Abstract
The prevention of neurodevelopmental disorders (NDD) of prenatal origin suffers from the lack of objective tools for early detection of susceptible individuals and the long time lag, usually in years, between the neurotoxic exposure and the diagnosis of mental dysfunction. Human data on the effects of alcohol, lead and mercury and experimental data from animals on developmental neurotoxins and their long term behavioral effects have achieved a critical mass, leading to the concept of the Developmental Origin of Health and Disease (DOHaD). However, there is currently no way to evaluate the degree of brain damage early after birth. We propose that Extracellular Vesicles (EVs) and particularly exosomes, released by brain cells into the fetal blood may offer us a non-invasive means of assessing brain damage by neurotoxins. We are inspired by the strategy applied by Alan Turing (a cryptanalyst working for the British government) who created a first computer to decrypt German intelligence communications during World War II. Given the growing evidence that miRNAs, which are among the molecules carried by EVs, are involved in cell-cell communication, we propose that decrypting messages from EVs can allow us to detect damage thus offering an opportunity to cure, reverse or prevent the development of NDD. This review summarizes recent findings on miRNAs associated with selected environmental toxicants known to be involved in the pathophysiology of NDD.
Keywords: Neurodevelopmental disorders, microRNAs, Extracellular vesicles, environmental exposure, biomarkers
Introduction
The development of specific non-invasive biomarkers of neurotoxicity in humans is a high priority for scientists, industry and governments, but accurate and efficient tools are lacking. Well characterized environmental toxicants such as lead or mercury, as well as chemical exposures such as alcohol, affect the developing brain [1–3] and predispose to future mental disorders [4–7]. The underlying mechanisms of developmental neurotoxicity are not well characterized but recent evidence implicates miRNAs. Indeed, miRNAs are key regulators of gene expression and environmental toxicants affect their expression [3, 8, 9]. NDD may have several causes but those caused by environmental exposures are challenging to demonstrate in humans mostly due to the time lag between exposure and the first symptoms but also because of the lack of objective markers. Currently, virtually all diagnoses of NDD are based on clinical observations which are usually made several years after the moment of initial brain damage. Growing evidence supports the potential of miRNAs as biomarkers of brain health because of their tissue-specific properties and their accessibility in the blood through their presence in Extracellular Vesicles (EVs) [10]. MiRNAs are a class of single stranded, small noncoding RNA molecules of ~ 22 nucleotides (nt) in length, evolutionarily well conserved and derived from genome-encoded stem-loop precursors [11]. MiRNAs induce gene silencing at the post-transcriptional level by recognizing target messenger RNA (mRNA) through complementary binding of 3′ or 5′ untranslated regions (3′ or 5′ UTR), and represent an important mechanism for regulating the expression of the mammalian genome. This recognition of mRNAs leads to inhibition of the translation by sequestration or degradation of the target mRNAs. The biogenesis of miRNAs and their mechanisms of action have been explained in excellent detailed reviews [12].
MiRNAs influence and reflect almost every cellular and developmental process investigated so far such as embryonic development, cell functions, immune reactions and adaptation to stress, as well as disease etiology [8], [13]. MiRNAs are implicated in several pathologies such as: immune disorders [14–17], cardiovascular disease [18–20], endocrine dysfunction [21–23], biogenesis pathways in cancer [24, 25], nephropathy [26], as well as in drug addiction [27], gynecological / reproductive diseases [28] and CNS disorders [29–33]. Since the discovery of miRNAs in Caenorhabditis elegans, approximately 2600 mature miRNAs have been identified in humans and, based on computational analysis, it is believed that all mRNAs are potential targets of miRNAs [34–36]. Interestingly, miRNAs are released from virtually all cell types and most are encapsulated in EVs (Extracellular Vesicles) which are found in all biological fluids [37].
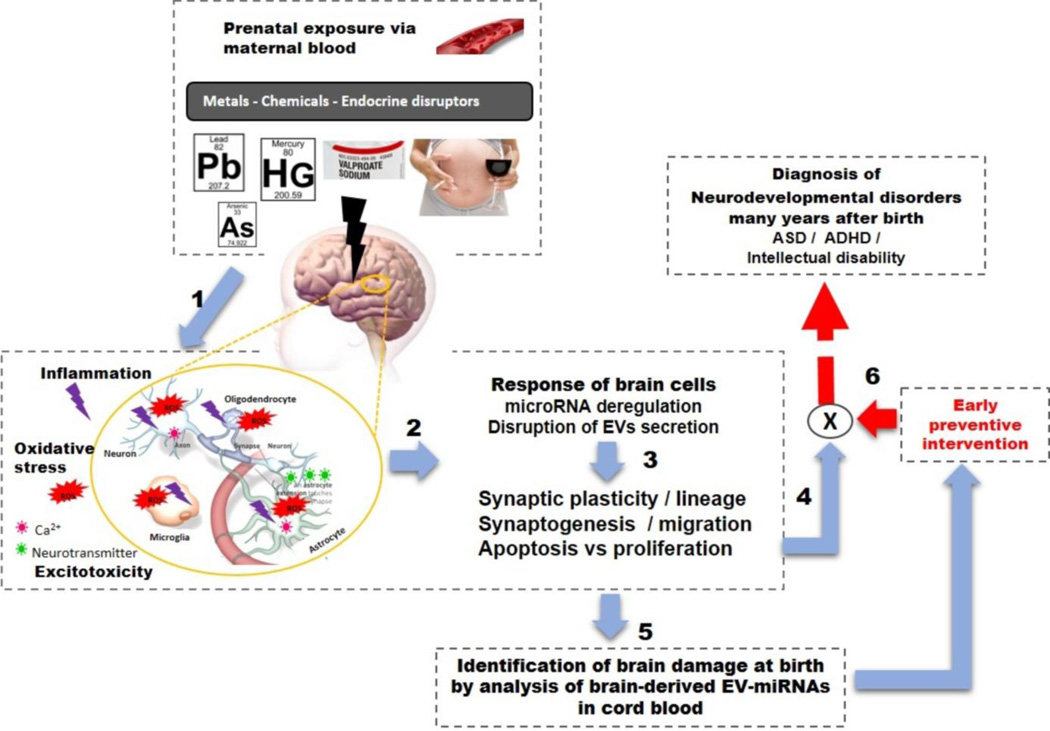
EVs are spherical-structures limited by a lipid bilayer, secreted by cells into extracellular spaces and are commonly classified into 3 categories, according to the International Society of Extracellular Vesicles [37, 38]. First, exosomes, nanovesicles of 40 – 100nm diameter, formed within the endosomal network and released by cells after the fusion of multivesicular bodies with the plasma membrane [38]. The second group includes microvesicles, microparticles, and ectosomes, larger than 100nm that are shed from the plasma membrane [37]. And finally, the third vesicle group comprises apoptotic bodies released when plasma membrane shedding occurs during apoptosis. This latter group is eliminated during EV isolation procedures due to their size (> 800nm). Currently, there is a need for additional specific markers for each EV type since some features are shared by two group (lipid composition; certain membrane markers).The biogenesis and secretions of EVs have been recently reviewed in detail [39]. EVs are released by cells in a constitutive manner but also as a consequence of cellular reactions to external signals or pathological states such as hypoxia, oxidative stress, cytokines, cancer and infection [37, 40, 41]. EVs and particularly exosomes are now recognized as an important mode of cell-cell communication and could be one of the means by which cells adapt to stimuli. Indeed, exosomes contain cytosolic cellular components such as proteins, RNAs (miRNAs, mRNAs) and lipids and are able to transfer their contents to targeted cells but can also induce cascade signaling in recipient cells by binding to the plasma membrane[37, 42]. Moreover, it appears that miRNAs are not passively loaded into EVs since their miRNA repertoire differs from that of the parent cells and certain miRNAs are usually excluded from EVs, indicating an active selection of miRNA [43–45]. Currently three online databases, EVpedia, Exocarta and Vesiclepedia provide an inventory of all proteins, lipids and RNAs found associated with EVs [46–50]. Interestingly, EVs have been found in virtually all biological fluids including urine, blood, breast milk, saliva, amniotic fluid, cerebrospinal fluids, which supports their potential utilization as biomarkers of the status of their cell of origin [37]. EVs released by brain cells were reported to be involved in CNS development as well as in the pathogenesis of certain mental disorders such as Alzheimer disease, fronto-temporal dementia, schizophrenia [51–56]. Currently, there are no studies on miRNA expression in EVs linked with environmental exposures, particularly neurotoxicants, relevant for neurological functions. Here, we summarize the rationale for testing the potential of EVs and their miRNAs as markers to identify damaged brains in newborns, who are destined to suffer from NDDs. We highlight the fact that environmental exposures affect miRNAs with relevant functions in brain physiology, as demonstrated both in-vitro and in-vivo where changes in cellular or blood miRNA levels were reported after neurotoxicant exposure. Moreover, several animal models of NDD, and human studies showed differential expression of certain miRNAs in patients compared to neurotypical subjects. EVs are secreted by brain cells, are found in biological fluids and contain cellular miRNAs. Thus, we suggest it should be possible to use a few well studied developmental neurotoxins which give rise to specific NDD, to decode the meaning of the messages present in the miRNAs within EVs released by brain cells (Fig. 1). i.e, the intended target cells and biological effects. Thus, EVs with their miRNAs represent a potential tool which should be urgently explored in molecular epidemiology focused on NDD.
