Abstract
Soybean is a common source of protein in many pet foods. Slow glucuronidation of soy-derived isoflavones in cats has been hypothesized to result in accumulation with adverse health consequences. Here we evaluated species’ differences in soy isoflavone glucuronidation using urine samples from cats and dogs fed a soy-based diet and liver microsomes from cats compared with microsomes from 12 other species.
Significant concentrations of conjugated (but not unconjugated) genistein, daidzein, and glycitein, and the gut microbiome metabolites, dihydrogenistein and dihydrodaidzein were found in cat and dog urine samples. Substantial amounts of conjugated equol were also found in cat urine but not in dog urine.
β-glucuronidase treatment showed that all these compounds were significantly glucuronidated in dog urine while only daidzein (11%) and glycitein (37%) showed any glucuronidation in cat urine suggesting that alternate metabolic pathways including sulfation predominate in cats.
Glucuronidation rates of genistein, daidzein, and equol by cat livers were consistently ranked within the lowest three out of 13 species’ livers evaluated. Ferret and mongoose livers were also ranked in the lowest four species.
Our results demonstrate that glucuronidation is a minor pathway for soy isoflavone metabolism in cats compared with most other species.
Keywords: UDP-glucuronosyltransferase, glucuronidation, liver, soy, isoflavone, genistein, daidzein, equol, cat, dog
1. Introduction
Soybean is commonly used as a source of vegetable protein in pet foods available in the United States. However, in addition to protein, soybean and many soybean-derived products also contain significant amounts of flavonoid compounds (isoflavones) that are known to have biological activity with the potential to affect animal health. In previous studies we have shown that 24 of 42 cat foods sampled and 12 of 24 dog foods sampled contained substantial levels of the soy isoflavones, predominantly genistein and daidzein (Cerundolo et al. 2004; Court and Freeman 2002).
One disease that may be related to soybean consumption is hyperthyroidism. Hyperthyroidism (also called toxic nodular goiter), first described in 1979, is currently the most common endocrine disease of cats in the United States, affecting as many as one in 300 animals, resulting in significant morbidity and mortality (Broussard et al. 1995; Gerber et al. 1994). In contrast, this disease is extremely rare in dogs and most other species (except for humans). Although the exact etiology of feline hyperthyroidism is unknown, most evidence to date suggests an important role for the feline diet (Edinboro et al. 2004; Kass et al. 1999; Martin et al. 2000). Specifically, it has been suggested that some commercial feline diets may contain a goitrogen, that with continued consumption can lead to the development of autonomous thyroid hormone secreting nodules (i.e. toxic nodular goiter).
Soybean has goitrogenic effects in a number of species, including cats (Divi and Doerge 1996; White et al. 2004). In prior work we found a marked effect on thyroid function in cats administered a soy diet for 3 months that resulted in significant elevations in thyroxine (T4) concentrations relative to triiodothyronine (T3) concentrations (White et al. 2004). Conversely, we conducted a similar study in dogs and found no effect of a soy-based diet on T4 concentrations (Cerundolo et al. 2009). One of the proposed mechanisms for this increase in T4 relative to T3 is inhibition by soy isoflavones of thyroid peroxidase the enzyme that converts T4 to T3, (Divi et al. 1997; Divi and Doerge 1996; White et al. 2004). While these studies indicate a possible link between dietary soy consumption and feline hyperthyroidism, it is not yet clear why dogs (and most other species except humans) do not develop hyperthyroidism as a result of consuming soy (Doerge et al. 2000). One possibility is species differences in soy isoflavone metabolism and excretion. Glucuronidation is the major metabolic mechanism responsible for effective elimination of soy isoflavones in all species evaluated to date (Court and Greenblatt 2000; Yasuda et al. 1994; Yasuda et al. 1996). Furthermore, it is well known that cats poorly eliminate many phenolic compounds by glucuronidation (Court 2013; Court and Greenblatt 2000). To date, only one study has reported the metabolism of soy isoflavones in cats (Whitehouse-Tedd et al. 2013). However that study focused on measuring unconjugated and sulfate conjugated metabolites of daidzein and genistein in the plasma of cats administered soy. They found substantial circulating concentrations of daidzein and genistein sulfate in most cats, but very low concentrations of unconjugated daidzein and genistein. Although they also reported being unable to detect daidzein or genistein glucuronide in any of the cat plasma samples, their assay was not validated for sensitivity, accuracy or precision in measurement of those compounds. Furthermore, it is possible that there is rapid clearance of soy isoflavone glucuronides from cat plasma into urine would minimize circulating glucuronide concentrations and thereby underestimate the extent of glucuronidation.
In this study, we quantified and compared the soy isoflavone metabolite profiles in urine samples collected from cats and dogs that were fed a soybean-based diet daily for 3 months or 12 months (respectively). These diets had been shown in previous studies to cause detectable effects on endocrine function, including elevated free T4 concentrations in the cats (White et al. 2004) and elevated estradiol concentrations without thyroid hormone effects in the dogs (Cerundolo et al. 2009). Since we found evidence for much lower (or no) concentrations of glucuronidated metabolites in urine from cats versus dogs, we also attempted to replicate these findings by in vitro glucuronidation assays using livers from cats and dogs, and also extend the findings to an additional 11 other mammalian species. Our results confirm the minor role of glucuronidation in soy isoflavone metabolism in cats and also indicate that a number of other species, including ferret and mongoose could also be sensitive to the biological effects of the soy isoflavones through slower elimination by glucuronidation. We also found evidence for formation of the intestinal metabolite equol in cats but not in dogs, possibly related to species differences in the intestinal microbiome.
2. Materials and Methods
2.1. Reagents
Substrates for the soy isoflavone glucuronidation assay, genistein (G-6055), daidzein (D-7878), and equol (E-5880), were purchased from LC laboratories, Woburn, MA. Biochanin-A (D2016) and UDP-glucuronic acid (U6751-1g) were from Sigma-Aldrich Co., St. Louis, MO. For the analysis of soy isoflavones and metabolites in urine, genistein (G6649), daidzein (D7802), glycitein (G2785), equol (45405), Helix pomatia β-glucuronidase/sulfatase mixed enzyme (G-0762) and bovine liver β-glucuronidase (G0501) were from Sigma-Aldrich Co., St. Louis, MO. Dihydrogenistein (D449710) and dihydrodaidzein (D449000) were from Toronto Research Chemicals, North York, Ontario, Canada. For the estradiol glucuronidation assay, β-estradiol (E8875) and phenacetin (77440-50G) were from Sigma-Aldrich Co., St. Louis, MO.
2.2. Urine samples from dogs and cats fed a soy-based diet
Urine samples from 10 healthy dogs, owned by students and staff at the University of Pennsylvania Veterinary Teaching Hospital, and 18 healthy cats, owned by students and staff at Tufts University, were used for this study as reported in detail previously (Cerundolo et al. 2009; White et al. 2004). These studies were conducted to determine the effect of dietary soy on endocrine function.
