Abstract
Arsenic is a human carcinogen, and also increases risk for non-cancer outcomes. Arsenic-induced epigenetic dysregulation may contribute to arsenic toxicity. Although there are several reviews on arsenic and epigenetics, these have largely focused on DNA methylation. Here, we review investigations of the effects of arsenic on global levels of histone posttranslational modifications (PTMs). Multiple studies have observed that arsenic induces higher levels of H3 lysine 9 dimethylation (H3K9me2), and also higher levels of H3 serine 10 phosphorylation (H3S10ph), which regulates chromosome segregation. In contrast, arsenic causes a global loss of H4K16ac, a histone PTM that is a hallmark of human cancers. Although the findings for other histone PTMs have not been entirely consistent across studies, we discuss biological factors which may contribute to these inconsistencies, including differences in the dose, duration, and type of arsenic species examined; the tissue or cell line evaluated; differences by sex; and exposure timing. We also discuss two important considerations for the measurement of histone PTMs: proteolytic cleavage of histones and arsenic-induced alterations in histone expression.
Keywords: Arsenic, posttranslational histone modifications
Introduction
Arsenic Exposure
Inorganic arsenic (InAs) has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer [1]. Exposure to InAs has also been associated with numerous non-cancer health outcomes, including cardiovascular disease, nonmalignant lung disease, and neurodevelopmental outcomes (reviewed in [2]). The primary source of exposure is naturally contaminated drinking water [2]. More than 200 million individuals are exposed to arsenic concentrations which exceed the World Health Organization guideline for safe drinking water, which is 10 µg/L [3]. Dietary arsenic is also a major source of exposure and contributes to a larger fraction of total arsenic exposure when concentrations in drinking water are low, e.g., <50 [4] or <10 µg/L [5]. InAs is metabolized via two sequential methylation reactions, yielding monomethylarsonous acid (MMA) and dimethylarsinic acid (DMA), respectively [6]. The trivalent forms (MMAIII and DMAIII) are the most cytotoxic (reviewed in [7]). However, DMAIII is highly unstable, and it is unclear if this metabolite is present in large quantities in vivo [8].
Although eliminating exposure to arsenic remains the primary public health strategy, cancer risks remain elevated decades after arsenic exposure has been reduced [9]. However, the mechanisms underlying this are unclear and likely multifactorial. While arsenic is not a traditional mutagen [10], there is increasing evidence that arsenic induces epigenetic dysregulation, including alterations in both DNA methylation and histone posttranslational modifications (PTMs) (previously reviewed in [11]), which has been implicated in cancers and other adverse health outcomes [12]. There is also interest in the molecular actions of arsenic, because arsenic trioxide (As2O3), in combination with all-trans retinoic acid, is currently the standard of care for acute promyelocytic leukemia (APL) [13].
Arsenic and Epigenetics
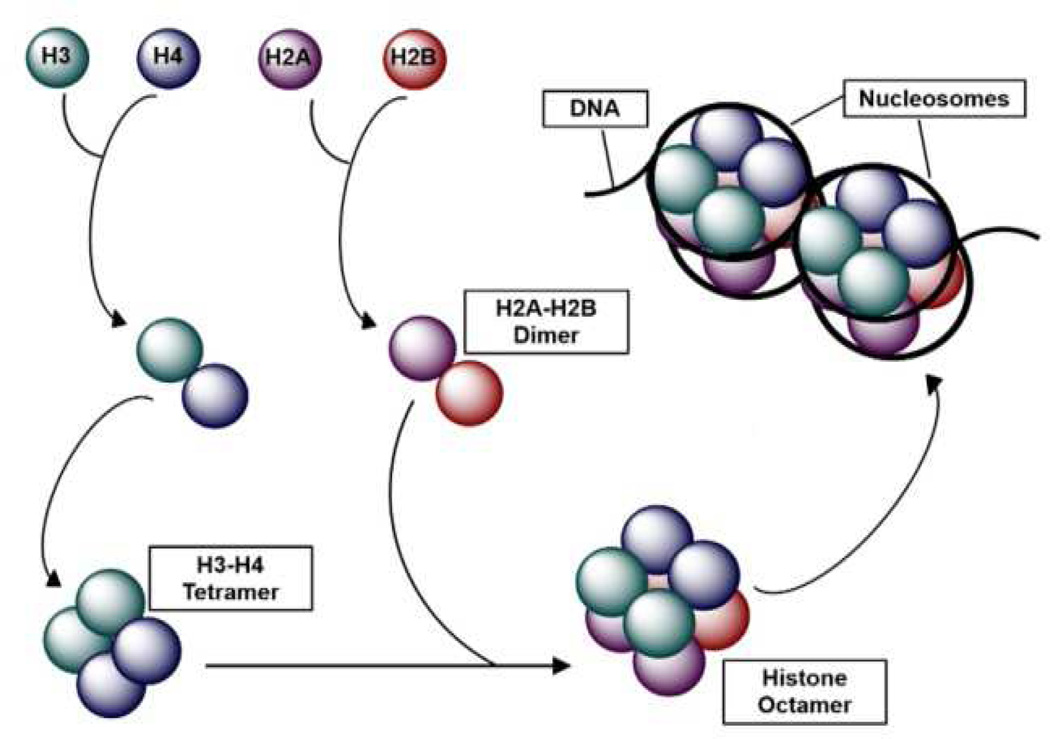
Epigenetics is defined as the study of stable and heritable changes in gene expression or cellular phenotypes that occur without changes to the underlying DNA sequence [14]. Genomic DNA is approximately 2 m in length and must fit inside the nucleus, which on average has a diameter of 6 µm [12]. This is accomplished by packaging DNA into chromatin. The major unit of chromatin is the nucleosome, which is composed of approximately 146 base pairs of DNA, wrapped 1.65 times around a histone protein octamer, consisting of two copies of the four canonical core histones: H2A, H2B, H3, and H4 (Figure 1) [15]. A fifth histone (H1), known as the linker histone, helps to stabilize the nucleosome and facilitates the folding of nucleosomes into higher-order structures [15]. Together, several types of epigenetic modifications regulate the accessibility of DNA for different biological processes, such as gene transcription, DNA replication, and DNA repair. The best-studied modification is DNA methylation [16]]. Histone proteins can also be modified, and these PTMs interact with DNA methylation to influence DNA accessibility [16].
Figure 1.
Nucleosome structure. The basic unit of chromatin is the nucleosome, which is composed of 147 base pairs of DNA wrapped around a histone octamer. The histone octamer is comprised of two copies of each of the four core histone proteins: H2A, H2B, H3, and H4. Histones H2A and H2B form two dimers, while two copies of histones H3 and H4 form a tetramer. (Adapted from [107] by permission from Macmillan Publishers Ltd.)
Histones have two major domains: a globular core and a long, unstructured N-terminal tail. Although numerous globular core PTMs have been identified by mass spectrometry, PTMs in the N-terminal tails have been more thoroughly studied [17]. This is largely due to the fact that histone PTMs were first identified by Edman degradation, a peptide sequencing technique which is limited to measuring the first 20 to 30 amino acids from the N-terminus [17]. The nomenclature of histone PTMs is based on the histone; the type of amino acid (indicated by its single letter abbreviation); the position of the modified amino acid in relation to the N-terminus (indicated by a number); the type of modification present; and, in the case of lysine methylation, the number of methyl groups present (0, 1, 2, or 3). Seventeen different types of PTMs on more than 30 amino acids have been identified for human H3 alone [18]. To date, the best described modifications include the acetylation (ac) and methylation (me) of lysine residues (K), and the phosphorylation (ph) of serine residues (S), particularly on H3 and H4. Histone PTMs can alter chromatin structure through both direct and indirect mechanisms. Since ac and ph groups are negatively charged, the addition of these moieties reduces the affinity of the positively charged histones for the negatively charged DNA, leading to a more open chromatin conformation [19]. Originally, it was proposed that the combinatorial patterns of simultaneously occurring PTMs formed a “histone code” [20]. More recently, a “language of histone marks” has been proposed, which is probably more appropriate given that there is significant crosstalk between distinct PTMs on the same, or different, core histones, which is important for the regulation of gene transcription and DNA damage repair (reviewed recently in [21].
