Abstract
Atlantic killifish, Fundulus heteroclitus, are adapted to creosote-based PAHs at the US EPA Superfund site known as Atlantic Wood (AW) on the southern branch of the Elizabeth River, VA USA. Subsequent to the discovery of the AW population in the early 1990s, these fish were shown to be recalcitrant to CYP1A induction by PAHs under experimental conditions, and even to the time of this study, killifish embryos collected from the AW site are resistant to developmental deformities typically associated with exposure to PAHs in reference fish. Historically, however, 90+% of the adult killifish at this site have proliferative hepatic lesions including cancer of varying severity. Several PAHs at this site are known to be ligands for the aryl hydrocarbon receptor (AHR). In this study, AHR-related activities in AW fish collected between 2011–2013 were re-examined nearly 2 decades after first discovery. This study shows that CYP1A mRNA expression is three-fold higher in intestines of AW killifish compared to a reference population. Using immunohistochemistry, CYP1A staining in intestines was uniformly positive compared to negative staining in reference fish. Livers of AW killifish were examined by IHC to show that CYP1A and AHR2 protein expression reflect lesions-specific patterns, probably representing differences in intrinsic cellular physiology of the spectrum of proliferative lesions comprising the hepatocarcinogenic process. We also found that COX2 mRNA expression levels were higher in AW fish livers compared to those in the reference population, suggesting a state of chronic inflammation. Overall, these findings suggest that adult AW fish are responsive to AHR signaling, and do express CYP1A and AHR2 proteins in intestines at a level above what was observed in the reference population.
Keywords: Atlantic killifish, AHR2, CYP1A, COX2, Adaptation to PAHs
1. Introduction
Between 1926 and 1992, Atlantic Wood Industries operated a wood-treating facility on the Southern Branch of the Elizabeth River in Portsmouth, VA USA. In 1990, the Atlantic Wood (AW) site was added to the National Priorities List (NPL) of most hazardous waste sites due to the high discharge of creosote and pentachlorophenol (PCP) into the river, the storage of treated wood, and environmental disposal of wastes, including the leasing of the property by the US Navy for waste disposal. A larger historical overview of the development and industrial use of the Elizabeth River, sources of pollution, establishment of the AW Superfund site, a review of toxicological studies at the AW site, and attempts at recovery can be found in a recent thorough review (Di Giulio and Clark, 2015). Additional information is provided by US EPA Mid-Atlantic Superfund (EPA).
Several studies were initiated in the early 1990s to determine environmental impacts of local contaminated sediments at the AW site, along with studies to examine the impact on the Elizabeth River at sites both proximal and distal to the areas of highest contamination. One series of studies focusing on a small non-migratory killifish, Fundulus heteroclitus, living at the AW site, showed extremely high prevalence of hepatic proliferative lesions including pre-cancerous altered hepatocellular foci (90%), the spectrum of liver neoplasia including hepatoblastoma (30%), cholangiocellular proliferations, and exocrine pancreatic neoplasia (Fournie and Vogelbein, 1994; Vogelbein et al., 1999; Vogelbein et al., 1990). In contrast, only a baseline of early pre-cancerous liver lesions (<5%) have been observed in uncontaminated reference sites, within the Elizabeth River and elsewhere. Concomitant with studies showing severe liver pathology in adult killifish, other studies demonstrated higher glutathione-s-transferase (GST) activity in intestines and livers (Van Veld et al., 1991). Another study indicated that inducibility of hepatic CYP1A was compromised in these chronically exposed fish (Van Veld and Westbrook, 1995). In that study, killifish from AW and a reference population at King's Creek (KC), VA, near Gloucester Point, received an i.p. injection of benzo-a-pyrene, (BAP), a potent CYP1A inducer, and hepatic CYP1A protein and enzymatic activity (EROD assay) were measured 24 and 48 h later. Responses in whole livers of AW fish were depressed compared to KC fish. Meanwhile, immunohistochemistry using a CYP1A antibody showed that livers from AW killifish exhibiting proliferative liver lesions did elaborate CYP1A activity, but with extremely heterogeneous tissue expression patterns, and also not uniformly across the severity spectrum of liver lesions indicative of carcinogenesis (Van Veld et al., 1992). Subsequent studies showed that embryos of AW killifish exhibited a striking acute toxicity resistance to the complex mixture of chemical contaminants in AW sediments, while embryos from a reference site developed severe cardiovascular terata leading to high mortality (Ownby et al., 2002; Williams, 1994). This phenomenon was heritable up through F2 generations (Meyer et al., 2002).
More recent studies have suggested that a “recalcitrant” CYP1A induction phenotype is the underlying mechanism of adaption (e.g., acute toxicity resistance) to chemical contaminant exposure in these fish (Wills et al., 2010b). Ongoing studies by others demonstrated that the toxicity and typical cardiovascular deformities in embryos resulting from exposure to select PAHs in AW killifish occurred at only high doses of compounds or high concentrations of sediment extracts (Clark et al., 2013a; Meyer et al., 2002; Wassenberg and Di Giulio, 2004a; Wassenberg and Di Giulio, 2004b; Wassenberg et al., 2002; Wills et al., 2010a; Wills et al., 2010b; Wills et al., 2009).
Because some of the PAHs, and many other compounds in creosote, found at the AW site are metabolized to teratogenic, carcinogenic, and immunotoxic intermediates through CYP1A/CYP1B-mediated pathways, the general consensus developing at the time was that altered CYP1A induction (lowered CYP1A activity mediated through the aryl hydrocarbon receptor [(AHR)], was the key to understanding the acute toxicity resistance phenomenon in AW killifish. Other studies demonstrated the physiological cost of resistance or adaptation to life in a harsh chemical environment in the killifish. For example, natural cytotoxic cell activity was reduced (Faisal et al., 1991), and both lymphoid and hepatic DNA-adduct formation were high in adult fish at the site (Rose et al., 2000; Rose et al., 2001). Immunotoxicological studies as recently as 2002 and 2003 revealed that immunoglobulins (IgM) were compromised in adult AW killifish in comparison to a reference site, and that indicators of innate and pro-inflammatory immune functions were elevated (Frederick et al., 2007).
In summary of what is known leading up this study, embryos of AW killifish are adapted to in situ pollutants due to down-regulated AHR pathways, altered phase I and II detoxification responses, and upregulated anti-oxidant defense (reviewed by (Di Giulio and Clark, 2015), while adults exhibit significant signs of toxicity, including altered immune function and survivability in clean water (Frederick et al., 2007), as well as lesions and carcinogenesis (Van Veld et al., 1991; Vogelbein et al., 1999; Vogelbein and Unger, 2006). However, even adult AW killifish are resistant to acute toxicity of AW sediments and PAHs.
Specific to teleostean fishes, gene duplications in early vertebrates yield AHR1, AHR2, and AHR-repressor (AHRR) in modern fishes, and there are paralogs (e.g., α, β, γ, δ etc) of each (Andreasen et al., 2002; Hahn et al., 2009; Hahn et al., 2004; Hansson and Hahn, 2008; Jenny et al., 2009; Karchner et al., 1999). At least in the zebrafish and Atlantic killifish models, AHR2 is involved in typical teratogenic responses of embryos to planar PCBs, dioxins, and PAHs, as can be demonstrated with anti-AHR2 morpholinos (Clark et al., 2010; Van Tiem and Di Giulio, 2011). Pertinent to the present study, developing killifish are highly sensitive to AHR-binding ligands, while developing zebrafish are one of the least sensitive aquatic models tested to date, as reviewed by Doering et al (Doering et al., 2013).
