Abstract
Purpose
To investigate the applicability of fusion biotoxins combining pore-forming toxins (PFTs) and ribosome-inactivating proteins (RIPs) for the anti-cancer treatment.
Methods
Membrane active PFTs tend to destabilize cell membranes of tumor cells, but lack a warhead inducing significant cause of cell death. Cell-impermeable RIPs possess a powerful warhead, yet not able to enter the tumor cells. To address these challenges for anti-tumor effects, we introduced a fusion strategy of conjugating melittin (a PFT) and gelonin (a type 1 RIP) via chemical and recombinant methods, followed by in vitro assays and in vivo animal studies.
Results
In vitro characterization results confirmed that the chimeric gelonin-melittin fusion proteins retained equivalent intrinsic activity to that of unmodified gelonin in inhibiting protein translation. However, chemically conjugated gelonin-melittin (cGel-Mel) and recombinant chimeric gelonin-melittin fusion (rGel-Mel) exhibited greater cell uptake, yielding a significantly enhanced cytotoxic activity over treatment of gelonin, melittin or physical mixture of gelonin and melittin. Remarkably, cGel-Mel and rGel-Mel displayed 32- and 10-fold lower IC50 than gelonin in the cell lines. The superior anti-tumor efficacy of multivalent cGel-Mel to monovalent rGel-Mel suggested that valency could be a crucial factor for the extent of melittin-mediated cell uptake. Tumoricidal effects observed from animal studies were in good accordance with our findings from the cellular assays.
Conclusions
This study successfully demonstrated that fusion of biotoxins could provide a simple yet effective way to synergistically augment their anti-tumor activity.
Keywords: Gelonin, Toxin, Ribosome Inactivating Protein, Melittin, Cancer
INTRODUCTION
Biotoxins are a group of proteins and peptides produced from various biological origins that play important roles in the host defense system for survival against predators (1, 2). Despite varied underlying mechanisms, these biotoxins are commonly cytotoxic and thus have been considered as attractive anti-cancer drug candidates (3, 4). Among the biotoxins, pore-forming toxins (PFTs) are known as membrane-active peptides possessing strong binding affinity to the plasma membranes and capable of forming transient pores (5). Melittin, a 26-amino acid peptide which is cationic and amphipathic, is the principal active component of the bee venom (6). As a typical PFT, melittin can destabilize and create pores inside the cell membranes. By this direct lytic action to the membranes and via additional mechanisms involved in apoptosis, cell cycle arrest or necrosis, melittin can kill tumor cells and significantly inhibit tumor growth (7, 8). These anti-tumor effects are, however, available only with high (above micro-molar) concentration of melittin. Apart from the membrane activity, melittin also possesses cell translocalizing ability, possibly through the pores it forms or via cell transduction mechanism of cell penetrating peptides due to their structural resemblance (cationicity and amphipathicity) (9). To date, there have been accumulating evidences that melittin could facilitate intracellular delivery of various attached cargos (e.g., genes, nanoparticles, etc.) (10, 11).
Another type of biotoxins is ribosome-inactivating proteins (RIPs) which are a group of protein toxins that are exceptionally potent in inhibiting protein synthesis (12, 13). Gelonin, first derived from the seeds of Gelonium Multiflora, is a type 1 RIP that possesses N-glycosidase activity (14). By cleavage of an adenine group from a preserved region of the 28S rRNA of eukaryotic ribosomes, gelonin can irreversibly inactivate the ribosomes. Because of their unmatched substrate specificity and repetitive mode of action (inactivation of more than 2000 ribosomes per min), it was reported that only several molecules of RIPs are required to kill a tumor cell, if they could have access to their substrates present in the cytosol (14, 15). However, there remains a formidable challenge for gelonin to become clinically available. Gelonin is a single chain glycoprotein which consists of only a catalytic domain without a cell binding domain. Hence, gelonin alone can barely internalize tumor cells and exhibit only low cytotoxicity (16, 17).
To date, there have been various attempts to enhance the anti-tumor activity of biotoxins (18). In recent studies, nanocarriers have been utilized to increase the local density of the PFTs for the cellular uptake (19, 20), while combination therapy with small molecule anti-cancer drugs could compensate their low cytocidal effects (21). On the other hand, for RIPs, conjugations with ligands specifically binding to tumor cells have been the most widely used strategy to overcome the cell membrane barrier (15, 22, 23). Yet, none of those approaches have achieved any clinical success.
To enhance the anti-tumor activity of melittin and gelonin, in this research, we adopted a simple yet effective way of strategically conjugating the two biotoxins. The fusion chimera of gelonin and melittin was synthesized by both chemical and genetic conjugation methods, and then cellular behavior and anti-tumor activity of the fusion proteins were examined in tumor cell lines. Lastly, we explored in vivo the feasibility whether this strategy could provide a way to significantly augment the tumoricidal effects of biotoxins with HeLa s.c. xenograft tumor bearing mice model. Overall results presented in this study suggested that the fusion of gelonin and melittin could potentially contribute to achieve more effective cancer therapy.
MATERIALS AND METHODS
Materials
Heterobifunctional polyethylene glycol (NHS-PEG-PDP; 2 kDa) was purchased from JenKem Technology USA Inc. (Plano, TX). E. coli strains (TOP10 and BL21star (DE3)), pEXP-5-NT/TOPO® TA expression kit, fetal bovine serum (FBS), PBS, Dulbecco's Modified Eagle Medium (DMEM) and Hoechst 33342 were purchased from Invitrogen (Carlsbad, CA). Traut's reagent (2-iminothiolane), Isopropyl-β-thiogalactopyranoside (IPTG) and kanamycin were from Fisher Scientific (Pittsburg, PA). T4 DNA ligase and DNA restriction enzymes (NheI and EcoRI) were purchased from New England Biolabs (Ipswich, MA), and PCR primers were from Integrated DNA Technologies Inc. (Coralville, IA). Rabbit reticulocyte lysate assay kit and luciferase assay kit were purchased from Promega Corporation (Madison, WI), and BCA assay kit was from Bio-Rad Laboratories (Hercules, CA). Melittin (from honey bee venom) and Rhodamine B isothiocyanate (TRITC) were obtained from Sigma-Aldrich (St. Louis, MO). Cell proliferation kit II (XTT) was purchased from Roche Applied Science (Indianapolis, IN).
