Abstract
IMPORTANCE
Adrenocortical carcinoma (ACC) is a rare but aggressive endocrine tumor, and the prognostic factors associated with long-term outcomes after surgical resection remain poorly defined.
OBJECTIVES
To define clinicopathological variables associated with recurrence-free survival (RFS) and overall survival (OS) after curative surgical resection of ACC and to propose nomograms for individual risk prediction.
DESIGN, SETTING, AND PARTICIPANTS
Nomograms to predict RFS and OS after surgical resection of ACC were proposed using a multi-institutional cohort of patients who underwent curative-intent surgery for ACC at 13 major institutions in the United States between March 17, 1994, and December 22, 2014. The dates of our study analysis were April 15, 2015, to May 12, 2015.
MAIN OUTCOMES AND MEASURES
The discriminative ability and calibration of the nomograms to predict RFS and OS were tested using C statistics, calibration plots, and Kaplan-Meier curves.
RESULTS
In total, 148 patients who underwent surgery for ACC were included in the study. The median patient age was 53 years, and 65.5% (97 of 148) of the patients were female. One-third of the patients (35.1% [52 of 148]) had a functional tumor, and the median tumor size was 11.2 cm. Most patients (77.7% [115 of 148]) underwent R0 resection, and 8.8% (13 of 148) of the patients had N1 disease. Using backward stepwise selection of clinically important variables with the Akaike information criterion, the following variables were incorporated in the prediction of RFS: tumor size of at least 12 cm (hazard ratio [HR], 3.00; 95% CI, 1.63–5.70; P < .001), positive nodal status (HR, 4.78; 95% CI, 1.47–15.50; P = .01), stage III/IV (HR, 1.80; 95% CI, 0.95–3.39; P = .07), cortisol-secreting tumor (HR, 2.38; 95% CI, 1.27–4.48; P = .01), and capsular invasion (HR, 1.96; 95% CI, 1.02–3.74; P = .04). Factors selected as predicting OS were tumor size of at least 12 cm (HR, 1.78; 95% CI, 1.00–3.17; P = .05), positive nodal status (HR, 5.89; 95% CI, 2.05–16.87; P = .001), and R1 margin (HR, 2.83; 95% CI, 1.51–5.30; P = .001). The discriminative ability and calibration of the nomograms revealed good predictive ability as indicated by the C statistics (0.74 for RFS and 0.70 for OS).
CONCLUSIONS AND RELEVANCE
Independent predictors of survival and recurrence risk after curative-intent surgery for ACC were selected to create nomograms predicting RFS and OS. The nomograms were able to stratify patients into prognostic groups and performed well on internal validation.
Adrenocortical carcinoma (ACC) is a rare disease, with an estimated annual incidence of 0.5 to 2.0 cases per million.1,2 For patients with localized ACC, complete surgical resection remains the best intervention for long-term survival.2–4 Unfortunately, ACC is a highly malignant tumor, with up to 70% to 85% of patients experiencing recurrence after surgical resection.3,5–7 In turn, 5-year overall survival (OS) estimates for patients with ACC range from 15% to 84% depending on the stage of disease at presentation.5,7–9 The results of studies10,11 have suggested that adjuvant mitotane therapy may improve the prognosis, although prospective data have been scarce because of the low incidence of ACC. While several clinicopathological factors have been associated with survival and the risk of recurrence, accurate prognostication among patients with ACC remains a challenge.9,12–14
Reliable prognostication in any cancer after surgical resection is critical to patients and treating physicians for making decisions regarding adjuvant treatment, type, and frequency of follow-up, as well as for providing patients and their families with helpful information about treatment modalities and short-term and long-term outcomes. The American Joint Committee on Cancer (AJCC)/International Union against Cancer (UICC) staging schema and the European Network for the Study of Adrenal Tumors (ENSAT) classification are the most widely used criteria to stage patients with ACC.1,12 These staging systems incorporate factors that assess the local extension of the primary tumor, lymph node involvement, and distant metastasis.1,12 Although the AJCC/UICC or ENSAT classification may be helpful for the general prediction of survival, risk stratification systems may not be as applicable to determine the prognosis of an individual patient.
Zini et al15 reported on the use of a nomogram to predict cancer-specific and all-cause mortality among patients with ACC based on Surveillance, Epidemiology, and End Results program data. Because this nomogram was constructed using a population-based cancer database, the availability of predictive factors was limited, and the data lacked granularity because of the administrative nature of the data set. Other studies6,16–20 have attempted to identify patient-related or tumor-related factors for estimating survival and recurrence risk after surgical resection of ACC, but these publications have been largely small, single-center reports. Therefore, the aim of the present study was to define clinicopathological factors associated with survival and the risk of recurrence after curative surgical resection using a large multi-institutional cohort of patients. In particular, we sought to create and internally validate nomograms to predict the individual risk of recurrence-free survival (RFS) and OS after resection of ACC.