Most studies with adult AW killifish have been limited to either lymphoid organs and plasma (Frederick et al., 2007; Kelly-Reay and Weeks-Perkins, 1994; Rose et al., 2000; Rose et al., 2001), or livers as the primary source of tissue as an indicator organ of toxicity (Vogelbein et al., 1999; Vogelbein et al., 1990; Vogelbein and Unger, 2006). Yet, other organs, and especially intestines, are in direct contact with environmental matrices since these fish consume both epi-fauna and interstitial prey items, and they “drink” water. To date, the intestines of AW fish have been examined for GST activity, but not for CYP1A expression and other AHR2-mediated functions. In this study, we have applied molecular tools and antibodies to determine if adult killifish from the AW site are refractory to CYP1A induction by PAHs exposed in situ, and to examine AHR-related activities in lymphoid tissues and intestines to compare with hepatic CYP1A expression. Secondarily, we wanted to revisit earlier studies from two decades ago showing that CYP1A expression varies across the severity spectrum of liver lesions observed in AW fish (Van Veld et al., 1992) as a result of exposure to a myriad of toxicants at the AW site, and to compare CYP1A protein expression to expression of AHR2. This study also provides an opportunity to examine more closely the relationship between exposure to the harsh sediments at the AW site and state of COX2 expression previously observed when examining immune function in these fish (Frederick et al., 2007).
2. Materials and methods
2.1. Animals and tissue collection
Adult Atlantic killifish were collected from Kings Creek, VA, Gloucester County, and at the Atlantic Wood Superfund site on the southern branch of the Elizabeth River, VA. For initial studies, 30 adult males (8–10 g) and 30 adult females (8-10 g) were collected in July 2011 at each site using baited minnow traps, transported to the laboratory at VIMS and quickly sedated with tricaine methanesulphonate (MS-222). Livers, intestines, and lymphoid organs (spleen and anterior kidney) were carefully dissected and placed in RNAZol. For gene expression analysis, three pools of five livers, three pools of five intestines, three pools of five lymphoid tissues for males, and the same for females from each site were processed for RNA isolation following the manufacturer's instructions. First strand cDNA synthesis was carried out using the RT2 Easy First Strand Kit (SABioscience Corporation) as described by the manufacturer. Genomic DNA from each pooled sample was removed using elimination mixture supplied by the manufacturer. Gene expression was analyzed by quantitative real-time PCR using a BioRad iQ5 detection system and RT2 SYBR green/ fluorescein master mix.
2.2. qRT-PCR
Primer sets were designed using Integrated DNA Technology (IDT) software, and examined for proper efficiency, lack of primer dimers, and proper melt curves prior to use. Primer sets for specific gene products, product size, and qPCR conditions are listed in Table 1. These specific genes were selected to represent basic AHR-related products associated with phase I and II detoxification enzymes, and COX2 as a marker of inflammation. The quantity of these mRNAs was expressed as fold-changes in gene expression compared to 18S expression using the Pfaffl method (Pfaffl, 2001). Expression data were compared between groups using ANOVA, followed by a Bonferroni's post- test using GraphPad5 statistical package. Also, when data revealed a difference between males and females at each site, these data were also graphically represented as a separate set of data.
Table 1.
Primer sets for qRT-PCR.
| Gene Name | Accession # | Primer Sequence (5’ → 3’) | Tm °C | Product size (bp) |
|---|---|---|---|---|
| AHR2 | U29679.3 | F: ATC GAC AGC AGT ATG CCA CCT CTT R: TTA GCA GGG AAG GAA GCG TTG ACT |
58 | 100 |
| AHRR | AF443441.1 | F: TTG TCT CGA AGC TGT ATG GCT CGT R: ATC TTA ATG GGC GGC ATT TCA GGC |
57 | 124 |
| CYP1A | AF 026800.1 | F: AAG CAA GAG GGA GAG AAG GTC CTT R: TGT GCT TCA TCG TGA GGC CAT ACT |
57 | 150 |
| CYP1B | AF235140.1 | F: CCA AAG AAT ACA CAG AGG CAA CGG R: ATG AAG GCA TCC AGG TAA GGC AT |
58 | 175 |
| UGT | AY725222.1 | F: TTA CCG TAA CAA CAT CCA GCG CCT R: CAG CTC CTT TGT TCC TGA TGT CGT |
59 | 100 |
| COX2 | AY532639.2 | F: TAC CCG CCA CTG GTT AAG GAT GTT R: TTG TGT TCA CGG AGC CAA ATG GTG |
57 | 146 |
| GSTα | AY725219.1 | F: TCT GAC AGA AAG CAC TGC GAT CCT R: TGA GCA GGA AGA CCT TTG AGC AGT |
57 | 150 |
| 18S | M91180.1 | F: TTC GTA TTG TGC CGC TAG AGG TGA R: TTC GAA CCT CCG ACT TTC GCT CTT |
57 | 125 |
2.3. Immunoblotting for liver and intestinal CYP1A protein expression
To follow up on gene expression data, killifish were collected again in July 2012 - 2013 at the same two sites using baited minnow traps. Although CYP1A expression profiles from the two populations at this second year of sampling would not be from the same fish as used for mRNA expression profiles, the use of individual animals from a robust population size at each site should be representative of fish previously sampled. Livers and intestines from 8 males and 8 females from each site were quickly removed, individually wrapped in foil, and flash frozen in liquid nitrogen, then stored at −80° C until further use. Liver and intestine tissues (400 mg) from were then individually homogenized in 1 mL homogenization buffer [25 mM MOPS (pH 7.5), 1 mM EDTA, 5 mM EGTA, 20 mM Na2MoO4, 1 mM DDT, 10% glycerol, 0.02% NaN3], containing 2 x of HALT protease inhibitor cocktail (Pierce) using a mini bead-beater (3110BX) with 1 mL of 1 mm glass beads (Biospec). Homogenizations were carried out in XXTuff 2 mL microvials (Biospec) at 4800 rpm over a 3 min period. Tubes were quickly placed on ice for 5 min then shaken again for 3 min, followed by another 5 min on ice, then one final 3 min shake. Tube contents were removed by pipetting and centrifuged in a clean 1.5 mL snap cap tube at 1000 × g for 3 min to pellet debris and organelles. The overlying lysate was then centrifuged at 16,000 × g to obtain S9 fractions for SDS-PAGE and immunoblotting. Thirty μg of protein from each sample were subjected to SDS-PAGE on 4-20% gels, then transferred to 0.45μM Immunlon (PVDF) membrane at 4° C overnight at 35 V.
Following a 5 min wash with 0.1 M phosphate buffered saline with 0.05% Tween-20 (PBS-Tw) the blot was covered with blocking buffer (10% FBS in PBS-Tw) and gently rocked for 2 h at room temperature. Following a 5 min wash with PBS-Tw, blots of intestine and liver proteins were incubated with mAb C10-7 against fish CYP1A (Rice et al., 1998). Blots were washed x 3 with PBS-Tw and further incubated with alkaline phosphatase-conjugated goat-anti-mouse Ig (h+l) (1:2000) for 1 hr at RT. After four washings with PBS-Tw, alkaline phosphatase activity was visualized using the chromogen NBT/BCIP (Fisher Scientific) in alkaline phosphatase buffer (100 mM NaCl, 5mM MgCl2, 100 mM Tris; pH 9.5).