Synthesis of Gelonin and Melittin Chemical Conjugates (cGel-Mel)
The scheme for chemical conjugation of gelonin and melittin is shown in Fig. 1a. Recombinant gelonin produced from E. coli was thiol-activated by mixing gelonin (5 mg/mL in 10 mM PBS, 2 mM EDTA, 50 mM triethanolamine; pH 8) with 5-fold molar excess of Traut's reagent and incubation at 4 ° C for 1 h. The unreacted Traut's reagent was removed by ultrafiltration (molecular weight cut-off (MWCO): 10 kDa), and the number of generated thiol groups per gelonin was determined by Ellman's assay. At the same time, melittin (2 mg/mL in 10 mM PBS; pH 7) was mixed with a heterobifunctional PEG (NHS-PEG-PDP; 2 kDa) at 1:5 molar ratio and incubated at room temperature for 2 h. The melittin-PEG-PDP was purified from unreacted PEG by a heparin column, and the melittin-to-PEG conjugation ratio was determined by quantification of the melittin and PEG from the conjugate peak fraction by using BCA and barium iodide assay, respectively. The prepared gelonin-SH and melittin-PEG-PDP were mixed together (at 1:5 molar ratio), and then incubated for overnight at 4°C. The gelonin-melittin chemical conjugates (cGel-Mel) were purified with a heparin column connected to the HPLC (Alltech 526 HPLC Pump, Deerfield, IL) using a salt gradient (0 to 1.4 M NaCl). Any melittin or melittin-PEG-PDP possibly contained in the cGel-Mel peak fraction was removed by ultrafiltration (MWCO: 10 kDa).
Figure 1.
Synthesis schemes of gelonin-melittin fusion proteins. (a) Scheme of chemical conjugation between gelonin and melittin and (b) schematic diagram of gelonin-melittin fusion gene contained in pET28a vector for engineering of recombinant gelonin-melittin fusion chimera (rGel-Mel).
Genetic Engineering of Recombinant Gelonin-Melittin Fusion Chimera (rGel-Mel)
Gene Construction
Partial schematic design of pET-Gel-Mel vector for the expression of rGel-Mel is shown in Fig. 1b. Melittin gene was generated by sequential PCR, and the final product was inserted into a TOPO-TA vector (Invitrogen, Carlsbad, CA). PCR primers were used as follows – forward primer: GAT AAC GGC CAG CTA GCG GAA ATT GA, reverse primer-1: GCT AAT CAG GGC CGG CAG ACC GGT GGT CAG CAC TTT CAG CAC GGC ACC AAT ACC ACC TTT CGG ATC TTT GTC GAC, reverse primer-2: GAG CTC GAA TTC TTA TTA CTG CTG ACG TTT ACG TTT AAT CCA GCT AAT CAG GGC CGG. Partial C-terminal gelonin and melittin encoding gene (655 bp) were doubled digested (NheI & EcoRI) from the TOPO-TA vector, and after purification on 1% agarose gel, ligated into linearized pET28a-Gel vector (kindly provided by Dr. Wolfgang E. Trommer in University of Kaiserslautern, Germany). The constructed pET-Gel-Mel (pET28a-Gel-Mel) vector was subjected to DNA sequencing analysis.
Expression and Purification
A single colony of BL21 (DE3) transformed of pET-Gel-Mel was picked and used to inoculate 20 mL of LB broth containing 80 μg/mL of kanamycin. This starter culture was incubated for overnight (at 37°C, 250 rpm) and then diluted to 1L of LB broth. When optical density at 600 nm reached about 1, Isopropyl-D-thiogalactoside (IPTG) was added (final concentration: 0.5 mM), and the culture was further incubated for 4 h. Then, cells were harvested by centrifugation and re-suspended in PBS (pH 7). Protease inhibitors (cOmplete, EDTA-free, Roche, Indianapolis, IN) were added into all buffers used for the purification. Cells were lysed by sonication (4 × 30 s, with 50% output), and the supernatant fraction after centrifugation was loaded onto Talon® metal affinity resins (Clontech, Mountain View, CA). After wash with PBS, 6×His tagged rGel-Mel was eluted with PBS containing 400 mM imidazole. The rGel-Mel was further purified on a heparin column using a salt gradient (0 to 2 M NaCl).
Protein Assays
The final products of cGel-Mel and rGel-Mel were examined by SDS-PAGE, and western blot assay was further carried out using mouse anti-6×His tag antibody (Abcam, Cambridge, MA) to confirm the expression of rGel-Mel, following the previously reported procedures (24, 25). The final cGel-Mel and rGel-Mel products were quantified by BCA assay, and the purity was determined by densitometry analysis (ImageJ software, National Institutes of Health, Bethesda, MD).
Assay for Inhibition of Protein Translation
The N-glycosidase activities of cGel-Mel and rGel-Mel were assessed in comparison with gelonin, following the identical procedures described in Shin et al (24).
Cell Culture
HeLa human cervical cancer, 9L rat glioma, U87 MG human glioblastoma-astrocytoma, CT26 murine colon cancer, LS174T human adenocarcinoma and MDCK (Madin-Darby canine kidney) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in DMEM medium containing 10% FBS and 1% penicillin-streptomycin antibiotics.
Cell Uptake
To assess the cellular behavior of cGel-Mel and rGel-Mel in comparison of gelonin, these gelonin samples were labeled with a fluorescence dye (rhodamine isothiocyanate; TRITC), and their uptake into HeLa cells were observed by confocal microscopy. Gelonin samples (2 mg/mL in 0.1 M bicarbonate buffer, pH 9.0) were mixed with 3-fold molar excess of TRITC and incubated for 2 h at room temperature. After incubation, TRITC-labeled gelonin samples were purified with dye removal resins (Bio-Rad, Hercules, CA). After purification, the contents of gelonin samples and labeled TRITC were quantified, respectively, by following the manufacturer's instruction (ThermoFisher Scientific, San Jose, CA). Based on the quantification results, the molar ratios of gelonin samples-to-TRITC were 1:1.9 for gelonin-TRITC, 1:2.1 for cGel-Mel-TRITC and 1:1.8 for rGel-Mel-TRITC, respectively.
