Abstract
Human aging is characterized by reductions in the ability to remember associations between items, despite intact memory for single items. Older adults also show less selectivity in task-related brain activity, such that patterns of activation become less distinct across multiple experimental tasks. This reduced selectivity, or dedifferentiation, has been found for episodic memory, which is often reduced in older adults, but not for semantic memory, which is maintained with age. We used functional magnetic resonance imaging (fMRI) to investigate whether there is a specific reduction in selectivity of brain activity during associative encoding in older adults, but not during item encoding, and whether this reduction predicts associative memory performance. Healthy young and older adults were scanned while performing an incidental-encoding task for pictures of objects and houses under item or associative instructions. An old/new recognition test was administered outside the scanner. We used agnostic canonical variates analysis and split-half resampling to detect whole brain patterns of activation that predicted item vs. associative encoding for stimuli that were later correctly recognized. Older adults had poorer memory for associations than did younger adults, whereas item memory was comparable across groups. Associative encoding trials, but not item encoding trials, were predicted less successfully in older compared to young adults, indicating less distinct patterns of associative-related activity in the older group. Importantly, higher probability of predicting associative encoding trials was related to better associative memory after accounting for age and performance on a battery of neuropsychological tests. These results provide evidence that neural distinctiveness at encoding supports associative memory and that a specific reduction of selectivity in neural recruitment underlies age differences in associative memory.
Introduction
Associative or relational memory, i.e., binding contextual information to stimulus attributes, is more vulnerable to aging than memory for single features or items (Old & Naveh-Benjamin, 2008). According to Naveh-Benjamin (2000), the associative deficit in aging consists of a reduced ability to link multiple units of information or representations. Impaired episodic memory for associations between items, but intact memory for single items, has been observed for scenes, word pairs, face-name pairs, face-location pairs and objects (Old & Naveh-Benjamin, 2008). These data illustrate the difficulty older adults have in remembering associations, regardless of the type of stimuli or paradigm used to test memory.
Functional magnetic resonance imaging (fMRI) has provided valuable insights into age differences in memory mechanisms. A number of areas, including the hippocampi, are often under-recruited in older adults during item (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Dennis et al., 2008; Gutchess et al., 2005) or associative (de Chastelaine, Wang, Minton, Muftuler, & Rugg, 2011; Leshikar, Gutchess, Hebrank, Sutton, & Park, 2010; Spreng, Wojtowicz, & Grady, 2010) encoding, and activity in the frontal lobes is less lateralized in older compared to younger adults during associative memory tasks (Leshikar et al., 2010; De Chastelaine et al., 2011). In addition to these reports of reduced task-related brain activity, studies have shown that older adults also have more diffuse patterns of activation (Cabeza, 2002), and/or less specificity in task-relevant regions (for reviews, see Grady, 2012; Grady, 2008). This was first noted among posterior visual structures, such as the parahippocampal place area and fusiform face area, that are primarily responsive to specific categories of visual stimuli (e.g., Carp, Park, Polk, & Park, 2011; Park et al., 2004), but also has been reported in other areas of cortex during visual tasks (Grady, 2002).
These findings of reduced selectivity of task-related activation have been argued to support the view that aging leads to a lack of specificity in the brain, known as dedifferentiation. Although the term dedifferentiation was originally proposed to account for the finding that behavioural measures across different cognitive tasks become more highly correlated with age (Lindenberger & Baltes, 1994), it has more recently been used to describe less distinct patterns of activity in the aging brain (Grady, 2002). Dedifferentiation of the neural signature for different categories of visual stimuli (e.g. Carp et al., 2011), could result in neural representations for these stimuli that are less distinct in older adults. In addition, the neural patterns that underlie memory tasks also become less selective in older adults (Carp, Gmeindl, & Reuter-Lorenz, 2010; Dennis & Cabeza, 2011; Sambataro et al., 2012; St-Laurent, Abdi, Bondad, & Buchsbaum, 2014; St-Laurent, Abdi, Burianova, & Grady, 2011). If neural activity, and the subsequent stimulus representations, becomes less distinct with age, this could result in memory deficits, specifically those that demand subtle differences or relations between stimuli to be identified. For example, work by St-Laurent and colleagues (2011) has shown that dedifferentiation in neural patterns is evident on tasks in which age differences are most apparent, such as episodic and autobiographical memory tasks, but not on tasks in which age differences are lacking, including semantic memory tasks. If it is the case that greater distinctiveness in neural activity, especially at encoding, would result in stronger memory representations, then dedifferentiation, or a lack of neural selectivity, in older adults should be more important for associative than item encoding, and could be a potential mechanism underlying the reduction in associative memory performance.
Thus, our main question of interest was to identify whether a brain effect could be found that shows the same pattern as the behavioral effect consistently reported in the literature, such that brain selectivity would differ across age groups for associative encoding, but not for item encoding. A number of studies have examined brain activity during associative tasks, but typically, with the exception of one study (Dennis et al., 2008), participants are provided with an associative encoding task and then tested later for their memory of the items and associations (Giovanello & Schacter, 2012; Jackson & Schacter, 2004; Kim & Giovanello, 2011; Kukolja, Thiel, Wilms, Mirzazade, & Fink, 2009; Rajah, Languay, & Valiquette, 2010). However, with this approach, the material is always encoded in an associative manner, leaving open the question of whether age differences exist in the processes needed to encode single items vs. associations between two items, and whether these differences would differentially impact later memory. Furthermore, Dennis and colleagues (2008), observed under-recruitment in older adults in visual processing regions (fusiform gyrus and parahippocampal gyrus) during item encoding, and in middle temporal and prefrontal regions during associative encoding, but they did not assess whether this under-recruitment results in a loss of distinctiveness in the neural patterns underlying different forms of encoding. In order to test the idea that age differences in associative memory are related directly to dedifferentiation during associative encoding, the experimental design would need to provide specific encoding instructions to allow for a direct comparison to be made between item and associative encoding, as well as provide a metric of measuring neural selectivity across the item and associative encoding conditions. Should age differences in neural activity be confined to associative memory, it would further support the view that aging produces deleterious effects for associative memory, whereas other forms of episodic memory, such as item memory, are relatively spared.
To address this question, we scanned younger and older adults while they were encoding pictures of houses or household objects, as well as house/object pairs, to assess item and associative memory, respectively. We determined how well we could predict when older and younger participants were engaged in associative or item encoding, based on their brain activity patterns. The rationale for this predictive modeling approach is as follows: 1) age-related dedifferentiation of brain activity during a particular type of cognitive processing should make it more difficult to predict the brain state that accompanies that processing in an older adult, and 2) this should be the case primarily for those types of processing that result in robust age effects, such associative encoding, but not for those accompanied by minimal age differences, such as item encoding. Predictive modeling has been used in a previous memory study to demonstrate that older adults lack neural selectivity during mental replay of memories (St-Laurent, et al., 2014), but has not been used to examine age differences during encoding. Here, we aimed to determine whether lack of neural selectivity when making an association between two items, compared to the processing of single items, is detrimental to older adults’ associative memory. We used a predictive modeling approach known as agnostic canonical variates analysis (aCVA), a variant of linear discriminant analysis (Evans, Todd, Taylor, & Strother, 2010). ACVA is a multivariate technique that identifies whole-brain patterns of neural activation that are predictive of different class labels. The class discrimination in the current study was between item and associative encoding events, allowing for a metric of prediction to be computed for each age group and each type of encoding.
