Abstract
Purpose
Fungizone® (AmB-SD), amphotericin B solubilized by sodium deoxycholate, contains a highly aggregated form of the antifungal agent that causes dose-limiting renal toxicity. With the aim of reducing the formulation’s toxicity by co-delivering monomeric amphotericin B (AmB) and sodium supplementation, we deaggregated AmB-SD with FDA-approved excipient PEG-DSPE in 0.9% NaCl-USP. Herein, we describe a reformulated AmB-SD with PEG-DSPE micelles that results in a less toxic drug with maintained antifungal activity.
Methods
We compared the aggregation state and particle size of AmB-SD alone or combined with PEG-DSPE micelles. In vitro hemolytic activity and in vivo renal toxicity were measured to determine the toxicity of different formulations. In vitro antifungal assays were performed to determine differences in efficacy among formulations.
Results
PEG-DSPE micelles in saline deaggregated AmB-SD. Deaggregated AmB-SD exhibited significantly reduced in vitro and in vivo toxicity. In vitro antifungal studies showed no difference in minimum inhibitory and fungicidal concentrations of AmB-SD combined with PEG-DSPE relative to the drug alone.
Conclusions
Reformulation of AmB-SD with PEG-DSPE micelles in saline facilitates co-delivery of monomeric AmB and sodium supplementation, potentially reducing the dose-limiting nephrotoxicity of AmB-SD. Ease of preparation and commercially available components lead us to acknowledge its potential for clinical use.
Keywords: Amphotericin B, PEG-DSPE, Aggregation State Hypothesis, Sodium Supplementation
Introduction
The potent antifungal drug amphotericin B (AmB) exerts fungicidal activity by binding to ergosterol, an important cell membrane lipid (1). AmB tends to self-aggregate, a property that contributes to loss of binding specificity and consequential toxicity (2). Severe nephrotoxicity has limited its clinical use, prompting researchers to focus on safely solubilizing and reducing toxicity of the drug (3–7). Attempts to reduce toxicity have resulted in formulations that are less nephrotoxic in comparison to the original AmB formulation, Fungizone® (AmB-SD).
Several lipid-based AmB formulations fulfill the need for less toxic antifungal therapy, but this is achieved with some caveats. Lipid AmB formulations require higher dosing to achieve antifungal efficacy similar to that of AmB-SD (8, 9). Additionally, they are considerably more costly (8). A pharmacoeconomic study on the cost of AmB-SD and liposomal AmB showed that AmB-SD is the more cost effective formulation, even when the cost associated with the original formulation’s greater incidence of renal failure was accounted for (10). Depending on the fungal infection, it may be necessary to treat a patient with AmB for several weeks. Taking this into consideration, the decreased antifungal efficacy compounded with the cost of lipid AmB can act as a barrier to patient accessibility. Furthermore, dose-limiting renal toxicity is still an issue with these improved lipid formulations (11).
Clearly, there is a need for development of a less costly formulation of AmB that causes minimal host toxicity without compromising antifungal efficacy. We address this issue by combining delivery of monomeric AmB and sodium supplementation, two promising strategies that have been shown to reduce host toxicity. AmB monomers form soluble aggregates above the critical aggregation concentration (CAC). Past studies have evaluated the effect of surfactants and cyclodextrins on the aggregation state of AmB (2, 12–19). These excipients interact with AmB soluble aggregates and deaggregate the drug. Some studies further characterized the different aggregation states of AmB, e.g. aggregated or monomeric, in relation to their impact on mammalian cell toxicity and affinity for ergosterol. Their results support the idea that aggregated AmB interacts with both fungal and mammalian cell membranes, while monomeric AmB selectively binds to ergosterol in fungal cell membranes. This “aggregation state” hypothesis attributes the drug’s toxicity to the aggregated form of AmB and suggests a solution, delivery of monomeric AmB. Salt supplementation, the administration of saline prior to or following AmB treatment, minimizes AmB-related damage to kidney function. Reduced glomerular filtration rate and sodium depletion have been ameliorated and even reversed by salt supplementation in both animal experiments and human trials (20–23). Although the mechanism behind the protective effect of salt loading is incompletely understood, sodium-related alterations to AmB-induced vasoconstriction and inhibition of the tubuloglomerular feedback mechanism that causes vasoconstriction of the afferent arterioles have been proposed (21, 24, 25). IV administration of saline cannot occur concurrently with AmB infusion, as AmB-SD preparation instructions specify that the drug should not be mixed with 0.9% NaCl. There is a risk that the drug will precipitate out of solution, introducing the potential for embolism. Therefore, patients must undergo a more time-consuming regimen to receive this toxicity-reducing therapy. Our group has explored the utility of PEG-lipid micelles for delivery of monomeric AmB. Deaggregation of AmB has been achieved with many different PEG-lipids, presumably due to a more favorable interaction with the lipid tail than with itself and sequestration of the hydrophobic molecule into the hydrophobic micelle core. Significant reductions in both in vitro hemolytic activity (26–29) and in vivo renal toxicity (30) have been demonstrated relative to AmB-SD. However, issues arise when the methods used to create these formulations are taken into consideration. One common way of preparing PEG-lipid micelles, the thin-film rehydration method, involves use of specialized equipment to produce a very small amount of drug. Oftentimes, preparation is achieved only after following lengthy and complex protocols. Considering the volume of drug that is needed for large-scale pre-clinical and clinical studies, issues encountered during drug production scale-up may present a formidable barrier to clinical relevance. To combat the issues of toxicity and scale-up, we combined commercially produced AmB-SD with the FDA-approved PEG-lipid poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) in saline. We sought to determine if PEG-DSPE micelles would (1) deaggregate AmB-SD, (2) impart stability in saline, (3) impact antifungal efficacy, and (4) reduce renal toxicity due to co-delivery of monomeric AmB and salt supplementation. Reformulation was accomplished by simple addition of AmB-SD to PEG-DSPE dissolved in saline (Figure 1). The absence of organic solvents removes the need for use of complex protocols and specialized equipment. Additionally, the commercial availability of AmB-SD reveals the lack of need for scale-up, and the FDA-approval of PEG-DSPE reduces resistance on the path to clinical trials. We implemented a wide variety of techniques and approaches to evaluate our hypothesis. To determine aggregation state and stability in saline, we physically characterized different formulations. We compared the different formulations’ in vitro toxicity with a hemolysis assay, as anemia is a side effect of AmB therapy. In vivo renal toxicity in rats was studied to determine whether or not the combination of monomeric AmB and saline actually reduced toxicity. Furthermore, we evaluated whether or not PEG-DSPE affected the in vitro fungicidal activity of AmB. Herein, we present a reformulation of AmB-SD with PEG-DSPE micelles in saline that is unique in both simplicity of preparation and potential for clinical application.