Figure 1. Conceptual model.
(1) Exposure to environmental neurotoxins during prenatal period provokes oxidative stress / neuron inflammation / excitotoxicity through neurotransmitter and CA2+ perturbation. (2) pathological states perturb both the secretion of EVs by brain cells and their miRNAs profiles; (3) MiRNAs and EVs are key players in brain development, homeostasis and function (i.e synaptic plasticity) and the miRNA profiles of EVs secreted by specific brain cells may affect brain development and reflect brain damage. (4) In NDD, synaptic plasticity, synaptogenesis, cell differentiation, and the balance between proliferation versus apoptosis are impaired which lead to NDD (4). (5) Since EVs and miRNAs are tissue-specific and reflect brain responses and phenotype, decoding of the information in EVs in cord blood could help us identify early brain damage to prevent the development NDD(6).
1. Known neurotoxic xenobiotics perturb miRNAs in the brain
It is clear that exposure to neurotoxic chemicals (metals, polychlorinated biphenyls, endocrine disruptors, volatile organic compounds) during early fetal development can cause brain injury predisposing to NDD as well as subclinical brain dysfunction[1, 57–59]. Sanders et al. reviewed epidemiological studies on perinatal and childhood exposure to metals (cadmium, lead, manganese, or their combination) and their effects on neurodevelopment with a focus on cognitive function and behavior [2]. Here we summarize the results of observational studies and in-vitro/in-vivo experiments determining the effects of a few recognized environmental neurotoxicants on miRNA expression, with a focus on NDD (Table 1).
Table 1.
Environmental exposures and microRNA expression relevant for CNS functions
| In-vitro and animals studies | ||||
|---|---|---|---|---|
| Environmental exposures | miRNAs | Functions / miRNAs targets | Species or cells lines or tissues | References |
| Metals | ||||
| Mercury |
(miR-302b, miR-367, miR-372, miR- 196b, miR-141) ↑ |
axon guidance, learning and memory processes |
neuronal/glial culture derived from NT2 cell line (carcinoma pluripotent stem cells) |
[72] |
| Methyl-mercury chloride | 12 miRNA deregulated | neuronal development | differentiating human pluripotent cells | [71] |
| Copper | let-7, miR-7a, miR-128, and miR-138 | neurological process, neurotoxicity | zebrafish | [170] |
| Lead | ↑(miR-204, miR-211, miR-448, miR- 449a, miR-34b, and miR-34c) ↓miR-494 |
Neural injury and neurodegeration, axon and synapse function, neural development and regeneration. |
rat (hippocampus) | [67] |
| Silver nanoparticles | (miR-297, miR-132, miR-22, miR-27b, miR-196*, miR-1226) deregulated |
oxidative stress, neuronal function, inflammatory response |
human embryonic stem cell(HES)-derived neural stem/ progenitor cells |
[75] |
| Aluminium | ↑ (miR-9, miR-125b, miR-128) | neuronal differentiation, inflammation | human neural (HN) cells | [74] |
| Aluminium and iron sulfate |
↑(miR-146a) | Down-regulation of inflammation repressor and neurotoxicity |
human neural (HN) cells | [73] |
| Chemicals | ||||
| Ethanol | ↑ (miR-10a, miR-10b) | Early embryo development. | mouse fetal brain | [77] |
| ↓ miR-21, miR-335, miR-9, and miR- 153 |
anti-apoptotic factor, pro-apoptotic, anti- mitogenic factor, |
ex-vivo model of fetal cerebral cortical neuroepithelium |
[78] | |
| ↓miR-153 | preventing premature Neural Stem Cells differentiation |
mouse fetal cortical Neural Stem Cells | [79] | |
| ↓miR-9, miR-29a, miR-29b and miR- 133, ↑miR-34b |
inflammation and neuronal apoptosis pro-apoptotic miRNA |
mice cerebellum granule neurons | [81] | |
| ↑miR-9 | neuronal plasticity | adult mammalian brain | [81] | |
| ↑miR-155 | neuroinflammation | mice cerebellum | [82] | |
| ↑miR-10a-5p, ↓miR-26a and miR-495 | target brain-derived neurotropic factor, neurodevelopment as well as in the fine- tuning of synaptic plasticity |
rat hippocampus | [172] | |
| ↓miR-9 | neural-enriched miRNA, neural progenitor cell migration and neurogenesis |
Zebrafish embryos, murine NSCs | [174] | |
| ↑ (let 7 family members, miR-34c, miR-146a, miR-194, miR-203, and miR-369) |
neuronal plasticity effectors targeted | Post mortem human prefrontal cortex (alcohol patients vs normal) |
[175] | |
| ↑ (miR-497, miR-302b, miR-34b) | neuronal cell death, mitochondria-dependent neuronal apoptosis |
human neuroblastoma cell line | [176] | |
| Valproic acid | ↑(mir-206, mir-133a and mir-10a) ↓ (mir-124a, mir-128 and mir-137) |
muscle-abundant miRNA neural specific miRNA, neural differentiation |
murine embryonic stem cells (mESCs) | [88] |
| (miR-16a, miR-18c, miR-122, miR-132, miR-457b, and miR-724) |
neurodevelopment | zebrafish | [87] | |
| Cocaïne | ↓ (miR-133b, miR-144, miR-191) ↑ (miR-134, miR-135, miR-181c, miR- 22, miR-26b, miR-382, miR-409-3p and miR-50, miR-30a) |
dopamine receptors, brain disorders, drug abused |
Rat hippocampus | [177] |
| Endocrine Disruptors | ||||
| Bisphenol A | ↑miR-146 | inflammation | HTR-8 and 3A Human placental cells | [178] |
| Trymethyltin | ↑miR-9* and miR-384-5p | Neurotoxicity | Rat serum and brain | [180] |
| Air pollution | ||||
| Cigarette smoking | ↓ (miR-16, miR-21, miR-93, miR- 146a) |
growth and developmental processes | Human placenta + TCL-1 cells | [181] |
| Ambient PM | ↓ (miR-1, miR-126, miR-135a, miR- 146a, miR-155, miR-21, miR-222, miR- 9) |
neuronal differentiation | leukocyte | [183] |
| Carbon tetrachloride | ↑(miR-122, miR-133a, and miR-124) | liver-, muscle- and brain-specific miRNAs | rat plasma | [185] |
| Others | ||||
| RDX | 13 miRNAs deregulated | neurotoxicity, carcinogenesis and toxicant- metabolizing enzymes |
Mice brain and liver | [186] |
| ↑ (miR-71, miR-27ab, miR-27b, miR- 98, miR-135a, miR-674-5p), ↓ (miR- 320, miR-129*, miR-342-3p) |
neurotoxicity | Rat brain | [187] | |
| Ionizing radiation | ↑miR-132, miR-212, miR-134 | synaptic actin remodeling, neurogenesis, neuroinflammation |
brain of neonatal mice | [188] |
| Human in-vivo studies | ||||
|
Environmental exposures |
||||
| Arsenic | ↑12 miRNAs | innate and adaptive immune signaling | Human cord blood | [67] |
| Bisphenol A | ↑miR-146a | immune inflammatory and neural disease pathways |
2ndtrimester human placentas | [179] |
| Cigarette smoking | ↓ (miR-129, miR-634), ↑(miR-340, miR-365) |
embryo development, particularly cell death and apoptosis |
Human spermatozoa | [182] |
| Benzene | ↑ (miR-34a, miR-205, miR-10b, let- 7d, miR-185 and miR-423-5p-2) ↓ (miR-133a, miR-543, hsa-miR-130a, miR-27b,miR-223, miR-142-5p and miR-320b) |
Regulation of transcription, axon guidance, nervous system development, and regulation of actin cytoskeleton organization. |
human peripheral blood mononuclear cells | [184] |
• Arsenic
Arsenic is one of the most common environmental pollutants and a worldwide health concern. Arsenic, a known risk factor for NDD, induces oxidative stress which affects in-vitro EV-secretion by astrocytes [60]. Moreover, arsenic, even at low concentrations, impairs CNS functions and induces cognitive dysfunction, including learning and memory deficits and mood disorders, and children are particularly vulnerable [60]. Rager et al. examined human cord blood samples (n=40) for miRNA expression changes associated with in utero arsenic exposure and reported an increased expression of 12 miRNAs [61]. This suggests that the target miRNAs in the recipient cells should generate fewer protein molecules, which should in turn alter the function of the recipient cells.
