Abstract
The diagnosis and treatment of prostate cancer (CaP) continues to be challenging, as prostate-specific antigen (PSA) appears to be overly sensitive and biopsy is the only reliable method for confirmation. Hence, the goal of the study is to identify a biomarker that could distinguish malignant cancer from benign prostatic hyperplasia (BPH) during the early diagnosis of the disease. We observed significant differential expression of miR-301a in CaP samples in comparison to BPH and adjacent benign samples. The overexpression of miR-301a activates the invasion/migration of CaP cells. In contrast, silencing miR-301a expression inhibited the colony-forming ability, adhesion, invasion and migration of CaP cells. Similarly, the overexpression of miR-301a increased cell motility in normal RWPE-1 prostate epithelial cells. Our results suggest that miR-301a is differentially expressed between BPH and CaP specimens and that the expression of miR-301a correlates with biochemical recurrence and/or metastasis in CaP patients. Hence, the expression of miR-301a could be a potential marker for metastasis in CaP patients. Detecting miR-301a expression during diagnosis will avoid wait and watch timelines, thus preventing morbidity.
Keywords: microRNA, metastasis, biomarker, diagnosis and prostate cancer
1. Introduction
Prostate cancer (CaP) is the most commonly diagnosed cancer in American men. In 2015, an estimated 220,800 new cases will be diagnosed, and 27,540 cancer deaths are projected in the US [1]. An elaborate understanding of the genetic basis of familial CaP has led to targeted screening of high-risk groups, particularly on the background of routine CaP screening after discussing the risks and benefits of screening. Early-stage CaP patients have a variety of treatment options, which include but are not limited to active surveillance, radical prostatectomy and radiation therapy [2]. Additionally, androgen deprivation therapy is the mainstay for the management of metastatic CaP, while chemotherapy or newer targeted agents are offered to castration-resistant metastatic CaP patients [3].
To date, CaP diagnosis is often based on the use of prostate-specific antigen (PSA), which changed the outlook for diagnosis and treatment [4]. However, the low specificity of PSA frequently causes unnecessary biopsies and misdiagnoses [5]. The limitations of the use of PSA include the following: PSA is not specific to CaP, especially not high-grade/risk disease, PSA can be raised or lowered due to other non-cancer related factors, and only 25% of patients with elevated PSA will have CaP [6]. In addition, trans-rectal ultrasound-guided prostate biopsy impacts patients psychologically and has its own complications [7]. Both biopsy- and histology-based diagnoses have limitations, as they may not suggest a prognosis for treatment. Hence, it is imperative to identify a reliable marker for distinguishing aggressive from non-aggressive CaP.
MicroRNAs (miRNAs) are small noncoding RNA molecules that are known to regulate several biological functions (proliferation, survival, apoptosis, metastasis and angiogenesis) in a cell-specific manner [8]. It was recently established that miRNAs regulate the initiation, promotion, and progression of the disease and causes chemo resistance in many cancer types. miRNAs target the 3′-untranslated regions (3′-UTRs) of genes and translationally repress specific signal transduction pathways. Several miRNAs were identified, and their expression levels were linked to the clinical outcome of CaP [9]. miR-143 was reported to be involved in the differentiation of CaP stem cells and to enhance the migration potential of CaP cell lines [10]. Similarly, higher expression levels of miRNA-375 and miRNA-141 were found in the sera of castration-resistant CaP (CRPC) patients in comparison to low-risk localized patients; however, the biological and clinical roles of these miRNAs were not explored [11].
miR-301a has gained more attention in cancer research because of its significant role in cancer-related differentiation, inflammation, apoptosis and pathogenesis [12]. In particular, stronger expression of miR-301a has been reported in pancreatic [13], breast [14], prostate [15], hepatocellular [16], and small cell lung cancers [17], which correlated with the invasion and migration of the disease.
Our results have demonstrated for the first time that increased expression of miR-301a may correlate with an aggressive phenotype of CaP. Further, the observed significantly higher expression of miR-301a in CaP patients indicates that this differential expression may be responsible for early-onset and aggressive disease. The detection of miR-301a could help to identify patients who have a higher risk for CaP and who may benefit from aggressive forms of treatment.
