Abstract
BACKGROUND
Human papillomavirus (HPV) vaccines prevent HPV infection and cervical precancers. The impact of vaccinating women with a current infection or after treatment for an HPV-associated lesion is not fully understood.
OBJECTIVES
To determine whether HPV-16/18 vaccination influences the outcome of infections present at vaccination and the rate of infection and disease after treatment of lesions.
STUDY DESIGN
We included 1711 women (18–25 years) with carcinogenic human papillomavirus infection and 311 women of similar age who underwent treatment for cervical precancer and who participated in a community-based trial of the AS04-adjuvanted HPV-16/18 virus-like particle vaccine. Participants were randomized (human papillomavirus or hepatitis A vaccine) and offered 3 vaccinations over 6 months. Follow-up included annual visits (more frequently if clinically indicated), referral to colposcopy of high-grade and persistent low-grade lesions, treatment by loop electrosurgical excisional procedure when clinically indicated, and cytologic and virologic follow-up after treatment. Among women with human papillomavirus infection at the time of vaccination, we considered type-specific viral clearance, and development of cytologic (squamous intraepithelial lesions) and histologic (cervical intraepithelial neoplasia) lesions. Among treated women, we considered single-time and persistent human papillomavirus infection, squamous intraepithelial lesions, and cervical intraepithelial neoplasia 2+. Outcomes associated with infections absent before treatment also were evaluated. Infection-level analyses were performed and vaccine efficacy estimated.
RESULTS
Median follow-up was 56.7 months (women with human papillomavirus infection) and 27.3 months (treated women). There was no evidence of vaccine efficacy to increase clearance of human papillomavirus infections or decrease incidence of cytologic/histologic abnormalities associated with human papillomavirus types present at enrollment. Vaccine efficacy for human papillomavirus 16/18 clearance and against human papillomavirus 16/18 progression from infection to cervical intraepithelial neoplasia 2+ were −5.4% (95% confidence interval −19,10) and 0.3% (95% confidence interval −69,41), respectively. Among treated women, 34.1% had oncogenic infection and 1.6% had cervical intraepithelial neoplasia 2+ detected after treatment, respectively, and of these 69.8% and 20.0% were the result of new infections. We observed no significant effect of vaccination on rates of infection/lesions after treatment. Vaccine efficacy estimates for human papillomavirus 16/18 associated persistent infection and cervical intraepithelial neoplasia 2+ after treatment were 34.7% (95% confidence interval −131, 82) and −211% (95% confidence interval −2901, 68), respectively. We observed evidence for a partial and nonsignificant protective effect of vaccination against new infections absent before treatment. For incident human papillomavirus 16/18, human papillomavirus 31/33/45, and oncogenic human papillomavirus infections post-treatment, vaccine efficacy estimates were 57.9% (95% confidence interval −44, 88), 72.9% (95% confidence interval 29, 90), and 36.7% (95% confidence interval 1.5, 59), respectively.
CONCLUSION
We find no evidence for a vaccine effect on the fate of detectable human papillomavirus infections. We show that vaccination does not protect against infections/lesions after treatment. Evaluation of vaccine protection against new infections and resultant lesions warrants further consideration in future studies.
Keywords: cervical cancer, clinical trial, human papillomaviruses, prevention, vaccines
The human papillomavirus (HPV) L1 virus-like particle vaccines first licensed in 2006 have been shown to be highly effective for prophylaxis of HPV infection, which in turn reduces rates of HPV-associated precancer lesions.1–3 The high efficacy of these vaccines is thought to be explained by the induction of neutralizing antibodies that protect against infection by binding to virions and preventing them from infecting human cells.4
Among HPV-infected individuals, neutralizing antibodies induced by vaccination could hypothetically bind to virions produced by infected cells, reducing spread of an existing infection by limiting the ability of the virus to infect new cells in the infected individual. It is also possible that vaccine-induced, cell-mediated immune responses to L1 promote clearance of infected cells.5 Initial efforts to evaluate such a “secondary” effect of the vaccine, however, suggested that vaccination did not lead to faster clearance or reduced persistence of prevalent infections.6–8
More recently, findings from both randomized and nonrandomized studies have suggested a possible benefit of vaccination to prevent the recurrence of genital lesions after excisional treatment of prevalent disease, once again raising the possibility of a beneficial effect of vaccination of individuals already infected with HPV.9–11 One study of women treated for cervical or vaginal HPV-associated disease observed that rates of disease after treatment were reduced among those who were vaccinated before treatment (46.2% vaccine efficacy against recurrent HPV-related disease), an effect that was stronger for cervical than noncervical disease.9 A similar effect was observed in a nonrandomized study that vaccinated women after treatment for cervical disease was completed (65.3% reduction in the rate of recurrent cervical intraepithelial neoplasia type 2-3, or CIN2-3).10 A third study of men who have sex with men treated for high-grade anal intra-epithelial neoplasia reported that rates of disease after treatment also were reduced among those who were vaccinated before treatment (55.7% reduction in the rate of recurrent high-grade anal intra-epithelial lesions).11 The later study was not randomized, and unvaccinated men had more time than vaccinated men from treatment to enrollment into the study, raising the possibility that efficacy estimates were biased in favor of the vaccine.
Of note, none of the aforementioned reports evaluate whether the observed effects resulted from (1) a therapeutic effect on residual infection directly or through reduced ability of residual virus to infect new cells (“secondary” effect) or (2) benefit of vaccination on de novo primary lesions caused by a new HPV infections after treatment (prophylactic effect).