2.4. Development and characterization of mAbs against killifish AHR2α
A C-terminus portion of Fundulus heteroclitus AHR2 cDNA was previously cloned into a pQE80/82 6-HIS expression plasmid (Qiagen) and used to transfect the BL21-CodonPlus (RP) strain of E. coli for protein expression (Merson et al., 2006), and provided to us as a gift from Dr. Mark E. Hahn, WHOI. The expression plasmid for recombinant protein was isolated using a GeneJet Plasmid Miniprep Kit (Thermofisher), and used to transform DE3 E. coli (Arctic Express system, Agilent) using directions provided by the manufacturer. The transformed cells were then prepared for growth and induction of recombinant proteins as previously described (Merson et al., 2006). The culture was then centrifuged at 4,000 × g for 20 min at 4° C to obtain cell pellets containing recombinant proteins.
Following the directions provided by a Ni-NTA Fast Start Kit (Qiagen), the above pellets were then suspended in 20 mL of lysis buffer for denaturing conditions under constant shaking overnight at 4° C. Next, the mixture was centrifuged at 14,000 × g for 30 min and the supernatant collected. Per instructions from the kit, recombinant proteins were isolated over Ni-agarose columns. The purity of recombinant protein throughout washing and elution steps was determined visually by SDS-PAGE on 4-20% Criterion™ gels (Biorad) stained with Comassie blue stain, followed by de-staining to visualize separated proteins. The presence of HIS-tag on recombinant protein was verified by repeating the above SDS-PAGE using washing and elution fractions and transferring proteins to Immulon PVDF membranes (Fisher) and probing with Ni-HRP as part of a commercially available kit (SuperSignal, Pierce). HRP activity was visualized using 4-chloro-1-napthol as a substrate.
The most visually pure elution of recombinant protein was used to immunize 6 – 8 week old female Balb/c mice at the Godley-Snell Animal facilities at Clemson University, and under IACUC approved conditions. Following one primary and three boosting immunizations given 14, 35, and 56 days later, spleen cells were isolated and fused with SPO2/14 myeloma cells (ATCC, Manassas VA, USA) using general techniques previously described (Rice et al., 1998). Subsequent primary hybridoma supernatants were first screened by ELISA for reactivity against recombinant proteins. Cells from positive wells were transferred to 24 well plates and grown to near confluence, and 1 mL of supernatant from each well was screened by SDS-PAGE and immunoblotting using a multi-screen apparatus (Biorad, Richmond CA, USA). Cells from wells testing positive for their respective protein were cloned by limiting dilution to obtain monoclonal hybridoma lines (mAbs).
To test for reactivity against full length native proteins, 10 adult killifish were collected using baited minnow traps in a salt marsh near the Belle Baruch Marine Lab, Georgetown, SC, and maintained as a laboratory population as tissue donors. These lab fish were euthanized in tricaine (MS-222) and livers quickly removed, pooled homogenized and centrifuged to obtain tissue S9 fraction protein. Livers from fish collected at KC and AW sites were examined as well as pooled liver samples (10 livers pooled) per site. Thirty μg of liver S9 fraction protein from pooled samples, 10 μg each of COS-7 cell (African green monkey kidney fibroblast) lysates expressing either full length AHR1 or AHR2 (both were gifts from Dr. Mark E. Hahn, WHOI) were subjected to SDS-PAGE/immunoblotting on 10% gels probed with supernatants as a source of antibody.
2.5 Liver histopathology and IHC
Additional livers, intestines, and lymphoid organs were collected during the same July 2012 - 2013 trapping from the AW and KC sites, then placed in 10% aqueous buffered zinc formalin (Z-Fix, Anatech, Ltd, Battle Creek, MI, USA) for 5 days, and the fixative replaced with 70% ethanol until processing. Organs were processed by routine methods for paraffin histology (Prophet et al., 1992), either by the Clemson University Histology Facility, or at the Virginia Institute of Marine Science, College of William and Mary, Histology Facility. Sections were cut at 5 μM on a rotary microtome from individual tissues from each population. One section was stained with hematoxylin and eosin (H&E) for liver lesion diagnosis and subsequent serial sections were processed for immunohistochemistry.
Select livers, based on diagnosis and variety of liver lesion types, were probed with mAb against AHR2 (5B6) (this study) and CYP1A (C10-7) (Rice et al., 1998), Slides were heated in Tris-EDTA buffer, pH 9, by microwave on 100% power for 5 min followed by cooling for 5 min, followed by a final 5 min 100% power, and a final 20 min rest in the container. Tissues on slides were encircled with a Liquid Blocker Super mini pen to separate tissue slices, then the appropriate antibody, as hybridoma supernatant diluted 1:20 in PBS, was added and incubated overnight at 4° C. Each slide with tissue slices contained one slice receiving secondary antibody only as a control. Additional screening assays included isotype controls for the mAbs. The slides were then washed and endogenous peroxidases quenched with 3% hydrogen peroxide, and an avidin-biotin blocking step (Vector Labs) was included. Tissues were further processed using a horse-anti-mouse IgG Vectastain ABC-Ultra kit as directed by the kit. Antibody labeling was detected with Nova Red staining, and counter-stained with hematoxylin (Vector Labs).
3.0 Results
3.1 qRT-PCR results
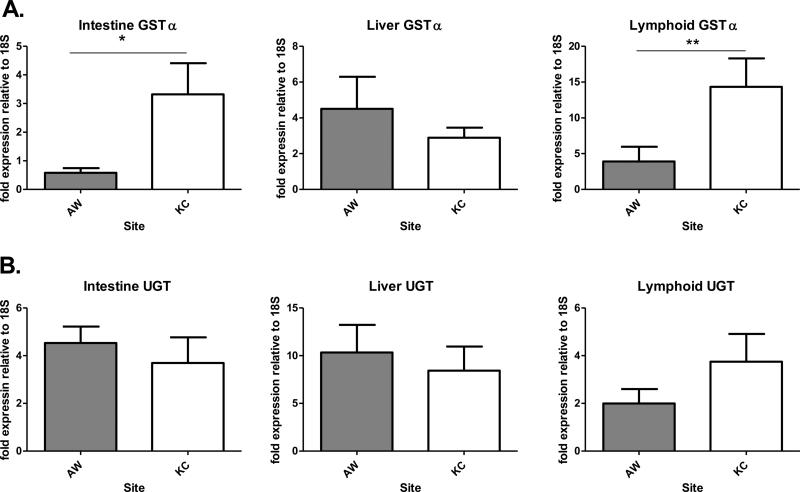
mRNA expression levels of CYP1A and CYP1B, two gene products under the direct control of the AHR, were higher in AW killifish compared to KC reference fish, but only for intestine CYP1A and lymphoid CYP1B (Figure 1A, 1B). CYP1A gene expression was highest in intestine, with lymphoid and liver expression being comparatively low and not significantly different from each other. An analysis of AHR2 mRNA expression showed no differences between populations, or between organs (Figure 2A). Given that AHRR is often studied as a marker of AHR activation (Mimura et al., 1999; Tigges et al., 2013), expression of AHRR mRNA levels were evaluated as well. Liver and lymphoid AHRR mRNA levels were higher in AW fish than in KC fish (Figure 2B). mRNA expression levels of GSTα differed in lymphoid tissues and intestines between the two populations, with AW fish expressing less (Figure 3a). Of the three tissues examined, lymphoid GSTα mRNA was expressed at a higher level than for livers and intestines, even in KC fish. There were no differences in mRNA expression levels of UGT between the two populations, though liver expression was higher in both populations than in lymphoid and intestine tissues (Figure 3b).