Methods
Patient Population and Data Collection
Patients were identified from a retrospective, multi-institutional database consisting of 265 patients who underwent surgery for ACC between March 17, 1994, and December 22, 2014, at the following 13 major institutions in the United States: The Johns Hopkins University School of Medicine, Baltimore, Maryland; Stanford University School of Medicine, Stanford, California; Emory University, Atlanta, Georgia; Medical College of Wisconsin, Milwaukee; New York University School of Medicine, New York; The Ohio State University, Columbus; Washington University School of Medicine in St Louis, Missouri; University of Wisconsin School of Medicine and Public Health, Madison; University of California, San Diego; University of Texas Southwestern Medical Center, Dallas; University of California, San Francisco; Vanderbilt University, Nashville, Tennessee; and Wake Forest School of Medicine, Winston-Salem, North Carolina. The dates of our study analysis were April 15, 2015, to May 12, 2015. Only patients who underwent curative-intent surgery were included in the study group. Patients with metastatic disease at presentation or those with a macroscopically positive (R2) resection margin were excluded. Patients who were younger than 18 years or those with missing values on relevant predictors or follow-up data were not included in the study. To avoid the inclusion of deaths due to postoperative complications, patients who died within 30 days of surgery were excluded. Based on the inclusion and exclusion criteria, 148 patients were included in the analytic cohort. The institutional review boards at each participating institution approved this study. No additional patient informed consent that was specific to this study was required given its retrospective nature.
Demographic and clinicopathological data were collected, including age, sex, race, tumor size, tumor laterality (ie, left or right), tumor function (ie, hormone secreting or non-secreting), the presence or absence of capsular invasion, and the final T, N, and M stages of disease. Tumor size was defined as the maximal diameter of the tumor in the resected specimen. The functionality of the tumor was categorized as glucocorticoid, mineralocorticoid, or virilizing/feminizing hormonal hypersecreting tumors vs nonsecreting tumors. Resection margin status (negative [R0] or microscopically positive [R1]) and lymph node status (no metastasis [N0] or lymph node metastasis [N1]) were ascertained based on the final pathological assessment. Treatment and operative details included the surgical approach (open abdominal or posterior, minimally invasive surgery, or thoracoabdominal surgery), as well as information on adjuvant chemotherapy, radiation therapy, and mitotane therapy. Minimally invasive surgery was defined as robotic, laparoscopic, retroperitoneoscopic, or hand-assisted procedures. The primary outcomes of interest were long-term RFS and OS.
Statistical Analysis
Categorical variables were reported as whole numbers and proportions, and continuous variables were reported as medians with interquartile ranges (IQRs) unless indicated otherwise. The RFS and OS for the study population were generated using the Kaplan-Meier method, and differences in RFS and OS were examined using the log-rank test. Clinicopathological variables associated with recurrence risk and survival were assessed a priori based on clinical importance, scientific knowledge, and predictors identified in previously published articles.9,21,22 A correlation matrix was used to evaluate all explanatory variables for collinearity, and plausible interaction terms were tested, including interactions between age, sex, tumor size, nodal status, T stage, resection margin, and capsular invasion. No significant interaction was found; therefore, no interaction term was included in the multivariable analysis. Continuous predictors (ie, age and tumor size) were categorized after being assessed using restricted cubic splines to relax the linear relationship assumptions between continuous predictors and recurrence or death risks.23 The risk of recurrence and death was increased based on tumor size (approximately 14 and 12 cm, respectively). To be comparable with previous data,24 tumor size was modeled in the nomograms as a categorical variable (<12 vs ≥12 cm). The associations of relevant clinicopathological variables with RFS and OS were assessed using Cox proportional hazards regression models. Backward stepwise selection with the Akaike information criterion (AIC) was used to identify variables for the multivariable Cox proportional hazards regression models. Hazard ratios (HRs) were presented with their 95% CIs.25 Selected variables were incorporated in the nomograms to predict the probability of 3-year and 5-year RFS and OS rates after curative-intent surgical resection of ACC using statistical software (rms in R, version 3.0.3; http://www.r-project.org).26 For allocating points in the nomograms, the regression coefficients were applied to each individual observation to define the linear predictor.27
The model performance was evaluated by the predictive accuracy for individual outcomes (discriminating ability) and by the accuracy of point estimates of the survival function (calibration). The performance of the nomograms was evaluated using the C statistics by Harrell et al.28 The C statistic estimates the probability of concordance between predicted and observed outcomes in rank order and is equivalent to the area under the receiver operating characteristic curve.28 A C statistic of 0.5 indicates the absence of discrimination, whereas a C statistic of 1.0 indicates perfect separation of patients with different outcomes. Calibration was evaluated using a calibration plot, a graphic representation of the relationship between the observed outcome frequencies and the predicted probabilities, with a bootstrapped sample of the study group. In a well-calibrated model, the predictions should fall on a 45-degree diagonal line. Last, we plotted Kaplan-Meier curves over the tertiles of patients stratified by the scores predicted by the nomograms in the data set to further assess calibration. The model was validated using bootstrapped resampling to quantify any overfitting. Statistical analyses were performed with software programs (Stata, version 14.0; StataCorp LP and R, version 3.0.3; http://www.r-project.org). All tests were 2 sided, and P < .05 was considered statistically significant.