In terms of the brain regions whose activity would predict the two types of encoding, we hypothesized that the brain regions where activity would predict item vs. associative encoding would be the same regions shown by prior studies to be active during item and associative encoding. That is, activity in areas along the ventral visual stream that are active when people encode houses and objects (e.g., Grady, McIntosh, Rajah, & Craik, 1998; Grill-Spector, Kushnir, Edelman, Itzchak, & Malach, 1998; Haxby et al., 2001) should predict item encoding trials, and activity within the medial temporal lobe and prefrontal cortex, known to be active during associative memory (e.g. Dennis, et al., 2008; Kim, 2011), should predict trials involving encoding of item pairs.
Materials and Methods
Participants
Twenty right-handed young adults and 20 older adults participated in the study. All participants were healthy and had no reported history of untreated hypertension, diabetes or stroke. Structural magnetic resonance images (MRIs) were inspected for abnormalities or severe white matter changes by a neuroradiologist, and based on this inspection 3 young adults and 1 older adult were removed due to incidental findings. In addition, 1 young adult and 1 older adult were excluded based on poor behavioural performance (i.e., >2 SD on recognition tests), leaving a final sample of 16 young adults and 18 older adults (see Table 1). Informed consent was obtained prior to the experiment and participants received monetary compensation for their participation. This study was approved by the Research Ethics Board of Baycrest Centre.
Table 1.
Participant demographic data and scores on neuropsychological, encoding and recognition tests. Mean values are reported with standard deviations. Differences across age groups (p <.05) are indicated with asterisks. Age differences determined via independent sample t-test or chi-square test for categorical variables.
| Demographic data | Young | Old |
|---|---|---|
| N | 16 | 18 |
| Males | 7 | 8 |
| Age | 21.1 (2.2) | 69.2 (4.2)* |
| Education | 14.9 (1.1) | 15.9(2.8) |
| MMSE | 29.6 (0.7) | 29.2 (1.2) |
| Extended Range Vocabulary Test | 22.9 (8.3) | 31.7 (8.3)* |
|
| ||
| Neuropsychological scores | Young | Old |
|
| ||
| Memory | ||
| Logical Memory Immediate Recall | 28.8 (5.7) | 25.8 (4.9) |
| Logical Memory Delayed Recall | 27.3 (7.1) | 22.5 (6.8) |
| Verbal Paired Associates Immediate Recall | 45.2 (9.2) | 31.0 (10.1)* |
| Verbal Paired Associates Delayed Recall | 12.7 (1.9) | 10.8 (7.8) |
| Designs Immediate Recall | 86.5 (13.9) | 65.1 (14.1)* |
| Designs Delayed Recall | 76.3 (17.6) | 53.3 (12.0)* |
| CVLT-Short Delayed Recall | 13.0 (3.2) | 12.1 (2.9) |
| CVLT-Long Delayed Recall | 13.5 (2.5) | 12.3 (2.9) |
| Attention | ||
| Trails A (s) | 15.3 (3.5) | 25.8 (10.9)* |
| Digit Span Forward | 12.2 (2.7) | 11.2 (3.1) |
| Processing Speed | ||
| Symbol Search | 46.6 (7.2) | 30.3 (6.8)* |
| Digit Symbol Coding | 87.1 (12.1) | 61.6 (12.3) |
| Working Memory | ||
| Trails B (s) | 34.4 (9.8) | 59.3 (18.2)* |
| Digit Span Backward | 10.6 (2.2) | 10.0 (2.2) |
| Executive Function | ||
| Verbal Fluency | 44.3 (9.0) | 43.2 (11.0) |
| Design Fluency | 11.7 (2.6) | 9.0 (2.6)* |
| Stroop Colour Word Interference | 54.1 (6.9) | 38.5 (6.0)* |
| Mood | ||
| PANAS Positive | 32.8 (7.2) | 39.9 (5.7)* |
| PANAS Negative | 17.6 (6.6) | 13.2 (2.8)* |
|
| ||
| fMRI encoding task | Young | Old |
|
| ||
| % Responses | ||
| Modern Decisions | .57 (.06) | .56 (.07) |
| Likely Decisions | .62 (.07) | .55 (.07)* |
| Task RT (ms) | ||
| Item Encoding | 1288 (303) | 1828 (415)* |
| Associative Encoding | 1911 (329) | 2579 (423)* |
|
| ||
| Recognition task | Young | Old |
|
| ||
| % Hits | ||
| Item Recognition | .83 (.10) | .85 (.08) |
| Associative Recognition | .84 (.09) | .72 (.15)* |
| % False Alarms | ||
| Item Recognition | .10 (.07) | .12 (.09) |
| Associative Recognition | .12 (.09) | .22 (.12)* |
All participants completed a battery of neuropsychological tests to establish the cognitive profile for young and older adults. Years of education were comparable across age groups (Table 1). The Mini-Mental State Exam (MMSE), with a cut-off score of 26, was used as a screening test for dementia (Folstein, Folstein, & McHugh, 1975). Additional neuropsychological tests assessed the domains of: 1) memory, using Wechsler Memory Scale’s (WMS-IV) Logical Memory, Verbal Paired Associates and Designs (Wechsler, 2009), California Verbal Learning Test (CVLT-II, Delis, Kramer, Kaplan, & Ober, 1987); 2) attention and processing speed, using Trails A (Reitan, 1958), Wechsler Adult Intelligence Scale’s (WAIS-R) Digit Span Forward (Wechsler, 2008), WAIS-R Symbol Search and Digit Symbol Coding (Wechsler, 2008); 3) working memory/executive function, assessed with Trails B (Reitan, 1958), WAIS-R Digit Span Backward (Wechsler, 2008), FAS Controlled Verbal Fluency Test (Benton & Hamsher, 1976), Delis Kaplan Executive Function System (DK-EFS) Design Fluency (Delis, Kaplan, & Kramer, 2001), and the Stroop Color-Word Test (Golden, 1978); and 4) vocabulary, using the Extended Range Vocabulary Test (Ekstrom, French, & Harman, 1976). Mood was measured using the Positive and Negative Affect Scale (PANAS, Watson, Clark, & Tellegen, 1988).
Experimental Design
Participants performed item (10 min 4 sec) and associative (10 min 4 sec) incidental encoding runs in the scanner, both of which were preceded and followed by resting-state runs (5 min 4 sec; data from the resting runs will not be reported here). A practice trial was given prior to the encoding runs. The order of item and associative encoding runs was counterbalanced across participants, such that half of the participants received item and the other half received associative encoding first. A surprise recognition test was given outside the scanner.
