Abstract
Background
Exceeding the Institute of Medicine's guidelines for pregnancy weight gain increases childhood and adolescent obesity. However, it is unknown if these effects extend to mid-life.
Objective
To determine if exceeding the Institute of Medicine's guidelines for pregnancy weight gain increases risk of obesity in daughters 40 years later.
Study Design
This cohort study is based on adult offspring in The Child Health and Development Studies and the Collaborative Perinatal Population pregnancy cohorts originally enrolled in the 1960s. In 2005-2008, 1,035 daughters in their 40s were recruited to the Early Determinants of Mammographic Density study. We classified maternal pregnancy weight gain as greater than vs. less than or equal to the 2009 clinical guidelines. We used logistic regression to compare the odds ratios of daughters being overweight/obese (body mass index ≥25) at a mean age of 44 between mothers who did not gain or gained more than pregnancy weight gain guidelines , accounting for maternal pre-pregnant body mass index, and daughter's body size at birth and childhood. We also examined potential family related confounding through a comparison of daughter siblings using generalized estimating equations, clustered on sibling units and adjusted for maternal age and race.
Results
Mothers who exceeded guidelines for weight gain in pregnancy were more likely to have daughters who were overweight/obese in their 40s (odds ratio [OR], 3.4; 95% confidence interval {CI}, 2.0–5.7). This magnitude of association translates to a relative risk (RR) increase of 50% (RR = 1.5; 95% CI, 1.3-1.6). The association was of the same magnitude when examining only the siblings whose mother exceeded guidelines in 1 pregnancy and did not exceed the guidelines in the other pregnancy. The association was stronger with increasing maternal prepregnancy BMI (P trend < .001). Compared to mothers with BMI <25 who did not exceed guidelines, the relative risks (RR) for having an overweight/obese adult daughter were 1.3 (95% CI, 1.1-1.7), 1.7 (95% CI, 1.4-2.1) and 1.8 (95% CI, 1.5-2.1), respectively, if mothers exceeded guidelines and their prepregnancy BMI was <25, overweight (BMI 25-<30), or obese (BMI >30). This pattern held irrespective of daughters’ weight status at birth, at age 4 years, or at age 20 years.
Conclusion
Our findings support that obesity prevention before pregnancy and strategies to maintain weight gain during pregnancy within the Institute of Medicine guidelines might reduce the risk of being overweight in midlife for the offspring.
Keywords: gestational weight gain, obesity, life course
Introduction
The prevalence of overweight (BMI≥25- <30 kg/m2) and obesity (BMI≥30 kg/m2) in American women of childbearing age has increased greatly over the last 30 years.1 The U.S. Institute of Medicine (IOM) has targeted pregnancy weight gain as a possible modifiable risk factor for reducing obesity in future generations.1 Earlier versions of the IOM guidelines, established in 19702 and first revised in 19903, were originally designed to prevent small for gestational age children and to make sure that mothers gained enough weight during pregnancy. Their current (2009) guidelines recognize the increasing rates of overweight/obesity in women of childbearing age and provide pre-pregnancy BMI-specific guidelines. For singleton pregnancies, the IOM recommends that mothers with a body mass index (BMI) of 18.5 to <25 kg/m2 should gain no more than 16 kg.1 Underweight (BMI<18.5 kg/m2), overweight and obese mothers should gain no more than 18, 11.5 and 9 kg, respectively.1
Observational studies demonstrate that mothers who gain in excess of the current recommended guidelines are more likely to have offspring with higher birth weights4–6 and higher childhood BMI7–13, than mothers who gain within the recommended ranges. A recent meta-analysis of seven studies found that pregnancy weight gain outside the ranges recommended by IOM1 led to a higher risk for childhood overweight/obesity between the ages of 2 and 20 [combined adjusted odds ratio (OR) of 1.43 (95 % confidence interval (CI) 1.24–1.65)].14 It is currently unknown, however, whether the association between excessive pregnancy weight gain and offspring's risk of being overweight extends to midlife. We hypothesized that exceeding guidelines is associated with daughters’ risk of overweight/obesity independently of shared environmental or behavioral factors between the mother and daughter that may persist into adulthood. We tested this hypothesis, using both cohort and sibling-based analyses, by applying current IOM pregnancy weight gain guidelines to a cohort of women born in the 1960s, when pregnant women were discouraged from gaining much weight at all (4-6 kg).2,15