Dogs were eligible for the study based on a medical history, physical, and dermatologic examinations, and clinicopathologic tests. Dogs were required to be between two and 8 years of age, neutered, and free from endocrine or dermatologic diseases. Dogs were fed a diet with high isoflavone content via a hydrolyzed soy isolate-base according to the prior specifications (Cerundolo et al. 2009). The isoflavone content of the canine diet (measured after hydrolysis in aglycone equivalents) was 28 mg of daidzein/kg dry weight, 68 mg of genistein/kg dry weight, and 14 mg glycitein/kg dry weight. Urine samples used in this study were collected from each dog at 12 months following diet initiation.
Cats were required to live indoors and be between ages one and ten years. The cats were subjected to physical examinations, CBC, serum biochemical profiling, total T4 concentration analysis, and urinalysis to determine eligibility. Cats with endocrine or metabolic diseases were excluded. Cats were fed a formulated soy diet for three months balanced with fair iodine and taurine content according to specifications listed previously (White et al. 2004). The isoflavone content of the feline soy diet (measured after hydrolysis in aglycone equivalents) was 182 mg daidzein/kg dry weight, 198 mg genistein/kg dry weight, and 29 mg glycitein/kg dry weight. Urine samples used in this study were collected from cats via cystocentesis after three months of being on the soy diet.
2.3 Analysis of soy isoflavone and metabolites in urine
Soy isoflavone and metabolite concentrations were measured in pooled urine samples in quadruplicate using a previously published method with minor modifications (Cerundolo et al. 2009). Values were normalized to urine creatinine concentration (Creatinine Assay Kit, MAK080, Sigma Aldrich). Briefly, 500 µL of pooled dog urine (n=10) or pooled cat urine (n=18) samples were mixed with 1.25 µg of biochanin A (internal standard), and either 500 µL 1M phosphate buffer (pH 5.0) with 2,500 units of Helix pomatia β-glucuronidase/sulfatase mixed enzyme or 500 µL 1M phosphate buffer (pH 7.0) with 2,500 units of bovine liver β-glucuronidase. Control incubations containing no enzyme were conducted in parallel. After incubating overnight at 37°C, 500 µL of 1M NaOH was added, vortex mixed, extracted once into 5 mL of ethyl acetate, centrifuged, and the organic layer dried in a centrifugal vacuum. The dried samples were reconstituted in 200 µL of mobile phase solution and analyzed by HPLC-MS (Thermo Finnigan Deca XP Plus, Thermo Fisher Scientific, Waltham, MA) for genistein, daidzein, glycitein, dihydrogenistein, dihydrodaidzein, equol, dehydroequol, 6-hydroxy-O-desmethylangolensin, and O-desmethylangolensin content. Concentrations of genistein, daidzein, glycitein, dihydrogenistein, dihydrodaidzein, and equol were determined by comparison to standard curves (50 ng/mL– 5 µg/mL) generated using pure standards diluted in blank urine. Results were presented as the mean and standard deviation of quadruplicate determinations. Equol concentrations were also determined using individual dog urine samples.
2.4 Liver microsomes
Liver samples from human donors with no known liver disease were provided by the International Institute for the Advancement of Medicine (Exton, PA), the Liver Tissue Procurement and Distribution System (University of Minnesota, Minneapolis), or the National Disease Research Interchange (Philadelphia, PA). These were de-identified samples that had been initially obtained under the approval of the Human Investigation Review Committee for the responsible institution. For animal species comparisons, livers had been obtained previously from 16 dogs (mixed-breed), 8 ferrets (domestic from MIT colony), four mice (CD-1 strain), 16 cats (domestic short-hair), cow (unknown breed), horse (Quarter horse), pig (Large White), rabbit (New Zealand White), rat (Wistar strain), monkey (Crab-eating Macaque), fox (Northeastern United States wild), and two mongoose (Hawaii wild). These tissues were obtained following euthanasia of the animals for unrelated studies under the approval of the Institutional Animal Care and Research Committee of Tufts University. Liver microsomes were prepared individually (except mouse livers were pooled from four animals) from frozen liver as previously described (von Moltke et al. 1993). In brief microsomes were prepared through ultracentrifugation; microsomal pellets were resuspended in 0.1M potassium phosphate buffer containing 20% glycerol and stored at 80°C until use. Total protein concentrations were determined by a bicinchoninic acid protein assay (BCA assay; Pierce Chemical, Rockford, IL) with bovine serum albumin as a standard.
2.5 Glucuronidation assay
In vitro glucuronidation assays were developed to measure the formation of glucuronide from genistein, daidzein, and equol separately based on the method previously published for these compounds (Doerge et al. 2000). Briefly, 500 µL polypropylene microcentrifuge tubes were prepared containing substrate (35 µM genistein, daidzein, or equol), pooled liver microsomes (0.5 mg/mL), and phosphate buffer (50mM, pH 7.5) to a final volume of 100 µL. UDPGA solutions were prepared containing UDP-glucuronic acid (10 mM), MgCl2 (10 mM), and alamethicin (50 µg/mg protein) and the reaction started by addition of 50 µL this mixture. The tubes were placed in a water bath for 30 min at 37°C. Reactions were stopped by addition of 100 µL cold acetonitrile containing 1% acetic acid and 5 µM biochanin A (internal standard) and placed on ice. Tubes were then centrifuged at 14,000 rcf for 5 minutes and the supernatant was transferred to HPLC vials to be dried in a vacuum oven at 40°C to dry off excess acetonitrile. Once samples were completely dried 100 µL of a 25% acetonitrile/75% water mixture was added to the vials and analyzed by HPLC.
The HPLC apparatus (Agilent 1100) consisted of a gradient capable pump run at 1 mL/min, autoinjector, 250 × 4.6 reverse phase C18 column, and UV absorbance detector set at 270 nm. The mobile phase consisted of 50 mM phosphate buffer (pH 2.2) mixed with acetonitrile starting at 5% and increased to 50% over 30 minutes. Glucuronide peaks on the chromatogram were identified by comparing to control samples that excluded UDPGA or were not incubated. In all species a major glucuronide peak was identified for daidzein, genistein, and equol, which was identified as the 7-OH glucuronide based on published data for human liver microsomes (Pritchett et al. 2008). A second glucuronide peak eluting after the 7-OH glucuronide peak was found in incubations of liver microsomes with daidzein and genistein (but not with equol). This was identified as the 4’-OH glucuronide based on published data for human liver microsomes (Pritchett et al. 2008). Glucuronide peaks were quantified using a standard curve generated with substrate and internal standard (biochanin A). Consequently data is expressed as nmole equivalents of glucuronide formed per minute per mg microsomal protein.
Limits of linearity of glucuronide formation and minimization of substrate consumption (less than 10%) were verified with respect to incubation time (up to 60 minutes) and microsomal protein concentration (up to 1 mg/mL) for each substrate and for each species. Based on these preliminary studies, for genistein glucuronidation the protein concentrations used were 0.01 mg / mL (rabbit, horse, cow, pig, mouse, monkey, dog, human, rat, ferret) and 0.04 mg / mL (cat, mongoose, fox), while incubation times were 5 minutes (rabbit, horse), 10 minutes (cow, pig, mouse, monkey), 30 minutes (dog, human, rat, ferret), and 60 minutes (cat). For daidzein glucuronidation the protein concentrations used were 0.001 mg / mL (monkey), 0.01 mg / mL (horse, pig, rabbit), and 0.05 mg / mL (cat, human, dog, cow, rat, ferret, mouse, mongoose, fox), while incubation times were 5 minutes (horse), 10 minutes (dog, cow, pig, rabbit), 30 minutes (human, rat, monkey, ferret, mouse), and 60 minutes (mouse, cat, mongoose, fox). For equol glucuronidation the protein concentrations used were 0.01 (monkey, horse, pig, rabbit, human, dog, cow, rat, mouse) and 0.1 mg / mL (cat, ferret, mongoose, fox), while incubation times were 10 minutes (pig, monkey), and 30 minutes (horse, rabbit, human, dog, cow, rat, mouse, cat, ferret, mongoose, fox).
