Abstract
The calyx of Held synapse plays an important role in the auditory system, relaying information about sound localization via fast and precise synaptic transmission, which is achieved by its specialized structure and giant size. During development, the calyx of Held undergoes anatomical, morphological, and physiological changes necessary for performing its functions. The large dimensions of the calyx of Held nerve terminal are well suited for direct electrophysiological recording of many presynaptic events that are difficult, if not impossible to record at small conventional synapses. This unique accessibility has been used to investigate presynaptic ion channels, transmitter release, and short-term plasticity, providing invaluable information about basic presynaptic mechanisms of transmission at a central synapse. Here, we review anatomical and physiological specializations of the calyx of Held, summarize recent studies that provide new mechanisms important for calyx development and reliable synaptic transmission, and examine fundamental presynaptic mechanisms learned from studies using calyx as a model nerve terminal.
1. Introduction
Sound localization, a sensory processing vital for most animals, requires precise and specialized neuronal circuitry. Among several synapses with unique properties to accommodate this processing, the calyx of Held synapse receives special attention because of its morphological and electrophysiological characteristics that facilitate precise transmission of signal information. This synapse is composed of a single large nerve terminal, which originates from the globular bushy cells in the ventral cochlear nucleus (VCN), and the cell body of the principal neuron in the medial nucleus of the trapezoid body (MNTB) (Borst et al., 2012a; Borst et al., 2012b; Schneggenburger et al., 2006). Prior to hearing onset, which occurs around postnatal day (P) 11-12 in rats (Blatchley et al., 1987; Geal-Dor et al., 1993; Kamiya et al., 2001), the calyx of Held undergoes rapid morphological and functional transformation (Hoffpauir et al., 2006; Iwasaki et al., 2001; Taschenberger et al., 2000; Taschenberger et al., 2002) that ensures fast and dependable relay of sound localization information. Interestingly, most of these developmental steps occur before the onset of hearing in mice, supporting the view that sensory activity does not play a major role in the formation of the calyx synapse, and its maturation is rather guided by intrinsic signaling mechanisms (Erazo-Fischer et al., 2007; Hoffpauir et al., 2006; Rodriguez-Contreras et al., 2008; Youssoufian et al., 2008). However, in other rodent species, sensory activity might play a bigger role in calyx development, suggesting species-specific differences in the role of genetic and activity-dependent components (Felmy et al., 2004; Ford et al., 2009).
The calyx of Held is characterized by its giant size, covering a large area of the soma of the postsynaptic principal neuron in the MNTB of an adult rat or mouse (Schneggenburger et al., 2006). The axosomatic nature of this synapse is thought to account for high speed and efficiency of synaptic transmission, setting it apart from small conventional synapses (Borst et al., 2012a). The calyx nerve terminal harbors several hundred active zones, which are the sites of synaptic contact where transmitter release occurs (Sätzler et al., 2002; Taschenberger et al., 2002). A single action potential (AP) fired by the globular bushy cell in the VCN can release a large number of synaptic vesicles containing glutamate, resulting in rapid and effective activation of the postsynaptic neuron in the MNTB (Borst et al., 1996). Release strength and plasticity are efficiently controlled by the amount of calcium (Ca2+) influx via the voltage-gated Ca2+ channels (VGCCs) and dependent on the Ca2+ channel number, composition, and arrangement within the active zone (Hoffpauir et al., 2006; Iwasaki et al., 1998; Meinrenken et al., 2002; Nakamura et al., 2015; Sheng et al., 2012; Wu et al., 1998; Wu et al., 1999b). These specialized structural and functional properties of the calyx of Held nerve terminal discussed here and postsynaptic MNTB neuron discussed in greater details in other reviews (Borst et al., 2012a; Kopp-Scheinpflug et al., 2011) guarantee high fidelity transmission of acoustic signal information for further processing by the auditory circuits (Trussell, 1999).
The large synapse formed by the calyx of Held presynaptic terminal onto principal cell of the MNTB allows for direct electrophysiological analysis of presynaptic calcium currents, mechanisms of vesicle release and recycling, and for simultaneous recordings of corresponding changes in excitatory postsynaptic currents (EPSCs) (Borst et al., 1995; Chuhma et al., 1998; Forsythe, 1994; Sun et al., 2001; Wong et al., 2003). The unique accessibility of the calyx of Held to whole-cell recording has also been used for combined electrophysiological and calcium-imaging studies, investigating presynaptic Ca2+ dynamics in a single nerve terminal (Bollmann et al., 2000; Bollmann et al., 1998; Schneggenburger et al., 2000; Xu et al., 2005). Using calyx as a model, molecules important for control of vesicle exocytosis such as synaptotagmins have been determined (Kochubey et al., 2011a; Kochubey et al., 2011b; Lou et al., 2005; Sun et al., 2007). Endocytosis, a process of vesicle membrane retrieval after exocytosis, has also been studied in great detail using the calyx of Held (Renden et al., 2007; Sun et al., 2001; Sun et al., 2002; Wu et al., 2014a; Wu et al., 2009b; Yamashita et al., 2005). Here, we review morphological and physiological properties of the calyx of Held and their changes during development that make this synapse suitable for its auditory function and a useful model for studying presynaptic mechanisms of synaptic transmission and plasticity.
2. Specialized Morphology and Structure of the calyx of Held
2.1 Anatomy of the MNTB and its input and output projections
In the adult, the globular bushy cells of the VCN send large-diameter axons (measured at 2-3 um in diameter (Ford et al., 2015), which cross the midline of the brainstem and terminate with the calyx-type nerve terminals at the soma of principal neurons of the contralateral MNTB (Harrison et al., 1966; Smith et al., 1991; Spirou et al., 1990). A single MNTB principal neuron receives input from only one calyx of Held, although multiple calyceal inputs are occasionally observed (Bergsman et al., 2004; Hoffpauir et al., 2006; Kuwabara et al., 1991b; Rodriguez-Contreras et al., 2006). The MNTB neurons receive additional non-calyceal excitatory and inhibitory inputs of unclear origins and functions (Hoffpauir et al., 2006; Rodriguez-Contreras et al., 2006; Smith et al., 1991). It has been shown that an inhibitory influence may originate from the ipsilateral ear and can undergo substantial modifications during the course of postnatal development (Green et al., 2005). In addition, a recent study has identified the ventral nucleus of the trapezoid body as one of the major sources of glycinergic inhibitory input to the MNTB (Albrecht et al., 2014).
The MNTB principal cells provide inhibitory glycinergic projections to adjacent nuclei in the superior olivary complex, including the lateral superior olive (Kuwabara et al., 1991a; Tollin, 2003) and the medial superior olive, which comprise circuitries important for computing interaural intensity levels and time differences, respectively, and which are the first nuclei where information from both ears converges (Banks et al., 1992; Brand et al., 2002; Couchman et al., 2010; Goldberg et al., 1968; Kuwabara et al., 1992). In addition, the MNTB neurons project to the superior paraolivary nuclei (Banks et al., 1992; Sommer et al., 1993) and to the ventral and dorsal nuclei of the lateral lemniscus (Kelly et al., 2009; Siveke et al., 2007; Smith et al., 1998; Sommer et al., 1993). In summary, the calyx of Held relays acoustic information to multiple target nuclei for further computation of sound location.