Materials and Methods
Study design and population
This study is based on adult daughters who were born in the 1960s to women enrolled in the Child Health and Development Studies (CHDS)16 and National Collaborative Perinatal Study (NCPP) pregnancy cohorts.17 In 2005 to 2008, 40-50 years after birth, daughters (N= 1,134) participated in a new follow-up, Early Determinants of Mammographic Density (EDMD).18 Pregnant women who received prenatal care at the Kaiser Family Health Plan in Oakland, California, were eligible for the CHDS, and were recruited in California between 1959 and 1967.16 Pregnant women, who received prenatal care at one of the teaching hospitals of Harvard Medical School or Brown University, were approached to take part in the New England sites of the Collaborative Perinatal Project (also known as the New England Family Study), which was conducted between 1959 and 1966.17 We used a subset of the CHDS and CPP based on adult follow-up eligibility (for details of EDMD18). Both studies were designed to study prenatal and familial factors influencing child growth and development. In a subsample of pregnancies in the same mother, we also employed a sibling study design, consisting of 247 sibling pairs, to control for family level confounding. The Institutional Review Boards at Columbia University Medical Center, Kaiser Permanente, Brigham and Women's Hospital and Brown University approved the EDMD study. Informed consent was obtained from the mothers in the original cohorts and daughters in the follow-up study.
Data collection
Baseline maternal and childhood data
Maternal interviews collected information on pre-pregnancy body mass index (BMI), smoking during pregnancy and maternal education at registration.16,17,19 Weight gain during pregnancy was abstracted from the records of prenatal visits and was determined as the difference between the last pre-delivery weight and the first recorded weight.16 A trained interviewer enquired after the first day of the last menstrual period at the prenatal registration interview to calculate gestational age, which is the time period between the last menstrual period and the day of delivery.
We categorized pregnancy weight gain into a binary variable according to the current IOM's clinical guidelines: mothers either did not gain more weight than the upper limit of the BMI-specific guidelines, currently established as 16 kg, 11.5 kg and 9 kg for women whose pre-pregnancy BMI is 18.5-<25, 25<30 and >30, respectively, or mothers exceeded the upper limit of the guidelines.1
We assessed growth in height and weight measurements at 4 years of age because children were measured at different time-points in the CPP and CHDS and these were the common time points between the two cohorts. In the CHDS, serial growth measurements were abstracted from medical records.16 In the CPP, trained clinical staff measured childhood height and weight at 4 years.17 Because the actual dates of the clinic visit differed for each individual and did not correspond to exactly 4 years, we performed interpolations of height and weight measurements using individual cubic interpolation splines and then calculated BMI percentiles according to the Centers for Disease Control (CDC) growth chart reference percentiles.
Adult daughter interviews
When daughters were in their forties, we conducted a 45 minute computer-assisted telephone interview, gathering data on personal health, medication, socio-demographic factors and anthropometric measures.18 We calculated BMI at age 20 years and current BMI from self-reported height and weight. A subgroup of the daughters participating in the EDMD study also participated in the Early Determinants of Adult Health (EDAH19,20) study where current adult height and weight was measured clinically. Inter-observer agreement between self-reported and clinically measured BMI was high (n=190; kappa=0.81). The mean and standard deviation of BMI in the subset (27.7 (7.0) kg/m2) was no different from the BMI in the overall cohort (27.2 (6.5) kg/m2) suggesting that there would be minimal differential measurement error between the subset and the overall cohort due to body size.
