Abstract
Purpose
We determined commonly experienced symptoms reported by adult patients with cancer admitted to urban, ethnically diverse hospice settings and identified symptom clusters.
Methods
We used hierarchical cluster analysis of 150 patients (41% male, 20–92 years [M=59, SD=13.3], 51% African American, 37% Caucasian, 12% other). Using pen-tablet computers, participants completed the Symptom Distress Scale (SDS), a sleep quality item and listed analgesics consumed in the previous 24 hours.
Results
Four symptom clusters were identified: Cluster 1 (Pain-Fatigue) consisted of pain frequency, fatigue, and pain intensity; Cluster 2 (Ingestion-Elimination) consisted of appetite and bowel problems; Cluster 3 (General Well-Being) consisted of insomnia, appearance, and outlook; Cluster 4 (Respiratory-Nausea-Concentration) consisting of breathing, cough, nausea frequency, nausea intensity, and concentration. There were no significant differences between Caucasians and African Americans on total SDS scores, analgesic consumption, sleep quality or most cluster scores.
Conclusion
This is the first symptom cluster analysis in a U.S. sample with a sizeable proportion of minority hospice/palliative care patients with cancer. Further research to determine the stability of identified symptom clusters over time and discovery of the biological interactions of symptoms within the cluster may lead to symptom management therapies designed for the alleviation of all clustered symptoms.
Keywords: Symptom Clusters, Cancer, Hospice, Palliative Care, Hierarchical Cluster Analysis, Symptom Distress Scale, Pittsburgh Sleep Quality Index
INTRODUCTION
Cancer symptom research has focused upon single symptoms such as pain or fatigue until researchers demonstrated that symptoms are multifaceted phenomena often occurring in groups (1) leading to investigation of the symptom cluster phenomenon. Knowledge of symptom clusters expands our comprehension of the cancer symptom experience and potentially leads to improved symptom management and reduced symptom distress. Despite recent advances in symptom cluster research, insufficient information exists describing the existence of symptom clusters among cancer patients in the hospice/palliative care setting, especially those from minority groups.
Over 80% of hospice cancer patients have unrelieved symptoms (2) and they often experience pain at higher levels than the general hospice population (3). Common symptoms include pain, fatigue, anorexia, insomnia, constipation, dyspnea, cough, nausea, memory problems, and diarrhea (4). The source of these aforementioned symptoms may be the result of the cancer, the lingering effects of treatment, comorbidities, and/or medication side effects. Sleep quality concerns are prevalent throughout the cancer trajectory and remain a valid concern as life is ending (5). Reasons for poor sleep quality include demographic, lifestyle, and psychological variables along with disease and treatment related variables. Additionally, several medications (opioids and NSAIDS) commonly prescribed to patients with cancer effects sleep quality. This broad spectrum of cancer related symptoms validates the fact that hospice patients with cancer rarely experience single symptoms (6) and supports the investigation of multiple symptoms in cancer populations at the end of life (7).
Hospice and palliative care patients with cancer report access to a variety of medications for alleviation of symptoms including NSAIDS and adjuvant drugs such as gabapentin and amitriptyline (8). Opioid analgesics such as morphine sulfate, oxycodone, hydromorphone, fentanyl, and codeine with acetaminophen are commonly prescribed. Equianalgesic conversion formulas allow clinicians and researchers to adjust for opioid potency differences (9) therefore, opioids are commonly converted to oral morphine sulfate equalivants (MSEQs). Analgesic consumption may alleviate symptoms thereby alternating symptom cluster composition. Therefore, it is important to investigate symptom clusters in relation to analgesic consumption.
Cancer symptom research has shifted from a single symptom focus to the investigation of symptom clusters. Researchers investigating symptom clusters have proposed two distinctive definitions. The initial definition focused on three or more coexisting symptoms (10, 11). Other investigators propose that a symptom cluster occurs when two symptoms significantly correlate with a patient outcome (6, 12). Nevertheless, the investigation of symptom clusters in patients with cancer especially at end of life is relatively new, and all definitions, supported by definitive evidence, deserve exploration.
Current symptom cluster research in patients with cancer has yielded inconsistent results. Researchers have used different symptom measurement tools and different statistical clustering techniques (13). Unequal gender and ethnic/racial study populations with homogenous or heterogeneous cancer types have yielded two to eight symptom clusters. This lack of uniformity adds uncertainty regarding the validity of the symptom clusters identified. Until a consensus regarding the definition of what constitutes a symptom cluster is reached, which symptom measurement tool or what symptom data are to be collected, and which clustering techniques are utilized, the outcomes of symptom cluster research may remain ambiguous.
The investigation of symptom clusters in patients with cancer has focused primarily on patients undergoing cancer treatment. Information exists regarding symptom clusters in patients with cancer in the hospice/palliative care setting. However, this information does not address gender, or race/ethnicity issues, sleep quality, or analgesic consumption. To overcome these knowledge gaps we aimed to: 1) identify symptom clusters based on item ratings from the Symptom Distress Scale (SDS); and 2) to examine symptom clusters for differences by gender, race/ethnicity, analgesic consumption, and sleep quality.