Figure 1.
Relative fold expression of CYP1A and CYP1B in the intestines, livers, and lymphoid tissues of Atlantic killifish, Fundulus heteroclitus, collected from AW and KC (reference population) sites. Data represent fold mRNA expression relative to 18S levels using the Pfaffl method. Error bars show ± SEM, standard error of the mean of 3 pooled samples of five organs per pool for males, and the same for females (n = 6 pooled samples for each collection site). *Indicates statistically significant difference between populations (p≤0.05).
Figure 2.
Relative fold expression of AHR2 and AHRR in the intestines, livers, and lymphoid tissues of Atlantic killifish collected from AW and KC sites. Data represent fold mRNA expression relative to 18S levels using the Pfaffl. Error bars show ± SEM, standard error of the mean of 3 pooled samples of five organs per pool for males, and the same for females (n = 6 pooled samples per site). * Indicates statistically significant difference between populations (p≤0.05).
Figure 3.
Relative fold expression of GSTα and UGT in the intestines, livers, and lymphoid tissues of Atlantic killifish collected from AW and KC sites. Data represent fold mRNA expression relative to 18S levels using the Pfaffl method. Error bars show ± SEM, standard error of the mean of 3 pooled samples of five organs per pool for males, and the same for females (n = 6 pooled samples per site). * Indicates statistically significant difference between populations (p≤0.05).
When mRNA expression data were further analyzed for sex differences, and statistical interactions, there were differences between male and female expression of GSTα in AW fish; males express higher GSTα mRNA (Figure 4A), and the same is true for liver AHRR mRNA (Figure 4B). GSTα mRNA expression is higher in KC males than in AW males, while AHRR mRNA expression is higher in AW males than KC males. Based on previous work in our lab demonstrating that COX2 protein expression is higher in AW vs. KC killifish (Frederick et al., 2007), the expression of COX2 mRNA expression was examined in intestine, liver, and lymphoid tissues. Liver COX2 mRNA was three-fold higher in AW than KC killifish (Figure 5). Of particular note, liver COX2 expression in AW fish was also nearly three times higher than in other tissues, regardless of population.
Figure 4.
Sex differences in the relative fold expression of GSTα and AHRR in the intestines, livers, and lymphoid tissues of male vs female Atlantic killifish collected from the AW and KC sites. Data represent fold mRNA expression relative to 18S levels using the Pfaffl method. Error bars show ± SEM, standard error of the mean of 3 pooled samples of five organs per pool for males, and the same for females (n = 3 pooles samples per sex). * Indicates statistically significant difference between populations (p≤0.05).
Figure 5.
Relative fold expression of COX2 in the intestines, livers, and lymphoid tissues of Atlantic killifish, Fundulus heteroclitus, collected from AW and KC sites. Data represent fold mRNA expression relative to 18S levels using the Pfaffl method. Error bars show ± SEM, standard error of the mean of 3 pooled samples of five organs per pool for males, and the same for females (n = 6 pooled samples per site). * Indicates statistically significant difference between populations (p≤0.05).
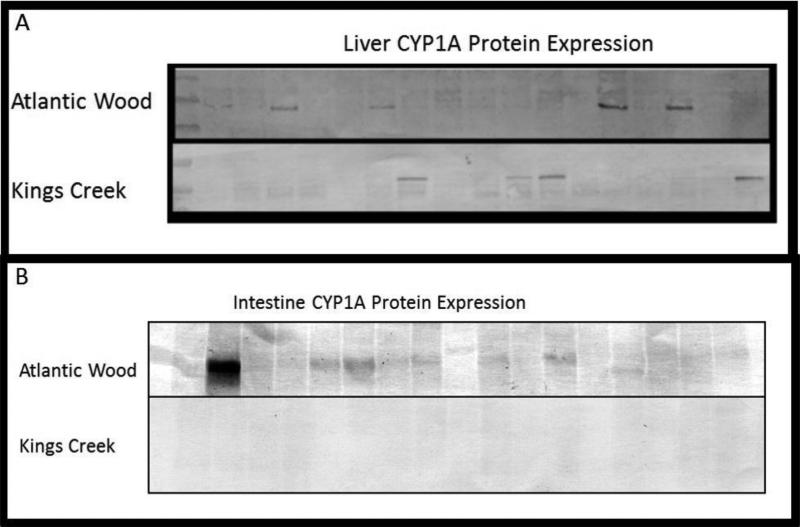
Upon examination of whole liver S9 fractions, CYP1A protein expression was clearly detected in some, but not all fish examined (Figure 6). Even though KC killifish are considered a reference population, CYP1A is expressed in some individuals as well. Intestine CYP1A expression is fairly uniform in all AW fish, with some individuals exhibiting high expression (Figure 6B). No intestine CYP1A protein was observed in KC fish.
Figure 6.
CYP1A protein expression in homogenates from 16 mummichog livers (A) and 16 intestines (B) collected from the AW and KC sites. Proteins were probed with mAb C10-7 for CYP1A detection from 8 adult males (shown at the left of the molecular weight marker) and 8 females (shown in the further right). Homogenates (30 μg per individual sample) were subjected to SDS-PAGE and immunoblotting.
3.2 Monoclonal antibody production and characterization results
Our results show that mAb 5B6 (IgG1κ) is specific to AHR2, (Figure S1), and displays no cross-reactivity with AHR1. This antibody also recognizes protein in both KC and AW killifish, as well as a Clemson University laboratory-reared population. It is noted that AHR2 protein is more highly expressed in fish collected directly from the field than a population reared in the lab (these were all pooled samples). As with mammalian AHR, AHR2 is expressed in all tissues of killifish, as previously reported by others (Merson et al., 2006).
3.3 Liver and intestine CYP1A and AHR2 IHC results
Considering that liver CYP1A is expressed at both the gene and protein level in all adult AW killifish intestines examined, localization of intestinal CYP1A was determined by IHC. And because CYP1A activity is mechanistically related to AHR2 activation, this protein was also examined by IHC. Intestinal AHR2 is equally and abundantly expressed in both populations of killifish, while CYP1A is expressed only in AW fish (Figure 7), thereby supporting gene expression data.
Figure 7.
Representative mmunohistochemistry of CYP1A and AHR2 expression in intestines from killifish collected at the King's Creek reference site (A, B, C) and the Atlantic Wood site (D, E. F). Tissue sections were prepared for IHC and probed with backgroumd stain only, mAb C10-7 for CYP1A, or mAb 5B6 for AHR2 as described in methods. Postive staining is noted by a dark red-brown intensity.
3.4 Liver histopathology and IHC results
Liver sections from 94 AW and 90 KC killifish were examined by microscopy upon H&E staining, as well as AHR2 and CYP1A expression by IHC. As can be seen in representative tissue examples, CYP1A expression patterns are different in representative liver tissues of AW fish livers. For example, within the same liver there may be both upregulation and downregulation of CYP1A, with intense staining in local foci (Figure 8a). At least in one liver examined, an altered hepatocellular focus undetected by H&E staining becomes clear when stained for CYP1A (Figure 8b,c,d), though AHR2 staining is either faint or lacking. Staining for CYP1A and AHR2 reveals that eosinophilic foci express high levels of CYP1A, and little AHR2, while basophilic foci express no CYP1A, but a mild upregulation of AHR2 (Figure 8 h,i, j).