• Lead
Lead is a well-studied developmental neurotoxin which induces adverse effects on neuronal differentiation, synaptic plasticity, learning and memory, neurogenesis and neuroregeneration, essential functions which are linked to the development of several NDD [62–63]. In addition, early life exposure to lead is related to increased risk of Schizophrenia [64]. Epidemiological studies provide sound evidence that even relatively low blood levels of Pb have adverse effects on the developing brain, especially during fetal life and childhood [63, 65, 66]. Recently, An et al. have reported that chronic exposure to lead alters the miRNA expression profile in the adult rat hippocampus and bioinformatics analysis as well as mRNA profiling analysis showed that the target genes of these affected miRNAs are involved in neural injury, neurodegeneration, axon and synapse function, neural development, and regeneration and apoptosis regulation [67].
• Mercury
Mercury is a well-recognized developmental neurotoxin, which induces learning disabilities, reduced cognitive function, and several neurological disorders, especially when the exposure occurs in utero even at very low doses [68–70]. Using differentiating human pluripotent cells repeatedly treated with non-cytotoxic doses of methylmercury chloride (MeHgCl), Nerini-Molteni et al. reported significant changes in the expression of 12 miRNAs associated with neural development [71]. In a mixed neuronal/glial culture, derived from NT2 cells, exposure to methyl mercury chloride during neuronal differentiation induced overexpression of 5 miRNAs (miR-302b, miR-367, miR-372, miR-196b and miR-141) [72].These miRNAs were associated with developmental processes and cellular responses to stress, and pathway analysis revealed possible links to CNS development including axon guidance, learning and memory processes [72]. The data suggest that miRNA profiling could provide a functional evaluation of the toxicity pathways involved in developmental neurotoxicity [72]
• Other neurotoxic metals
Pogue et al. provided evidence that in primary cultures of human neural (HN) cells, exposure to iron- and aluminum-sulfate (ROS-generating neurotoxic metal sulfates) induced up-regulation of miR-9, miR-125b, miR-128 and miRNA-146a. MiRNA-146a down-regulates the expression of complement factor H, a repressor of inflammation, leading to increased neuroinflammation [73,74]. These results suggest that miRNA-146a may aggravate the inflammatory response in stressed HN cells [73].
Silver nanoparticles (NPs) are commonly found in medical devices, pharmaceuticals and cosmetics and may cause adverse effects on the CNS [75]. Oh et al. performed the first comprehensive profiling of genes as well as miRNAs indicating epigenetic regulation in silver NP-treated neural stem/progenitor cells (NPCs) derived from embryonic stem cells (hESC). This cell model showed neural cell differentiation capacity allowing them to determine the ‘global’ effects of silver NPs on the entire neural cell system. In the NPCs, 6 miRNAs were deregulated (miR-297, miR-132, miR-22, miR-27b, miR-196b, miR-1226) and neuronal function, such as synaptic long-term potentiation, and axonal guidance signaling, were strongly regulated by the miRNA targeted genes in response to silver NPs [18]
In a zebrafish model, copper induced deregulation of miRNAs (from olfactory system tissues) involved in neurogenesis (e.g., let-7, miR-7a, miR-128, and miR-138), which suggests a role for miRNAs in copper-mediated toxicity via interference with neurogenesis processes [76].
• Ethanol
Ethanol is highly toxic for the developing brain. Prenatal exposure to ethanol leads to a myriad of developmental disorders known as fetal alcohol spectrum disorder, often characterized by intellectual disability, central nervous system damage, and specific craniofacial dysmorphic features. Studies in both animals and cultured cells reported that exposure to ethanol during the in-utero period, or during brain cell differentiation affected the expression of brain miRNAs involved in functions which are often altered in NDD (Table 1). Briefly, Wang et al. reported that ethanol caused both reduced cognitive function in offspring mice and deregulation of several miRNA including the miR-10 family which was significantly up-regulated in the fetal mouse brain but could be restored to normal with folic acid treatment [77]. In rodent cultured neurons [78–80] and fetal neural stem cells [79], ethanol treatment altered the expression of miR-9 (targeted calcium- and voltage-activated potassium channel in the brain) and miR-153, an miRNA that protects neurons from cell death. A recent study also revealed that miR-29b (a miRNA that prevents cell death by regulating Bcl-2 expression) was decreased in cerebrum and cerebellum granule neurons isolated from mice exposed to ethanol, which may explain the ethanol-induced neuronal apoptosis in the developing cerebellum [81]. Ethanol increased the expression of pro-inflammatory cytokines in the brain of mice due to miR-155 overexpression [82]. In humans, comprehensive analysis of miRNA levels in the post-mortem frontal cortex of alcoholic patients showed deregulation of 35 miRNAs involved in cell cycle regulation, differentiation and CNS development. Thus, miRNA deregulation may either be part of the mechanism of brain injury or may be a reflection of a damaged brain.
• Valproic acid
Valproic acid (VPA) is a widely prescribed drug to control epilepsy and pain. VPA is a recognized teratogen which increases the risk of malformations but also of neurodevelopmental disorders such as ASD [83–85] and ADHD [86]. For example, the risk of ASD is increased 5-fold in mothers who took VPA prior to conception [85]. Animal models suggest that VPA induces apoptosis, altered neurotransmitter profiles, and impaired synaptogenesis which are some of the mechanisms responsible for its cognitive and behavioral effects [85]. Aluru et al, reported that exposure to VPA altered the miRNA expression profiles in the developing embryo of zebrafish [87]. The targets of the miRNAs included several genes which are involved in the normal functioning of the CNS. In murine embryonic stem cells, VPA changes the miRNA expression profile during neural differentiation of neurons and astrocytes, in addition to the shifts in the lineage specification from neural to myogenic differentiation [88]. Concomitantly, the expression of myogenic regulatory factors as well as muscle-specific genes were elevated, while genes involved in neurogenesis were repressed [88]. Considering that prenatal exposure to VPA induces autistic-like features in rodents [83, 89] as well as deregulation of miRNAs involved in CNS development, it is possible that this deregulation contributes to the development of ASD-like features.
Investigating the role of miRNAs in NDD disorders may provide molecular diagnostic tools for psychiatric disorders and further studies focusing on brain miRNA deregulation following in utero exposure to environmental toxicants will help to identify mechanisms underlying the neurotoxic effects. Excitingly, miRNAs and brain-EVs show great potential as biomarkers and studies in neuro-oncology and neurodegenerative diseases support the potential use of EVs as biomarkers of NDD.
Apoptosis, calcium intracellular regulation, neurotransmitter secretion and neuro-inflammation, which have been reported to be affected by neurotoxins, are critical for the secretion of EVs by brain cells which led us to hypothesize that such neurotoxic xenobiotics will affected both miRNA content and EV secretion within the developing brain.
2. Release of extracellular vesicles (EV) by brain cells is mediated by neurotransmitters and the activation of inflammatory/oxidative stress pathways
EVs are now recognized as key actors in neuron-glia communication and their release by neurons and glial cells appears to be controlled by neurotransmitters (e.g. serotonin [90], glutamate [91]), intracellular calcium, but also, oxidative stress, microglia activation and neuro-inflammation [91–93]. The secretion of EVs by neurons, oligodendrocytes (ODC), astrocytes, microglia, and Schwan cells has been reported in-vitro and in-vivo [94–100]. Astrocytes, essential for trophic support of neurons but also very involved in cellular communications, release EVs [96, 101]. Under hyperthermia and oxidative stress, cultured astrocytes released EVs carrying the heat shock protein Hsp70 and synapsine I conferring pro-survival effects on neurons [102]. ODC treated with a calcium ionophore increase the release of exosomes containing myelin components [95].
The secretion of exosomes by ODC also depends on the electrical activity of neurons and appears to be controlled by the neurotransmitter glutamate [103]. Indeed, exosomes produced by ODC regulate, in an autocrine fashion, the membrane expansion, axonal myelination and myelin formation in response to trophic factors of neuronal origin but do not influence the differentiation of their precursors [104]. Finally, exosomes derived from ODC augmented neuron integrity and survival, axonal maintenance and immune surveillance by transferring myelin proteins, lipids and miRNAs to neurons and microglia [103, 105].
Microglia, the resident macrophages of the brain, were reported to secrete exosomes exhibiting similar protein content as B cell-derived exosomes and this in-vitro secretion is up-regulated following microglia activation with interferon treatment [97]. Serotonin released by neurons induced exosome secretion by microglia (both in murine microglial BV-2 cells and mouse primary microglia), suggesting that a neurotransmitter can control exosome release [90]. Microglia activation is normally neuroprotective, allowing the pruning of inactive synapses through phagocytosis of ODC-derived exosomes. However, their activation can also cause neurodegeneration, and propagation of inflammation by a mechanism which involves the uptake of microglia-derived exosomes [92].