METHODS
Sample and Setting
For this correlational, cross-sectional, secondary data analysis, we analyzed baseline data collected from the first 150 consecutive participants enrolled in an ongoing randomized clinical trial (RCT). Eligibility requirements for the primary study included patients with cancer who were: (a) admitted to home care level of hospice or palliative care service; (b) experiencing pain on a daily basis; (c) able to speak, read and write English; (d) at least 18 years of age; and (e) evaluated by hospice admission nurses to have a Palliative Performance Scale (PPS) score ≥ 40. The Institutional Review Board (IRB) of the University of Illinois at Chicago granted approval for both studies.
Instruments
Palliative Performance Scale (PPS)
The PPS measures the physical condition of hospice and palliative care patients by the clinician assessing ambulation status, activity, and evidence of disease, self-care capabilities, the process of food and fluid intake, and the state of consciousness. PPS scores range from 0 (death) to 100 (full capabilities) in 10 point increments (14). Several research groups (15–18) demonstrated its usefulness for predicting survival in hospice patients. Patients with a PPS score ≥ to 40 upon admission to hospice generally have a median survival of 18 (18) to 28 (16) days.
Symptom Distress Scale
The Symptom Distress Scale (SDS) is a 13-item valid and reliable tool for the measurement of the degree of symptom distress experienced by patients with cancer (19). The SDS includes 11 items that measure the level of symptom distress related to nausea, appetite, insomnia, pain, fatigue, bowel pattern, concentration, appearance, breathing, outlook, and cough and 2 items that measure the intensity of pain and nausea. Responses to SDS questions are in a Likert-type format ranging from “1” representing normal or usual symptom occurrence to “5” indicating severe or constant distress. Participants rate the intensity of pain and nausea using a similar Likert scale where “1” represents no distress to “5” representing extensive or unbearable distress. Participants’ responses to items are summed. Demonstrated validity and reliability exist for the SDS in patients with cancer (19). Cronbach’s α ranges from .67 (20) to .85 (21). Cronbach’s α for this study was .76.
Sleep Quality
We measured sleep quality with a single item from the Pittsburgh Sleep Quality Index (PQSI). Buysse and colleagues (22) developed the content for the PQSI from their experience with sleep disorder patients, a literature review of established sleep disorder questionnaires and 18 months of field testing. Buysse and colleagues (22) reported initial internal consistency reliability with a Cronbach’s alpha of .83. Participants in our study rated their sleep quality in the past 24 hours on a 4-point scale (“4” = very good, “3” = fairly good, “2” = fairly bad, and “1” = very bad).
Demographics and Analgesic Consumption
PAINReportIt® (23) is a software program (Nursing Consult LLC, Seattle, WA) with a demographic data form to document age, gender, ethnicity, medication consumption histories, pain histories and other items not relevant to this study. On average, patients require 10 to 18 minutes to complete these demographic screens (24). Participants reported pain medications and dosages consumed in the past 24 hours using lists presented on the PAINReportIt® screens. PAINReportIt®-Plus (24) contains the SDS, and the one item from the PQSI.
Procedures
Enrolled participants received instruction on the data collection procedures and completed the demographic data, SDS, PSQI-6 item, medication consumption histories, and additional study measures using pen-tablet computers with PAINReportIt® Plus. Data analysis began when 150 participants completed baseline data for the parent study.
Analyses
Power Analysis
Sample size and power for the study were based on detecting one or more symptom clusters to reject a hypothesis of randomness (24). A cluster analysis with a sample size of 150 participants and 13 potential symptoms achieves 85% power at 0.05 to detect one or more orthogonal symptom clusters.
Statistical Analysis
The data had a normal distribution therefore parametric methods were used for these analyses with a significance level of < 0.05. Descriptive statistics were used to summarize demographic and disease characteristics and sleep quality. We calculated SDS mean item and SDS total scale scores, a Cronbach’s alpha to determine the internal consistency of the SDS scale, and correlations between SDS items. We categorized SDS total scale scores as mild distress (13–24), moderate distress (25–32), and severe distress (33–65) (18).
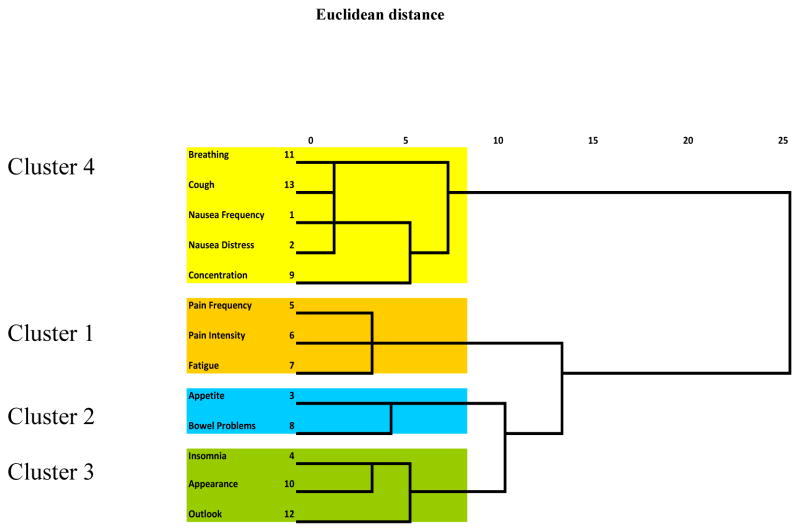
We performed a hierarchical cluster analysis using squared Euclidean distances with item-level symptom data obtained from the 13-item SDS with SPSS 17 for Windows® (SPSS®, Chicago, IL). Groupings are based on Euclidean distances, such that symptoms in the same cluster are more similar to each other than to symptoms found in other clusters (25). Ward’s method (25) was chosen to link clusters together based on the degree of similarity between observations within the same cluster. A dendogram (Figure 1) displays the results showing variables in each group and the distance between the groupings.
