Abstract
This study aimed to determine whether age at introduction of solid foods was associated with feeding difficulties at three years. The study was carried out using data from the Southampton Women’s Survey (SWS). Women enrolled in the SWS who subsequently became pregnant were followed up during pregnancy and postpartum, and the offspring have been studied through childhood. Maternal sociodemographic and anthropometric data, and child anthropometric and feeding data, were collected through interviews and self-complete questionnaires. When the children were three years, mothers/carers rated six potential child feeding difficulty questions on a four-point Likert scale, including one general question and five specific feeding difficulty questions. Age at introduction of solids as a predictor of feeding difficulties was examined in 2,389 mother-child pairs, adjusting for child (age last breastfed, sex, gestation) and maternal characteristics (parity, pre-pregnancy body mass index, age, education, employment, parenting difficulties, diet quality). The majority of mothers/carers (61%) reported some feeding difficulties (general feeding difficulty question) at three years; specifically with their child eating enough food (61%), eating the right food (66%), and being choosy with food (74%). Children who were introduced to solids ≥ six months had a lower risk of feeding difficulties (RR=0.73 (95%CI=0.59;0.91), p=0.004) than children who were introduced to solids between four and six months. No other significant associations were found. There were few associations between feeding difficulties in relation to age at introduction of solid foods. However, general feeding difficulties were less common among infants introduced to solid foods ≥ six months of age.
Keywords: introduction of solid foods, feeding difficulties, infants, child
Introduction
The recommended age at which solid foods should be introduced to infants has changed over time (1). For example, solid foods were recommended to be introduced to infants from two months of age in the 1950’s whereas they were recommended from nine months in the early 1900’s (1). The optimal age is still a current topic of debate (2–4). In the United Kingdom (UK), infant feeding guidelines were changed in 2003 to recommend exclusive breastfeeding for the first six months of life, with solid foods introduced from then on alongside continued breastfeeding (5); prior to that the advice was to introduce solid foods between four and six months of age (6). This change followed the Kramer and Kakuma systematic review for the World Health Organisation (WHO) (7) and aligned UK recommendations with international infant feeding guidance.
Concerns have been expressed on the appropriateness of the revised infant feeding guidance in a developed and industrialised context, such as the UK (3, 4). Some research indicates that there may be ‘critical windows’ in infancy when children are receptive to new food flavours and textures (8–10), suggesting that delaying the introduction of solid foods may lead to an aversion to certain flavours and textured foods, and possibly feeding difficulties in later childhood (10–16). There is also evidence showing that delaying the introduction of ‘lumpy solids’ to nine or 10 months of age is associated with feeding difficulties in childhood (10, 17). However, to our knowledge, differences in age at introduction of any solid foods around varying ages in mid-infancy, and later risk of feeding difficulties has not been evaluated.
The aim of this study was to determine whether the introduction of solid foods at or after six months of age is associated with feeding difficulties in later childhood. The study was carried out using data from the Southampton Women’s Survey (SWS), that spanned the change in UK infant feeding guidance in 2003 (5), and the infants have been followed up in childhood. It provides an opportunity to examine differences in infant feeding practice in relation to risk of feeding difficulties assessed the same way in a large population of UK children.
Methods
The Southampton Women’s Survey (SWS)
The SWS is an ongoing, prospective cohort study of 12,583 non-pregnant, women aged 20–34 years, living in the city of Southampton, UK (18). Assessments of lifestyle, diet and anthropometry were performed at study entry (April 1998 – December 2002). Women enrolled in the SWS who subsequently became pregnant were followed up during pregnancy and postpartum, and the offspring have been studied through infancy and childhood. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Southampton and South West Hampshire Local Research Ethics Committee (06/Q1702/104). Written informed consent was obtained from all participating women and by a parent or guardian with parental responsibility on behalf of their children.
Measures
Maternal data
Prior to pregnancy, maternal sociodemographic and anthropometric data were collected through face-to-face interviews and self-complete questionnaires. Maternal educational attainment was defined in six groups according to highest academic qualification; i) no academic qualification, ii) General Certificate of Secondary Education (GCSE, ~16 years of age) grade D or below, iii) GCSE grade C or above, iv) Advanced level (A-level, ~18 years of age) or equivalent, v) Higher National Diploma (HNDs) or equivalent, and vi) Degree. Pre-pregnancy height (cm) was measured to the nearest 0.1cm using a portable stadiometer (Harpenden, CMS Weighing Equipment Ltd., London, UK), and weight (kg) to the nearest 0.1kg using a portable scale (Seca, Hamburg, Germany). Women were asked to remove their shoes and any heavy items of clothing or jewellery prior to measurements. Pre-pregnancy maternal diet was measured using an interviewer-administered, 100-item Food Frequency Questionnaire (FFQ), to assess habitual dietary intake over the previous three months (19). Principal Components Analysis (PCA) was performed on reported frequencies of consumption of 48 foods and food groups derived from the FFQ, based on the correlation matrix to adjust for unequal variances of the original variables score (20). The first principal component identified a pattern that was consistent with UK dietary recommendations (21, 22). From this pattern ‘prudent’ diet scores before pregnancy were calculated by multiplying the coefficients from the PCA by each woman’s standardized reported frequencies of pre-pregnancy consumption and were interpreted as a measure of diet ‘quality’ (20). Among women who became pregnant, smoking status (yes, no) in pregnancy was ascertained at the 11- and 34-week interviews. Maternal employment was ascertained at the two year follow-up, with women asked whether they were ‘in paid employment or self-employment in the week ending last Sunday’. Information on parenting difficulties was collected at 3 years using a 30-item Child-Parent Relationship Scale (23). The questionnaire responses were summed to obtain a ‘closeness’ score and a ‘conflicts’ score.