2. Material and Methods
2.1. Prostate tissue specimens
A total of 58 formalin-fixed paraffin-embedded (FFPE) specimens with matching controls, 4 paired metastatic tumors, 6 fresh tumor tissues and 13 BPH samples were selected for this study based on the associated clinical diagnoses. Of the 75 samples, 23 samples were low-grade (Gleason <6), and 24 samples were moderate-grade (Gleason 7). The mean age of the patients with CaP was 53 (range: 46-88) years. The prostate specimens were collected from the Department of Pathology under the approval of the Committee for the Protection of Human Subjects in Research and the Institutional Review Board at the University of Louisville, and the experiments were carried out in accordance with the approved guidelines. Both the cancer and the adjacent benign hyperplasic margins were outlined by the pathologist (on the slides and corresponding paraffin blocks), which were subjected to microdissection and RNA extraction after histological confirmation of all of the prostate samples by a board-certified pathologist.
2.2. RNA isolation, cDNA synthesis and quantitative real-time reverse transcription PCR
Total RNA was extracted from the tumors and from adjacent and benign hyperplasia samples by using the miRVana kit (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. cDNA was synthesized with the miScript II kit (Qiagen, Cambridge, MA, USA). Real-time PCR was performed with an Applied Biosystems system using the miScript RT II SYBR kit. The mature form of the miRNAs was detected using the miRNA qPCR quantitation assay according to the manufacturer's instructions. 18S/U6 was used as an internal control, and primers were purchased from Ambion. Fold changes were calculated by normalizing based on BPH values. Each experiment was performed in triplicate.
2.3. Cell lines and culture
Human CaP (PC-3, LNCaP, and 22Rv1) and normal prostate epithelial (RWPE-1) cell lines were purchased from ATCC and cultured according to the associated guidelines. C4-2B cells were obtained from ViroMed Laboratories (Minneapolis, MN, USA).
2.4. Scrambled control, inhibitor and mimic of miRNA-301 and transfection
The miR-301a inhibitor and mimic and the negative controls were purchased from Qiagen (Cambridge, MA, USA). Small interfering RNAs (siRNAs) that targeted human sequences were designed according to previously validated oligonucleotides and synthesized by Qiagen. Oligonucleotide transfection was performed with Mirus SiQuest Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer's instructions.
2.5. Cell viability assays
CaP and normal prostate epithelial cells were transfected with an miR-301a mimic or inhibitor or the scrambled miR-301a control for 48 h. Cell viability was quantified using the trypan blue exclusion method or the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide (MTT) assay, as described previously [18].
2.6. Soft agar colony formation assay
A colony formation assay was performed on both PC-3 and LNCaP cells to monitor anchorage-independent growth by using the CytoSelect™ 96-Well In Vitro Tumor Sensitivity Assay kit (Cell Biolabs, Inc., San Diego, CA, USA), as we described previously [19].
2.7. Wound healing, migration and invasion assays
PC3, LNCaP or RWPE-1 cells were transfected with miR-301a sequences (scrambled, mimic and inhibitor) for 48 h, and the cells were replated in six-well plates, as described previously for migration assays. Similarly, for invasion assays, the transfected cells were plated and evaluated using Boyden chambers equipped with polyethylene terephthalate membranes with 8-μm pores (BD Biosciences), as described previously [20]. Briefly, Transwell chambers containing 8-μm pores (BD Falcon) were coated with Matrigel (BD Scientific), and extracellular matrix proteins were layered on top of the Transwell membrane. The CaP cells were trypsinized and resuspended in incomplete medium. These cells were transfected with the miR-301a mimic and inhibitor and allowed to migrate into the Boyden chamber coated with Matrigel. The cells that remained in the upper chamber were removed with a cotton swab, and the Boyden chamber with a PET membrane was stained with crystal violet.
