 | 3,225 | 90 | 10,815 | 87 | 1,559 | 94 | 142,843 | 93a |
| Yes | 6,599 | 4.9 | 277 | 7.7 | 1,087 | 8.8 | 88 | 5.3 | 8,051 | 5.3 |
| Unknown | 1,605 | 1.2 | 90 | 2.5 | 497 | 4.0 | 4 | 0.2 | 2,195 | 1.4 |
| Fixation | ||||||||||
| Cementless | 92,150 | 65 | 747 | 20.5 | 4,135 | 33 | 1,464 | 91 | 98,496 | 62a |
| Hybrid | 3,519 | 2.5 | 272 | 7.5 | 3,538 | 28 | 19 | 1.2 | 7,348 | 4.6 |
| Cemented | 41,878 | 30 | 2,515 | 69 | 2,390 | 19 | 75 | 4.7 | 46,858 | 29 |
| Reversed hybrid | 3,898 | 2.8 | 106 | 2.9 | 2,589 | 20 | 43 | 2.7 | 6,636 | 4.2 |
| Unknown | 125 | 0.1 | 23 | 0.6 | 37 | 0.3 | 0 | 0.0 | 164 | 0.1 |
| Diameter of head | ||||||||||
| 22–28 mm | 53,543 | 38 | 2,806 | 77 | 7,523 | 58 | 509 | 29 | 64,381 | 40a |
| 32 mm | 59,911 | 42 | 293 | 8.0 | 4,011 | 31 | 1,193 | 67 | 65,408 | 41 |
| 36 mm | 26,302 | 18 | 232 | 6.3 | 1,343 | 10 | 35 | 2.0 | 27,912 | 17 |
| ≥ 38 mm | 3,207 | 2.2 | 323 | 8.8 | 83 | 0.6 | 36 | 2.0 | 3,649 | 2.3 |
| Articulation | ||||||||||
| Metal-on-metal | 6,411 | 4.5 | 87 | 2.4 | 16 | 0.1 | 0 | 0.0 | 6,514 | 4.0a |
| Metal-on-PE | 48,107 | 34 | 1,957 | 54 | 3,672 | 28 | 134 | 7.7 | 53,870 | 33 |
| Ceramic-on-PE | 66,704 | 47 | 1,245 | 34 | 7,417 | 57 | 1,551 | 89 | 76,917 | 48 |
| Ceramic-on-ceramic | 11,962 | 8.5 | 349 | 9.6 | 1,275 | 9.9 | 52 | 3.0 | 13,638 | 8.5 |
| Other | 7,913 | 5.6 | 12 | 3.3 | 447 | 3.5 | 3 | 0.2 | 8,375 | 5.2 |
| Period | ||||||||||
| 2007–2009 | 35,241 | 24 | 939 | 26 | 4,256 | 33 | 923 | 52 | 41,359 | 26a |
| 2010–2011 | 37,907 | 27 | 1,452 | 40 | 3,814 | 29 | 748 | 42 | 43,921 | 27 |
| 2012–2014 | 69,816 | 49 | 1,272 | 35 | 4,890 | 38 | 102 | 5.8 | 76,080 | 47 |
p < 0.0001.
Numbers do not add up to total due to unknown or missing components.
Statistics
The 4 groups of non-mixed, mixed stem-head, mixed head-cup, and mixed stem-head-cup components were taken separately and compared using chi-square test to test differences in patient and prosthesis characteristics, including manufacturer.
Survival time (with 95% confidence interval (CI)) was calculated as the time from primary THA to first revision arthroplasty for any reason (Nelissen et al. 1992), death of the patient, or January 1, 2015 (the end of follow-up). Standard survival analysis treats death simply as censored information, but this approach overestimates revision rates (Lacny et al. 2015, Wongworawat et al. 2015). Thus, crude cumulative incidence of revision was calculated using competing risk analysis, where death was considered to be a competing risk. Crude revision percentages within 1 year and 6 years were estimated according to the mixed-component group. Furthermore, revision rates within 6 years according to the reason for revision were estimated for non-mixed THAs and mixed THAs. The mixed-THA group contained all the mixed THAs, including mixed stem-head THAs, mixed head-cup THAs, and mixed stem-head-cup THAs. Differences in revision rates were compared using chi-square test. Crude and multivariable Cox proportional hazards regression analyses were performed. Adjustments were made for possible confounding variables, e.g. age at surgery, gender, ASA score, diagnosis (osteoarthritis vs. non-osteoarthritis), previous operation, fixation, head diameter, articulation, and period of surgery, to discriminate independent risk factors for revision. For all covariates added to the multivariate Cox proportional hazards regression analyses, the proportional hazards assumption was checked and met. Any p-values less than 0.05 were considered to be statistically significant. All analyses were performed using SPSS 22.0.