Children’s data
At birth, infant sex was recorded and each baby was weighed, to the nearest gram, on calibrated digital scales (Seca, Birmingham, UK). Gestational age at birth was determined using a computerised algorithm based on menstrual data or, when these were uncertain, with ultrasound assessment of fetal anthropometry in early pregnancy (24). Each mother-child pair was visited within two weeks of the infants’ 6-month birthday, and within a period of two weeks before and up to three weeks after their 12-month birthday, when the primary caregiver was interviewed by a trained research nurse. Details of the infant’s milk feeding history over the preceding six months and the age or date on which solid foods were first introduced into the infant’s diet were recorded at these 6- and 12- month visits. Duration of breastfeeding was defined according to the date of the last breastfeed.
When the children were aged three years, data were collected on the number of eating occasions (meals) per day, and dietary intake over the preceding three months was assessed using an 80-item FFQ (25) completed by the child’s main carer. Prompt cards were used to show the foods included in each food group, to ensure standardised responses to the FFQ. The average frequency of consumption of the listed foods was recorded, and a prudent diet score was calculated for each child using the same procedure as for the mothers’ diets (25). The scores describe compliance with the ’prudent’ dietary pattern (characterised by high consumption of fruit, vegetables, water and wholemeal cereals), and used as an indicator of the quality of the children’s diets (25).
Child outcome data
Data on child feeding difficulty at three years was collected through a questionnaire developed for the Avon Longitudinal Study of Parents and Children (ALSPAC) study (26). In the questionnaire, mothers/carers were asked to rate six questions on potential feeding difficulties of their child on a four-point Likert scale; including one general question; (1. whether they felt there had been difficulties feeding their child) and five more specific feeding difficulty questions (2. not eating sufficient amounts of food, 3. refusal to eat the right food, 4. being choosy with food, 5. over-eating, and 6. being difficult to get in to a feeding routine). Possible response options included; (1) ‘yes, worried me greatly’, (2) ‘yes, worried me a bit’, (3) ‘yes, but did not worry me’, and (4) ‘no, did not happen’, which were converted into a binary score to indicate whether feeding difficulties did (1-3) or did not occur (4). Weight was measured using portable scales (Seca, Germany) to the nearest 0.1kg and height using Leicester Height measurer to the nearest 0.1cm at three years. Child BMI (weight (kg)/height (m2)) was calculated. Overweight and obesity was defined according to the International Obesity Task Force (IOTF) child cut-points (27), and collapsed to a binary variable: ‘overweight/obese’ and ‘not overweight/obese’.
Statistical analysis
All statistical analyses were performed using Stata version 14.1 (Statacorp LP, College Station, TX, USA). Descriptive data are presented as mean (standard deviation) or median (interquartile range) for continuous variables, and percentages of subjects for categorical variables. Significance levels were set at p<0.05. Children were categorised into three groups according to whether they were introduced to solid foods prior to four months of age, between four and less than six months (reference group), and at or after six months of age. T-tests (for normally distributed variables), Mann-Whitney U-Tests (for non-normally distributed variables) and chi-squared tests (for categorical variables) were used to compare the characteristics of mothers and children included in the analysis, with those for live singleton term births not in the study. Unadjusted associations between maternal and childhood characteristics and age at introduction of solids were made using Pearson’s correlation (for normally distributed variables), Spearman’s correlation (for non-normally distributed variables) and t-tests (for binary variables). The six feeding difficulty questions were assessed separately. In regression analyses age at introduction of solids was considered as a categorical variable (with ≥4 and <6 months as the reference) and a continuous variable in weeks. Age at introduction of solids as a predictor of feeding difficulties was examined by fitting a poisson regression model with robust standard errors, adjusting for age last breastfed, child sex, gestation, parity, pre-pregnancy maternal BMI, maternal age, maternal education, maternal employment, parenting difficulties, and maternal diet quality. A Directed Acyclic Graphic (DAG; a graphical representation of causal assumptions) was used to identify potential confounding variables (see supplementary material File 1). Relative risk and 95% confidence intervals are presented.