Statistical analysis
We assessed the association between maternal pregnancy weight gain and daughter overweight/obese status (BMI≥25 kg/m2) at time of interview (mean age 44, range 39-49). We also defined daughter body size categories at three previous times in the life course as stratifying variables: high birth weight (>4000g), overweight/obese (BMI>85th percentile) at age 4, overweight/obese (BMI≥25 kg/m2) at age 20. We adjusted for race/ethnicity, education, age at delivery, smoking during pregnancy, and gestational age, and compared models with and without these potential confounders or intermediary variables. We retained covariates in the models if they affected the key exposure associations (exceeded guidelines) by more than 10% (none of the variables met criterion), were an independent predictor of the outcome (race/ethnicity) or were based on a priori hypotheses (maternal age).21,22
We use generalized logistic regression to regress daughter overweight status at interview on maternal weight gain status (exceeded vs. did not exceed) both before and after stratifying by pre-pregnancy BMI. The number of mother/daughter pairs with complete data for pre-pregnancy BMI, pregnancy weight gain and BMI was 885. We also ran relative risk regressions for the final models to place in context the magnitude of the odds ratios in terms of relative risks. After the analysis of all daughters, we limited the analysis to daughters who were not high birth weight, or overweight/obese at age 4 or 20. We also stratified by parity of the daughter. In sensitivity analyses, we also applied the cut-offs from previous guidelines established before and after 1970.1–3
We further examined potential family related confounding through a comparison of daughter siblings. Sibling pairs were concordant for pregnancy weight gain if the mother did not exceed or exceeded guidelines in both pregnancies; sibling pairs were discordant if the mother only exceeded guidelines in one pregnancy. We used general estimating equations among the discordant siblings adjusted for maternal age and race, and accounted for the shared correlation between siblings, to calculate the ORs and 95%CI of being overweight if a mother exceeded the guidelines and stratified by pre-pregnancy BMI.
Results
Table 1 shows descriptive characteristics of mothers and daughters included in the analysis and compares these characteristics by maternal weight gain status according to the current guidelines.1 Maternal age at delivery, education, smoking habits, and race/ethnicity were similar between mothers who did and did not exceed guidelines. Compared to mothers with a BMI<25 kg/m2, who on average gained 9.8 kg during pregnancy, overweight and obese mothers gained less weight (8.8 kg and 6.6 kg respectively; data not shown). However, because BMI-specific guidelines are lower for overweight and obese women, mothers who exceeded guidelines had higher mean pre-pregnancy BMI compared to mothers who did not. Mothers who exceeded guidelines also had longer gestations and daughters with higher birth weights, birth lengths, and BMIs at ages four and 20 years. These characteristics of the cohort are similar to national data of women during the 1960s.23,24
Table 1.
Study Characteristics of the Early Determinants of Mammographic Density cohort (n=1045) according to maternal weight gain in pregnancy.
| Did not exceed IOM guidelines1 (n=942) | Exceeded IOM guidelines1 (n=103) | P-value | |||
|---|---|---|---|---|---|
| Maternal Characteristics | Mean or % | SD | Mean or % | SD | |
| Pre-pregnancy BMI (kg/m2) | 26.1 | ±3·5 | 26.9 | ±4.3 | p<0.0001 |
| <25 | 81% | 34% | |||
| 25 to <30 | 15% | 44% | |||
| 30+ | 4% | 22% | |||
| Age at delivery (years) | 26.1 | ±5·7 | 25.7 | ±5.6 | 0.48 |
| Education (more than high school) | 35% | 27% | 0.22 | ||
| Smoking during pregnancy (yes vs. no) | 41% | 33% | 0.18 | ||
| Race/Ethnicity | |||||
| Non-Hispanic White | 83% | 79% | 0.52 | ||
| Non-Hispanic Black | 11% | 17% | 0.31 | ||
| Hispanic, Asian, Native American, Other | 6% | 4% | 0.70 | ||
| Daughter Characteristics | |||||
| Gestational age (weeks) | 40.1 | ±2.0 | 40.6 | ±2.0 | p<0.01 |
| Birth weight (kg) | 3.4 | ±0.5 | 3.7 | ±0.5 | p<0.0001 |
| Birth length (cm) | 51.2 | ±2.8 | 52.5 | ±2.3 | p<0.0001 |
| BMI at age 4 years (kg/m2) | 16.4 | ±1.6 | 17.1 | ±2.5 | p<0.0001 |
| BMI at age 20 years (kg/m2) | 22.1 | ±3.9 | 24.8 | ±5.8 | p<0.0001 |
| BMI at interview (kg/m2) | 26.7 | ±6.1 | 31.3 | ±7.5 | p<0.0001 |
| Age at interview (years) | 44.1 | ±1.9 | 44.1 | ±1.9 | 0.93 |
| Parous (%) | 86.9% | 88.2% | 0.70 | ||
Classification from 2009 Institute of Medicine pregnancy weight gain guidelines
Table 2 shows the odds ratio of having an overweight, middle-aged daughter for mothers who exceeded IOM guidelines, unadjusted and adjusted for maternal age and race/ethnicity. Mothers who exceeded guidelines were about three-times more likely to have an overweight, middle-aged daughter (OR=3.4; 95%CI=2.0- 5.7) than mothers who did not exceed guidelines. The magnitude of association translates to a relative risk (RR) increase of 50% (RR=1.5; 95% CI, 1.3-1.6). The association of pregnancy weight gain with daughters BMI was present and of the same magnitude when examining only the siblings whose mother exceeded guidelines in one pregnancy and did not exceed the guidelines in the other pregnancy (OR=3.6; 95%CI=1.2- 10.6).