Figure 1.
Dendograms using Ward’s Method (minimum sum of squares)
To examine cluster scores for differences by gender and race/ethnicity, we computed sub-scale scores for each symptom cluster by summing the individual SDS item scores identified in each cluster. Distribution assumptions for cluster scores were checked and all cluster scores met the assumption of normality. We then computed Cronbach’s alpha for each newly identified sub-scale. We calculated Pearson r correlations for each sub-scale. We used t-tests and analysis of variance (ANOVA) to investigate gender and race/ethnicity differences in cluster scores of each of the four clusters.
The subjects’ self-reported analgesic medications were classified according to the World Health Organization’s (WHO) analgesic ladder. We calculated MSEQs for opioid medications. We categorized MSEQs into three categories based on the frequency distribution. We used Pearson r to determine correlations between cluster sub-scale scores and total MSEQs consumed.
RESULTS
Participant Characteristics
Demographic Characteristics
Our sample consisted of 150 participants, 104 (69/3%) admitted to hospice and 45 (30.7%) admitted to palliative care. Participants (Table 1) ranged between 20 and 93 years of age with a mean of 59 (SD = 13) years. Patients with lung cancer (n=40, 27%) represented the largest group of participants. Palliative Performance Scale scores ranged from 40 to 90 (Mean = 52.3, SD=8.30).
Table 1.
Participant Characteristics (N = 150)
| Variable | N | % |
|---|---|---|
| Gender | ||
| Male | 62 | 41.3 |
| Female | 88 | 58.7 |
| Race/Ethnicity | ||
| Caucasian | 56 | 37.3 |
| African American | 77 | 51.3 |
| Hispanic | 9 | 6.0 |
| Asian | 3 | 2.0 |
| Native American | 1 | 0.7 |
| Other | 4 | 2.7 |
| Marital Status | ||
| Single | 49 | 32.0 |
| Married | 50 | 33.3 |
| Widowed | 18 | 12.0 |
| Divorced | 22 | 14.7 |
| Separated | 2 | 1.3 |
| Unknown | 10 | 7.6 |
| Education | ||
| <= 8th Grade | 15 | 10.0 |
| <=12th Grade | 58 | 38.7 |
| Vocational | 21 | 14.0 |
| Associate’s Degree | 23 | 15.3 |
| Bachelor’s Degree | 14 | 9.3 |
| Master’s Degree | 8 | 5.3 |
| Doctoral Degree | 3 | 2.0 |
| Unknown | 8 | 5.3 |
| Income | ||
| <$10,000 | 52 | 34.7 |
| $10–20,000 | 27 | 18.0 |
| $21–30,000 | 11 | 7.3 |
| $31–40,000 | 11 | 7.3 |
| $41–50,000 | 16 | 10.7 |
| > $50,000 | 14 | 9.3 |
| Unknown | 19 | 12.7 |
| Cancer Types | ||
| Lung | 40 | 26.7 |
| Colon | 21 | 14.0 |
| Pancreatic | 17 | 11.3 |
| Gastrointestinal | 16 | 10.7 |
| Gynecological | 10 | 6.7 |
| Prostate | 8 | 5.3 |
| Breast | 6 | 4.0 |
| Genitourinary | 3 | 2.0 |
| Others* | 22 | 14.7 |
| Unknown | 7 | 4.7 |
| Sleep Quality (4 point scale) | ||
| Very Bad (1) | 29 | 19.3 |
| Fairly Bad (2) | 28 | 18.7 |
| Fairly Good (3) | 62 | 41.3 |
| Very Good (4) | 27 | 18.0 |
Analgesic Characteristics
Responses indicated that 32% had access to adjuvant drugs, 45.3% to NSAIDS, 40% to WHO Step 2 opioid drugs, and 78.7% to WHO Step 3 opioid drugs. Morphine sulfate immediate release (44%), morphine sulfate extended release (32%), fentanyl (27%), and hydromorphone (23%) were the most frequently reported WHO Step 3 drugs. WHO Step 2 drugs reported included hydrocodone (Vicodin) (11%) and acetaminophen with codeine (11%). Non-opioid prescriptions included acetaminophen (34%), ibuprophen (13%), naproxen (13%), and aspirin (10%). Commonly reported adjuvant drugs included dexamethasone (11%), gabapentin (6%), and amitriptyline (2%). MSEQs ranged from 0 to 405 (Median = 23.0, Mean = 46.52, SD = 70.5). We categorized MSEQ values into three groups; participants reporting no opioid medications consumed (n=28, 19%) (MSEQ = 0), those reporting consuming opioid medications from 1 to 41.5 MSEQ (n= 75, 50%), and those consuming more than 41.6 MSEQ (n=47, 31%).
Symptom Distress
Total SDS scores (Table 2) ranged from 15 to 54 (Mean = 35.27, SD = 8.24). Examination of the rank order for the symptoms at the item level indicates that SDS values of ‘3’, ‘4’, or ‘5’ were frequently reported by 85.2% of the sample for the frequency of pain, by 80.5% of the sample for fatigue, and by 73% of the sample for pain distress. Contrasting these highly rated symptoms is the frequency of participants reporting values of ‘3’, ‘4’, or ‘5’ for breathing (25.7%), frequency of nausea (26.4%), and cough (24.3%). We found statistically significant, mild to moderate correlations between most SDS items (Table 3).