Results
A total of 3,158 live births were recorded in the SWS. Of these, there were eight neonatal deaths and seven babies born with major congenital growth abnormalities. Two-hundred babies were born pre-term, leaving 2,943 term (after 37 weeks’ gestation) live singleton births. Of these, 194 babies had no information about age at starting solids, either because the 6-month questionnaire had not been completed (n=161) or information about the age at starting solids was not reported in either the 6- or 12-month questionnaire (n=33). One mother reported that her child started solid foods at one year of age, which was considered an outlier and removed from the analysis. Of these 2,748 babies, 359 had no information on feeding behaviours, leaving 2,389 in the final sample. Of the final sample, 55% (n=1319) reached four months of age (former recommended age to introduce solids) prior to the change in guidance in May 2003. Mother-child pairs excluded from the analysis were more likely to have a lower maternal education level (p<0.001), be multiparous (p=0.009), have smoked during pregnancy (p<0.001) and to be slightly younger (p=0.006); infants were less likely to have been breastfed for at least four months (p<0.001) compared with mother-child pairs included in the analysis (Table 1).
Table 1.
Characteristics of mothers and children in study compared with term live singleton births not in study.
| In study | |||
|---|---|---|---|
| Characteristic | No (n = 554*) | Yes (n = 2389) | P-value |
| Mother | |||
| Education (≥A-levels), n (%) | 278 (51%) | 1452 (61%) | <0.001 |
| Primiparous, n (%) | 251 (45%) | 1231 (52%) | 0.009 |
| Smoking in pregnancy, n (%) | 112 (23%) | 334 (14%) | <0.001 |
| Pre-pregnancy BMI, median (IQR) | 24.1 (22.0-27.3) | 24.2 (21.9-27.4) | 0.87 |
| Age at child’s birth, years, mean (SD) | 30.3 (4.0) | 30.8 (3.8) | 0.006 |
| Child | |||
| Birthweight, g, mean (SD) | 3486 (487) | 3506 (471) | 0.35 |
| Males, n (%) | 265 (48%) | 1160 (49%) | 0.85 |
| Breastfed for ≥ 4 months, n (%) | 120 (32%) | 968 (42%) | <0.0001 |
| Child at 3 years | |||
| Height, cm, mean (SD) | 95.2 (3.7) | 95.8 (3.5) | 0.16 |
| Weight, kg, median (IQR) | 14.8 (13.6-15.9) | 14.9 (13.8-16.1) | 0.70 |
| Overweight/obese, n (%) | 10 (14%) | 322 (14%) | 0.97 |
| Meals per day, median (IQR) | 5.0 (4.0-5.0) | 5.0 (4.0-6.0) | 0.61 |
n=number, BMI=body mass index, IQR=interquartile range, SD=standard deviation.
n for some analyses much lower, particularly for three year characteristics where it is about 70.
Maternal and child characteristics and the age at introduction of solids
The distribution of the age at introduction of solids before and after the change in feeding guidance in May 2003 is shown in the supplementary material (File 2). There was a small shift in the distribution of the age at introduction of solids before and after the infant feeding guidelines changed. Forty-five percent (n=1,070) of children were born prior to May 2013. Prior to May 2003, 61% of infants were introduced to solid foods between four and six months, and 39% prior to four months. Few infants (0.1%) were introduced to solid foods at or after six months of age. After the guidelines were revised, a greater proportion of infants were introduced to solids at or after six months (8%), however a larger proportion of infants were introduced to solids between four and six months (75%), and the proportion introduced to solids before 4 months fell to 17%. Overall, ninety-five percent of mothers reported introducing solids before six months of age. The infants were grouped according to their age at introduction of solids; maternal and child characteristics according to these groups are found in Table 2. All maternal and child factors considered were associated with the timing of introducing solids, with the exception of the proportion of children who were overweight or obese at three years. Earlier introduction of solids was observed among younger, multiparous mothers with lower educational attainment who continued to smoke in pregnancy. Earlier age at introduction of solid foods was associated with shorter duration of breastfeeding and was more common in boys and among babies of higher birth weight; and, after accounting for sex, the association with birth weight remained (p<0.001; not reported in table). Differences in feeding practice at three years were found, such that earlier introduction solid foods was associated with poorer diet quality and with small differences in eating frequency at this age.
Table 2.