Results
161,360 THAs were included in the analysis. 11% of those performed in the period 2007–2014 were composed of mixed components (n = 18,396). This included 2.3% with a mixed stem and head, 8.0% with a mixed head and cup, and 1.1% with a mixed stem, head, and cup (Table 1).
Mixed stem-head THAs
Mixing of stem and head components from different manufacturers was found in 3,663 (2.3%) of the THAs. Almost 40% of the patients with a THA with a mixed stem and head were aged 75 years or older. A relatively large proportion of 22- to 28-mm diameter head components (77%), cemented THAs (69%), and metal-on-polyethylene articulations (54%) were used in THAs with a mixed stem and head (Table 1).
Mixed head-cup THAs
The most frequent combination of mixed components used in THA was between the femoral head and the acetabular component (n = 12,960; 8.0%), with a relatively large proportion of patients aged under 60 years. The number of mixed head-cup THAs remained relatively constant over the periods 2007–2009, 2010–2011, and 2012–2014. Similar to the mixing of stem and head, a relatively large proportion of 22- to 28-mm diameter head components (58%) were used in head-acetabulum mixed THAs, while this group contained a relatively small number of cemented THAs (19%) (Table 1).
Mixed stem-head-cup THAs
Mixing of femur, femoral head, and cup was found in 1,773 cases (1.1%). Most of these patients had a low ASA score (I or II in 89%) and no previous operations on the affected hip joint (94%). A relatively large proportion of 32-mm diameter head components (67%), ceramic-on-polyethylene articulations (89%), and cementless fixations (91%) were used in THAs with mixed femur, femoral head, and acetabulum. In the most recent time period (2012–2014), only 102 patients received a THA with a mixed stem, head, and cup (Table 1).
Manufacturers
The implanted THAs were manufactured by 21 different manufacturers. The femoral stem components were manufactured by 16 different manufacturers, femoral head components by 17, and the acetabular components by 19 companies. Manufacturers with more than 500 THAs (n = 8) are listed in Table 2. For these manufacturers, the percentages of non-mixed implants varied between 65% (Link) and 98% (Mathys Medical). A mixed stem and head component (n = 3,663) varied from 0% (Stryker) to 15% (Link) between different manufacturers. Mixed femoral head and acetabular components manufactured by Mathys Medical were rarely combined with acetabular components from other manufacturers (2%). Head-cup mixing was more common in THAs with femoral stem and head components from the manufacturers Link (20%) and Wright Medical (16%). Mixing of femoral stem, femoral head, and cup was detected in 1,773 THAs, ranging from 0% (Stryker and DePuy J&J) to 5% (Smith and Nephew) (Table 2). Many different combinations of manufacturers were seen in all the mixed-THA groups, with the most frequently used combinations of manufacturers being the same for mixed stem-head THAs and mixed head-cup THAs (Table 3).
Table 2.