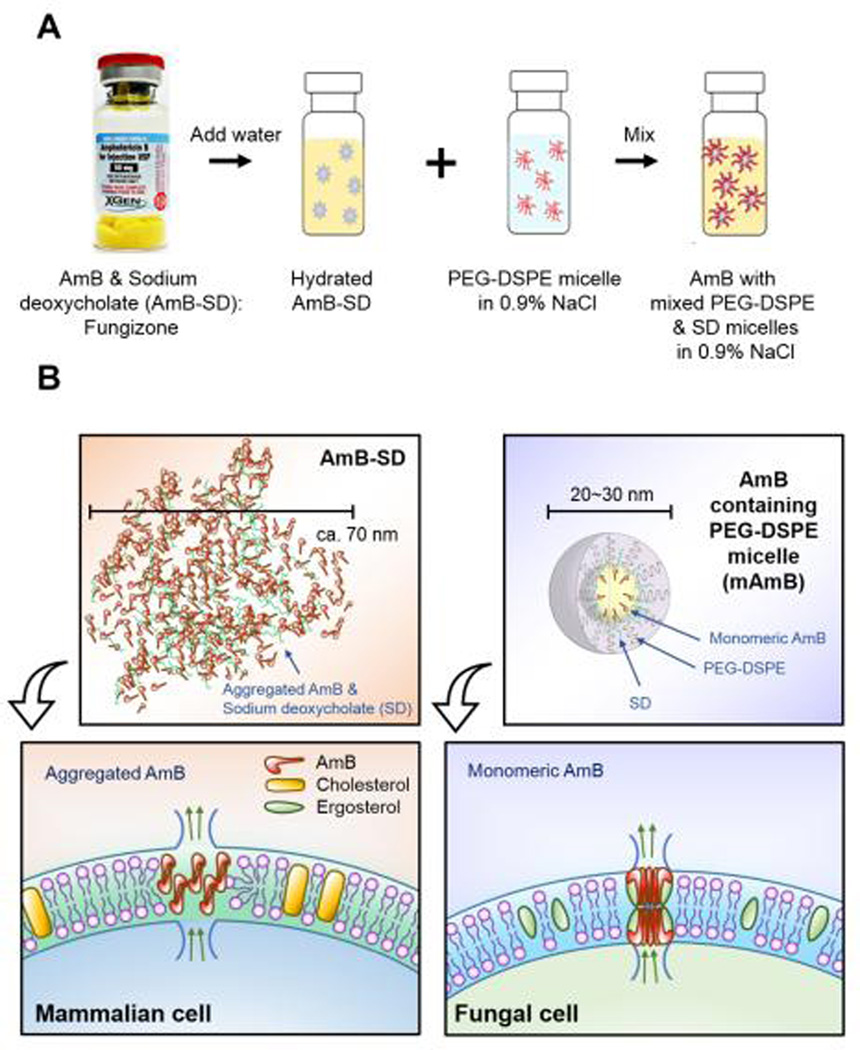
Figure 1.
Reformulation of AmB-SD with PEG-DSPE micelles in saline (A). Depiction of a proposed membrane-level interaction of aggregated and monomeric AmB (B).
Materials and Methods
Preparation of AmB-SD with PEG-DSPE Micelles in Saline
Amphotericin B for Injection USP (X-GEN Pharmaceuticals, Horseheads, NY) was rehydrated with 10 ml sterile water for injection without a bacteriostatic agent USP (Baxter, Deerfield, IL), according to manufacturer’s instructions, resulting in a 5 mg/ml stock solution. PEG-DSPE (Avanti Polar Lipids, Alabaster, AL) was dissolved in 0.9% NaCl-USP (Baxter, Deerfield, IL) to the desired concentration. Three formulations containing AmB-SD and PEG-DSPE micelles in mole to mole ratios of 1:20, 1:40, and 1:90 were analyzed in the following studies. From here on, these formulations will be referred to as mAmB-20, mAmB-40, and mAmB-90.
Degree of AmB Aggregation – IV/I Ratio Determination
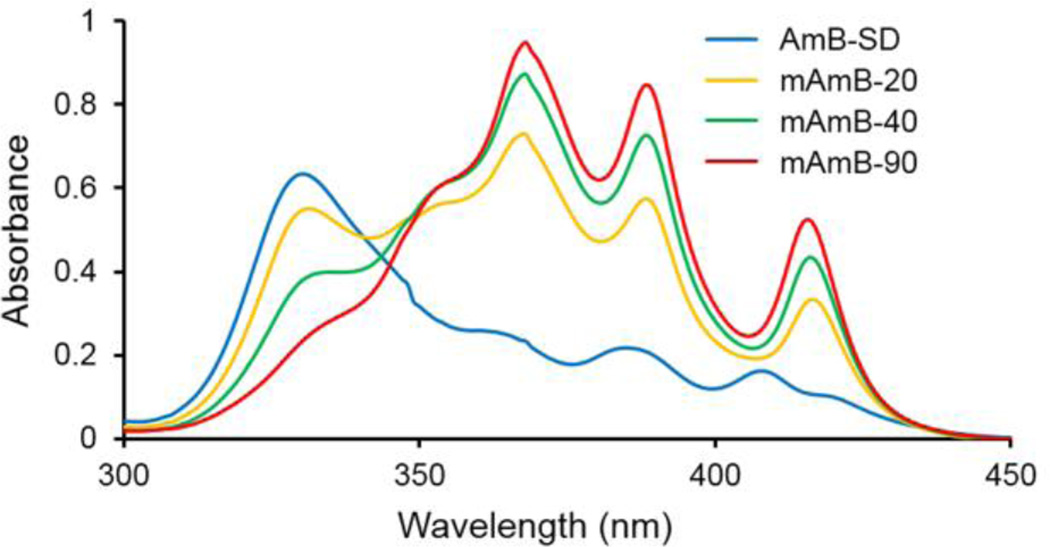
Measuring absorbance of AmB at distinct wavelengths using UV-vis spectroscopy provides insight into its degree of self-aggregation (2, 14, 26, 31). Samples were prepared at 0.1 mg/ml, the infusion solution of AmB-SD administered clinically. To evaluate the aggregation state of AmB, samples were diluted 10-fold in 0.9% NaCl-USP in a quartz cuvette with a 1 mm path length (Varian, Palo Alto, CA) for UV-visible analysis using a CARY 100 Bio UV-visible spectrophotometer (Varian, Palo Alto, CA). Absorbance spectra were recorded from 300–450 nm. The IV/I ratio was determined as the ratio of the fourth (~409 nm) and the first peak (~328 nm). Measurements were repeated in triplicate over a period of four hours on three separate occasions using independent stocks of AmB-SD and PEG-DSPE solutions. Samples were stored protected from light at room temperature during the experiment.