Characteristics of 2389 mother-child pairs according to age at introduction of solid foods in infancy.
| Age at introduction of solid food | |||||||
|---|---|---|---|---|---|---|---|
| <4 months (n = 642) | ≥ 4 & < 6 months (n = 1637) | ≥ 6 months (n = 110) | P-value | ||||
| Mother | |||||||
| Education (≥A-levels) (n, %) | 349 | 54% | 1038 | 64% | 65 | 60% | <0.001 |
| Primiparous (n, %) | 277 | 43% | 886 | 54% | 68 | 62% | <0.001 |
| Smoked in pregnancy (n, %) | 124 | 20% | 205 | 13% | 5 | 5% | <0.001 |
| Age at child’s birth (years) (mean, SD) | 29.7 | 3.8 | 31.0 | 3.7 | 32.8 | 3.8 | <0.001 |
| Pre-pregnancy BMI (kg/m2) (median, IQR) | 24.8 | 22.4-28.0 | 24.0 | 21.8-27.2 | 23.8 | 21.3-25.8 | 0.006 |
| Child | |||||||
| Birthweight (g) (mean, SD) | 3587 | 503 | 3483 | 455 | 3382 | 445 | <0.001 |
| Male (n, %) | 389 | 61% | 791 | 48% | 49 | 45% | <0.001 |
| Breastfed for ≥ 4 months (n, %) | 187 | 30% | 725 | 46% | 56 | 54% | <0.001 |
| Overweight/obese, n (%) | 103 | 17% | 209 | 13% | 10 | 10% | 0.31 |
| Meals per day at 3 years (median, IQR) | 5 | 4-6 | 5 | 4-5 | 5 | 4-5.5 | 0.002 |
| Prudent diet score at 3 years (mean, SD) | -0.21 | 1.0 | 0.12 | 0.96 | 0.39 | 0.86 | <0.001 |
n=number, BMI=body mass index, SD=standard deviation, IQR=interquartile range.
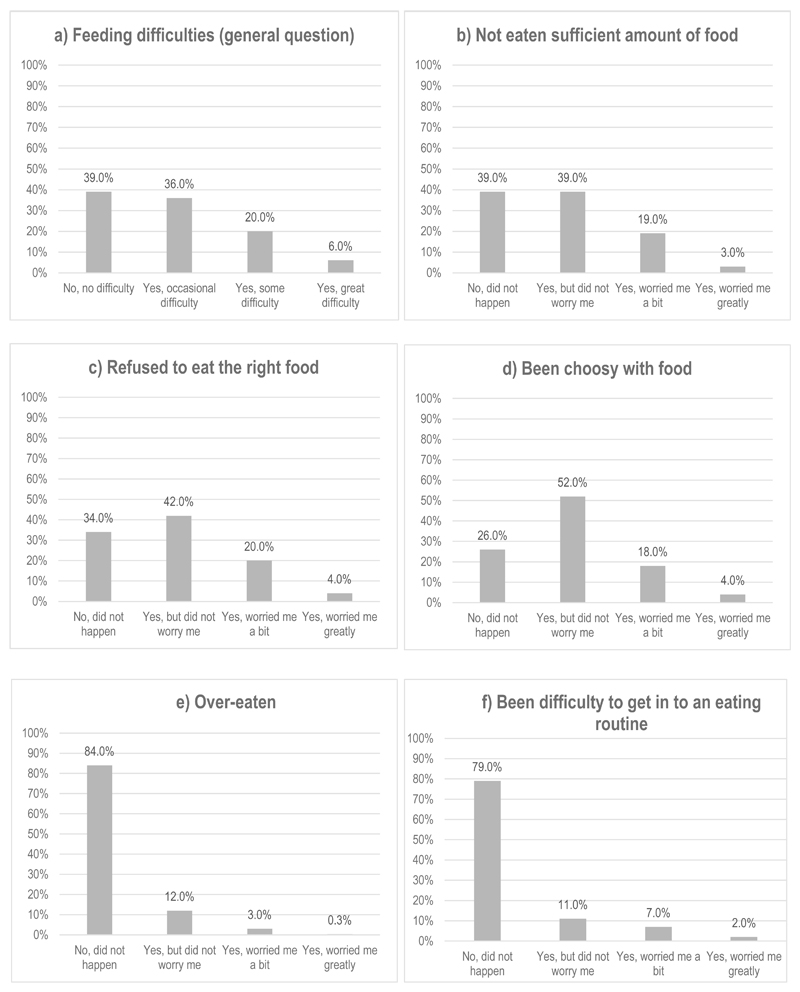
Feeding difficulties at three years
Rates of feeding difficulties are reported in Figure 1. The majority of mothers/carers (61%) reported some feeding difficulties in their child at three years. In response to questions about specific aspects of feeding difficulties, the majority of mothers/carers reported difficulties with their child eating enough food (61%), eating the right food (66%), and being choosy with food (74%). However, of those who did report difficulty for these feeding aspects, the majority of mothers/carers indicated that they weren’t worried about the feeding issue. Over-eating and problems with establishing a routine were less common, with just 16% and 21% of mothers/carers reporting these feeding difficulties, respectively.
Figure 1.
Proportion of reported child feeding issues at 3 years of age.