Manufacturers of total hip prostheses represented in the groups with non-mixed or mixed components (n = 161,360)
| Non-mixed THA (n = 142,964) |
Mixed stem- head THA (n = 3,663) |
Mixed head- cup THA (n = 12,960) |
Mixed stem- head-cup THA (n = 1,773) |
Totala (n = 161,360) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | |
| Zimmer | 38,883 | 89 | 403 | 0.9 | 4,388 | 10 | 53 | 0.1 | 44,837 |
| Stryker | 22,572 | 94 | 10 | 0.0 | 1,456 | 6.1 | 6 | 0.0 | 24,121 |
| Biomet | 28,649 | 94 | 221 | 0.7 | 1,272 | 4.2 | 295 | 1.0 | 30,664 |
| Smith and Nephew | 19,225 | 88 | 147 | 0.7 | 1,200 | 5.5 | 1,171 | 5.4 | 21,874 |
| DePuy J&J | 13,672 | 96 | 33 | 0.2 | 528 | 3.7 | 3 | 0.0 | 14,329 |
| Link | 11,826 | 65 | 2,734 | 15 | 3,659 | 20 | 30 | 0.2 | 18,370 |
| Mathys Medical | 6,016 | 98 | 23 | 0.4 | 108 | 1.8 | 17 | 0.3 | 6,199 |
| Wright Medical | 831 | 79 | 53 | 5.0 | 167 | 16 | 2 | 0.2 | 1,053 |
The total included prostheses with unknown or missing components.
A mixed prosthesis was categorized according to the manufacturer of the most distal component.
Manufacturers with <500 THAs are not shown.
Table 3.
The 5 most frequently registered combinations of manufacturers of THA components in each mixed-component group (n = 18,396)
| Mixed stem-head THA (n = 3,663) |
Mixed head-cup THA (n = 12,960) |
Mixed stem-head-cup THA (n = 1,773) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stem | Head | n | Head | Cup | n | Stem | Head | Cup | n | |
| 1 | a | b | 2,623 | a | b | 2,377 | f | d | f | 945 |
| 2 | c | d | 207 | c | d | 2,147 | b | a | b | 92 |
| 3 | b | e | 117 | c | e | 867 | f | h | g | 63 |
| 4 | f | e | 66 | g | b | 753 | g | a | i | 32 |
| 5 | c | b | 63 | c | f | 631 | c | f | c | 23 |
The letters represent anonymized manufacturers of hip arthroplasty components.
Revision
The overall 1-year revision rates of non-mixed THAs and mixed THAs for all causes were similar (1.3% (CI: 1.3–1.4) for non-mixed THAs and 1.4% (CI: 1.2–1.5) for mixed THAs). The overall 6-year revision rate for all causes was not significantly different for non-mixed THAs and for mixed THAs (3.5% (CI: 3.4–3.7) for non-mixed THAs and 3.4% (CI: 3.1–3.7) for mixed THAs). No statistically significant differences were found between the mixed-component groups (Table 4).
Table 4.
Cumulative incidence of revision in THAs performed in the period 2007–2014 in the Netherlands (n = 161,360)
| Revision for any reason: | Non-mixed THA (n = 142,964) |
Mixed THAa (n = 18,396) |
||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| 1 year | 1.3 | 1.3–1.4 | 1.4 | 1.2–1.5 |
| 6 years | 3.5 | 3.4–3.7 | 3.4 | 3.1–3.7 |
This group includes mixed stem-head THAs, mixed head-cup THAs, and mixed stem-head-cup THAs.
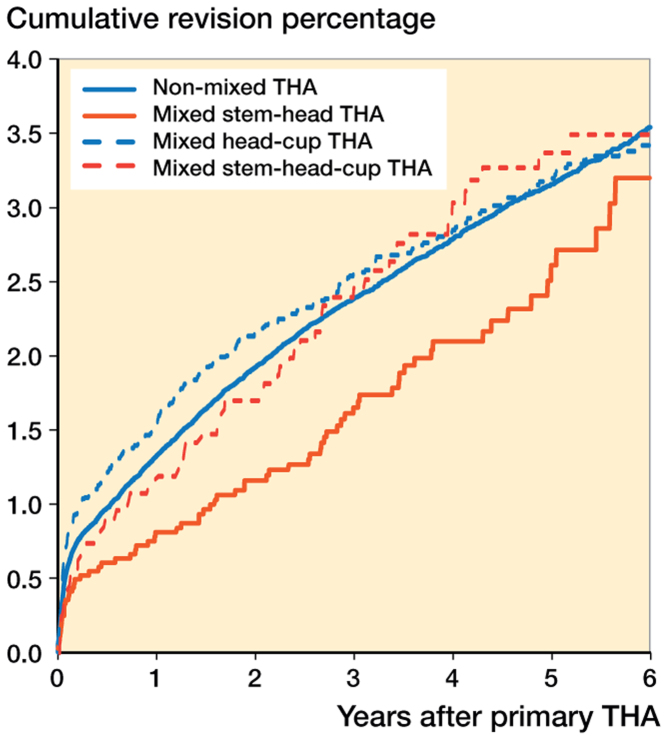
Cumulative incidence of revision in the mixed-component groups showed no statistically significant difference in revision rate, although the revision rate was somewhat lower in the first years in the group with mixed stem-head components (Figure 2).