2.6 Estradiol glucuronidation assay
Glucuronidation of the endogenous substrate β-estradiol at the 3’-OH position was determined as a positive control using a previously published method (Court 2005). This activity is primarily catalyzed by UGT1A1 in humans (Court 2005). β-estradiol substrate concentration was 100 µM, and species optimized microsomal protein concentrations were 0.01 mg / mL (pig, rabbit) and 0.05 mg / mL (all other species), while the incubation time was 60 minutes (all species).
2.7 Statistical analysis
Results for each species were expressed as a mean value ± standard deviation. Kinetics data were analyzed utilizing Sigmaplot (version 5.0) to derive Michaelis-Menten constant (Km) and maximal velocity (Vmax) values for each species. Correlations in glucuronidation activities between species and between individual cat livers were determined using Spearman correlation. Rs values over 0.7 were considered as strong correlations with p-values <0.05 considered as significant.
3. Results
3.1 Dog and cat urine soy isoflavone and metabolite concentrations
Table 1 shows the results of quantification of soy isoflavones and metabolites in dog and cat urine receiving soy based diets. Figure 1 shows the structures of unconjugated isoflavones that were detected in the urine samples. For all analytes only very low or no amounts of unconjugated compounds were found. Concentrations of total (conjugated plus unconjugated) genistein, daidzein, and glycitein were generally similar to each other in dog urine. However, in cat urine glycitein levels were much lower (10 fold or more) than genistein and daidzein. Furthermore total genistein and daidzein concentrations were higher in cat urine than in dog urine. These differences may simply reflect differences in the soy isoflavone composition of the food in that the relative amount of glycitein in the cat food (7.1% of total isoflavones) was nearly half that in dog food (12.7% of total isoflavones). However the total measured amount of isoflavones in cat food (409 mg/kg dry weight) was nearly four times higher than in dog food (110 mg/kg dry weight).
Table 1.
Soy isoflavone and metabolite concentrations (normalized to creatinine content) in pooled urine samples collected from dogs (n= 10) and cats (n= 18) fed a soy-based diet. Samples were also assayed for dehydroequol, O-desmethylangolensin, and 6-hydroxy-O-desmethylangolensin but these metabolites were not detected. Results are presented as the mean and standard deviation (SD) of quadruplicate measurements of pooled dog (n=10) and cat (n=18) urine samples.
| Urinary concentration (pmoles / mg creatinine) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genistein | Daidzein | Glycitein | Dihydrogenistein | Dihydrodaidzein | Equol | |||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Dog urine (n = 10) | ||||||||||||
| Unconjugated(a) | 31 | (7) | 20 | (5) | 9 | (1) | 0 | (0) | 60 | (12) | 0 | (0) |
| Conjugated(b) | 1025 | (273) | 1099 | (344) | 573 | (192) | 1919 | (487) | 822 | (263) | 1.2 | (0.9) |
| Total(c) | 1056 | (257) | 1119 | (305) | 582 | (143) | 1919 | (487) | 883 | (229) | 1.2 | (0.9) |
| Glucuronides(d) | 530 | (129) | 587 | (118) | 312 | (55) | 631 | (146) | 259 | (39) | 0.5 | (0.3) |
| % Glucuronides(e) | 50 | (12) | 52 | (12) | 54 | (11) | 33 | (8) | 29 | (6) | 39 | (27) |
| Cat urine (n = 18) | ||||||||||||
| Unconjugated(a) | 29 | (21) | 37 | (4) | 10 | (1) | 0 | (0) | 0 | (0) | 0 | (0) |
| Conjugated(b) | 3431 | (202) | 1442 | (133) | 140 | (11) | 2998 | (363) | 278 | (57) | 190 | 23 |
| Total(c) | 3460 | (1371) | 1478 | (153) | 150 | (16) | 2998 | (363) | 278 | (57) | 190 | 23 |
| Glucuronides(d) | 11 | (2) | 168 | (20) | 55 | (10) | 0 | (0) | 0 | (0) | 0 | (0) |
| % Glucuronides(e) | 0.3 | (0.1) | 11 | (1) | 37 | (5) | 0 | (0) | 0 | (0) | 0 | (0) |
Concentrations without enzyme treatment
Concentrations after treatment with Helix pomatia glucuronidase/sulfatase enzymes
Sum of free and conjugated concentrations
Concentrations after treatment with bovine liver β-glucuronidase
Percentage of free and conjugated isoflavones that are glucuronide
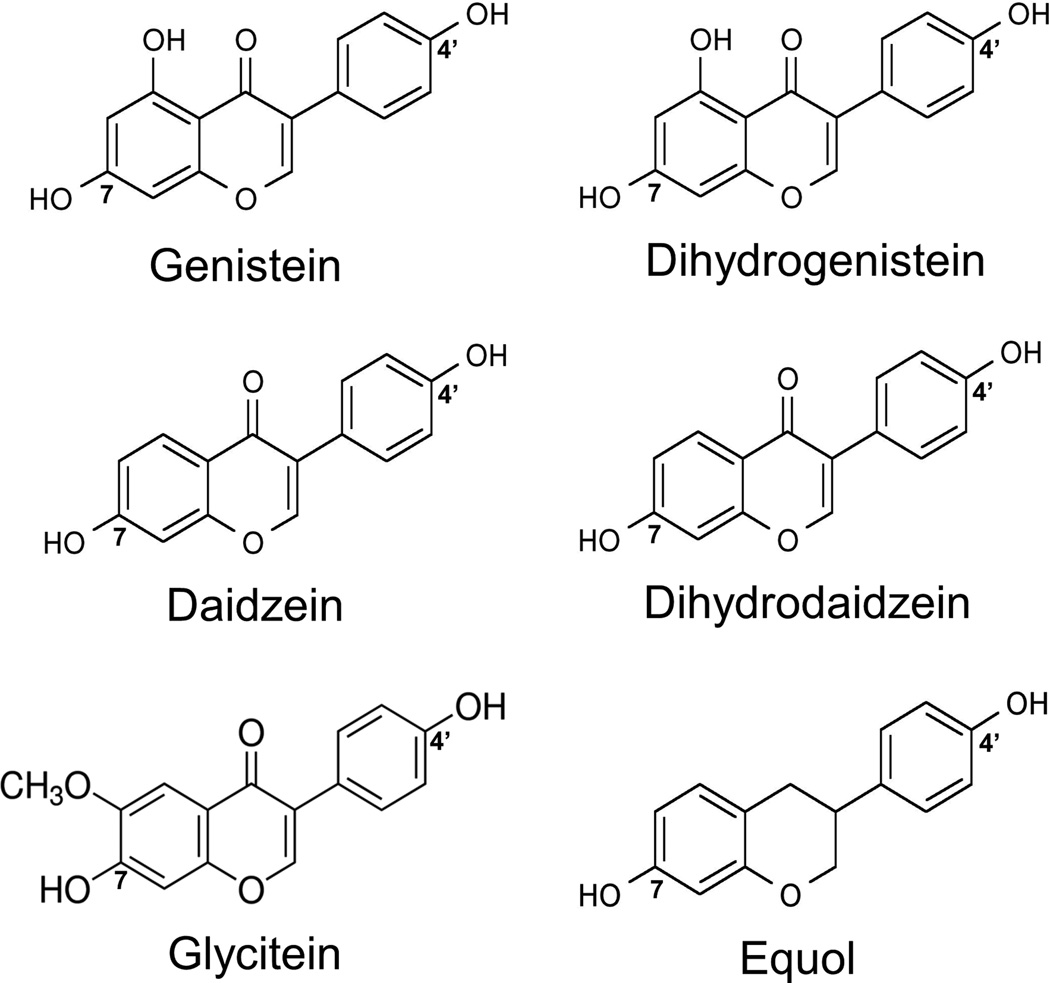
Figure 1.