Similarly, miR-301a transfectants were plated in six-well plates and cultured until they reached confluency. A linear wound was gently created in the monolayer of cells with a 200 μl sterile pipette tip. The cells were then washed with PBS/growth medium to remove the detached cells, and fresh medium was added gently. The wound gap distance was photographed at the same site at the 0, 24 and 48 h time points. The wound gap was measured by using the NIS-Element AR software (Nikon Instruments, Inc., Melville, NY, USA), and the distance between the opposing edges of the wound was measured in micrometers.
2.8. Statistics
Statistical analysis was performed with the GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 15.0 software (IBM SPSS Statistics, IBM, Ehningen, Germany). Differences between treatments groups were analyzed either using a two-sample Student's t-test for normally distributed variables or the Wilcoxon rank sum test for non-normally distributed variables. P values below 0.05 were considered to be significant. All differences denoted by asterisks were statistically significant and were encoded in the figures (*P<0.05, **P<0.01 and ***P<0.001). The data were presented as the mean+sd of a minimum of three independent experiments unless stated otherwise.
3. Results
3.1. Differential expression of miR-301a in CaP tissues
A total of 75 FFPE samples (4 paired metastatic tumors and 6 fresh tumor tissues with matching controls and 10 adjacent benign tissues) and 13 BPH samples were selected based on their clinical diagnoses. Of the 75 samples, 23 were low-grade (Gleason <6), 24 were moderate-grade (Gleason 7) and 17 were highly aggressive (Gleason 8-10). The mean age of the patients with CAP was 53 (range: 46-88) years. Based on the Hematoxylin and Eosin (H&E) staining of the CaP tumor tissues, Gleason Score 6 (3+3) [small infiltrative glands, with open lumen and circumscribed by stromal] and Gleason score 7 (4+3) [the glands are fused, cribriform or are poorly defined] tumors were identified (Fig-1A-up). The identified tumor portions and adjacent controls in the paraffin sections were micro-dissected, followed by RNA extraction [21] and the detection of miR-301a expression via real-time PCR, with 18S and U6 as controls. CaP patients with Gleason 6 and Gleason 7 tumors exhibited higher expression of miR-301a than BPH and normal adjacent benign tissues (Fig-1A-down). We observed a stronger induction of miR-301a expression (∼50-fold) in comparison to BPH. To determine whether miR-301a could be a potential metastatic marker, we also analyzed the primary tumor, adjacent benign tissue and paired metastasis samples from the same patients. The results revealed that both tumor and bone metastases exhibited significantly higher expression of miR-301a (10 folds) than benign tissues (Fig-1B). A similar trend toward higher expression of miR-301a was also evident at other metastatic sites, such as the lymph nodes (Fig-1B) and the left neck (data not shown). The bone metastatic cells were positive for PSA, which further confirms that the tumor cells metastasized from the prostate (Fig-1B). Next, we calculated the sensitivity and specificity of miR-301a expression between paired tumor and adjacent benign tissues. The Receiver Operating Characteristic (ROC) curve predicted a sensitivity of 85% and a specificity of 60% at optimal values. The smaller sample size contributed to the low specificity. ROC analysis yielded an AUC value of 78% (data not shown).
Figure 1.
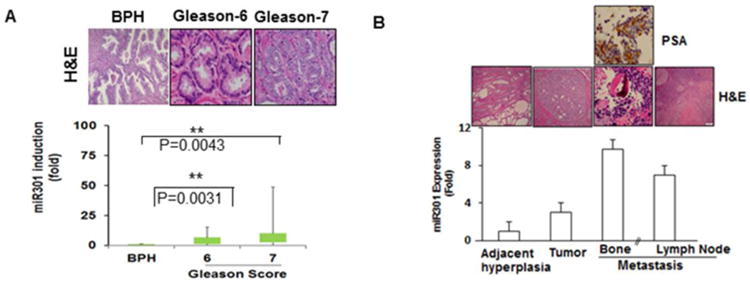
Human CaP tissues illustrating the expression of miR-301a. (A) H&E staining in human BPH and Gleason 6 and Gleason 7 CaP tissues (top). MiR301a expression profile in CaP patients, as indicated by real-time PCR, showing significant differences between BPH and different stages of CaP. (B) miR-301a expression in tissues from adjacent hyperplasia, primary tumor and bone (same patient) and lymph node metastases.