3. EVs released by brain cells contain miRNAs which are involved in brain development and function, and the pathogenesis of mental disorders
Since the discovery of the first miRNA, lin-4, in C.elegans [36] and the identification of miRNAs conserved across species [106], our understanding of the role of miRNAs in brain development and function has been steadily growing. In mammals, more than 600 miRNA species have been discovered in the brain with specific temporo-spatial expression patterns [107, 108]. In the developing human brain, Moreau et al. uncovered distinct temporal expression patterns of miRNAs in post-mortem brain tissues from the fetus, early postnatal and adult brain tissue and constructed a miRNA expression atlas of the developing human brain [108]. Patterns of expression of miRNAs were studied during brain development in neurons, oligodendrocytes, astrocytes and microglia [109]. For example, in rodents, miR-124 and miR-128 are primarily expressed in neurons, whereas miR-23, miR-26, and miR-29 are present in large amounts in astrocytes [110]. Ziats et al. discovered differentially expressed miRNAs across both temporal and spatial dimensions, and between the male and female prefrontal cortex. The targets of the specific miRNAs were highly enriched for gene sets related to ASD, schizophrenia, bipolar disorder but not for adult-onset psychiatric diseases [107]. It is clear that miRNAs contribute to the maintenance of normal neuronal function and homeostasis which are often affected in NDD [111].
miRNAs and EVs in brain physiology
• miRNAs as key regulators of brain development
In one of the first studies using mice to examine the role of miRNAs in neuron integrity, it was reported that the depletion of all mature miRNAs (by interfering within the miRNA biogenesis pathway using a knock out strategy to target a key protein for miRNAs biogenesis) resulted in a progressive neurodegenerative phenotype characterized by loss of motor control [112]. For example, results using Drosophilia showed that disruption of the Argonaute protein (the ribonuclease needed for miRNA biogenesis) results in miRNAs depletion and caused developmental defects with malformation of the CNS in embryos [113]
Subsequent studies in developmental models in several species (i.e. zebrafish, mice, rat, C.elegans) and in-vitro (human neural stem cells) reported that the depletion of miRNAs reduces the number of neurons and glial cells, impairs axon guidance and neuronal differentiation, and induces cerebellum and midbrain malformations [112, 114–117]. MiRNAs regulate the transition of pluripotent stem cells to the neural lineage [50]. For example in mice, miR-9 and miR-124a, which were reported to be altered by neurotoxicants such as aluminium, methyl-mercury chloride or ethanol, have been implicated in the decision of neural precursors to differentiate into neurons or glial cells [117]. Moreover, when miR-124a (expressed in neurons but not in astrocytes) is experimentally expressed in non-neuronal cells it promotes a neuronal-like mRNA profile [117] and additional studies showed that miRNAs can regulated the shift from neuronal to glial fate and promote the generation of astrocytes and oligodendrocytes [50, 118, 119]. Evidence for the implication of EVs in neuroembryology comes from the discovery of exosomes in rodent and human embryonic CSF (eCSF) that contain evolutionarily conserved molecules important for coordinating intracellular pathways [120]. Feliciano et al. showed that eCSF served as a medium for molecules that regulate embryonic neural stem cell amplification during corticogenesis through the distribution of exosomes [120]. Recently, Lopez- Verrilli et al. showed that EVs from Schwann cells were selectively captured by neurons (in vitro) which resulted in a substantial neurite outgrowth, a sharp increase in axonal regeneration and a change in the RNA profile of neurons. Analysis of the contents of EVs showed that miRNAs and mRNAs were selectively packaged into EVs and some mRNAs coded for neuron specific proteins [98]. Thus, these results, confirmed by other studies, revealed the roles of miRNAs in developmental processes of the CNS such as tissue morphogenesis, neural patterning, neuronal specification and differentiation, and axonal path finding [111, 121].
• Role of EVs and miRNAs in synaptic plasticity
Synaptic plasticity is a critical process in learning and memory and its disruption triggers NDD such as ASD or ADHD [122, 123]. In vitro studies in rodent cells reported that release of EVs by neurons at somato-dendritic (post-synaptic) sites is modulated by glutaminergic synaptic activity. The EVs contained subunits of neurotransmitter receptor suggesting roles for EVs in homeostatic synaptic-scaling. Moreover, neuronal EVs contained synaptic-plasticity-associated protein and miRNAs and preferentially interacted with targeted neurons at the pre-synaptic terminal. This suggests that EVs act as vehicles for both anterograde and retrograde information transfer, consistent with a role in synaptic plasticity and memory [51, 91, 124].
Considering the vital role of miRNAs in post-transcriptional regulation, their widespread expression in different brain regions and their regulation by neuronal activity, miRNAs are increasingly considered as central players in synaptic plasticity. Bak et al showed that several miRNAs are expressed specifically in the hippocampus and cortex, and Kye et al, reported that a small number of miRNAs including miR-26a are highly enriched in dendrites compared to the cell body in mouse brain [125, 126]. For example, miR-132, is required for memory acquisition, spatial memory formation, and it regulats the visual cortex plasticity in a developmental and experience-dependent manner [127, 128]. Karpova et al. in a review focused on the epigenetic control of the brain-derived neurotrophic factor (BDNF), a central player in neuronal plasticity, reported that miRNAs, such as miR-206, targeted BDNF transcripts resulting in memory impairment and were up-regulated in neurodegenerative disorders [129]. Several reviews summarize the regulatory mechanisms of miRNAs in synaptic plasticity and learning memory and the picture that emerges is that miRNAs are ideally suited to contribute to the regulation of long-term potentiation-related gene expression, that miRNAs are pleiotropic, synaptically located, tightly regulated, and function in response to synaptic activity [130]–[133]. The first direct links between miRNA dysfunction and synaptic pathologies are emerging, raising the interest in these molecules as potential biomarkers and therapeutic targets in NDD.
• miRNA, EVs and neuroprotection
In rodent models as well as in humans, changes in miRNA expression after ischemic stroke have been reported [134]. Ischemia in rodents induced miRNA changes (192 miRNAs up-regulated and 95 miRNAs down-regulated) in cortical tissue [135]. These results suggest that certain miRNAs (those with altered expression following neurotoxic xenobiotic exposure) may act in a neuroprotective manner. This is further supported by studies in which individual miRNAs (e.g. neuron-enriched miR-29, Mir-132, and miR-124; astrocyte-enriched miR-146a and miR181) were shown to reduce neuronal death and to improve neurological outcomes after ischemia [134]. In addition, an in vitro study showed that EVs derived from ODC maintained neuron and axon integrity and exhibited pro-survival effects on neurons [93].
miRNAs and EVs in CNS disorders: focus on NDD disorders
Recent evidence supports the essential roles of EVs and miRNAs in the pathogenesis of neurodegenerative disorders such as prion disease, Alzheimer disease, multiple sclerosis, CNS infections and brain tumorigenesis. Deregulation of miR-124, the most abundant brain miRNA, has been linked to neurodegeneration, neuroimmune disorders and CNS stress among others [136]. In the context of mental disorders, it appears that EVs and miRNAs could represent the underlying mechanisms of disorders such as bipolar affective disorder, schizophrenia, major depression and anxiety disorders [137–140]. Whether miRNA deregulation is a cause or a consequence of such disorders is not clear and the majority of studies which examined the expression of miRNAs in mental disorders are case-control studies. In addition, one must control for the influence of psychotropic drugs on miRNA expression since several studies showed that lithium, haloperidol or valproate induced changes of the miRNA profiles in brain [141, 142]. For NDDs such as ASD, ADHD and intellectual deficiency, little is known about the role of miRNAs in the pathogenesis of these disorders and surprisingly no publication reports the analysis of EVs for these disorders.
Autism spectrum disorder (ASD)
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders caused by the interaction between genetic vulnerability and environmental factors [143]. Direct evidence for miRNA involvement in ASDs is currently lacking and the existing human studies were case-control studies which reported altered cellular or serum miRNA (i.e. not in EVs) levels in tissues from patients with ASD. The miRNA profile of olfactory cells of autistic patients, revealed differential expression of several miRNAs relevant for the pathophysiology of autism such as: miR-146a, miR-221, miR-654-5p, and miR-656 [143]. In post-mortem cerebellar cortex and temporal cortex, two studies showed deregulated miRNAs and among their predicted targets there were genes that are known genetic causes of autism, such Neurexin and SHANK3 [144, 145]. Three other studies reported that in lymphoblastoid cells, miRNAs were differentially expressed in ASD [146–148]. Finally, more recently, two studies reported differentially expressed miRNAs in the blood of children and adults with ASD compared to controls [149,150]. Unfortunately, no studies have compared the miRNAs levels in different tissues of the same patients; however, changes in the levels of certain miRNAs (miR-106b, miR-663 and miR-23a) have been reported in both post-mortem cortex, lymphoblastoid cells and serum of ASD patients. Some of these miRNAs (miR-23a, miR-132, miR-134 and miR-146b) have been shown to regulate dendritic spine structure and morphology, synaptic connectivity and maturation (two process altered in ASD), were related to anxiety behavior, and were affected by alcohol, metals and VPA exposure [151, 152].