2.2 Calyx ultrastructure and its relation to transmitter release
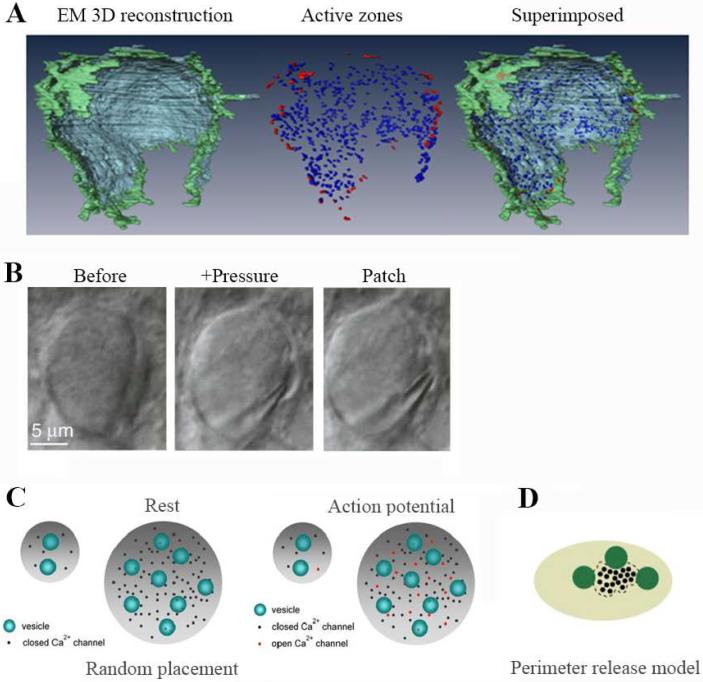
The calyx nerve terminal possesses highly specialized ultrastructural properties as well as universal components of the basic release machinery (Borst et al., 2012a). Its large size, covering ~50% of the soma of the postsynaptic neuron in the MNTB, permits to harbor 300-700 of active zones (Figure 1A), which are morphologically similar to those observed in conventional small nerve terminals (Sätzler et al., 2002; Taschenberger et al., 2002). The active zones in the calyx have an average surface area of 0.1 μm2 and are separated by ~0.6 μm from their nearest neighbors (Sätzler et al., 2002), similar to the dimensions estimated in hippocampal (Schikorski et al., 1997) and cerebellar excitatory synapses (Xu-Friedman et al., 2003; Xu-Friedman et al., 2001).
Figure 1. Calyx ultrastructure.
A. Three-dimensional reconstruction of the calyx of Held with electron microscopy, showing rim and center of the calyx release face (left), active zones at the rim (red) and central region (blue) (middle; each spot represents an active zone) and the two images superimposed (right). The calyx vertical length is ~18 μm (adapted from Satzler et al, 2002 and Sheng et al, 2012). B. Differential infrared contrast (DIC) image of a calyx before (left) and after pressure application (middle) via a pipette that partially separated the calyx from the postsynaptic neuron, and after the cell-attached patch at the calyx release face (right, same pipette as in the middle). C. A schematic drawing showing that an active zone with a small number of Ca2+ channels (left) has fewer RRVs at rest (Rest) and ~1 Ca2+ channel open during an AP (Action Potential). Whereas at an active zone with many channels (right), the RRV number and the Ca2+ channel number per RRV increases (Rest), and the open calcium channel number during an AP (Action Potential) increases to more than 10. D. Perimeter release model, where releasable synaptic vesicles (green) are positioned at the perimeter of a VGCC cluster (adapted with permission from Nakamura et al., 2015).
Synaptic transmission in the calyx of Held is mediated by glutamate release via Ca2+-dependent vesicle exocytosis. Like in other synapses, vesicles in the calyx terminal can be categorized into the readily releasable pool (RRP), the reserve pool, and the recycling pool (Rizzoli et al., 2005). The RRP vesicles are usually docked in the proximity of active zone membrane and undergo exocytosis upon a brief train of stimulation. Indeed, the RRP vesicles at the calyx can be depleted by 25 - 50 APs or a single 20 – 50 ms depolarization pulse (Sakaba et al., 2001b; Schneggenburger et al., 1999; Sun et al., 2001; Wu et al., 1999a; Xu et al., 2005). The RRP’s parameters such as its size, composition, and probability of vesicle release along with mechanisms of their regulation have been extensively studied in the calyx of Held (Chen et al., 2015; Neher, 2015; Sakaba, 2006; Sheng et al., 2012; Thanawala et al., 2013; Wolfel et al., 2007). Heterogeneity of release probability between vesicles in the RRP, which contribute to a fast and a slow component of release, might be influenced by the differences in the intrinsic Ca2+ sensitivity (Wolfel et al., 2007). Vesicles of the reserve pool are thought to be located further in the cytoplasm and do not participate in release under normal conditions (Rizzoli et al., 2005). The recycling pool refers to vesicles being constantly recycled for exocytosis via endocytosis. Earlier studies show that only a small fraction of all vesicles in the calyx (5-20%) participates in recycling in vitro (de Lange et al., 2003) or even less (1-5%) in vivo (Denker et al., 2011). However, recent studies show that most vesicles in the calyx terminal participate in recycling and can be mobilized for exocytosis during repetitive firing (Qiu et al., 2015; Xue et al., 2013). There are more than 200,000 vesicles in the recycling pool (Qiu et al., 2015; Xue et al., 2013), a number similar to the total number of vesicles in the calyx terminal, as estimated by electron microscopy (de Lange et al., 2003; Qiu et al., 2015). Thus, the calyx maximizes its capacity in maintaining high fidelity synaptic transmission by using essentially all vesicles for recycling (Qiu et al., 2015; Xue et al., 2013). The experimental data supporting this conclusion are mostly from P7-9 rats or mice at room temperature. While it is conceivable that the results may apply to older animals in physiological temperature, evidence is needed to confirm this possibility.
Speed and precision of synaptic transmission at the calyx of Held are dependent on the ultrastructural arrangement of the release machinery within the active zone, such as the topography and number of the VGCCs, and their relation to the readily releasable vesicles (RRVs) (Hoffpauir et al., 2006; Nakamura et al., 2015; Sheng et al., 2012; Wang et al., 2008). Effective coupling between VGCCs and vesicles plays an important role in determining synaptic strength of the calyx of Held synapses (Chen et al., 2015; Fedchyshyn et al., 2005; Sheng et al., 2012; Wu et al., 1998; Wu et al., 1999b). A recent study uses an advanced technique to patch predominantly single active zones at the release face of the calyx nerve terminal in P8-10 rats (Figure 1B) (Sheng et al., 2012). This study reports that a single active zone contains 5-218 calcium channels with an average of 42, and 1–10 RRVs with an average of 5. An AP opens 1-35 calcium channels with a mean of 7 channels, indicating that multiple VGCCs are open at single active zones to control release. The large variation in the number of VGCCs per active zone and thus the number of open calcium channels per AP causes a large difference in release strength by regulating the release probability of the RRVs (Figure 1C) (Sheng et al., 2012). An active zone with higher density of VGCCs has higher release probability of the RRVs, which may lead to multi-vesicular release during single AP, but if more than one AP is repeated at higher frequency, it may lead to short-term depression of release (Sheng et al., 2012). Evidently, the density of calcium channels at the active zone controls the release strength, multi-vesicular release, and the properties of short-term synaptic plasticity.
An electron microscopy study by Wimmer et al., 2006 showed a “donut”-like formation of synaptic vesicles housing a central cluster of interconnected mitochondria (Wimmer et al., 2006). Interestingly, “donuts”-like assemblies only appear during maturation of the calyx of Held with the onset of hearing. This structural specialization of the release apparatus and mitochondria might represent an optimal spatial arrangement, which may contribute to the changes in functional properties known to accompany postnatal calyx maturation. This ultrastructural arrangement might be advantageous for sustaining high rates of transmitter release during the high frequency firing that occurs physiologically at the calyx (Wimmer et al., 2006).