Association between age at introduction of solids and risk of feeding difficulties at three years
The relative risks of feeding difficulties at three years according to the age at introducing solid foods are presented in Table 3. Infants were grouped according to whether they were introduced to solid foods i) prior to four months, ii) between four and six months (reference group), iii) and at or after six months of age. The model adjusted for potential confounding variables in childhood (age last breastfed, gestation, sex), as well as maternal variables (pre-pregnancy BMI, age, parity, education, employment, parenting difficulties, and diet quality). There were no differences between the three feeding groups for the five specific feeding difficulties of not eating sufficient foods, refusing to eat the right food, being choosy with food, overeating or being difficult to get in to an eating routine in the adjusted model. However, a significant association between the general feeding difficulty question and age of introducing solids was found. After taking account of potential confounding factors, children who were introduced to solid foods at or after six months had a lower relative risk of feeding difficulties (RR=0.73 (95%CI=0.59; 0.91), p=0.004) than children who were introduced to solids between four and six months.
Table 3. Relative risk of feeding difficulties at 3 years according to age at introduction of solid.
| Age at introduction of solid foods | Unadjusted RR | 95% CI | P-value | Adjusted RR* | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Feeding difficulties | ||||||
| <4 months | 0.97 | 0.91, 1.05 | 0.50 | 0.96 | 0.89, 1.04 | 0.36 |
| ≥4 & <6 months | 1.00 | - | - | 1.00 | - | - |
| ≥6 months | 0.82 | 0.68, 0.99 | 0.04 | 0.73 | 0.59, 0.91 | 0.004 |
| Not eaten sufficient amount of food | ||||||
| <4 months | 1.02 | 0.95, 1.10 | 0.55 | 1.01 | 0.94, 1.10 | 0.72 |
| ≥4 & <6 months | 1.00 | - | - | 1.00 | - | - |
| ≥6 months | 0.94 | 0.80, 1.11 | 0.50 | 0.90 | 0.75, 1.08 | 0.27 |
| Refused to eat the right food | ||||||
| <4 months | 0.99 | 0.93, 1.06 | 0.86 | 0.98 | 0.91, 1.05 | 0.61 |
| ≥4 & <6 months | 1.00 | - | - | 1.00 | - | - |
| ≥6 months | 0.98 | 0.85, 1.13 | 0.76 | 0.96 | 0.83, 1.11 | 0.57 |
| Been choosy with food | ||||||
| <4 months | 0.99 | 0.94, 1.04 | 0.71 | 1.00 | 0.95, 1.06 | 0.90 |
| ≥4 & <6 months | 1.00 | - | - | 1.00 | - | - |
| ≥6 months | 0.94 | 0.83, 1.06 | 0.32 | 0.91 | 0.80, 1.04 | 0.17 |
| Over-eaten | ||||||
| <4 months | 1.12 | 0.91, 1.38 | 0.27 | 1.13 | 0.91, 1.39 | 0.27 |
| ≥4 & <6 months | 1.00 | - | - | 1.00 | - | - |
| ≥6 months | 1.19 | 0.79, 1.80 | 0.41 | 1.19 | 0.75, 1.87 | 0.46 |
| Been difficult to get into an eating routine | ||||||
| <4 months | 1.20 | 1.01, 1.43 | 0.03 | 1.12 | 0.94, 1.35 | 0.21 |
| ≥4 & <6 months | 1.00 | - | - | 1.00 | - | - |
| ≥6 months | 1.12 | 0.78, 1.62 | 0.54 | 1.00 | 0.66, 1.52 | 0.99 |
| foods in infancy | ||||||
RR=relative risk, 95% CI=95% confidence intervals.
Model adjusted for age last breastfed, gestation, maternal BMI, maternal age, maternal education, maternal employment, parenting difficulties, parity, sex and maternal diet.
Discussion
This study aimed to assess whether age at introduction of solid foods was associated with feeding difficulties in a large population of children aged three years old. The principal finding was that general feeding difficulties were reported to be less common among infants who were introduced to solid foods at or after six months of age; this association was not explained by differences in maternal and background characteristics. There were no other significant associations between the age of introducing solids and risk of difficulties in specific aspects of feeding at three years. Male and larger babies were more likely to be introduced to solid foods earlier; consistent with findings from the Millennium babies study(28). The tendency to introduce solid foods earlier to boys may be partly due to their larger size and consequently higher energy requirements and feeding drive (28), although after accounting for sex, the association with birth weight remained. The magnitude of the change in distribution of the age at introduction of solids following the change in infant feeding guidelines in May 2003 was small but distinct. Although the majority of mothers/carers still introduced solids between four and six months (pre May 2003=61%; post May 2003=75%), fewer infants were introduced solids prior to four months (from 39% to 17%) and more infants introduced to solids at or after six months (from 0.1% to 8%).