Chemical structures of genistein, dihydrogenistein, daidzein, dihydrodaidzein, glycitein, and equol. Also shown for reference are the positions of hydroxyl groups (7-hydroxyl and 4’-hydroxyl) that may be glucuronidated.
Both cat and dog urine contained substantial concentrations of conjugated (but not unconjugated) dihydrogenistein and dihydrodaidzein. However cat urine showed much higher concentrations (over 10 times more) of conjugated equol compared to dog urine. Individual dog urines were also analyzed for equol content and only 3 of 10 dogs had detectable concentrations of conjugated equol (and no unconjugated equol). Unfortunately there were insufficient cat urine samples to evaluate differences in equol content between individual animals.
The percentage of total isoflavone conjugates that were glucuronides was much higher in dog urine versus cat urine for all the compounds tested. In dog urine, the percent as glucuronides ranged from 29% (dihydrodaidzein) to 54% (glycitein). In cat urine, the percentages as glucuronides were undetectable for equol, dihydrogenistein, and dihydrodaidzein, and were very low for genistein (0.3%), but somewhat higher for daidzein (11%) and glycitein (37%).
Urine samples were also assayed for dehydroequol, O-desmethylangolensin, and 6-hydroxy-O-desmethylangolensin but these metabolites were not detected before or after treatment with deconjugation enzymes.
3.2 Species differences in hepatic isoflavone and estradiol glucuronidation
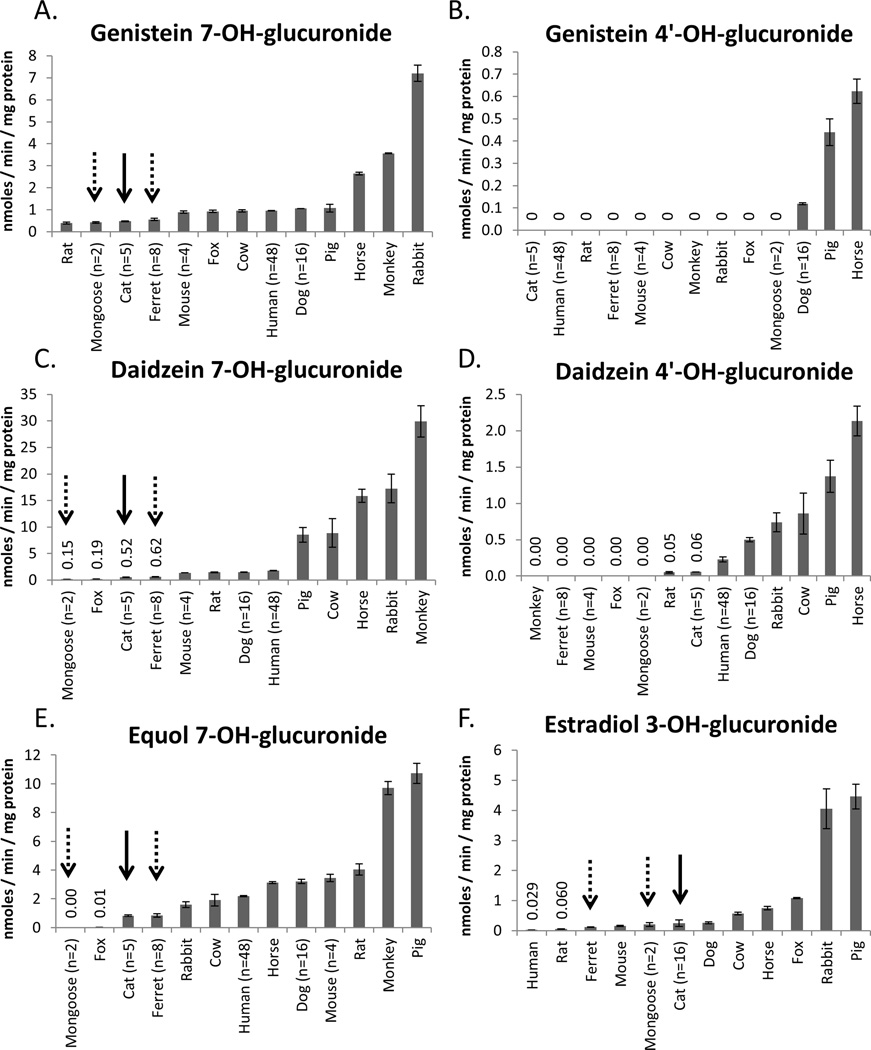
Species differences in the rates of hepatic glucuronidation of genistein, daidzein, and equol at 35 µM substrate concentration, and estradiol at 100 µM substrate concentration ranked from lowest to highest activities are shown in Figure 2. In all species glucuronidation at the 7-OH position of the isoflavone far exceeded glucuronidation at the 4’-OH position by at least 10 fold. Daidzein 4’-OH-glucuronidation could not be measured in five of the 13 species evaluated, genistein 4’-OH-glucuronidation was not detected in 10 of the 13 species, and equol 4’-OH-glucuronidation was not detected in any of the species evaluated. Glucuronidation of isoflavones at the 7-OH position was detected in all species livers’ evaluated except for glucuronidation of equol by mongoose liver.
Figure 2.
Comparison of the rates of glucuronidation of genistein (A: 7-OH-glucuronidation, B: 4’-OH-glucuronidation), daidzein (C: 7-OH-glucuronidation, D: 4’-OH-glucuronidation), equol (E: 7-OH-glucuronidation), and estradiol (F: 3-OH-glucuronidation) by liver microsomes prepared from 13 different species. Species are ranked from lowest (left) to highest (right) for each glucuronidation activity. Glucuronidation of the endogenous substrate, estradiol, was included as a positive control. In all species, 7-OH-glucuronidation of genistein and daidzein predominated over 4’-OH-glucuronidation, while equol 4’-OH-glucuronidation was not detected. Cat liver microsomes (indicated by solid arrow) consistently ranked in the lowest 3 species for 7-OH-glucuronidation of all the isoflavones, while they were the middle ranked species in estradiol glucuronidation. Other species that also consistently ranked low in isoflavone 7-OH-glucuronidation included ferret and mongoose (indicated by dashed arrows). Each bar represents the mean and error bars are the standard deviation of triplicate measurements of pooled liver microsomal samples. The numbers of individual animals contributing to each microsomal pool are indicated on the x-axis of each plot.