Particle Size Determination - Dynamic Light Scattering
Particle size was determined by dynamic light scattering (DLS) using a ZETASIZER Nano-ZS (Malvern Instruments Inc., Worcestershire, UK) with a He-Ne laser light source (4mW, 633 nm) and a 173° angle scattered light collection configuration to determine the hydrodynamic diameters of AmB formulations. Samples were prepared at 0.1 mg/ml, diluted 5-fold in 0.9% NaCl-USP, and allowed to equilibrate to 25°C before DLS analysis. Each sample was subjected to ten measurements repeated in triplicate over a period of four hours. Measurements were taken on three separate occasions using independent stocks of AmB-SD and PEG-DSPE solutions. In order to calculate the Z-average particle diameter and PDI from the Stokes-Einstein equation and correlation function, respectively, the Cumulants analysis method was used to curve fit the correlation function. Samples were stored protected from light at room temperature during the experiment.
Hemolysis assay
To determine the hemolytic activity of different formulations, bovine red blood cells (Innovative Research, Novi, MI) were washed three times in 1× PBS (Corning cellgro, Manassas, VA), diluted 200-fold in PBS, and further diluted in a 1:1 (v/v) ratio with AmB-SD alone or in the presence of PEG-DSPE micelles. Samples were incubated at 37°C, shaking at approximately 200 × g. After 30 minutes of incubation, samples were placed on ice for 5 minutes to halt hemolysis, then centrifuged at 13,000 × g for 30 seconds. Supernatant was transferred to a 96-well plate and analyzed for absorbance at 405 nm. The negative control consisted of cells in a 1:1 dilution with 1× PBS. The positive control sample, in which total lysis (TL) of cells was achieved, was prepared with cells in the presence of 25 µg/ml AmB-SD in PBS. In addition to different AmB concentrations, the hemolytic activity of PEG-DSPE alone was tested. Experiments were performed in triplicate. Percent hemolysis was calculated using the following equation in which PBS and TL represent the negative and positive controls, respectively: Percent Hemolysis = ((Sample-PBS)/(TL-PBS))*100%.
Renal toxicity
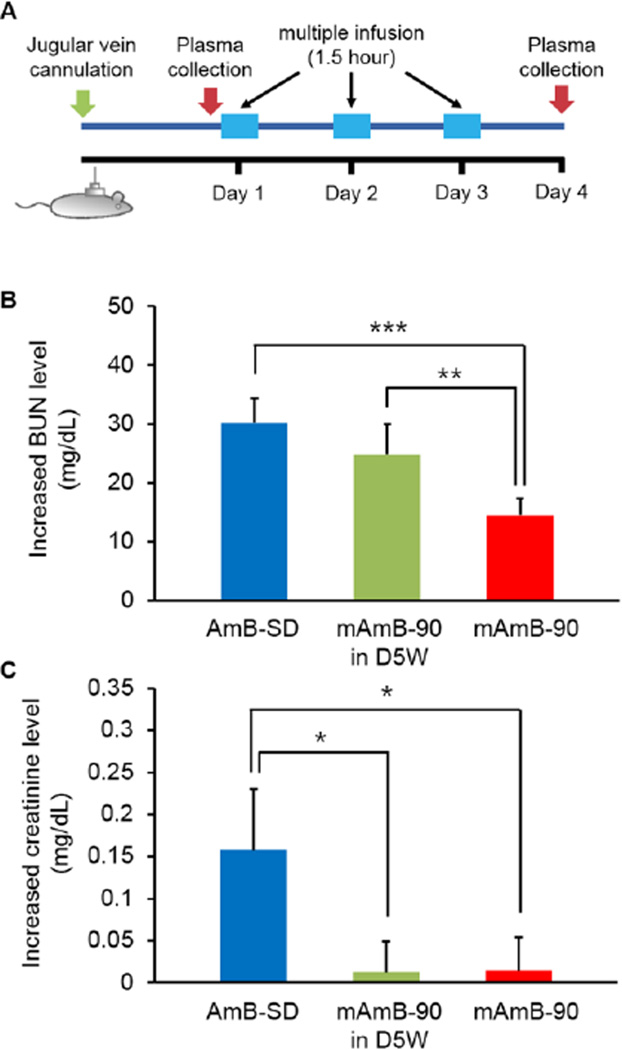
This infusion study was performed to determine the renal toxicity of different AmB formulations after a three day dosing regimen in rats. Jugular vein-cannulated male Sprague-Dawley rats (250–300 kg) were purchased from Charles River (Raleigh, NC). Animals were housed individually to avoid displacement of cannula, and had free access to water and rat chow in a temperature and light-controlled room for at least one week before the first infusion. The cannulated rats were divided into three groups: AmB-SD in D5W-USP (Fun), mAmB-90 (M), and mAmB-90 in D5W-USP (M-D). Using rodent infusion kits (SAI Technologies, Lake Vila, IL), each rat was connected to an Elite Infusion Pump 11 (Harvard apparatus, Holliston, MA). Each group received a daily 1.5-hour infusion of 2 mg/kg AmB for three days. The total volume of each infusion was 7.5 mL. Before beginning the study and 24 hours after the final infusion, approximately 1.5 mL blood was collected from cannula and transferred into heparinized tubes (BD Vacutainer®, Franklin Lakes, NJ). Whole blood samples were centrifuged at 2000 × g for 10 minutes to allow for separation and collection of plasma. Plasma samples were immediately taken to UW-Madison’s Veterinary Care Center for kidney toxicity analysis, where levels of creatinine and blood urea nitrogen (BUN) were determined. In accordance with the US Public Health Service Policy on Human Care and Use of Laboratory Animals, all animal handling and care procedures complied with a University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC) approved protocol.