HeLa cells were seeded onto 24-well plates at 5×104 cells/well and incubated in complete DMEM medium (with 10% FBS). After incubation for overnight, TRITC-labeled gelonin samples: 1) gelonin 2) gelonin and melittin mixture (1:1 molar ratio), 3) cGel-Mel and 4) rGel-Mel were added to different wells and incubated at 37°C for 2 h. The final concentrations of the gelonin samples were 1 μM of gelonin-TRITC, 0.86 μM of cGel-Mel-TRITC and 0.89 μM of rGel-Mel-TRITC. The concentrations of cGel-Mel-TRITC and rGel-Mel-TRITC were decided as above, as, at those concentrations, they showed equivalent fluorescence intensity to the 1 μM of gelonin-TRITC. After counterstaining their nuclei with Hoechst 33342, the cells were washed with PBS, and the cell images were acquired by using a Nikon A1R-A1 confocal microscope. Cell images were analyzed using NIS-elements software (Nikon Instruments Inc., Melville, NY).
For quantitative analysis of the cell uptake, HeLa cells prepared on 24-well plates as above were incubated with TRITC-labeled gelonin samples up to 4 h, and at certain time points (0, 0.5, 1 and 2 h post-treatment) lysed with 250 μL lysis buffer. The lysates were collected, transferred to 96 well-plates, and then the fluorescence intensities were measured.
Cellular Protein Inhibition Assay
HeLa cells were seeded onto 12-well plates (at a density of 105 cells per well), and incubated for overnight. After cells were attached to the bottom of the plates, the cells were incubated with either 1) PBS (control), 2) gelonin, 3) mixture of gelonin and melittin (at 1:1 molar ratio), 4) cGel-Mel or 5) rGel-Mel at a final concentration of 100 nM or 1 μM. After 24 h incubation, cells were washed twice with PBS, and then lysed with 1% triton-X 100 solution. The cell lysates were collected by using a scraper and centrifuged at 12,000 rpm for 10 min. The total protein amounts in the supernatants were quantified by the BCA protein assay and the relative cellular protein levels (%) were calculated by dividing the proteins contents of the treated cells by those of the control cells with PBS.
Cytotoxicity Assay
HeLa, 9L, U87 MG, CT26, LS174T and MDCK cells were dispensed into 96-well plates (at a density of 104 cells per well), and, incubated overnight. After incubation, gelonin, melittin, mixture of gelonin and melittin (at 1:1 molar ratio), cGel-Mel and rGel-Mel were separately added to the wells at varying final concentrations (10−11 – 10−5 M), and the cells were further incubated for 48 h. The relative cell viability was determined by XTT assay (Roche Applied Science, Indianapolis IN) and the IC50 of gelonin samples were calculated using Prism software (Prism version 5.0, GraphPad, San Diego, CA).
Tumor Growth Inhibition by Gelonin-Melittin Fusion Chimeras
Athymic nude mice (male, 6 weeks old, Charles River Laboratories) were randomly divided into 8 groups (N = 5), and, at day 0 (3 days after arrival of animals), HeLa cells were implanted to the left hind region of the legs (107 cells in 100 μL). When the average tumor size reached 200 mm3 (on day 12), the mice were treated with either: 1) PBS, 2) gelonin (total injected dose: 10 μg; 14 nmoles/kg), 3) melittin (1 μg; 14 nmoles/kg), 4) mixture of gelonin and melittin (10 μg : 1 μg; both 14 nmoles/kg), 5) cGel-Mel (5 μg; 5.4 nmoles/kg), 6) cGel-Mel (10 μg; 11 nmoles/kg), 7) rGel-Mel (5 μg; 6.3 nmoles/kg) or 8) rGel-Mel (10 μg; 13 nmoles/kg). Samples were administered every third day for total 5 times starting at day 12 (on days 12, 15, 18, 21 and 24) directly into the tumor, and tumor sizes were monitored daily. The tumor volume (mm3) was calculated following the equation of V = (x2 × y)/2, where x is the width and y is the length of the tumor. Animal experiments were conducted according to the protocol approved by the University of Michigan Committee on Use and Care of Animals (UCUCA; PRO00005294).
Statistical Analysis
Data were expressed as mean ± standard deviation (S.D.). Statistically significant differences among groups were determined by 1-way ANOVA with adopting the Tukey's multiple comparison test as the post hoc test (Prism version 5.0, GraphPad, San Diego, CA). Data with P-values less than 0.05 were considered statistically significant.
RESULTS
Synthesis of Gelonin-Melittin Chemical Conjugate (cGel-Mel)
The cGel-Mel was prepared by chemical conjugation via a reducible disulfide bond. The disulfide bond in cGel-Mel was formed between thiol activated-gelonin (gelonin-SH) and PDP introduced-melittin (melittin-PEG-PDP). Ellman's assay results indicated that average 5 thiols were generated per gelonin molecule, while P2T assay results showed 1:3 conjugation ratios for melittin and NHS-PEG-PDP (bifunctional PEG; 2kDa). After incubation of the mixture of gelonin-SH and melittin-PEG-PDP (at 1:5 molar ratios), slight aggregation occurred, but it was readily removed by brief centrifugation (3000 rpm for 3 min). The cGel-Mel was purified from unreacted gelonin-SH by using a heparin column with a salt gradient (0 to 1.4 M NaCl). While gelonin-SH was eluted at 0.35 M NaCl (retention time: 35 min), cGel-Mel was eluted at significantly higher 0.65 M NaCl (retention time: 65 min); presumably due to the basic nature of melittin (data not shown). The final cGel-Mel product was acquired after removal of any unreacted melittin-PEG-PDP contained in the conjugation peak fraction by ultracentrifugation (molecular weight cut-off: 10 kDa). SDS-PAGE results confirmed the synthesis of cGel-Mel (avg. 2-3 melittin conjugated to a gelonin molecule) with purity of higher than 90% based on Image J analysis (Fig. 2a).