Further evidence for the role of miRNAs in the pathogenesis of ASD comes from the VPA rat model of ASD where elevated miR-181c levels in the amygdala were observed. Experimental suppression of miR-181c attenuated neurite outgrowth and branching and reduced synaptic density in primary amygdalar neurons to levels which were similar to controls [153].
Attention deficit/hyperactivity disorders (ADHD)
ADHD is one of the most prevalent NDD, often associated with cognitive handicap and learning deficit [154]. Although the etiology and pathogenesis remain unknown, several theories suggest that it results both from genetic and environmental factors [154]. The blood of ADHD patients has been reported to contain altered levels of miRNAs involved in differentiation from pluripotency to neurogenesis and in the maintenance of pluripotency, somatic cell reprogramming, tissue regeneration, and neural stem cell plasticity [154, 155].
Numerous studies support a role for miRNAs in environmentally-related disease since neurotoxins affect miRNA expression. In parallel, is it clear that miRNAs/EVs play a crucial role in CNS development and particularly in processes such as synaptogenesis, synaptic plasticity, neural differentiation and fate which are often impaired in NDDs. On the other hand, in NDDs like ASD and ADHD where there is a crucial lack of human studies, a reasonable hypothesis is that exposure to environmental toxins during prenatal life leads to NDD through deregulation of the release of EVs or alterations in their miRNA content.
4. miRNA profiles of brain EVs as potential biomarkers for neurodevelopmental disorders?
The miRNA content of EVs is recognized to be a fingerprint of the releasing cell type and its physiological state [37]. Studies focused on miRNA changes in the context of brain physiopathology support the possibility of their use as biomarkers of CNS disorders. Most of the studies have been dedicated to neurodegenerative diseases and neuro-oncology [156–158].
Indeed, while the Blood Brain Barrier (BBB) as well the blood-CSF barrier are generally considered to have a highly selective permeability to blood compounds or drugs [159, 160], recent studies suggest that exosomes have the ability to cross the BBB in both directions, although the route of transfer remains unclear [161–164]. Moreover, exosomes were shown to be able to enter the brain parenchyma at the choroid plexus and were internalized both in neurons and glial cells (in-vitro and in-vivo rodent studies) [99]. The most promising finding comes from Kapogiannis et al. who successfully isolated neuronal-EVs in plasma using a specific marker of neuronal origin, NCAM/ L1-CAM [165]. Moreover, accumulating experimental and clinical evidence indicates that a number of brain diseases (i.e glioblastoma) are associated with BBB dysfunctions resulting in elevated barrier permeability that may enable leakage of EVs from the brain. Indeed, Shao et al. successfully detected and quantified glioma markers in glioma-derived exosomes in the blood circulation, with a detection rate of > 90%, suggesting the possibility of using EVs derived from brain tumors as an effective diagnosis platform [166]. In ASD, often associated with immune dysfunction, the detection of autoantibodies against neural cells of the cerebellum in the plasma suggests the existence of BBB disruption [167]. Moreover, environmental neurotoxicant induced neuroinflammation may participate both in BBB disruption and EV release [92–168].
EVs have been found in cord blood and taking into account the BBB immaturity during the first year of life [169], it is possible that EVs arising from brain cells can be detected in cord blood.
Conclusion
There is an urgent need to develop a molecular tool to evaluate brain damage at birth, whether it results from exposure to neurodevelopmental toxins or others causes. On the basis of the information compiled in this review, we propose that the miRNA profiles of EVs released by brain cells and present in cord blood may reveal the degree of brain damage (Fig. 1). EVs are a messenger for intercellular communications among the community of cells within the brain. The miRNA profiles of EVs play important roles in virtually all aspects of brain development and functioning; however, we are not yet able to decode the information contained in the miRNA profiles. Yes, we know which target mRNAs in the recipient cells will be degraded or sequestered and thus not be translated into proteins, but what will be the resulting effects on the brain? This is a far more complex situation than analyzing the effects of a given neurotransmitter and this is why we use the term “decoding” and make reference to the remarkable achievement of Alan Turing. We may be able to use well studied neurotoxins, such as lead, mercury and ethanol, which predispose to NDD, as “Rosetta stones”, to allow us to decode EV messages and thus to identify damaged brains at birth, thus opening the possibility of preventive / therapeutic interventions to reduce the incidence of NDD. Finally, there are an increasing number of environmental chemicals to which fetuses are exposed and for which there are no neurodevelopmental toxicity data. A molecular tool to evaluate brain damage at birth would be a major advance for the field of molecular epidemiology by permitting timely, non-invasive detection of neurodevelopmental toxins in humans.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Virginie Gillet, Darel Hunting, and Larissa Takser declare that they have no conflict of interest.
Contributor Information
Virginie Gillet, Faculté de Médecine et Sciences de la Santé de l’Université de Sherbrooke, Département Pédiatrie, 3001, 12ème avenue Nord, Sherbrooke (Québec) Canada J1H 5N4, Virginie.Gillet@USherbrooke.ca.
Darel Hunting, Faculté de Médecine et Sciences de la Santé de l’Université de Sherbrooke, Département Radiobiologie, 3001, 12ème avenue Nord, Sherbrooke (Québec) Canada J1H 5N4, Darel.Hunting@USherbrooke.ca.
Larissa Takser, Faculté de Médecine et Sciences de la Santé de l’Université de Sherbrooke, Département Pédiatrie, 3001, 12ème avenue Nord, Sherbrooke (Québec) Canada J1H 5N4, Larissa.Takser@USherbrooke.ca.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of outstanding importance
- 1.Ratnaike R. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003 Jul.79(933):391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders AP, Claus Henn B, Wright RO. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr. Environ. Health Rep. 2015 Sep.2(3):284–294. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natarajan SK, Pachunka JM, Mott JL. Role of microRNAs in Alcohol-Induced Multi-Organ Injury. Biomolecules. 2015;5(4):3309–3338. doi: 10.3390/biom5043309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froehlich TE, Anixt JS, Loe IM, et al. Update on Environmental Risk Factors for Attention-Deficit/Hyperactivity Disorder. Curr. Psychiatry Rep. 2011 Oct.13(5):333–344. doi: 10.1007/s11920-011-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle LR, Mattson SN. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Review of Evidence and Guidelines for Assessment. Curr. Dev. Disord. Rep. 2015 Sep.2(3):175–186. doi: 10.1007/s40474-015-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandjean P, Weihe P, Debes F, et al. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol. Teratol. 2014 Jun.43:39–44. doi: 10.1016/j.ntt.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forns J, Fort M, Casas M, Cáceres A, Guxens M, Gascon M, Garcia-Esteban R, Julvez J, Grimalt JO, Sunyer J. Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology. 2014 Jan.40:16–22. doi: 10.1016/j.neuro.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8. Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect. 2015 May;123(5):399–411. doi: 10.1289/ehp.1408459. This reviewed provides recent state of knowledge regarding the effects of environmental exposures on miRNAs expression. This suggested that miRNAs could be use as new biomarkers to detect environmental exposures-induced physiological changes.