Figure 8.
Immunohistochemical expression of CYP1A and AHR2 in liver of mummichog, Fundulus heteroclitus, inhabiting a creosote-contaminated environment. a) heterogeneous expression of CYP1A protein in mummichog liver exhibiting proliferative liver lesions. UR: up-regulation of CYP1A expression, DR: down-regulation of CYP1A expression, arrows: high CYP1a expression in focal proliferative liver lesions. b) Cryptic altered hepatocellular focus (CF) not apparent in H&E stained section, c) CYP1A over-expression of same CF, d) Lack of AHR2 immunostaining in same CF. e) Eosinophilic altered hepatocellular focus (EF), arrows indicate borders, H&E stain. f) High up-regulation of CYP1a expression in same EF, g) lack of AHR2 expression in same EF. h) Basophilic altered hepatocellular focus (BF) in H&E stained section, i) down-regulation of CYP1A expression in same BF, j) mild up-regulation of AHR2 expression in same BF.
As can be seen by immunohistochemistry, the same liver can harbor multiple lesions, including hepatocellular adenoma and an eosinophilic altered hepatoceullar foci, both of which exhibit upregulation of CYP1A, with lack of AHR2 in either (Figure 9 a,b,c). A hepatocellular carcinoma shows mild expression of CYP1A, with up regulation in a nearby eosinophilic foci, but no AHR2 expression (Figure 9 d, e, f). A fairly large globular hepatocellular carcinoma can be seen exhibiting little CYP1A, except for edges which express high levels of CYP1A, and there is mild expression of AHR2 (Figure 9 g, h, i). Similar lesions were not observed in any of the KC fish livers. No tissue tumors or gross lesions were found in intestine tissues of either population.
Figure 9.
Immunohistochemical expression of CYP1A and AHR2 in livers of mummichog, Fundulus heteroclitus, inhabiting a creosote-contaminated environment. a) hepatocellular adenoma (ADN) and eosinophilic altered hepatocellular foci (EF) in liver parenchyma, H&E stain. b) up-regulation of CYP1A expression within adenoma and altered foci depicted in a). c) Lack of expression of AHR2 in adenoma and altered foci depicted in a). d) Hepatocellular carcinoma (HCC) in liver parenchyma, H&E stain. e) mild focal CYP1A expression within HCC depicted in d) and elevated expression within altered focus (EF). f) Lack of AHR2 expression in carcinoma depicted in d). g) lobular hepatocellular carcinoma (HCC) in liver parenchyma, H&E stain. h) lack of CYP1A expression within carcinoma (HCC) depicted in g). Note highly elevated CYP1A expression in peripheral non-neoplastic parenchyma and high CYP1A expression in some neoplastic hepatocytes (arrows). i) Mild AHR2 expression within carcinoma depicted in g). Note higher expression of non-neoplastic parenchyma at periphery of neoplasm (arrows).
4.0 Discussion
To our knowledge, this study is the first to examine the expression of a battery of genes related to the known toxicity of AHR-active PAHs on the US EPA national priority list simultaneously in intestines, livers, and lymphoid tissues of AW killifish. The most significant observations were that intestine CYP1A and liver COX2 mRNA expression levels are three-fold higher in AW killifish compared to reference fish. In contrast, intestine and lymphoid GSTα gene expression was much lower than in KC fish. At the time of primer design and application in qPCR assays for this study, GSTα was the only killifish GST sequence we were aware of, and therefore the results are interpreted only within the context of this subclass. Subsequent to this study and reporting, it became apparent that killifish GSTmu has been examined, and shown be elevated in killifish residing in Sidney tar ponds of Nova Scotia, Canada (Paetzold et al., 2009a). The full suite of GST subclasses in killifish has not yet been described and characterized.
Multiple classes of GSTs have been identified in other species of teleostean fish, including alpha-, mu-, pi-, and rho-type proteins, each generally being co-expressed, and at high levels (Trute et al., 2007). The difference between classes seems to be related to different oxidized substrates and which types of chemicals induce oxidative stress. In terms of GST activity in Atlantic Wood killifish, previous work demonstrated higher total activity in livers and intestines (Van Veld et al., 1991), and a unique GST protein was isolated from these killifish not present in reference fish (Armknecht et al., 1998). Unfortunately, the particular class of that unique GST described by Armknecht et al. was not identified or further investigated. It is possible that GSTs other than the alpha class are elevated in AW killifish, while alpha is either suppressed or selected against. As previously addressed, GSTmu in killifish has been reported (Paetzold et al., 2009b). Now that the genome of killifish has been sequenced, and annotation continues, it will be possible to later revisit this issue of GST forms in fish within the Elizabeth River system.
At the time of this study, only UDP-UGT2A (UGT-2A) was available. Though not statistically significant, there was a trend towards higher UGT expression in lymphoid tissues of KC, indicating the possible presence of phenolic intermediates as substrates in these tissues. It is difficult to explain the mechanisms behind reduced phase II enzymes in AW killifish intestines and lymphoid tissues, and elevated intestinal CYP1A, when each of these enzymes are under the direct control of the AHR as part of the battery of genes expressed. Much of what is known about AHR activity is based on either in vitro studies, or on whole animal studies beginning with unexposed animals and following activation of the AHR over time after exposure. In a field study like the one described herein, exposures are ongoing, and fish are exposed to multiple abiotic and biotic factors and stressors, and some factors may activate, while others inhibit gene expression.
One clue to understanding the data lies in sex differences in GSTα activity, with male AW fishing expressing more than females. These fish were caught and tissues collected on the full moon, which coincides with lunar spawning cycles in killifish, when estrogen is highest in females. The cross talk between estrogen receptors and AHR has been studied extensively, with the understanding that activation of one can inhibit the other by co-opting co-activators (Matthews and Gustafsson, 2006; Safe and Wormke, 2003) But ultimately it is the expressed protein and function that yield the phenotype observed at the time of sampling, and therefore either function or protein expression should be evaluated in future studies.
mRNA expression for AHRR was also different by site and sex, with AW fish expressing more, and females expressing more than males. Why AW fish express more AHRR is unclear at this point, but one can speculate that AHR2 is indeed functional in this population and that AHRR is induced as would be expected. Why the sex difference in AHRR mRNA expression is also unclear, and it may be related to the amount of estrogen in circulation at the time of sampling (spawning), which may inhibit the expression of AHR-related signaling (Elskus, 2004). Future studies should consider examining hepatic cellular sub fractions (e.g., membrane, cytoplasm, nuclear, bile canaliculi) fractions for AHRR protein in these killifish.