Results
Demographic and Clinicopathological Characteristics
The median patient age was 53 years (IQR, 44–62 years), and 65.5% (97 of 148) of the patients were female (eTable in the Supplement). Most patients (81.8% [117 of 143]) were of white race, and the median body mass index (calculated as weight in kilograms divided by height in meters squared) was 28.0 (IQR, 24.0–33.0). The ACC lesions were equally distributed on the right side (48.6% [71 of 146]) and the left side (51.4% [75 of 146]). In total, 35.1% (52 of 148) of all tumors were functional: among them, most were glucocorticoid (18.9% [28 of 148]) or virilizing/feminizing (10.8% [16 of 148]) hormone-secreting tumors, while only 5.4% (8 of 148) of the patients had a mineralocorticoid hormone-secreting type. In total, 64.6% (95 of 147) of the patients underwent surgery with an open abdominal or posterior approach, with the remaining patients having minimally invasive surgery (19.0% [28 of 147]) or thoracoabdominal surgery (16.3% [24 of 147]). On the final pathology report, most patients (77.7% [115 of 148]) underwent R0 resection, and the remaining 22.3% (33 of 148) of the patients had an R1 margin. The median tumor size was 11.2 cm (IQR, 8.0–15.0 cm), and 57.8% (63 of 109) had capsular invasion. Most tumors were advanced, with 37.8% (56 of 148) being stage III tumors and 10.8% (16 of 148) being stage IV tumors. Lymph node metastasis was observed in 8.8% (13 of 148) of the patients. In total, 31.1% (46 of 148) of the patients received mitotane therapy after surgery, and 10.4% (15 of 148) of the patients received adjuvant radiation therapy.
At a median follow-up of 23.0 months (range, 1.1–207.4 months), 54.1% (80 of 148) of the patients had recurrence of their disease, and 34.5% (51 of 148) of the patients had died. The unadjusted median RFS was 23.2 months (95% CI, 16.0–38.3 months), and the unadjusted median OS was 86.3 months (95% CI, 47.3–189.9 months). The 1-year, 3-year, and 5-year RFS percentages were 65.5% (95% CI, 56.5%–73.2%), 40.7% (95% CI, 31.2%–50.0%), and 30.4% (95% CI, 21.4%–40.3%), respectively, while the OS percentages were 87.7% (95% CI, 80.6%–92.3%), 67.7% (95% CI, 57.7%–75.9%), and 54.5% (95% CI, 43.2%–64.5%), respectively (eFigure in the Supplement).
Model Specifications and Predictors of RFS and OS
Established risk factors, as well as demographic and tumor characteristics of clinical importance, were selected as candidate variables for the prediction model. Backward stepwise selection using the AIC in the Cox proportional hazards regression modeling identified the following 5 variables that had the strongest association with recurrence risk: tumor size of at least 12 cm, positive nodal status, stage III/IV, cortisol-secreting tumor, and capsular invasion (Table 1). Similarly, backward step-wise selection using the AIC in the Cox proportional hazards re-gressionmodelingidentifiedthefollowing3variablesassociated with OS: tumor size of at least 12 cm, positive nodal status, and R1 margin (Table 2). On multivariable analysis, tumor size of at least 12 cm (HR, 1.78; 95% CI, 1.00–3.17; P = .05), positive nodal status (HR, 5.89; 95% CI, 2.05–16.87; P = .001), and R1 margin (HR, 2.83; 95% CI, 1.51–5.30; P = .001) were each independently associated with OS.
Table 1.