An arrangement of the release machinery components in the active zone has been recently investigated at a nanometer scale (Nakamura et al., 2015). This study demonstrates that vesicular release at the calyx of Held is driven by Ca2+ channel clusters with an average of 20–30 channels per cluster and that at the majority of release sites multiple VGCCs contribute to the release of each vesicle following an AP. Moreover, experimental data together with simulation suggest a topographical arrangement, termed perimeter release model (Figure 1D), where most releasable vesicles are located 15–30 nm from the outer perimeter of VGCC clusters (Keller et al., 2015; Nakamura et al., 2015). Further evidence such as direct visualization of the anatomical relation between released vesicles and opened VGCCs is needed to support this model and would be an interesting research direction in the future.
3. Development and maturation of the calyx of Held
3.1 Axonal growth and guidance
During embryonic and postnatal development, the calyx of Held undergoes significant transformation in order to possess morphological and functional properties necessary for performing its major role in relaying acoustic information. During embryonic development, axons grow from the VCN towards the midline, cross the midline by embryonic day (E) 15 into the region of the presumptive MNTB (Michalski et al., 2013), and form collateral branches that will terminate in calyces of Held (Hoffpauir et al., 2006). A combination of attractive and repulsive molecules guides the axons across the midline to the contralateral MNTB and guarantees formation of proper synaptic contacts (Yu et al., 2014). For instance, Netrin-1, an attractive cue molecule (Mitchell et al., 1996) is expressed at the brainstem midline and the VCN axons express its receptor, DCC (Howell et al., 2007). Deletion of either Netrin-1 or DCC leads to a failure of the VCN axons to reach the midline, suggesting that midline attraction is mediated by Netrin/DCC signaling (Howell et al., 2007). On the other hand, deletion of Robo3, which is known to mediate repulsion, causes the VCN axons to be prematurely repelled from the midline, resulting in ipsilateral mistargeting (Renier et al., 2010).
Before crossing the midline, the VCN axons must ignore analogous targets in the ipsilateral MNTB. This feature may depend on the activation of either Eph receptors expressed by the VCN axons, or their specific membrane-bound ephrin ligands located in the MNTB (Hsieh et al., 2010). Interestingly, deletion of ephrin ligand, ephrin B2, and not its EphB2 receptor, leads to the appearance of abnormal ipsilateral calyces, suggesting that “reverse” signaling through ephrin B2 inhibits formation of ipsilateral calyces in the MNTB (Hsieh et al., 2010). Taken together, multiple guidance cues are required to control the VCN axons reaching their appropriate targets.
3.2 Formation of a giant synapse
In rodents, immature calyces of Held appear in the MNTB during the first few days of postnatal life (Kandler et al., 1993). At the beginning of this period, principal neurons are innervated by several fibers arriving from the VCN, which form a number of small glutamatergic nerve terminals (Hoffpauir et al., 2006; Hoffpauir et al., 2010; Rodriguez-Contreras et al., 2006). Between P2 and P4, calyces begin to appear and form synapses on the majority of principal cells. During the next developmental stage, which lasts until the onset of hearing, the calyces continue to grow and the collaterals gradually disappear, resulting in elimination of competing synaptic inputs and formation of one major synaptic contact (Hoffpauir et al., 2006; Rodriguez-Contreras et al., 2006; Rodriguez-Contreras et al., 2008). During the second and third postnatal weeks, morphological maturation of the calyx involves appearance of many digit-like processes, protruding from the presynaptic membrane (Kandler et al., 1993), a process called fenestration (Ford et al., 2009). Notably, formation and maturation of the calyx of Held are mostly activity-independent (Nakamura et al., 2011), as studies using deaf mouse mutants or suppression of spontaneous activity in the prehearing auditory system do not show any major deficits in the calyx of Held development (Clause et al., 2014; Robertson et al., 1998; Youssoufian et al., 2008). Thus, morphological changes of the calyx of Held may not rely on the external stimuli and neuronal activity and are likely dependent on the intrinsic signals.
A recent study demonstrates that proper establishment of the key features of the calyx of Held such as giant size, number of active zones, elimination of competing synaptic inputs, developmental gain of rapid transmitter release and high release efficacy, depend on a target-derived family of bone morphogenetic proteins (BMPs) (Xiao et al., 2013). Both BMP receptors and BMP ligands are expressed in the MNTB (Xiao et al., 2013). Deletion of BMP receptors leads to smaller-size terminals, reduced number of active zones and docked vesicles per active zone, and reduced EPSC, suggesting abnormal morphology and function in the absence of BMP signaling (Xiao et al., 2013). Moreover, in the absence of BMP signaling, MNTB neurons are innervated by several nerve endings of smaller size, suggesting that BMP signaling is also important for elimination of competing synaptic inputs (Xiao et al., 2013).
3.3 Refinement of synaptic functions
The physiological properties of the calyx develop in parallel to morphological changes, ensuring that synaptic transmission is fast and reliable (Borst et al., 2012a; Schneggenburger et al., 2006). Between the first and second postnatal weeks, the waveform of the presynaptic AP becomes faster and shorter (Figure 2A), which may lead to a reduction in release probability and a decrease in synaptic delays, allowing the calyx to fire at very high frequency (Taschenberger et al., 2000). Changes in the presynaptic AP waveform are in part due to the developmental changes in the voltage-gated sodium and potassium channels, such as the speed up of inactivation and recovery of sodium currents and increased density and faster activation kinetics of potassium currents (Elezgarai et al., 2003; Leao et al., 2005; Nakamura et al., 2007).
Figure 2. Calyx synapse development.
A. Current-clamp whole-cell recordings of presynaptic APs in brainstem slices at P7, P10, and P14. APs were evoked by afferent fiber stimulation (adapted with permission from Taschenberger and Von Gersdorff, 2000). B. A schematic drawing showing developmental changes in the calyx synapse. As synapse mature, vesicle size and number increase; and a mixture of loosely coupled N-, R-, and P/Q-type VGCCs is replaced by tightly coupled P/Q-type VGCCs, which switches release modality from “micro-” to “nanodomain” (modified from Fedchyshyn and Wang, 2005).
As the AP becomes faster and briefer (Taschenberger et al., 2000) , fewer VGCCs open, thereby reducing the amount of presynaptic Ca2+ influx and decreasing the probability of release (Borst et al., 1998b; Yang et al., 2006). To compensate for these changes, the size of the RRP increases (Iwasaki et al., 2001; Taschenberger et al., 2000; Taschenberger et al., 2002). Another important presynaptic adaptation during calyx maturation is a switch in calcium channel subtype composition. Transmitter release in immature calyces at P7-10 is mediated by a mixture of N-, R-, and P/Q-type Ca2+ channels (Figure 2B) (Wu et al., 1998; Wu et al., 1999b). As calyces mature, transmission becomes entirely dependent on P/Q-type Ca2+ channels after P13 (Iwasaki et al., 1998; Iwasaki et al., 2000). N- and R-type Ca2+ channels are more distant from the release site than P/Q-type Ca2+ channels (Wu et al., 1999b), and therefore require more overlapping Ca2+ domains to trigger transmitter release. In contrast, P/Q type channels are thought to be more tightly coupled to synaptic vesicles (Figure 2B) (Inchauspe et al., 2007; Wu et al., 1999b). It has been suggested that during this developmental period, the release modality switches from “microdomain,” involving cooperative action of many loosely coupled N-, R-, and P/Q-type VGCCs, to “nanodomain,” in which opening of fewer tightly coupled P/Q-type VGCCs effectively induces vesicle release (Fedchyshyn et al., 2005; Wang et al., 2008). Filamentous protein Septin 5 may be involved in mediating this switch (Yang et al., 2010).