Table 2.
Symptom Distress Scale: Mean, Standard Deviations, and Item Frequencies
| N=150 | Response Item Frequencies | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | 1 | 2 | 3 | 4 | 5 | |
|
|
|||||||
| Nausea Frequency | 2.07 | 1.08 | 52 | 59 | 24 | 7 | 8 |
| Nausea Intensity | 2.18 | 1.19 | 63 | 24 | 42 | 16 | 5 |
| Appetite | 2.83 | 1.27 | 30 | 34 | 27 | 48 | 11 |
| Insomnia | 2.80 | 1.38 | 39 | 28 | 33 | 30 | 20 |
| Pain Frequency | 3.90 | 1.12 | 4 | 18 | 25 | 48 | 55 |
| Pain Intensity | 3.18 | 1.12 | 12 | 28 | 50 | 41 | 19 |
| Fatigue | 3.64 | 1.19 | 5 | 25 | 38 | 33 | 49 |
| Bowel | 2.73 | 1.48 | 38 | 46 | 15 | 21 | 30 |
| Concentration | 2.27 | 1.22 | 52 | 45 | 19 | 29 | 5 |
| Appearance | 2.98 | 1.32 | 30 | 19 | 49 | 28 | 24 |
| Breathing | 2.11 | 1.08 | 47 | 65 | 18 | 14 | 6 |
| Outlook | 2.68 | 1.41 | 44 | 30 | 26 | 31 | 19 |
| Cough | 1.95 | 1.11 | 68 | 46 | 16 | 16 | 4 |
| SDS Total | 35.27 | 8.24 | |||||
Table 3.
Symptom Distress Scale Correlations
| Nausea Frequency | Nausea Intensity | Appetite | Insomnia | Pain Frequency | Pain Intensity | Fatigue | Bowel | Concentration | Appearance | Breathing | Outlook | Cough | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nausea Frequency | 1.00 | ||||||||||||
| Nausea Intensity | .37* | 1.00 | |||||||||||
| Appetite | 0.17 | .97* | 1.00 | ||||||||||
| Insomnia | .19* | .22* | 0.17 | 1.00 | |||||||||
| Pain Frequency | 0.11 | 0.03 | 0.14 | .32* | 1.00 | ||||||||
| Pain Intensity | 0.12 | .30* | 0.09 | .32* | .41* | 1.00 | |||||||
| Fatigue | .27* | .30* | .32* | .25* | .27* | .34* | 1.00 | ||||||
| Bowel | .20* | .33* | .38* | 0.14 | .26* | 0.14 | .32* | 1.0 | |||||
| Concentration | 0.07 | .22* | 0.13 | .22* | 0.05 | 0.08 | .19* | 0.1 | 1.00 | ||||
| Appearance | .27* | .25* | .26* | .42* | .23* | .18* | .28* | .21 | .31* | 1.00 | |||
| Breathing | 0.04 | .20* | 0.09 | .28* | 0.04 | .23* | .39* | 0.0 | 0.00 | .23* | 1.00 | ||
| Outlook | 0.14 | .18* | .21* | .31* | 0.11 | .18* | .19* | 0.0 | .17* | .43* | .31* | 1.0 | |
| Cough | 0.14 | 0.03 | .18* | 0.12 | 0.14 | 0.09 | .19* | 0.1 | 0.08 | .17* | .44* | .17 | 1.0 |
Correlation is significant at the 0.01 Level (2-tailed)
Correlation is significant at the 0.05 Level (2-tailed)
Cluster Analyses
Final designation of symptom clusters was determined via hierarchal cluster analysis. We identified four symptom clusters (Figure 1). Cluster 1 (Pain-Fatigue) consisted of SDS items pain frequency, fatigue, and pain intensity (α = .70); Cluster 2 (Ingestion-Elimination) consisted of SDS items appetite and bowel problems (α = .74); Cluster 3 (General Well-Being) consisted of SDS items insomnia, appearance, and outlook (α = .60); Cluster 4 (Respiratory-Nausea-Concentration) consisted of SDS items breathing, cough, nausea frequency, nausea intensity and concentration (α = .60). Cluster 1 yielded statistically significant (p< .01) but weak (.27) to moderate (.41) linear correlations among items. Cluster 2 yielded a .38 correlation (p<.01). Cluster 3 yielded statistically significant (p< .01) but weak (.31) to moderate (.43) correlations. Cluster 4 yielded no (.00) to moderate (.44) linear correlations (p<.05); concentration demonstrated the lowest correlations with other items in this cluster. The fatigue item, although part of the Pain-Fatigue cluster, was correlated at statistically significant levels with all other SDS items (r=.19 to .39, p<.01).
Gender
We found no significant gender differences in cluster scores. The t-tests for Cluster 1 (Pain-Fatigue) t (148)=−.36, p>.72, Cluster 2 (Ingestion-Elimination) t (148) = −1.47, p > .16, Cluster 3 (General Well-Being) t (148) −.18, p > .86, and Cluster 4 (Respiratory-Nausea-Concentration) t (148) = .04, p > .97 indicate no significant differences by gender.