Existing evidence on the timing of introducing solid foods in infancy and later risk of feeding difficulties is limited and a current topic of debate. There is growing evidence on the programming of flavour preferences and its influence on later food choices, particularly flavour preferences developed through exposure to breastmilk (29) or formula milk in early life (9, 30, 31). However, much less is known about children with feeding difficulties specifically in relation to the timing of introducing solid foods. The evidence base on feeding difficulties includes animal experiments and a human case study (11), and observational studies prone to confounding issues (10, 14). Follow-up studies of feeding difficulties have been conducted in children who were tube fed prior to introducing solids (12), however these findings are unlikely to be generalisable to a healthy population. Caution should be taken in drawing conclusions from this evidence base.
There are therefore very few studies that can be compared directly with the SWS. The most relevant data have come from the Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC), in which feeding difficulties in childhood were assessed using the same questions, although the follow-up studies were conducted at different ages (6 and 15 months (10) and 7 years (14)). Additionally, an important difference in the ALSPAC analyses was that the infant feeding exposure used was the age at which lumpy solids were introduced (<6 months; 6-9 months; 10+ months) (10), whereas the present analyses considered introduction of any solid foods. Introduction of lumpy solid foods prior to six months in ALSPAC was associated with a lower likelihood of reporting four of the specific feeding difficulties at 15 months of age, when compared with introduction between six and nine months (10), but the relative risk of over-eating in this group was higher (10). When the children were seven years old, reported feeding difficulties were most common in relation to late (10+ months) introduction of solid foods (14); there were few differences between the children fed lumpy foods before six months when compared with the 6-9 month group. The authors suggest that the data provide evidence to support a sensitive period in the first year, when infants may be more likely to accept tastes and textures. These findings are in contrast with the present study, in which there was no evidence of differences in feeding difficulties among children who were introduced to (any) solid foods later in infancy. Infants who complied with the latest feeding guidance, starting on solid foods at 6 months, had the ‘healthiest’ dietary patterns at three years (Table 2), and were reported to have fewer feeding difficulties when compared with children who had been introduced to solid foods earlier in infancy.
A high proportion of mothers/carers indicated that their child displayed some degree of feeding difficulty; however they were ‘not worried about it’. This raises a couple of questions; firstly whether the mother/carer was not concerned as the specific feeding difficulty was infrequent, or whether the feeding difficulty was regularly encountered but the mother/carer was not concerned about the issue. If the latter, then it would be interesting to understand why some mothers/carers are not concerned about feeding difficulties in their child. While there were significant associations between the timing of introducing solids and risk of feeding difficulties assessed through the general question, no significant associations were detected through the five more specific feeding difficulty questions. It may be that an additive effect was observed; in that there were small differences in each of the specific feeding difficulties, which only led to a significant association when assessed through the general feeding difficulty question. Or it may be that there was a specific aspect of feeding difficulties that was not assessed through the individual specific questions (e.g. a child taking a considerable amount of time to eat a meal).
Recommendations for practice
Although 86% of UK mothers report a good understanding of the WHO infant feeding recommendations (32) and the majority express an initial desire to comply, some mothers report that waiting to introduce solids until six months is challenging (33, 34). The 2010 UK Infant Feeding Survey found that 94% of mothers reported introducing solids before six months of age (35), consistent with the SWS findings (95%). Similar trends have been reported in other developed countries that have adopted the WHO infant feeding recommendation, including the United States (36) and Australia (37). The small proportion of mothers meeting the infant feeding recommendation internationally indicates that additional efforts and resources are required to support mothers. Evidence from the SWS and other studies indicate that younger mothers, with a lower education level, who have a higher pre-pregnancy BMI, and smoked during pregnancy, are more likely to introduce solid foods to infants earlier than recommended (10, 37–39), and are a high risk subgroup who could benefit from additional support during the first six months of motherhood.
Strengths and weaknesses
The study has several strengths. In the SWS, young women were recruited from the general population regardless of whether they were planning a pregnancy, making the SWS study unique in the western world. The SWS provided a novel opportunity to examine differences in age at introduction of solid foods within a longitudinal study that spanned the 2003 change in infant feeding recommendations in the UK, thus providing a wide range of ages of introduction of solids. The study has a large sample size, and assessed the outcome of feeding difficulties in children using a previously developed questionnaire (10), enabling the comparison of findings between feeding difficulty studies. However, it is a limitation that a binary outcome to indicate the presence or absence of each feeding problem was used, in order to avoid any subjective reporting bias associated with perceived severity of the feeding difficulty. Future studies that use other feeding difficulty questionnaires, and alternate methods of classifying the presence of a feeding difficulty, will be needed to confirm and extend our findings. Limitations of the study also need to be acknowledged. As with other infant feeding studies (10, 14), parental report data on infant feeding methods and feeding difficulties were collected which could be prone to misreporting and a social desirability bias. Eighty-one percent of the pregnant cohort who gave birth to healthy, term, live singleton births were included in the study, and there were significant differences between mother-child pairs that were included and excluded from the analysis. Because of the change in infant feeding policy, almost all infants in the ‘at or after 6 month’ group were born later in the study, which may have implications for the findings. Only a small proportion of mothers reported introducing solid foods to infants at or after six months of age (5%, n=110). It will be important in future studies to extend and replicate these findings in a more balanced analysis, with similar numbers of children in each group. Future studies could also examine the association between weaning method (e.g. baby-led weaning) and risk of feeding difficulties which was not assessed in the SWS. Care should be taken in interpreting the findings as they may not be generalisable outside the UK. Despite adjusting for potential confounders, some confounders may have been missed. For example, the model adjusted for duration of breastfeeding, but we did not consider whether effects differed between infants who were partially or exclusively breastfed, which should be addressed in future studies. A causal pathway cannot be assumed due to the observational nature of the SWS. Further research may be needed to ascertain causal mechanisms to determine the optimum age to introduce solid foods in relation to other infant outcomes (such as allergies, asthma, overweight and obesity, and iron status) that were outside the scope of this study.