PTMs of histones can also indirectly alter chromatin structure by recruiting chromatin modifiers [19]. Since methyl groups have a neutral charge, histone methylation largely influences chromatin by recruiting or blocking the binding of chromatin modifiers, and each methylation state (i.e., me1, me2, or me3) can have distinct effects [19]. Gene-specific levels of histone PTMs are highly dynamic as they play active roles in regulating gene transcription. In contrast, global levels of histone PTMs are thought to be relatively stable over time. For example, global levels of histone PTMs remain unchanged in mouse mesenchymal stem cells during adipogenesis, despite alterations at specific genes [22]. Also, global levels of several histone PTMs measured in peripheral blood mononuclear cells (PBMCs) from human participants did not fluctuate measurably across three time points measured one week apart [23].
There have been several comprehensive reviews on arsenic and epigenetics that focus primarily on DNA methylation [11, 24–27]. Here we summarize the growing literature on arsenic and global levels of histone PTMs and discuss some of the major challenges in the field.
Influences of arsenic on global histone PTMs
The majority of studies on arsenic and histone PTMs have been conducted in vitro [28–47]. However, there are a few supporting studies in rodents [48, 49] and human populations [50–54]. Collectively, these studies provide evidence that arsenic alters many different PTMs at the global level. Details of each study are summarized in Table 1 and are also described below.
Table 1.
Summary of studies examining the effects of arsenic on global levels of histone PTMs
| Reference | Cell Line, Mouse Strain, or Population |
Sexa | Exposure; Doseb |
Dose converted to µg/Lc |
Duration | Technique | PTMs examined |
Findings |
|---|---|---|---|---|---|---|---|---|
| Cell Culture Studies | ||||||||
| Arrigo (1983) [28] |
Kc 161 (D. Melanogaster, embryonic) |
? | NaAsO2 (AsIII); 50 µM |
6496 µg/L | 4 hours | Radiolabeling | H3, H4, H2A, H2B methylation and acetylation |
Methylation: ↓H3, H4, ↑H2B Acetylation: ↓H3, H4, H2A, H2B |
| Desrosiers and Tanguay (1986) [29] |
Schneider and Kc III cells (D. Melanogaster, embryonic) |
? | NaAsO2 (AsIII); 50 µM |
6496 µg/L | 4 hours | Radiolabeling | H3, H4, and H2B methylation and acetylation |
Methylation: ↓H3, H4, ↑H2B Acetylation: ↓H3, H4, H2B |
| Cobo et al. (1995) [46] |
CHO (Chinese hamster, ovary) |
F | NaAsO2 (AsIII); 10 µM |
1299 µg/L | 2 hours | Radiolabeling | H1, H2A, H3, H4 phosphorylation |
Phosphorylation: ↓H1 and H3 No effects on H2A or H4 |
| Perkins et al. (2000) [37] |
HL-60 (human, APL) and K562 (human, CML) |
F | As2O3 (AsIII); 1, 2 µM/2 µM |
198, 396 µg/L /396 µg/L |
7 days/24 hours |
Western blotting |
H3 and H4 acetylation |
↑Acetylation for all doses and durations |
| Li et al. (2002) [30] |
NB4 (human, APL) |
F | As2O3 (AsIII); 0.4, 0.8, 1.6 µM |
79, 158, 317 µg/L |
24 hours | Western blotting |
H3S10PhK14ac, H3S10ph, H3K14ac, H3K9acK14ac |
0.8 and 1.6 µM ↑H3S10ph and H3S10phK14ac No effect on H3K14ac or H3K9acK14ac |
| Kannan- Thulasiraman et al. (2006) [45] |
KT-1 (human, CML)/ NB4 (human, APL) |
M/F | As2O3 (AsIII); 2 µM |
396 µg/L | 20 minutes | Western blotting |
H3S10ph | ↑H3S10ph |
| Ramirez et al. (2008) [31] |
HepG2 (human, liver cancer) |
M | NaAsO2 (AsIII); 7.5 µM |
974 µg/L | 24 hours | Western blotting |
H3K4me2, me3, H3K9ac, H3K9me2, me3, H3K27me3, H4K20me3 |
↑H3K9ac No effects on methylation marks |
| Zhou et al. (2008) [32] |
A549 (human, lung cancer)/BEAS-2B (human, healthy lung, SV40- transformed) |
M/M | NaAsO2 (AsIII); 2.5, 5 µM/1, 2 µM |
325, 650 µg/L/130, 260 µg/L |
24 hours | Western blotting |
H3K4me1, me2, me3, H3K9me, me2, me3 H3K27me3, H3K36me2, me3/ H3K9me2 |
↑H3K9me2, me3, no effect on H3K9me1, ↑H3K4me2, me3, ↓H3K4me1, ↓H3K27me3, ↑H3K36me3, ↓H3K36me2/↑H3K9me2 |
| Zhou et al. (2009) [33] |
A549 (human, lung cancer) |
M | NaAsO2 (AsIII); 1, 5 µM |
130, 650 µg/L | 24 hours | Western blotting |
H3K4me1, me2, me3 |
↑H3K4me2, me3 ↓H3K4me1 |
| Jo et al. (2009) [40] |
UROTsa (human, healthy urothelium, SV40- transformed) |
F | NaAsO2 (AsIII); 3 µM MMAIIIO (MMAIII); 1 µM |
390 µg/L | 7 days | Western blotting |
H4K16ac | ↓H4K16ac for both AsIII and MMAIII |
| Suzuki et al. (2009) [42] |
HepG2 (human, liver cancer) |
M | NaAsO2 (AsIII); 60 µM C2H7AsI (DMAIII); 0.5 µM |
7795 µg/L | 0.5, 1.5, 3, 5, 7 hours |
Western blotting |
H3S10ph | ↑H3S10ph |
| Chu et al. (2011) [34] |
UROTsa (human, healthy urothelium, SV40- transformed) |
F | NaAsO2 (AsIII); 1 nM, 3 and 10 µM/ MMAIIIO (MMAIII); 0.3, 1, 3 µM |
0.1, 390, 1299 µg/L |
24 hours, 7 days |
Mass spectrometry |
H3 and H4 acetylation |
10 µM AsIII ↓H4K16ac, ↓H3 acetylation/ 3 µM MMAIII ↓H4K16ac, ↓H3 acetylation |
| Treas et al. (2012) [39] |
RWPE1 (human, healthy prostate) |
M | NaAsO2 (AsIII); 100 pg/mL +/− E2, 100 ng/mL +/− E2 |
0.1, 100 µg/L | Every 6 days for 6 months |
Western blotting |
H3ac, H3K4me3 |
↑H3ac with AsIII or E2 alone, even greater ↑ for AsIII + E2 (100 ng/mL) ↓H3ac for combination of AsIII + E2 (100 pg/mL) ↑H3K4me3 for combination of AsIII + E2 (100 ng/mL) |
| Kim et al. (2012) [41] |
3T3 cells (BALB/c mouse, embryo fibroblasts) |
F | As2O3 (AsIII); 0.5 µM) |
99 µg/L | 2, 4 weeks | Western blotting |
H3K27me3 | ↑H3K27me3 |
| Suzuki et al. (2013) [43] |
HepG2 (human, liver cancer) |
M | As2O3 (AsIII); 0.5 µM) |
99 µg/L | 0.5, 1, 2, 5 hours |
Western blotting |
H3S10ph | ↑H3S10ph |
| Ge et al. (2013) [44] |
UROTsa (human, healthy urothelium, SV40- transformed) |
F | CH3AsI2 (MMAIII); 50 nM |
NA | 12 weeks | Western blotting |
H4K5ac, H4K8ac, H4K12ac, H4K16ac |
↓H4K12ac and H4K16ac No effects on H4K5ac or H4K8ac |
| Herbert et al. (2014) [35] |
Primary human neonatal keratinocytes |
? | Arsenic source unspecified (AsIII); 0.5 µM |
Source unspecified |
1, 12, 24, 48 days |
Western blotting |
H4K16ac | ↑H4K16ac for all durations |
| Liu et al. (2015) [47] |