We previously reported on the lack of efficacy of HPV-16/18 vaccination on clearance of infections present at vaccination from a large, community-based randomized clinical trial in rural Costa Rica.6 Given the recent, previously summarized data suggesting a possible role for HPV vaccination after treatment of cervical lesions, we report herein a reevaluation of data from Costa Rica by expanding the study in 3 important ways. First, we evaluated the effect of vaccination on HPV clearance in a larger number of participants followed for a longer period of time. Second, we evaluated the impact of vaccination on the rate of progression of prevalent infection to precancer (not available in our previous report). Finally, we evaluated the impact of vaccination on the rate of infection and disease after excisional treatment for cervical disease. For the latter analysis, we further evaluated whether lesions occur in a background of unresolved infection and/or new infections after treatment, in an attempt to better understand the underlying mechanism of any observed vaccine effects (ie, recurrence caused by residual infection vs primary prevention).
Methods
Women included in the present evaluation were participants in a larger community-based randomized clinical trial of 7466 Costa Rican women 18–25 years of age at enrollment (June 2004 to December 2005) that evaluated the efficacy of the HPV-16/18 virus–like particle vaccine formulated with the AS04 adjuvant system (Cervarix; GSK Biologicals, Rixensart, Belgium) against persistent type-specific infection with HPV and HPV-associated precancerous lesions. The design and methods for this trial have been described in detail previously and involved randomization, vaccination (3 doses offered over 6 months), active annual (or more frequently if clinically indicated) follow-up, referral to colposcopy of high-grade precancers and/or persistent low-grade cervical lesions, and treatment by loop electrosurgical excisional procedure (LEEP) when clinically indicated.12
For the present analyses, focused on the evaluation of vaccine efficacy among women with current HPV infection at the time of vaccination, we included 2 groups of women. The first consisted of participants who, at entry into the trial, were infected with 1 or more of 12 oncogenic HPV types (defined herein), and who were randomized, vaccinated, and followed for a period of approximately 4.75 years (median follow-up 56.7 months; interquartile range 52.4–66.5). The second consisted of participants who were randomized and vaccinated, who at some time during follow-up received LEEP treatment for cervical precancer, and who were followed after treatment (median follow-up 27.3 months; interquartile range 15.3–39.8) as part of their participation in the larger trial. This latter group should not be considered formally randomized, because randomization occurred at entry into the main trial and not at the time of LEEP treatment. For both groups of women, we performed analyses at the infection level, as described herein. The trial was reviewed and approved by the US National Cancer Institute and Costa Rica INCIENSA Institute (Instituto Costarriciense de Investigacion y Ensenanza en Nutricion y Salud) human subjects review committees. Participants provided written informed consent.
At enrollment and during the follow-up period, risk factor questionnaires were administered and pelvic examinations performed on sexually experienced women. Specimens were collected at those visits to allow for ThinPrep cytology (Hologic, Marlborough, MA) and HPV DNA testing. Cytology was interpreted via use of the Bethesda system.13 Women with cytologic evidence of high-grade precancer and those with evidence of persistent low-grade cervical lesions were referred to colposcopy for further evaluation and disease ascertainment. For those referred to colposcopy, biopsies were collected when cervical lesions were evident and treatment by LEEP performed when clinically indicated. Hematoxylin and eosin slides prepared from formalin-fixed and paraffin-embedded materials were used to grade the lesions by the study pathologist in Costa Rica, followed by independent review by a second pathologist in the United States. When grades were discrepant, a third US pathologist blindly evaluated the slides to adjudicate and establish the final diagnosis for analytical purposes.