The tighter spatial association of VGCCs and synaptic vesicles achieves higher local intracellular Ca2+ concentrations sensed by vesicle, which enhances release efficiency and thus quantal output, ensuring reliable transmission in the mature calyx (Chen et al., 2015; Fedchyshyn et al., 2005; Kochubey et al., 2009; Wang et al., 2008). In addition, it may help to minimize asynchronous release and boost synchronous release as calyces mature (Scheuss et al., 2007; Taschenberger et al., 2000). Thus, a tighter coupling between vesicles and VGCCs in the calyx helps to facilitate fast, reliable synaptic transmission and maintain release during high-frequency firing.
4. Calyx as a model to study synaptic transmission and plasticity
The large dimensions of the calyx are well suited for direct electrophysiological recordings and imaging (Borst et al., 1995; Forsythe, 1994; Schneggenburger et al., 2006; Von Gersdorff et al., 2002). For this reason, calyx is a model of choice to study presynaptic ion channels, exocytosis, endocytosis, and short-term plasticity (Borst et al., 1995; Forsythe, 1994; Schneggenburger et al., 2006; Sun et al., 2004; Von Gersdorff et al., 2002). Using brain slice preparation containing the MNTB in the auditory brainstem (Paradiso et al., 2007), a variety of advanced biophysical techniques have been used to study critical steps of synaptic transmission in great detail. For example, presynaptic whole-cell recordings of the AP or calcium currents can be combined with simultaneous postsynaptic EPSC recordings to study how AP or calcium channels control release (Borst et al., 1995; Wu et al., 1998; Wu et al., 2004). Measurements of Ca2+ concentration increase, induced by photolysis of caged Ca2+compounds, are combined with recordings of EPSCs to reveal the sensitivity of calcium sensor located on the release machinery to local Ca2+ concentrations (Bollmann et al., 2000; Schneggenburger et al., 2000; Sun et al., 2007). Whole-cell recordings of the calyx membrane capacitance, a parameter proportional to the surface membrane area, combined with postsynaptic EPSC recordings, are used to study vesicular exo- and endocytosis (Sun et al., 2001; Sun et al., 2002; Yamashita et al., 2005). Cell-attached capacitance recordings at the calyx release face (Figure 1B), particularly at single active zones, combined with calcium channel recordings, are used to study the modes of fusion and how calcium channels control release at single active zones (He et al., 2007; He et al., 2006; Sheng et al., 2012). These advanced techniques have revealed tremendous insights into critical steps of synaptic transmission.
4.1 Exocytosis
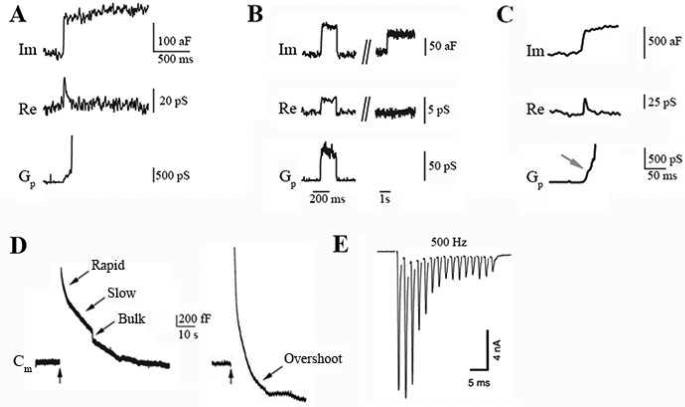
Exocytosis occurs via vesicle fusion with the plasma membrane and involves formation and dilation of the fusion pore and transmitter release into the synaptic cleft. Cell-attached recordings of membrane capacitance and fusion pore conductance at the release face of the calyx (Figure 1B) revealed three fusion modes. The first form is full-collapse fusion that opens a large pore to allow rapid and complete release of transmitter (Figure 3A) (He et al., 2006) The second form involves rapid fusion pore opening and closure, called ‘kiss-and-run’ (Figure 3B) (He et al., 2006), which allows for rapid vesicle recycling. Depending on the pore size, kiss-and-run may serve to limit the rate of transmitter release, although most kiss-and-run events observed at the calyx have a large fusion pore to allow for rapid and complete release of transmitter (He et al., 2006). The third fusion mode involves fusion among vesicles that forms a large compound vesicle, followed by fusion of the compound vesicle with the plasma membrane, termed compound exocytosis (Figure 3C) (He et al., 2009). Compound exocytosis increases the quantal size and thus synaptic strength. It contributes to the generation of a widely observed form of synaptic plasticity, the post-tetanic potentiation, induced by prolonged repetitive nerve firing (He et al., 2009; Xue et al., 2010).
Figure 3. Synaptic transmission and plasticity at the calyx of Held.
A-C. Im (imaginary component of the admittance, reflecting capacitance), Re (real component of the admittance, reflecting conductance), and the fusion pore conductance (Gp). A. The non-flicker up step, not accompanied by detectable Re changes, suggests full collapse fusion. B. The capacitance flicker with a detectable fusion pore conductance indicates kiss-and-run fusion. C. A giant capacitance up step indicates compound exocytosis (adapted from He et al., 2006 and He et al., 2009) D. Four forms of endocytosis (arrows): rapid, slow, bulk, and overshoot endocytosis, were observed by whole-cell capacitance (Cm) recordings at the calyx of Held (vertical arrows indicatestimulus). E. High-frequency stimulation at 500 Hz induces severe short-term depression in the postsynaptic MNTB neuron at P14 (adapted with permission from Taschenberger and Von Gersdorff, 2000).
Like other synapses, the calyx of Held synapse can release either synchronously or asynchronously (Taschenberger et al., 2005). It has been demonstrated that synaptotagmin 2 (Syt2), a member of a family of transmembrane proteins, functions as a Ca2+ sensor for fast synchronous release at calyces (Kochubey et al., 2011a; Kochubey et al., 2011b; Lou et al., 2005; Sun et al., 2007). Deletion of Syt2 abolishes synchronous release from calyx terminals, but leaves a slower, asynchronous form of Ca2+-triggered release intact (Sun et al., 2007). A proposed dual Ca2+sensor model suggests that at high Ca2+ concentrations the release is mediated by the Ca2+-triggering function of Syt2, whereas at the very low Ca2+ concentrations, release is driven by a different unknown Ca2+ sensor (Sun et al., 2007; Xu et al., 2007a).