HeLa (human, cervical cancer)/HEK293T (human, embryonic kidney) |
F/F | As2O3 (AsIII); 0.2–0.8 µM |
40–158 µg/L | 24, 48, 72 hours |
Western blotting |
H4K16ac for both cell lines |
↓H4K16ac in both cell lines |
| Rahman et al. (2015) [36] |
HEK293T (human, embryonic kidney)/UROtsa (human, healthy urothelium, SV40- transformed) |
F/F | As2O3 (AsIII); 1, 5 µM As2O3 (AsIII); 0.5, 2.5 µM |
198, 989/99, 495 µg/L |
72 hours 3 hours |
Western blotting |
H3K9ac, H4K12ac, H4K16ac |
↓H3K9ac (UROTsa only after 72 h, both doses) No effect at shorter duration or for other PTMs |
| Ray et al. (2015) [38] |
HaCaT (human, keratinocytes from healthy skin, SV40- transformed) |
M | NaAsO2 (AsIII); 0, 1, 5, 10, 25 µM/10 µM |
0, 130, 650, 1299, 3248 µg/L/1299 µg/L |
8 hours/0- 24 hours |
Western blotting |
H3S10ph | ↑H3S10ph in dose- and time- dependent manner |
| Ma et al. (2016) [53] |
HEK293T (human, embryonic kidney)/HaCaT (human, keratinocytes from healthy skin, SV40- transformed) |
F/M | NaAsO2 (AsIII); Single treatment: 0, 1.56, 3.13, 6.25 µM/0, 2.5, 5, 10 µM Repeated treatment: 0.3 µM (HEK293T only) |
0, 203, 407, 812 µg/L/0, 325, 650, 1299 µg/L 39 µg/L |
Single treatment: 8 hours/4 hours Single treatment different durations (6.25 µM/10 µM): 0, 2, 4, 8, 12 hours Repeated treatment: 84 days |
Western blotting |
H3K18ac, H3K9me2, H3K36me3 |
Single treatment: ↑H3K18ac in dose-dependent manner ↓H3K36me3 and H3K9me2 in dose- dependent manner (both cell types) Single treatment multiple durations: Initial ↑H3K18ac then decrease Initial ↓H3K9me2 then increase ↓H3K36me3 in time-dependent manner (both cell types) Repeated treatment: ↑H3K18ac after 1 week of treatment and ↓ after that |
| Pournara et al. (2016) [54] |
Jurkat (human, acute T cell leukemia)/CCRF- CEM (human, ALL) |
M/M/F | NaAsO2 (AsIII); 0.1, 1, 100 µg/L |
0.1, 1, 100 µg/L |
48 hours, 72 hours |
Western blotting |
H3K9me3, H3K9ac |
↑H3K9me3 at 1 µg/L and ↑H3K9ac with increasing doses of arsenic, beginning with 0.1 µg/L/↑H3K9me3 at 100 µg/L and ↑H3K9ac with increasing doses of arsenic beginning at 1 µg/L |
| Mouse Studies | ||||||||
| Cronican et al. 2013 [48] |
C57BL6/J (brain, cortex and hippocampus) |
Both, combined |
NaAsO2 (AsIII); 100 µg/L |
100 µg/L | (1 week before conception until birth) |
ChIP-seq | H3K9ac | ↓H3K9ac |
| Tyler et al. 2015 [49]e |
C57BL/6 (brain, dentate gyrus and frontal cortex) |
Both, separate |
Na3AsO4 (AsV); 50 µg/L |
50 µg/L | (10 days prior to pregnancy –weaning) |
Western blotting |
H3K4me3, H3K9ac, H3K9me3 |
M dentate gyrus: ↑H3K4me3 and ↑H3K9ac F dentate gyrus: ↓H3K4me3 and ↓H3K9ac M frontal cortex: ↑H3K4me3 and ↓H3K9ac F frontal cortex: No change in H3K4me3 or H3K9ac No effects on H3K9me3 in either brain region in either sex |
| Population Studies | ||||||||
| Cantone et al. 2011 [50] |
Adults, occupationally exposed via inhalation, Italy (PBLs) (n = 63) |
M | Arsenic in particulate matter (0.01 – 0.31 µg/m3) |
NA | Chronic (years) |
Sandwich ELISA |
H3K4me2, H3K9ac |
↑H3K4me2 |
| Chervona et al. 2012 [51] |
Adults, exposed via contaminated drinking water, Bangladesh (PBMCs) (n = 40, 50% M) |
Both, separate |
Water As (primarily AsIII) (50 – 500 µg/L) |
50–500 µg/L | Chronic (years) |
Sandwich ELISA |
H3K4me3, H3K9ac, H3K9me2, H3K18ac, H3K27ac, H3K27me3 |
Whole sample: ↑H3K9me2, ↓H3K9ac. M: ↓H3K4me3 and H3K27me3. ↑H3K27ac. F: ↑H3K4me3 and H3K27me3. ↓H3K27ac |
| Howe et al. 2016 [52] |
Adults, exposed via contaminated drinking water, Bangladesh (PBMCs) (n = 317, 50% M) |
Both, separate |
uAsCr and bAs | NA | Chronic (years) |
Sandwich ELISA |
H3K36me2, H3K36me3, H3K79me2 |
M: ↑H3K36me2 No significant associations with H3K36me3 or H3K79me2 in either sex |
| Ma et al. 2016 [53] |
Adults with and without arsenicosis, exposed via diet and inhalation due to use of arsenic- contaminated coal for indoor cookstoves, China (lymphocytes) (n = 215, 44% M) |
Both, combined |
uAsCr and hair arsenic |
NA | Chronic (years) |
Sandwich ELISA |
H3K9ac, H3K14ac, H3K18ac, H3K9me2, H3K36me3, H3K79me2 |
↓H3K18ac and H3K9me2 ↑H3K14ac and H3K36me3 |
| Pournara et al. 2015 [54] |
Adults, exposed via contaminated drinking water, Argentina (sorted CD4+ and CD8+ cells) (n = 28) |
F | uAs | NA | Chronic (years) |
Western blotting |
H3K9ac, H3K9me3 |
↓H3K9me3 in CD4+ cells No significant association with H3K9me3 in CD8+ cells No significant association with H3K9ac in either cell type |
Abbreviations used: APL, acute promyelocytic leukemia; ALL, acute lymphoblastic leukemia; bAs, blood arsenic; CML, chronic myelogenous leukemia; E2, estradiol; PBL, peripheral blood leukocyte; PBMC, peripheral blood mononuclear cell; uAs, urinary arsenic; uAsCr, urinary arsenic adjusted for urinary creatinine; wAs, water arsenic
With respect to cell culture studies, sex refers to the biological sex of the animal or patient from which the cell line was derived
Units are reported as they were in the original reference.
Units were only converted to µg/L for inorganic arsenic sources, since these are the predominant arsenic species in drinking water
Total methylation of histone proteins
Two early studies observed that total methylation levels of H2B were increased by 50 µM trivalent InAs (AsIII), while those of H3 and H4 were reduced, in cells derived from Drosophila melanogaster embryonic tissues [28, 29]. These studies used tritiated S-adenosylmethionine or methionine to measure methyl incorporation into histones and thus did not evaluate the methylation states of specific lysine residues, which are regulated by distinct enzymes and often have unique biological roles [19].
Methylation of H3K4
Methylation at H3K4 has been associated with actively transcribed genes [19]. H3K4me1 is a marker which best predicts enhancer regions, while H3K4me3 mainly localizes to transcription start sites [19]. For some genomic regions, all three methylation states of H3K4 show overlapping patterns [55–57]. All three methylation states have been examined in relation to arsenic exposure.