Upon examination of liver CYP1A protein expression by immunoblotting, it was clear that several individuals from both sites had appreciable levels of expression. This is probably due to different reasons; expression in reference fish is more than likely the result of random exposure to motor boat oil, or creosote leaking from a small bridge near the collection site (personal observation). In Atlantic Wood fish, induction of liver CYP1A is probably due to either AHR2-related signaling from environmental exposures to AHR-ligands, or intrinsic aspects of lesions and tumors (as further discussed below). Perhaps a contributing factor in reduced liver CYP1A activity in response to BAP in adult fish noted in earlier studies (Van Veld and Westbrook, 1995) is liver damage already present in adult AW fish, and this may be the case in some of the fish sampled in this study. To this point, it is well known that liver damage reduces expression and function of CYP genes and proteins (Rodighiero, 2012). However, nearly twenty years ago the same profile of liver lesion expression of CYP1A observed in this study was observed histologically (Van Veld et al., 1992), though they did not examine AHR2 expression at the time. It was speculated that progression of tumor types follows a predictable course of over-expression of CYP1A in early lesions (pre-cancerous altered hepatocellular foci), and reduction or absence in more advanced lesions (adenoma, carcinoma, hepatoblastomas), as has been well documented in rat liver tumor models (Roomi et al., 1985).
From another perspective, recent studies show that some human breast cancers constitutively express high levels of CYP1A1 (Rodriguez and Potter, 2013), and that knocking down CYP1A1 in breast cancer cells lines results in reduced cell cycling, growth rates, and intracellular signaling related to proliferation. Though not examined in the Rodriquez and Potter study, constitutive expression of CYP1A1 would suggest a likewise constitutive expression and activity of AHR, which is known to be the case in several tumor types and in inflammation (Moennikes et al., 2004; Tauchi et al., 2005). As noted in our AW liver lesions, eosinophilic altered hepatocellular foci express CYP1A over surrounding normal parenchyma, but little AHR2, while basophilic hepatocellular foci express little or no CYP1A, but higher levels of AHR2 than in surrounding cells, and especially in cells bordering the basophilic lesion and normal cells. Whether or not the expression of CYP1A by certain lesions contributes to the resistance phenotype of AW fish is unknown, and our study does not offer additional answers to whether or not tumor-expression of CYP proteins is adaptive or not. However, at the whole organ level, total CYP1A gene and protein may be minimal, which may explain low levels of liver CYP1A protein expression in some individuals examined by immunoblotting.
One of the more interesting findings from this study is a strong association between CYP1A and liver COX2 mRNA expression. Elevated COX2 mRNA expression in Atlantic Wood livers is a novel finding, in that a correlation between COX2 and liver pathology in fish has not been published, at least to our knowledge. COX2 expression is known to be directly linked to AHR activity (Degner et al., 2009; Dong et al., 2010; Vogel et al., 2007), is a prognostic indicator in human colon and liver cancer (Eberhart et al., 1994; Kondo et al., 1999), and may have a role in prostate cancer (Song et al., 2002). The significance of high COX2 expression in Atlantic Wood fish livers may be linked to hepatotoxicity (Luster et al., 2001), as well as carcinogenesis (Ognjanovic and Hainaut, 2010). Elevated COX2 expression in AW livers may also play a role in PAH metabolism, leading to further toxic metabolite formation, as COX2 can metabolize some PAHs to diol-epoxide intermediates (Eling et al., 1990; Parkinson, 2001). Moreover, CYP1A1 may metabolize prostaglandin endoperoxide to the mutagen malondialdehyde (Plastaras et al., 2000), which would further damage local tissues.
Elevated COX2 expression in Atlantic Wood fish livers suggests a state of chronic inflammation-like conditions. Chronic inflammation in mammals is linked to hepatotoxicity (Luster et al., 2001), as well as carcinogenesis (Ognjanovic and Hainaut, 2010), and tumor associated macrophages may actually promote tumor progression (DeNardo and Coussens, 2007; DeNardo et al., 2008; Sica and Mantovani, 2012). Unfortunately, we do not yet have a killifish-specific COX2 antibody that recognizes COX2 by current IHC procedures used in our labs.
5. Conclusions
From this study we conclude that Atlantic killifish inhabiting the waters and sediments of the AW superfund site have active AHR2-related signaling capabilities. This is demonstrated in a uniformly elevated CYP1A expression in intestines of AW fish compared to the reference population, as well as in some of the livers, though in most cases, liver expression may be related to intrinsic biology of lesion type and stage of hepatocarcinogenesis. Also found in this study, is elevated expression of COX2 in AW killifish livers, and this may be the novelist finding. At least in terms of liver lesions, there are many carcinogenic compounds within AW sediments that are not known as AHR ligands, but are associated with liver toxicity and carcinogenesis under experimental conditions, including arsenic, pentachlorophenol, and several PAHs (Bunton, 1996; Weisburger, 1978).
Future studies with PAHs on the southern branch of the Elizabeth River will need to focus on other sites because Atlantic Wood superfund site is now officially sealed off (Di Giulio and Clark, 2015). As recently described, another creosote-contaminated site on the Elizabeth River, referred to as “Republic” also has high levels of sediment PAHs, and killifish from this site also exhibit a resistance phenotype, at least with developing embryos (Clark et al., 2013b). This site may allow researchers to continue with this line of research. Two very key questions should be answered going forward; what are the GST classes expressed in creosote-adapted killifish, and what are their regulatory restraints, and; are there unique AHR2 nsSNPs in these fish, thus allowing them to not respond to typical PAH ligands as suggested by recent studies with fish adapted to PCBs (Reitzel et al., 2014)? These two questions can be answered with information now available from the killifish genome project, and using recently published approaches (Reitzel et al., 2014).
Supplementary Material
Highlights.