Figure 2.
Cumulative incidence of revision according to category of mixing of THA components in the Netherlands in the period 2007–2014 (n = 161,360)
Revision of a primary THA due to loosening of the acetabulum was more common in mixed THAs (16% for mixed THAs and12% for non-mixed THAs). Revision due to a symptomatic metal-on-metal bearing was less common in mixed THAs, although this was mainly due to the fact that the proportion of metal-on-metal THAs was much higher in the non-mixed-component group (1.7% vs. 6.6%) (Table 5).
Table 5.
Reasons for revision or reoperation in revised THAs performed in the period 2007–2014 in the Netherlands (n = 3,879)
| Non-mixed THA (n = 3,403) |
Mixed THAa (n = 476) |
Total (n = 3,879) |
||||
|---|---|---|---|---|---|---|
| Revision within 6 years for | n | % | n | % | n | % |
| Infection | 379 | 11 | 57 | 12 | 436 | 11 |
| Periprosthetic fracture | 450 | 13 | 62 | 13 | 512 | 13 |
| Symptomatic MoMb bearing | 223 | 6.6 | 8 | 1.7d | 231 | 6.0 |
| Dislocation | 969 | 29 | 152 | 32 | 1,121 | 29 |
| Loosening of femur | 712 | 21 | 84 | 18 | 796 | 21 |
| Loosening of acetabulum | 421 | 12 | 77 | 16c | 498 | 13 |
| Cup/liner wear | 119 | 3.5 | 16 | 3.4 | 135 | 3.5 |
| Periarticular ossification | 71 | 2.1 | 14 | 2.9 | 85 | 2.2 |
| Girdlestone | 141 | 4.1 | 19 | 4.0 | 160 | 4.1 |
| Other | 549 | 16 | 78 | 16 | 627 | 16 |
This group includes mixed stem-head THAs, mixed head-cup THAs, and mixed stem-head-cup THAs.
MoM: metal-on-metal.
p < 0.05
p < 0.0001.
The crude survival analysis showed that patients with a mixed stem-head THA had a lower risk of revision than those with non-mixed THAs (hazard ratio (HR) = 0.78, 95% CI: 0.62–0.98) (Table 6). However, after adjustment for confounders there was no statistically significant difference in revision rate between the different mixed-component groups and non-mixed THAs. Younger patients (< 60 years), those with previous operation of the affected hip, patients with an ASA score of II–IV, those with a diagnosis other than osteoarthritis—and also those with a reversed hybrid THA, a small femoral head component (22–28 mm), a large femoral head component (≥ 38 mm), a metal-on-metal or metal-on-polyethylene articulation, or a THA implanted in the period 2012–2014 were more frequently revised. THAs with cemented fixation and ceramic-on-ceramic articulation resulted in a lower frequency of revision (Table 6).
Table 6.