Antifungal Efficacy
Minimum inhibitory concentration (MIC)
To determine the MIC, the lowest concentration of AmB in a given formulation that considerably inhibited visible fungal growth, S. cerevisiae strain 9763 (American Type Culture Collection, Manassas, VA) or C. albicans strain K1 colonies cultured for approximately 24 hours on Sabouraud dextrose agar (SDA) plates were diluted to a 0.5 McFarland standard in sterile normal saline and diluted 100-fold in yeast peptone dextrose (YPD). Inoculum was added to serial dilutions of AmB in 96-well plates, following the microplate dilution method. Plates were sealed with Breathe Easier membranes (Sigma Aldrich, St. Louis, MO). ATCC 9763 or K1 was incubated at 30°C or 37°C, respectively, for 18–20 hours. Experiments were repeated in triplicate on three separate occasions with distinct ATCC 9763 or K1 colonies and independent stocks of AmB-SD and PEG-DSPE. SDA was prepared with 1% peptone, 4% glucose, and 1.5% agar in deionized water. YPD media was prepared with 1% yeast extract, 2% peptone, and 2% glucose in deionized water. Autoclaving was used to sterilize media.
Minimum fungicidal concentration (MFC)
The MFC was interpreted as the lowest concentration of drug that inhibited ≥ 95% fungal growth. From the MIC experiment, 50 µl of each concentration above the MIC was plated in triplicate on a new 96-well plate with wells filled with 150 µl YPD. Plates were sealed as before and incubated for approximately 24 hours. Results were read by eye and turbidometrically with the Spectramax M2 (Molecular Devices, Sunnyvale, CA) set to detect absorbance at 580 nm. Experiments were repeated in triplicate with independent colonies and stocks of AmB-SD and PEG-DSPE.
Time kill assay
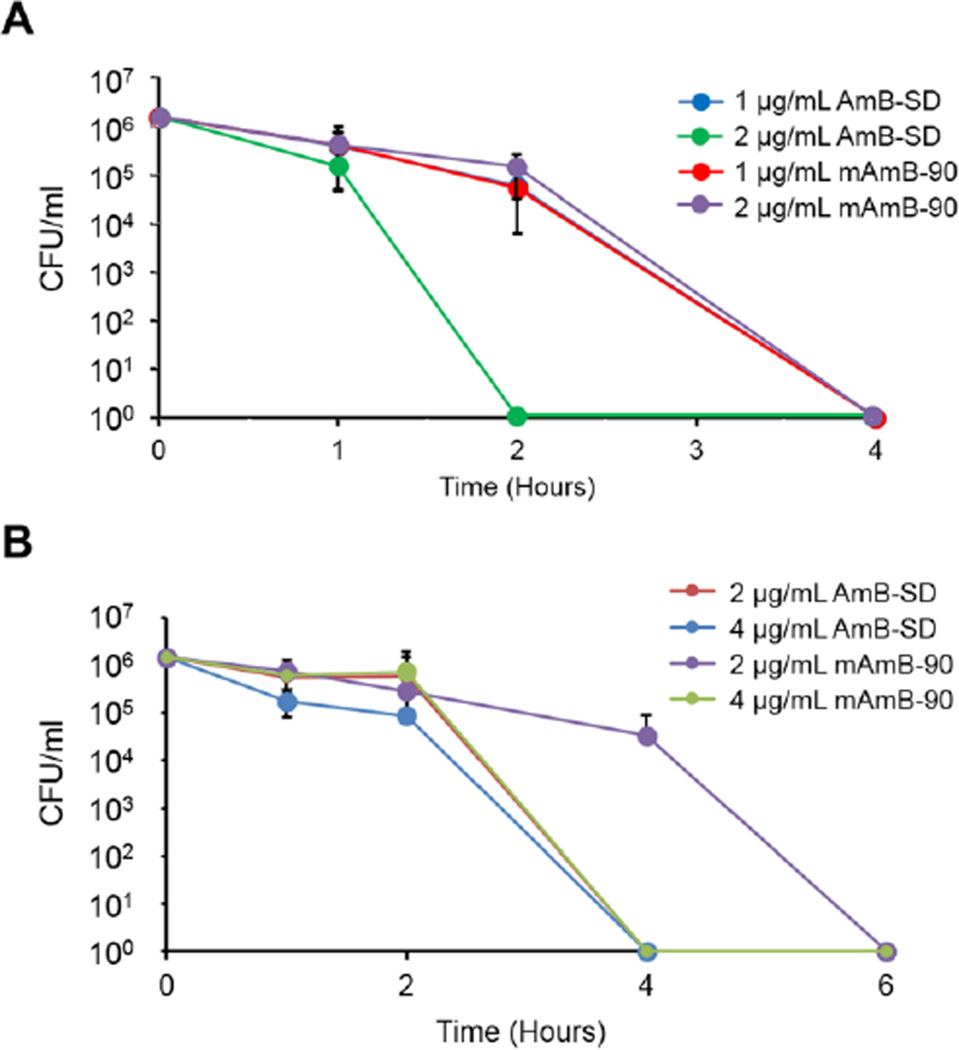
Time kill assays were used to determine the antifungal activity of different formulations at various AmB concentrations relative to one another over time. ATCC 9763 or K1 colonies were diluted to a 0.5 McFarland standard in sterile normal saline, diluted 1000-fold in YPD, and added to AmB formulations in sterile test tubes. Samples were incubated at 30°C or 37°C, shaking at approximately 200×g. At predetermined timepoints, 100 µl sample was removed, diluted five-fold in 0.9% NaCl, and spread upon an SDA plate. Colonies were counted after 48 hours of incubation. Again, experiments were repeated in triplicate with independent colonies and stocks of AmB-SD and PEG-DSPE.
Results
Deaggregation of AmB-SD by PEG-DSPE Micelles in 0.9% NaCl-USP
We found that PEG-DSPE micelles deaggregate AmB-SD. Even in the presence of saline, PEG-DSPE micelles deaggregate AmB-SD over time, and monomer to aggregate ratio measurements reveal that equilibrium may be reached within four hours (data not shown). Addition of PEG-DSPE at increasing mole ratios and the resultant deaggregation of AmB-SD was visualized by UV-vis spectroscopy and evidenced by increased absorbance at ~409 nm and decreased absorbance at ~328 nm (Figure 2). AmB-SD alone was highly aggregated, having an average IV/I ratio of <0.20. Increased absorbance at peak IV and a IV/I ratio approaching 0.80 was observed from mAmB-20, indicating partial deaggregation. More extensive deaggregation of mAmB-40 and m-AmB-90 formulations was evidenced by average IV/I ratios exceeding 3.0 and 5.0, respectively (Table I). In a short-term stability study over 50 hours, at storage temperatures of both 4°C and room temperature (~25°C), the monomer to aggregate ratio of mAmB-90 slightly increased over time (data not shown). As a frame of reference, completely deaggregated AmB in DMSO has a IV/I ratio of approximately 7.