Table 2.
OR (95% CI) for daughters being overweight (BMI≥25) as an adult from mothers who exceeded BMI-specific IOM guidelines for gestational weight gain1
| Overall Cohort | Mothers of siblings discordant for exceeding guidelines | ||||
|---|---|---|---|---|---|
| N | Unadjusted model | Adjusted model for maternal age, and race | |||
| Pregnancy Weight Gain | Daughters BMI<25 | Daughters BMI≥25 | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| not more than guidelines | 444 | 493 | 1 | 1 | 1 |
| more than guidelines | 20 | 78 | 3.5 (2.1-5.9) | 3.4 (2.0-5.7) | 3.6 (1.2-10.6) |
Because women cannot modify their prepregnancy BMI once pregnant, it is helpful for women to know the benefits of staying within weight gain guidelines, even if they are overweight or obese prior to pregnancy. Compared to mothers with the same prepregnancy BMI who did not exceed guidelines, the odds ratio (95% CI) for having an overweight/obese daughter was 2.6 (1.1–6.2) in overweight mothers who exceeded guidelines and 1.6 (0.4–6.7) in obese mothers who exceeded guidelines, although the latter was not statistically significant.
Figure 1 also shows the increasing risk of having an overweight, middle-aged daughter by pre-pregnancy BMI categories in reference to mothers with BMI<25kg/m2 who did not exceed guidelines. Overweight mothers were about two to five times more likely to have an overweight middle-aged daughter if they did not exceed (OR= 2.2; 95%CI=1.5- 3.3) or exceeded (OR= 5.6; 95% CI= 2.4-12.7) guidelines. The odds ratio increased for obese mothers who did not exceed (OR=4.8; 95%CI= 2.0-23.8) and those that exceeded guidelines (OR=6.9; 95%CI= 2.0-23.8). To translate the magnitude of these odds ratios into relative risk, the figure presents the results from the relative risk regression models adjusting for the same covariates. Although, the sample size is limited, this association was also present, although not statistically significant, in mothers of siblings who exceeded the guidelines in one pregnancy but not the other [(pre-pregnancy BMI<25 kg/m 2: OR=2.5; 95%CI=0.5 to 11.3) and (pre-pregnancy BMI=25-<30 kg/m 2: OR=7.0; 95%CI=0.9 to 56.9)]. We did not have sufficient numbers of discordant siblings to examine the association in obese mothers.
Figure 1. Risk of having an overweight/obese adult daughter by maternal pre-pregnancy BMI and gestational weight gain guidelines.
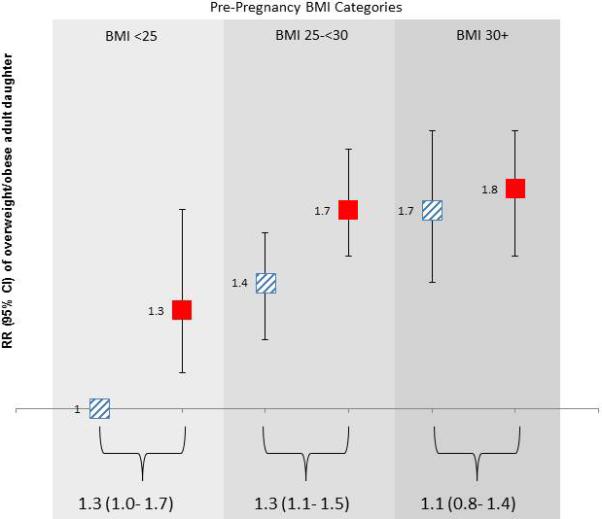
Relative risk of having an overweight/obese adult daughter in mothers who exceeded (solid, red) and did not exceed (striped, blue) Institute of Medicine 2009 guidelines for gestational weight gain, adjusted for maternal age and race. Risk ratios (RR) and 95% confidence interval (CI) depicted as boxes and bars are in reference to mothers with body mass index (BMI) <25, who did not exceed guidelines. P trend < .001. Strata specific RR and 95% CI are noted beneath brackets.