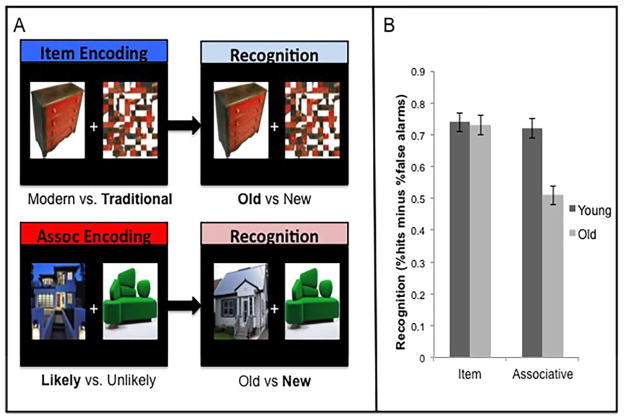
For item encoding trials participants were presented with a picture of an object or a house placed on the right or left side of a fixation cross (Figure 1A). The opposite side of the display was the same image but scrambled to ensure that item and associative encoding tasks had the same amount of visual input. Scrambled images were created using Photoshop 8.0 (www.photoshop.com). Associative encoding trials had an image of an object and a house situated on either side of a fixation cross (Figure 1A). The images (house/object or scrambled image for item; house or object for associative) were equally likely to occur on the right and left side of the fixation cross. Half of the pictures were modern in design and the other half traditional. All the objects could be found within a house. Pictures for item and associative tasks were drawn from online sources. E-prime (www.psynet.com) was used to present the task and collect responses.
Figure 1.
A. Examples of pictures presented during encoding and recognition. B. Performance (hits minus FA) during item and associative recognition tests. Error bars represent standard error of the mean (S.E.M.).
During item encoding participants were instructed to: “Please indicate if the style of the house or object is modern or traditional”. During associative encoding, for each house/object pair participants were asked to indicate: “Based on the style of the object and house, how likely would it be for the object to be found in the house?” Participants made a modern versus traditional response or likely versus unlikely response, via a button press using their dominant right-hand. They were told that that there was no correct answer to the question and that their decision should be subjective. The purpose of these decisions was to focus attention either on the object attributes during item encoding, or to facilitate an associative link between the house and object during associative encoding trials. Half of the image pairs in associative encoding were similar in design (i.e., modern with modern or traditional with traditional), whereas half were different in design (i.e., traditional with modern). Practice trials consisted of the same parameters but included only 5 trials for each type of encoding using stimuli not seen during scanning. Responses and reaction times were recorded.
Each encoding trial began with a fixation cross (with a duration that varied from 1–5 sec), followed by the presentation of the stimulus (5 sec). There were a total of 75 trials in each encoding run: 48 initial presentations, 12 repetitions (to ensure an adequate number of correct recognitions for later analysis), and 15 null events (fixation cross 5 sec in duration, preceded by a jittered inter-stimulus interval).
A surprise memory test was given outside the scanner consisting of separate recognition tests for item and associative memory. An approximate 30 min delay occurred between presentation and recognition for each memory type, as the recognition tests were given in the same order as encoding runs. The item recognition test consisted of 32 old items and 32 new items. Participants were instructed to make their decision based solely on the intact image (see Figure 1A). For item memory, participants were instructed to indicate if the item had been seen during scanning (“old”) or not (“new”). Associative recognition consisted of 32 intact pairs, 16 rearranged pairs and 16 new pairs. For associative memory, participants were instructed to indicate if the paired items had been seen together during scanning (“old” for intact pairs) or not (“new” for rearranged or new pairs). Memory was computed as the proportion of hits (correct old judgments) minus the proportion of false alarms (FA; incorrect old judgments) for item and associative tests. A repeated samples analysis of variance (ANOVA) was used to identify differences between age groups.
fMRI Data Acquisition and Analyses
Participants were scanned using a Siemens Trio 3T scanner. The scanning session was approximately 1 hour and 23 min, which included set-up and removal of participants, as well a high resolution structural scan, three resting state runs, two encoding runs, and diffusion tensor acquisition. Anatomical scans were acquired with a 3D MP-RAGE sequence (TR=2s, TE=2.63 ms, FOV=25.6 cm2, 256 × 256 matrix, 160 slices of 1mm thickness). Functional runs were obtained with an EPI sequence (300 volumes for the encoding runs and 150 volumes for the resting state runs, TR=2 s, TE=30 ms, flip angle=70N, FOV=20 cm2, 64 × 64 matrix, 30 interleaved axial slices of 5mm thickness, no gap). Pulse and respiration were measured throughout the scan.
Preprocessing of the images was performed with Analysis of Functional Neuroimages (AFNI, Cox, 1996). The following steps were carried out: realignment of the subject’s functional volume to their structural volume, physiological motion correction, slice timing correction, rigid-body motion correction, spatial normalization to the Montreal Neurological Institute (MNI) template, smoothing with an 8 mm Gaussian filter (final voxel size was 4 mm isotropic). We regressed out white matter, ventricular and large blood vessel signal, as well as the six-motion parameter estimates from each voxel time series, for each participant’s run (Grady et al., 2010). The first 2 TRs from each run were also dropped to avoid signal instability.
Lastly, to further reduce the influence of head motion, as proposed in Siegel et al. (2014), we employed a method that removes images considered to be outliers based on motion parameter estimates recorded for each person and on measurement of voxel intensity changes in each brain volume, across each time course. We identified and removed volumes that were outliers in both the 6 rigid-body motion parameter estimates (MPEs), and in the fMRI signal using a multivariate approach (for details see Campbell, Grigg, Saverino, Churchill, & Grady, 2013; Churchill, Spring, Afshin-Pour, Dong, & Strother, 2015). This involved the decomposition of the motion parameter estimates and fMRI signal intensity data matrices for each run and participant using a principal component (PC) analysis. For each of the two PC-space data sets the median PC-space coordinate vector was computed and the degree of displacement for each data point was measured. The data points that deviated using a Gamma probability distribution at p<.05 for both the image and motion medians were then removed and replaced by interpolating voxel values from adjacent volumes, using cubic splines. This controls for potential spikes, while minimizing discontinuities in the fMRI time courses due to removal of outliers. The number of scans dropped did not differ between groups (older adults M=10.8, SD=3.5; younger adults M=8.7, SD=3.8, p=.11).
Only stimuli (items or pairs) that were correctly identified as “old” at recognition were included in the analyses so that our results would be based only on those stimuli for which encoding was sufficient for accurate recognition. An average of 38 events for item encoding (young M=37.6, SD=3.8; old M=38.2, SD=3.3) and 32 events for associative encoding (young M=34.6, SD=3.2; old M=29.9, SD=5.9) were included. We included both first presentation trials and second presentation trials to obtain maximal statistical power and the most robust effects. The analysis described below was event-related and assessed activity in each event across 8 lags or time points (i.e., a 16 second interval) post-stimulus onset. For the predictive modeling analysis we chose to perform a combined analysis of all our data to allow for age-dependent differences in expression of common neural substrates to emerge in a data driven fashion, supported by measures of prediction and spatial pattern reproducibility across training and test groups.
