Abstract
Background
Systematic evaluation and estimation of growth trajectories in twins require ultrasound measurements across gestation, performed in controlled clinical settings. Currently there are few such data for contemporary populations. There is also controversy about whether twin fetal growth should be evaluated using the same benchmarks as singleton growth.
Objectives
Our objective was to empirically define the trajectory of fetal growth in dichorionic twins using longitudinal two-dimensional ultrasonography and to compare the fetal growth trajectories for dichorionic twins with those based on a growth standard developed by our group for singletons.
Study Design
A prospective cohort of 171 women with twin gestations was recruited from eight U.S. sites from 2012 to 2013. After an initial sonogram at 11w0d–13w6d where dichorionicity was confirmed, women were randomized to one of two serial ultrasonology schedules. Growth curves and percentiles were estimated using linear mixed models with cubic splines. Percentiles were compared statistically at each gestational week between the twins and 1,731 singletons, after adjustment for maternal age, race/ethnicity, height, weight, parity, employment, marital status, insurance, income, education and infant sex. Linear mixed models were used to test for overall differences between the twin and singleton trajectories using likelihood ratio tests of interaction terms between spline mean structure terms and twin-singleton indicator variables. Singleton standards were weighted to correspond to the distribution of maternal race in twins. For those ultrasound measurements where there were significant global tests for differences between twins and singletons, we tested for week-specific differences using Wald tests computed at each gestational age. In a separate analysis, we evaluated the degree of reclassification in small for gestational age, defined as below the 10th percentile that would be introduced if fetal growth estimation for twins was based upon an unweighted singleton standard.
Results
Women underwent a median of 5 ultrasounds. The 50th percentile abdominal circumference and estimated fetal weight trajectories of twin fetuses diverged significantly beginning at 32 weeks, while biparietal diameter in twins was smaller from 34 through 36 weeks. There were no differences in head circumference or femur length. The mean head circumference/abdominal circumference ratio was progressively larger for twins compared with singletons beginning at 33 weeks, indicating a comparatively asymmetric growth pattern. At 35 weeks, the average gestational age at delivery for twins, the estimated fetal weights for the 10th, 50th and 90th percentiles were 1960, 2376, and 2879 g for dichorionic twins and 2180, 2567, and 3022 g for the singletons. At 32 weeks, the initial week when the mean estimated fetal weight for twins was smaller than that of singletons, 34% of twins would be classified as small for gestational age using a singleton, non-Hispanic white standard. By 35 weeks, 38% of twins would be classified as small for gestational age.
Conclusions
The comparatively asymmetric growth pattern in twin gestations, initially evident at 32 weeks, is consistent with the concept that the intrauterine environment becomes constrained in its ability to sustain growth in twin fetuses. Near term, nearly 40% of twins would be classified as small for gestational age based on a singleton growth standard.
Keywords: Dichorionic, Estimated fetal weight, Fetal growth, Multiple gestation, Twin
Introduction
Twin gestations represented 3.4% of U.S. births in 2014.1 The infant mortality rate is higher in twins versus singletons (23.6 versus 5.4 per 1,000 live births) as is the rate of cerebral palsy (7 versus 1.6 per 1,000 live births).2, 3 Cross-sectional U.S. natality data demonstrate that after 28 weeks of gestation, twins are born with lower mean birth weights, and that the difference between twins and singletons progressively widens with increasing gestational age, implying that growth slows at the beginning of the third trimester in twin gestations.4 However, these findings reflect birth size and do not convey the longitudinal pattern of in utero fetal growth from early in pregnancy. Such cross-sectional studies based on birth weight cannot adequately assess early onset growth abnormalities because the data are inherently biased by preterm deliveries associated with complications that may also affect fetal growth or by iatrogenic preterm deliveries that result from suspected growth restriction.
Systematic evaluation and estimation of growth trajectories in twins require ultrasound measurements across gestation. Currently there are few such data for contemporary populations.5, 6 Furthermore, no prior study of twins performed in multiple clinical centers with a rigorous design, including training of sonographers, standardization of ultrasound measurements and assessment of quality control has been conducted.