- 9.Hou L, Wang D, Baccarelli A. Environmental chemicals and microRNAs. Mutat. Res. 2011 Sep.714(1–2):105–112. doi: 10.1016/j.mrfmmm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015 Feb.13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat. Rev. 2012;13(5):358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014 Aug.15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 13.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008 Mar.9(3):219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 14.Simpson LJ, Ansel KM. MicroRNA regulation of lymphocyte tolerance and autoimmunity. J Clin. Invest. 2015 Jun.125(6):2242–2249. doi: 10.1172/JCI78090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heegaard NHH, Carlsen AL, Skovgaard K, Heegaard PMH. Circulating Extracellular microRNA in Systemic Autoimmunity. EXS. 2015;106:171–195. doi: 10.1007/978-3-0348-0955-9_8. [DOI] [PubMed] [Google Scholar]
- 16.Lee H-M, Nguyen DT, Lu L-F. Progress and challenge of microRNA research in immunity. Front. Genet. 2014;5:178. doi: 10.3389/fgene.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S, Pan W, Qian Y. MicroRNA in immunity and autoimmunity. J Mol. Med. Berl. Ger. 2013 Sep.91(9):1039–1050. doi: 10.1007/s00109-013-1043-z. [DOI] [PubMed] [Google Scholar]
- 18.Arunachalam G, Upadhyay R, Ding H, Triggle CR. MicroRNA Signature and Cardiovascular Dysfunction. J Cardiovasc. Pharmacol. 2015 May;65(5):419–429. doi: 10.1097/FJC.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer P, Werner N, Jansen F. Role and Function of MicroRNAs in Extracellular Vesicles in Cardiovascular Biology. BioMed Res. Int. 2015;2015:161393. doi: 10.1155/2015/161393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elia L, Condorelli G. RNA (Epi)genetics in cardiovascular diseases. J Mol. Cell. Cardiol. 2015 Dec.89(Pt A):11–16. doi: 10.1016/j.yjmcc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Kadamkode V, Banerjee G. Micro RNA: an epigenetic regulator of type 2 diabetes. MicroRNA Shāriqah United Arab Emir. 2014;3(2):86–97. doi: 10.2174/2211536603666141118232514. [DOI] [PubMed] [Google Scholar]
- 22.Park S-Y, Jeong H-J, Yang W-M, et al. Implications of microRNAs in the pathogenesis of diabetes. Arch. Pharm. Res. 2013 Feb.36(2):154–166. doi: 10.1007/s12272-013-0017-6. [DOI] [PubMed] [Google Scholar]
- 23.Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J Endocrinol. 2005 Dec.187(3):327–332. doi: 10.1677/joe.1.06426. [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer. 2015 Jun.15(6):321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38. doi: 10.1186/s12935-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung AC, Yu X, Lan HY. MicroRNA and nephropathy: emerging concepts. Int. J. Nephrol. Renov. Dis. 2013;6:169–179. doi: 10.2147/IJNRD.S37885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues Clin. Neurosci. 2014 Sep.16(3):335–344. doi: 10.31887/DCNS.2014.16.3/pkenny. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santamaria X, Taylor H. MicroRNA and gynecological reproductive diseases. Fertil. Steril. 2014 Jun.101(6):1545–1551. doi: 10.1016/j.fertnstert.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Tan L, Yu J-T, Tan L. Causes and Consequences of MicroRNA Dysregulation in Neurodegenerative Diseases. Mol. Neurobiol. 2015 Jun.51(3):1249–1262. doi: 10.1007/s12035-014-8803-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Ji B, Cheng B, et al. Neuroprotection of microRNA in neurological disorders (Review) Biomed. Rep. 2014 Sep.2(5):611–619. doi: 10.3892/br.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henshall DC. MicroRNA and epilepsy: profiling, functions and potential clinical applications. Curr. Opin. Neurol. 2014 Apr.27(2):199–205. doi: 10.1097/WCO.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su W, Aloi MS, Garden GA. MicroRNAs mediating CNS inflammation: Small regulators with powerful potential. Brain. Behav. Immun. 2015 Jul. doi: 10.1016/j.bbi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu B, Karayiorgou M, Gogos JA. MicroRNAs in psychiatric and neurodevelopmental disorders. Brain Res. 2010 Jun.1338:78–88. doi: 10.1016/j.brainres.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011 Jan.39:D152–D157. doi: 10.1093/nar/gkq1027. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman RC, Farh KK-H, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009 Jan.19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec.75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 37. Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. This recent reviewed written by experts in the field of EVs provides a thorough description of EVs, about their properties, functions, and current or ongoing research for clinical utilization.
- 38.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015 Jun.25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 40.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011;3:15–15. doi: 10.3410/B3-15. Journal Article, Epub 2011 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007 Jun.9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 43.Nolte-’t Hoen ENM, Buermans HPJ, Waasdorp M, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012 Oct.40(18):9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics. 2011 Nov.12(Suppl 3):S18. doi: 10.1186/1471-2164-12-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathivanan S, Fahner CJ, Reid GE, et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012 Jan.40:D1241–D1244. doi: 10.1093/nar/gkr828. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D-K, Kang B, Kim OY, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Ramirez MA, Nicoli S. Role of miRNAs and epigenetics in neural stem cell fate determination. Epigenetics. 2014 Jan.9(1):90–100. doi: 10.4161/epi.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stappert L, Roese-Koerner B, Brüstle O. The role of microRNAs in human neural stem cells, neuronal differentiation and subtype specification. Cell Tissue Res. 2015 Jan.359(1):47–64. doi: 10.1007/s00441-014-1981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem. Soc. Trans. 2013 Feb.41(1):241–244. doi: 10.1042/BST20120266. [DOI] [PubMed] [Google Scholar]
- 52.Frühbeis C, Fröhlich D, Kuo WP, et al. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma P, Schiapparelli L, Cline HT. Exosomes function in cell-cell communication during brain circuit development. Curr. Opin. Neurobiol. 2013 doi: 10.1016/j.conb.2013.08.005. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajendran L, Bali J, Barr MM, et al. Emerging roles of extracellular vesicles in the nervous system. J Neurosci. Off. J. Soc. Neurosci. 2014 Nov.34(46):15482–15489. doi: 10.1523/JNEUROSCI.3258-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banigan MG, Kao PF, Kozubek JA, et al. Differential Expression of Exosomal microRNAs in Prefrontal Cortices of Schizophrenia and Bipolar Disorder Patients. PLoS ONE. 2013 Jan.8(1):e48814. doi: 10.1371/journal.pone.0048814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi P, Benussi L, Furlan R, et al. Extracellular vesicles in Alzheimer’s disease: friends or foes? Focus on aβ-vesicle interaction. Int. J. Mol. Sci. 2015;16(3):4800–4813. doi: 10.3390/ijms16034800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014 Mar.13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet Lond. Engl. 2006 Dec.368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 59.Hill DS, Cabrera R, Wallis Schultz D, et al. Autism-Like Behavior and Epigenetic Changes Associated with Autism as Consequences of In Utero Exposure to Environmental Pollutants in a Mouse Model. Behav. Neurol. 2015;2015:426263. doi: 10.1155/2015/426263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rager JE, Bailey KA, Smeester L, et al. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ. Mol. Mutagen. 2014 Apr.55(3):196–208. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caito S, Aschner M. Neurotoxicity of metals. Handb. Clin. Neurol. 2015;131:169–189. doi: 10.1016/B978-0-444-62627-1.00011-1. [DOI] [PubMed] [Google Scholar]
- 63.Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. BioMed Res. Int. 2014;2014:840547. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guilarte TR, Opler M, Pletnikov M. Is lead exposure in early life an environmental risk factor for Schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology. 2012 Jun.33(3):560–574. doi: 10.1016/j.neuro.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain J. Neurol. 2003 Jan.126(Pt 1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 66.Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environ. Res. 2006 Feb.100(2):284–293. doi: 10.1016/j.envres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 67.An J, Cai T, Che H, et al. The changes of miRNA expression in rat hippocampus following chronic lead exposure. Toxicol. Lett. 2014 Aug.229(1):158–166. doi: 10.1016/j.toxlet.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Solan TD, Lindow SW. Mercury exposure in pregnancy: a review. J Perinat. Med. 2014 Nov.42(6):725–729. doi: 10.1515/jpm-2013-0349. [DOI] [PubMed] [Google Scholar]
- 69.Bose-O’Reilly S, McCarty KM, Steckling N, et al. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care. 2010 Sep.40(8):186–215. doi: 10.1016/j.cppeds.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taber KH, Hurley RA. Mercury Exposure: Effects Across the Lifespan. J Neuropsychiatry Clin. Neurosci. 2008 Oct.20(4):iv–389. doi: 10.1176/jnp.2008.20.4.iv. [DOI] [PubMed] [Google Scholar]
- 71.Nerini-Molteni S, Mennecozzi M, Fabbri M, et al. MicroRNA profiling as a tool for pathway analysis in a human in vitro model for neural development. Curr. Med. Chem. 2012;19(36):6214–6223. [PubMed] [Google Scholar]
- 72.Pallocca G, Fabbri M, Sacco MG, et al. miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol. Toxicol. 2013 Aug.29(4):239–257. doi: 10.1007/s10565-013-9250-5. [DOI] [PubMed] [Google Scholar]