HPV HC2 testing (Digene Corporation, Silver Spring, MD) with the use of PreservCyt solution (Cytyc Corporation, Marlborough, MA) was performed as per the manufacturer’s instructions14 on all enrollment (prevaccination) specimens, all specimens collected at the final blinded visit, and to triage equivocal cytology results (atypical cells of undetermined significance) during follow-up. Broad-spectrum polymerase chain reaction–based HPV DNA testing was performed on cells stored in Preservcyt solution at all study timepoints using a previously described procedure based on amplification with the SPF10 primers, a DNA enzyme immunoassay detection of amplimers, and typing using the LiPA line blot detection system (LiPA HPV InnoLiPA HPV genotyping assay SPF10 system, version 1, Labo Bio-medical Products, Rijswijk, the Netherlands).15–17 The LiPA detects 25 HPV genotypes, including the 12 oncogenic HPV types of interest for this evaluation (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59). To ensure that HPV-16 and HPV-18 infections were not missed, all specimens that screened positive for HPV DNA via the SPF10 DNA enzyme immunoassay but that were negative for HPV-16 or HPV-18 by LiPA were tested for the presence of HPV-16 and HPV-18 DNA with the use of type-specific primers, as previously described.17
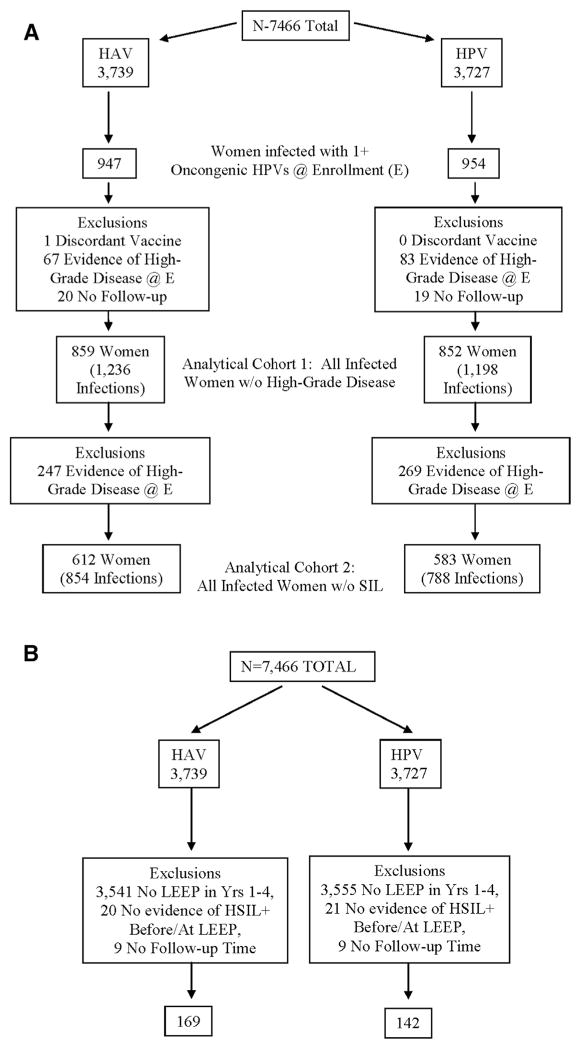
A total of 7466 women were enrolled and randomized in our trial. Of these, the 1901 (954 HPV arm; 947 control arm) who tested positive for 1 or more of 12 oncogenic HPV types at randomization were considered for the infection-level analysis that evaluated the fate of HPV infections present at the time of vaccination (Figure 1, A). We further excluded from this analysis 1 woman who received a discordant vaccine (0 HPV arm; 1 control arm), 150 women who had evidence of high-grade disease requiring treatment at enrollment (83 HPV arm; 67 control arm), and 39 women with no follow-up time after enrollment (19 HPV arm; 20 control arm).
Figure 1. Flow charts.
A, Consort – evaluation of prevalent HPV infections. B, Consort – evaluation of recurrence after LEEP.
HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LEEP, loop electrosurgical excisional procedure.
Women included in this analytical cohort (N=1711; 8588 person years of follow-up) had a median of 7 study visits after enrollment (7, interquartile range 5–8 HPV arm; 7, interquartile range 5–8 control arm) and were followed for a median of 56.7 months (56.4, inter-quartile range 52.0–66.1 HPVarm; 57.0, interquartile range 52.7–66.8 control arm). They had a total of 2434 oncogenic HPV infections at enrollment (1198 HPVarm; 1236 control arm). Compared with women included in our previous publication on this topic,6 this analysis included an additional 106 women (6.6% increase) and 7136 person years of follow-up (491% increase). Comparison of this analytical cohort by study arm revealed balance with respect to age, lifetime number of partners, cytology results, and HPV status (single vs multiple infections; HPV type) at enrollment (Table 1).
TABLE 1.
Enrollment characteristics of women included in the prevalent infection cohort, by vaccination arm
| Variables | Control arm, n (%) | HPV arm, n (%) |
|---|---|---|
| Number of women | 859 (50.2%) | 852 (49.8%) |
| Age at entry, years | ||
| <20 | 261 (30.4%) | 228 (26.8%) |
| 20–21 | 213 (24.8%) | 234 (27.5%) |
| 22–23 | 206 (24.0%) | 206 (24.2%) |
| >23 | 179 (20.8%) | 184 (21.6%) |
| Lifetime no. partnersa | ||
| 1 | 244 (28.6%) | 249 (29.3%) |
| 2 | 231 (27.0%) | 237 (27.8%) |
| 3+ | 379 (44.4%) | 365 (42.9%) |
| Costa Rica cytology | ||
| Normal | 612 (71.2%) | 583 (68.4%) |
| Abnormal | 247 (28.8%) | 269 (31.6%) |
| Histology | ||
| Normal | 819 (95.3%) | 820 (96.2%) |
| CIN1+ | 40 (4.7%) | 32 (3.8%) |
| HPV infection status | ||
| Single | 583 (67.9%) | 590 (69.2%) |
| Multiple | 276 (32.1%) | 262 (30.8%) |
| HPV analysis group (nonexclusive groups) | ||
| HPV-16/18 | 299 (34.8%) | 256 (30.0%) |
| HPV-31/33/45 | 207 (24.1%) | 197 (23.1%) |
| HPV-Oncogenic | 859 (100%) | 852 (100%) |
CIN1, cervical intraepithelial neoplasia type 1; HPV, human papillomavirus.
Six women missing lifetime number of partners information (5 control; 1 HPV) were excluded.