Figure 2.
UV-visible absorption spectra of AmB-SD alone and with PEG-DSPE micelles in 0.9% NaCl-USP. Samples concentrated to 0.1 mg/ml AmB and diluted 10-fold in 0.9% NaCl-USP for analysis.
Table I.
Physical Properties of AmB-SDa alone and Combined with PEG-DSPE Micelles and Antifungal Activity against S. cerevisiae
| Formulation | IV/I Ratiob | Z-Average Particle Diameter (nm)c |
PDIc | MIC (µg/ml) |
MFC (µg/ml) |
|---|---|---|---|---|---|
| AmB-SD | 0.181±0.041 | 3310 | 0.398 | 0.25 | 0.5–1 |
| mAmB-20 | 0.785±0014 | 498 | 0.679 | 0.25 | 0.5–1 |
| mAmB-40 | 3.42±0.31 | 27.2 | 0.312 | 0.25 | 0.5–1 |
| mAmB-90 | 5.16±1.4 | 23.2 | 0.175 | 0.25 | 0.5–1 |
The original concentration of AmB for each formulation was 0.10 mg/ml. Monomer to aggregate ratio, particle size, and PDI values are measurements taken four hours after formulation preparation.
IV/I ratio presented as mean ± standard deviation (n=3).
Mean Z-average particle diameter and PDI presented (n=3).
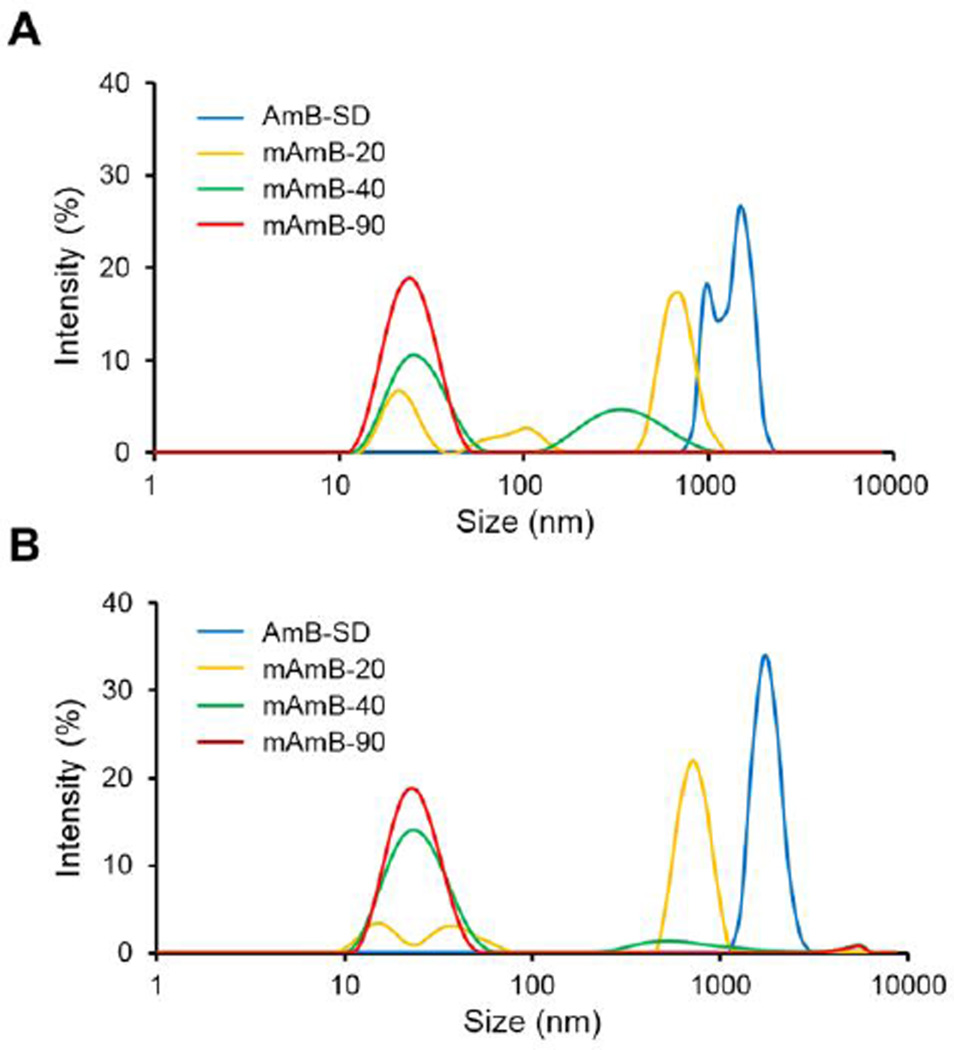
Particle Size Determination
AmB-SD formed large, micron-sized aggregates in 0.9% NaCl-USP. However, addition of PEG-DSPE decreased particle size to nanoscale. Reduced particle size population heterogeneity was observed from mAmB-40 and mAmB-90 formulations (Table I). MAmB-20 and mAmB-40 analysis revealed multimodal size distributions that evolved over a four hour period. MAmB-20 trended towards larger particle size and higher polydispersity while size and polydispersity of mAmB-40 decreased over time. We observed a unimodal size distribution that was maintained over four hours from mAmB-90 (Figure 3). In a short-term stability study with storage at both 4°C and room temperature (~25°C), particle size of mAmB-90 remained approximately 20 nm and PDI did not exceed 0.13 (data not shown).
Figure 3.
Z-average particle diameter of AmB-SD alone and with PEG-DSPE micelles in 0.9% NaCl-USP immediately after sample preparation (A) and four hours after sample preparation (B). Samples concentrated to 0.1 mg/ml AmB and diluted 5-fold in 0.9% NaCl-USP for analysis.