3.2. Stronger expression of miR-301a targets in CaP cell lines
To understand the biological function of miR-301a, we analyzed the basal levels of miR-301a expression in CaP cells. Stronger expression of miR-301a was observed (1.5- to 8-fold) in CaP (PC-3, DU-145, LNCaP, C4-2B, and 22Rv1) cell lines in comparison to normal prostate epithelial cells (RWPE-1) (Fig-2A) (PrpEC, PzHPV-7 and RWPE-2) (data not shown). These results correlated with our clinical findings, and we believe that these cell lines are suitable for our proposed study. Based on the observed higher expression of miR-301a, the PC-3 and LNCaP cell lines were selected as representative models for our studies.
Figure 2.
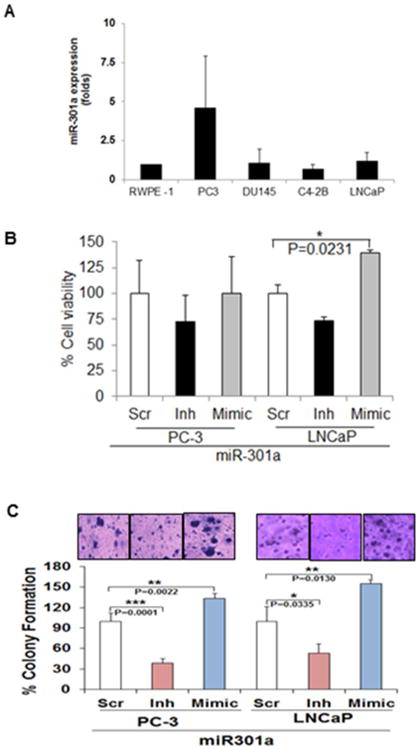
MiR-301a expression in CaP cell lines: (A) Real-time PCR analysis of the expression levels of miR-301a in a normal prostate epithelial cell line (RWPE-1) and cancer (PC-3, DU-145, C4-2B and LNCaP) cell lines. U6 and 18S were used as controls for this analysis. (B) Overexpression of miR-301 increased the viability and (C) colony-forming ability of PC-3 and LNCaP cells after 48 h of transfection. Silencing miR-301a inhibits the colony-forming ability of PC-3 and LNCaP cells.
3.3. The biological role of miR-301a in castration-resistant prostate cancer (CRPC) cell lines
To understand the biological role of miR-301a in CaP cells, we transiently overexpressed (mimic) or silenced (inh) miR-301a expression in CaP (PC-3 and LNCaP) cells for 48 h. Overexpression and silencing of miR-301a were confirmed by qPCR. Overexpression increased miR-301a levels in the cells by approximately 2,000- to 3,000-fold in comparison to cells transfected with scrambled siRNA (data not shown). Similarly, cells transfected with silenced miR-301a showed a significant inhibition of miR-301a in comparison to controls, as indicated by qPCR (data not shown). miR-301a overexpression led to increased proliferation in PC-3 (10%) and LNCaP (40%) cells after 48 h of transfection, signifying that miR-301a plays a promoting role in CaP cells (Fig-2B). However, no significant inhibition of proliferation was seen in miR-301a-silenced PC-3 and LNCaP cells (Fig-2B). Further, we determined the colony-forming ability of CaP cells, and our results suggested that the overexpression of miR-301a significantly increased the ability of PC-3 (p=0.0022) and LNCaP (p=0.0130) cells to form colonies. In contrast, miR-301a knockdown minimized both the number and size of the colonies (Fig-2C), emphasizing the therapeutic role of miR-301a in CaP (PC-3: p=0.0001 and LNCaP: p=0.033) cells.