Whole brain predictive modeling
Non-parametric prediction, activation, influence, and reproducibility resampling (NPAIRS, Strother et al., 2002; 2004; 2010) was used to determine whether specific brain patterns were predictive of item and associative encoding, respectively (Java version available at: http://code.google.com/p/plsnpairs/). With this multivariate approach, which applied aCVA (a multi-class linear discriminant) within a split-half cross-validation framework, we determined if the brain states associated with encoding items or item pairs could be predicted in each participant at above chance levels, and assessed the reproducibility of the spatial patterns associated with these brain states across participants. Prediction is based on the ability of a model generated from a training subset of the data to predict the two brain states from an independent test subset of the data, using iterative split-half resampling. Reproducibility, on the other hand, is a measure of how well a spatial pattern seen in the training model can be reproduced using the independent test datasets.
NPAIRS-CVA starts by reducing the dimensionality of the dataset using principal component analysis (PCA) decomposition. This first PCA provides a computational speedup by providing PCs as features, instead of voxels, in subsequent data analysis stages. Split-half resampling was then applied to the set of PCs from the combined group of young and old participants. These data were partitioned 50 times into two independent subject datasets (i.e., N=M/2 where M=34) to produce training and test datasets, which consist of both item and associative encoding runs per subject (the partition is based on subjects and not runs). The first subset of subjects was used to train a classifier with 16 classes, defined by the 8 lags from the item and associative encoding HRFs, and the second set was used to test the classifier’s accuracy. In the current study younger and older adults were both included in the analysis to provide a common set of brain patterns underlying the prediction and reproducibility metrics, from which we could then determine age differences. After splitting the data, the dimensions/components of each split fMRI dataset were reduced using a second PCA. This acts as an independent denoising step for each split dataset and is needed to produce stable, non-singular data matrices that can be subsequently analyzed using aCVA (Strother et al., 2004). The number of principal components was varied from 2 to 25 in steps of 1 to produce a curve reflecting prediction-reproducibility tradeoffs (Rasmussen, Hansen, Madsen, Churchill, & Strother, 2012). Canonical variables (CVs) were generated from the aCVA to identify condition-consistent covariance in the data across subjects. Each CV was associated with an eigenvalue that represents the signal-to-noise-ratio (SNR) of that variable. The percentage of variance accounted for can be computed by dividing the eigenvalue of the CV by the sum of all eigenvalues for all CVs (we retained two CVs based on the amount of variance accounted for, see Results). Canonical eigenimages express the spatial patterns in the brain associated with each CV, and for each participant a CV score was calculated for each HRF time point, per condition, indicating the participant’s expression of the spatial patterns.
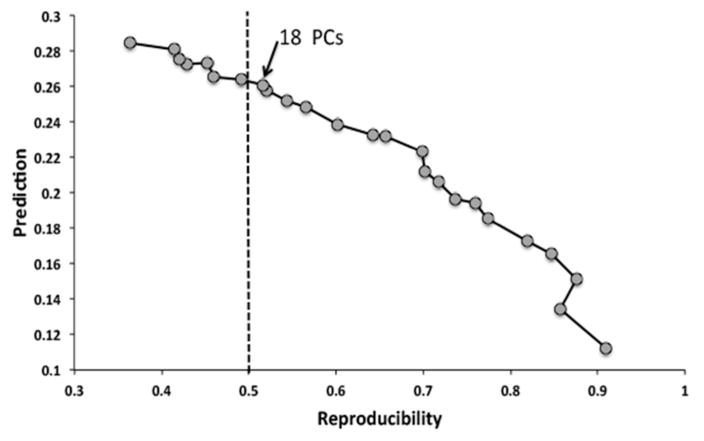
Based on the eigenimages, reproducibility was computed for each independent pair of split-half data sets as the average correlation coefficient (r) between voxels within these paired canonical eigenimages. The median r is calculated across the 50 split half partitions as the measure of overall reproducibility. Prediction is measured in the test set of subjects as the posterior probability of each scan’s true class membership for each subject (i.e., the HRF time point for item or associative encoding brain states) based on the training set parameters (Strother, et al., 2004). The number of PCs retained in the solution reported here was 18, and was chosen because this was the model with maximum prediction for a reproducibility > 0.5 (refer to Figure 2). To ensure the stability of this result, we also examined the results obtained with fewer PCs (13 PCs, which would emphasize reproducibility over prediction) and more PCs (23 PCs, which would emphasize prediction over reproducibility). See Rasmussen et al. (2012) for a validation of this approach in identifying the known left and right network components of the motor system in an alternating finger tapping task. The age differences in prediction that we report below were also found with these additional solutions, indicating that our results were not biased by our choice of 18 PCs.
Figure 2.
The NPAIRS results depicting the prediction (mean probability of correctly classifying a scan’s encoding type) by reproducibility (mean correlation between voxels across split half partitions) curve spanning across solutions with 1PC (high reproducibility but low prediction-far right) to 25 PCs (low reproducibility but high prediction-far left). The 18 PC solution lies just to the right of the line at reproducibility = 0.5, which represents the point of maximal prediction at a level of 0.5 reproducibility.
A repeated measures ANOVA with condition (item vs. associative) and time (i.e., 8 time points in the analysis window) as within subjects factors and age group (young vs. old) as a between subject factor, was used to determine whether significant condition, time, and group differences were present in prediction probabilities. An additional repeated measures ANOVA was carried out on the CV scores from both CVs to look for age differences in brain patterns, since both CVs contributed to the prediction. For this analysis of CV scores we focused on the time points in the event where prediction peaked for item (time point 3) and associative (time point 4) conditions. In this analysis, condition (item and associative), CV (CV1 vs. CV2), and time (time points 3 and 4) were entered as within subjects factors, with age group as the between subjects factor.
To examine the effect of mean NPAIRS-CVA prediction on item and associative recognition accuracy we carried out a hierarchical regression analysis. Given the age differences in scores on some of the neuropsychological tests, we thought it would be important to control for these variables, as well as age, in our exploration of this relation. In order to reduce the number of variables in the regression, we first ran a series of PCA analyses using the neuropsychological tests to compute factors for the cognitive domains that were represented. These consisted of an immediate memory factor (derived using immediate memory from the Logical Memory, Verbal Paired Associates, Designs and CVLT scales), a delayed memory factor (delayed memory scores from the aforementioned memory tests), an executive function factor (Trails B, Digit Span Backward, Verbal Fluency, Design Fluency, Stroop Interference) and an attention/speed of processing factor (Trails A, Digit Span Forward, Symbol Search, and Digit Symbol Coding). We then calculated the correlations between item or associative recognition accuracy and these factor scores, as well as vocabulary, and the positive and negative mood scores from the PNAS, across the entire sample of participants. Variables that were significantly correlated were included in the subsequent regression model to predict memory performance. Hierarchical regression analyses were done separately for the item and associative conditions, with age group entered first, followed by the neuropsychological scores that correlated with performance (using a forward regression), and with associative brain prediction and the interaction of this variable with age entered as the third step (also with forward regression). Thus, these analyses determined whether prediction at the brain level could predict memory performance after accounting for age and differences in neuropsychological scores, and whether any such relation characterized memory performance regardless of age, or was influenced by age (i.e., if the interaction term was significant).