Figure 2.
Purification results of gelonin-melittin fusion proteins. (a) SDS-PAGE results of the final gelonin-melittin chemical conjugate (cGel-Mel) products purified by heparin column purification. Lane M: protein molecular weight standard (Mark 12™ standard, Invitrogen), Lane NR: cGel-Mel with non-reducing condition. Lane R: cGel-Mel with reducing condition. (b) SDS-PAGE and western blot results of recombinant gelonin-melittin fusion chimera (rGel-Mel) purification. Lane M: protein molecular weight standard (Mark 12™ standard, Invitrogen), Lane CL: supernatant of cell lysates. Lane FT: flow through fraction. Lane W: wash fraction. Lane E: elution fraction representing rGel-Mel. Lane WB: western blot results for final rGel-Mel product by utilizing anti-His antibody.
Expression and Purification of Recombinant Gelonin-Melittin Fusion Chimera (rGel-Mel)
As shown in Fig. 2b, the rGel-Mel was expressed as a soluble protein from the E. coli and successfully purified by loading to a Talon metal affinity column. The eluent from the Talon column purification was again purified by using a heparin column to improve the purity. The yield of the final rGel-Mel product averaged 3 mg per L culture, and the purity was above 90% (based on Image J analysis).
Protein Synthesis Inhibition by Gelonin-Melittin Fusion Proteins
Rabbit reticulocyte lysate assay results are shown in Fig. 3. As shown, gelonin, cGel-Mel and rGel-Mel exerted similar inhibitory profiles for the translation of luciferase mRNA in the cell-free translational systems. The IC50 values for gelonin, cGel-Mel and rGel-Mel were 13.5 ± 2 pM, 13.2 ± 3.9 pM, and 12.6 ± 6.6 pM, respectively. These results indicated that conjugation of melittin to gelonin, whether by a chemical or recombinant method, did not affect the intrinsic activity of gelonin significantly.
Figure 3.
Rabbit reticulocyte lysate assay results of gelonin (circle), cGel-Mel (triangle) and rGel-Mel (reverse triangle). By using a cell-free translational system, inhibition of protein translation by gelonin samples were measured, and the translated luciferase was quantified using a chemiluminescent assay. (N = 3). The luminescence intensity vs. gelonin concentration curves were fitted by adopting nonlinear regression model (Prism, GraphPad).
Cellular Uptake
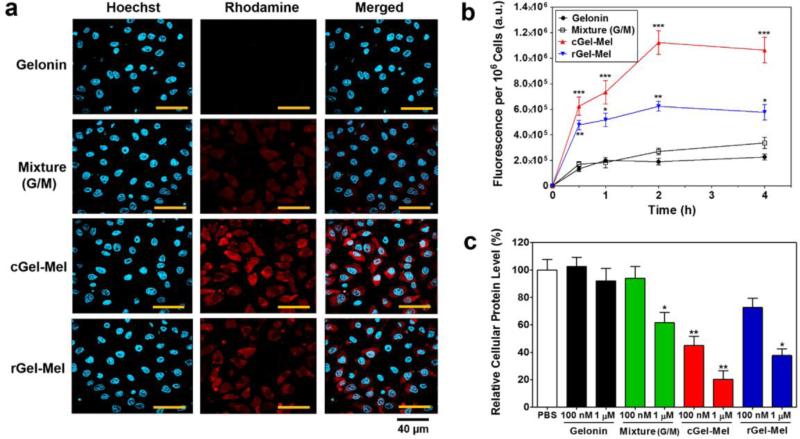
Fig. 4a displays the fluorescent images of HeLa cells incubated with either 1) rhodamine labeled-gelonin, 2) mixture of labeled-gelonin and melittin, 3) labeled-cGel-Mel or 4) labeled-rGel-Mel, and Fig. 4b shows the relative amounts of cell-associated gelonin samples along the time course. As shown in Fig. 4a, while gelonin incubated cells showed almost no fluorescence inside the cells, fluorescence signal was clearly observed from the cells incubated with either cGel-Mel or rGel-Mel. However, cells incubated with the mixture of gelonin and melittin exhibited little fluorescence. Among these cells, the cells incubated with cGel-Mel showed higher fluorescence signals than with rGel-Mel. Quantitative analysis data (Fig. 4b) provided similar results to the observation of the microscopic images. At 4 h post-incubation, the fluorescence intensities of the cells incubated with the mixture of gelonin and melittin, cGel-Mel or rGel-Mel were 1.2-, 4.6- and 2.3-fold greater than cells treated with gelonin, respectively.
Figure 4.
Cell uptake and protein synthesis inhibition assay results of gelonin-melittin fusion proteins on HeLa cells. (a) Confocal microscopic images of live HeLa cells after incubation with TRITC-labeled gelonin, 1:1 mixture of gelonin and melittin, cGel-Mel or rGel-Mel for 2 h at 37°C. After incubation, the cells were washed with PBS and then the nuclei were counterstained with Hoechst 33342. Fluorescence images were acquired using rhodamine (red), Hoechst (blue) channels and merged. (b) Quantitative analyses of the cell uptake along the time course up to 4 h post-incubation of gelonin samples. (c) Cellular protein synthesis inhibition assay results after Hela cells were incubated with either gelonin, mixture of gelonin and melittin, cGel-Mel or rGel-Mel for 24 h. The final concentrations of gelonin samples tested were 100 nM and 1 μM. Statistical differences among groups were compared by 1-way ANOVA with Tukey's multiple comparison test using Prism software (GraphPad). *P < 0.05, **P < 0.01, ***P < 0.001. (cGel-Mel: gelonin-melittin chemical conjugate, rGel-Mel: recombinant gelonin-melittin fusion chimera)
Cellular Protein Synthesis Inhibition Assay
Fig. 4c shows the cellular protein synthesis inhibition assay results after 24 h-incubation of HeLa cells with various gelonin samples. As seen, compared with the PBS control group, gelonin did not show any inhibition of protein synthesis at either 100 nM or 1 μM (relative protein level: 103 ± 12% at 100 nM and 92 ± 16% at 1 μM, respectively). In comparison, cells treated with mixture of gelonin and melittin or rGel-Mel exhibited significant inhibition at 1 μM (62 ± 13% and 38 ± 9%, respectively). However, the most prominent inhibitory effects were observed from the cGel-Mel treated cells (45 ± 12% at 100 nM and 20 ± 11% at 1 μM).