For the infection-level analysis that evaluated recurrence after treatment, we considered 370 women (172 HPV arm; 198 control arm) who were treated with LEEP during the follow-up period (Figure 1, B). We further excluded 41 women (21 HPV arm; 20 control arm) who had a LEEP performed but for whom there was no evidence of high-grade disease before the procedure and 18 women (9 HPV arm; 9 control arm) who had no follow-up after treatment. A total of 311 women were therefore included in the analysis (142 HPV arm; 169 control arm). A total of 130 of these women also were included in the analytical cohort of women infected at entry that was described in the preceding paragraph. Women included in this analytical cohort had a median of 3 study visits after treatment (4, interquartile range 3–5 HPV arm; 3, interquartile range 2–5 control arm) and were followed for a median of 27.3 months after treatment (31.8, interquartile range 19.6–39.8 HPV arm; 23.9, interquartile range 11.5–39.8 control arm). These women had a total of 156 oncogenic HPV infections detected after treatment (71 HPV arm; 85 control arm). Comparison of this analytical cohort by study arm revealed balance with respect to age at enrollment (Table 2).
TABLE 2.
Enrollment characteristics of women included in the recurrence cohort, by vaccination arm
| Variables | Control arm, n (%) | HPV arm, n (%) |
|---|---|---|
| Number of women | 169 | 142 |
| Age at entry, years | ||
| <20 | 37 (21.9%) | 31 (21.8%) |
| 20–21 | 38 (22.5%) | 36 (25.3%) |
| 22–23 | 53 (31.4%) | 38 (26.8%) |
| >23 | 41 (24.3%) | 37 (26.1%) |
| Lifetime no. partners | ||
| Unknown | 2 (1.2%) | 0 (0.0%) |
| Virgin | 15 (8.9%) | 3 (2.1%) |
| 1 | 48 (28.4%) | 33 (23.2%) |
| 2 | 40 (23.7%) | 42 (29.6%) |
| 3+ | 64 (37.9%) | 64 (45.1%) |
| Cytology result | ||
| Inadequate | 1 (0.6%) | 2 (1.4%) |
| Normal | 97 (57.4%) | 57 (40.1%) |
| LSIL | 31 (18.3%) | 36 (25.4%) |
| HSIL+ | 40 (23.7%) | 47 (33.1%) |
| HPV DNA (Hierarchical - HPV-16/18, HPV-31/33/45, Other-onco, Non-onco, Negative) | ||
| Negative | 34 (20.1%) | 13 (9.2%) |
| HPV 16/18-positive | 64 (37.9%) | 55 (38.7%) |
| HPV 31/33/45-positive | 19 (11.2%) | 24 (16.9%) |
| Other oncogenic-positive | 33 (19.5%) | 36 (25.4%) |
| Nononcogenic-positive | 19 (11.2%) | 14 (9.9%) |
HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Given the high efficacy of the HPV-16/18 vaccine against new infections and associated lesions, women in the HPV arm for this analytical cohort were relatively more likely to have a LEEP early in the study to treat a condition that was prevalent at enrollment and conversely less likely to have a LEEP later in the study to treat an incident condition arising from an infection initiating after vaccination (23.9% and 39.6% for HPV arm and Control arm had LEEP performed to treat lesions caused by incident infections, respectively). As a result, the median number of months between enrollment and treatment was 28.2 for those in the HPV arm (interquartile range 15.2–44.6 months) and 38.1 for women in the control arm (interquartile range 22.4–50.5 months). We also observed a greater proportion of women in the HPV arm (compared with the control arm) who reported 2+ partners and who had an abnormal cytology at enrollment, and a lower proportion of women in the HPVarm (compared with the control arm) who were HPV DNA negative at enrollment (Table 2).
We chose to use an infection rather than a woman as the unit of analysis because of our interest in the fate of individual HPV infections. A total of 31.4% of infected women had more than 1 HPV type at enrollment. We evaluated the following HPV categories: HPV-16/18 (HPV-16 and/or HPV-18—the primary vaccine types), HPV-31/33/45 (HPV-31 and/or HPV-33 and/or HPV-45—HPV types for which evidence for cross-protection has been documented), and oncogenic types (any of the 12 oncogenic HPV types).
For evaluation of infections and lesions occurring among women infected at entry, we considered the following outcomes: type-specific viral clearance and development of histologically confirmed cervical lesions (CIN1+ and CIN2+). In a subanalysis restricted to infected women with normal cytology, we also considered as an outcome the development of cytologically evident lesions (low-grade squamous intra-epithelial lesion+ and high-grade squamous intraepithelial lesion+).
For the evaluation of women treated by LEEP, we considered the following outcomes after LEEP: HPV infection, persistent HPV infection (defined as detection of type-specific HPV at 2 or more consecutive visits after treatment), squamous intraepithelial lesion (SIL), and CIN2+. We also conducted sub-analyses that considered outcomes associated with HPV types present and absent before treatment, separately. Sensitivity analyses were performed restricted to women whose LEEP was performed to treat conditions prevalent at enrollment, to ensure that findings were not driven by the differential proportion of incident outcomes observed between study arms. As results from these sensitivity analyses were similar to those observed for our main analyses, they are not presented further.
Vaccine efficacy, a measure of the percent reduction (or increase) in outcome rates observed when the HPV arm is compared against the control arm, was computed, along with the corresponding 95% confidence interval (95% CI) around vaccine efficacy estimates. The generalized estimating equations method was used to account for possible lack of independence between clearance in analysis of more than one infection in the same woman. The estimates of rates of infection that clear from the generalized estimating equations analysis can, therefore, be slightly different from the crude percentages. We present cumulative vaccine efficacy against persistence for several HPV categories overall and restricted to women who received all vaccine doses and to those with evidence of a single HPV type at entry. In addition to cumulative analyses, curves depicting the fate of prevalent infections over time were also developed.