Results
Neuropsychological test results
Table 1 summarizes the results from the neuropsychological test battery. Consistent with the literature on cognitive aging (see review by Old & Naveh-Benjamin, 2008), older adults performed worse on tests of associative memory (Verbal Paired Associates and Designs), but were comparable to younger adults on tests of item memory (CVLT). Also consistent with previous findings of normal aging (Park et al., 2002), older adults were better than younger adults on tests that measure crystallized intelligence (e.g., vocabulary) and poorer on tests that assess fluid intelligence (e.g., symbol search). In regards to mood, older adults had higher ratings of positive affect and lower ratings of negative affect compared to younger adults, as a number of studies have reported (e.g., Carstensen et al., 2011; Charles, Reynolds, & Gatz, 2001).
fMRI Behavioural Results
Encoding
Differences across groups for item (modern/traditional) and associative (likely/unlikely) responses during encoding were explored via an independent samples t-test. Modern decisions were equal across groups during item encoding [p=.825], whereas younger adults made more likely decisions during associative encoding [t(32)=2.93, p=.006, Table 1]. A repeated measures ANOVA with Age as a between subjects factor and Memory Task as a within subjects factor (item or associative) was performed for encoding RT. RTs were unavailable for one older adult during associative encoding. Significant effects of Age [F(1,31)=26.1, p<.001] and Task [F(1, 31)=31.0, p<.001] were found for RT, such that older adults were slower to make decisions than younger adults, and both groups were slower to make associative (likely/unlikely judgments) than item decisions (modern/traditional; t >9.0, p<.001; Table 1). The interaction of age and task was not significant; indicating that the additional time needed for the associative judgment was equivalent in the two age groups.
Recognition
Using a similar 2×2 repeated measures design for recognition scores, we found significant effects of Age [F(1, 32)=8.8, p=.006], Task [F(1, 32)=44.5, p<.001] and a significant interaction between Age and Task type [F(1, 32)=29.9, p<.001] (Figure 1B). Older adults performed worse on associative relative to item recognition tests [t(17)=9.4, p<.001], but no difference between item and associative recognition tests was found for young adults [t(15)=.78, p=.45; paired t-test]. Performance was equal across age groups for item recognition [t(32)=.32, p=.75], but older adults performed more poorly on associative recognition [t(32)=4.8, p<.001; independent samples t-test]. Compared to the younger adults, the older group had a lower number of hits [t(32)=2.7, p=.012] and a higher number of FAs [t(32)=−2.9, p=.007] for associative trials (Table 1).
To test whether the number of likely decisions at encoding had any influence on associative recognition scores, an additional 2×2 repeated measures analysis was performed with age as a between subject factor and decision type as a within subjects factor (% likely vs. unlikely decisions that led to hits). A significant effect of Age [F(1, 31)=10.23, p=.003] was found, whereby older adults had fewer associative hits than younger adults. However, neither the effect of a likely/unlikely decision [F(1, 31)=.01, p=.93) nor the interaction between Age and Decision type [F(1, 31)=.25, p=.62] was significant. This demonstrates that associative decisions at encoding do not account for the age difference in associative memory.
fMRI Results
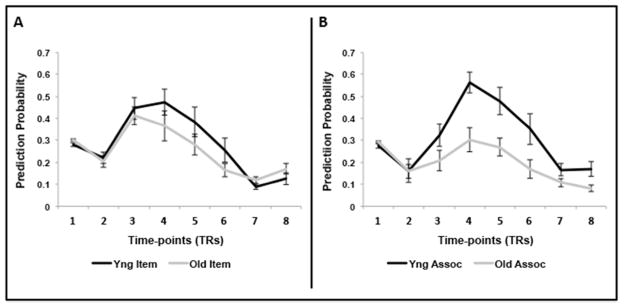
We turn next to the question of whether predictive modeling would reveal a neural effect that is similar to the associative deficit seen in behavior (i.e., age differences in prediction for associative memory but not for item memory). Prediction probabilities, averaged across subjects for each of the 8 time points for each condition and group, are shown in Figure 3. These plots indicate that maximal prediction was found at the third and fourth time points for the item condition (Figure 3A) and the fourth for the associative condition (Figure 3B). First, we tested whether overall prediction was significantly greater than chance (1/16 or 0.07) in both groups. Both young and older adults had robust overall prediction (young M=0.30±0.08, old M=0.23± 0.07; one-sample t-tests, t’s>10.0, p’s<0.001 for both contrasts). Next, we entered the prediction values into a repeated-measures ANOVA with group, condition, and time as factors. Prediction was higher overall in younger than in older adults (significant effect of age F(1, 32)=8.02, p=.008). However, this age difference was qualified by significant interactions between age group and condition (F(1,32)=7.63, p=.009) and age group and time (F(7,224) =5.17, p=.001). Separate analyses for item and associative conditions showed that prediction for item encoding did not differ across age groups (F(1,32)=1.19, p=.284), whereas for associative encoding prediction was significantly weaker in older relative to younger adults (F(1,32)=14.91, p=.001). There also was a significant interaction of group and time for associative prediction (F(7,224)=3.14, p=.013), suggesting that the group difference was largest when prediction was maximal. Thus, prediction of brain patterns linked to item encoding was comparable across age groups, whereas brain patterns related to associative encoding were more distinct and predictable in younger than in older adults.
Figure 3.
A. NPAIRS prediction values for item encoding events. B. NPAIRS prediction values for associative encoding events. Prediction values are shown for all 8-time points, for young and older adults. Note that maximal prediction occurs at time points 3 or 4. Error bars represent S.E.M. Similar plots for the 13 and 23 PC solution can be found on our website (https://sites.google.com/site/gradylabgroup/).
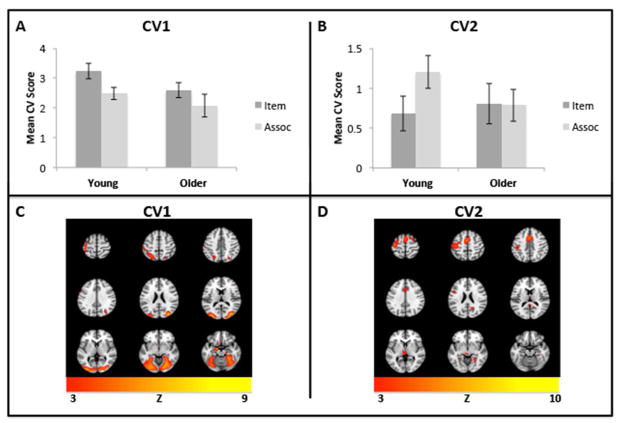
The spatial patterns that underlie the prediction of item and associative brain states are represented primarily by the canonical eigenimages of the first two CVs, which together accounted for 86% of the variance in the data (63% and 23%, respectively) and will be reported here (Figure 4). To determine differences across conditions and groups in the activity expressed in these CVs, we focused on the time where prediction was maximal, i.e., time points 3 and 4 (see Figure 3). We entered the scores from these times for both CVs into an ANOVA with group, condition, CV score and time as factors (data from both CVs were entered into the analysis because the combined effect of these spatial patterns contributes to prediction). The critical 4-way interaction was significant (F[1,32]=3.98, p=0.05), so we followed up with separate analyses of each age group. Here, we were primarily interested in the main effect of condition and the interaction of CV score and condition, which would indicate whether activity for the item and associative conditions was differentially expressed in the two spatial patterns within each group. In young adults the effect of condition was not significant (F<1) but the interaction of CV score and condition was significant (F[1,15]=7.00, p=0.018), reflecting greater activity for item encoding on the first CV score and greater activity for associative encoding on the second CV score (Figure 4A and B). In older adults neither the effect of condition (F[1,17]=1.03, p=0.324) nor the CV score by condition interaction (F[1,17]=1.57, p=0.227) was significant, although there was a trend for higher activity during item encoding on CV1 in the older group (Figure 4A). This group difference in expression of the CV patterns suggests that the reduced prediction in older adults, especially for associative encoding, is related to a reduction in the differential expression of the activity patterns that characterize item vs. associative encoding in younger adults.