Of the 13 species evaluated, cat livers ranked relatively low for all isoflavone glucuronidation activities measured. Other species livers that also consistently ranked relatively low in isoflavone glucuronidation activities were ferret and mongoose. Species that ranked relatively low in isoflavone glucuronidation with somewhat less consistency included rat (except with equol), fox (except with genistein), and human (except with equol). In contrast, cat livers ranked somewhat higher for estradiol glucuronidation (sixth lowest). Mongoose and ferret livers were the fifth and third lowest in estradiol glucuronidation activities, respectively.
Table 2 shows Spearman correlation coefficients and p-values for comparisons in glucuronidation activities between species for each substrate. Such correlations may indicate whether similar UGT enzyme isoforms may be involved in the glucuronidation of these substrates. A strong correlation was observed between genistein and daidzein 7-OH-glucuronidation activities (Rs=0.84; p<0.001). Moderate correlations were also observed between daidzein and equol 7-OH-glucuronidation activities (Rs=0.54; p=0.03), and between genistein 7-OH-glucuronidation and estradiol glucuronidation activities (Rs=0.61; p=0.03). All other correlations between glucuronidation activities were not significant (Rs<0.5; p>0.05). Correlations with and between isoflavone 4’-OH glucuronidation activities were not evaluated since rates were not measurable for the majority of species.
Table 2.
Spearman correlation (Rs) coefficients and p-values for comparisons between rates of 7-OH-glucuronidation of daidzein, genistein, and equol, and 3-OH-glucuronidation of estradiol measured using liver microsomes from 13 different species.
| Daidzein | Equol | Estradiol | |
|---|---|---|---|
| Genistein | 0.841 (<0.001) | 0.379 (0.2) | 0.608 (0.03) |
| Daidzein | - | 0.599 (0.03) | 0.364 (0.2) |
| Equol | - | - | −0.049 (0.9) |
3.3 Dog, cat, and human liver glucuronidation enzyme kinetics
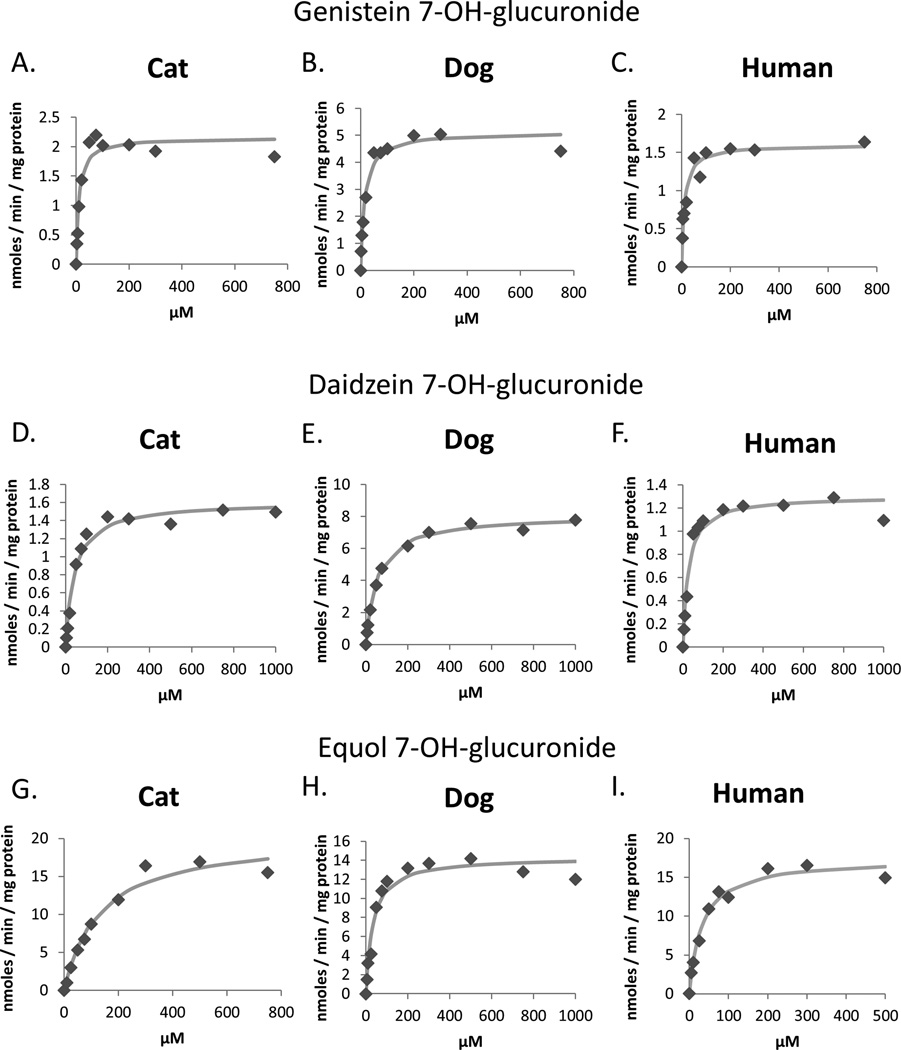
Table 3 shows enzyme kinetic parameters determined for 7-OH-glucuronidation of genistein, daidzein, and equol in pooled dog (n=16), cat (n=5), and human (n=48) liver microsomes. Plots of measured data points and fitted curves are shown in Figure 3. Data for all species were well described by a one-enzyme Michaelis-Menten model. Km values for genistein and daidzein glucuronidation were generally similar for the three species. However, Km values for equol glucuronidation were over four times higher in the cat livers compared with dog and human livers. Cat livers consistently showed lower intrinsic clearance (Vmax/Km) values than dog livers for all substrates. Intrinsic clearance values were also lower for cat livers compared to human livers for daidzein and equol, however they were higher than human livers for genistein. Isoflavone glucuronidation at the 4’-OH position were too low to accurately derive enzyme kinetic values.
Table 3.
Enzyme kinetic parameters determined for 7-OH-glucuronidation of genistein, daidzein, and equol using pooled liver microsomes from dog (n=16), cat (n=5), and human (n=48).
| Genistein | |||
|---|---|---|---|
| Species | Vmax (nmoles/min/mg protein) |
Km (µM) |
Vmax/Km (nmoles/min/mg/µM) |
| Dog | 5.13 ± 0.2 | 15.3 ± 2.4 | 0.335 ± 0.07 |
| Cat | 2.15 ± 0.1 | 10.3 ± 2.5 | 0.209 ± 0.07 |
| Human | 1.60 ± 0.07 | 11.7 ± 2.4 | 0.137 ± 0.06 |
| Daidzein | |||
|---|---|---|---|
| Species | Vmax (nmoles/min/mg protein) |
Km (µM) |
Vmax/Km (nmoles/min/mg/µM) |
| Dog | 8.10 ± 0.1 | 55.4 ± 3.8 | 0.146 ± 0.02 |
| Cat | 1.61 ± 0.06 | 41.9 ± 6.6 | 0.038 ± 0.04 |
| Human | 1.30 ± 0.05 | 26.6 ± 5.1 | 0.049 ± 0.04 |
| Equol | |||
|---|---|---|---|
| Species | Vmax (nmoles/min/mg protein) |
Km (µM) |
Vmax/Km (nmoles/min/mg/µM) |
| Dog | 14.3 ± 0.6 | 32.6 ± 6.9 | 0.439 ± 0.09 |
| Cat | 20.4 ± 1.4 | 132 ± 26 | 0.155 ± 0.08 |
| Human | 17.4 ± 0.7 | 31.8 ± 5.1 | 0.547 ± 0.08 |
Figure 3.