Results
Impact of HPV-16/18 vaccination on infections prevalent at vaccination
We first evaluated whether rates of viral clearance and/or progression to cervical preneoplastic lesions differed by vaccination status. Results are summarized in Table 3. We found no evidence that HPV-16/18 vaccination altered the fate of an HPV infection present at the time of vaccination. When we evaluated HPV-16/18 infections, for example, vaccine efficacy was −5.4% (95% CI −19, 10) for clearance, −15.5% (95% CI −86, 28) for progression to CIN1+, and 0.3% (95% CI −69, 41) for progression to CIN2+ in the cohort of women with HPV infection but without precancer. A similar lack of vaccination effect was observed when we evaluated infections with HPV-31/33/45 (HPV types for which evidence for partial cross-protection against incident infections and lesions was previously demonstrated) or infections with any oncogenic HPV. These results were consistent when the more restricted cohort of women without cytologic abnormalities at vaccination was evaluated, and among this more restricted cohort when progression to low-grade or high-grade SIL was evaluated. Time-dependent curves depicting the fate of prevalent infections are provided as Supplemental Figures 1 and 2 and, again, do not suggest any measurable effect of vaccination on either clearance or progression of prevalent infections.
TABLE 3.
Impact of HPV-16/18 vaccination on infections prevalent at vaccination
| HPV Types | Cohort | Outcome | Arm | No. infections | No. events | No. person-years | Rate per 1000 | % vaccine efficacy | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| HPV-16/18 | Infected women without precancer | Clearance | HPV | 268 | 243 | 492 | 489 | −5.4% | [−19, 10] |
| Ctrl | 313 | 298 | 569 | 517 | |||||
| CIN1+ | HPV | 244 | 32 | 1200 | 28.0 | −15.5% | [−86, 28] | ||
| Ctrl | 295 | 35 | 1427 | 24.2 | |||||
| CIN2+ | HPV | 258 | 25 | 1301 | 20.2 | 0.3% | [−69, 41] | ||
| Ctrl | 308 | 31 | 1504 | 20.3 | |||||
| Infected women without cytologic abnormalities | Clearance | HPV | 173 | 160 | 326 | 481 | −3.3% | [−19, 16] | |
| Ctrl | 216 | 208 | 403 | 497 | |||||
| LSIL+ | HPV | 171 | 62 | 642 | 77.2 | −3.5% | [−85, 42] | ||
| Ctrl | 216 | 71 | 861 | 74.6 | |||||
| HSIL+a | HPV | 161 | 23 | 744 | 30.9 | −48.6% | [−172, 19] | ||
| Ctrl | 200 | 20 | 961 | 20.8 | |||||
| CIN1+ | HPV | 171 | 23 | 835 | 28.5 | 5.5% | [−60, 44] | ||
| Ctrl | 214 | 32 | 1035 | 30.2 | |||||
| CIN2+ | HPV | 171 | 17 | 849 | 20.7 | 22.6% | [−40, 57] | ||
| Ctrl | 215 | 29 | 1050 | 26.8 | |||||
| HPV-31/33/45 | Infected women without precancer | Clearance | HPV | 214 | 206 | 323 | 617 | 4.0% | [−13, 24] |
| Ctrl | 222 | 211 | 348 | 594 | |||||
| CIN1+ | HPV | 201 | 17 | 938 | 18.3 | 14.0% | [−62, 54] | ||
| Ctrl | 210 | 22 | 1020 | 21.3 | |||||
| CIN2+ | HPV | 208 | 14 | 986 | 14.4 | 17.4% | [−65, 59] | ||
| Ctrl | 218 | 19 | 1070 | 17.4 | |||||
| Infected women without cytological abnormalities | Clearance | HPV | 144 | 138 | 219 | 617 | 9.3% | [−12, 35] | |
| Ctrl | 159 | 151 | 261 | 564 | |||||
| LSIL+ | HPV | 144 | 32 | 578 | 52.4 | −4.2% | [−79, 39] | ||
| Ctrl | 158 | 41 | 662 | 50.3 | |||||
| HSIL+ | HPV | 144 | 13 | 653 | 20.1 | 4.2% | [−101, 54] | ||
| Ctrl | 158 | 17 | 749 | 21 | |||||
| CIN1+ | HPV | 144 | 14 | 656 | 21.5 | 17.6% | [−63, 58] | ||
| Ctrl | 157 | 20 | 753 | 26.1 | |||||
| CIN2+ | HPV | 144 | 12 | 663 | 18.2 | 15.7% | [−76, 60] | ||
| Ctrl | 157 | 17 | 763 | 21.6 | |||||
| Oncogenic HPV | Infected women without precancer | Clearance | HPV | 1198 | 1149 | 1761 | 599 | 4.8% | [−3.7, 14] |
| Ctrl | 1236 | 1184 | 1881 | 571 | |||||
| CIN1+ | HPV | 1110 | 83 | 5487 | 14.0 | −0.9% | [−42, 28] | ||
| Ctrl | 1149 | 89 | 5646 | 13.9 | |||||
| CIN2+ | HPV | 1160 | 62 | 5817 | 9.6 | 3.1% | [−45, 35] | ||
| Ctrl | 1208 | 68 | 6004 | 9.9 | |||||
| Infected women without cytologic abnormalities | Clearance | HPV | 788 | 758 | 1195 | 575 | 1.0% | [−8.8, 12] | |
| Ctrl | 854 | 822 | 1311 | 569 | |||||
| LSIL+ | HPV | 779 | 191 | 3242 | 45.2 | −10.7% | [−45, 16] | ||
| Ctrl | 847 | 197 | 3615 | 40.8 | |||||
| HSIL+ | HPV | 779 | 64 | 3750 | 14.3 | −19.2% | [−84, 23] | ||
| Ctrl | 847 | 62 | 4128 | 12 | |||||
| CIN1+ | HPV | 779 | 62 | 3814 | 15.5 | 6.7% | [−35, 35] | ||
| Ctrl | 842 | 76 | 4130 | 16.6 | |||||
| CIN2+ | HPV | 779 | 46 | 3862 | 10.8 | 15.0% | [−31, 45] | ||
| Ctrl | 845 | 59 | 4188 | 12.8 |
CI, confidence interval; CIN1, cervical intraepithelial neoplasia type 1; CIN2, cervical intraepithelial neoplasia type 2; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Excludes 5 individuals from the HPV arm and 8 individuals from the control arm with both HPV16 and HPV18 infections to allow for model conversion.