Figure 4.
NPAIRS results for CV1 and CV2 by age group and condition. A. Mean canonical scores for CV1. B. Mean canonical scores for CV2. C. The Z-scored eigenimage corresponding to CV1, showing regions that were more active for item encoding in both groups. D. The Z-scored eigenimage corresponding to CV2, showing regions that were more active for associative encoding in young adults. Error bars represent S.E.M. A minimum Z of 3 was used for all images (equivalent to p <.005). Eigenimages are displayed using Mango (Research Imaging Institute, UTHSCSA).
The spatial patterns represented by CV1 and CV2 are displayed in Figure 4C and D, respectively. CV1 was associated with more activity in regions typically activated during object and house perception (middle occipital gyrus) and motor processing (pre and post central gyrus). Activity on CV2 (Table 2) was higher in left inferior frontal gyrus, anterior cingulate cortex, left motor/premotor cortex, and fusiform gyrus extending into the medial temporal lobe (MTL).
Table 2.
Brain regions that related to greater predictive power for successful item events for both age groups (CV 1) and successful associative events (CV 2) in young adults. Coordinates are in MNI space.
| Brain Regions | Left | Right | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| X | Y | Z | Z-score | X | Y | Z | Z-score | |
| CV1 | ||||||||
| Middle occipital gyrus | 36 | −84 | 16 | 9.17 | ||||
| Parahippocampal gyrus | −12 | −4 | −24 | 7.72 | ||||
| Mammillary body | 0 | −12 | −40 | 6.83 | ||||
| Rectal gyrus | 0 | 20 | −20 | 6.42 | ||||
| Inferior parietal lobe | −32 | −60 | 56 | 5.39 | ||||
| Postcentral gyrus | −40 | −32 | 60 | 5.37 | ||||
| Precentral gyrus | −36 | −8 | 60 | 5.27 | ||||
|
| ||||||||
| CV2 | ||||||||
| Precentral gyrus | −32 | −20 | 64 | 9.74 | ||||
| Middle cingulate cortex | 0 | 4 | 44 | 6.74 | ||||
| Thalamus | 0 | −24 | −8 | 4.86 | ||||
| Inferior temporal gyrus | 44 | −24 | −28 | 4.59 | ||||
| Retrosplenium | 20 | −56 | 16 | 4.42 | ||||
| Fusiform gyrus | −28 | −48 | −12 | 3.59 | 28 | −44 | −16 | 4.03 |
| Posterior cingulate cortex | 4 | −40 | 4 | 3.79 | ||||
| Inferior frontal gyrus | −44 | 12 | 20 | 4.11 | 44 | 8 | 20 | 3.30 |
| Cerebellum | −28 | −64 | −56 | 3.22 | 0 | −36 | −60 | 3.38 |
| Precuneus | −8 | −64 | 44 | 3.16 | ||||
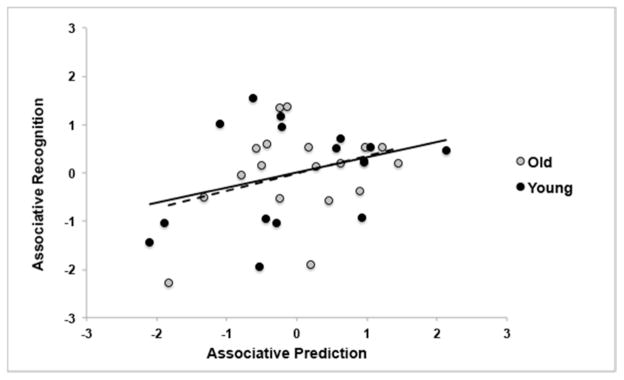
Finally, we determined whether item or associative prediction values in the brain were predictive of recognition accuracy. A number of the neuropsychological covariates were correlated with associative memory. Therefore, we carried out a hierarchical regression analysis using forward stepwise regression, to predict associative recognition accuracy. Age group was entered as the first step, followed by the neuropsychological scores that correlated with associative recognition (this included the factor scores for immediate memory, delayed memory, executive function/working memory, and attention/speed of processing, and the PANAS negative mood scores) as the second step, and mean associative prediction values and the interaction of this variable with age were entered as the last step. The only significant predictors of associative memory were age (F(1,32)=23.04, p<0.001, R2=.42) and associative prediction in the brain (Fchange(1,31)=4.21, p=.049, R2change=.07). A scatterplot of the relation between associative prediction and recognition (residuals after controlling for age are plotted in Figure 5), shows an increase in associative memory accuracy as associative prediction in the brain increases. In contrast, item recognition accuracy was not significantly correlated with any of the covariates, and a similar hierarchical regression carried out to predict item recognition from age, brain prediction and the interaction of these two variables was also not significant (F<1).
Figure 5.
Scatterplot of associative prediction values and recognition scores (hits minus FA) for young and older adults using the residuals obtained when controlling for age. The solid line shows the slope for younger adults and the dashed line represents the older adults.
Discussion
This study examined the neural correlates of the associative memory deficit in older adults by examining brain activity that was predictive of memory encoding conditions, across young and older adults. As hypothesized, an associative deficit was observed in both behavioural and neuroimaging findings. Age differences in prediction of brain activity associated with item vs. associative encoding provide evidence of dedifferentiation of brain activation patterns in aging, but this was specific to associative encoding. Critically, the ability to predict the brain activity accompanying associative encoding was related to better associative memory, even after accounting for age and neuropsychological performance, suggesting that this measure of neural specialization is important for supporting associative memory.
Consistent with earlier work, we found that older adults had worse memory for associations but intact memory for items compared to young adults. The associative deficit in the current study was driven by a lower number of hits and a higher number of FAs, as reported by others (Bender, Naveh-Benjamin, & Raz, 2010). It has been suggested that the age-related reduction in episodic memory can be accounted for by greater reliance on familiarity rather than recollective processes (Yonelinas, 2002). Relying on familiarity would lead to correct recognition for items, but doing so for associations would result in a high number of FAs. Although we did not assess recollection and familiarity directly, our results support the view that the associative memory deficit is in part attributable to an age related increase in familiarity-based recognition.