Enzyme kinetic plots showing the effect of increasing substrate concentration on 7-OH-glucuronide formation rates measured by HPLC from genistein (A–C), daidzein (D–F), and equol (G–I) for pooled liver microsomes from five cats (A,D,G), 16 dogs (B,E,H), and 48 humans (C,F,I).
3.4 Variability in isoflavone and estradiol glucuronidation among 16 cat livers
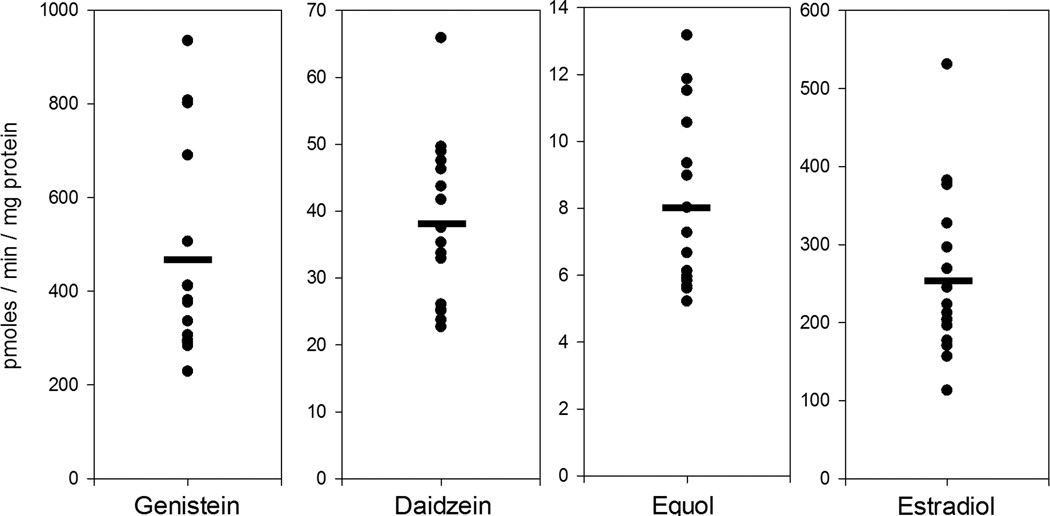
Individual differences in isoflavone glucuronidation (at the 7-OH position) and estradiol glucuronidation measured in 16 different cat livers are shown in Figure 4. Mean (standard deviation) glucuronidation activities in pmoles/min/mg protein were 460 (223), 38 (12), 7.9 (2.6), and 255 (106) for genistein, daidzein, equol, and estradiol (respectively). Coefficients of variation were highest for genistein glucuronidation (48%), lowest for daidzein (32%) and equol glucuronidation (33%), and moderately high for estradiol glucuronidation (42%).
Figure 4.
Variability in glucuronide formation rates between liver microsomes prepared from 16 different cats. Each filled circle represents the average of duplicate determinations of glucuronide formation rates (7-OH-glucuronidation for isoflavones and 3-OH-glucuronidation for estradiol) for an individual cat measured using 35 µM genistein, daidzein, or equol, or 100 µM estradiol substrate concentration.
Table 4 shows Spearman correlation coefficients and p-values for comparisons in glucuronidation activities between individual cat livers for each substrate. Such correlations may indicate whether similar UGT enzyme isoforms may be involved in the glucuronidation of these substrates. Moderate correlations were observed between genistein and daidzein (Rs=0.67; p=0.005), and between genistein and equol (Rs=0.67; p=0.005) 7-OH-glucuronidation activities. Weaker although significant correlations were observed between daidzein and equol (Rs=0.54; p=0.03) 7-OH-glucuronidation and between daidzein 7-OH-glucuronidation and estradiol (Rs=0.60; p=0.01) glucuronidation activities. All other activity correlations were not significant (Rs<0.05; p>0.05).
Table 4.
Spearman correlation (Rs) coefficients and p-values for comparisons between rates of 7-OH glucuronidation of daidzein, genistein, and equol, and 3-OH glucuronidation of estradiol measured using liver microsomes prepared from 16 different cats.
| Daidzein | Equol | Estradiol | |
|---|---|---|---|
| Genistein | 0.668 (0.005) | 0.668 (0.005) | 0.459 (0.07) |
| Daidzein | - | 0.535 (0.03) | 0.603 (0.01) |
| Equol | - | - | 0.274 (0.298) |
4. Discussion
To the best of our knowledge, this is the first study to report soy isoflavone and metabolite concentrations in urine of cats and dogs receiving a soy-based diet. Our results indicate that food derived soy isoflavones are extensively conjugated in both dogs and cats, either directly, or after reduction to the dihydro derivatives, followed by excretion into the urine. There were, however, species differences in the type of conjugation. In dog urine, about half of the parent isoflavones (genistein, daidzein, glycitein) and a third of the microbial metabolites (dihydrogenistein, dihydrodaidzein, equol) were excreted as glucuronides with presumably the majority of the remainder undergoing sulfation. These findings are similar to prior reports of soy isoflavones in human urine, which indicate that the glucuronide conjugates of daidzein and genistein are predominantly found with lower amounts of sulfate conjugates and essentially no unconjugated isoflavones (Cimino et al. 1999; Shelnutt et al. 2002). In contrast, in cat urine we were unable to detect glucuronide metabolites of dihydrogenistein, dihydrodaidzein, or equol, and only trace amounts of genistein. However, there were somewhat higher amounts of daidzein (11%) and glycitein (37%) that were glucuronidated.
These differences in the ability of cats to glucuronidate the different substrates may reflect differences in the number and substrate selectivity of UGTs in cats versus other species. Studies with human recombinant UGTs have shown that genistein and daidzein are glucuronidated predominantly by UGT1A9 with some contribution by UGT1A1 (particularly for daidzein), while glycitein is glucuronidated mainly by UGT1A1 (Tang et al. 2009). However cats only have two UGT1A sub family isoforms, including UGT1A1 and UGT1A2, and completely lack UGT1A9. Consequently, this lack of UGT1A9 in cats may explain the relatively low amounts of genistein and daidzein glucuronides found in cat urine. However glucuronidation of glycitein (and to a lesser extent daidzein) by cat UGT1A1 may explain the higher amounts of glucuronides of these isoflavones found in cat urine. This could be tested in the future when recombinant cat UGTs become available for research. Regardless, glycitein is not the most abundant isoflavone found in soy and has not been associated with significant effects on biological activity.