Race/ethnicity
Analysis of variance (ANOVA) indicated that there were no significant differences in cluster scores based on race/ethnicity except for Cluster 1 (Pain-Fatigue). Participants categorized as other race/ethnicity (Hispanic, Asian, and other) (n=17) reported higher pain scores (Mean = 12.0, SD = 2.57) than Caucasian (Mean = 10.8, SD = 2.79) and African American (Mean = 10.8, SD − 2.49) participants [F (2,147) = 3.49, p < 0.3]. Bonferroni post hoc criterion confirmed that the group mean cluster score for “other” race/ethnicity was significantly higher that either Caucasian or African American groups, but that the means for these latter two groups were not statistically different.
Analgesic Medications
There were weak and statistically non-significant correlations identified among three of the four cluster scores and MSEQ doses. However, the General Well-Being Cluster [r (150) = .17, p<.04) demonstrated a statistically significant but weak correlation with MSEQ. We interpret this finding as those participants who reported less distress with SDS items insomnia, appearance, and outlook, which formed the General Well-Being Cluster, used less analgesics than other participants.
Sleep Quality
Sixty-four participants (44%) in this study reported fairly good sleep quality and 22 participants (18%) reported very good sleep quality. We found statistically significant differences in the Pain-Fatigue, general well-being, and respiratory-nausea-concentration clusters with sleep quality. These findings indicate that participants reporting better sleep quality may experience less symptom distress.
DISCUSSION
This is the first symptom cluster analysis in a sample from the United States with a sizeable proportion of minority patients with cancer admitted to the hospice/palliative care setting. Our results indicate that hospice/palliative care patients with cancer continue to experience distress from numerous symptoms. Using the Symptom Distress Scale we were able to identify the presence and severity of symptoms frequently experienced by patients with cancer. By measuring symptoms in two dimensions (level of distress and intensity) we found similar symptom clusters when only presence was used to investigate symptom clustering. Identifying symptom clusters in this patient population is the first step to developing symptom management interventions aimed at addressing all symptoms within a cluster.
We identified four symptom clusters using hierarchical cluster analysis techniques. Our clusters are similar to clusters found by other researchers; specifically, our Pain-Fatigue cluster (25–32). However, pain and fatigue do not always cluster together (33). In a 100% lung cancer sample (34), fatigue clustered with decreased appetite, drowsiness, sleep disturbances, dry mouth and distress symptoms. In a heterogeneous cancer sample (6), fatigue clustered with weakness, anorexia, lack of energy, dry mouth, early satiety, weight loss, and taste change. Variation in symptom cluster results appears to be related to the specific sample investigated, the symptom measurement tool utilized, and statistical clustering technique used rather than the interaction of the symptoms themselves.
Henoch and colleagues (35) explored the use of two symptom assessment tools and two analytical techniques to determine symptom clusters in 400 adults (41% stage IV) with newly diagnosed inoperable lung cancer. The SDS and 11 of 30 items from the European Organization for Research and Treatment of Cancer (EORTC) provided the symptom data. The investigators used hierarchical cluster analysis using average linkage between groups and factor analysis to demonstrate three similar symptom clusters between both symptom collection tools. A respiratory cluster including symptoms of dyspnea and cough along with mood cluster consisting of mood, outlook, concentration, and insomnia were similarly identified. A difference between the two symptom data collection tools emerged in the pain cluster. When analyzing symptom data from the SDS, fatigue and appetite did not cluster with pain, nausea, or bowel problems as they did with symptom data from the EORTC. Using factor analysis with symptom items, similar results emerged using the SDS and EORTC data collection tools. However, fatigue loaded on both the pain cluster and respiratory cluster in both symptom data collection tools.
In our study, fatigue clustered with pain presence and pain severity and was significantly but weakly correlated with all SDS items except breathing and cough. These findings are similar to Tsai’s and colleagues’ (36) investigation of fatigue in Asian hospice patients with cancer where fatigue was significantly correlated with nausea, vomiting, lack of appetite, sleep disturbance, dyspnea, dry mouth, restlessness, and problems of concentration. These findings are expected as fatigue has been determined to be the most prevalent (37) and distressing (38) cancer related symptom that persists for long periods.
Previous symptom cluster research in patients with cancer varied in study purpose and sample characteristics. Few studies focused on patients with advanced cancers (33, 39). Most studies investigating the presence of symptom clusters enrolled 83% to 97% Caucasian (34, 40–43) or 100% Asian (25, 29–32, 34, 44) participants. It is important to note that our in our study African Americans comprised 51% of our 150 participants.
Symptom Distress
The high degree of symptom distress experienced by participants of this study is consistent with previous findings. The inclusion criteria of patients experiencing pain on a daily basis could explain some of the high prevalence of reported pain. Considering that participants in this study are at the end of the cancer trajectory because their disease has progressed beyond benefit of curative treatment(s) and current therapeutic cancer therapies are no longer beneficial nor desired, it is not unrealistic to expect that previous symptom management has alleviated some symptom distress. Therefore, the need for rapid, effective symptom recognition and management primarily focusing on highly prevalent symptoms is paramount in this patient population.