Cox Proportional Hazards Regression Model Showing the Association of Variables With Recurrence-Free Survival
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Factors Selected | ||||
| Tumor size, cm | ||||
| <12 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥12 | 1.56 (1.02–2.47) | .04 | 3.00 (1.63–5.70) | <.001 |
| Nodal status | ||||
| Negative | 1 [Reference] | NA | 1 [Reference] | NA |
| Positive | 3.44 (1.47–8.07) | .004 | 4.78 (1.47–15.50) | .01 |
| Not harvested | 1.05 (0.63–1.75) | .84 | 1.56 (0.79–3.05) | .20 |
| T stage | ||||
| I/II | 1 [Reference] | NA | 1 [Reference] | NA |
| III/IV | 2.26 (1.43–3.57) | <.001 | 1.80 (0.95–3.39) | .07 |
| Cortisol-secreting tumor | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 2.57 (1.52–4.34) | <.001 | 2.38 (1.27–4.48) | .01 |
| Capsular invasion | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 2.28 (1.30–4.00) | .004 | 1.96 (1.02–3.74) | .04 |
| Factors Not Selected | ||||
| Age, y | ||||
| <65 | 1 [Reference] | NA | NA | NA |
| ≥65 | 0.79 (0.43–1.44) | .44 | NA | NA |
| Sex | ||||
| Male | 0.96 (0.60–1.52) | .85 | NA | NA |
| Female | 1 [Reference] | NA | NA | NA |
| Functional tumor | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 1.53 (0.98–2.39) | .06 | NA | NA |
| Margin | ||||
| R0 | 1 [Reference] | NA | NA | NA |
| R1 | 1.54 (0.91–2.58) | .11 | NA | NA |
Abbreviations: HR, hazard ratio; NA, not applicable.
Table 2.
Cox Proportional Hazards Regression Model Showing the Association of Variables With Overall Survival
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Factors Selected | ||||
| Tumor size, cm | ||||
| <12 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥12 | 1.47 (0.84–2.58) | .17 | 1.78 (1.00–3.17) | .05 |
| Nodal status | ||||
| Negative | 1 [Reference] | NA | 1 [Reference] | NA |
| Positive | 5.54 (2.01–15.27) | .001 | 5.89 (2.05–16.87) | .001 |
| Not harvested | 1.55 (0.74–3.25) | .25 | 1.44 (0.65–3.22) | .37 |
| Resection margin | ||||
| R0 | 1 [Reference] | NA | 1 [Reference] | NA |
| R1 | 2.94 (1.62–5.33) | <.001 | 2.83 (1.51–5.30) | .001 |
| Factors Not Selected | ||||
| Age, y | ||||
| <65 | 1 [Reference] | NA | NA | NA |
| ≥65 | 1.10 (0.55–2.18) | .79 | NA | NA |
| Sex | ||||
| Male | 1.09 (0.67–1.78) | .73 | NA | NA |
| Female | 1 [Reference] | NA | NA | NA |
| Functional tumor | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 1.48 (0.84–2.59) | .17 | NA | NA |
| Cortisol-secreting tumor | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 1.75 (0.89–3.46) | .11 | NA | NA |
| T stage | ||||
| I/II | 1 [Reference] | NA | NA | NA |
| III/IV | 1.96 (1.08–3.54) | .03 | NA | NA |
| Capsular invasion | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 2.45 (1.18–5.11) | .02 | NA | NA |
Abbreviations: HR, hazard ratio; NA, not applicable.
Nomograms and Model Performance
Nomograms to predict RFS and OS of the patients with ACC after surgical resection are shown in Figure 1. The nomogram to predict RFS was created based on the following 5 independent prognostic factors: tumor size (<12 or ≥12 cm), nodal status (N0, N1, or Nx), T stage (I/II or III/IV), cortisol-secreting tumor, and capsular invasion. The nomogram to predict OS was created based on the following 3 independent prognostic factors: tumor size (<12 or ≥12 cm), nodal status (N0, N1, or Nx), and resection margin (R0 or R1). Higher total points based on the sum of the assigned number of points for each factor in the nomograms were associated with a worse prognosis. For example, a patient with a large (≥12 cm), advanced T-stage (III/IV) ACC without lymph node metastasis, evidence of cortisol secretion, or capsular invasion would have a total of 109.5 points (72 points for tumor size, 0 points for N0, 37.5 points for stage III/IV, 0 points for no cortisol-secreting tumor, and 0 points for no capsular invasion), for a predicted 3-year and 5-year RFS of 46.0% and 34.0%, respectively. Similarly, a patient who was seen with at least a 12-cm ACC without lymph node metastasis with a microscopically positive resection margin would have a total of 90 points (32.5 points for tumor size, 0 points for N0, and 57.5 points for R1 margin). For this patient, the predicted 3-year OS was 48.0%, and the predicted 5-year OS was 30.0%.
Figure 1. Nomograms Predicting Survival in Patients After Resection of Adrenocortical Carcinoma.