In addition to determining concentrations of the isoflavones derived directly from soybeans (genistein, daidzein, glycitein) and their conjugates in dog and cat urine, we also assayed for a range of reductive metabolites of the isoflavones (dihydrogenistein, dihydrodaidzein, equol, dehydroequol, O-desmethylangolensin, and 6-hydroxy-O-desmethylangolensin) and their conjugates. These latter metabolites are derived from metabolism by the gut microbiota. In humans it has been shown that there are individual differences in the ability to form these certain metabolites. For example dihydrogenistein and dihydrodaidzein are found in 80–90% of people, while equol is only formed in 30–50% of people (Atkinson et al. 2005). Interestingly we found concentrations of dihydrogenistein and dihydrodaidzein in both species that were similar to or higher than concentrations of the parent isoflavones. These concentrations are about five times higher than reported before for humans (Zhang et al. 2012), which may reflect differences in the gut microbiota. It is not known whether dihydrogenistein or dihydrodaidzein have biological importance.
Equol, which is derived from daidzein, has a higher affinity for estrogen receptors, unique antiandrogenic properties, and a greater antioxidant capacity than daidzein (Setchell et al. 2002; Yuan et al. 2007). Interestingly we found relatively high conjugated equol concentrations in pooled cat urine that were greater than 10% of the parent daidzein concentration, while pooled dog urine showed very low conjugated equol concentrations that were only 0.1% of the daidzein concentrations. This difference was reflected by our in vitro metabolism studies of equol that showed significantly lower intrinsic clearance for equol glucuronidation in livers from cats compared with dog and human livers. In humans a urinary equol to daidzein ratio of at least 0.018 (1.8%) is considered a positive equol producing phenotype (Zhang et al. 2012). Consequently cats may be exposed to relatively high concentrations of equol resulting in possible biological effects. Unfortunately we were unable to evaluate differences in equol concentrations between urine samples from individual cats. However we were able to evaluate individual dog urine samples and found that only three of ten samples had detectable equol concentrations, although not as high as the pooled cat urine samples with equol to daidzein ratios 0.004–0.017. Interestingly two of the three equol positive dog urine samples were from dogs that shared the same household, which suggests that they may have shared the same intestinal microbiome.
We also performed in vitro glucuronidation experiments using liver microsomes and were able to recapitulate our urinary metabolite findings in that cats consistently showed lower glucuronidation of genistein, daidzein, and equol in comparison to dogs and humans. We were also able to broaden our investigation to include 10 other species and found that ferret and mongoose livers behaved similar to cat livers in having consistently low glucuronidation of genistein, daidzein, and equol. We also evaluated the 3-OH glucuronidation of estradiol, which is primarily mediated by UGT1A1 in humans. This was included as a positive control since all species to date have UGT1A1, probably because this enzyme is responsible for detoxification and elimination of bilirubin (Court 2005). We observed estradiol-3-OH glucuronidation activities for cat, ferret, and mongoose livers that were similar to those found in humans and dogs suggesting that low levels of glucuronidation of isoflavones were not a result of problems with liver quality or storage.
Correlation analysis between glucuronidation activities measured for each substrate for the different species showed a high correlation between daidzein and genistein glucuronidation suggesting that these substrates are glucuronidated by the same enzymes probably reflecting their structural similarities. We also found relatively high correlations between genistein, daidzein, and equol glucuronidation activities for the 16 different cat livers, again suggesting that the same feline UGTs may be involved in glucuronidation of these compounds. Interestingly estradiol glucuronidation activity in the 16 cat livers was most highly correlated with daidzein glucuronidation. This may be a reflection of the importance of UGT1A1 in glucuronidation of both compounds that was previously shown using human recombinant UGTs (Tang et al. 2009).
Our observation of low glucuronidation of the soy isoflavones by cat, ferret, and mongoose livers is consistent with a general theory that contends an animal’s natural diet is an important determinant of their ability to metabolize and detoxify compounds that they could ingest. In previous work we have determined that mongoose, ferrets, and cats can be characterized as hypercarnivores, in that they get more than 70% of their diet from animal matter and very little from plants (Shrestha et al. 2011). Plant derived diets, such as those containing soybeans, frequently contain compounds that if not effectively metabolized and eliminated can result in an adverse impact on an animal’s health. Hypercarnivores have avoided the necessity to metabolize these compounds and through evolutionary adaptation have lost the necessary genes and their encoded enzymes. The best example of this is the finding that UGT1A6, which normally encodes for a highly efficient phenol glucuronidation enzyme, has become a pseudogene in domestic cats and all other species in the Felidae family (Shrestha et al. 2011). In a previous study we confirmed that glucuronidation of acetaminophen was poor in ferret liver microsomes, but this was not caused by genetic defects in the UGT1A6 gene (Court 2001). Similarly, we determined that the UGT1A6 gene was present and not a pseudogene in mongoose (Shrestha et al. 2011). As indicated above, a number of other UGT1A sub-family enzymes normally found in other species such as UGT1A9 are not found in the domestic cat presumably through the same evolutionary process. Consequently it is possible that ferrets and mongoose also may lack UGT1A9. This could be explored through evaluation of the whole genome sequence for these species once they become available.
We found that glucuronidation at the 7-OH position of daidzein, genistein, and equol was consistently more efficient than glucuronidation at the 4’-OH position in all species that we evaluated. This is consistent with reports in humans and rats (Doerge et al. 2000). A prior study showed the same difference using recombinant human UGTs suggesting that the 4’-OH site is less amenable to glucuronidation than the 7-OH site (Doerge et al. 2000). Genistein also possesses a potential glucuronidation site at the 5-OH position but we found no evidence for glucuronidation at that site based on attempts to identify additional HPLC peaks with the same mass spectrum as the 7-OH- and 4’-OH-glucuronide peaks.
There are several limitations to this study that should be mentioned. The soy-based diets differed in soy isoflavone content with a somewhat higher content in the cat diet versus the dog diet, which limits direct comparisons of urinary isoflavone metabolite concentrations between species. However, this difference is unlikely to have affected our main conclusion that cats poorly glucuronidate soy isoflavones given that both relative (expressed as a percentage of total isoflavones excreted) and absolute concentrations of soy isoflavone glucuronides were dramatically lower in cat versus dog urine, despite the higher amounts of isoflavones consumed by the cats. Another limitation was that the urine samples were pooled prior to analysis because of limited sample availability. However since we included samples from 10 dogs and 18 cats, it is likely that our results are a good representation of the population mean value, despite losing information regarding population variability. We also analyzed urine samples using an indirect method involving enzyme hydrolysis to determine overall concentrations of conjugated isoflavones. Consequently, our results should be confirmed in future studies by measuring the glucuronide and sulfate conjugates directly.
Finally, although the majority of the studies here were focused on glucuronidation, our urine metabolite results and the plasma metabolite results reported by Whitehouse-Tedd et al. (2013) indicate that sulfation of soy isoflavones is probably the major metabolic pathway in cats. Consequently differences in the rates of sulfation of isoflavones between cats may be of substantial importance in determining differences in exposure and the resulting effects of these compounds in individual cats.
In conclusion the results of this study demonstrate that glucuronidation is only a minor pathway in the elimination of the soy isoflavones in cats and probably several other species including ferret and mongoose. We also found evidence for significant formation of equol, a biologically active intestinal metabolite of daidzein, in cats but not in dogs, possibly related to species differences in the intestinal microbiome.