Table 3 shows that the association between exceeding guidelines and daughters’ risk of being overweight at midlife persists even in daughters that were not overweight in previous life stages. The odds ratio for being overweight at midlife were similar between daughters that were not overweight at age four (column 2) and all daughters (column 1). The associations of exceeding guidelines remained in daughters who were not high birth weight nor overweight at ages four (column 4) and 20 (column 6). The overall association of exceeding guidelines and daughters overweight/obesity was also present in daughters who were nulliparous (OR=10.7; 95%CI=1.3 to 86.4; n=124), parous (OR=2.8; 95%CI=1.7 to 4.8) with 1-2 children (OR=4.5; 95%CI=1.5 to 13.4) or 3-7 children (OR=2.3; 95%CI=1.3 to 4.3). The association also remained in parous daughters who were not overweight at age 20 with 1-2 children (OR=5.3; 95%CI=1.5 to 18.7) or 3-7 children (OR=2.0; 95%CI=1.0 to 3.9), although the latter association was not statistically significant.
Table 3.
OR (95% Confidence Intervals) for being overweight (BMI ≥25) at age 40, stratified by body size across the lifecourse, EDMD cohort
| In all daughters | In daughters who were not high birthweight (≤4,000g) | In daughters who were not overweight at age 4 years (BMI<85th percentile) | In daughters who were not high birthweight or overweight at age 4 years | In daughters who were not overweight at age 20 years (BMI<25 kg/m2) | In daughters who were not high birthweight or overweight at ages 4 or 20 years (BMI<25 kg/m2) | |
|---|---|---|---|---|---|---|
| N | 1035 | 767 | 731 | 631 | 764 | 555 |
| Pre-pregnancy BMI | ||||||
| <25 | ||||||
| did not exceed guidelines (≤16 kg) | 1 | 1 | 1 | 1 | 1 | 1 |
| exceeded guidelines (>16 kg) | 2.1 (1.0-4.6) | 1.4 (0.6-3.2) | 2.1 (0.9-4.6) | 1.5 (0.6-3.4) | 2.1 (0.9-4.6) | 1.4 (0.6-3.5) |
| ≥25 to <30 (overweight) | ||||||
| did not exceed guidelines (≤11.5 kg) | 2.2 (1.5-3.3) | 2.3 (1.5-3.6) | 2.0 (1.3-3.2) | 2.3 (1.4-3.7) | 2.3 (1.5-3.4) | 2.2 (1.3-3.8) |
| exceeded guidelines (>11.5 kg) | 5.6 (2.4-12.7) | 6.0 (2.0-17.7) | 4.2 (1.7-9.9) | 4.6 (1.5-14.2) | 3.9 (1.5-10.0) | 3.4 (0.9-13.5) |
| ≥30 (obese) | ||||||
| did not exceed guidelines (≤9 kg) | 4.8 (2.2-10.8) | 5.2 (2.2-12.2) | 8.1 (3.1-21.4) | 10.0 (3.4-29.4) | 3.9 (1.6-9.0) | 8.4 (2.7-25.9) |
| exceeded guidelines (>9 kg) | 6.9 (2.0-23.8) | 9.2 (2.1-40.4) | 6.1 (1.7-21.6) | 8.7 (1.9-39.0) | 5.4 (1.5-19.7) | 7.2 (1.5-34.4) |
* all models adjusted for maternal age and race
Comment
Daughters whose mothers exceeded the IOM guidelines1 were about three times more likely to be overweight at midlife than daughters whose mothers did not exceed guidelines. The association of exceeding guidelines on daughters body size was especially clear for overweight mothers, irrespective of their daughter being born high birth weight(>4000 grams) or overweight at age four or 20. Our sibling analyses confirmed that the findings were independent of fixed family-level confounding. Our results also show that exceeding pregnancy weight gain guidelines has a long-term effect on adult BMI which is not due to body size tracking across the life course.