Our NPAIRS-CVA approach demonstrated that the dissociation observed in the behavioural literature is also apparent in the brain. We were able to successfully predict neural activity for item and associative conditions across groups; however, older adults had reduced prediction of associative trials. These results are consistent with dedifferentiation, i.e., less task selectivity, specifically for the associative encoding condition. Critically, the associative prediction probabilities were also related to associative recognition, whereas item prediction in the brain was not associated with item memory. Reduced selectivity of brain activity in older adults during associative encoding and the relation between predictability of the associative “brain state” and subsequent memory for stimulus pairs both support the idea that age-related dedifferentiation plays a critical role in the associative deficit. To our knowledge this is the first report of a direct link between a specific age difference in brain activity during associative encoding (i.e., the ability to predict brain activity during associative encoding) and reduced associative memory in older adults. Although others have examined predictive patterns of brain activity during associative encoding, these studies were restricted to young adult participants (for examples refer to Duncan, Tompary, & Davachi, 2014; Kuhl, Johnson, & Chun, 2013).
Our result adds to previous literature on dedifferentiation in aging, which found that older adults have less distinct neural representations across stimulus categories in visual (Carp, Park, Polk, et al., 2011; Park, et al., 2004; Payer et al., 2006), motor (Carp, Park, Hebrank, Park, & Polk, 2011), and auditory (Grady, Charlton, He, & Alain, 2011) systems. Specific to memory, brain activity is also less distinct in older compared to younger adults when contrasting implicit and explicit memory (Dennis & Cabeza, 2011), episodic, semantic and autobiographical retrieval (St-Laurent, et al., 2011), episodic encoding and working memory (Sambataro, et al., 2012), and perception and mental replay (St-Laurent et al., 2014). Taken together with earlier work, our result provides compelling evidence to support the idea that older adults have less task selectivity in regions that are task-specific in young adults, and that this dedifferentiation has a negative impact on associative memory in older adults.
The patterns obtained from the NPAIRS-CVA analyses characterizing item and associative encoding revealed regions that were commonly identified in the literature to be linked to encoding of items or associations. Areas that differentiated item encoding from associative encoding were related to activity in visual processing areas, such as the middle occipital gyrus, which are highly responsive to the presentation of objects and houses (e.g., Gutchess et al., 2005; Dennis et al., 2008). On the other hand, activity in the precuneus and fusiform gyrus/MTL (see Kim, 2011) accompanied associative encoding, at least in younger adults. The precuneus may predict associative encoding because it plays a pivotal role in source memory effects, particularly for pictorial information (Kim, 2011), and is an important area for mental imagery (Cavanna & Trimble, 2006). The fusiform gyrus/MTL is of interest in this context as it has been identified as a structure with reduced specificity in older adults on tasks of memory encoding (St-Laurent et al., 2014) and visual perception (Carp, Park, Polk, et al., 2011; Lee, Grady, Habak, Wilson, & Moscovitch, 2011). In addition, our finding that left inferior frontal gyrus activity was predictive of associative encoding in young adults, but not in older adults, is consistent with numerous reports that this region is active during encoding of multiple types of stimuli in young adults (for a meta-analysis, see Spaniol et al., 2009), but often is less so in older adults (e.g., Grady et al., 1995; Logan, Sanders, Snyder, Morris, & Buckner, 2002). Taken together, these results suggest that activity in brain regions typically seen during encoding, even if the encoding is incidental, can be differentially predictive of specific encoding processes that are sensitive to age differences.
Given the evidence for dedifferentiation of brain activity in older adults, and our finding of less predictable brain activity for associative encoding, one might wonder about the underlying mechanism of this effect. One possibility is that the object and house representations in visual cortex are less distinct in older adults and this overlap in information is propagated to other cortical areas involved in associative encoding. The prior reports of reduced category-specific activity in visual areas in older adults mentioned above and broader tuning curves in visual cortex in monkeys (Yu, Wang, Li, Zhou, & Leventhal, 2006) would support this interpretation. Alternatively, the reduction of associative-specific neural activity in older adults and more overlap in activity during item and associative encoding, reflected in poorer prediction, may reflect a broader and less-specific engagement of neural resources in older individuals, relative to younger adults, to complete the task. Consistent with this view is that older adults often show greater activity in the frontal lobes than young adults during cognitive tasks (e.g. Cabeza, 2002; Park & Reuter-Lorenz, 2009). Thus, it may be that older adults need to pool their neural resources to perform the same task as younger adults, particularly for associative encoding, resulting in less distinction across conditions and poorer prediction.
It is important to note that additional factors also could have influenced age differences in the level of prediction across conditions. For instance, previous work has argued that the associative deficit in aging may result from differences in attention or inhibitory control, whereby older adults fail to inhibit irrelevant information and consequently encode too much information compared to younger adults (“hyperbinding”; Campbell, Hasher & Thomas, 2010). Although this is an intriguing idea, it is unclear whether encoding too much information would lead to improvements or declines in neural specificity. Furthermore, eye-movements are known to differ between young and older adults during associative encoding. A recent study by Kamp and Zimmer (2015) found age differences in the time course of fixation transitions between items encoded as a pair, suggesting differences in visual scanning while making associations between items. We did not measure eye movements during encoding so cannot rule out whether differences in such movements are contributing factors to the current findings. However, if eye movements were involved in predicting associative encoding, we would expect to see activity in CV2 corresponding to the frontal eye fields (FEF), which are linked to eye movements (Choi & Henderson, 2015). Although activity in dorsal motor/premotor cortex predicted associative encoding trials (see CV2 in Figure 4) this activity is not consistent with the location of the FEF (e.g., Paus, 1996; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008) and is lateralized to the left hemisphere, so may correspond to motor activity related to the button press response. In addition, activity in other regions such as the fusiform/MTL and cingulate cortex, is not easily explained by eye movements, suggesting that eye-movements are not a major contributor of differences in prediction between age groups.
In conclusion, our results provide evidence that dedifferentiation contributes to reductions in associative memory performance in older adults. Even though younger and older adults were found to activate the same neural networks during item and associative encoding, we found that neural activity across encoding conditions was more selective and predictable in younger compared to older adults. The neuroimaging results mirror the behavioural effect observed in the literature: older adults had less selective activity, as measured by less predictable brain activity, for associative but not for item encoding. In addition, greater specificity in the brain, such that different patterns of brain activity predicted item vs. associative conditions, was associated with better relational memory. This suggests that predictably selective activity during different kinds of encoding is important for subsequent memory, perhaps because it leads to more distinct memory representations, and that a lack of distinctiveness in neural recruitment is specifically detrimental to associative processing in older age.
Acknowledgments
This work was supported by: CIHR (MOP14036 to CG, and MOP 84483 to SCS), NSERC (CGS to CS), Canada Research Chairs program, Ontario Research Fund, Canadian Foundation for Innovation, the Canadian Partnership for Stroke Recovery, and the Ontario Brain Institute. We would also like to thank Lynn Hasher and Nicole Anderson for their helpful comments, and for the staff at the imaging centre for technical assistance, and for the generosity of Jack & Anne Weinbaum, Sam & Ida Ross, Joseph & Sandra Rotman in support of the imaging centre at Baycrest.