Two studies by Zhou et al. demonstrated that 1–5 µM AsIII reduced global levels of H3K4me1 and increased global levels of H3K4me2 in A549 cells [32, 33], which were originally derived from a male human lung tumor. The finding for H3K4me2 was consistent with an occupational study of male steel workers (n = 63), which observed a positive association between inhalation of arsenic-contaminated particulate matter and H3K4me2, in peripheral blood leukocytes (PBLs) [50]. Zhou et al. also found that global levels of H3K4me3 were increased after exposure to AsIII [32, 33], consistent with a study which utilized RWPE1 cells, which were originally derived from a healthy human prostate [39]. However, the latter finding was only observed when RWPE1 cells were simultaneously exposed to estradiol (E2) and the highest dose of arsenic examined (100 ng/mL) [39]. Interestingly, two studies have also observed that arsenic influences H3K4me3 differentially by sex [49, 51]. In an epidemiological study in Bangladeshi adults (n = 40), our group observed that the association between water arsenic (wAs) exposure (50–500 µg/L) and H3K4me3, in PBMCs, was negative among men, but positive among women [51]. In contrast, 50 µg/L of pentavalent InAs (AsV) increased H3K4me3 in the dentate gyrus and frontal cortex of male mice and reduced this PTM in the dentate gyrus of female mice [49].
Methylation of H3K9
H3K9 methylation has generally been associated with transcriptional repression [58]. There is evidence that H3K9me1 primes certain regions of the genome for heterochromatin formation [58], although this PTM has also been associated with active genes [56]. In contrast, H3K9me3 is important for maintaining heterochromatin and genomic stability [58].
Zhou et al. examined the effects of 1–5 µM AsIII on global levels of all three methylation states of H3K9 in A549 cells [32]. Although H3K9me1 levels were not altered by AsIII, H3K9me2 was increased compared to controls [32]. Similarly, our group observed that wAs exposure was positively associated with H3K9me2 in PBMCs from Bangladeshi men and women [51]. In contrast with these findings, an epidemiological study in China (n = 218) observed a negative association between creatinine-adjusted urinary arsenic (uAsCr) and H3K9me2 in lymphocytes [53]. However, the same group also evaluated the effect of AsIII on H3K9me2 in vitro, using female-derived human embryonic kidney cells (HEK293T) and male-derived human keratinocytes (HaCaT); for both cell lines they observed that AsIII initially reduced H3K9me2, but that longer durations of exposure (8 or 12 hours) increased this PTM, consistent with the findings by Zhou et al. and our epidemiological study in Bangladesh [53].
Similar to their findings for H3K9me2, Zhou et al. observed that AsIII increased H3K9me3 [32]. Another group also observed that AsIII increased H3K9me3 in CD4+ cell lines (Jurkat and CCRF-CEM); these cells were derived from male patients with acute lymphoblastic leukemia [54]. However, the same group observed an inverse association between uAs and H3K9me3, measured in sorted CD4+ cells, in a small epidemiological study of Argentinian women (n = 28) [54] In contrast with these studies, alterations in H3K9me3 were not observed in either the dentate gyrus or the frontal cortex of adult male and female mice exposed to 50 µg/L AsV during the perinatal period [49]. None of these studies investigated the potential mechanisms by which arsenic might alter H3K9me3. However, one group speculated that differences in cell type, dose, or timing of exposure may have contributed to some of the inconsistencies across studies [49].
Methylation of H3K27
H3K27me3 is a repressive mark that is important for gene regulation and X chromosome inactivation [59]. Although several studies have examined the effects of arsenic on H3K27me3, the findings have been inconsistent; this is in spite of the fact that these studies all used antibody-based techniques to measure H3K27me3. One study did not observe that 7.5 µM AsIII altered H3K27me3 in HepG2 cells, which were originally derived from a male human liver tumor [31]. However, Zhou et al. observed that 2.5 and 5 µM µg/L AsIII reduced H3K27me3 in A549 cells [32]. Consistent with this, our group observed an inverse association between wAs exposure and H3K27me3 in PBMCs among Bangladeshi men [51]. In contrast, wAs was positively associated with H3K27me3 among women [51], similar to an in vitro study which utilized female-derived mouse embryonic fibroblasts and observed that 0.5 µM AsIII increased this PTM [41].
Methylation of H3K36
H3K36 methylation is important for both transcriptional activation and transcriptional elongation [60]. There is also evidence that H3K36me2 and H3K36me3 play important roles in DNA repair [61], and that both PTMs are dysregulated in cancers; a global increase in H3K36me2 has been associated with oncogenic programming [62], while a global loss of H3K36me3 has been observed in many cancer types [63].
Although 2.5 and 5 µM AsIII reduced H3K36me2 in A549 cells [32], we recently observed a positive association between uAsCr and H3K36me2, measured in PBMCs, among men in an epidemiological study of Bangladeshi adults (n = 317) [52]; since H3K36me2 antagonizes H3K27me3 [59], this finding was consistent with our previous finding that wAs was inversely associated with H3K27me3 among men from the same population [51]. Similarly, we found that uAsCr was positively, although not significantly, associated with H3K36me3 among Bangladeshi men [52], consistent with a previous finding that AsIII increases H3K36me3 in A549 cells [32]. An epidemiological study of Chinese adults also observed a positive association between uAsCr and H3K36me3, measured in lymphocytes [53]. However, in contrast with their population-based findings, the same group observed that AsIII reduced H3K36me3 in both a dose- and time-dependent manner in two different cell lines (HEK293T and HaCaT) [53].
Methylation of H3K79
H3K79 is located in the globular core domain of histone H3. Of the PTMs located on this residue, only H3K79me2 has been examined in relation to arsenic exposure. This PTM plays important roles in transcriptional activation and elongation [57, 64]. It is also dysregulated in cancers and is currently a promising target for the treatment of MLL-fusion leukemia [65]. Two epidemiological studies in adults, one by our group in Bangladeshi adults and another in Chinese adults, observed null associations between uAsCr and H3K79me2, measured in peripheral blood [52, 53]. This is surprising, given that uAsCr was previously found to be inversely associated with H3K27me3, a PTM that is mutually exclusive with H3K79me2 [66].
H3 and H4 acetylation
The addition of acetyl groups to histone proteins reduces their affinity for DNA, leading to a more open chromatin conformation [67]. Acetylation at specific lysine residues can also recruit bromodomain-containing proteins, such as the SWI/SNF complex, which remodels the chromatin to a more open conformation to allow for active transcription [67].
Three studies have examined the effects of arsenic on pan-acetylation levels. Two early studies, which used D. melanogaster cell lines, found that 50 µM AsIII reduced total acetylation levels of the core histones; these were determined by measuring the incorporation of acetyl groups derived from tritiated acetic acid [28, 29]. In contrast, 1 and 2 µM AsIII increased H3 and H4 pan-acetylation levels, measured by Western blot, in human myeloid leukemia cell lines [37].
Several other studies have examined the influence of arsenic on specific histone PTMs and observed differences by lysine residue. Arsenic has not been shown to alter H4K5ac [44, 47] or H4K8ac [36, 44]. Furthermore, AsIII did not alter H3K14ac in APL cell lines [30]. However, an epidemiological study in China observed a positive association between uAsCr and H3K14ac in lymphocytes [53].
The findings for H3K9ac have also been inconsistent. The same epidemiological study in China did not observe an association between uAsCr and H3K9ac in lymphocytes [53], and a small epidemiological study of Argentinian women did not observe a significant association between uAs and H3K9ac, measured in sorted CD4+ and CD8+ cells. However, 7.5 µM AsIII increased H3K9ac in HepG2 cells [31] and 0.1–100 µM AsIII also increased this PTM in a dose-dependent manner in Jurkat and CCRF-CEM cells. In contrast, AsIII (0.5–5 µM) decreased this PTM in female-derived human embryonic kidney cells and in UROTsa cells, which were derived from a female human ureter [36]. Similarly, an inverse association between wAs and H3K9ac, measured in PBMCs, was observed in both Bangladeshi men and women [51]. Furthermore, 100 µg/L AsIII reduced genome-wide levels of H3K9ac, measured by ChIP-seq, in both the hippocampus and cortex of mice (males and females combined) [48]. However, importantly, AsV (50 µg/L) has also been shown to alter H3K9ac in both a region- and sex-dependent manner in the mouse brain [49].