Impact of HPV-16/18 vaccination on rate of HPV infections and cervical lesions after LEEP
Next, we evaluated whether rates of cervical infections and lesions after excisional treatment by LEEP differed by vaccination status. Overall, 34.1% of the 311 women included in this analysis had 1+ oncogenic HPV infections and 1.6% had CIN2+ detected after treatment, respectively. Among this subset, 69.8% of infection and 20.0% of CIN2+ lesions were the result of new HPV infections absent before treatment. This finding highlights the effectiveness of LEEP as a treatment for cervical precancers and the fact that disease recurrence is a rare event after treatment. We observed no evidence for a significant effect of vaccination when we evaluated HPV-16/18 infections and/or HPV-16/18 associated cytologic/histologic lesions after LEEP (Table 4). We observed no evidence for a positive vaccine efficacy for HPV-16/18 events when cytologic/histologic outcomes were evaluated. Similar patterns were observed when we evaluated HPV-31/33/45, oncogenic HPV, and outcomes irrespective of HPV type, with the exception of a significant vaccine efficacy observed for any HPV-31/33/45 infection outcome (vaccine efficacy 56.9%; 95% CI 8.2, 80) (Table 4).
TABLE 4.
Impact of HPV-16/18 vaccination on recurrence of HPV infections and cervical lesions after LEEP
| HPV types | Outcome | Arm | Overall analysis
|
Analysis restricted to newly detected HPV infections after treatment
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. events (# Prevalent/ # Incident) | No. person-years | Rate per 1000 | % vaccine efficacy | 95% CI | Rate per 1000 | % vaccine efficacy (incident) | 95% CI (incident) | |||
| HPV-16/18 | HPV infection | HPV | 12 (8/4) | 678 | 18.2 | 25.2% | [−58, 65] | 7.6 | 57.9% | [−43, 88] |
|
| ||||||||||
| Ctrl | 16 (8/8) | 664 | 24.4 | 18.0 | ||||||
|
| ||||||||||
| Persistent HPV | HPV | 4 (4/0) | 689 | 5.9 | 34.7% | [−131, 82] | 0.0 | 100.0% | N/A | |
|
| ||||||||||
| Ctrl | 6 (4/2) | 665 | 9.1 | 4.5 | ||||||
|
| ||||||||||
| LSIL+ | HPV | 4 (4/0) | 302 | 13.2 | −8.0% | [−339, 73] | 0.0 | 100.0% | N/A | |
|
| ||||||||||
| Ctrl | 4 (2/2) | 326 | 12.3 | 6.1 | ||||||
|
| ||||||||||
| HSIL+ | HPV | 3 (3/0) | 348 | 8.6 | −58.0% | [−857, 74] | 0.0 | 100.0% | N/A | |
|
| ||||||||||
| Ctrl | 2 (1/1) | 367 | 5.4 | 2.7 | ||||||
|
| ||||||||||
| CIN2+ | HPV | 3 (3/0) | 362 | 8.3 | −211.0% | [−2901, 68] | 0.0 | N/A | N/A | |
|
| ||||||||||
| Ctrl | 1 (1/0) | 375 | 2.7 | 0.0 | ||||||
|
| ||||||||||
| HPV-31/33/45 | HPV infection | HPV | 9 (4/5) | 1038 | 9.1 | 56.9% | [8.2, 80] | 5.7 | 72.9% | [29, 90] |
|
| ||||||||||
| Ctrl | 20 (3/17) | 989 | 21.1 | 21.0 | ||||||
|
| ||||||||||
| Persistent HPV | HPV | 2 (2/0) | 1049 | 2.0 | 76.0% | [−11, 95] | 0.0 | 100.0% | N/A | |
|
| ||||||||||
| Ctrl | 8 (1/7) | 1000 | 8.2 | 8.6 | ||||||
|
| ||||||||||
| LSIL+ | HPV | 5 (3/2) | 302 | 16.5 | −8.0% | [−275, 69] | 6.6 | 46.0% | [−194, 90] | |
|
| ||||||||||
| Ctrl | 5 (1/4) | 326 | 15.3 | 12.3 | ||||||
|
| ||||||||||
| HSIL+ | HPV | 3 (3/0) | 348 | 8.6 | 0.0 | N/A | N/A | |||
|
| ||||||||||
| Ctrl | 0 (0/0) | 367 | 0.0 | 0.0 | ||||||
|
| ||||||||||
| CIN2+ | HPV | 0 (0/0) | 362 | 0.0 | 0.0 | N/A | N/A | |||
|
| ||||||||||
| Ctrl | 0 (0/0) | 375 | 0.0 | 0.0 | ||||||
|
| ||||||||||
| Oncogenic HPV | HPV infection | HPV | 71 (25/46) | 4097 | 14.1 | 24.3% | [−7.7, 47] | 11.2 | 36.7% | [1.5, 59] |
|
| ||||||||||
| Ctrl | 85 (20/65) | 3969 | 18.7 | 17.7 | ||||||
|
| ||||||||||
| Persistent HPV | HPV | 22 (14/8) | 4156 | 4.7 | 31.7% | [−19, 61] | 2.2 | 65.5% | [23, 85] | |
|
| ||||||||||
| Ctrl | 30 (9/21) | 4001 | 6.9 | 6.5 | ||||||
|
| ||||||||||
| LSIL+ | HPV | 19 (10/9) | 302 | 62.9 | −57.9% | [−225, 23] | 29.8 | 2.8% | [−141, 61] | |
|
| ||||||||||
| Ctrl | 13 (3/10) | 326 | 39.8 | 30.6 | ||||||
|
| ||||||||||
| HSIL+ | HPV | 7 (6/1) | 348 | 20.1 | −145.8% | [−859, 37] | 2.9 | 47.3% | [−480, 95] | |
|
| ||||||||||
| Ctrl | 3 (1/2) | 367 | 8.2 | 5.4 | ||||||
|
| ||||||||||
| CIN2+ | HPV | 3 (3/0) | 362 | 8.3 | −55.5% | [−834, 74] | 0.0 | 100.0% | N/A | |
|
| ||||||||||
| Ctrl | 2 (1/1) | 375 | 5.3 | 2.7 | ||||||
|
| ||||||||||
| Irrespective of HPV | LSIL+ | HPV | 33 | 302 | 109.2 | −15.0% | [−89, 30] | |||
|
| ||||||||||
| Ctrl | 31 | 326 | 95.0 | |||||||
|
| ||||||||||
| HSIL+ | HPV | 10 | 348 | 28.7 | −163.3% | [−742, 18] | ||||
|
| ||||||||||
| Ctrl | 4 | 367 | 10.9 | |||||||
|
| ||||||||||
| CIN2+ | HPV | 3 | 362 | 8.3 | −55.5% | [−834, 74] | ||||