3.4. MiR-301a regulates epithelial to mesenchymal transition (EMT) in CaP cells
As the above results guided us to determine the role of miR-301a in EMT, we further analyzed the expression of EMT markers and phenotypic changes in miR-301a-transfected CaP cells. EMT is a multistep process, in which adhesion plays an important role in cancer cell dissemination [22] and the inhibition of adhesion molecules attenuates metastasis [23]. The adhesion of miR-301a-transfected cells was approximately 169% in PC-3 cells and 162% in LNCaP cells, which is higher than that observed in cells transfected with the scrambled sequence (Fig-3A). To confirm whether miR-301a was responsible for the increased adhesion levels in CaP Cells, we measured cell adhesion after the knockdown of miR-301 expression in both PC-3 and LNCaP cells. As expected, a significant decrease (61% in PC-3 cells and 59% in LNCaP cells) in adhesion occurred in miR-301a-silenced cells, suggesting the involvement of miR-301a in CaP cell adhesion (Fig-3A).
Figure 3.
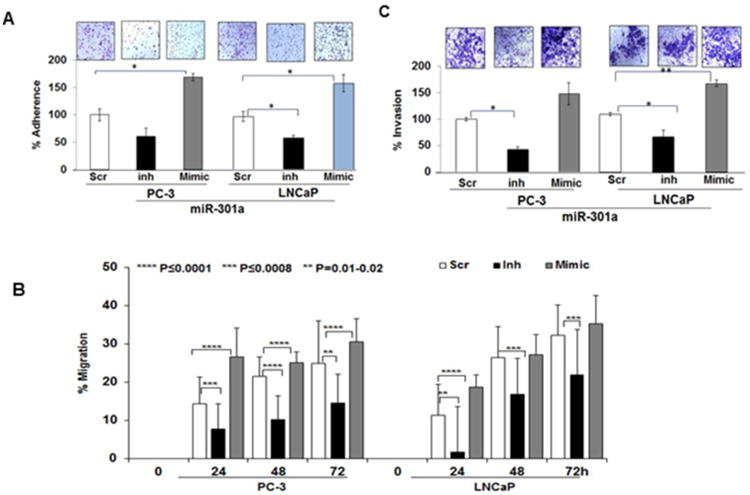
Overexpression of miR-301a increased the (A) adhesion, (B) migration (C) invasive ability of PC-3 and LNCaP cells after 48 h of transfection. Silencing miR-301a fails to inhibit the adhesion, (A) migration (B) and invasion (C) of PC-3 and LNCaP cells.
To determine the anti-migratory and anti-invasive effects of miR-301a, we performed in vitro Transwell invasion and migration assays on PC-3 and LNCaP cells for up to 24 h. As observed in Fig-3B, miR-301a-transfected cells exhibited an increase in migration from 24-72 h in comparison to PC-3 and LNCaP cells transfected with the scrambled sequence. Similarly, miR-301a-silenced CaP cells exhibited less migration than the controls and miR-301a-overexpressing cells (Fig-3B). As shown in Fig-3C, silencing miR-301a expression significantly impeded the invasion of CaP cells (PC-3: 42.5% and LNCaP: 53%) in comparison to the scrambled transfectant (100%). In contrast, the overexpression of miR-301a promoted cell invasion in both PC-3 (149%) and LNCaP (153%) cells. Thus, these results confirmed the metastatic role of miR-301a in CaP cells.
Next, we determined the role of miR-301a in the regulation of EMT markers, such as β-catenin and E-cadherin expression, via immunofluorescent analysis to confirm the localization of β-catenin and E-cadherin in miR-301a-transfected CaP cells (Fig-4A). Intense staining in the cell membrane suggests the shuttling of β-catenin to the cell membrane and cellular junctions in miR-301a-overexpressing cells, while less staining in silenced miR-301a cells suggests the downregulation of β-catenin (Fig-4B). The disruption of E-cadherin/β-catenin might contribute to aberrant tumor morphogenetic effects. The loss of E-cadherin expression or normal localization at cell-cell contacts is consistently observed at sites of EMT during tumor progression. E-cadherin expression levels are often inversely correlated with tumor malignancy. On the other hand, the complete inhibition of E-cadherin expression in mimic-transfected cells in comparison to scrambled-transfected cells was observed (Fig-4B), and the inhibition of miR-301a reversed the basal expression of E-cadherin in PC-3 cells (Fig-4B).
Figure 4.