Footnotes
Disclosure Statement
The authors declare no competing financial interests.
References
- Bender AR, Naveh-Benjamin M, Raz N. Associative deficit in recognition memory in a lifespan sample of healthy adults. Psychology and Aging. 2010;25:940–948. doi: 10.1037/a0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher Kd. Multilingual Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Frontiers in Aging Neuroscience. 2013;5 doi: 10.3389/fnagi.2013.00073. Article 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-binding: A unique age effect. Psychological Science. 2010;21(3):399–405. doi: 10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Gmeindl L, Reuter-Lorenz PA. Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Frontiers in Human Neuroscience. 2010;4:217. doi: 10.3389/fnhum.2010.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk TA. Age-related neural dedifferentiation in the motor system. PLoS One. 2011;6:e29411. doi: 10.1371/journal.pone.0029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Polk TA, Park DC. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage. 2011;56:736–743. doi: 10.1016/j.neuroimage.2010.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, et al. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychology and Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology. 2001;80:136–151. [PubMed] [Google Scholar]
- Choi W, Henderson JM. Neural correlates of active vision: An fMRI comparison of natural reading and scene viewing. Neuropsychologia. 2015;75:109–118. doi: 10.1016/j.neuropsychologia.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Churchill NW, Spring R, Afshin-Pour B, Dong F, Strother SC. An Automated, Adaptive Framework for Optimizing Preprocessing Pipelines in Task-Based Functional MRI. PLoS One. 2015;10:e0131520. doi: 10.1371/journal.pone.0131520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cerebral Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX: Pearson; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test-Research Edition. New York: Psychological Corporation; 1987. [Google Scholar]
- Dennis NA, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiology of Aging. 2011;32:2318, e2317–2330. doi: 10.1016/j.neurobiolaging.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychololgy: Learning Memory and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Tompary A, Davachi L. Associative encoding and retrieval are predicted by functional connectivity in distinct hippocampal area CA1 pathways. Journal of Neuroscience. 2014;34:11188–11198. doi: 10.1523/JNEUROSCI.0521-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH. Educational Testing Service Kit of factor-referenced cognitive tests. Princeton, NJ: ETS; 1976. [Google Scholar]
- Evans JW, Todd RM, Taylor MJ, Strother SC. Group specific optimisation of fMRI processing steps for child and adult data. Neuroimage. 2010;50:479–490. doi: 10.1016/j.neuroimage.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini Mental State”- a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schacter DL. Reduced specificity of hippocampal and posterior ventrolateral prefrontal activity during relational retrieval in normal aging. Journal of Cognitive Neuroscience. 2012;24:159–170. doi: 10.1162/jocn_a_00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago: Stoelting; 1978. [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature reviews Neuroscience. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Age-related differences in face processing: A meta-analysis of three functional neuroimaging experiments. Canadian Journal of Experimental Psychology. 2002;56:208–220. doi: 10.1037/h0087398. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Charlton R, He Y, Alain C. Age differences in FMRI adaptation for sound identity and location. Frontiers in Human Neuroscience. 2011;5:24. doi: 10.3389/fnhum.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Craik FIM. Neural correlates of the episodic encoding of pictures and words. Proceedings of the National Academy of Science, USA. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron. 1998;21:191–202. doi: 10.1016/s0896-6273(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial temporal activity. Journal of Cognitive Neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Jackson O, 3rd, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Kamp SM, Zimmer HD. Contributions of attention and elaboration to associative encoding in young and older adults. Neuropsychologia. 2015;75:252–264. doi: 10.1016/j.neuropsychologia.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim SY, Giovanello KS. The effects of attention on age-related relational memory deficits: fMRI evidence from a novel attentional manipulation. Journal of Cognitive Neuroscience. 2011;23:3637–3656. doi: 10.1162/jocn_a_00058. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Johnson MK, Chun MM. Dissociable neural mechanisms for goal-directed versus incidental memory reactivation. Journal of Neuroscience. 2013;33:16099–16109. doi: 10.1523/JNEUROSCI.0207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Wilms M, Mirzazade S, Fink GR. Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiology of Aging. 2009;30:630–645. doi: 10.1016/j.neurobiolaging.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grady CL, Habak C, Wilson HR, Moscovitch M. Face processing changes in normal aging revealed by fMRI adaptation. Journal of Cognitive Neuroscience. 2011;23:3433–3447. doi: 10.1162/jocn_a_00026. [DOI] [PubMed] [Google Scholar]
- Leshikar ED, Gutchess AH, Hebrank AC, Sutton BP, Park DC. The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex. 2010;46:507–521. doi: 10.1016/j.cortex.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychology and Aging. 2008;23:104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Science USA. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Payer D, Marshuetz C, Sutton B, Hebrank A, Welsh RC, Park DC. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17:487–491. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Valiquette L. Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex. 2010;46:535–549. doi: 10.1016/j.cortex.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Hansen L, Madsen K, Churchill N, Strother S. Pattern reproducibility, interpretability, and sparsity in classification models in neuroimaging. Pattern Recognition. 2012;45:2085–2100. [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Sambataro F, Safrin M, Lemaitre HS, Steele SU, Das SB, Callicott JH, et al. Normal aging modulates prefrontoparietal networks underlying multiple memory processes. European Journal of Neuroscience. 2012;36:3559–3567. doi: 10.1111/j.1460-9568.2012.08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, et al. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neuroscience and Biobehavioral Reviews. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Bondad A, Buchsbaum BR. Memory reactivation in healthy aging: evidence of stimulus-specific dedifferentiation. Journal of Neuroscience. 2014;34:4175–4186. doi: 10.1523/JNEUROSCI.3054-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Burianova H, Grady CL. Influence of Aging on the Neural Correlates of Autobiographical, Episodic, and Semantic Memory Retrieval. Journal of Cognitive Neuroscience. 2011;23:4150–4163. doi: 10.1162/jocn_a_00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother SC, Anderson J, Hansen LK, Kjems U, Kustra R, Sidtis J, et al. The quantitative evaluation of functional neuroimaging experiments: the NPAIRS data analysis framework. Neuroimage. 2002;15:747–771. doi: 10.1006/nimg.2001.1034. [DOI] [PubMed] [Google Scholar]
- Strother S, La Conte S, Kai Hansen L, Anderson J, Zhang J, Pulapura S, et al. Optimizing the fMRI data-processing pipeline using prediction and reproducibility performance metrics: I. A preliminary group analysis. Neuroimage. 2004;23(Suppl 1):S196–207. doi: 10.1016/j.neuroimage.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Strother SC, Oder A, Spring R, Grady C. The NPAIRS computational statistics framework for data analysis in neuroimaging. Paper presented at the 19th International Conference of Computational Statistics: Keynote, Invited and Contributed Papers.2010. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 4. San Antonio, TX: Psychological Corporation; 2008. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 4. San Antonio, TX: Psychological Corporation; 2009. [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140:1023–1029. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]