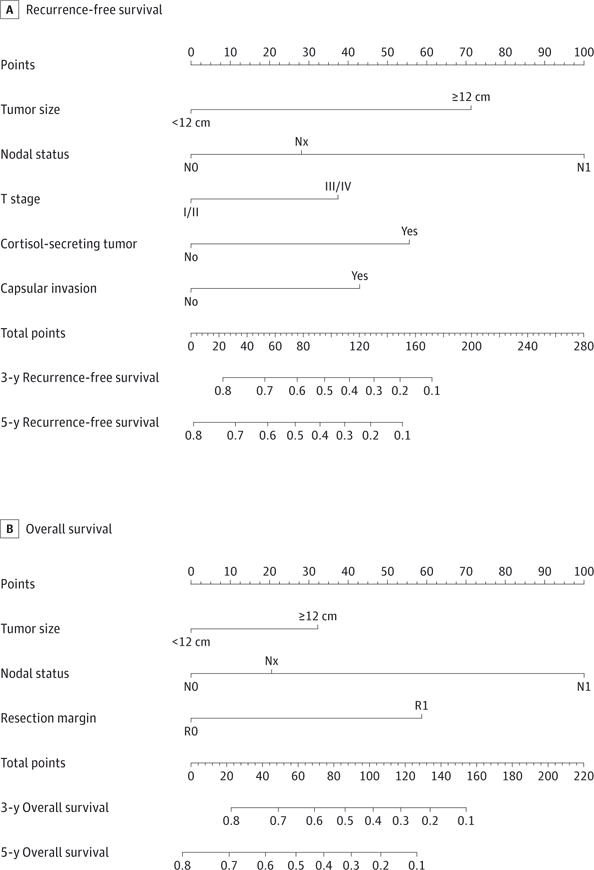
The nomogram to predict recurrence-free survival was created based on 5 independent prognostic factors, and the nomogram to predict overall survival was created based on 3 independent prognostic factors (see the Model Specifications and Predictors of RFS and OS subsection of the Methods section).
To further assess the discriminative ability of the model, the predicted probability of RFS and OS was then plotted as Kaplan-Meier curves stratified by the tertile of the predicted probability calculated from the nomograms (Figure 2). Patients with the lowest predicted 5-year RFS (tertile 3) had a substantially worse outcome (4.9% 5-year RFS) compared with patients in tertiles 1 and 2 (64.5% and 28.9% 5-year RFS, respectively) (P < .001). Compared with actual survival based on Kaplan-Meier tables, the median 5-year RFS predicted by the nomogram revealed good estimation of 4.8%, 28.5%, and 61.9% in tertiles 1, 2, and 3, respectively (P < .001). Similarly, patients with the lowest predicted 5-year OS (tertile 3) had a substantially worse outcome (31.1% 5-year OS) compared with patients in tertiles 1 and 2 (72.8% and 51.0% 5-year OS, respectively) (P < .001). The median 5-year OS values predicted by the nomogram were 70.1%, 53.1%, and 23.4% in tertiles 1, 2, and 3, respectively (P < .001). The discriminative ability of the final model for RFS and OS was also assessed using the C statistics (0.74 for RFS and 0.70 for OS). The accuracy of the model and potential model overfit were assessed by bootstrap validation with 200 resamplings. The 30-sample bootstrapped calibration plot for the prediction of 5-year RFS and OS is shown in Figure 3.
Figure 2. Kaplan-Meier Curves Demonstrating Survival in Patients After Resection for Adrenocortical Carcinoma According to Tertiles of Predicted Survival.
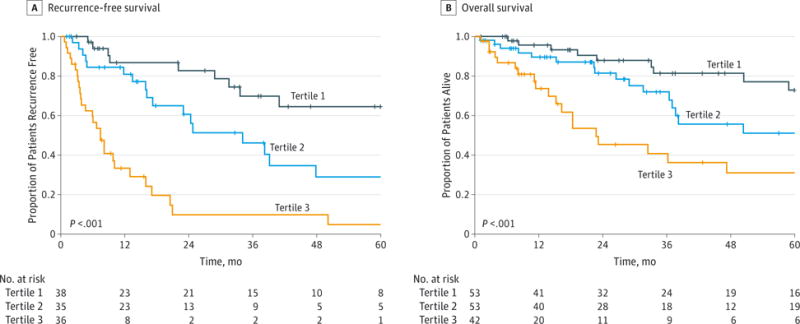
P values are by the log-rank test.
Figure 3. Calibration Plot Comparing Predicted and Actual Survival Probabilities at 5-y Follow-up.