Multivariate survival analysis of patients who underwent THA in the period 2007–2014 in the Netherlands (n = 161,360)
| Crude hazard ratio for revision (95% CI) | Adjusted hazard ratioa (95% CI) | |
|---|---|---|
| THA mixing category | ||
| Non-mixed | 1.0 | 1.0 |
| Mixed stem-head | 0.78 (0.62–0.98)b | 0.80 (0.63–1.03) |
| Mixed head-cup | 1.03 (0.92–1.15) | 1.11 (0.97–1.27) |
| Mixed stem-head-cup | 1.01 (0.77–1.31) | 1.02 (0.77–1.37) |
| Age at surgery, years | ||
| < 60 | 1.42 (1.32–1.54)b | 1.20 (1.11–1.31)b |
| 60–74 | 1.0 | 1.0 |
| ≥ 75 | 0.84 (0.78–0.91)b | 0.93 (0.85–1.01) |
| Sex | ||
| Male | 1.20 (1.13–1.28)b | 1.08 (1.01–1.16)b |
| Female | 1.0 | 1.0 |
| ASA score | ||
| I | 1.0 | 1.0 |
| II | 0.99 (0.92–1.06) | 1.18 (1.09–1.27)b |
| III–IV | 1.18 (1.06–1.31)b | 1.46 (1.31–1.64)b |
| Diagnosis | ||
| Osteoarthritis | 1.0 | 1.0 |
| Non-osteoarthritis | 1.31 (1.20–1.42)b | 1.18 (1.07–1.29)b |
| Previous operation | ||
| No | 1.0 | 1.0 |
| Yes | 1.36 (1.20–1.54)b | 1.19 (1.08–1.32)b |
| Fixation | ||
| Cementless | 1.0 | 1.0 |
| Hybrid | 0.72 (0.61–0.85)b | 0.78 (0.65–0.94) |
| Cemented | 0.58 (0.53–0.63)b | 0.63 (0.57–0.69)b |
| Reversed hybrid | 1.19 (1.03–1.37)b | 1.17 (0.99–1.37) |
| Unknown | 1.21 (0.58–2.54) | 1.18 (0.44–3.14) |
| Diameter of head | ||
| 22–28 mm | 1.07 (0.99–1.15) | 1.12 (1.03–1.21)b |
| 32 mm | 1.0 | 1.0 |
| 36 mm | 1.15 (1.04–1.27) | 1.11 (0.99–1.23) |
| ≥ 38 mm | 4.23 (3.78–4.73)b | 2.86 (2.44–3.35)b |
| Articulation | ||
| Metal-on-metal | 2.92 (2.65–3.22)b | 1.71 (1.49–1.97)b |
| Metal-on-PE | 1.01 (0.94–1.09) | 1.16 (1.07–1.26)b |
| Ceramic-on-PE | 1.0 | 1.0 |
| Ceramic-on-ceramic | 1.04 (0.92–1.18) | 0.88 (0.77–1.01) |
| Other | 0.82 (0.69–0.97)b | 0.93 (0.78–1.11) |
| Period | ||
| 2007–2009 | 1.14 (1.05–1.23)b | 1.06 (0.97–1.15) |
| 2010–2011 | 1.0 | 1.0 |
| 2012–2014 | 1.16 (1.06–1.26)b | 1.28 (1.17–1.40)b |
Adjusted for age at surgery, gender, ASA score, diagnosis, previous operation, fixation, head diameter, articulation, and period.
p < 0.0001.
Discussion
There is an ongoing debate about the use of components from different manufacturers in assembling a total hip arthroplasty. Based on a nationwide register, we found similar short-term survival of mixed and non-mixed THAs. These findings are supported by recent research from the NJR of England and Wales, in which even lower revision rates were found in patients with mixed cemented stems with polyethylene cups from another manufacturer (Tucker et al. 2015).
It has been hypothesized that mixing and matching of components from different manufacturers can lead to adverse effects due to unforeseen size mismatching of heads and tapers, and between heads and cups (Ljung et al. 1989, Barrack et al. 1993, Morlock et al. 2001, Andrew et al. 2008). Moreover, mixing and matching of components from different manufacturers may result in an alloy mismatch (Morlock et al. 2001). Recent awareness of taper corrosion has revealed that dissimilar alloy pairing is associated with increased taper damage at the modular interfaces (Higgs et al. 2013). Although ceramic femoral heads on metal tapers appear to reduce taper fretting corrosion compared to metal heads (Kurtz et al. 2013), there is very little literature on the long-term results. However, the recent research from the NJR of England and Wales revealed that mixing of a cemented stem with a polyethylene cup from a different manufacturer did not result in higher revision rates (Tucker et al. 2015).