Analgesics
In our study, analgesic medications available to participants reflect commonly prescribed medications in hospice (45). However, analgesic consumption in MSEQs appears to be lower than other studies in the hospice population. Portenoy and colleagues (46) investigated 725 hospice patients reported 640 participants (88.3%) received intravenous morphine equivalent doses (Mean = 65.7, SD = 45.6). In a study of 435 patients with cancer 396 (91%) received an oral morphine dose of 5–299 mg (47). Lundorff and colleagues (48) reported median oral morphine doses of 120 mg/day (range 10–720 mg/day) in opioid treated cancer patients in a hospital setting. There are many reasons for low analgesic consumption including reluctance of clinicians, patients, and caregivers to use high MSEQ doses. The low MSEQ doses that we observed may be a major factor in the high pain distress reported by our participants.
Sleep Quality
In our study participants reported high SDS total scores, yet 59% (n = 89) also reported relatively good sleep quality scores. Future research utilizing a similar sample and capturing sleep medications will facilitate determining if high symptom distress and worst pain scores with relatively good sleep quality scores is unique to this study population or routinely occurs in the hospice/palliative care setting.
IMPLICATIONS FOR FUTURE RESEARCH
The identification of symptom clusters in the hospice/palliative care setting is the first step toward developing symptom management techniques targeting all symptoms within the cluster. Cluster analysis is an exploratory statistical technique used to identify similar symptom profiles (49) which can provide subgroups of persons at risk for variations in the symptoms. Instead of this approach, we clustered symptoms rather than persons and provide descriptive evidence for the presence of symptom clusters. The composition of symptoms within the cluster will add additional options for outcome measures that could be considered for future research studies. Knowing which symptoms cluster together may trigger an investigation of other symptoms within the cluster thereby improving clinical assessment techniques and potentially alleviating all symptoms within the cluster.
Addressing symptom clusters over time and the development of interventions to address all symptoms in a cluster is the next logical next step in symptom cluster research. However, there are several methodological issues when conducting a longitudinal study, including attrition due to death, that require innovative approaches when investigating patients with cancer in the hospice/palliative care setting. We demonstrated the feasibility of investigating symptom clusters in the hospice/palliative care setting at one time point. Future research to explore the identified symptom clusters over time would increase the understanding of the symptom experience in patients with cancer in the hospice/palliative care setting.
LIMITATIONS
There are limitations to this study. First, this was a secondary data analysis of data collected as near as possible to admission to hospice/palliative care. Using a cross-sectional research design limits the ability to determine improvement or deterioration of study participants, thereby potentially altering symptom cluster results. Enrollment criteria stipulated participants have a Palliative Performance Scale (PPS) score ≥ 40, thereby eliminating a significant portion of potential hospice patients with PPS scores of 10, 20, or 30 and potentially altering the number and composition of symptom clusters. Enrollment criteria required participants to be experiencing pain on a daily basis thereby potentially altering symptom cluster results. Secondly, we collected symptom data via the SDS scale. It is impossible to capture all potential symptoms without over burdening this vulnerable population with data collection. Therefore, not all symptoms patients with cancer may experience are measurable with the SDS or any other symptom collection tool. Thirdly, although we were able to capture a racial/ethnic mix that relatively mirrors the population figures for Chicago and are significantly different from investigations comprised of ≥ 80% Caucasian samples, there was an underrepresentation of Hispanic patients. Hence, the symptom experience and symptom clustering in Hispanic patients with cancer admitted to hospice/palliative care settings remains inadequately determined. Future research targeted towards obtaining participation from all ethnic groups comprising the population mix should be conducted.
CONCLUSIONS
This analysis is the first to describe the presence of symptom clusters in an ethnically diverse hospice/palliative care setting in the U.S. The four identified clusters are similar to those found in advanced cancer patients receiving treatment demonstrating patients with cancer in hospice/palliative care continue to have multiple, distressing symptoms. These results add valuable insight by providing a greater understanding of the symptom experience in patients at the end of the cancer trajectory who are receiving hospice/palliative care.
Determining the stability of identified symptom clusters over time and discovering the biological interactions of all symptoms within the cluster may lead to cluster management therapies designed to alleviate all clustered symptoms thereby decreasing the symptom burden. We also found that symptom distress was high despite the use of analgesics, but whether this was due to insufficient dosing or a general lack of effective analgesics for cancer pain cannot be determined.
With insufficient symptom cluster research in existence, we provide evidence that adds to the growing body of knowledge specifically in the hospice/palliative care setting. Development of aggressive pain and symptom management strategies to alleviate symptom distress is needed. Knowing which symptoms cluster together may facilitate development of management strategies that target at all the symptoms within each cluster.
Acknowledgments
Grant Number 1F31NR010048 from the National Institutes of Health, National Institute of Nursing Research, supported this publication. Grant Number R01 NR009092 from the National Institutes of Health, National Institute of Nursing Research supported data collection and P30 NR010680 supported manuscript preparation. Its contents are solely the responsibility of the authors and do not represent the official views of the National Institute of Nursing Research. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
Contributor Information
Stephen J. Stapleton, Email: sstaple@ilstu.edu, Assistant Professor, Mennonite College of Nursing, Illinois State University, Normal, Illinois 61790, Phone: 309-438-4644, Fax: 309-438-4410.
Janean Holden, Email: holdenje@umich.edu, Professor, School of Nursing, University of Michigan, 400 North Ingalls Building, Ann Arbor, MI 48109-5482, 734-763-7667.
Joel Epstein, Email: jepstein@coh.org, Director of Oral Health Services, Division of Head and Neck Surgery, Professor of Oral Medicine, Department of Surgery, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles CA, City of Hope National Medical Center, 1500 Duarte Rd, Duarte, CA 91010, 626-256-4673.