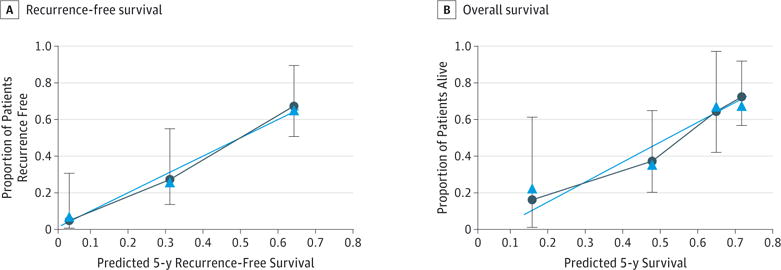
The 30-sample bootstrapped calibration plot for the prediction of 5-y recurrence-free survival and overall survival is shown. The blue line represents the ideal fit; circles represent nomogram-predicted probabilities; triangles represent the bootstrap-corrected estimates; and error bars represent the 95% CIs of these estimates.
Discussion
While complete surgical resection remains the treatment modality of choice for ACC, the risk of recurrence with surgery can be significant, with as many as 50% to 70% of the patients experiencing recurrence.21 In fact, because ACC often manifests at an advanced stage, 5-year OS remains guarded, ranging from 20% to 45%.29 However, survival after surgery for ACC can vary widely depending on the tumor stage,1,8 reflecting the prognostic heterogeneity associated with this disease.12 Accurate prognostication after surgery for ACC is important not only to select patients for adjuvant treatment but also to inform patients accurately about their long-term prognosis. While the AJCC/UICC and ENSAT staging systems are most commonly used for predicting outcomes,1 the prognostic factors reported to be associated with long-term survival have varied, and the optimal method for risk stratification of the patients with ACC is unclear.14,21 In this study, we created 2 nomograms that numerically predicted an individual’s RFS and OS after surgical resection for ACC based on patient-related and tumor-related factors. This information can be used to inform the prognosis of the patients, as well as to make individualized decisions regarding the treatment and surveillance. The present study is important because the nomograms were developed using a large multi-institutional group of patients who underwent surgery for ACC. In addition, in contrast to the previously proposed nomogram based on the Surveillance, Epidemiology, and End Results program database,15 the performance of the present nomograms was rigorously assessed and internally validated.
Another particular strength of the present study is that it took into account a wide array of variables previously reported to be associated with the prognosis after surgical resection of ACC.21,24,30 Factors reported to be associated with outcomes have varied widely, with a relative lack of consensus regarding which factors are key to determining the prognosis.17,21,22 For example, some studies17,18,21,24,29 have reported that age, sex, high tumor grade, hormone-secreting tumors, and tumor size are associated with worse outcomes, and other studies31–34 have demonstrated an association between cortisol-secreting ACC tumors and a poor outcome. In contrast, other researchers have reported no correlation between age, sex, tumor size, or functionality of the tumor and long-term survival.11,16,19,20,22,35,36 In the present study, we similarly noted no association of sex, age, or functionality of the tumor with RFS or OS (Table 1 and Table 2). In contrast, most data on ACC have demonstrated that the completeness of surgery (ie, R0 vs R1) and the tumor stage at diagnosis have been most consistently associated with a worse prognosis.1,17,18,21,29 Indeed, we also noted that these 2 factors were strongly linked to outcomes because the risk of death was 3-fold higher among patients with an R1 margin, and the risk of recurrence was 2-fold higher among patients with stage III/IV disease. To our knowledge, the present study is the first risk stratification model to consider previously proposed risk variables and assess them independently for their inclusion in formal nomograms for ACC prognosis.
Accurate risk stratification of the patients with tumors such as ACC is important because the prognosis of the patients may be heterogeneous.5,8,9,37 Instead of using staging information from the AJCC/UICC or ENSAT classification, which is derived based on population-based or large cohort data, nomograms may provide a more individualized manner to provide prognostic information to patients. Zini et al15 previously proposed a nomogram among patients with ACC that incorporated age and tumor stage in the model, indicating that distant metastasis was the most potent predictive factor for mortality. The inclusion of the patients with metastasis is problematic and is not particularly helpful because it is self-evident that distant disease largely drives the prognosis. As such, we chose to limit our analytic cohort to only patients who had no evidence of metastatic disease to provide prognostic information to those patients most likely undergoing surgical management of ACC (ie, those classified as M0). Indeed, when stratified into tertiles, the proposed nomograms were able to identify distinct groups of the patients who were at different risks of recurrence and death (Figure 2). Most important, our proposed nomograms demonstrated good discriminative ability, with a C statistic of 0.74 for predicting RFS and a C statistic of 0.70 for predicting OS (Figure 1). In addition, when the median 5-year survival was predicted by the nomograms, it was similar to the actual survival calculated from the Kaplan-Meier curves. Collectively, the data strongly suggest that the proposed nomograms can provide patient-specific information on the risk of recurrence and survival for patients with ACC after surgery.