Our registry study should be considered in the light of having certain limitations. First of all, the validity of the LROI has not been 100% since its introduction, but has been improving over the years. The validity of the registry increased from 88% completeness for THAs in 2009 to 98% in 2012 (van Steenbergen et al. 2015). Secondly, patient-reported outcome measures (PROMs) will be reported in the very near future, and the LROI does not yet report on surgeon experience. Retrieval analysis is the only method of confirming size mismatch, and may therefore be underrepresented in national joint registries that record the diagnosis for revision at the time of revision. Finally, our study had a limited follow-up time of 8 years. We acknowledge that possible complications of mixing components of different manufacturers, e.g. osteolysis, may have resulted in adverse events that would not be detected within the 8-year follow-up period.
Revision of primary THAs due to loosening of the acetabulum appeared to be more common in mixed THAs. Theoretically, loosening of components in the mixed-component group may be explained by increased trunnion wear due to a taper mismatch.
Manufacturers generally issue warnings and precautions regarding their products, cautioning against mixing of components from different manufacturers. However, surgeons prefer combinations that have the highest Orthopaedic Data Evaluation Panel (ODEP) rating, but will ask for combinations to fulfill certain criteria that may not be within the reach of many manufacturers. For example, a cemented stem suitable for an anterior approach combined with an uncemented cup with a bearing type that is only possible by combining one manufacturer’s highly ODEP-rated cup with a stem from a competitor. However, the question remains as to whether this is allowable by law. Orthopedic surgeons should comply with all the regulations that are set by manufacturers—such as instructions for product surveillance, vigilance, and maintenance to avoid restrictions based on civil law (Michel 2009). Legally, a THA that has been tested for its configuration and has been approved by a declaration of conformity is modified when components from different manufacturers are mixed. With the replacement or substitution of an incompatible component, the declaration of conformity of the original manufacturer expires (Michel 2009). The implications of these laws are not foreseeable yet, but surgeons should be cautioned to check whether mixing of the products is not restricted in the precaution sheets of the prostheses they use. With the recent merger of Biomet and Zimmer, and the manufacture of ceramic heads for several different companies by CeramTec, extra care should be applied to interpretation of the precaution sheets for newly released prosthesis combinations.
Mixed THAs are also described as off-label arthroplasties. “Offlabel use” refers to use of medical devices for purposes or subpopulations other than those approved by the United States Food and Drug Administration (Malcolm et al. 2015). Malcolm et al. (2015) demonstrated that the prevalence of offlabel THAs and TKAs was 30% and 37%, respectively, in the USA. They predicted an increase in the prevalence of off-label arthroplasties in the future. Tucker et al. (2015) described over 90,000 cases recorded between 2003 and 2013. In half of these cases, stems and heads from one manufacturer were mixed with a polyethylene cemented cup from another manufacturer. These numbers emphasize the differences between countries regarding the frequency of mixing of different components, as only 1% of the Dutch implants are used with this mixed combination.
The use of different taper sizes by the different manufacturers has made it difficult for surgeons to combine the right combination of stem and head junction, especially in revision hip arthroplasty. Manufacturers often have extensive overviews of which stems (male taper) can be combined with which heads (female taper), as these may differ in shape, roughness, inclination, and angle (Werner et al. 2015). Another issue is that manufacturers have changed tapers over the years in the same stem, e.g. Omnifit stems produced before the year 1991 had a Morse taper, but nowadays they have a C-taper (D’Lima et al. 1999). With very few literature overviews on taper dimensions of components, more research efforts towards unraveling the clinical significance of the potential mismatches are required.
In conclusion, 11% of THAs in the Netherlands were composed of mixed components, with similar medium-term revision rates to those of non-mixed THAs. Further studies on the use of mixed components in THA are needed, and they should be performed with a similar nationwide or international cohort with long-term follow-up.
RMP and AHH contributed to the study design and study protocol, gathering of data, analysis of data, writing of the initial draft, and preparation of the final draft. LNS contributed to gathering of data, analysis of data, writing of the initial draft, and preparation of the final draft. SKB, AVZ, and RES contributed to the study design and study protocol, writing of the initial draft, and preparation of the final draft. RWP contributed to the study design and study protocol, analysis of data, writing of the initial draft, and preparation of the final draft.