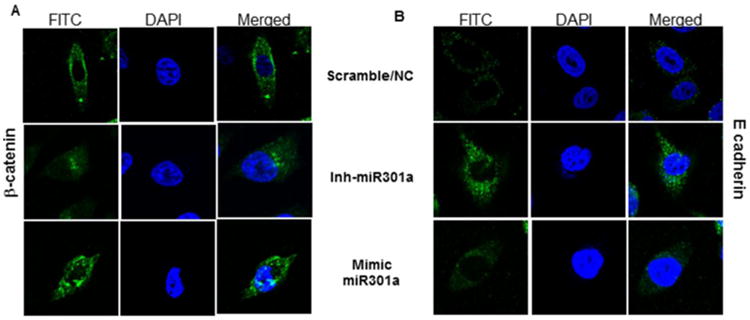
Silencing and overexpression of miR-301 a in PC-3 cells. After 48 h of transfection, the CaP cells were subjected to subcellular localization of β-catenin (A) and E-cadherin (B). In PC-3 cells transfected with the miR-301a-inhibitor or the miR-301a-mimic, as indicated, localization was examined by immunofluorescence staining.
4. Discussion
Our results clearly suggest that miR-301a plays a crucial role in disease progression and that miR-301a could be a reliable metastatic maker for CaP. Our delineation of the biological function of miR-301a suggests that this miRNA is a master driver for EMT that initiates a cascade of metastatic events in CaP.
It is estimated that one in six men will be diagnosed with CaP during their lifetimes; however, estimates of the risk of death from CaP are one in thirty-seven. CaP is heterogeneous in nature, and no promising prognostic and predictive markers are available to differentiate high-risk individuals, who tend to develop CaP and are more likely to benefit from treatment, from indolent CaP patients, who may be best treated with active surveillance. Many groups have been working to develop genetic markers for the diagnosis, prognosis, chemoresistance and metastasis of CaP [24]. To date, PSA expression is the most widely used biomarker [25]; however, PSA is not specific to CaP. Elevated levels of PSA could be altered due to other non-cancer related factors, and only 25% of patients with elevated PSA will have CaP [6]. More recently, multiple professional organizations and the United States Preventive Services Task Force (USPSTF) recommended against routine PSA screening, which initiated intense public debate [26]. This recommendation was criticized because the number of African American (AA) CaP patients in the studies reviewed by the USPSTF was very low. Many recommendations that suit Caucasian American (CA) CaP patients may not be appropriate for AA CaP patients. For example, active surveillance of low-grade cancers may work well for CA CaP patients but not for AA men. AA men with low-risk disease according to prostate biopsy and who subsequently chose immediate surgery rather than observation showed significant upstaging of the disease upon examination of radical prostatectomy specimens [26]. It is desirable to stratify the risk in this cohort. Furthermore, the current diagnosis of CaP is controversial, as elevated levels of PSA lead to a risk of overdetecting the disease, which will eventually cause unnecessary biopsies and patient anxiety associated with the diagnosis of low-risk/indolent prostate cancer [27].
In the present study, we observed stronger expression of miR-301a in Gleason 6 and 7 tumors in comparison to BPH, which implies that miR-301a could distinguish between indolent and aggressive CaP. Detecting miR-301a expression at an early stage of diagnosis may avoid morbidity and mortality in CaP patients. Our findings demonstrate that miR-301a could act as a metastatic marker; in comparison to benign tissues, tumor and metastatic sites exhibited a significant 10-fold higher expression of miR-301a. In fact, the ROC curve predicted a sensitivity of 85% sensitivity and a specificity of 60% at optimal values. The observed low specificity values were due to the smaller sample size. ROC analysis yielded an AUC value of 78% (data not shown).
We found low levels of miR-301a expression in high-grade tumors (Gleason-8 to10) in comparison to Gleason 6 and 7 tumors; however, the expression was higher than that observed in BPH or adjacent benign hyperplasia samples. This difference could be due to the presence of undifferentiated cells as a characteristic of aggressive tumors and due to the fact that miR-301a expression may present predominantly in epithelial cells. We understand that the sample size is limitation of the study; however, this is the first study to demonstrate the differential expression of miR-301 in different stages of CaP.