Cytotoxicity Assay
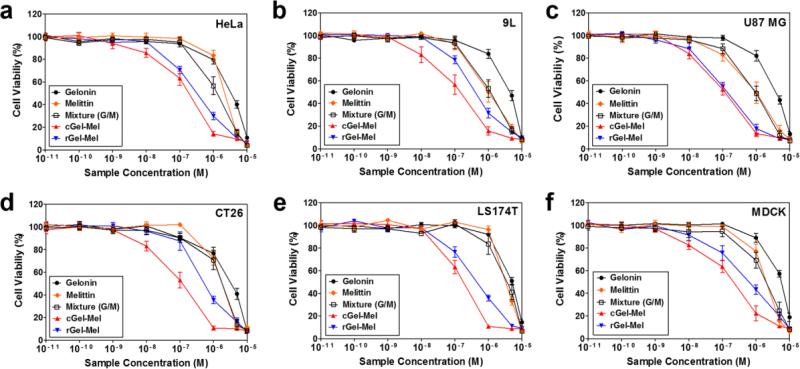
The cytotoxic activities of 1) gelonin, 2) melittin, 3) mixture of gelonin and melittin, 4) cGel-Mel and 5) rGel-Mel were examined on various cancer cell lines (HeLa, 9L, U87 MG, CT26 and LS174T) and noncancerous MDCK cells via XTT assay. The XTT assay results are shown in Fig. 5 and the calculated IC50 values for each cell line are summarized in Table I. As shown, gelonin exerted only mild cytotoxicity against all the tested cell lines (avg. IC50: 3100 - 4800 nM), while melittin induced significantly higher cytotoxicity (avg. IC50: 990 - 4100 nM). The cytotoxicity level of the mixture of gelonin and melittin (avg. IC50: 890 - 3600 nM) was similar to that of melittin. However, both the cGel-Mel (avg. IC50: 88 - 180 nM) and rGel-Mel (avg. IC50: 120 - 570 nM) showed significantly augmented cytotoxicity, compared with other samples.
Figure 5.
Cytotoxicity assay results. Cancerous cell lines ((a) HeLa, (b) 9L, (c) U87 MG, (d) CT26 and (e) LS174T) and a noncancerous cell line ((f) MDCK) were treated with a varying final concentration (10−11 – 10−5 M as gelonin) of gelonin, melittin, 1:1 mixture of gelonin and melittin, cGel-Mel and rGel-Mel. Then, the relative cell viability was measured after incubation for 48 h by XTT assay. N=3. (cGel-Mel: gelonin-melittin chemical conjugate, rGel-Mel: recombinant gelonin-melittin fusion chimera)
Table I.
Cytotoxic activity (IC50) of gelonin, melittin, gelonin/melittin mixture and melittin-fused gelonins
| Cell Line | IC50a, nM |
||||
|---|---|---|---|---|---|
| Gelonin | Melittin | Mixture (G/M) | cGel-Mel | rGel-Mel | |
| HeLa | 3300 ± 1100 | 2200 ± 680 | 1300 ± 480 | 150 ± 50** | 370 ± 130** |
| 9L | 4300 ± 1300 | 1100 ± 310 | 1200 ± 330 | 120 ± 60** | 390 ± 140** |
| U87 MG | 4200 ± 1000 | 990 ± 300 | 890 ± 190 | 92 ± 36*** | 120 ± 60*** |
| CT26 | 3100 ± 1200 | 1300 ± 210 | 1500 ± 350 | 88 ± 42*** | 530 ± 170* |
| LS174T | 4800 ± 560 | 4100 ± 580 | 3600 ± 990 | 130 ± 28*** | 420 ± 140*** |
| MDCK | 4700 ± 1000 | 1800 ± 360 | 1900 ± 580 | 180 ± 60** | 570 ± 71* |
IC50 values were calculated by nonlinear regression using Prism software (GraphPad).
For all the experiments, N=3. Statistical differences among the IC50 values of gelonin, melittin, mixture (G/M), cGel-Mel and rGel-Mel were compared by 1-way ANOVA (Tukey's multiple comparison test as the post hoc test).
P < 0.05
P < 0.01
P < 0.001.
(Mixture G/M: mixture of gelonin and melittin at 1:1 molar ratio, cGel-Mel: gelonin and melittin chemical conjugate, rGel-Mel: recombinant gelonin-melittin fusion chimera)
Tumor Growth Inhibition by Gelonin-Melittin Fusion Proteins
Preliminary animal studies were carried out to examine the anti-tumor activities of gelonin-melittin fusion proteins using HeLa s.c. xenograft tumor bearing mice. The growth profiles of the tumors are shown in Fig. 6a and the average tumor volumes of each group at day 30 (30 days after tumor implantation) are shown in Fig. 6b. As seen in Fig. 6a, while PBS, gelonin, melittin and mixture of gelonin and melittin groups showed continuous growth of tumor, significant inhibition of tumor growth was observed in the mice treated with cGel-Mel and rGel-Mel. At day 30, compared with the PBS treated-control group (tumor size: 1340 mm3), mice treated with gelonin (1280 mm3, 5% inhibition), melittin (1010 mm3, 24% inhibition) and mixture of gelonin and melittin (910 mm3, 32% inhibition) displayed insignificant effects on the tumor growth inhibition. In a sharp contrast, cGel-Mel and rGel-Mel significantly exerted tumoricidal effects, inhibiting the tumor growth by 84% (size: 210 mm3) and 77% (310 mm3), respectively, at 10 μg dose. Even at 5 μg dose, cGel-Mel and rGel-Mel led to tumor growth inhibition by 65% (470 mm3) and 42% (770 mm3), respectively.