|
| ||||||||||
| Ctrl | 2 | 375 | 5.3 | |||||||
CI, confidence interval; CIN2, cervical intraepithelial neoplasia type 2; Ctrl, control; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LEEP, loop electrosurgical excisional procedure; LSIL, low-grade squamous intraepithelial lesion; N/A, not available.
Because the HPV-16/18 vaccine has been proven to protect against new HPV infections and their associated lesions, we next evaluated whether there was evidence for vaccine efficacy against infection and cytologic/histologic lesions after treatment for infections that were newly detected after treatment (ie, excluding infections and lesions associated with infections that were evident before treatment) (Table 4; Supplemental Tables 1 and 2). Although numbers and statistical power were limiting, vaccine efficacy estimates were consistently positive in this restricted analysis. In some instances, the vaccine efficacy observed reached statistical significance. When HPV infection was examined as the outcome, for example, we observed a nonsignificant but positive vaccine efficacy estimate of 57.9% (95% CI −43, 88) for HPV-16/18, a significant positive vaccine efficacy estimate of 72.9% (95% CI 29, 90) for HPV-31/33/45, and a significant positive vaccine efficacy estimate of 36.7% (95% CI 1.5, 59) for oncogenic HPVs.
Discussion
The ability of HPV virus-like particle vaccines to prevent new infections and their associated lesions has been clearly demonstrated.1–3 This high prophylactic efficacy is likely explained by the induction of neutralizing antibodies that protect against infection by binding to virions and preventing them from infecting human cells.4 Whether HPV vaccination benefits individuals already infected with HPV is less well understood. Initial reports suggested that vaccination of women already infected with HPV did not affect the fate of detectable infections,6–8 but more recent reports have raised the possibility of benefit in another group of women with a history of infection—women who receive treatment for HPV-associated disease.9–11 To what extent vaccination helps prevent infection and disease after treatment and whether any benefit of vaccination in this group of women is explained by prevention of new infections after treatment vs protection against recurrence of already existing infections has not been determined.
To clarify these issues, we conducted a comprehensive evaluation within a large randomized clinical trial of young adult women in Costa Rica in which we evaluated both the impact of vaccination on prevalent infection and the effect of vaccination on post-treatment infections/disease. Our results reinforce the notion that HPV vaccination is prophylactic and does not provide secondary benefit. First, we confirmed that there are no differences in rates of viral clearance or progression among women infected at the time of vaccination. Second, we report no evidence for a reduction in the rates of post-LEEP infections and/or disease after vaccination. Additional analyses did suggest one subgroup of women that might benefit from vaccination—women exposed to new HPV infections after treatment. For this subgroup, our evaluation observed significant vaccine efficacy against the development of new infections associated with HPV-31/33/45 and oncogenic infections, and nonsignificant but suggestive evidence for vaccine efficacy against new infections associated with HPV-16/18. We were unable to convincingly demonstrate similar protection against cervical lesions (SIL and CIN2+) associated with these new HPV infections, given the small number of such events in our study; nonetheless, vaccine efficacy estimates were suggestive of protection against lesions caused by new HPV infections after treatment, a topic that deserves consideration in future studies. It warrants mention that, consistent with the high curative rate of LEEP, our evaluation highlights that rates of cervical disease after treatment are low and that only a small fraction of this disease is caused by new infections that were not already evident at the time of treatment. This finding suggests that, in contrast to the proven benefit of vaccinating young adolescents to prevent initial HPV infection and subsequent disease, any potential impact of vaccination targeting women who either have a detectable HPV infection and/or receive treatment for HPV-associated disease is likely small at best.