Conclusions
Since the revision of the infant feeding recommendations 13 years ago, there has been continued debate on the evidence behind the change in recommendations. Questions have been raised as to whether the delayed introduction of solid foods to six months of age leads to an aversion to certain flavours and textured foods, and possibly feeding difficulties in later childhood. Evidence from the SWS showed few associations between age at introduction of solid foods and feeding difficulties in childhood, although general feeding difficulties were less common among children who were introduced to solid foods at or after six months of age, in line with current UK feeding policy.
Supplementary Material
Acknowledgements
We are grateful to the women of Southampton and their children who gave their time to take part in these studies, and to the research nurses and other staff who collected and processed the data.
Footnotes
Financial Support
This work was supported by grants from the UK Medical Research Council, the British Heart Foundation, UK Foods Standard Agency, the Dunhill Medical Trust, the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre, the European Union's Seventh Framework Programme (FP7/2007-2013), and projects EarlyNutrition and ODIN (under grant agreement numbers 289346 and 613977). JLH received an Endeavour Research Fellowship funded by the Australian Government (Department of Education and Training).
Conflicts of Interest
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products; KMG, HMI and CC are part of an academic consortium that has received research funding from Abbot Nutrition, Nestec and Danone. None of the other authors had any potential conflicts of interest.
Authorship
JLH, SRC, HMI, CC, KMG and SMR were responsible for the design of the study and formulated the research question. SRC analysed the data, and JLH drafted the initial paper. All authors are responsible for drafting and revising the manuscript and have approved the final version.
References
- 1.Krebs NF. Food choices to meet nutritional needs of breast-fed infants and toddlers on mixed diets. J Nutr. 2007;137:511S–7S. doi: 10.1093/jn/137.2.511S. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JJ, Wells JC. Duration of exclusive breast-feeding: introduction of complementary feeding may be necessary before 6 months of age. Br J Nutr. 2005;94:869–72. doi: 10.1079/bjn20051601. [DOI] [PubMed] [Google Scholar]
- 3.Fewtrell M, Wilson DC, Booth I, et al. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2011;342:c5955. doi: 10.1136/bmj.c5955. [DOI] [PubMed] [Google Scholar]
- 4.Koplin J, Allen K. Optimal timing for solids introduction–why are the guidelines always changing? Clin Exp Allergy. 2013;43:826–34. doi: 10.1111/cea.12090. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health. Infant feeding recommendation. [accessed 15th December 2015];2003 http://webarchive.nationalarchives.gov.uk/20130107105354/http://vww.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_ 4096999.pdf
- 6.Department of Health and Social Security. Present Day Practice in Infant Feeding. London: HMSO; 1988. [Google Scholar]
- 7.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. 2002;1:CD003517. doi: 10.1002/14651858.CD003517. [DOI] [PubMed] [Google Scholar]
- 8.Mennella JA, Beauchamp GK. Early flavor experiences: research update. Nutr rev. 1998;56:205–11. doi: 10.1111/j.1753-4887.1998.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 9.Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics. 2004;113:840–5. doi: 10.1542/peds.113.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Northstone K, Emmett P, Nethersole F. The effect of age of introduction to lumpy solids on foods eaten and reported feeding difficulties at 6 and 15 months. J Hum Nutr Diet. 2001;14:43–54. doi: 10.1046/j.1365-277x.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 11.Illingworth RS, Lister J. The critical or sensitive period, with special reference to certain feeding problems in infants and children. J Pediatr. 1964;65:839–48. doi: 10.1016/s0022-3476(64)80006-8. [DOI] [PubMed] [Google Scholar]
- 12.Mason SJ, Harris G, Blissett J. Tube Feeding in Infancy: Implications for the Development of Normal Eating and Drinking Skills. Dysphagia. 2005;20:46–61. doi: 10.1007/s00455-004-0025-2. [DOI] [PubMed] [Google Scholar]
- 13.Blossfeld I, Collins A, Kiely M, et al. Texture preferences of 12-month-old infants and the role of early experiences. Food Quality and Preference. 2007;18:396–404. [Google Scholar]
- 14.Coulthard H, Harris G, Emmett P. Delayed introduction of lumpy foods to children during the complementary feeding period affects child’s food acceptance and feeding at 7 years of age. Matern Child Nutr. 2009;5:75–85. doi: 10.1111/j.1740-8709.2008.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulthard H, Harris G, Fogel A. Exposure to vegetable variety in infants weaned at different ages. Appetite. 2014;78:89–94. doi: 10.1016/j.appet.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Clark BJ, Laing S. Infant feeding: a review of weaning. J Hum Nutr Diet. 1990;3:11–8. [Google Scholar]