Only two studies, both of which utilized UROTsa cells, have evaluated the effect of arsenic on H4K12ac. One group observed that 50 nM MMAIII reduced H4K12ac [44], while the other group did not observe alterations in this PTM after exposure to 0.5–2.5 µM AsIII [36]. Both studies measured H4K12ac by Western blot, using antibodies purchased from Millipore. It is therefore unlikely that differences in laboratory methods explain these inconsistencies. Alternatively, it is possible that MMAIII and AsIII differentially influence certain PTMs, such as H4K12ac. However, this will need to be confirmed in future studies, which examine both arsenic species under identical laboratory conditions.
The effects of arsenic on H4K16ac have been more consistent. In addition to its role in regulating nucleosome-level interactions, which are essential for the formation of 30 nm chromatin fibers, H4K16ac influences interactions between chromatin fibers, which determine the higher order tertiary structure of the chromatin [68]. Four studies, three of which utilized UROTsa cells, measured this PTM by either Western blot or mass spectrometry, and observed that AsIII and MMAIII, across a range of doses (0.2–10 µM) induced a global loss of H4K16ac [34, 40, 44, 47], which is a hallmark of most human cancers [68]. However, two studies, both of which measured H4K16ac by Western blot, have been less consistent. One of these studies used primary human neonatal keratinocytes (donor sex unspecified) and observed that AsIII increased H4K16ac [35], and another study, which used UROTsa cells and female-derived human embryonic kidney cells, did not observe alterations in this PTM after treatment with AsIII at doses ranging from 0.5–2.5 µM [36].
Only one group has examined the effects of AsIII on H3K18ac, which were found to be both dose- and time-dependent, with short durations (4 or 8 hours) increasing H3K18ac in HEK293T and HaCaT cells, but longer durations (84 days) reducing this PTM [53]. The same group also observed that H3K18ac was associated with the expression of several oxidative stress response genes and inversely associated with urinary 8-hydroxy-2-deoxyguanosine, a biomarker of oxidative stress [53]. It was therefore hypothesized that arsenic induces an adaptive response to oxidative stress which may be mediated by H3K18ac [53]. However, this response appears to be transient and may ultimately be inhibited by long-term exposure to arsenic [53]. Consistent with this, the same group observed an inverse association between uAsCr and H3K18ac in lymphocytes from Chinese adults who had been chronically exposed to arsenic [53].
Histone phosphorylation and phosphoacetylation
Histone phosphorylation is important for transcriptional activation and chromatin compaction during mitosis and meiosis [69]. One study observed that a very high dose of AsIII (10 µM) reduced total phosphorylation levels of both H1 and H3 in Chinese hamster ovary cells [46]. However, multiple studies using various cell lines, have consistently observed that different doses and durations of arsenic, including both AsIII and DMAIII, induce higher global levels of H3S10ph [30, 38, 42, 43, 45], which is critical for regulating chromosome segregation during mitosis [70]. One study also observed that AsIII induces H3S10phK14ac in APL cells; this phosphoacetylation mark is thought to play an important role in regulating As2O3-induced apoptosis in this cell type [30].
Challenges in the field
Although several studies have examined the effects of arsenic on global levels of histone PTMs, the findings have been largely inconsistent. These discrepancies may be attributed to many factors, including differences in the dose, duration, and type of arsenical examined; the particular tissues or cell lines utilized; the timing of exposure; the potential modifying effects of sex; and possible measurement error.
Dose, duration and type of arsenical examined
Although three studies have examined MMAIII or DMAIII in addition to AsIII and observed consistent effects [34, 40, 42], the majority have solely evaluated AsIII [28–33, 35–39, 41, 43, 45–47]. Additional studies will therefore be needed to evaluate whether arsenic metabolites differentially influence histone PTMs. Furthermore, while both As2O3 and NaAsO2 have been used to generate AsIII in experimental studies, there is evidence that As2O3 is more toxic [71]. Thus, the source of arsenic may be another important consideration. However, there may also be inconsistencies across studies which use the same source of arsenic, since cell lines differ in their capacity to metabolize arsenic ([72] and reviewed in [10]). Similarly, interspecies differences in arsenic metabolism may contribute to inconsistencies between in vivo studies [73].
The impact of arsenic on histone PTMs may also vary based on the dose and duration used. Two studies observed that certain PTMs were only altered at the higher doses of arsenic examined [30, 34], and a third study found that arsenic had completely opposite effects at a lower (100 pg/mL) versus higher (100 ng/mL) dose [39]. Furthermore, while six experimental studies examined exposure durations of weeks or months [39, 41, 44, 48, 49, 53], the majority evaluated durations of 24 hours or less [28–33, 38, 42, 43, 45, 46]. Theses shorter durations may have profoundly different effects than the chronic exposures experienced by most human populations [50, 51, 53, 54]. In support of this, one group found that a single treatment of AsIII for 8 hours increased H3K18ac, while repeated exposure to AsIII for 84 days led to an eventual reduction in this PTM; the latter finding was consistent with their epidemiological study of Chinese adults who had been chronically exposed to arsenic [53].
Tissue Differences
While some studies have examined the effects of arsenic on multiple cell lines or in different tissue types and observed similar effects [32, 37, 45, 47, 48, 53], the majority have evaluated a single cell line or tissue [28–31, 33–35, 38–44, 46, 50, 51]. Furthermore, one study observed that arsenic influenced H3K4me3 and H3K9ac in a region-dependent manner in the mouse brain [49], and another observed that arsenic was associated with lower levels of H3K9me3 in CD4+, but not CD8+, lymphocytes [54]. These findings suggest that arsenic may have tissue-specific effects, at least for some histone PTMs. Arsenic may also have differential effects in normal vs. transformed/cancer cell lines. However, this has not been well-studied. One group did observe that arsenic increased H3K9me2 in cells derived from both normal human lung tissue (BEAS-2B) and a human lung tumor (A549) [32]. However, the BEAS-2B cells were found to be much more sensitive to arsenic and were therefore exposed to lower doses [32].
Timing of exposure
Thus far, epidemiological studies have exclusively examined the effects of arsenic on histone PTMs during adulthood [50–54]. However, the prenatal period is thought to be particularly susceptible to epigenetic dysregulation [74]. While two studies in mice have examined the effects of pre- or perinatal arsenic exposure, respectively, on histone PTMs in the brains of pups [48] or adult animals [49], this was not compared with postnatal or adulthood exposures, which may also impact histone PTMs.
Potential modifying effects of sex
There is evidence that susceptibility to arsenic toxicity differs by sex (reviewed in [2]). For some outcomes, such as skin lesions and skin cancer, males are more susceptible [75, 76], while for other outcomes, such as certain developmental outcomes and, potentially, cardiovascular disease, females may be more susceptible [77–79]. All three of the studies which stratified by sex observed that arsenic influenced histone PTMs in a sex-dependent manner [49, 51, 52]. This is consistent with previous studies which have demonstrated sex-specific effects of arsenic on DNA methylation patterns [80–83]. Although many of the systemic sex differences that exist in vivo cannot be easily replicated in vitro, potential contributions from hormones and sex chromosomes can at least be considered. One in vitro study demonstrated that arsenic influences histone PTMs differently based on the presence or absence of E2 [39]. Androgens have not been similarly evaluated. However, many histone modifying enzymes bind androgen receptors, and this can alter their function [84]. For example, histone demethylase LSD1 has been shown to demethylate H3K4 in the absence of androgen, but demethylates H3K9 in the presence of androgen [85]. Although few studies have systematically evaluated potential interactions between arsenic and sex hormones, numerous in vitro studies may have been influenced by such interactions, as cell culture media is commonly supplemented with fetal bovine serum, which contains sex steroid hormones [86]. Genetic sex differences between tissues and cell lines may also be important considerations, as some histone demethylases are X-encoded, and others are Y-encoded [87].