Diana J. Wilkie, Email: diwilkie@ufl.edu, Professor, Prairieview Trust – Earl and Margo Powers Endowed Professor, and Director, Center of Excellence in Palliative Care Research, Department of Biobehavioral Nursing Science, College of Nursing, University of Florida, 1225 Center Drive, Room 2203, Gainesville, FL 32610, Phone: 352-273-6401.
References
- 1.Dodd M, Miaskowski C, Lee K. Occurrence of symptom clusters. Journal of the National Cancer Institute Monographs. 2004;(32):76–8. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- 2.Ng K, von Gunten CF. Symptoms and attitudes of 100 consecutive patients admitted to an acute hospice/palliative care unit. J Pain Symptom Manage. 1998;16(5):307–16. doi: 10.1016/s0885-3924(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 3.McMillan S, Small B. Symptom distress and quality of life in patients with cancer newly admitted to hospice home care. Oncol Nurs Forum. 2002;29(10):1421–8. doi: 10.1188/02.ONF.1421-1428. [DOI] [PubMed] [Google Scholar]
- 4.Potter VT, Wiseman CE, Dunn SM, Boyle FM. Patient barriers to optimal cancer pain control. Psychooncology. 2003 Mar-Apr;12(2):153–60. doi: 10.1002/pon.627. [DOI] [PubMed] [Google Scholar]
- 5.Hearson B, Sawatzky J. Sleep disturbance in patients with advanced cancer. Int J Palliat Nurs. 2008;14(1):30–7. doi: 10.12968/ijpn.2008.14.1.28150. [DOI] [PubMed] [Google Scholar]
- 6.Walsh D, Rybicki L. Symptom clustering in advanced cancer. Supportive care in cancer. 2006;14(8):831–6. doi: 10.1007/s00520-005-0899-z. [DOI] [PubMed] [Google Scholar]
- 7.Miaskowski C, Cooper B, Paul S, Dodd M, Lee K, Aouizerat B, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33(5):E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 8.Ferrell B, Levy M, Paice J. Managing pain from advanced cancer in the palliative care setting. Clin J Oncol Nurs. 2008;12(4):575–81. doi: 10.1188/08.CJON.575-581. [DOI] [PubMed] [Google Scholar]
- 9.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–87. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 10.Beck S. Symptom clusters: impediments and suggestions for solutions. Journal of the National Cancer Institute Monographs. 2004;(32):137–8. doi: 10.1093/jncimonographs/lgh032. [DOI] [PubMed] [Google Scholar]
- 11.Paice J. Assessment of symptom clusters in people with cancer. Journal of the National Cancer Institute Monographs. 2004;(32):98–102. doi: 10.1093/jncimonographs/lgh009. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, McGuire D, Tulman L, Barsevick A. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–82. doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Dong ST, Butow PN, Costa DSJ, Lovell MR, Agar M. Symptom Clusters in Patients with Advanced Cancer: A Systematic Review of Observational Studies. Journal of Pain and Symptom Management. 2014;48(3):411–450. doi: 10.1016/j.jpainsymman.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12(1):5–11. [PubMed] [Google Scholar]
- 15.Harrold J, Rickerson E, Carroll J, McGrath J, Morales K, Kapo J, et al. Is the palliative performance scale a useful predictor of mortality in a heterogeneous hospice population? J Palliat Med. 2005;8(3):503–9. doi: 10.1089/jpm.2005.8.503. [DOI] [PubMed] [Google Scholar]
- 16.Head B, Ritchie C, Smoot T. Prognostication in hospice care: can the palliative performance scale help? J Palliat Med. 2005;8(3):492–502. doi: 10.1089/jpm.2005.8.492. [DOI] [PubMed] [Google Scholar]
- 17.Lau F, Downing GM, Lesperance M, Shaw J, Kuziemsky C. Use of Palliative Performance Scale in end-of-life prognostication. J Palliat Med. 2006;9(5):1066–75. doi: 10.1089/jpm.2006.9.1066. [DOI] [PubMed] [Google Scholar]
- 18.Weng L, Huang H, Wilkie D, Hoenig N, Suarez M, Marschke M, et al. Predicting survival with the Palliative Performance Scale in a minority-serving hospice and palliative care program. J Pain Symptom Manage. 2009;37(4):642–8. doi: 10.1016/j.jpainsymman.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCorkle R, Cooley ME, Shea J. A User’s Manual for the Symptom Distress Scale. New Haven, CT: Yale School of Nursing; 1995. Unpublished manuscript. [Google Scholar]
- 20.Degner LF, Henteleff PD, Ringer C. The relationship between theory and measurement in evaluations of palliative care services. J Palliat Care. 1987;(2):8–13. [PubMed] [Google Scholar]
- 21.Cowan LF, Young-Graham K, Loveys-Cochrane B. Comparison of a Theory of Quality of Life Between Myocardial Infarction and Malignant Melanoma: A Pilot Study. Progress in Cardiovascular Nursing. 1992;7(1):18–28. [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Wilkie D, Judge MKM, Berry D, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25(3):213–24. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 24.Wilkie D, Kim Y, Suarez M, Dauw C, Stapleton S, Gorman G, et al. Extending computer technology to hospice research: interactive pentablet measurement of symptoms by hospice cancer patients in their homes. J Palliat Med. 2009;12(7):599–602. doi: 10.1089/jpm.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Lin C. Cancer symptom clusters: a validation study. J Pain Symptom Manage. 2007;34(6):590–9. doi: 10.1016/j.jpainsymman.