Our findings extend the literature on pregnancy weight gain guidelines and adult body size to midlife. Another study reported a U-shape association with gestational weight gain (not using the guideline cut points) and BMI in mid adulthood25. The effect of exceeding the clinical guidelines only previously has been investigated until age 20. For example, two meta-analyses14,26 and at least eight other studies investigated pregnancy weight gain according to the IOM guidelines and weight in postnatal life, at birth27–31, childhood32 and in young adulthood until 20 years of age.33 Six studies showed that exceeding gestational weight guidelines was associated with a near twofold risk in having an overweight child, either by stratifying or controlling for pre-pregnancy BMI.27–31,34 Our findings are consistent with another study that examined the association of exceeding IOM guidelines with BMI in young adulthood at 20 years of age, controlling for pre-pregnancy BMI, showing a positive association, but twenty years earlier than our outcome.33 In 146,894 sibling sets, Lawlor et al.35 found that overweight mothers with elevated weight gain from around 10 weeks of gestation, had sons with higher BMI at age 18 compared to mothers with a BMI <25 kg/m2. We show the same association, but in daughters that were 20 years older than this Swedish sample, and moreover, in daughters that were not overweight at age 20. While using recalled BMI at age 20 is a limitation of our study, we were able to compare the self-reported measures of BMI at 40 with the clinical measurements in a sub-cohort and found the same inference (data not shown). Furthermore, the association also persisted in daughters who were not overweight in childhood when their height and weight were measured at clinic visits. The persistent association between exceeding guidelines and overweight/obesity in daughters even when they were not overweight or obese at previous stages in life suggests that the underlying mechanisms linking pregnancy weight gain to offspring's body size is not due to maternal and daughter body size tracking and is most likely independent of shared family characteristics.
Pregnancy weight gain and pre-pregnancy BMI has increased in these last thirty years.1 A possible limitation of the study might be that current guidelines were applied to a population of pregnant women who were on average younger (26.1 years) than current mothers, had a lower pre-pregnancy BMI (23.2 m/kg2) than current mothers, and most likely were following different advice from their doctors 40 years ago than current day guidelines. Before 1970, doctors recommended that mothers of all BMIs should gain no more than 6.4 kg (14lbs).2 In an effort to not gain weight, it was common for women to suppress their appetite by smoking and taking medications, extreme measures to regulate weight by modern standards.36 Nevertheless, the majority of women in our cohort (80%) gained more than what was commonly recommended in the 1960s (4 to 6 kg or 10-14 lbs.).1,2,15 After 1970 and until 1990, mothers were encouraged to gain up to 11.4 kg (25lbs), regardless of their pre-pregnancy BMI.3 The association of pregnancy weight gain with adult overweight status persisted when we applied the 1960, 1970 and 1990 guidelines. The overall consistency in our findings using the different thresholds for pregnancy weight gain suggests that gestational weight gain above 11.4kg is associated with overweight/obesity in daughters at midlife. This robust finding has intergenerational implications and we applied estimates from our data to predict implications for future generations. When they were in their forties, 37% of adult daughters in our cohort were overweight/obese and this matches the percentage of US women who were overweight/obese at their first full-term birth in 2010. When we apply the relative risks generated by our cohort to the more recent distributions of pre-pregnancy BMI and pregnancy weight gain in the US, we estimate that 51% of their daughters will be overweight/ obese in 40 years’ time (see Supplemental Figure).
We did not investigate the effect of maternal of paternal diet nor paternal BMI as these were not measured. Epidemiological studies show that paternal BMI is positively associated with childhood BMI, however, there are only limited studies of paternal BMI and offspring adult obesity.37,38 Animal studies indicate that paternal diet affects offspring obesity by paternal environmental exposures influencing sperm development which might induce epigenetic alterations in their offspring39 (reviewed in40). In addition, genetic studies show that paternally inherited SNPs are associated with adult BMI in large human studies.41
Our study offers further support of the importance of counseling all women regarding their weight during pregnancy. If mothers gained weight within guidelines, this may help reduce their offspring's as well as their own risk of overweight and obesity42,43 as well as lowering the risk of adverse pregnancy outcomes.44 Unfortunately, at this time interventions to reduce gestational weight gain have been challenging.45,46 Better understanding of the determinants of weight gain in pregnancy is likely required to intervene effectively. A 2012 review of intervention studies in pregnant women during the gestational period concluded that while some diet and exercise interventions are able to modify GWG in the mothers, very few studies have shown an impact on birth size in the offspring. However, none of the interventions followed the offspring past delivery.47 Given our data showing that exceeding guidelines predicts overweight/obesity even in women that were not large at birth or during childhood, longer term follow-up of the intervention studies is warranted.