Figure 6.
Tumor growth inhibition by gelonin-melittin fusion proteins in HeLa s.c. xenograft tumor mouse model. (a) Tumor growth profiles. Tumor size was measured daily since tumor inoculation. When the average tumor size reached 200 mm3 (at day 12; 12 days after tumor implantation at day 0), mice were divided into 8 groups (N=5) and treated with PBS (circle), gelonin (square; 14 nmoles/kg), melittin (diamond; 14 nmoles/kg), mixture of gelonin/melittin (open square; both 14 nmoles/kg), cGel-Mel (closed or open triangle for 5 or 10 μg (5.4 or 11 nmoles/kg)) or rGel-Mel (closed or open reverse triangle for 5 or 10 μg (6.3 or 13 nmoles/kg)) by intra-tumor injection at days 12, 15, 18, 21 and 24. (b) Average tumor size of each group at day 30. Statistically significant differences of the tumor sizes among groups were compared by 1-way ANOVA with Tukey's multiple comparison test using Prism software (GraphPad). *P < 0.05, ***P < 0.001. (cGel-Mel: gelonin-melittin chemical conjugate, rGel-Mel: recombinant gelonin-melittin fusion chimera)
DISCUSSION
Biotoxins, such as pore-forming toxins (PFTs) and ribosome-inactivating proteins (RIPs), are attractive anti-cancer drug candidates, yet, for their clinical utilization, there remain formidable challenges (e.g., low efficacy, poor pharmacokinetic profiles, and toxicity, etc.) (7, 26-28). Among the hurdles, low anti-tumor efficacy is a critical and imminent problem that should be addressed. The PFTs such as melittin are membrane active peptides possessing lytic properties but mild cytocidal activity (9, 29), while gelonin, a typical type of RIPs, is an extremely potent protein synthesis inhibitor lacking of a cell binding moiety to internalize tumor cells (15, 30). As melittin requires a powerful warhead, while gelonin is in need of a carrier that facilitates cell internalization, coupling melittin and gelonin appeared to be a simple yet effective way to achieve the goal.
In this study, fusion of gelonin and melittin were successfully accomplished by both chemical conjugation and genetic recombination methods. The gelonin-melittin chemical conjugate (cGel-Mel) was synthesized via a reversible disulfide bond using a bifunctional PEG (NHS-PEG-PDP; 2 kDa) as the cross-linker (Fig. 1). Because of the reversible linkage, once internalized tumor cells, gelonin could be released into the reducible environment of the cytosol. This might be necessary for exerting the full activity of gelonin, if the melittin tends to remain on the surface of the cells rather than internalization. The final cGel-Mel products consisted of a mixture of 1:2 and 1:3 molar ratio of gelonin and melittin conjugates (Fig. 2a). On the other hand, the recombinant gelonin and melittin fusion chimera (rGel-Mel) was acquired as a single product with gelonin and melittin coupled covalently at a 1:1 molar ratio (Fig. 2b). Compared with the chemical conjugation method, the major advantages of this genetic recombination method include efficiency and reproducibility of the production process, while the utmost disadvantage might be that reducible linkage is not available.
After successful preparation of the gelonin-melittin fusion proteins, proper functioning of both components were examined by in vitro assays and microscopic imaging. The rabbit reticulocyte lysate assay results clearly evidenced that the intrinsic activity of gelonin for inhibiting protein translation was little affected by either chemical or genetic modification of gelonin; presumably, due to small size of melittin and the inherent stability of the gelonin protein structure (Fig. 3). Confocal microscopic data provided proofs for the membrane activity of the gelonin-melittin fusion proteins (Fig. 4). Gelonin-melittin fusion proteins were able to internalize HeLa cells, while gelonin could not, and significantly higher fluorescence intensity (F.I.) was observed for both cGel-Mel (avg. 4.3-fold) and rGel-Mel (2.6-fold) compared with gelonin. Interestingly, mixture of melittin and gelonin (G/M) provided no enhancement on the cell internalization of gelonin. This difference in the cell uptake profiles between the fusion proteins and the physical mixture might be explained by the ‘transient’ nature (lasting for only milliseconds) of the pore formation by melittin (31), requiring cargos to be located in vicinity of the pores to be delivered into the cells. Another important finding from the confocal data was that greater uptake was examined from the cGel-Mel treated cells (avg. 1.8-fold higher F.I.) than the rGel-Mel treated cells, suggesting that the number of melittin molecules conjugated on the gelonin molecule (i.e., valency of melittin) could be a crucial factor for the cell internalization. This valency-dependent cell penetration might be better understood considering the pore structures generated by melittins (32, 33). Huang et al. reported that the pores formed by melittins are more likely an architecture of the gathering of 4 – 7 monomers and, when melittins are dispersed, randomly distribute on the plasma membrane surface (34). Thus, it appeared that by possessing 2 – 3 melittins bound on 1 gelonin molecule, cGel-Mel would have a greater opportunity than monomeric rGel-Mel to create intact pores on the cell membrane.