- 17.Coulthard H, Harris G, Emmett P. Long-term consequences of early fruit and vegetable feeding practices in the United Kingdom. Public Health Nutr. 2010;13:2044–51. doi: 10.1017/S1368980010000790. [DOI] [PubMed] [Google Scholar]
- 18.Inskip HM, Godfrey KM, Robinson SM, et al. Cohort profile: the Southampton women's survey. Int J Epidemiol. 2006;35:42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson S, Godfrey K, Osmond C, et al. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur J Clin Nutr. 1996;50:302–8. [PubMed] [Google Scholar]
- 20.Crozier SR, Robinson SM, Borland SE, et al. Dietary patterns in the Southampton Women's Survey. Eur J Clin Nutr. 2006;60:1391–9. doi: 10.1038/sj.ejcn.1602469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department of Health Committee on Medical Aspects of Food Policy. Nutritional aspects of cardiovascular disease. London: HMSO; 1994. [Google Scholar]
- 22.Department of Health Committee on Medical Aspects of Food Policy. Nutritional aspects of the development of cancer. London: The Stationery Office; 1998. [Google Scholar]
- 23.Pianta RC. Child-parent relationship scale. Unpublished measure, University of Virginia. 1992 [Google Scholar]
- 24.Robinson SM, Crozier SR, Harvey NC, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101:368–85. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarman M, Fisk CM, Ntani G, et al. Assessing diets of 3-year-old children: evaluation of an FFQ. Public Health Nutr. 2014;17:1069–77. doi: 10.1017/S136898001300102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Avon Longitudinal Study of Parents and Children Questionnaires. [accessed 4th January 2016];Child-based questionnaires. http://www.bristol.ac.uk/alspac/researchers/questionnaires/ [Google Scholar]
- 27.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright CM, Parkinson KN, Drewett RF. Why are babies weaned early? Data from a prospective population based cohort study. Arch Dis Child. 2004;89:813–6. doi: 10.1136/adc.2003.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forestell CA, Mennella JA. Early determinants of fruit and vegetable acceptance. Pediatrics. 2007;120:1247–54. doi: 10.1542/peds.2007-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mennella JA, Beauchamp GK. Flavor experiences during formula feeding are related to preferences during childhood. Early Hum Dev. 68:71–82. doi: 10.1016/s0378-3782(02)00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mennella JA, Forestell CA, Morgan LK, et al. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr. 2009;90:780S–8S. doi: 10.3945/ajcn.2009.27462O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore AP, Milligan P, Goff LM. An online survey of knowledge of the weaning guidelines, advice from health visitors and other factors that influence weaning timing in UK mothers. Matern Child Nutr. 2014;10:410–21. doi: 10.1111/j.1740-8709.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh A, Kearney L, Dennis N. Factors influencing first-time mothers’ introduction of complementary foods: a qualitative exploration. BMC public health. 2015;15:939. doi: 10.1186/s12889-015-2250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoddinott P, Craig LC, Britten J, et al. A serial qualitative interview study of infant feeding experiences: idealism meets realism. BMJ open. 2012;2:e000504. doi: 10.1136/bmjopen-2011-000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Health and Social Care Information Centre IFF Research. Infant Feeding Survey 2010. The Health and Social Care Information Centre; 2012. [Google Scholar]
- 36.Clayton HB, Li R, Perrine CG, et al. Prevalence and reasons for introducing infants early to solid foods: variations by milk feeding type. Pediatrics. 2013;131:e1108–e14. doi: 10.1542/peds.2012-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott JA, Binns CW, Graham KI, et al. Predictors of the early introduction of solid foods in infants: results of a cohort study. BMC pediatr. 2009;9:60. doi: 10.1186/1471-2431-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiess S, Grote V, Scaglioni S, et al. Introduction of complementary feeding in 5 European countries. J Pediatr Gastroenterol. 2010;50:92–8. doi: 10.1097/MPG.0b013e31819f1ddc. [DOI] [PubMed] [Google Scholar]
- 39.Rebhan B, Kohlhuber M, Schwegler U, et al. Infant feeding practices and associated factors through the first 9 months of life in Bavaria, Germany. J Pediatr Gastroenterol. 2009;49:467–73. doi: 10.1097/MPG.0b013e31819a4e1a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.