In vitro Hemolysis Assay
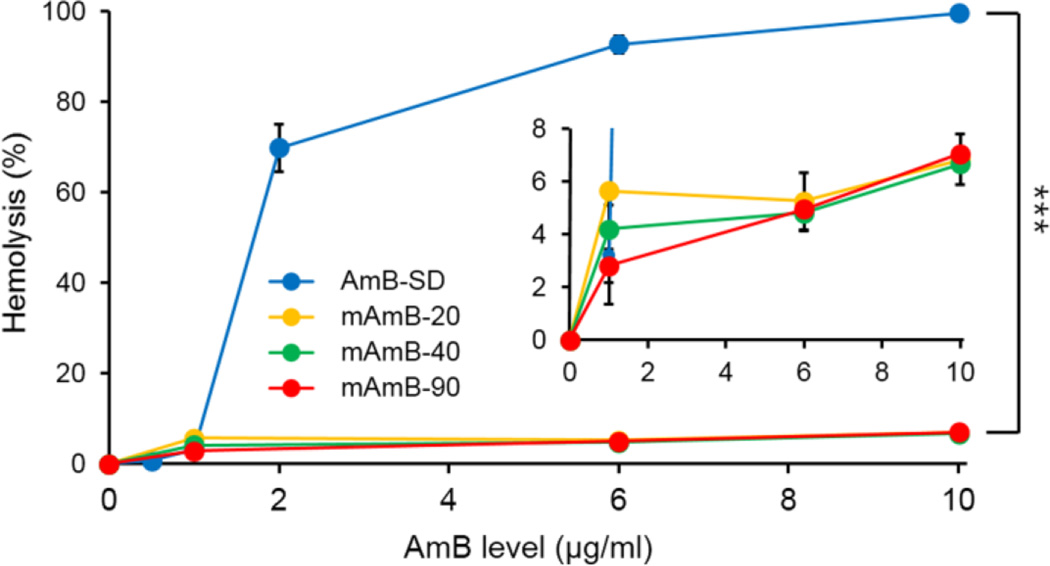
Hemolytic activity was used to assess in vitro mammalian cell membrane toxicity of AmB-SD and mAmB formulations using bovine red blood cells as a model. MAmB-20, mAmB-40, and mAmB-90 did not exceed 10% hemolysis at any tested AmB concentration (Figure 4, Inset). AmB-SD was highly hemolytic, reaching nearly complete hemolysis at concentrations as low as 2 µg/ml (Figure 4). Above 1 µg/ml, AmB-SD was significantly more hemolytic than each mAmB formulation (P <0.0001). PEG-DSPE alone exhibited negligible hemolytic activity (<1%).
Figure 4.
Hemolytic activity of AmB-SD alone and with PEG-DSPE micelles (A). Enlargement of hemolytic activity of AmB-SD with PEG-DSPE micelles (Inset). Data presented as mean ± standard deviation (n=3).
Renal Toxicity
Increases in BUN and creatinine after daily infusions of 2 mg/kg AmB as AmB-SD in D5W, mAmB-90 in D5W, or mAmB-90 (in saline) over three days were monitored to determine renal toxicity (Figure 5A). While mAmB-90 and the other mAmB formulations discussed previously were prepared with saline, mAmB-90 prepared with D5W was included in this experiment to determine whether or not saline plays a role in reducing renal toxicity. Respectively, BUN increases following treatment with AmB-SD and mAmB-90 in D5W were approximately 2-fold and 1.5-fold greater than BUN levels following mAmB-90 treatment (Figure 5B). MAmB-90 in D5W caused considerably higher levels of BUN than mAmB-90; this was interpreted as significant (P <0.01). The increase in creatinine following AmB-SD treatment was over 10-fold greater than the creatinine increase observed after mAmB-90 in D5W and mAmB-90 treatment (Figure 5C). Average creatinine increases following mAmB-90 in D5W or mAmB-90 treatment were similar and quite low at less than 0.015 mg/dL.
Figure 5.
Renal Toxicity. Study methods schematic (A). BUN increase after treatment (B). Creatinine increase after treatment (C). Data presented as mean ± standard error of the mean (n=9).
Antifungal Efficacy
The MICs and MFCs of AmB-SD alone or in the presence of PEG-DSPE micelles dissolved in 0.9% NaCl-USP were maintained across the different formulations (Table 1). In ATCC 9763 time kill studies (Figure 6A) at 1 µg/ml AmB, AmB-SD and mAmB-90 resulted in complete absence of CFU after four hours. At 2 µg/ml AmB, no CFUs were evident after two hours of treatment with AmB-SD, while mAmB-90 treatment reduced the CFU count to zero after four hours. The MIC of AmB-SD and mAmB-90 against K1 ranged from 0.25–0.5 µg/ml, and the MFC ranged from 0.5–1 µg/ml. K1 time kill studies (Figure 6B) in which 4 and 2 µg/ml AmB as AmB-SD and 4 µg/ml AmB as mAmB-90 decreased CFU counts to zero within four hours. MAmB-90 at 2 µg/ml AmB resulted in absence of CFUs between four and six hours.
Figure 6.
ATCC 9763 time kill curves of AmB-SD alone and with PEG-DSPE in 0.9% NaCl-USP at 1 µg/ml and 2 µg/ml AmB (A). K1 time kill curves of AmB-SD alone and with PEG-DSPE in 0.9% NaCl-USP at 2 µg/ml and 4 µg/ml AmB (B). Data presented as mean ± standard deviation (n=3).
Discussion
Physical Properties
Simple addition of PEG-DSPE micelles in saline deaggregated AmB-SD without methods requiring complex protocols or use of specialized equipment. For IV infusion in clinical settings, 5 mg/ml AmB-SD is diluted to 0.1 mg/ml in D5W-USP, resulting in a highly aggregated form of the drug. Immediately after dilution in D5W, particle size is under 100 nm. However, as confirmed by a previous study, this size increases over time (6). When AmB-SD is diluted to 0.1 mg/ml in 0.9% NaCl, AmB remains highly aggregated and particle size is even larger, reaching micron size. Adding increasing amounts of PEG-DSPE micelles reduced the degree of aggregation and decreased particle size (Table 1). After it was clear that PEG-DSPE deaggregated AmB-SD to various extents, we evaluated the interaction between sodium deoxycholate (SD) and PEG-DSPE. The two were combined in mole ratios simulating the different mAmB formulations. A single peak was evident after DLS analysis, leading us to suggest that SD and PEG-DSPE form mixed micelles (data not shown). Deaggregation may be achieved from disruption of AmB aggregates by formation of mixed PEG-DSPE and SD micelles followed by partitioning of monomeric AmB into the mixed micelle core (Figure 1). While deaggregation to a similar extent as that of mAmB-90 was achieved in a previously described formulation at a 1:20 mole ratio of free AmB to PEG-DSPE, the thin-film rehydration method used to produce the formulation poses challenges to scale-up (27).