4.2 Endocytosis
Following exocytosis, membrane is retrieved via endocytosis for recycling and maintaining an adequate rate of synaptic transmission during repetitive firing. Endocytosis influences exocytosis by affecting vesicle availability for release (Wu et al., 2014a) and by membrane clearance at the release sites (Hosoi et al., 2009; Wu et al., 2009b). Using cell-attached capacitance measurements, it has been shown that only a small percentage of fusing vesicles is retrieved via kiss-and-run mechanism in the calyx of Held (He et al., 2006). Whole-cell capacitance recordings from the entire calyx revealed four kinetically different forms of endocytosis: 1) slow endocytosis with a time constant around 10 – 20 s (Sun et al., 2002) , which depends on clathrin (Hosoi et al., 2009; Wu et al., 2009b) and dynamin (Lou et al., 2008; Xu et al., 2008; Yamashita et al., 2005); 2) a rapid form of endocytosis with a time constant of ~1-2 s (Sun et al., 2002; Wu et al., 2005), which depends on GTP hydrolysis and dynamin (Xu et al., 2008); 3) bulk endocytosis which retrieves a vesicle much larger than a regular vesicle (Wu et al., 2007; Wu et al., 2009b); and 4) an endocytosis overshoot, which retrieves more membrane than exocytosed (Figure 3D) (Renden et al., 2007; Wu et al., 2007; Wu et al., 2009b). Whether rapid endocytosis is mediated by kiss-and-run at the single vesicle level remains to be determined in the future. The four different endocytic pathways described above co-exist at the same terminals and contribute to membrane retrieval in an activity- and Ca2+-dependent manner (Wu et al., 2014a; Wu et al., 2005; Wu et al., 2009b). A recent in vivo study examined the mechanisms of vesicle recycling, under physiological patterns of activity and found two forms of endosomal structures that differ in size: small synaptic vesicles and large cisternal endosomes (Korber et al., 2012). The authors suggested that cisternal endosomes serve as intermediates of the vesicle cycle, and that clathrin-mediated endocytosis and endosomal budding are the predominant mechanisms of synaptic vesicle recycling in a tonically active central synapse in vivo (Korber et al., 2012).
Ca2+ influx through the VGCCs increases intracellular Ca2+ concentrations, which initiates endocytosis at calyces (Midorikawa et al., 2014; Sun et al., 2010; Wu et al., 2009b; Xue et al., 2012). Pharmacological studies suggest that calmodulin functions as Ca2+ sensor, mediating calcium-triggered endocytosis at the calyx (Wu et al., 2009b). This is further supported by a study showing that knockdown of calmodulin inhibits endocytosis at hippocampal synapses (Sun et al., 2010). One of the downstream targets of calmodulin is calcineurin, which may dephosphorylate endocytic proteins (Cousin et al., 2001). Studies, using calcineurin knockout mice, demonstrate its involvement in both rapid and slow endocytosis at calyces (Sun et al., 2010; Wu et al., 2014b). However, the use of calcineurin blockers has been controversial as their effect on endocytosis varies between different cell types, developmental stages, and endocytic forms (Smillie et al., 2005; Sun et al., 2010). Our recent study showed that calcineurin and calmodulin blockers slowed down endocytosis at a small calcium influx or in the presence of exogenous calcium buffer, but did not inhibit endocytosis at a large calcium influx, which may explain the discrepancies among previous pharmacological studies (Wu et al., 2014b). Pharmacological evidence also suggests that myosin light chain kinase functions downstream of calmodulin to phosphorylate myosin in accelerating endocytosis at the calyx (Yue et al., 2014). A role of myosin II in endocytosis has also been indicated in hippocampal neurons, where both acute inhibition and genetic knockout of myosin II impair the compensatory endocytosis (Chandrasekar et al., 2013). There are other factors that can potently regulate endocytosis. For example, temperature has been shown to speed up endocytosis, partly because of its facilitation of calcium influx (Renden et al., 2007). Developmental changes lead to faster endocytosis kinetics at the calyx, probably by tighter coupling between endocytosis machinery and the VGCCs (Yamashita et al., 2010). Endocytosis at mature calyx is also accelerated by Rho kinase (Taoufiq et al., 2013) and is subject to retrograde modulation by protein kinase G, which acts through postsynaptic NO pathway (Eguchi et al., 2012). Brain-derived neurotrophic factor inhibits slow and rapid forms of endocytosis at immature and mature calyces (Baydyuk et al., 2015). In addition to proteins, endocytosis also depends on lipid signaling via PIP2 (Taoufiq et al., 2013) and lipid components such as cholesterol (Yue et al., 2015).
Vesicles regenerated via endocytosis are not recycled directly into the RRP for immediate use, but join the recycling vesicle pool, because complete block of rapid and slow endocytosis does not affect the RRP size and replenishment of the RRP (Wu et al., 2009a). The RRP replenishment is inhibited by blockers of calcium influx, calmodulin, dynamin, and AP2, all of which inhibit endocytosis without affecting the RRP size or the calcium current, suggesting that endocytosis is involved in the RRP replenishment (Hosoi et al., 2009; Wu et al., 2009b). The clearance of the exocytic proteins and membranes, produced by exocytosis at the active zone, is therefore proposed as an additional role of endocytosis. A recent study shows that cleavage of two SNARE proteins, SNAP25 and syntaxin, critical for exocytosis, inhibits endocytosis and the RRP replenishment at the calyx. These results suggest the involvement of SNARE proteins in facilitation of vesicle replenishment to the RRP, likely by clearance of exocytotic materials from active zones (Xu et al., 2013). Given that the RRP replenishment depends on calcium and calmodulin (Sakaba et al., 2001a), calcium-triggered endocytosis and active zone clearance are therefore suggested to mediate calcium-dependent facilitation of the RRP replenishment (Wu et al., 2009b). Although the studies described above are done in synapses of animals prior to hearing onset, endocytosis is essential for vesicle recycling at all developmental stages. Moreover, the mechanisms of membrane clearance from active zones may be crucial for recovering synaptic transmission from short-term depression of release induced by repetitive firing during sound stimulation.
4.3 Short-term plasticity
Electrophysiological recordings from calyx of Held synapse have revealed three forms of short-term synaptic plasticity: 1) short-term depression induced by various frequencies of firing (Figure 3E); 2) short-term facilitation induced by very brief high frequency firing train; and 3) post-tetanic potentiation induced by prolonged high frequency firing (Von Gersdorff et al., 2002). Each of these three forms is caused by multiple mechanisms with mostly presynaptic origins. Short-term depression can be caused by depletion of the RRP (Wu et al., 1999a), decrease of the release probability of the RRP vesicles (Wu et al., 1999a) due to calcium current inactivation (Forsythe et al., 1998; Xu et al., 2005), and postsynaptic glutamate receptor desensitization (Erazo-Fischer et al., 2007; Koike-Tani et al., 2008; Wong et al., 2003). Short-term depression is predominantly caused by Ca2+/calmodulin-induced presynaptic calcium channel inactivation during 0.2 to 30 Hz of firing (Xu et al., 2005) and after 0.2 to 100 Hz of firing (Forsythe et al., 1998; Xu et al., 2005), whereas during 100 Hz or higher frequencies of firing, depletion of the RRP is dominant (Xu et al., 2005). Short-term facilitation is caused by residual Ca2+, low level of calcium persistent after an AP or AP train, which may induce an increase in vesicle release probability (Felmy et al., 2003), Ca2+ channel facilitation (Borst et al., 1998a; Cuttle et al., 1998; Xu et al., 2005), and partial saturation of cytosolic Ca2+ buffers (Muller et al., 2008; Muller et al., 2007; Xu et al., 2007b). Post-hearing calyces show increased resistance to synaptic depression (Koike-Tani et al., 2008) and facilitation (Crins et al., 2011), likely due to developmental changes in morphological and functional properties, such as tight coupling of Ca2+ channels and vesicles, increase in vesicle pool, and reduced release probability (Iwasaki et al., 2001; Taschenberger et al., 2000; Taschenberger et al., 2002; Von Gersdorff et al., 2002).