Potential sources of histone PTM measurement error
There are many possible causes of histone PTM measurement error. However, two particular sources of concern include: 1) enzymatic cleavage of histone proteins and 2) arsenic-induced alterations in histone expression.
Histone cleavage has been the topic of several recent reviews, and is a phenomenon that occurs in many cell types and species [88–90]. Although the causes and consequences of histone cleavage remain largely unclear, many potential biological functions have been hypothesized (reviewed in [88–90]). Importantly, H3 cleavage has been shown to influence the measurement of certain histone PTMs [91]. This is likely true for other histone proteins, which can also be clipped [88]. While some studies have screened samples for histone cleavage by Western blot before measuring affected PTMs [49, 52], the majority have not considered this phenomenon.
Importantly, arsenic may also increase expression of the canonical histones [92]. Therefore, histone PTM measures may be overestimated in tissues exposed to higher concentrations of arsenic, unless PTM measures are normalized to total expression levels of the respective histone protein.
Other considerations
Histone variants, which replace the canonical histone proteins, pose an additional challenge, as these variants often harbor distinct PTMs [93, 94]. The effects of arsenic on specific histone variants remain largely unknown. However, in multiple cell types, arsenic has been shown to induce γ-H2AX [95–97], a phosphorylated variant of H2A which is an established mark of double-strand breaks [94]. There is also evidence that arsenic induces aberrant polyadenylation of H3.1, leading to increased expression of this canonical histone and potential displacement of the H3.3 variant [92, 98]. Furthermore, a recent study demonstrated that mRNA and protein levels of 10 different H2B variants were altered in HeLa and BEAS-2B cells transformed by AsIII (0.5 and 1 µm for 5 weeks) [99]. Interestingly, two of the H2B variants were highly downregulated (H2B1B and H2B10), while two were highly upregulated (H2B1C and H2B1K), compared with controls [99].
Importantly, folate and other nutritional methyl donors may differentially influence particular histone PTMs [100–104], and nutritional status has been shown to modify the effects of arsenic on DNA methylation [82, 105, 106]. Therefore, differences in the nutritional composition of cell culture media and mouse chow, as well as inter-individual differences in nutritional status, may also contribute to inconsistencies across studies.
Conclusions
Numerous studies have observed that arsenic alters global levels of histone PTMs, which are dysregulated in cancers and other outcomes. For example, there is sufficient evidence that AsIII increases global levels of H3S10ph, a mark that is important for chromosome segregation. There is also substantial evidence that arsenic causes a global reduction in H4K16ac, which is a hallmark of many human cancers. However, the findings for other PTMs have been inconsistent or sparsely studied. Inconsistencies are likely due in part to the complexity of the relationship between arsenic and PTMs, which may vary based on the type, dose, and duration of exposure; the particular tissue examined; differences by sex; and the importance of exposure timing, but may also be due to potential sources of measurement error, including enzymatic cleavage of histone proteins and arsenic-induced alterations in histone expression. Moving forward, these factors will need to be considered to better understand and reduce some of the discrepancies between studies. Since histone PTMs are reversible and are promising targets of epigenetic therapeutics, a more complete characterization of the effects of arsenic on PTMs, and the factors which modify these relationships, may improve the ability to design interventions which reduce disease burden in arsenic-exposed populations. Future studies which examine the downstream implications of arsenic-induced alterations in histone PTMs, including changes in other epigenetic modifications and gene expression, and their potential effects on clinical outcomes, will also be critical for a better overall understanding of the mechanisms underlying arsenic-induced health outcomes.
Acknowledgments
This work was supported by NIH grants P42 ES010349, RO1 CA133595, RO1 ES017875, T32 ES007322, P30 ES009089, and F31ES025100
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain primary data from any studies with animal or human subjects performed by the authors.
Conflict of Interest
Caitlin G. Howe and Mary V. Gamble declare they have no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as
• Of importance
•• Of major importance
- 1.World Health Organization. Arsenic and arsenic compounds. IARC Monographs. 2012;100C [Google Scholar]
- 2.National Research Council. Critical Aspects of EPA's IRIS Assessment of Inorganic Arsenic. National Research Council Interim Report. 2013 [Google Scholar]
- 3.World Health Organization. Arsenic Fact Sheet No 372. 2012
- 4.Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman Q, Rahman M. Dietary arsenic exposure in bangladesh. Environ Health Perspect. 2007;115(6):889–893. doi: 10.1289/ehp.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurzius-Spencer M, Burgess JL, Harris RB, et al. Contribution of diet to aggregate arsenic exposures—An analysis across populations. J Expo Sci Environ Epidemiol. 2014;24(2):156–162. doi: 10.1038/jes.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challenger F. Biological methylation. Chem Rev. 1945;36(3):315–361. [Google Scholar]
- 7.Hall MN, Gamble MV. Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol. 2012;2012 doi: 10.1155/2012/595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raml R, Rumpler A, Goessler W, et al. Thio-dimethylarsinate is a common metabolite in urine samples from arsenic-exposed women in Bangladesh. Toxicol Appl Pharmacol. 2007;222(3):374–380. doi: 10.1016/j.taap.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 9. Steinmaus CM, Ferreccio C, Romo JA, et al. Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;22(4):623–630. doi: 10.1158/1055-9965.EPI-12-1190. This study demonstrated that cancer risks persist decades after arsenic exposure has been reduced, indicating the need for public health interventions which complement arsenic remediation efforts.
- 10.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533(1):37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Ren X, McHale CM, Skibola CF, et al. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect. 2011;119(1):11. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brookes E, Shi Y. Diverse epigenetic mechanisms of human disease. Annu Rev Genet. 2014;48:237–268. doi: 10.1146/annurev-genet-120213-092518. [DOI] [PubMed] [Google Scholar]
- 13.Falchi L, Verstovsek S, Ravandi-Kashani F, et al. The evolution of arsenic in the treatment of acute promyelocytic leukemia and other myeloid neoplasms: Moving toward an effective oral, outpatient therapy. Cancer. 2015 doi: 10.1002/cncr.29852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Luger K, Mader AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 16.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10(5):295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 17.Mersfelder EL, Parthun MR. The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006;34(9):2653–2662. doi: 10.1093/nar/gkl338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu YM, Du JY, Lau AT. Posttranslational modifications of human histone H3: an update. Proteomics. 2014;14(17–18):2047–2060. doi: 10.1002/pmic.201300435. This review summarizes all of the posttranslational histone modifications that have been identified on histone H3.