In vitro Toxicity
Previously, our group revealed the extensive hemolytic activity of AmB above its CAC, approximately 1 µg/ml, and the significant reduction of such activity upon incorporation with polymeric micelles, formulations in which monomeric AmB was dominant (26–29, 32). Consistent with these studies, mAmB formulations deaggregated to different extents exhibited significantly reduced hemolytic activity compared to AmB-SD alone, which was highly hemolytic at concentrations as low as 2 µg/ml (Figure 4). Although mAmB-20 exhibited strong absorbance at the characteristic aggregation wavelength (Figure 2), its hemolytic activity was similar to the other less aggregated formulations (Figure 4). This led us to question whether aggregation state was the only determining factor of hemolytic activity. PEG-DSPE could also play a role in reducing hemolysis. We propose that introduction of monomeric AmB, the consequential shift away from aggregated AmB as the dominant species, and the PEG brush shielding mixed PEG-DSPE and SD micelles from the surrounding environment may all contribute to the significant reduction in hemolytic activity of the PEG-DSPE formulations.
In vivo Toxicity
BUN and creatinine levels, markers of renal function, were significantly greater in the AmB-SD treated group than in both mAmB-90 in D5W and mAmB-90 groups. This suggests that renal function is damaged by aggregated AmB but not monomeric AmB. Interestingly, BUN in rats treated with mAmB-90 in D5W was significantly greater than BUN in mAmB-90 treated rats, even though the physical properties of mAmB-90 in D5W are similar to those of mAmB-90. The particle size and monomer to aggregate ratio of each formulation is similar over a four hour period (data not shown). This reinforces previous studies’ reports on the protective effect of saline on renal function. However, the negligible difference in increased creatinine between mAmB-90 in D5W and mAmB-90 treated groups did not highlight the protective effect of saline. A difference was indiscernible from the very slight measured increase in creatinine from both groups, and it is unknown whether a difference may have emerged after a lengthier treatment regimen or one exceeding the dosage of 2 mg/kg AmB used in this study.
Fungicidal Efficacy
Addition of PEG-DSPE micelles did not affect AmB-SD’s fungicidal activity in long-term endpoint assays, i.e. MIC and MFC experiments. Our formulation’s maintenance of in vitro antifungal activity can be contrasted against the reduced potency of AmBisome, a liposomal AmB preparation that is used clinically, compared to AmB-SD (33). In dynamic dose-response time-kill assays, we observed maintained fungal activity with between AmB-SD and mAmB-90 formulations. An interesting observation revealed by time-kill assays was the longer time required for 2 µg/ml AmB as mAmB-90 to reduce CFU count to zero relative to AmB-SD at the same concentration (Figure 6). It’s possible that release of AmB is slower from a mixed micelle of PEG-DSPE and sodium deoxycholate than from sodium deoxycholate alone.
Conclusion
PEG-DSPE micelles deaggregate and stabilize AmB-SD in 0.9% NaCl. Consistent with prior work based on the aggregation state hypothesis, deaggregated AmB-SD reduced hemolytic activity and renal toxicity without decreasing antifungal efficacy relative to AmB-SD. The opportunity to co-administer saline along with monomeric AmB presents a unique method of AmB-SD preparation and delivery with potential to minimize AmB-induced nephrotoxicity and reduce patient treatment time. Deaggregation of AmB-SD by PEG-DSPE micelles is achieved without organic solvents or complicated procedures, simply by mixing AmB-SD and PEG-DSPE solutions. Therefore, formulation preparation does not pose a formidable challenge to scale-up. Additionally, the commercial availability and FDA-approval of both AmB-SD and PEG-DSPE are attractive features that suggest clinical relevance of this formulation for antifungal therapy.
Acknowledgments
This work was supported by the NIH (RO1 AI-43346). Special thanks to Dr. Tim Bugni for kindly providing C. albicans strain K1.
Abbreviations
- AmB
Amphotericin B
- BUN
Blood Urea Nitrogen
- CAC
Critical Aggregation Concentration
- AmB-SD
Fungizone
- MFC
Minimum Fungicidal Concentration
- MIC
Minimum Inhibitory Concentration
- SDA
Sabouraud Dextrose Agar
- SD
Sodium Deoxycholate
- TL
Total Lysis
- YPD
Yeast Peptone Dextrose
References
- 1.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(7):2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barwicz J, Christian S, Gruda I. Effects of the aggregation state of amphotericin-b on its toxicity to mice. Antimicrobial Agents and Chemotherapy. 1992;36(10):2310–2315. doi: 10.1128/aac.36.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: Time for a new "gold standard". Clinical Infectious Diseases. 2003;37(3):415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 4.Cleary JD, Rogers PD, Chapman SW. Variability in polyene content and cellular toxicity among deoxycholate amphotericin B formulations. Pharmacotherapy. 2003;23(5):572–578. doi: 10.1592/phco.23.5.572.32209. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DRJ, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clinical Infectious Diseases. 2001;32(5):686–693. doi: 10.1086/319211. [DOI] [PubMed] [Google Scholar]
- 6.Lamyfreund MT, Schreier S, Peitzsch RM, Reed WF. Characterization and time-dependence of amphotericin-B - deoxycholate aggregation by quasi-elastic light-scattering. Journal of Pharmaceutical Sciences. 1991;80(3):262–266. doi: 10.1002/jps.2600800314. [DOI] [PubMed] [Google Scholar]
- 7.Inselmann G, Balaschke M, Heidemann HT. Enzymuria following amphotericin B application in the rat. Mycoses. 2003;46(5–6):169–173. doi: 10.1046/j.1439-0507.2003.00856.x. [DOI] [PubMed] [Google Scholar]
- 8.Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. Amphotericin B formulations and drug targeting. Journal of Pharmaceutical Sciences. 2008;97(7):2405–2425. doi: 10.1002/jps.21179. [DOI] [PubMed] [Google Scholar]