Originally, physicians and scientists created IOM guidelines to safeguard women for a healthy pregnancy and ensure that their children had optimal birth outcomes. Our study confirms that these guidelines are important both to ensure adequate weight gain but also to facilitate clinicians in counseling pregnant couples to prevent the high risk for obesity in their children.
Supplementary Material
Acknowledgements
This study is funded in part by the National Cancer Institute's R01CA104842 and K07CA90685, and the National Institute on Aging, P01AG023028. We also thank Jasmine A. McDonald for reviewing drafts of this manuscript.
Sources of the study: The Child Health and Development Studies, the Collaborative Perinatal Population pregnancy cohorts, and the Early Determinants of Mammographic Density study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: We declare we have no conflicts of interest.
References
- 1.Institute of M, National Research Council Committee to Reexamine IOMPWG . The National Academies Collection: Reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press (US)National Academy of Sciences; Washington (DC): 2009. [PubMed] [Google Scholar]
- 2.Research CN, editor. Maternal Nutrition and the Course of Pregnancy. 1970 [Google Scholar]
- 3.Institute of Medicine NA of S . Nutrition during pregnancy. National Academy Press; Washington, D.C.: 1990. [Google Scholar]
- 4.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008:1–223. [PMC free article] [PubMed] [Google Scholar]
- 5.Hutcheon JA, Platt RW, Meltzer SJ, Egeland GM. Is birth weight modified during pregnancy? Using sibling differences to understand the impact of blood glucose, obesity, and maternal weight gain in gestational diabetes. Am J Obs Gynecol. 2006;195:488–94. doi: 10.1016/j.ajog.2006.01.107. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–90. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj. 2000;320:967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowan NA, Stember ML. Parental risk factors for infant obesity. MCN Am J Matern Child Nurs. 2000;25:234–40. doi: 10.1097/00005721-200009000-00004. quiz 241. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obes (Silver Spring) 2007;15:760–71. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- 10.Moreira P, Padez C, Mourao-Carvalhal I, Rosado V. Maternal weight gain during pregnancy and overweight in Portuguese children. Int J Obes. 2007;31:608–14. doi: 10.1038/sj.ijo.0803582. [DOI] [PubMed] [Google Scholar]
- 11.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obs Gynecol. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87:1818–24. doi: 10.1093/ajcn/87.6.1818. [DOI] [PubMed] [Google Scholar]
- 13.Sridhar SB, Darbinian J, Ehrlich SF, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obs Gynecol. 2014;211:259, e1–8. doi: 10.1016/j.ajog.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tie HT, Xia YY, Zeng YS, et al. Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: a meta-analysis. Arch Gynecol Obs. 2014;289:247–57. doi: 10.1007/s00404-013-3053-z. [DOI] [PubMed] [Google Scholar]
- 15.Eastman NJ, Hellman LM. Williams Obstetrics. Appleton-Century-Crofts. 1966 [Google Scholar]
- 16.Berg van den BJ. The California Child Health and Development Studies. In: Medinick SA, Finello KM, HM, editors. Handbook of Longitudinal Research. Praeger Publishers; New York: 1984. [Google Scholar]
- 17.Broman S. The Collaborative Perinatal Project: an overview. In: Medinick SA, Finello KM, HM, editors. Handbook of Longitudinal Research. Praeger Publishers; New York: 1984. [Google Scholar]
- 18.Terry MB, Schaefer CA, Flom JD, et al. Prenatal smoke exposure and mammographic density in mid-life. J Dev Orig Heal Dis. 2011;2:340–52. doi: 10.1017/S2040174411000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susser E, Buka S, Schaefer CA, et al. The Early Determinants of Adult Health Study. J Dev Orig Heal Dis. 2011;2:311–21. doi: 10.1017/S2040174411000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumey LH, Susser E, Andrews H, Gillman MW. Birth size and adult size in same-sex siblings discordant for fetal growth in the Early Determinants of Adult Health study. J Dev Orig Heal Dis. 2011;2:330–9. doi: 10.1017/S2040174411000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80:502–11. [PubMed] [Google Scholar]
- 22.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta- analysis. PLoS One. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flegal KM, Harlan WR, Landis JR. Secular trends in body mass index and skinfold thickness with socioeconomic factors in young adult women. Am J Clin Nutr. 1988;48:535–43. doi: 10.1093/ajcn/48.3.535. [DOI] [PubMed] [Google Scholar]