-AHR-related activities in creosote-adapted adult killifish were examined
-Creosote-adapted adult killifish have elevated intestine CYP1A
-Creosote-adapted adult killifish have elevated liver COX2 mRNA expression
-Most creosote-adapted adult killifish have lesions varying in severity
-Liver lesions in creosote-adapted adult killifish express CYP1A and AHR2 proteins
Acknowledgments
The authors thank Dr. Mark Hahn and Dr. Sibel Karchman at the Woods Hole Oceanographic Institutions for the recombinant AHR2a expression plasmid. Feedback and continued discussions with Dr. Rich Di Giulio at Duke University regarding the Elizabeth River system and the resistance phenotype in Atlantic Wood killifish were greatly appreciated. Finally, a sincere appreciation is extended to Dr. Pete van Veld for his help, both in the field and in the lab, and for his pioneering work with the AW killifish population. This work was funded by grant NIH grant 1R15ES016905-01 to CDR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
References
- Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The Zebrafish (Danio rerio) Aryl Hydrocarbon Receptor Type 1 Is a Novel Vertebrate Receptor. Mol Pharmacol. 2002;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- Armknecht SL, Kaattari SL, Van Veld PA. An elevated glutathione S-transferase in creosote-resistant mummichog (Fundulus heteroclitus). Aquatic Toxicology. 1998;41:1–16. [Google Scholar]
- Bunton TE. Review Article: Experimental Chemical Carcinogenesis in Fish. Toxicol Pathol. 1996;24:603–618. doi: 10.1177/019262339602400511. [DOI] [PubMed] [Google Scholar]
- Christine Paetzold S, Ross NW, Richards RC, Jones M, Hellou J, Bard SM. Up-regulation of hepatic ABCC2, ABCG2, CYP1A1 and GST in multixenobiotic-resistant killifish (Fundulus heteroclitus) from the Sydney Tar Ponds, Nova Scotia, Canada. Mar Environ Res. 2009;68:37–47. doi: 10.1016/j.marenvres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Clark B, Bone AJ, Giulio RT. Resistance to teratogenesis by F1 and F2 embryos of PAH-adapted Fundulus heteroclitus is strongly inherited despite reduced recalcitrance of the AHR pathway. Environ Sci Pollut Res. 2013a:1–11. doi: 10.1007/s11356-013-2446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound- and Mixture-Specific Differences in Resistance to Polycyclic Aromatic Hydrocarbons and PCB-126 among Fundulus heteroclitus Subpopulations throughout the Elizabeth River Estuary (Virginia, USA). Environmental Science & Technology. 2013b;47:10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus). Aquatic Toxicology. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3′-diindolylmethane in breast cancer cells. J Nutr. 2009;139:26–32. doi: 10.3945/jn.108.099259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast cancer research : BCR. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer metastasis reviews. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- Di Giulio RT, Clark BW. The Elizabeth River Story: A Case Study in Evolutionary Toxicology. Journal of Toxicology and Environmental Health, Part B. 2015:1–40. doi: 10.1080/15320383.2015.1074841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering J, Giesy J, Wiseman S, Hecker M. Predicting the sensitivity of fishes to dioxin-like compounds: possible role of the aryl hydrocarbon receptor (AhR) ligand binding domain. Environ Sci Pollut Res. 2013;20:1219–1224. doi: 10.1007/s11356-012-1203-7. [DOI] [PubMed] [Google Scholar]
- Dong W, Matsumura F, Kullman SW. TCDD Induced Pericardial Edema and Relative COX-2 Expression in Medaka (Oryzias Latipes) Embryos. Toxicological Sciences. 2010;118:213–223. doi: 10.1093/toxsci/kfq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Eling TE, Thompson DC, Foureman GL, Curtis JF, Hughes MF. Prostaglandin H Synthase and Xenobiotic Oxidation. Annual Review of Pharmacology and Toxicology. 1990;30:1–45. doi: 10.1146/annurev.pa.30.040190.000245. [DOI] [PubMed] [Google Scholar]
- Elskus AA. Estradiol and estriol suppress CYP1A expression in rainbow trout primary hepatocytes. Marine environmental research. 2004;58:463–467. doi: 10.1016/j.marenvres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- EPA, U. EPA Superfund Program. Atlantic Wood industries, Inc.; Portsmouth VA USA: http://www.epa.gov/reg3hwmd/npl/VAD990710410.htm. [Google Scholar]
- Faisal M, Weeks BA, Vogelbein WK, Huggett RJ. Evidence of aberration of the natural cytotoxic cell activity in Fundulus heteroclitus (Pisces: Cyprinodontidae) from the Elizabeth River, Virginia. Veterinary Immunology and Immunopathology. 1991;29:339–351. doi: 10.1016/0165-2427(91)90024-7. [DOI] [PubMed] [Google Scholar]
- Fournie JW, Vogelbein WK. Exocrine Pancreatic Neoplasms in the Mummichog (Fundulus heteroclitus) from a Creosote-Contaminated Site. Toxicol Pathol. 1994;22:237–247. doi: 10.1177/019262339402200302. [DOI] [PubMed] [Google Scholar]
- Frederick LA, Van Veld PA, Rice CD. Bioindicators of Immune Function in Creosote-adapted Estuarine Killifish,Fundulus heteroclitus. Journal of Toxicology and Environmental Health, Part A. 2007;70:1433–1442. doi: 10.1080/15287390701382910. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem Pharmacol. 2009;77:485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Franks DG, Merson RR. Aryl hydrocarbon receptor polymorphisms and dioxin resistance in Atlantic killifish (Fundulus heteroclitus). Pharmacogenetics and Genomics. 2004;14:131–143. doi: 10.1097/00008571-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Hansson MC, Hahn ME. Functional properties of the four Atlantic salmon (Salmo salar) aryl hydrocarbon receptor type 2 (AHR2) isoforms. Aquat Toxicol. 2008;86:121–130. doi: 10.1016/j.aquatox.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny MJ, Karchner SI, Franks DG, Woodin BR, Stegeman JJ, Hahn ME. Distinct Roles of Two Zebrafish AHR Repressors (AHRRa and AHRRb) in Embryonic Development and Regulating the Response to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicological Sciences. 2009;110:426–441. doi: 10.1093/toxsci/kfp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleos tFundulus heteroclitus : Evidence for a novel subfamily of ligand-bidning basic helix loop helix-per-PER-ARNT-SIM (bHLH-PAS) factors. Journal of Biological Chemistry. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- Kelly-Reay K, Weeks-Perkins BA. Determination of the macrophage chemiluminescent response in Fundulus heteroclitus as a function of pollution stress. Fish & Shellfish Immunology. 1994;4:95–105. [Google Scholar]
- Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K, Matsuura N, Wakasa K.-i., Nakamori S, Sakon M, Monden M. Increased Expression of COX-2 in Nontumor Liver Tissue Is Associated with Shorter Disease-free Survival in Patients with Hepatocellular Carcinoma. Clinical Cancer Research. 1999;5:4005–4012. [PubMed] [Google Scholar]
- Luster MI, Simeonova PP, Gallucci RM, Bruccoleri A, Blazka ME, Yucesoy B. Role of inflammation in chemical-induced hepatotoxicity. Toxicology Letters. 2001;120:317–321. doi: 10.1016/s0378-4274(01)00284-3. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson J-Å. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nuclear Receptor Signaling. 2006;4:e016. doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson RR, Franks DG, Karchner SI, Hahn ME. Development and characterization of polyclonal antibodies against the aryl hydrocarbon receptor protein family (AHR1, AHR2, and AHR repressor) of Atlantic killifish Fundulus heteroclitus. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:85–94. doi: 10.1016/j.cbpc.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in Killifish (Fundulus heteroclitus): Heritability of Altered Expression and Relationship to Survival in Contaminated Sediments. Toxicological Sciences. 2002;68:69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes & Development. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L, Schwarz M. A Constitutively Active Dioxin/Aryl Hydrocarbon Receptor Promotes Hepatocarcinogenesis in Mice. Cancer Research. 2004;64:4707–4710. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- Ognjanovic S, Hainaut P. 14.19 - Inflammation in Carcinogenesis. In: McQueen CA, editor. Comprehensive Toxicology (Second Edition) Elsevier; Oxford: 2010. pp. 401–415. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environmental Toxicology and Chemistry. 2002;21:1897–1902. [PubMed] [Google Scholar]