Post-tetanic potentiation, the third form of short-term plasticity observed in calyx, is caused by a long-lasting increase in the presynaptic Ca2+ concentration following tetanus. Prolonged calcium increase may potentiate synapses by increasing the RRP size, the RRP release probability (Habets et al., 2005), the vesicle sensitivity to calcium (Korogod et al., 2007), and quantal size via compound fusion (He et al., 2009). Protein kinase C (PKC) is a calcium sensor mediating post-tetanic potentiation of vesicle release (Fioravante et al., 2011; Fioravante et al., 2014; Korogod et al., 2007), whereas a PKC-independent mechanism facilitates post-tetanic potentiation of quantal size (Xue et al., 2010). At calyces prior to hearing onset, PKCγ mediates post-tetanic potentiation by increasing the release probability (Chu et al., 2014). After hearing onset, PKCβ becomes the dominant PKC isoform, which mediates post-tetanic potentiation by increasing the size of RRP (Chu et al., 2014). Thus, the molecular and cellular mechanisms, underlying different forms of synaptic plasticity, shift during calyx development to modify the pattern of short-term plasticity.
5. Concluding remarks
The auditory circuits are well equipped with morphological and functional properties to maximize synaptic transmission across a wide range of activity levels. The unique characteristics of the calyx of Held described in this review serve a crucial role in precise and timely computation of sound information. Developmental changes in morphology and function following the initial establishment of large calyces help prepare the calyx synapse to function as a fast and dependable relay of neuronal firing signals. A remarkable body of works using calyx synapse as a model to study mechanisms of synaptic transmission and plasticity has revealed precise organization of the release machinery and discovered mechanisms controlling crucial steps of transmitter release.
Highlights.
We review anatomical and physiological specializations of the calyx of Held.
Summarize recent studies that provide new mechanisms important for calyx development and reliable synaptic transmission.
Examine fundamental presynaptic mechanisms learned from studies using calyx as a model nerve terminal.
Acknowledgments
This work was supported by the NINDS Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- Albrecht O, Dondzillo A, Mayer F, Thompson JA, Klug A. Inhibitory projections from the ventral nucleus of the trapezoid body to the medial nucleus of the trapezoid body in the mouse. Frontiers in neural circuits. 2014;8:83. doi: 10.3389/fncir.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. J.Neuorsci. 1992;12:2819–2837. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydyuk M, Wu XS, He L, Wu LG. Brain-derived neurotrophic factor inhibits calcium channel activation, exocytosis, and endocytosis at a central nerve terminal. J Neurosci. 2015;35:4676–82. doi: 10.1523/JNEUROSCI.2695-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsman JB, De Camilli P, McCormick DA. Multiple large inputs to principal cells in the mouse medial nucleus of the trapezoid body. J Neurophysiol. 2004;92:545–52. doi: 10.1152/jn.00927.2003. [DOI] [PubMed] [Google Scholar]
- Blatchley BJ, Cooper WA, Coleman JR. Development of auditory brainstem response to tone pip stimuli in the rat. Brain Res. 1987;429:75–84. doi: 10.1016/0165-3806(87)90140-4. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Helmchen F, Borst JGG, Sakmann B. Postsynaptic Ca2+ influx mediated by three different pathways during synaptic transmission at a calyx-type synapse. J.Neuorsci. 1998;15:10409–10419. doi: 10.1523/JNEUROSCI.18-24-10409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, Soria van Hoeve J. The calyx of held synapse: from model synapse to auditory relay. Annu Rev Physiol. 2012a;74:199–224. doi: 10.1146/annurev-physiol-020911-153236. [DOI] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Facilitation of presynaptic calcium currents in the rat brainstem. J.Physiol. 1998a;513:149–155. doi: 10.1111/j.1469-7793.1998.149by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J.Physiol. 1998b;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JGG, Rusu SI. The Calyx of Held Synapse. Synaptic Mechanisms in the Auditory System. 2012b;41:95–134. [Google Scholar]
- Borst JGG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J.Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–7. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Chandrasekar I, Huettner JE, Turney SG, Bridgman PC. Myosin II regulates activity dependent compensatory endocytosis at central synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:16131–45. doi: 10.1523/JNEUROSCI.2229-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Das B, Nakamura Y, DiGregorio DA, Young SM., Jr. Ca2+ channel to synaptic vesicle distance accounts for the readily releasable pool kinetics at a functionally mature auditory synapse. J Neurosci. 2015;35:2083–100. doi: 10.1523/JNEUROSCI.2753-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Fioravante D, Leitges M, Regehr WG. Calcium-dependent PKC isoforms have specialized roles in short-term synaptic plasticity. Neuron. 2014;82:859–71. doi: 10.1016/j.neuron.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Ohmori H. Postnatal development of phase-locked high-fidelity synaptic transmission in the medial nucleus of the trapezoid body of the rat. J.Neuorsci. 1998;18:512–520. doi: 10.1523/JNEUROSCI.18-01-00512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clause A, Kim G, Sonntag M, Weisz CJ, Vetter DE, Rubsamen R, Kandler K. The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neuron. 2014;82:822–35. doi: 10.1016/j.neuron.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman K, Grothe B, Felmy F. Medial superior olivary neurons receive surprisingly few excitatory and inhibitory inputs with balanced strength and short-term dynamics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:17111–21. doi: 10.1523/JNEUROSCI.1760-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Crins TT, Rusu SI, Rodriguez-Contreras A, Borst JG. Developmental changes in short-term plasticity at the rat calyx of Held synapse. J Neurosci. 2011;31:11706–17. doi: 10.1523/JNEUROSCI.1995-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol. 1998;512(Pt 3):723–9. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange RP, de Roos AD, Borst JG. Two modes of vesicle recycling in the rat calyx of Held. J.Neurosci. 2003;23:10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Bethani I, Krohnert K, Korber C, Horstmann H, Wilhelm BG, Barysch SV, Kuner T, Neher E, Rizzoli SO. A small pool of vesicles maintains synaptic activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17177–82. doi: 10.1073/pnas.1112688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K, Nakanishi S, Takagi H, Taoufiq Z, Takahashi T. Maturation of a PKG-dependent retrograde mechanism for exoendocytic coupling of synaptic vesicles. Neuron. 2012;74:517–29. doi: 10.1016/j.neuron.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Elezgarai I, Diez J, Puente N, Azkue JJ, Benitez R, Bilbao A, Knopfel T, Donate-Oliver F, Grandes P. Subcellular localization of the voltage-dependent potassium channel Kv3.1b in postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience. 2003;118:889–98. doi: 10.1016/s0306-4522(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Erazo-Fischer E, Striessnig J, Taschenberger H. The role of physiological afferent nerve activity during in vivo maturation of the calyx of Held synapse. J Neurosci. 2007;27:1725–37. doi: 10.1523/JNEUROSCI.4116-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of held synapse. J Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F, Schneggenburger R. Developmental expression of the Ca2+-binding proteins calretinin and parvalbumin at the calyx of Held of rats and mice. The European journal of neuroscience. 2004;20:1473–82. doi: 10.1111/j.1460-9568.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–11. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Chu Y, Myoga MH, Leitges M, Regehr WG. Calcium-dependent isoforms of protein kinase C mediate posttetanic potentiation at the calyx of Held. Neuron. 2011;70:1005–19. doi: 10.1016/j.neuron.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Chu Y, de Jong AP, Leitges M, Kaeser PS, Regehr WG. Protein kinase C is a calcium sensor for presynaptic short-term plasticity. Elife. 2014;3:e03011. doi: 10.7554/eLife.03011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ford MC, Grothe B, Klug A. Fenestration of the calyx of Held occurs sequentially along the tonotopic axis, is influenced by afferent activity, and facilitates glutamate clearance. The Journal of comparative neurology. 2009;514:92–106. doi: 10.1002/cne.21998. [DOI] [PubMed] [Google Scholar]
- Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nature communications. 2015;6:8073. doi: 10.1038/ncomms9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J.Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–42. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Functional organization of the dog superior olivary complex: an anatomical and electrophysiological study. Journal of neurophysiology. 1968;31:639–56. doi: 10.1152/jn.1968.31.4.639. [DOI] [PubMed] [Google Scholar]