- 24.Kleinman JC, Kopstein A. Smoking during pregnancy, 1967-80. Am J Public Health 1987. 77:823–5. doi: 10.2105/ajph.77.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuebe a M, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre- pregnancy body mass index, and obesity in the daughter. Int J Obes (Lond) 2009;33:743–52. doi: 10.1038/ijo.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia MZ, Park CK, Beyene J, Giglia L, Maxwell C, McDonald SD. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and meta-analysis. Obes Rev. 2015;16:189–206. doi: 10.1111/obr.12238. [DOI] [PubMed] [Google Scholar]
- 27.Badon SE, Dyer AR, Josefson JL, Group HSCR Gestational weight gain and neonatal adiposity in the Hyperglycemia and Adverse Pregnancy Outcome study-North American region. Obes (Silver Spring) 2014;22:1731–8. doi: 10.1002/oby.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berggren EK, Stuebe AM, Boggess KA. Excess Maternal Weight Gain and Large for Gestational Age Risk among Women with Gestational Diabetes. Am J Perinatol. 2015;32:251–6. doi: 10.1055/s-0034-1383848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraro ZM, Barrowman N, Prud’homme D, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med. 2012;25:538–42. doi: 10.3109/14767058.2011.638953. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JM, McGowan CA, Mahony RM, Foley ME, McAuliffe FM. Obstetric and metabolic implications of excessive gestational weight gain in pregnancy. Obes (Silver Spring) 2014;22:1594–600. doi: 10.1002/oby.20753. [DOI] [PubMed] [Google Scholar]
- 31.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obs Gynecol 2013. 121:969–75. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ensenauer R, Chmitorz A, Riedel C, et al. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: results from a retrospective cohort study. Int J Obes. 2013;37:505–12. doi: 10.1038/ijo.2012.226. [DOI] [PubMed] [Google Scholar]
- 33.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obs Gynecol. 2010;202:574, e1–8. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Liu E, Guo J, et al. Maternal prepregnancy body mass index and gestational weight gain on offspring overweight in early infancy. PLoS One. 2013;8:e77809. doi: 10.1371/journal.pone.0077809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor DA, Lichtenstein P, Fraser A, Langstrom N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr. 2011;94:142–8. doi: 10.3945/ajcn.110.009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sothern MS. Tobacco, Formula, and Frequent Pregnancies: The Obesity Trinity. Child Obes. 2010;6:166. doi: 10.1089/chi.2010.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleten C, Nystad W, Stigum H, et al. Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am J Epidemiol. 2012;176:83–92. doi: 10.1093/aje/kws134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patro B, Liber A, Zalewski B, Poston L, Szajewska H, Koletzko B. Maternal and paternal body mass index and offspring obesity: A systematic review. Ann Nutr Metab. 2013;63:32–41. doi: 10.1159/000350313. [DOI] [PubMed] [Google Scholar]
- 39.Ng S-F, Lin RCY, Laybutt DR, Barres R, Owens J a, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–6. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 40.Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. BioEssays. 2014;36:359–71. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoggart CJ, Venturini G, Mangino M, et al. Novel Approach Identifies SNPs in SLC2A10 and KCNK9 with Evidence for Parent-of-Origin Effect on Body Mass Index. PLoS Genet. 2014;10:1–12. doi: 10.1371/journal.pgen.1004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herring SJ, Rose MZ, Skouteris H, Oken E. Optimizing weight gain in pregnancy to prevent obesity in women and children. Diabetes Obes Metab. 2012;14:195–203. doi: 10.1111/j.1463-1326.2011.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen AK, Chaffee BW, Rehkopf DH, Coyle JR, Abrams B. Excessive gestational weight gain over multiple pregnancies and the prevalence of obesity at age 40. Int J Obes. 2014;38:714–8. doi: 10.1038/ijo.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.L.M. H a. T, J.R. B. The Institute of Medicine Guidelines for Gestational Weight Gain after a Diagnosis of Gestational Diabetes and Pregnancy Outcomes. Am J Perinatol. 2014 doi: 10.1055/s-0034-1383846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agha M, Agha R a., Sandell J. Interventions to reduce and prevent obesity in pre- conceptual and pregnant women: A systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095132. DOI:10.1371/journal.pone.0095132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinnunen TI, Pasanen M, Aittasalo M, et al. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. Eur J Clin Nutr. 2007;61:884–91. doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- 47.Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9:1263–307. doi: 10.3390/ijerph9041263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