- 73.Pogue AI, Li YY, Cui J-G, et al. Characterization of an NF-kappaB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg. Biochem. 2009 Nov.103(11):1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg. Biochem. 2007 Sep.101(9):1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh J-H, Son M-Y, Choi M-S, et al. Integrative analysis of genes and miRNA alterations in human embryonic stem cells-derived neural cells after exposure to silver nanoparticles. Toxicol. Appl. Pharmacol. 2015 Nov. doi: 10.1016/j.taap.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Bammler TK, Beyer RP, et al. Copper-induced deregulation of microRNA expression in the zebrafish olfactory system. Environ. Sci. Technol. 2013 Jul.47(13):7466–7474. doi: 10.1021/es400615q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L-L, Zhang Z, Li Q, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 2009 Mar.24(3):562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- 78.Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. Off. J. Soc. Neurosci. 2007 Aug.27(32):8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsai P-C, Bake S, Balaraman S, et al. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biol. Open. 2014;3(8):741–758. doi: 10.1242/bio.20147765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miranda RC. MicroRNAs and Fetal Brain Development: Implications for Ethanol Teratology during the Second Trimester Period of Neurogenesis. Front. Genet. 2012;3:77. doi: 10.3389/fgene.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qi Y, Zhang M, Li H, et al. MicroRNA-29b regulates ethanol-induced neuronal apoptosis in the developing cerebellum through SP1/RAX/PKR cascade. J Biol. Chem. 2014 Apr.289(14):10201–10210. doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lippai D, Bala S, Catalano D, et al. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol. Clin. Exp. Res. 2014 Aug.38(8):2217–2224. doi: 10.1111/acer.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Degroote S, Hunting D, Sébire G, et al. Autistic-like traits in Lewis rats exposed perinatally to a mixture of common endocrine disruptors. Endocr. Disruptors. 2014 Jan.2(1):e976123. [Google Scholar]
- 84.Wood AG, Nadebaum C, Anderson V, et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015 Jul.56(7):1047–1055. doi: 10.1111/epi.13007. [DOI] [PubMed] [Google Scholar]
- 85.Velez-Ruiz NJ, Meador KJ. Neurodevelopmental effects of fetal antiepileptic drug exposure. Drug Saf. 2015 Mar.38(3):271–278. doi: 10.1007/s40264-015-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen MJ, Meador KJ, Browning N, et al. Fetal antiepileptic drug exposure: Adaptive and emotional/behavioral functioning at age 6years. Epilepsy Behav. EB. 2013 Nov.29(2):308–315. doi: 10.1016/j.yebeh.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aluru N, Deak K, Jenny MJ, et al. Developmental exposure to valproic acid alters the expression of microRNAs involved in neurodevelopment in zebrafish. Neurotoxicol. Teratol. 2013;40:46–58. doi: 10.1016/j.ntt.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smirnova L, Block K, Sittka A, et al. MicroRNA profiling as tool for in vitro developmental neurotoxicity testing: the case of sodium valproate. PloS One. 2014;9(6):e98892. doi: 10.1371/journal.pone.0098892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ranger P, Ellenbroek BA. Perinatal Influences of Valproate on Brain and Behaviour: An Animal Model for Autism. Curr. Top. Behav. Neurosci. 2015 Oct. doi: 10.1007/7854_2015_404. [DOI] [PubMed] [Google Scholar]
- 90.Glebov K, Löchner M, Jabs R, et al. Serotonin stimulates secretion of exosomes from microglia cells. Glia. 2015 Apr.63(4):626–634. doi: 10.1002/glia.22772. [DOI] [PubMed] [Google Scholar]
- 91.Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell. Vesicles. 2014;3:24722. doi: 10.3402/jev.v3.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brites D, Fernandes A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 2015 Dec.9 doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pegtel DM, Peferoen L, Amor S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014 Sep.369(1652) doi: 10.1098/rstb.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fauré J, Lachenal G, Court M, et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006 Apr.31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Krämer-Albers E-M, Bretz N, Tenzer S, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin. Appl. 2007 Nov.1(11):1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 96.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. Vienna Austria 1996. 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 97.Potolicchio I, Carven GJ, Xu X, Stipp C, et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. Baltim. Md 1950. 2005 Aug.175(4):2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- 98.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013 Nov.61(11):1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 99.Grapp M, Wrede A, Schweizer M, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat. Commun. 2013;4:2123. doi: 10.1038/ncomms3123. no. Journal Article. [DOI] [PubMed] [Google Scholar]
- 100.Street JM, Barran PE, Mackay CL, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl. Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang G, Dinkins M, He Q, Zhu G, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol. Chem. 2012 Jun.287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor AR, Robinson MB, Gifondorwa DJ, et al. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev. Neurobiol. 2007 Nov.67(13):1815–1829. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- 103.Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013 Jul.11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol. Chem. 2011 Jan.286(1):787–796. doi: 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fröhlich D, Kuo WP, Frühbeis C, et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014 Sep.369(1652) doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pasquinelli AE, Reinhart BJ, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000 Nov.408(6808):86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 107.Ziats MN, Rennert OM. Identification of differentially expressed microRNAs across the developing human brain. Mol. Psychiatry. 2014 Jul.19(7):848–852. doi: 10.1038/mp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moreau MP, Bruse SE, Jornsten R, et al. Chronological changes in microRNA expression in the developing human brain. PloS One. 2013;8(4):e60480. doi: 10.1371/journal.pone.0060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015 Jan.48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Smirnova L, Gräfe A, Seiler A, et al. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005 Mar.21(6):1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 111.Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim. Biophys. Acta. 2008 Aug.1779(8):471–478. doi: 10.1016/j.bbagrm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Schaefer A, O’Carroll D, Tan CL, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp. Med. 2007 Jul.204(7):1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kataoka Y, Takeichi M, Uemura T. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells Devoted Mol. Cell. Mech. 2001 Apr.6(4):313–325. doi: 10.1046/j.1365-2443.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- 114.Giraldez AJ, Cinalli RM, Glasner ME, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005 May;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 115.Huang T, Liu Y, Huang M, et al. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J Mol. Cell Biol. 2010 Jun.2(3):152–163. doi: 10.1093/jmcb/mjq008. [DOI] [PubMed] [Google Scholar]
- 116.Hengst U, Cox LJ, Macosko EZ, et al. Functional and Selective RNA Interference in Developing Axons and Growth Cones. J Neurosci. 2006 May;26(21):5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krichevsky AM, Sonntag K-C, Isacson O, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells Dayt. Ohio. 2006 Apr.24(4):857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hou Q, Ruan H, Gilbert J, et al. MicroRNA miR124 is required for the expression of homeostatic synaptic plasticity. Nat. Commun. 2015;6:10045. doi: 10.1038/ncomms10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011 Aug.476(7359):228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feliciano DM, Zhang S, Nasrallah CM, et al. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PloS One. 2014;9(2):e88810. doi: 10.1371/journal.pone.0088810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun E, Shi Y. MicroRNAs: Small molecules with big roles in neurodevelopment and diseases. Exp. Neurol. 2015 Jun.268:46–53. doi: 10.1016/j.expneurol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 122.Hui Z, Yongchao Z, Yongqing Z. Recent progresses in molecular genetics of autism spectrum disorders. Yi Chuan Hered. Zhongguo Yi Chuan Xue Hui Bian Ji. 2015 Sep.37(9):845–854. doi: 10.16288/j.yczz.15-281. [DOI] [PubMed] [Google Scholar]
- 123.Washbourne P. Synapse Assembly and Neurodevelopmental Disorders. Neuropsychopharmacology. 2015 Jan.40(1):4–15. doi: 10.1038/npp.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lachenal G, Pernet-Gallay K, Chivet M, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011 Feb.46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Bak M, Silahtaroglu A, Møller M, et al. MicroRNA expression in the adult mouse central nervous system. RNA. 2008 Mar.14(3):432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kye M-J, Liu T, Levy SF, et al. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. RNA N. Y. N. 2007 Aug.13(8):1224–1234. doi: 10.1261/rna.480407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mellios N, Sugihara H, Castro J, et al. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat. Neurosci. 2011 Oct.14(10):1240–1242. doi: 10.1038/nn.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hansen KF, Karelina K, Sakamoto K, Wayman GA, Impey S, Obrietan K. miRNA-132: a dynamic regulator of cognitive capacity. Brain Struct. Funct. 2013 May;218(3):817–831. doi: 10.1007/s00429-012-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karpova NN. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology. 2014 Jan.76(Pt C):709–718. doi: 10.1016/j.neuropharm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 130.Aksoy-Aksel A, Zampa F, Schratt G. MicroRNAs and synaptic plasticity--a mutual relationship. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014 Sep.369(1652) doi: 10.1098/rstb.2013.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ryan B, Joilin G, Williams JM. Plasticity-related microRNA and their potential contribution to the maintenance of long-term potentiation. Front. Mol. Neurosci. 2015;8:4. doi: 10.3389/fnmol.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Earls LR, Westmoreland JJ, Zakharenko SS. Non-coding RNA regulation of synaptic plasticity and memory: implications for aging. Ageing Res. Rev. 2014 Sep.17:34–42. doi: 10.1016/j.arr.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Codocedo JF, Inestrosa NC. Environmental control of microRNAs in the nervous system: Implications in plasticity and behavior. Neurosci. Biobehav. Rev. 2016 Jan.60:121–138. doi: 10.1016/j.neubiorev.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Saugstad JA. Non-Coding RNAs in Stroke and Neuroprotection. Front. Neurol. 2015;6:50. doi: 10.3389/fneur.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lusardi TA, Farr CD, Faulkner CL, et al. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010 Apr.30(4):744–756. doi: 10.1038/jcbfm.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun Y, Luo Z-M, Guo X-M, et al. An updated role of microRNA-124 in central nervous system disorders: a review. Front. Cell. Neurosci. 2015;9:193. doi: 10.3389/fncel.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abdolmaleky HM, Zhou J-R, Thiagalingam S. An update on the epigenetics of psychotic diseases and autism. Epigenomics. 2015;7(3):427–449. doi: 10.2217/epi.14.85. [DOI] [PubMed] [Google Scholar]