- Paetzold S, Ross N, Richards R, Jones M, Hellou J, Bard S. Up-regulation of hepatic ABCC2, ABCG2, CYP1A1, and GST in multixenobiotic-resistant killifish (fundulus heteroclitus) from the Sydney Tar ponds, nova Scotia, Canada. Mar Environ Res. 2009a;68:37–47. doi: 10.1016/j.marenvres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Paetzold SC, Ross NW, Richards RC, Jones M, Hellou J, Bard SM. Up-regulation of hepatic ABCC2, ABCG2, CYP1A1 and GST in multixenobiotic- resistant killifish (Fundulus heteroclitus) from the Sydney Tar Ponds, Nova Scotia, Canada. Mar Environ Res. 2009b;68:37–47. doi: 10.1016/j.marenvres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Parkinson A. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett and Doull's toxicology; The basic science of poisons. McGraw-Hill Medical; New York: 2001. pp. 133–224. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Research. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastaras JP, Guengerich FP, Nebert DW, Marnett LJ. Xenobiotic metabolizing Cytochromes P450 Convert Prostaglandin Endoperoxide to Hydroxyheptadecatrienoic Acid and the Mutagen, Malondialdehyde. Journal of Biological Chemistry. 2000;275:11784–11790. doi: 10.1074/jbc.275.16.11784. [DOI] [PubMed] [Google Scholar]
- Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory Methods in Histotechnology. Armed Forces Institute of PathologyAmerican Registry of Pathology; Washington, DC.: 1992. [Google Scholar]
- Reitzel A, Karchner S, Franks D, Evans B, Nacci D, Champlin D, Vieira V, Hahn M. Genetic diversity at aryl hydrocarbon receptor loci in PCB sensitive and PCB resistant populations of Atlantic killifish (Fundulus heteroclitus). BMC Evol Biol. 2014;14:6. doi: 10.1186/1471-2148-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CD, Schlenk D, Ainsworth J, Goksøyr A. Cross-reactivity of monoclonal antibodies against peptide 277–294 of rainbow trout CYP1A1 with hepatic CYP1A among fish. Mar Environ Res. 1998;46:87–91. [Google Scholar]
- Rodighiero V. Effects of Liver Disease on Pharmacokinetics. Clinical Pharmacokinetics. 2012;37:399–431. doi: 10.2165/00003088-199937050-00004. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Potter DA. CYP1A1 Regulates Breast Cancer Proliferation and Survival. Molecular Cancer Research. 2013;11:780–792. doi: 10.1158/1541-7786.MCR-12-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi MW, Ho RK, Sarma DSR, Farber E. A Common Biochemical Pattern in Preneoplastic Hepatocyte Nodules Generated in Four Different Models in the Rat. Cancer Research. 1985;45:564–571. [PubMed] [Google Scholar]
- Rose WL, French BL, Reichert WL, Faisal M. DNA adducts in hematopoietic tissues and blood of the mummichog (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, Virginia. Mar Environ Res. 2000;50:581–589. doi: 10.1016/s0141-1136(00)00252-x. [DOI] [PubMed] [Google Scholar]
- Rose WL, French BL, Reichert WL, Faisal M. Persistence of benzo[a]pyrene–DNA adducts in hematopoietic tissues and blood of the mummichog, Fundulus heteroclitus. Aquatic Toxicology. 2001;52:319–328. doi: 10.1016/s0166-445x(00)00125-9. [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory Aryl Hydrocarbon Receptor–Estrogen Receptor α Cross-Talk and Mechanisms of Action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Lin H-P, Johnson AJ, Tseng P-H, Yang Y-T, Kulp SK, Chen C-S. Cyclooxygenase-2, Player or Spectator in Cyclooxygenase-2 Inhibitor- Induced Apoptosis in Prostate Cancer Cells. Journal of the National Cancer Institute. 2002;94:585–591. doi: 10.1093/jnci/94.8.585. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Hida A, Negishi T, Katsuoka F, Noda S, Mimura J, Hosoya T, Yanaka A, Aburatani H, Fujii-Kuriyama Y, Motohashi H, Yamamoto M. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–9368. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Weighardt H, Wolff S, Gotz C, Forster I, Kohne Z, Huebenthal U, Merk HF, Abel J, Haarmann-Stemmann T, Krutmann J, Fritsche E. Aryl Hydrocarbon Receptor Repressor (AhRR) Function Revisited: Repression of CYP1 Activity in Human Skin Fibroblasts Is Not Related to AhRR Expression. J Invest Dermatol. 2013;133:87–96. doi: 10.1038/jid.2012.259. [DOI] [PubMed] [Google Scholar]
- Trute M, Gallis B, Doneanu C, Shaffer S, Goodlett D, Gallagher E. Characterization of hepatic glutathione S-transferases in coho salmon (Oncorhynchus kisutch). Aquatic Toxicology. 2007;81:126–136. doi: 10.1016/j.aquatox.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tiem LA, Di Giulio RT. AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio). Toxicol Appl Pharmacol. 2011;254:280–287. doi: 10.1016/j.taap.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veld P, Ko U, Vogelbein W, Westbrook D. Glutathione S-transferase in intestine, liver and hepatic lesions of mummichog (Fundulus heteroclitus) from a creosote-contaminated environment. Fish Physiol Biochem. 1991;9:369–376. doi: 10.1007/BF02265157. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Vogelbein WK, Smolowitz R, Woodin BR, Stegeman JJ. Cytochrome P450IA1 in hepatic lesions of a teleost fish (Fundulus heteroclitus) collected from a polycyclic aromatic hydrocarbon-contaminated site. Carcinogenesis. 1992;13:505–507. doi: 10.1093/carcin/13.3.505. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Westbrook DJ. Evidence for depression of cytochrome P4501A in a population of chemically resistant mummichogs, (Fundulus heteroclitus). Environ Sci. 1995;3:221–234. [Google Scholar]
- Vogel CFA, Li W, Sciullo E, Newman J, Hammock B, Reader JR, Tuscano J, Matsumura F. Pathogenesis of Aryl Hydrocarbon Receptor-Mediated Development of Lymphoma Is Associated with Increased Cyclooxygenase-2 Expression. Am J Pathol. 2007;171:1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelbein WK, Fournie JW, Cooper PS, Van Veld PA. Hepatoblastomas in the mummichog, Fundulus heteroclitus (L.), from a creosote-contaminated environment: a histologic, ultrastructural and immunohistochemical study. Journal of Fish Diseases. 1999;22:419–431. [Google Scholar]
- Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ. Hepatic Neoplasms in the Mummichog Fundulus heteroclitus from a Creosote-contaminated Site. Cancer Research. 1990;50:5978–5986. [PubMed] [Google Scholar]
- Vogelbein WK, Unger MA. Liver carcinogenesis in a non-migratory fish: The association with polycyclic aromatic hydrocarbon exposure. Bull. Eur. Ass. Fish. Pathol. 2006;26:11–20. [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic Embryotoxicity of Polycyclic Aromatic Hydrocarbon Aryl Hydrocarbon Receptor Agonists with Cytochrome P4501A Inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004a;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res. 2004b;58:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Swails EE, Di Giulio RT. Effects of single and combined exposures to benzo(a)pyrene and 3,3′4,4′5-pentachlorobiphenyl on EROD activity and development in Fundulus heteroclitus. Mar Environ Res. 2002;54:279–283. doi: 10.1016/s0141-1136(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Weisburger EK. Mechanisms of Chemical Carcinogenesis. Annual Review of Pharmacology and Toxicology. 1978;18:395–415. doi: 10.1146/annurev.pa.18.040178.002143. [DOI] [PubMed] [Google Scholar]
- Williams CA. Toxicity resistance in mummichog (Fundulus heteroclitus) from a chemically contaminated environment. 1994 [Google Scholar]
- Wills LP, Jung D, Koehrn K, Zhu S, Willett KL, Hinton DE, Di Giulio RT. Comparative chronic liver toxicity of benzo[a]pyrene in two populations of the atlantic killifish (Fundulus heteroclitus) with different exposure histories. Environ Health Perspect. 2010a;118:1376–1381. doi: 10.1289/ehp.0901799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquatic Toxicology. 2010b;99:33–41. doi: 10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Zhu S, Willett KL, Di Giulio RT. Effect of CYP1A inhibition on the biotransformation of benzo[a]pyrene in two populations of Fundulus heteroclitus with different exposure histories. Aquat Toxicol. 2009;92:195–201. doi: 10.1016/j.aquatox.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.