Our knock-in and knock-out experiments clearly demonstrated that miR-301a not only promotes cell survival but also activates EMT by changing the kinetics of β-catenin and E-cadherin expression. However, in our study, silencing miR-301a resulted in the downregulation of E-cadherin, while the overexpression of miR-301a upregulated the same protein in CaP cells. Despite the contradiction with the existing theory that the downregulation of E-cadherin is a hallmark of EMT, similar trends toward higher expression of E-cadherin were reported in well-differentiated prostate tumors, while low levels of E-cadherin were observed in poorly differentiated tumors [28]. Another study also revealed the differential expression of E-cadherin between the center of tumors (higher expression) and invasive margins (lower expression) in bone metastasis of CaP [29]. Therefore, all of these studies suggests that quantifying the expression of E-cadherin by western blotting analysis is not ideal, and determining the localization signals may further suggest the functional role of E-cadherin in cancer.
Recently, Xie et al. reported that miR301 increases the invasiveness of CaP cells by activating TGF-β1/Smad/MMP9 signaling [30], and another cohort study confirmed that the expression of miR301 could be a predictive marker for the recurrence and metastasis of CaP [31]. These studies highlight the importance of miR301a, and analyzing more samples and identifying potential targets of miR-301a may suggest whether miR-301a could be prognostic marker for prostate cancer.
To conclude, our study clearly reveals that miR-301a expression could be a relevant biomarker and a predictive metastatic maker for CaP, despite our study's limitations concerning sample size, clinical follow-up and the overall survival of CaP patients. Although, one-third of the prostate specimens in our study were needle biopsy samples, we were successfully able to microdissect the tumor from the adjacent hyperplasia. Hence, these studies are feasible and may be translated to the clinical setting. Overcoming the limitations of this study by investigating a larger sample size and performing prospective studies may define whether miR-301a could be a predictive, prognostic or metastatic marker for CaP.
Highlights.
This study is the first of its kind that demonstrates the differential expression of miR-301a between CaP (Gleason-6 and Gleason-7) vs benign prostate hyperplasia (BPH) or adjacent benign tissues.
Correlating the expression of miR-301a with progression (metastasis) and clinical outcome (biochemical recurrence) of the disease suggests the translational potential of the studies.
This is the foremost study to delineate the role of miR-301a in CaP and dissecting the molecular axis of miR-301a may suggest the potentials of miR-301a as a possible therapeutic target of CaP besides its biomarker role.
Developing a novel predicting metastasis marker is the highlight of the study.
Acknowledgments
Financial support: This work was supported by R01CA140605, 1R01CA185972-01 and R01CA138797 (to C. D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chi K, Hotte SJ, Joshua AM, North S, Wyatt AW, Collins LL, et al. Treatment of mCRPC in the AR axis-targeted therapy resistant state. Ann Oncol. 2015 doi: 10.1093/annonc/mdv267. [DOI] [PubMed] [Google Scholar]
- 3.van der Poel H, Klotz L, Stief CG. Management of low- and intermediate-risk prostate cancer. World J Urol. 2015 doi: 10.1007/s00345-015-1618-0. [DOI] [PubMed] [Google Scholar]
- 4.Riegman PH, Vlietstra RJ, Klaassen P, van der Korput JA, Geurts van Kessel A, Romijn JC, et al. The prostate-specific antigen gene and the human glandular kallikrein-1 gene are tandemly located on chromosome 19. FEBS Lett. 1989;247:123–6. doi: 10.1016/0014-5793(89)81253-0. [DOI] [PubMed] [Google Scholar]
- 5.Esfahani M, Ataei N, Panjehpour M. Biomarkers for Evaluation of Prostate Cancer Prognosis. Asian Pac J Cancer Prev. 2015;16:2601–11. doi: 10.7314/apjcp.2015.16.7.2601. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N Engl J Med. 2001;344:1373–7. doi: 10.1056/NEJM200105033441806. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol. 1998;160:2115–20. doi: 10.1097/00005392-199812010-00045. [DOI] [PubMed] [Google Scholar]