After confirming that both gelonin and melittin were properly functioning in the gelonin-melittin fusion proteins, we evaluated the cellular protein synthesis inhibitory effects (Fig. 4c) and anti-tumor activity of those fusion proteins with various cancerous cell lines (Fig. 5). Not surprisingly, the cellular protein synthesis inhibition assay results were in good correlation with the cell uptake study results. While gelonin showed little inhibition effects for the protein synthesis; presumably due to the incapability of gelonin to internalize cells at submicromolar concentration, both rGel-Mel and cGel-Mel were able to significantly arrest the protein synthesis in the tested HeLa cells. These results clearly indicated that melittin-fused gelonins were able to internalize cells at a higher extent and further reach the cytosol where the ribosome substrates are present. Both cGel-Mel (avg. 31- and 15-fold, respectively) and rGel-Mel (avg. 10- and 4.8-fold, respectively) exhibited significantly higher cytotoxicity than unmodified gelonin or melittin, while the mixture of gelonin and melittin did not exhibit any improved cytotoxicity compared with treatment of melittin alone. These results were in good accordance with the cell uptake assay and the cellular protein synthesis inhibition assay results, and clearly evidenced our hypothesis that the fusion strategy could synergistically augment the anti-tumor activity of gelonin and melittin by compensating each other's major disadvantages. Also noteworthy was that multivalent cGel-Mel not only showed higher cell uptake than the monovalent rGel-Mel, but also a 3-fold greater cytotoxic effect. These results again exhibited the importance of multivalency when considering constructing melittin-fused proteins for enhancing their intracellular uptake (35). Another important finding was that enhanced cytotoxicity of the gelonin-melittin fusion proteins was observed from all the tested cancer cell lines. This result was not surprising, as the cell entry mechanism of melittin (possibly via formation of a transient pore or transduction) appears not discriminative of the cell types (29). Indeed, this universal membrane activity of melittin could be a favorable property, as it can expand the application of gelonin-melittin fusion proteins to the treatment of a variety of cancer-types. However, it should be also noted that this universal activity of melittin on every cell membrane could also provoke severe toxicity if it would allow internalization of the attached drugs into not only tumor cells, but also normal cells after systemic administration; as evidenced by the augmented cytotoxicity of noncancerous MDCK cells by incubation with cGel-Mel or rGel-Mel, compared with unmodified gelonin.
Encouraged by the cellular characterization results, a preliminary animal study with a HeLa s.c. xenograft tumor bearing nude mice was carried out to evaluate the in vivo anti-tumor efficacy of the gelonin-melittin fusion proteins. The results turned out promising as only the gelonin-melittin fusion proteins showed significant tumoricidal effects compared with unmodified gelonin, melittin or mixture of gelonin and melittin (Fig. 6). Remarkably, with only 5 μg dose of either cGel-Mel or rGel-Mel, the tumor growth could be profoundly inhibited. Nevertheless, because of nonselective mode of action, gelonin-melittin fusion proteins could potentially induce severe toxicity after systemic administration. Furthermore, maximum tolerable doses (MTDs) of the gelonin-melittin fusion proteins were significantly lower (avg. 0.3 μmole/kg; 5-fold) than that of gelonin, when determined by i.v. administration of a bolus single dose, while, on the other hand, the plasma half-lives of the gelonin-melittin fusion proteins were pretty short (avg. 3.5 min) with rapid elimination via renal clearance, and thus only limited amount of fusion proteins were able to reach the tumor site (less than 2% ID/g tissue) (data not shown). Overall, despite possessing enhanced anti-tumor activity, yet, a drug delivery system that enables targeting of the gelonin-melittin fusion proteins to the tumor site appeared as an imminent task to be addressed for their successful clinical application. Therefore, currently, we are developing a drug delivery system for the effective yet safe administration of the gelonin-melittin fusion proteins. In addition, further studies are underway to configure the optimal number of melittin on gelonin molecules to acquire the maximal cell uptake and anti-tumor activity.
CONCLUSION
Despite both melittin and gelonin possessing high potentials to be utilized as anti-cancer drugs, low cytotoxic activity of melittin and poor intracellular uptake of gelonin have hindered their clinical applications. To overcome these obstacles, in this research, we engineered gelonin-melittin fusion proteins by both chemical conjugation and genetic recombination methods. Cellular and in vivo results confirmed that gelonin-melittin fusion proteins could internalize tumor cells and further exert significantly augmented anti-tumor activities, compared with either of unmodified gelonin, melittin or mixture of gelonin and melittin. Overall, this study demonstrated that fusion of two biotoxins with complementary properties could provide a simple yet effective way to enhance their drugabilities.
ACKNOWLEDGMENTS
This work was partially supported by the NSFC 2013 A3 Foresight Program (81361140344), National Key Basic Research Program of China (2013CB932502) and National Natural Science Foundation of China (NSFC, 81402856). In addition, this work was also supported in part by National Institutes of Health R01 Grants CA114612 and grants from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1A6A3A01020598 & NRF-2015R1C1A1A02036781). We thank Dr. Wolfgang E. Trommer (University of Kaiserslautern, Germany) for the gelonin expression vector (pET28a-Gel).
ABBREVIATIONS
- PFTs
Pore-forming toxins
- RIPs
Ribosome-inactivating proteins
- cGel-Mel
Chemically conjugated gelonin-melittin
- rGel-Mel
Recombinant gelonin-melittin fusion chimera
- NHS-PEG-PDP
Pyridyldithio polyethylene glycol succinimidylpropionate
- FBS
Fetal bovine serum
- DMEM
Dulbecco's Modified Eagle Medium
- IPTG
Isopropyl-β-D-thiogalactopyranoside
- TRITC
Rhodamine B isothiocyanate
- EDTA
Ethylenediaminetetraacetic acid
- HPLC
High performance liquid chromatography
- MWCO
Molecular weight cut-off
- Gelonin-SH
Thiol activated-gelonin
- G/M
Mixture of gelonin and melittin
- F.I.