- 19.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 21.Dai H, Wang Z. Histone modifications patterns and their responses to environment. Curr Environ Health Rep. 2014;1(1):11–21. [Google Scholar]
- 22.Zhang Q, Ramlee MK, Brunmeir R, Villanueva CJ, Halperin D, Xu F. Dynamic and distinct histone modifications modulate the expression of key adipogenesis regulatory genes. Cell Cycle. 2012;11(23):4310–4322. doi: 10.4161/cc.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arita A, Niu J, Qu Q, et al. Global levels of histone modifications in peripheral blood mononuclear cells of subjects with exposure to nickel. Environ Health Perspect. 2012;120(2):198–203. doi: 10.1289/ehp.1104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2(1):87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1(3):222–228. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul S, Giri AK. Epimutagenesis: A prospective mechanism to remediate arsenic-induced toxicity. Environ Int. 2015;81:8–17. doi: 10.1016/j.envint.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Argos M. Arsenic Exposure and Epigenetic Alterations: Recent Findings Based on the Illumina 450K DNA Methylation Array. Curr Environ Health Rep. 2015;2(2):137–144. doi: 10.1007/s40572-015-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arrigo AP. Acetylation and methylation patterns of core histones are modified after heat or arsenite treatment of Drosophila tissue culture cells. Nucleic Acids Res. 1983;11(5):1389–1404. doi: 10.1093/nar/11.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desrosiers R, Tanguay RM. Further characterization of the posttranslational modifications of core histones in response to heat and arsenite stress in Drosophila. Biochem Cell Biol. 1986;64(8):750–757. doi: 10.1139/o86-102. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Chen P, Sinogeeva N, et al. Arsenic trioxide promotes histone H3 phosphoacetylation at the chromatin of CASPASE-10 in acute promyelocytic leukemia cells. J Biol Chem. 2002;277(51):49504–49510. doi: 10.1074/jbc.M207836200. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez T, Brocher J, Stopper H, Hock R. Sodium arsenite modulates histone acetylation, histone deacetylase activity and HMGN protein dynamics in human cells. Chromosoma. 2008;117(2):147–157. doi: 10.1007/s00412-007-0133-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29(9):1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Li Q, Arita A, Sun H, Costa M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol Appl Pharmacol. 2009;236(1):78–84. doi: 10.1016/j.taap.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu F, Ren X, Chasse A, et al. Quantitative mass spectrometry reveals the epigenome as a target of arsenic. Chem Biol Interact. 2011;192(1):113–117. doi: 10.1016/j.cbi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbert KJ, Holloway A, Cook AL, Chin SP, Snow ET. Arsenic exposure disrupts epigenetic regulation of SIRT1 in human keratinocytes. Toxicol Appl Pharmacol. 2014;281(1):136–145. doi: 10.1016/j.taap.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Rahman S, Housein Z, Dabrowska A, Mayan MD, Boobis AR, Hajji N. E2F1-mediated FOS induction in arsenic trioxide-induced cellular transformation: effects of global H3K9 hypoacetylation and promoter-specific hyperacetylation in vitro. Environ Health Perspect. 2015;123(5):484–492. doi: 10.1289/ehp.1408302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins C, Kim CN, Fang G, Bhalla KN. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-xL. Blood. 2000;95(3):1014–1022. [PubMed] [Google Scholar]
- 38.Ray PD, Huang BW, Tsuji Y. Coordinated regulation of Nrf2 and histone H3 serine 10 phosphorylation in arsenite-activated transcription of the human heme oxygenase-1 gene. Biochim Biophys Acta. 2015;10(88):18. doi: 10.1016/j.bbagrm.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treas JN, Tyagi T, Singh KP. Effects of chronic exposure to arsenic and estrogen on epigenetic regulatory genes expression and epigenetic code in human prostate epithelial cells. PLoS One. 2012;7(8):e43880. doi: 10.1371/journal.pone.0043880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jo WJ, Ren X, Chu F, et al. Acetylated H4K16 by MYST1 protects UROtsa cells from arsenic toxicity and is decreased following chronic arsenic exposure. Toxicol Appl Pharmacol. 2009;241(3):294–302. doi: 10.1016/j.taap.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HG, Kim DJ, Li S, et al. Polycomb (PcG) proteins, BMI1 and SUZ12, regulate arsenic-induced cell transformation. J Biol Chem. 2012;287(38):31920–31928. doi: 10.1074/jbc.M112.360362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T, Miyazaki K, Kita K, Ochi T. Trivalent dimethylarsenic compound induces histone H3 phosphorylation and abnormal localization of Aurora B kinase in HepG2 cells. Toxicol Appl Pharmacol. 2009;241(3):275–282. doi: 10.1016/j.taap.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Kita K, Ochi T. Phosphorylation of histone H3 at serine 10 has an essential role in arsenite-induced expression of FOS, EGR1 and IL8 mRNA in cultured human cell lines. J Appl Toxicol. 2013;33(8):746–755. doi: 10.1002/jat.2724. [DOI] [PubMed] [Google Scholar]
- 44.Ge Y, Gong Z, Olson JR, Xu P, Buck MJ, Ren X. Inhibition of monomethylarsonous acid (MMA III)-induced cell malignant transformation through restoring dysregulated histone acetylation. Toxicology. 2013;312:30–35. doi: 10.1016/j.tox.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Kannan-Thulasiraman P, Katsoulidis E, Tallman MS, Arthur JS, Platanias LC. Activation of the mitogen- and stress-activated kinase 1 by arsenic trioxide. J Biol Chem. 2006;281(32):22446–22452. doi: 10.1074/jbc.M603111200. [DOI] [PubMed] [Google Scholar]
- 46.Cobo JM, Valdez JG, Gurley LR. Inhibition of mitotic-specific histone phosphorylation by sodium arsenite. Toxicol In Vitro. 1995;9(4):459–465. doi: 10.1016/0887-2333(95)00038-a. [DOI] [PubMed] [Google Scholar]
- 47.Liu D, Wu D, Zhao L, et al. Arsenic Trioxide Reduces Global Histone H4 Acetylation at Lysine 16 through Direct Binding to Histone Acetyltransferase hMOF in Human Cells. PLoS One. 2015;10(10):e0141014. doi: 10.1371/journal.pone.0141014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cronican AA, Fitz NF, Carter A, et al. Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic. PloS one. 2013;8(2):e53478. doi: 10.1371/journal.pone.0053478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tyler CR, Hafez AK, Solomon ER, Allan AM. Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region-and sex-specific manner in the adult mouse brain. Toxicology and applied pharmacology. 2015;288(1):40–51. doi: 10.1016/j.taap.2015.07.013. This is the first animal study to examine the effects of arsenic on PTMs separately by sex and in different tissue types.
- 50.Cantone L, Nordio F, Hou L, et al. Inhalable Metal-Rich Air Particles and Histone H 3 K 4 Dimethylation and H 3 K 9 Acetylation in a Cross-sectional Study of Steel Workers. Environ Health Perspect. 2011;119(7):964–969. doi: 10.1289/ehp.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chervona Y, Hall MN, Arita A, et al. Associations between arsenic exposure and global posttranslational histone modifications among adults in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2252–2260. doi: 10.1158/1055-9965.EPI-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Howe CG, Liu X, Hall MN, et al. Associations between blood and urine arsenic concentrations and post-translational histone modifications in Bangladeshi men and women. Environ Health Perspect. 2016 doi: 10.1289/ehp.1510412. Epub ahead of print. This is the first large human study to evaluate the effects of arsenic exposure, due to arsenic-contaminated drinking water, on PTMs separately by sex.
- 53. Ma L, Li J, Zhan Z, et al. Specific histone modification responds to arsenic-induced oxidative stress. Toxicol Appl Pharmacol. 2016 doi: 10.1016/j.taap.2016.03.015. Epub ahead of print. This is the first large human study to evaluate the effects of arsenic exposure, due to use of arsenic-contaminated coal, on histone PTMs. This study also evaluated the effect of short versus long duration arsenic exposures on the same PTMs in vitro.
- 54. Pournara A, Kippler M, Holmlund T, et al. Arsenic alters global histone modifications in lymphocytes in vitro and in vivo. Cell Biol Toxicol. 2016 doi: 10.1007/s10565-016-9334-0. Epub ahead of print. This is the first human study to evaluate the effects of arsenic exposure on histone PTMs in sorted lymphocytes.