Strengths of our study include the random assignment of women into study arms, its community-based design, the large size of our cohort (7466 women total), and the blinded, active follow-up of participants over 4 years after vaccination. Limitations of our study include the modest number of women who required treatment for cervical precancer during the study period and the imbalances by study arm created during follow-up due to the high prophylactic efficacy of the vaccine and the resultant smaller proportion of women who required treatment for incident disease on the HPV compared to control arm of the study. This latter limitation is mitigated by the fact that sensitivity analyses restricted to women treated for precancer lesions identified at enrollment (ie, at randomization) yielded similar results.
In summary, our analysis supports a lack of secondary/therapeutic benefit from HPV vaccination and indicates that benefit of vaccinating women who receive treatment for HPV-associated disease suggested by previous reports is likely limited to the small number of women with disease caused by new HPV infections after treatment.
Supplementary Material
Acknowledgments
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project; we would like to specifically acknowledge the meaningful contributions by Loreto Carvajal, Rebecca Ocampo, Cristian Montero, Diego Guillen, Jorge Morales, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS), responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr. Diane Solomon (CVT: medical monitor and QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for the expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Amber D’Souza, Richard Roden, and Peter Gilbert).
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study. John T. Schiller and Douglas R. Lowy report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Registered with Clinicaltrials.gov NCT00128661.
Appendix
Investigators in the Costa Rica Vaccine Trial (CVT) group
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica—Bernal Cortés (specimen and repository manager), Paula González (co-investigator), Roland Herrero (co-principal investigator), Silvia E. Jiménez (trial coordinator), Carolina Porras (co-investigator), Ana Cecilia Rodríguez (co-investigator).
United States National Cancer Institute, Bethesda, MD—Allan Hildesheim (co-principal investigator & NCI co-project officer), Aimée R. Kreimer (co-investigator), Douglas R. Lowy (HPV virologist), Mark Schiffman (medical monitor & NCI co-project officer), John T. Schiller (HPV virologist), Mark Sherman (QC pathologist), Sholom Wacholder (statistician).
Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD—Ligia Pinto (head, HPV immunology laboratory), Troy Kemp (immunologist).
Georgetown University, Washington, DC—Mary Sidawy (histopathologist).
DDL Diagnostic Laboratory, Netherlands—Wim Quint (virologist, HPV DNA testing), Leen-Jan van Doorn (HPV DNA testing).
University of California, San Francisco, CA—Joel M. Palefsky (expert on anal HPV infection and disease diagnosis and management), Teresa M. Darragh (pathologist and clinical management).
University of Virginia, Charlottesville, VA—Mark H. Stoler (QC pathologist).
Footnotes
The authors report no conflict of interest.
References
- 1.Kjaer SK, Sigurdsson K, Iversen OE, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res. 2009;2:868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 2.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PAT-RICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 3.Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 Vaccine: Final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 Vaccine Trial. Vaccine. 2014;32:5087–97. doi: 10.1016/j.vaccine.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10:681–92. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bruijn MLH, Greenstone HL, Vermeulen H, et al. L1-specific protection from tumor challenge elicited by HPV16 virus-like particles. Virology. 1998;250:371–6. doi: 10.1006/viro.1998.9372. [DOI] [PubMed] [Google Scholar]
- 6.Hildesheim A, Herrero R, Wacholder W, et al. Effect of human papillomavirus 16/18 L1 virus-like particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 7.Haupt RM, Wheeler CM, Brown DR, et al. Impact of an HPV6/11/16/18 L1 virus-like particle vaccine on progression to cervical intraepithelial neoplasia in seropositive women with HPV16/18 infection. Int J Cancer. 2011;129:2632–42. doi: 10.1002/ijc.25940. [DOI] [PubMed] [Google Scholar]
- 8.Szarewski A, Poppe WAJ, Skinner SR, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131:106–16. doi: 10.1002/ijc.26362. [DOI] [PubMed] [Google Scholar]
- 9.Joura EA, Garland SM, Paavonen J, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after LEEP effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013;130:264–8. doi: 10.1016/j.ygyno.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papilloma-virus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891–8. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 12.Herrero R, Herrero R, Hildesheim A, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2006;26:4795–808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayar R, Solomon D. Second edition of ‘The Bethesda System for reporting cervical cytology’ – atlas, website, and Bethesda interobserver reproducibility project. Cytojournal. 2004;1:4. doi: 10.1186/1742-6413-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst. 2000;92:397–402. doi: 10.1093/jnci/92.5.397. [DOI] [PubMed] [Google Scholar]
- 15.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37:2508–17. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153:1731–9. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:3292–8. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.