Accurate data on the prognosis for patients with ACC may be important to treating physicians for several reasons. While the role of adjuvant chemotherapy or radiation therapy in ACC treatment has not been extensively studied because of the relative rarity of the disease,9,30 some studies3,11,19,38–40 have reported that adjuvant systemic mitotane therapy may provide RFS and OS benefit for select patients with localized disease. While only about 1 in 3 patients received adjuvant mitotane, individualized risk prediction models, such as the present nomograms, may have a role in selecting and guiding postoperative treatment in the future.
The present study had several limitations. Despite combining the experience of 13 large health care centers, the sample size was still small. As such, some analyses may have been limited. In addition, the data on certain factors, such as nuclear grade and mitotic index, were unavailable; therefore, their effect or potential incorporation in the nomograms could not be assessed. While collaborating with multiple institutions undoubtedly led to a lack of standard diagnostic or treatment approaches, the multicenter nature of the present study was also a strength because it improves the generalizability of our findings. Finally, although our nomograms were internally validated using bootstrap validation, future studies are needed to externally validate the proposed nomograms.
Conclusions
Using a large multicenter data set of patients who underwent resection of ACC, several independent prognostic variables were identified to predict RFS and OS. The proposed nomograms were able to stratify patients into distinct prognostic groups regarding recurrence and overall long-term outcomes. In addition, the nomograms performed well on internal validation. Future studies are needed to externally validate the proposed nomograms to establish their value in predicting the long-term prognosis after curative resection for ACC.
Supplementary Material
Footnotes
CME Quiz at jamanetworkcme.com
Author Contributions: Drs Kim and Pawlik had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kim, Evans, Hatzaras, Fields, Jin, Weber, Salem, Gad, Yopp, Mansour, Poultsides, Pawlik.
Acquisition, analysis, or interpretation of data: Kim, Margonis, Prescott, Tran, Postlewait, Maithel, Wang, Hatzaras, Shenoy, Phay, Keplinger, Fields, Jin, Weber, Salem, Sicklick, Gad, Yopp, Duh, Seiser, Solorzano, Kiernan, Votanopoulos, Levine, Poultsides, Pawlik.
Drafting of the manuscript: Kim, Margonis, Evans, Shenoy, Gad.
Critical revision of the manuscript for important intellectual content: Kim, Prescott, Tran, Postlewait, Maithel, Wang, Hatzaras, Phay, Keplinger, Fields, Jin, Weber, Salem, Sicklick, Yopp, Mansour, Duh, Seiser, Solorzano, Kiernan, Votanopoulos, Levine, Poultsides, Pawlik.
Statistical analysis: Kim, Fields.
Administrative, technical, or material support: Tran, Postlewait, Maithel, Evans, Shenoy, Keplinger, Fields, Jin, Weber, Salem, Gad, Poultsides, Pawlik. Study supervision: Margonis, Hatzaras, Sicklick, Yopp, Mansour, Levine, Poultsides, Pawlik.
Conflict of Interest Disclosures: None reported.