- 55.Ernst J, Kheradpour P, Mikkelsen TS, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Zang C, Rosenfeld JA. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera C, Saavedra F, Alvarez F, et al. Methylation of histone H3 lysine 9 occurs during translation. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv929. gkv929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J Biol Chem. 2011;286(10):7983–7989. doi: 10.1074/jbc.M110.194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012;13(2):115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jha DK, Pfister SX, Humphrey TC, Strahl BD. SET-ting the stage for DNA repair. Nat Struct Mol Biol. 2014;21(8):655–657. doi: 10.1038/nsmb.2866. [DOI] [PubMed] [Google Scholar]
- 62.Kuo AJ, Cheung P, Chen K, et al. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol Cell. 2011;44(4):609–620. doi: 10.1016/j.molcel.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfister SX, Markkanen E, Jiang Y, et al. Inhibiting WEE1 Selectively Kills Histone H3K36me3-Deficient Cancers by dNTP Starvation. Cancer Cell. 2015;28(5):557–568. doi: 10.1016/j.ccell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 2011;25(13):1345–1358. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daigle SR, Olhava EJ, Therkelsen CA, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122(6):1017–1025. doi: 10.1182/blood-2013-04-497644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernt KM, Zhu N, Sinha AU, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation b DOT1L. Cancer Cell. 2012;20(1):66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 68.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311(5762):844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 69.Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37(4):391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 70.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116(18):3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 71.Jiang X, Chen C, Zhao W, Zhang Z. Sodium arsenite and arsenic trioxide differently affect the oxidative stress, genotoxicity and apoptosis in A549 cells: an implication for the paradoxical mechanism. Environ Toxicol Pharmacol. 2013;36(3):891–902. doi: 10.1016/j.etap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Styblo M, Del Razo LM, Vega L. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74(6):289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 73.Vahter M. Species differences in the metabolism of arsenic compounds. Appl Organomet Chem. 1994;8(3):175–182. [Google Scholar]
- 74.Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6(7):791–797. doi: 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahsan H, Chen Y, Parvez F, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol. 2006;163:1138–1148. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y-C, Guo Y-LL, Su H-JJ, et al. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45(241–248) doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- 77.Gardner RM, Kippler M, Tofail F, et al. Environmental exposure to metals and children’s growth to age 5 years: a prospective cohort study. Am J Epidemiol. 2013;177(12):1356–1367. doi: 10.1093/aje/kws437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamadani JD, Tofail F, Nermell B, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40(6):1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- 79.Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Broberg K, Ahmed S, Engstrom K, et al. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J Dev Orig Health Dis. 2014;5(04):288–298. doi: 10.1017/S2040174414000221. This study examined the effects of arsenic on genome-wide DNA methylation in cord blood separately by sex and observed that the effects were more pronounced among boys.
- 81. Pilsner JR, Hall MN, Liu X, et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PloS One. 2012;7(5):e37147. doi: 10.1371/journal.pone.0037147. This study examined the effects of arsenic on global DNA methylation levels of DNA methylation in cord blood separately by sex and observed that the effects were in opposite directions for boys and girls.
- 82. Niedzwiecki MM, Liu X, Hall MN. Sex-specific associations of arsenic exposure with global DNA methylation and hydroxymethylation in leukocytes: results from two studies in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1748–1757. doi: 10.1158/1055-9965.EPI-15-0432. This was the first human study to examine the effects of arsenic on both 5-methylcytosine and 5-hydroxymethylcytosine. Differences by sex were evaluated and the associations between arsenic and these marks differed between men and women.
- 83.Nohara K, Baba T, Murai H, et al. Global DNA methylation in the mouse liver is affected by methyl deficiency and arsenic in a sex-dependent manner. Arch Toxicol. 2011;85(6):653–661. doi: 10.1007/s00204-010-0611-z. [DOI] [PubMed] [Google Scholar]
- 84.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28(7):778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 85.Metzger E, Wissmann M, Yin N, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 86.Miller VM, Kaplan JR, Schork NJ. Strategies and methods to study sex differences in cardiovascular structure and function: a guide for basic scientists. Biol Sex Differ. 2011;2:14–14. doi: 10.1186/2042-6410-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bellott DW, Hughes JF, Skaletsky H, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508(7497):494. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dhaenens M, Glibert P, Meert P, Vossaert L, Deforce D. Histone proteolysis: a proposal for categorization into ‘clipping’and ‘degradation’. Bioessays. 2015;37(1):70–79. doi: 10.1002/bies.201400118. This is a very comphrensive review of all of studies which have identified histone proteolysis and it provides important discussion about the difficulties in distinguishing between biological histone clipping versus histone degradation.
- 89.Azad GK, Tomar RS. Proteolytic clipping of histone tails: the emerging role of histone proteases in regulation of various biological processes. Mol Biol Rep. 2014;41(5):2717–2730. doi: 10.1007/s11033-014-3181-y. [DOI] [PubMed] [Google Scholar]
- 90.Zhou P, Wu E, Alam HB, Li Y. Histone cleavage as a mechanism for epigenetic regulation: current insights and perspectives. Curr Mol Med. 2014;14(9):1164–1172. doi: 10.2174/1566524014666141015155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Howe CG, Gamble MV. Enzymatic cleavage of histone H3: a new consideration when measuring histone modifications in human samples. Clin Epigenetics. 2015;7(1):1. doi: 10.1186/s13148-014-0041-5. This letter presents evidence that histone H3 is cleaved in human peripheral blood mononuclear cells and demonstrates that this influences the measurement of certain PTMs.
- 92. Brocato J, Fang L, Chervona Y, et al. Arsenic induces polyadenylation of canonical histone mRNA by down-regulating stem-loop-binding protein gene expression. J Biol Chem. 2014;289(46):31751–31764. doi: 10.1074/jbc.M114.591883. This study demonstrated that arsenic increases the expression of the canonical histone proteins.
- 93.Hake SB, Garcia BA, Duncan EM, et al. Expression patterns and post-translational modifications associated with mammalian histone H3 variants. J Biol Chem. 2006;281(1):559–568. doi: 10.1074/jbc.M509266200. [DOI] [PubMed] [Google Scholar]
- 94.Thambirajah AA, Li A, Ishibashi T, Ausio J. New developments in post-translational modifications and functions of histone H2A variants. Biochem Cell Biol. 2009;87(1):7–17. doi: 10.1139/O08-103. [DOI] [PubMed] [Google Scholar]
- 95.Yih LH, Hsueh SW, Luu WS, Chiu TS, Lee TC. Arsenite induces prominent mitotic arrest via inhibition of G2 checkpoint activation in CGL-2 cells. Carcinogenesis. 2005;26(1):53–63. doi: 10.1093/carcin/bgh295. [DOI] [PubMed] [Google Scholar]
- 96.Xie H, Huang S, Martin S, Wise JP., Sr Arsenic is cytotoxic and genotoxic to primary human lung cells. Mut Res Genet Toxicol Environ Mutagen. 2014;760:33–41. doi: 10.1016/j.mrgentox.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rehman K, Fu YJ, Zhang YF, et al. Trivalent methylated arsenic metabolites induce apoptosis in human myeloid leukemic HL-60 cells through generation of reactive oxygen species. Metallomics. 2014;6(8):1502–1512. doi: 10.1039/c4mt00119b. [DOI] [PubMed] [Google Scholar]
- 98.Brocato J, Chen D, Liu J, Fang L, Jin C, Costa M. A Potential New Mechanism of Arsenic Carcinogenesis: Depletion of Stem-Loop Binding Protein and Increase in Polyadenylated Canonical Histone H3.1 mRNA. Biol Trace Elem Res. 2015;166(1):72–81. doi: 10.1007/s12011-015-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rea M, Jiang T, Eleazer R, et al. Quantitative mass spectrometry reveals changes in histone H2B variants as cells undergo inorganic arsenic-mediated cellular transformation. Mol Cell Proteomics. 2016 doi: 10.1074/mcp.M116.058412. Epub ahead of print. This is the first study to demonstrate that arsenic differentially alters H2B variants.
- 100.Sadhu MJ, Guan Q, Li F, et al. Nutritional control of epigenetic processes in yeast and human cells. Genetics. 2013;195(3):831–844. doi: 10.1534/genetics.113.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garcia BA, Luka Z, Loukachevitch LV, Bhanu NV, Wagner C. Folate deficiency affects histone methylation. Med Hypotheses. 2016;88:63–67. doi: 10.1016/j.mehy.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lambrot R, Xu C, Saint-Phar S, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24(1):184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284(4):1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pilsner JR, Liu X, Ahsan H, et al. Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. Am J Clin Nutr. 2007;86(4):1179–1186. doi: 10.1093/ajcn/86.4.1179. [DOI] [PubMed] [Google Scholar]
- 106.Lambrou A, Baccarrelli A, Wright RO, et al. Arsenic exposure and DNA methylation among elderly men. Epidemiology. 2012;23(5):668. doi: 10.1097/EDE.0b013e31825afb0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen R, Kang R, Fan XG, et al. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]