- Green JS, Sanes DH. Early appearance of inhibitory input to the MNTB supports binaural processing during development. J Neurophysiol. 2005;94:3826–35. doi: 10.1152/jn.00601.2005. [DOI] [PubMed] [Google Scholar]
- Habets RL, Borst JG. Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol. 2005;564:173–187. doi: 10.1113/jphysiol.2004.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JM, Irving R. Ascending connections of the anterior ventral cochlear nucleus in the rat. J Comp Neurol. 1966;126:51–63. doi: 10.1002/cne.901260105. [DOI] [PubMed] [Google Scholar]
- He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–455. doi: 10.1016/j.tins.2007.06.012. [DOI] [PubMed] [Google Scholar]
- He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, Adachi R, Wu LG. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature. 2009;459:93–97. doi: 10.1038/nature07860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA. Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. J Neurosci. 2006;26:5511–23. doi: 10.1523/JNEUROSCI.5525-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Kolson DR, Mathers PH, Spirou GA. Maturation of synaptic partners: functional phenotype and synaptic organization tuned in synchrony. J Physiol. 2010;588:4365–85. doi: 10.1113/jphysiol.2010.198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Howell DM, Morgan WJ, Jarjour AA, Spirou GA, Berrebi AS, Kennedy TE, Mathers PH. Molecular guidance cues necessary for axon pathfinding from the ventral cochlear nucleus. J Comp Neurol. 2007;504:533–49. doi: 10.1002/cne.21443. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Nakamura PA, Luk SO, Miko IJ, Henkemeyer M, Cramer KS. Ephrin-B reverse signaling is required for formation of strictly contralateral auditory brainstem pathways. J Neurosci. 2010;30:9840–9. doi: 10.1523/JNEUROSCI.0386-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe CG, Forsythe ID, Uchitel OD. Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels. J Physiol. 2007;584:835–51. doi: 10.1113/jphysiol.2007.139683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J.Physiol. 1998;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol. 2001;534:861–71. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J.Neuorsci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Takahashi K, Kitamura K, Momoi T, Yoshikawa Y. Mitosis and apoptosis in postnatal auditory system of the C3H/He strain. Brain research. 2001;901:296–302. doi: 10.1016/s0006-8993(01)02300-9. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. The Journal of comparative neurology. 1993;328:161–84. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Keller D, Babai N, Kochubey O, Han Y, Markram H, Schurmann F, Schneggenburger R. An Exclusion Zone for Ca2+ Channels around Docked Vesicles Explains Release Control by Multiple Channels at a CNS Synapse. PLoS Comput Biol. 2015;11:e1004253. doi: 10.1371/journal.pcbi.1004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, van Adel BA, Ito M. Anatomical projections of the nuclei of the lateral lemniscus in the albino rat (Rattus norvegicus). The Journal of comparative neurology. 2009;512:573–93. doi: 10.1002/cne.21929. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Schneggenburger R. Synaptotagmin increases the dynamic range of synapses by driving Ca(2)+-evoked release and by clamping a near-linear remaining Ca(2)+ sensor. Neuron. 2011a;69:736–748. doi: 10.1016/j.neuron.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Han Y, Schneggenburger R. Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held. J Physiol. 2009;587:3009–3023. doi: 10.1113/jphysiol.2009.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochubey O, Lou X, Schneggenburger R. Regulation of transmitter release by Ca(2+) and synaptotagmin: insights from a large CNS synapse. Trends Neurosci. 2011b;34:237–246. doi: 10.1016/j.tins.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Koike-Tani M, Kanda T, Saitoh N, Yamashita T, Takahashi T. Involvement of AMPA receptor desensitization in short-term synaptic depression at the calyx of Held in developing rats. J Physiol. 2008;586:2263–75. doi: 10.1113/jphysiol.2007.142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Steinert JR, Forsythe ID. Modulation and control of synaptic transmission across the MNTB. Hearing research. 2011;279:22–31. doi: 10.1016/j.heares.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Korber C, Horstmann H, Satzler K, Kuner T. Endocytic structures and synaptic vesicle recycling at a central synapse in awake rats. Traffic. 2012;13:1601–11. doi: 10.1111/tra.12007. [DOI] [PubMed] [Google Scholar]
- Korogod N, Lou X, Schneggenburger R. Posttetanic potentiation critically depends on an enhanced Ca(2+) sensitivity of vesicle fusion mediated by presynaptic PKC. Proc.Natl.Acad.Sci.U.S.A. 2007;104:15923–15928. doi: 10.1073/pnas.0704603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, Zook JM. Classification of the principal cells of the medial nucleus of the trapezoid body. The Journal of Comparative Neurology. 1991a;314:707–720. doi: 10.1002/cne.903140406. [DOI] [PubMed] [Google Scholar]
- Kuwabara N, Zook JM. Projections to the medial superior olive from the medial and lateral nuclei of the trapezoid body in rodents and bats. The Journal of comparative neurology. 1992;324:522–38. doi: 10.1002/cne.903240406. [DOI] [PubMed] [Google Scholar]
- Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. The Journal of Comparative Neurology. 1991b;314:684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Leao RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von GH. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J.Neurosci. 2005;25:3724–3738. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- Lou X, Paradise S, Ferguson SM, De Camilli P. Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1. Proc Natl Acad Sci U S A. 2008;105:17555–60. doi: 10.1073/pnas.0809621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of Held governed by nonuniform channel-vesicle topography. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1648–67. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski N, Babai N, Renier N, Perkel DJ, Chedotal A, Schneggenburger R. Robo3-driven axon midline crossing conditions functional maturation of a large commissural synapse. Neuron. 2013;78:855–68. doi: 10.1016/j.neuron.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Midorikawa M, Okamoto Y, Sakaba T. Developmental changes in Ca2+ channel subtypes regulating endocytosis at the calyx of Held. J Physiol. 2014 doi: 10.1113/jphysiol.2014.273243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–15. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Muller M, Felmy F, Schneggenburger R. A limited contribution of Ca2+ current facilitation to paired-pulse facilitation of transmitter release at the rat calyx of Held. J Physiol. 2008;586:5503–20. doi: 10.1113/jphysiol.2008.155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Felmy F, Schwaller B, Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of Held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci. 2007;27:2261–71. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura PA, Cramer KS. Formation and maturation of the calyx of Held. Hear Res. 2011;276:70–8. doi: 10.1016/j.heares.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Takahashi T. Developmental changes in potassium currents at the rat calyx of Held presynaptic terminal. J Physiol. 2007;581:1101–12. doi: 10.1113/jphysiol.2007.128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS, Shigemoto R, Silver RA, DiGregorio DA, Takahashi T. Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron. 2015;85:145–58. doi: 10.1016/j.neuron.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Merits and Limitations of Vesicle Pool Models in View of Heterogeneous Populations of Synaptic Vesicles. Neuron. 2015;87:1131–42. doi: 10.1016/j.neuron.2015.08.038. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Wu W, Wu LG. Methods for patch clamp capacitance recordings from the calyx. J.Vis.Exp. 2007:244. doi: 10.3791/244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Zhu Q, Sun J. Quantitative analysis of vesicle recycling at the calyx of Held synapse. Proc Natl Acad Sci U S A. 2015;112:4779–84. doi: 10.1073/pnas.1424597112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, Von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J.Neurophysiol. 2007;98:3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- Renier N, Schonewille M, Giraudet F, Badura A, Tessier-Lavigne M, Avan P, De Zeeuw CI, Chedotal A. Genetic dissection of the function of hindbrain axonal commissures. PLoS Biol. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat.Rev.Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Robertson NG, Lu L, Heller S, Merchant SN, Eavey RD, McKenna M, Nadol JB, Jr., Miyamoto RT, Linthicum FH, Jr., Lubianca Neto JF, Hudspeth AJ, Seidman CE, Morton CC, Seidman JG. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat Genet. 