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Chow E, Fan G, Hadi S, Wong J, Kirou Mauro A, Filipczak L. Symptom clusters in cancer patients with brain metastases. Clin Oncol. 2008;20(1):76–82. doi: 10.1016/j.clon.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Ivanova M, Ionova T, Kalyadina S, Uspenskaya O, Kishtovich A, Guo H, et al. Cancer-related symptom assessment in Russia: validation and utility of the Russian M. D. Anderson Symptom Inventory. J Pain Symptom Manage. 2005;30(5):443–53. doi: 10.1016/j.jpainsymman.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Okuyama T, Wang X, Akechi T, Mendoza T, Hosaka T, Cleeland C, et al. Japanese version of the MD Anderson Symptom Inventory: a validation study. J Pain Symptom Manage. 2003;26(6):1093–104. doi: 10.1016/j.jpainsymman.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Tsai C, Chen B, Lin C. Symptom clusters and relationships to symptom interference with daily life in Taiwanese lung cancer patients. J Pain Symptom Manage. 2008;35(3):258–66. doi: 10.1016/j.jpainsymman.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Wang Y, Guo H, Mendoza T, Hao X, Cleeland C. Chinese version of the M. D. Anderson Symptom Inventory: validation and application of symptom measurement in cancer patients. Cancer. 2004;101(8):1890–901. doi: 10.1002/cncr.20448. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Laudico A, Guo H, Mendoza T, Matsuda M, Yosuico V, et al. Filipino version of the M. D. Anderson Symptom Inventory: validation and multisymptom measurement in cancer patients. J Pain Symptom Manage. 2006;31(6):542–52. doi: 10.1016/j.jpainsymman.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Fodeh SJ, Lazenby M, Bai M, Ercolano E, Murphy T, McCorkle R. Functional Impairments as Symptoms in the Symptom Cluster Analysis of Patients Newly Diagnosed With Advanced Cancer. J Pain Symptom Manage. 2013;46(4):500–510. doi: 10.1016/j.jpainsymman.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Fairclough D, Liao Z, Komaki R, Chang J, Mobley G, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. Journal of clinical oncology. 2006;24(27):4485–91. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 35.Henoch I, Ploner A, Tishelman C. Increasing stringency in symptom cluster research: a methodological exploration of symptom clusters in patients with inoperable lung cancer. Oncol Nurs Forum. 2009;36(6):E282–92. doi: 10.1188/09.ONF.E283-E292. [DOI] [PubMed] [Google Scholar]
- 36.Tsai L, Li I, Lai Y, Liu C, Chang T, Tu C. Fatigue and its associated factors in hospice cancer patients in Taiwan. Cancer Nurs. 2007;30(1):24–30. doi: 10.1097/00002820-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Hofman M, Ryan J, Figueroa Moseley C, Jean Pierre P, Morrow G. Cancer-related fatigue: the scale of the problem. Oncologist. 12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. 200. [DOI] [PubMed] [Google Scholar]
- 38.Cheville A. Cancer-related fatigue. Phys Med Rehabil Clin N Am. 2009;20(2):405–16. doi: 10.1016/j.pmr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Cheung W, Le L, Zimmermann C. Symptom clusters in patients with advanced cancers. Supportive care in cancer. 2009;17(9):1223–30. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- 40.Gift A, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31(2):202–12. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 41.Sarna L, Brecht ML. Dimensions of symptom distress in women with advanced lung cancer: a factor analysis. Heart Lung. 1997;26(1):23–30. doi: 10.1016/s0147-9563(97)90006-6. [DOI] [PubMed] [Google Scholar]
- 42.Ridner S. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Supportive care in cancer. 2005;13(11):904–11. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 43.Fox S, Lyon D. Symptom clusters and quality of life in survivors of ovarian cancer. Cancer Nurs. 2007;30(5):354–61. doi: 10.1097/01.NCC.0000290809.61206.ef. [DOI] [PubMed] [Google Scholar]
- 44.Chen M, Tseng H. Symptom clusters in cancer patients. Supportive care in cancer. 2006;14(8):825–30. doi: 10.1007/s00520-006-0019-8. [DOI] [PubMed] [Google Scholar]
- 45.Morgan AE, Lindley CM, Berry JI. Assessment of pain and patterns of analgesic use in hospice patients. American journal of hospice palliative medicine. 1994;11(1):13, 9, 22. doi: 10.1177/104990919401100104. [DOI] [PubMed] [Google Scholar]
- 46.Portenoy R, Sibirceva U, Smout R, Horn S, Connor S, Blum R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32(6):532–40. doi: 10.1016/j.jpainsymman.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Bercovitch M, Adunsky A. Patterns of high-dose morphine use in a home-care hospice service: should we be afraid of it? Cancer. 2004;101(6):1473–7. doi: 10.1002/cncr.20485. [DOI] [PubMed] [Google Scholar]
- 48.Lundorff L, Peuckmann V, Sjogren P. Pain management of opioid-treated cancer patients in hospital settings in Denmark. Acta Anaesthesiol Scand. 2008;52(1):137–42. doi: 10.1111/j.1399-6576.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 49.Barsevick A, Whitmer K, Nail L, Beck S, Dudley W. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31(1):85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]