References
- 1.Fassnacht M, Johanssen S, Quinkler M, et al. German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009;115(2):243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 2.Sabolch A, Else T, Griffith KA, et al. Adjuvant radiation therapy improves local control after surgical resection in patients with localized adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2015;92(2):252–259. doi: 10.1016/j.ijrobp.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Terzolo M, Baudin AE, Ardito A, et al. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169(3):263–270. doi: 10.1530/EJE-13-0242. [DOI] [PubMed] [Google Scholar]
- 4.Tran TB, Liou D, Menon VG, Nissen NN. Surgical management of advanced adrenocortical carcinoma: a 21-year population-based analysis. Am Surg. 2013;79(10):1115–1118. [PubMed] [Google Scholar]
- 5.Gratian L, Pura J, Dinan M, et al. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Ann Surg Oncol. 2014;21(11):3509–3514. doi: 10.1245/s10434-014-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojadinovic A, Brennan MF, Hoos A, et al. Adrenocortical adenoma and carcinoma: histopathological and molecular comparative analysis. Mod Pathol. 2003;16(8):742–751. doi: 10.1097/01.MP.0000081730.72305.81. [DOI] [PubMed] [Google Scholar]
- 7.Kerkhofs TM, Verhoeven RH, Bonjer HJ, et al. Dutch Adrenal Network Surgery for adrenocortical carcinoma in The Netherlands: analysis of the national cancer registry data. Eur J Endocrinol. 2013;169(1):83–89. doi: 10.1530/EJE-13-0142. [DOI] [PubMed] [Google Scholar]
- 8.Carnaille B. Adrenocortical carcinoma: which surgical approach? Langenbecks Arch Surg. 2012;397(2):195–199. doi: 10.1007/s00423-011-0852-1. [DOI] [PubMed] [Google Scholar]
- 9.Erickson LA, Rivera M, Zhang J. Adrenocortical carcinoma: review and update. Adv Anat Pathol. 2014;21(3):151–159. doi: 10.1097/PAP.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 10.Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99(2):455–461. doi: 10.1210/jc.2013-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 12.Asare EA, Wang TS, Winchester DP, Mallin K, Kebebew E, Sturgeon C. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery. 2014;156(6):1378–1385. doi: 10.1016/j.surg.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Turbendian HK, Strong VE, Hsu M, Ghossein RA, Fahey TJ., III Adrenocortical carcinoma: the influence of large vessel extension. Surgery. 2010;148(6):1057–1064. doi: 10.1016/j.surg.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the International Union Against Cancer–staging system: a North American validation. Eur J Cancer. 2010;46(4):713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Zini L, Capitanio U, Jeldres C, et al. External validation of a nomogram predicting mortality in patients with adrenocortical carcinoma. BJU Int. 2009;104(11):1661–1667. doi: 10.1111/j.1464-410X.2009.08660.x. [DOI] [PubMed] [Google Scholar]
- 16.Canter DJ, Mallin K, Uzzo RG, et al. Association of tumor size with metastatic potential and survival in patients with adrenocortical carcinoma: an analysis of the National Cancer Database. Can J Urol. 2013;20(5):6915–6921. [PubMed] [Google Scholar]
- 17.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 18.Kendrick ML, Lloyd R, Erickson L, et al. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg. 2001;136(5):543–549. doi: 10.1001/archsurg.136.5.543. [DOI] [PubMed] [Google Scholar]
- 19.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322(17):1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenal cortical carcinoma. Cancer. 1989;64(3):765–769. doi: 10.1002/1097-0142(19890801)64:3<765::aid-cncr2820640333>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 22.Fassnacht M, Libé R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7(6):323–335. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Harrison LE, Gaudin PB, Brennan MF. Pathologic features of prognostic significance for adrenocortical carcinoma after curative resection. Arch Surg. 1999;134(2):181–185. doi: 10.1001/archsurg.134.2.181. [DOI] [PubMed] [Google Scholar]
- 25.Simon R. Confidence intervals for reporting results of clinical trials. Ann Intern Med. 1986;105(3):429–435. doi: 10.7326/0003-4819-105-3-429. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 27.Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14(14):4400–4407. doi: 10.1158/1078-0432.CCR-07-4713. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–2546. [PubMed] [Google Scholar]
- 29.Icard P, Louvel A, Chapuis Y. Survival rates and prognostic factors in adrenocortical carcinoma. World J Surg. 1992;16(4):753–758. doi: 10.1007/BF02067377. [DOI] [PubMed] [Google Scholar]
- 30.Icard P, Chapuis Y, Andreassian B, Bernard A, Proye C. Adrenocortical carcinoma in surgically treated patients: a retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery. 1992;112(6):972–979. [PubMed] [Google Scholar]
- 31.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91(7):2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 32.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12(3):657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro RC, Michalkiewicz EL, Figueiredo BC, et al. Adrenocortical tumors in children. Braz J Med Biol Res. 2000;33(10):1225–1234. doi: 10.1590/s0100-879x2000001000013. [DOI] [PubMed] [Google Scholar]
- 34.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25(7):891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 35.Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol. 2002;20(4):941–950. doi: 10.1200/JCO.2002.20.4.941. [DOI] [PubMed] [Google Scholar]
- 36.Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma: clinical outcome at the end of the 20th century. Cancer. 2001;92(5):1113–1121. doi: 10.1002/1097-0142(20010901)92:5<1113::aid-cncr1428>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579–2586. doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 38.Fassnacht M, Terzolo M, Allolio B, et al. FIRM-ACT Study Group Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 39.Abraham J, Bakke S, Rutt A, et al. A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. Cancer. 2002;94(9):2333–2343. doi: 10.1002/cncr.10487. [DOI] [PubMed] [Google Scholar]
- 40.Wängberg B, Khorram-Manesh A, Jansson S, et al. The long-term survival in adrenocortical carcinoma with active surgical management and use of monitored mitotane. Endocr Relat Cancer. 2010;17(1):265–272. doi: 10.1677/ERC-09-0190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