1998;20:299–303. doi: 10.1038/3118. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, de Lange RP, Lucassen PJ, Borst JG. Branching of calyceal afferents during postnatal development in the rat auditory brainstem. J Comp Neurol. 2006;496:214–228. doi: 10.1002/cne.20918. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, van Hoeve JS, Habets RL, Locher H, Borst JG. Dynamic development of the calyx of Held synapse. Proc Natl Acad Sci U S A. 2008;105:5603–8. doi: 10.1073/pnas.0801395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5863–71. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001a;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Quantitative relationship between transmitter release and calcium current at the calyx of held synapse. J Neurosci. 2001b;21:462–476. doi: 10.1523/JNEUROSCI.21-02-00462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sätzler K, Sohl L, Bollmann JH, Borst JGG, Frotscher M, Sakmann B, Lubke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuss V, Taschenberger H, Neher E. Kinetics of both synchronous and asynchronous quantal release during trains of action potential-evoked EPSCs at the rat calyx of Held. J Physiol. 2007;585:361–81. doi: 10.1113/jphysiol.2007.140988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J.Neuorsci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calclium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006;326:311–337. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Sheng J, He L, Zheng H, Xue L, Luo F, Shin W, Sun T, Kuner T, Yue DT, Wu LG. Calcium-channel number critically influences synaptic strength and plasticity at the active zone. Nat Neurosci. 2012;15:998–1006. doi: 10.1038/nn.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveke I, Leibold C, Grothe B. Spectral composition of concurrent noise affects neuronal sensitivity to interaural time differences of tones in the dorsal nucleus of the lateral lemniscus. Journal of neurophysiology. 2007;98:2705–15. doi: 10.1152/jn.00275.2007. [DOI] [PubMed] [Google Scholar]
- Smillie KJ, Evans GJ, Cousin MA. Developmental change in the calcium sensor for synaptic vesicle endocytosis in central nerve terminals. J Neurochem. 2005;94:452–458. doi: 10.1111/j.1471-4159.2005.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol. 1998;79:3127–3142. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Carney LH, Yin TC. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- Sommer I, Lingenhohl K, Friauf E. Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp Brain Res. 1993;95:223–239. doi: 10.1007/BF00229781. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Brownell WE, Zidanic M. Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. J.Neurophysiol. 1990;63:1169–1190. doi: 10.1152/jn.1990.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynatpic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu W, Jin SX, Dondzillo A, Wu LG. Capacitance measurements at the calyx of Held in the medial nucleus of the trapezoid body. J.Neurosci.Methods. 2004;134:121–131. doi: 10.1016/j.jneumeth.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, Yang W, Bai L, Qadri S, Molkentin JD, Yue DT, Wu LG. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30:11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoufiq Z, Eguchi K, Takahashi T. Rho-kinase accelerates synaptic vesicle endocytosis by linking cyclic GMP-dependent protein kinase activity to phosphatidylinositol-4,5-bisphosphate synthesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12099–104. doi: 10.1523/JNEUROSCI.0730-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity [In Process Citation]. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Scheuss V, Neher E. Release kinetics, quantal parameters and their modulation during short-term depression at a developing synapse in the rat CNS. J Physiol. 2005;568:513–37. doi: 10.1113/jphysiol.2005.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, Von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Thanawala MS, Regehr WG. Presynaptic calcium influx controls neurotransmitter release in part by regulating the effective size of the readily releasable pool. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4625–33. doi: 10.1523/JNEUROSCI.4031-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–43. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–96. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff H, Borst JGG. Short-term plasticity at the calyx of Held. Nature reviews Neuroscience. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Wang LY, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. J Neurosci. 2008;28:14450–14458. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Horstmann H, Groh A, Kuner T. Donut-like topology of synaptic vesicles with a central cluster of mitochondria wrapped into membrane protrusions: a novel structure-function module of the adult calyx of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:109–16. doi: 10.1523/JNEUROSCI.3268-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel M, Lou X, Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3198–210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J.Neurosci. 2003;23:4868–4877. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JGG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999a;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc.Natl.Acad.Sci.USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014a;76:301–31. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J.Neuorsci. 1999b;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wu LG. Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proc Natl Acad Sci U S A. 2007;104:10234–9. doi: 10.1073/pnas.0611512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–83. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J Neurosci. 2009a;29:11038–42. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- Wu XS, Zhang Z, Zhao WD, Wang D, Luo F, Wu LG. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 2014b;7:982–8. doi: 10.1016/j.celrep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat.Neurosci. 2009b;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G, Schneggenburger R. BMP signaling specifies the development of a large and fast CNS synapse. Nat Neurosci. 2013;16:856–64. doi: 10.1038/nn.3414. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007a;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Xu J, He L, Wu LG. Role of Ca(2+) channels in short-term synaptic plasticity. Curr.Opin.Neurobiol. 2007b;17:352–359. doi: 10.1016/j.conb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Xu J, Luo F, Zhang Z, Xue L, Wu XS, Chiang HC, Shin W, Wu LG. SNARE proteins synaptobrevin, SNAP-25, and syntaxin are involved in rapid and slow endocytosis at synapses. Cell Rep. 2013;3:1414–21. doi: 10.1016/j.celrep.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J Neurosci. 2003;23:2182–2192. doi: 10.1523/JNEUROSCI.23-06-02182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21:6666–6672. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Wu LG. Post-tetanic potentiation is caused by two signaling mechanisms affecting quantal size and quantal content. J Physiol. 2010 doi: 10.1113/jphysiol.2010.196964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep. 2012;1:632–8. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Sheng J, Wu XS, Wu W, Luo F, Shin W, Chiang HC, Wu LG. Most vesicles in a central nerve terminal participate in recycling. J Neurosci. 2013;33:8820–6. doi: 10.1523/JNEUROSCI.4029-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Eguchi K, Saitoh N, von Gersdorff H, Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+. Nature neuroscience. 2010;13:838–44. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Wang LY. Amplitude and kinetics of action potential-evoked Ca2+ current and its efficacy in triggering transmitter release at the developing calyx of Held synapse. J Neurosci. 2006;26:5698–708. doi: 10.1523/JNEUROSCI.4889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS, Wang LY. Septins Regulate Developmental Switching from Microdomain to Nanodomain Coupling of Ca(2+) Influx to Neurotransmitter Release at a Central Synapse. Neuron. 2010;67:100–115. doi: 10.1016/j.neuron.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian M, Couchman K, Shivdasani MN, Paolini AG, Walmsley B. Maturation of auditory brainstem projections and calyces in the congenitally deaf (dn/dn) mouse. J Comp Neurol. 2008;506:442–51. doi: 10.1002/cne.21566. [DOI] [PubMed] [Google Scholar]
- Yu WM, Goodrich LV. Morphological and physiological development of auditory synapses. Hear Res. 2014;311:3–16. doi: 10.1016/j.heares.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HY, Xu J. Myosin light chain kinase accelerates vesicle endocytosis at the calyx of Held synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:295–304. doi: 10.1523/JNEUROSCI.3744-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HY, Xu J. Cholesterol regulates multiple forms of vesicle endocytosis at a mammalian central synapse. Journal of neurochemistry. 2015;134:247–60. doi: 10.1111/jnc.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]