- 138.Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010 Jun.1338:89–99. doi: 10.1016/j.brainres.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015 Apr.16(4):201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- 140.Hommers LG, Domschke K, Deckert J. Heterogeneity and individuality: microRNAs in mental disorders. J Neural Transm. Vienna Austria 1996. 2015 Jan.122(1):79–97. doi: 10.1007/s00702-014-1338-4. [DOI] [PubMed] [Google Scholar]
- 141.Zhou R, Yuan P, Wang Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009 May;34(6):1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beveridge NJ, Gardiner E, Carroll AP, et al. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010 Dec.15(12):1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nguyen LS, Lepleux M, Makhlouf M, et al. Profiling olfactory stem cells from living patients identifies miRNAs relevant for autism pathophysiology. Mol. Autism. 2016 Jan.7 doi: 10.1186/s13229-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abu-Elneel K, Liu T, Gazzaniga FS, et al. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008 Jul.9(3):153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- 145.Ander BP, Barger N, Stamova B, et al. Atypical miRNA expression in temporal cortex associated with dysregulation of immune, cell cycle, and other pathways in autism spectrum disorders. Mol. Autism. 2015;6:37. doi: 10.1186/s13229-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Talebizadeh Z, Butler MG, Theodoro MF. Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Res. Off. J. Int. Soc. Autism Res. 2008 Aug.1(4):240–250. doi: 10.1002/aur.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghahramani Seno MM, Hu P, Gwadry FG, et al. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011 Mar.1380:85–97. doi: 10.1016/j.brainres.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 148.Sarachana T, Zhou R, Chen G, et al. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2(4):23. doi: 10.1186/gm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mundalil Vasu M, Anitha A, Thanseem I, et al. Serum microRNA profiles in children with autism. Mol. Autism. 2014;5:40. doi: 10.1186/2040-2392-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang F, Zhou T, Yao X, et al. miRNA profiling in autism spectrum disorder in China. Genomics Data. 2015 Dec.6:108–109. doi: 10.1016/j.gdata.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Banerjee-Basu S, Larsen E, Muend S. Common microRNAs Target Established ASD Genes. Front. Neurol. 2014 Oct.5 doi: 10.3389/fneur.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mellios N, Sur M. The Emerging Role of microRNAs in Schizophrenia and Autism Spectrum Disorders. Front. Psychiatry. 2012 Apr.3 doi: 10.3389/fpsyt.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Olde Loohuis NFM, Kole K, Glennon JC, et al. Elevated microRNA-181c and microRNA-30d levels in the enlarged amygdala of the valproic acid rat model of autism. Neurobiol. Dis. 2015 Aug.80:42–53. doi: 10.1016/j.nbd.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kandemir H, Erdal ME, Selek S, et al. Evaluation of several micro RNA (miRNA) levels in children and adolescents with attention deficit hyperactivity disorder. Neurosci. Lett. 2014 Sep.580:158–162. doi: 10.1016/j.neulet.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 155.Wu LH, Peng M, Yu M, et al. Circulating MicroRNA Let-7d in Attention-Deficit/Hyperactivity Disorder. Neuromolecular Med. 2015 Jun.17(2):137–146. doi: 10.1007/s12017-015-8345-y. [DOI] [PubMed] [Google Scholar]
- 156.Chistiakov DA, Chekhonin VP. Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2014 Sep.35(9):8425–8438. doi: 10.1007/s13277-014-2262-9. [DOI] [PubMed] [Google Scholar]
- 157.Gonda DD, Akers JC, Kim R, et al. Neuro-oncologic applications of exosomes, microvesicles, and other nano-sized extracellular particles. Neurosurgery. 2013 Apr.72(4):501–510. doi: 10.1227/NEU.0b013e3182846e63. [DOI] [PubMed] [Google Scholar]
- 158.Viaud S, Théry C, Ploix S, et al. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res. 2010 Feb.70(4):1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 159.Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front. Neurosci. 2015 Mar.9 doi: 10.3389/fnins.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moretti R, Pansiot J, Bettati D, et al. Blood-brain barrier dysfunction in disorders of the developing brain. Front. Neurosci. 2015;9:40. doi: 10.3389/fnins.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alvarez-Erviti L, Seow Y, Yin H, Betts C, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011 Apr.29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 162.Lakhal S, Wood MJA. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays News Rev. Mol. Cell. Dev. Biol. 2011 Oct.33(10):737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 163.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008 Dec.10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wood MJA, O’Loughlin AJ, Samira L. Exosomes and the blood-brain barrier: implications for neurological diseases. Ther. Deliv. 2011 Sep.2(9):1095–1099. doi: 10.4155/tde.11.83. [DOI] [PubMed] [Google Scholar]
- 165.Kapogiannis D, Boxer A, Abner E, et al. Neural origin plasma exosomes provide novel biomarkers for brain insulin resistance in Alzheimer’s disease (I11-5B) Neurology. 2015 Apr.84(14 Supplement):I11–I15B. [Google Scholar]
- 166.Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012 Dec.18(12):1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Theoharides TC, Zhang B. Neuro-inflammation, blood-brain barrier, seizures and autism. J Neuroinflammation. 2011 Nov.8:168. doi: 10.1186/1742-2094-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de Vries HE, Kooij G, Frenkel D, et al. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012 Nov.53(Suppl 6):45–52. doi: 10.1111/j.1528-1167.2012.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jia R, Li J, Rui C, et al. Comparative Proteomic Profile of the Human Umbilical Cord Blood Exosomes between Normal and Preeclampsia Pregnancies with High-Resolution Mass Spectrometry. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;36(6):2299–2306. doi: 10.1159/000430193. [DOI] [PubMed] [Google Scholar]
- 170.Wang L, Bammler TK, Beyer RP, et al. Copper-induced deregulation of microRNA expression in the zebrafish olfactory system. Environ. Sci. Technol. 2013 Jul.47(13):7466–7474. doi: 10.1021/es400615q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qi Y, Zhang M, Li H, et al. MicroRNA-29b regulates ethanol-induced neuronal apoptosis in the developing cerebellum through SP1/RAX/PKR cascade. J. Biol. Chem. 2014 Apr.289(14):10201–10210. doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pietrzykowski AZ, Friesen RM, Martin GE, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008 Jul.59(2):274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prins SA, Przybycien-Szymanska MM, Rao YS, et al. Long-term effects of peripubertal binge EtOH exposure on hippocampal microRNA expression in the rat. PloS One. 2014;9(1):e83166. doi: 10.1371/journal.pone.0083166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pappalardo-Carter DL, Balaraman S, Sathyan P, et al. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: evidence from zebrafish and murine fetal neural stem cell models. Alcohol. Clin. Exp. Res. 2013 Oct.37(10):1657–1667. doi: 10.1111/acer.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lewohl JM, Nunez YO, Dodd PR, et al. Up-regulation of microRNAs in brain of human alcoholics. Alcohol. Clin. Exp. Res. 2011 Nov.35(11):1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yadav S, Pandey A, Shukla A, et al. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol. Chem. 2011 Oct.286(43):37347–37357. doi: 10.1074/jbc.M111.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen C-L, Liu H, Guan X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. J Biomed. Sci. 2013;20:96. doi: 10.1186/1423-0127-20-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Avissar-Whiting M, Veiga KR, Uhl KM, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. Elmsford N. 2010 Jul.29(4):401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.De Felice B, Manfellotto F, Palumbo A, et al. Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med. Genomics. 2015;8:56. doi: 10.1186/s12920-015-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ogata K, Sumida K, Miyata K. Circulating miR-9* and miR-384-5p as potential indicators for trimethyltin-induced neurotoxicity. Toxicol. Pathol. 2015 Feb.43(2):198–208. doi: 10.1177/0192623314530533. [DOI] [PubMed] [Google Scholar]
- 181.Maccani MA, Avissar-Whiting M, Banister CE, et al. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010 Oct.5(7):583–589. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Marczylo EL, Amoako AA, Konje JC, et al. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012 May;7(5):432–439. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- 183.Fossati S, Baccarelli A, Zanobetti A, et al. Ambient particulate air pollution and microRNAs in elderly men. Epidemiol. Camb. Mass. 2014 Jan.25(1):68–78. doi: 10.1097/EDE.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bai W, Chen Y, Yang J, et al. Aberrant miRNA profiles associated with chronic benzene poisoning. Exp. Mol. Pathol. 2014 Jun.96(3):426–430. doi: 10.1016/j.yexmp.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 185.Laterza OF, Lim L, Garrett-Engele PW, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin. Chem. 2009 Nov.55(11):1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 186.Zhang B, Pan X. DX Induces Aberrant Expression of MicroRNAs in Mouse Brain and Liver. Environ. Health Perspect. 2009 Feb.117(2):231–240. doi: 10.1289/ehp.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Deng Y, Ai J, Guan X, Wang Z, et al. MicroRNA and messenger RNA profiling reveals new biomarkers and mechanisms for RDX induced neurotoxicity. BMC Genomics. 2014;15(Suppl 11):S1. doi: 10.1186/1471-2164-15-S11-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kempf SJ, Casciati A, Buratovic S, et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol. Neurodegener. 2014;9:57. doi: 10.1186/1750-1326-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]