- 9.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrobial Agents and Chemotherapy. 2002;46(3):828–833. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cagnoni PJ, Walsh TJ, Prendergast MM, Bodensteiner D, Hiemenz S, Greenberg RN, et al. Pharmacoeconomic analysis of liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients. Journal of Clinical Oncology. 2000;18(12):2476–2483. doi: 10.1200/JCO.2000.18.12.2476. [DOI] [PubMed] [Google Scholar]
- 11.Falci DR, da Rosa FB, Pasqualotto AC. Comparison of nephrotoxicity associated to different lipid formulations of amphotericin B: a real-life study. Mycoses. 2015;58(2):104–112. doi: 10.1111/myc.12283. [DOI] [PubMed] [Google Scholar]
- 12.Espuelas MS, Legrand P, Cheron M, Barratt G, Puisieux F, Devissaguet JP, et al. Interaction of amphotericin B with polymeric colloids - A spectroscopic study. Colloids and Surfaces B-Biointerfaces. 1998;11(3):141–151. [Google Scholar]
- 13.Kajtar M, Vikmon M, Morlin E, Szejtli J. Aggregation of amphotericin-b in the presence of gamma-cyclodextrin. Biopolymers. 1989;28(9):1585–1596. doi: 10.1002/bip.360280908. [DOI] [PubMed] [Google Scholar]
- 14.Tancrede P, Barwicz J, Jutras S, Gruda I. THE EFFECT OF SURFACTANTS ON THE AGGREGATION STATE OF AMPHOTERICIN-B. Biochimica Et Biophysica Acta. 1990;1030(2):289–295. doi: 10.1016/0005-2736(90)90305-8. [DOI] [PubMed] [Google Scholar]
- 15.Gruda I, Dussault N. Effect of the aggregation state of amphotericin-b on its interaction with ergosterol. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire. 1988;66(3):177–183. doi: 10.1139/o88-024. [DOI] [PubMed] [Google Scholar]
- 16.Gruda I, Gauthier E, Elberg S, Brajtburg J, Medoff G. Effects of the detergent sucrose monolaurate on binding of amphotericin-B to sterols and its toxicity for cells. Biochemical and Biophysical Research Communications. 1988;154(3):954–958. doi: 10.1016/0006-291x(88)90232-x. [DOI] [PubMed] [Google Scholar]
- 17.Gruda I, Milette D, Brother M, Kobayashi GS, Medoff G, Brajtburg J. Structure-activity study of inhibition of amphotericin-B (Fungizone) binding to sterols, toxicity to cells, and lethality to mice by esters of sucrose. Antimicrobial Agents and Chemotherapy. 1991;35(1):24–28. doi: 10.1128/aac.35.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolard J, Legrand P, Heitz F, Cybulska B. One-sided action of amphotericin-B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991;30(23):5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- 19.Legrand P, Romero EA, Cohen BE, Bolard J. Effects of aggregation and solvent on the toxicity of amphotericin-B to human erythrocytes. Antimicrobial Agents and Chemotherapy. 1992;36(11):2518–2522. doi: 10.1128/aac.36.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branch RA. Prevention of amphotericin-B induced renal impairment - a review on the use of sodium supplementation. Archives of Internal Medicine. 1988;148(11):2389–2394. [PubMed] [Google Scholar]
- 21.Llanos A, Cieza J, Bernardo J, Echevarria J, Biaggioni I, Sabra R, et al. Effect of salt supplementation on amphotericin-b nephrotoxicity. Kidney International. 1991;40(2):302–308. doi: 10.1038/ki.1991.214. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi A, Ohnishi T, Stevenhead W, Robinson RD, Glick A, Oday DM, et al. Sodium status influences chronic amphotericin-b nephrotoxicity in rats. Antimicrobial Agents and Chemotherapy. 1989;33(8):1222–1227. doi: 10.1128/aac.33.8.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer J, Doubek M, Doubek J, Horky D, Scheer P, Stepanek M. Reduced nephrotoxicity of conventional amphotericin B therapy after minimal nephroprotective measures: Animal experiments and clinical study. Journal of Infectious Diseases. 2002;186(3):379–388. doi: 10.1086/341662. [DOI] [PubMed] [Google Scholar]
- 24.Sawaya BP, Briggs JP, Schnermann J. Amphotericin-B nephrotoxicity - the adverse consequences of altered membrane-properties. Journal of the American Society of Nephrology. 1995;6(2):154–164. doi: 10.1681/ASN.V62154. [DOI] [PubMed] [Google Scholar]
- 25.Carlson MA, Condon RE. Nephrotoxicity of amphotericin-B. Journal of the American College of Surgeons. 1994;179(3):361–381. [PubMed] [Google Scholar]
- 26.Adams ML, Andes DR, Kwon GS. Amphotericin B encapsulated in micelles based on poly(ethylene oxide)-block-poly(L-amino acid) derivatives exerts reduced in vitro hemolysis but maintains potent in vivo antifungal activity. Biomacromolecules. 2003;4(3):750–757. doi: 10.1021/bm0257614. [DOI] [PubMed] [Google Scholar]
- 27.Diezi TA, Kwon G. Amphotericin B/Sterol Co-loaded PEG-Phospholipid Micelles: Effects of Sterols on Aggregation State and Hemolytic Activity of Amphotericin B. Pharmaceutical Research. 2012;29(7):1737–1744. doi: 10.1007/s11095-011-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu BG, Okano T, Kataoka K, Sardari S, Kwon GS. In vitro dissociation of antifungal efficacy and toxicity for amphotericin B-loaded poly(ethylene oxide)-block-poly(beta-benzyl-L-aspartate) micelles. Journal of Controlled Release. 1998;56(1–3):285–291. doi: 10.1016/s0168-3659(98)00095-9. [DOI] [PubMed] [Google Scholar]
- 29.Lavasanifar A, Samuel J, Sattari S, Kwon GS. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharmaceutical Research. 2002;19(4):418–422. doi: 10.1023/a:1015127225021. [DOI] [PubMed] [Google Scholar]
- 30.Diezi TA, Takemoto JK, Davies NM, Kwon GS. Pharmacokinetics and Nephrotoxicity of Amphotericin B-Incorporated Poly(Ethylene Glycol)-Block-Poly(N-Hexyl Stearate l-aspartamide) Micelles. Journal of Pharmaceutical Sciences. 2011;100(6):2064–2070. doi: 10.1002/jps.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolard J, Seigneuret M, Boudet G. Interaction between phospholipid-bilayer membranes and the polyene antibiotic amphotericin-b - lipid state and cholesterol content dependence. Biochimica Et Biophysica Acta. 1980;599(1):280–293. doi: 10.1016/0005-2736(80)90074-7. [DOI] [PubMed] [Google Scholar]
- 32.Aramwit P, Yu BG, Lavasanifar A, Samuel J, Kwon GS. The effect of serum albumin on the aggregation state and toxicity of amphotericin B. Journal of Pharmaceutical Sciences. 2000;89(12):1589–1593. doi: 10.1002/1520-6017(200012)89:12<1589::aid-jps10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Johnson EM, Ojwang JO, Szekely A, Wallace TL, Warnock DW. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrobial Agents and Chemotherapy. 1998;42(6):1412–1416. doi: 10.1128/aac.42.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]