References
- Andrew J G, Beard D, Nolan J, Murray D.. “Mix and match” of femoral and acetabular components in total hip replacement – no effect on initial clinical benefit of surgery. Presented at the British orthopaedic association annual congress. J Bone Joint Surg Br 2008; 90-B (SUPP II). [Google Scholar]
- Barrack R L, Burke D W, Cook S D, Skinner H B, Harris W H.. Complications related to modularity of total hip components. J Bone Joint Surg Br 1993; 75(5): 688–92. [DOI] [PubMed] [Google Scholar]
- D’Lima D D, Walker R H, Colwell C W Jr. Omnifit-HA stem in total hip arthroplasty. A 2- to 5-year followup. Clin Orthop Relat Res 1999; (363): 163–9. [PubMed] [Google Scholar]
- Graves S E. The value of arthroplasty registry data. Acta Orthop 2010; 81(1): 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G B, Hanzlik J A, MacDonald D W, Gilbert J L, Rimnac C M, Kurtz S M, Implant Research Center Writing . Is increased modularity associated with increased fretting and corrosion damage in metal-on-metal total hip arthroplasty devices?: a retrieval study. J Arthroplasty 2013; 28(8 Suppl): 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S M, Kocagoz S B, Hanzlik J A, Underwood R J, Gilbert J L, MacDonald D W, Lee G C, Mont M A, Kraay M J, Klein G R, Parvizi J, Rimnac C M.. Do ceramic femoral heads reduce taper fretting corrosion in hip arthroplasty? A retrieval study. Clin Orthop Relat Res 2013; 471(10): 3270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacny S, Wilson T, Clement F, Roberts D J, Faris P D, Ghali W A, Marshall D A.. Kaplan-Meier survival analysis overestimates the risk of revision arthroplasty: a meta-analysis. Clin Orthop Relat Res 2015; 473(11): 3431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung P, Lidgren L, Rydholm U.. Hip socket wear due to component mismatch. A case report. Acta Orthop Scand 1989; 60(2): 223–4. [DOI] [PubMed] [Google Scholar]
- Malcolm T, Szubski C R, Schiltz N K, Klika A K, Koroukian S M, Barsoum W K.. Prevalence and perioperative outcomes of off-label total hip and knee arthroplasty in the United States, 2000–2010. J Arthroplasty 2015; 30(11): 1872–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C A. Product-mismatch – what is permitted?” European Cells and Materials 2009; 17 (Suppl. 1): 1.19579210 [Google Scholar]
- Morlock M, Nassutt R, Janssen R, Willmann G, Honl M.. Mismatched wear couple zirconium oxide and aluminum oxide in total hip arthroplasty. J Arthroplasty 2001; 16(8): 1071–4. [DOI] [PubMed] [Google Scholar]
- Nelissen R G, Brand R, Rozing P M.. Survivorship analysis in total condylar knee arthroplasty. A statistical review. J Bone Joint Surg Am 1992; 74(3): 383–9. [PubMed] [Google Scholar]
- Tucker K, Pickford M, Newell C, Howard P, Hunt L P, Blom A W.. Mixing of components from different manufacturers in total hip arthroplasty: prevalence and comparative outcomes. Acta Orthop 2015; 86(6): 671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenbergen L N, Denissen G A, Spooren A, van Rooden S M, van Oosterhout F J, Morrenhof J W, Nelissen R G.. More than 95% completeness of reported procedures in the population-based Dutch Arthroplasty Register. Acta Orthop 2015; 86(4): 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vektis www.Vektis.nl. Accessed: 01-10-2015
- Werner P H, Ettema H B, Witt F, Morlock M M, Verheyen C C.. Basic principles and uniform terminology for the head-neck junction in hip replacement. Hip Int 2015; 25(2): 115–9. [DOI] [PubMed] [Google Scholar]
- Wongworawat M D, Dobbs M B, Gebhardt M C, Gioe T J, Leopold S S, Manner P A, Rimnac C M, Porcher R.. Editorial: Estimating survivorship in the face of competing risks. Clin Orthop Relat Res 2015; 473(4): 1173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]