- 8.Schirrmeister H, Guhlmann A, Kotzerke J, Santjohanser C, Kuhn T, Kreienberg R, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17:2381–9. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Li J, Ding Q, Cheng G, Zhou H, Tao L, et al. miR-152 controls migration and invasive potential by targeting TGFalpha in prostate cancer cell lines. Prostate. 2013;73:1082–9. doi: 10.1002/pros.22656. [DOI] [PubMed] [Google Scholar]
- 10.Fan X, Chen X, Deng W, Zhong G, Cai Q, Lin T. Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer. 2013;13:61. doi: 10.1186/1471-2407-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen HC, Xie W, Yang M, Hsieh CL, Drouin S, Lee GS, et al. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate. 2013;73:346–54. doi: 10.1002/pros.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YH, Na HS, Jeong SY, Jeong SH, Park HR, Chung J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell. 2011;35:43–9. [PubMed] [Google Scholar]
- 13.Schultz NA, Werner J, Willenbrock H, Roslind A, Giese N, Horn T, et al. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol. 2012;25:1609–22. doi: 10.1038/modpathol.2012.122. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey AC, Xu Z, Weinberg CR, Getts RC, Wade PA, DeRoo LA, et al. Serum microRNA expression as an early marker for breast cancer risk in prospectively collected samples from the Sister Study cohort. Breast Cancer Res. 2013;15:R42. doi: 10.1186/bcr3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–76. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, et al. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652–7. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 17.Miko E, Czimmerer Z, Csanky E, Boros G, Buslig J, Dezso B, et al. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res. 2009;35:646–64. doi: 10.3109/01902140902822312. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan S, Koduru S, Kumar R, Venguswamy G, Kyprianou N, Damodaran C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int J Cancer. 2009;125:961–7. doi: 10.1002/ijc.24419. [DOI] [PubMed] [Google Scholar]
- 19.Suman S, Kurisetty KV, Das TP, Vadodkar V, Ramos G, Lakshmanaswamy R, Damodaran C. Activation of AKT signaling promotes Epithelial-Mesenchymal Transition and tumor growth in colorectal cancer cells. MolCarcino. 2013 doi: 10.1002/mc.22076. [DOI] [PubMed] [Google Scholar]
- 20.Das TP, Suman S, Damodaran C. Reactive oxygen species generation inhibits epithelial-mesenchymal transition and promotes growth arrest in prostate cancer cells. Mol Carcinog. 2013 doi: 10.1002/mc.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eble John N, S G, Epstein Jonathan I, Sesterhenn Isabell A. WHO classification of tumors. Pathology and genetics, tumors of the Urinary system and male genital organs. 2004 [Google Scholar]
- 22.Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–30. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glinsky GV, Glinskii AB, Stephenson AJ, Hoffman RM, Gerald WL. Gene expression profiling predicts clinical outcome of prostate cancer. J Clin Invest. 2004;113:913–23. doi: 10.1172/JCI20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jr, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98:516–21. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 26.Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 27.Vainio P, Lehtinen L, Mirtti T, Hilvo M, Seppanen-Laakso T, Virtanen J, et al. Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins. Oncotarget. 2011;2:1176–90. doi: 10.18632/oncotarget.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryden AA, Freemont AJ, Clarke NW, George NJ. Paradoxical expression of E-cadherin in prostatic bone metastases. BJU Int. 1999;84:1032–4. doi: 10.1046/j.1464-410x.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- 29.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010;3:90–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Xie H, Li L, Zhu G, Dang Q, Ma Z, He D, et al. Infiltrated pre-adipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-beta1/Smad/MMP9 signals. Oncotarget. 2015;6:12326–39. doi: 10.18632/oncotarget.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam RK, Amemiya Y, Benatar T, Wallis CJ, Stojcic-Bendavid J, Bacopulos S, et al. Identification and Validation of a Five MicroRNA Signature Predictive of Prostate Cancer Recurrence and Metastasis: A Cohort Study. J Cancer. 2015;6:1160–71. doi: 10.7150/jca.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]