Acknowledgments
The authors would like to acknowledge Keith Dan DVM who contributed to this work under the sponsorship of the Agnes Varis Research Scholars program while a student at Tufts University Cummings School of Veterinary Medicine. This work was also supported by the US National Institutes of Health grant GM102130 (M.H.C.), the William R. Jones endowment to Washington State University College of Veterinary Medicine (M.H.C.). Dr. Shrestha was supported by a Fulbright Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. Normal human liver samples were obtained through the Liver Tissue Cell Distribution System, Minneapolis, Minnesota and Pittsburgh, Pennsylvania, which was funded by NIH Contract #N01-DK-7-0004 / HHSN267200700004C.
Abbreviations
- UGT
UDP-glucuronosyltransferase
- TSH
thyroid stimulating hormone
- HPLC
high pressure liquid chromatography
- HPLC-MS
high pressure liquid chromatography- mass spectrometry
- CBC
complete blood cell counts
- UDPGA
UDP-glucuronic acid
- UV
ultraviolet
- nmoles
nanomoles
- pmoles
picomoles
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
References
- Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- Broussard JD, Peterson ME, Fox PR. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. J Am Vet Med Assoc. 1995;206:302–305. [PubMed] [Google Scholar]
- Cerundolo R, Court MH, Hao Q, Michel KE. Identification and concentration of soy phytoestrogens in commercial dog foods. Am J Vet Res. 2004;65:592–596. doi: 10.2460/ajvr.2004.65.592. [DOI] [PubMed] [Google Scholar]
- Cerundolo R, Michel KE, Court MH, Shrestha B, Refsal KR, Oliver JW, Biourge V, Shofer FS. Effects of dietary soy isoflavones on health, steroidogenesis, and thyroid gland function in dogs. Am J Vet Res. 2009;70:353–360. doi: 10.2460/ajvr.70.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino CO, Shelnutt SR, Ronis MJ, Badger TM. An LC-MS method to determine concentrations of isoflavones and their sulfate and glucuronide conjugates in urine. Clin Chim Acta. 1999;287:69–82. doi: 10.1016/s0009-8981(99)00124-2. [DOI] [PubMed] [Google Scholar]
- Court MH. Acetaminophen UDP-glucuronosyltransferase in ferrets: species and gender differences, and sequence analysis of ferret UGT1A6. J Vet Pharmacol Ther. 2001;24:415–422. doi: 10.1046/j.1365-2885.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- Court MH. Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases. Methods Enzymol. 2005;400:104–116. doi: 10.1016/S0076-6879(05)00007-8. [DOI] [PubMed] [Google Scholar]
- Court MH. Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms. Vet Clin North Am Small Anim Pract. 2013;43:1039–1054. doi: 10.1016/j.cvsm.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court MH, Freeman LM. Identification and concentration of soy isoflavones in commercial cat foods. Am J Vet Res. 2002;63:181–185. doi: 10.2460/ajvr.2002.63.181. [DOI] [PubMed] [Google Scholar]
- Court MH, Greenblatt DJ. Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics. 2000;10:355–369. doi: 10.1097/00008571-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Divi RL, Chang HC, Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54:1087–1096. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- Divi RL, Doerge DR. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9:16–23. doi: 10.1021/tx950076m. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28:298–307. [PubMed] [Google Scholar]
- Edinboro CH, Scott-Moncrieff JC, Janovitz E, Thacker HL, Glickman LT. Epidemiologic study of relationships between consumption of commercial canned food and risk of hyperthyroidism in cats. J Am Vet Med Assoc. 2004;224:879–886. doi: 10.2460/javma.2004.224.879. [DOI] [PubMed] [Google Scholar]
- Gerber H, Peter H, Ferguson DC, Peterson ME. Etiopathology of feline toxic nodular goiter. Vet Clin North Am Small Anim Pract. 1994;24:541–565. doi: 10.1016/s0195-5616(94)50058-5. [DOI] [PubMed] [Google Scholar]
- Kass PH, Peterson ME, Levy J, James K, Becker DV, Cowgill LD. Evaluation of environmental, nutritional, and host factors in cats with hyperthyroidism. J Vet Intern Med. 1999;13:323–329. doi: 10.1892/0891-6640(1999)013<0323:eoenah>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Martin KM, Rossing MA, Ryland LM, DiGiacomo RF, Freitag WA. Evaluation of dietary and environmental risk factors for hyperthyroidism in cats. J Am Vet Med Assoc. 2000;217:853–856. doi: 10.2460/javma.2000.217.853. [DOI] [PubMed] [Google Scholar]
- Pritchett LE, Atherton KM, Mutch E, Ford D. Glucuronidation of the soyabean isoflavones genistein and daidzein by human liver is related to levels of UGT1A1 and UGT1A9 activity and alters isoflavone response in the MCF-7 human breast cancer cell line. J Nutr Biochem. 2008;19:739–745. doi: 10.1016/j.jnutbio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Reed JM, Starks PT, Kaufman GE, Goldstone JV, Roelke ME, O'Brien SJ, Koepfli KP, Frank LG, Court MH. Evolution of a major drug metabolizing enzyme defect in the domestic cat and other felidae: phylogenetic timing and the role of hypercarnivory. PLoS One. 2011;6:e18046. doi: 10.1371/journal.pone.0018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Singh R, Liu Z, Hu M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6:1466–1482. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke LL, Greenblatt DJ, Harmatz JS, Shader RI. Alprazolam metabolism in vitro: studies of human, monkey, mouse, and rat liver microsomes. Pharmacology. 1993;47:268–276. doi: 10.1159/000139107. [DOI] [PubMed] [Google Scholar]
- White HL, Freeman LM, Mahony O, Graham PA, Hao Q, Court MH. Effect of dietary soy on serum thyroid hormone concentrations in healthy adult cats. Am J Vet Res. 2004;65:586–591. doi: 10.2460/ajvr.2004.65.586. [DOI] [PubMed] [Google Scholar]
- Whitehouse-Tedd KM, Cave NJ, Ugarte CE, Waldron LA, Prasain JK, Arabshahi A, Barnes S, Hendriks WH, Thomas DG. Isoflavone metabolism in domestic cats (Felis catus): comparison of plasma metabolites detected after ingestion of two different dietary forms of genistein and daidzein. J Anim Sci. 2013;91:1295–1306. doi: 10.2527/jas.2011-4812. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Kano Y, Saito K, Ohsawa K. Urinary and biliary metabolites of daidzin and daidzein in rats. Biol Pharm Bull. 1994;17:1369–1374. doi: 10.1248/bpb.17.1369. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Mizunuma S, Kano Y, Saito K, Oshawa K. Urinary and biliary metabolites of genistein in rats. Biol Pharm Bull. 1996;19:413–417. doi: 10.1248/bpb.19.413. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol Nutr Food Res. 2007;51:765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gao YT, Yang G, Li H, Cai Q, Xiang YB, Ji BT, Franke AA, Zheng W, Shu XO. Urinary isoflavonoids and risk of coronary heart disease. Int J Epidemiol. 2012;41:1367–1375. doi: 10.1093/ije/dys130. [DOI] [PMC free article] [PubMed] [Google Scholar]