Fluorescence intensity
REFERENCES
- 1.Henriques ST, Melo MN, Castanho MA. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399(1):1–7. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rádis-Baptista G, Kerkis A, Prieto-Silva ÁR, Hayashi MAF, Kerkis I, Tetsuo Y. Membrane-translocating peptides and toxins: from nature to bedside. J Braz Chem Soc. 2008;19:211–225. [Google Scholar]
- 3.de Virgilio M, Lombardi A, Caliandro R, Fabbrini MS. Ribosome-inactivating proteins: from plant defense to tumor attack. Toxins (Basel) 2010;2(11):2699–2737. doi: 10.3390/toxins2112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258(1):72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Anderluh G, Lakey JH. Proteins: membrane binding and pore formation. Springer Science & Business Media; New York: 2011. [Google Scholar]
- 6.Terwilliger TC, Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982;257(11):6016–6022. [PubMed] [Google Scholar]
- 7.Gajski G, Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36(2):697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Huh JE, Kang JW, Nam D, Baek YH, Choi DY, Park DS, Lee JD. Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J Nat Prod. 2012;75(11):1922–1929. doi: 10.1021/np300446c. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki K, Yoneyama S, Miyajima K. Pore formation and translocation of melittin. Biophys J. 1997;73(2):831–838. doi: 10.1016/S0006-3495(97)78115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan H, Soman NR, Schlesinger PH, Lanza GM, Wickline SA. Cytolytic peptide nanoparticles (‘NanoBees’) for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(3):318–327. doi: 10.1002/wnan.126. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Sun M, Wang Y, Sun de J, Zhang S. Expression and functional characterization of a recombinant targeted toxin with an uPA cleavable linker in Pichia pastoris. Protein Expr Purif. 2011;76(2):184–189. doi: 10.1016/j.pep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Puri M, Kaur I, Perugini MA, Gupta RC. Ribosome-inactivating proteins: current status and biomedical applications. Drug Discov Today. 2012;17(13-14):774–783. doi: 10.1016/j.drudis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987;262(12):5908–5912. [PubMed] [Google Scholar]
- 14.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980;255(14):6947–6953. [PubMed] [Google Scholar]
- 15.Atkinson SF, Bettinger T, Seymour LW, Behr JP, Ward CM. Conjugation of folate via gelonin carbohydrate residues retains ribosomal-inactivating properties of the toxin and permits targeting to folate receptor positive cells. J Biol Chem. 2001;276(30):27930–27935. doi: 10.1074/jbc.M102825200. [DOI] [PubMed] [Google Scholar]
- 16.Weidle UH, Tiefenthaler G, Schiller C, Weiss EH, Georges G, Brinkmann U. Prospects of bacterial and plant protein-based immunotoxins for treatment of cancer. Cancer Genomics Proteomics. 2014;11(1):25–38. [PubMed] [Google Scholar]
- 17.Shin MC, Zhang J, Min KA, He H, David AE, Huang Y, Yang VC. PTD-modified ATTEMPTS for enhanced toxin-based cancer therapy: an in vivo proof-of-concept study. Pharm Res. 2015;32(8):2690–2703. doi: 10.1007/s11095-015-1653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Zong J, Liu Z, Li L, Zheng X, Wang B, Sun G. A novel melittin-MhIL-2 fusion protein inhibits the growth of human ovarian cancer SKOV3 cells in vitro and in vivo tumor growth. Cancer Immunol Immunother. 2013;62(5):889–895. doi: 10.1007/s00262-013-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou KK, Pan H, Ratner L, Schlesinger PH, Wickline SA. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano. 2013;7(10):8605–8615. doi: 10.1021/nn403311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Jin H, Qian Y, Qi S, Luo H, Luo Q, Zhang Z. Hybrid melittin cytolytic Peptide-driven ultrasmall lipid nanoparticles block melanoma growth in vivo. ACS Nano. 2013;7(7):5791–5800. doi: 10.1021/nn400683s. [DOI] [PubMed] [Google Scholar]
- 21.Sprintz M, Benedetti C, Ferrari M. Applied nanotechnology for the management of breakthrough cancer pain. Minerva Anestesiol. 2005;71(7-8):419–423. [PubMed] [Google Scholar]
- 22.Weyergang A, Selbo PK, Berg K. Photochemically stimulated drug delivery increases the cytotoxicity and specificity of EGF-saporin. J Control Release. 2006;111(1-2):165–173. doi: 10.1016/j.jconrel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Madhumathi J, Verma RS. Therapeutic targets and recent advances in protein immunotoxins. Curr Opin Microbiol. 2012;15(3):300–309. doi: 10.1016/j.mib.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Shin MC, Zhao J, Zhang J, Huang Y, He H, Wang M, Min KA, Yang VC. Recombinant TAT-gelonin fusion toxin: synthesis and characterization of heparin/protamine-regulated cell transduction. J Biomed Mater Res A. 2015;103(1):409–419. doi: 10.1002/jbm.a.35188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TY, Park YS, Garcia GA, Sunahara RK, Woods JH, Yang VC. Cell permeable cocaine esterases constructed by chemical conjugation and genetic recombination. Mol Pharm. 2012;9(5):1361–1373. doi: 10.1021/mp200623w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stirpe F, Battelli MG. Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci. 2006;63(16):1850–1866. doi: 10.1007/s00018-006-6078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thrush GR, Lark LR, Clinchy BC, Vitetta ES. Immunotoxins: an update. Annu Rev Immunol. 1996;14:49–71. doi: 10.1146/annurev.immunol.14.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Orsolic N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31(1-2):173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey CE. The actions of melittin on membranes. Biochim Biophys Acta. 1990;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblum MG, Marks JW, Cheung LH. Comparative cytotoxicity and pharmacokinetics of antimelanoma immunotoxins containing either natural or recombinant gelonin. Cancer Chemother Pharmacol. 1999;44(4):343–348. doi: 10.1007/s002800050987. [DOI] [PubMed] [Google Scholar]
- 31.Rex S, Schwarz G. Quantitative studies on the melittin-induced leakage mechanism of lipid vesicles. Biochemistry. 1998;37(8):2336–2345. doi: 10.1021/bi971009p. [DOI] [PubMed] [Google Scholar]
- 32.Smith R, Separovic F, Milne TJ, Whittaker A, Bennett FM, Cornell BA, Makriyannis A. Structure and orientation of the pore-forming peptide, melittin, in lipid bilayers. J Mol Biol. 1994;241(3):456–466. doi: 10.1006/jmbi.1994.1520. [DOI] [PubMed] [Google Scholar]
- 33.Allende D, Simon SA, McIntosh TJ. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys J. 2005;88(3):1828–1837. doi: 10.1529/biophysj.104.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MT, Sun TL, Hung WC, Huang HW. Process of inducing pores in membranes by melittin. Proc Natl Acad Sci U S A. 2013;110(35):14243–14248. doi: 10.1073/pnas.1307010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Han Y, Fu H, Liu M, Wu J, Chen X, Zhang S, Chen Y. Construction and expression of sTRAIL-melittin combining enhanced anticancer activity with antibacterial activity in Escherichia coli. Appl Microbiol Biotechnol. 2013;97(7):2877–2884. doi: 10.1007/s00253-012-4541-y. [DOI] [PubMed] [Google Scholar]