Understanding fetal growth in twin gestations is important given that fetal growth is an influential determinant of health and disease in the perinatal period, childhood and adult life, and that there is uncertainty about whether twin fetal growth should be evaluated similarly to singleton growth.7 Therefore, in collaboration with eight institutions, the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD), National Institutes of Health conducted a prospective cohort study of dichorionic twin gestations as a part of the NICHD Fetal Growth Studies. Our objective was to empirically define the predominant trajectory of fetal growth in dichorionic twins using longitudinal two-dimensional ultrasonography and to compare and contrast the fetal growth trajectories for dichorionic twins with the singleton growth standard previously developed by our group.8
Materials and Methods
A prospective cohort study was conducted in which women with dichorionic twin gestations, irrespective of the mode of conception, maternal medical history or obesity status, were enrolled. Heterogeneity of the population’s characteristics in selection of pregnant women was important to maximize the possibility that factors associated with twin fetal growth could be examined and that the study conclusions would be more generalizable to the population of twins in the U.S. Women were enrolled between 8w0d and 13w6d. Accurate dating was required for enrollment. Thus, the ultrasound estimate of gestational age had to match the last menstrual period (LMP)-based gestational age (for the larger twin) according to the following criteria: 1) LMP-date and ultrasound date matched within five days for gestation estimates between 8w0d and 10w6d; 2) six days for those between 11w0d and 12w6d; and 3) seven days for those between 13w0d and 13w6d. For women with an in vitro fertilization (IVF) conception, a calculated LMP was determined using the date of transfer and embryo age at transfer. Ultrasound determination of chorionicity was established at the initial ultrasound examination. The pregnancy was classified as dichorionic if two gestational sacs were present with a thick intervening membrane and twin peak or lambda sign. If chorionicity was unable to be determined, the patient was determined to be ineligible. Chorionicity was confirmed by ultrasound at the subsequent visit. Information on chorionicity was also abstracted from the clinical placenta pathology report.
Inclusion criteria were: maternal age 18 to 45 years and anticipated delivery at the participating hospital. Study participants were excluded if fetal reduction was planned, or if the first trimester sonogram indicated congenital anomalies (structural or chromosomal), increased nuchal translucency (3.5 mm or more) in either twin, monochorionic twins, or crown-rump length discordance >10% because there is an increased risk of adverse perinatal outcomes with these conditions.9, 10 Recruitment began February 1, 2012 and continued through January 31, 2013, with final data collection completed on September 30, 2014. Institutional Review Board approval was obtained for the NICHD and all participating clinical institutions, and the data and imaging coordinating centers.
A standardized ultrasound protocol was developed and sonographers underwent extensive training and credentialing to ensure high quality ultrasound images. All study scans were performed on Voluson E8 (GE Healthcare; Milwaukee, WI) machines with standard operating procedures specified. Study data were collected into a customized application of the ViewPoint (GE Healthcare) clinical system with modifications to meet the study goals and electronically uploaded into a web-based data collection system designed for study purposes using Clinical Trial Processor (CTP) software from the Radiological Society of North America. Measurements were subsequently manually entered into a web-based data collection system.
After written informed consent was obtained, women underwent an enrollment visit. The initial study ultrasound was scheduled between 11w0d and 13w6d. First trimester parameters included crown-rump length, biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), humerus length (HL), femur length (FL), placental location, determination of chorionicity and twin designation. Study Twin 1 was defined and labelled as the fetus closest to the cervical os. In-person interviews were conducted to obtain information on sociodemographic characteristics, medical, reproductive and pregnancy histories, and health and lifestyle behaviors. Women were then randomized to receive sonograms according to one of the following two schedules: schedule A: 16, 20, 24, 28, 32 and 35 weeks or schedule B: 18, 22, 26, 30, 34 and 36 weeks. Women were expected to have a sonogram scheduled within ±1 week of the targeted gestational age. Women were randomized to one of two schedules to ensure adequate representation of gestational weeks for statistical modeling. Care was undertaken to allow the research ultrasounds to report to the clinical side if needed, recognizing the high risk status of twin pregnancies. Follow-up sonograms included core biometric measurements (BPD, HC, AC, FL). The HC/AC ratio was calculated for examination of trends in growth patterns over time. Estimated fetal weight (EFW) was calculated using the Hadlock formula, that incorporates HC, AC, and FL.11
Several steps were performed to maintain consistency of study twin designation. At each follow up visit, a series of identifying information was collated at the end of the examination including fetal sex, identifying anatomic differences, placental location and cord insertion, fetal position, size, location, and presentation/lie. The list was auto-populated for each fetus A or B. The sonographer then assigned each twin to the corresponding Study Twin Identification number (1 or 2) based on information from the prior sonogram. At delivery, care was taken to match same-sex twins with their designation in the longitudinal component of the study using an established protocol: a designation form was completed that collected information on birth date and time, neonatal sex, birth weight, presentation at birth (vertex, breech or unable to determine), whether an external or internal version performed prior to delivery, and identifying anatomic differences. Placental samples, or buccal swabs if the placenta was not available, were obtained for zygosity determination using standard single tandem repeat identifier kits (Applied Biosystems® AmpFLSTR® Identifiler® PCR Amplification Kit), for all same-sex twin pairs.
A 10% random sample of ultrasound images was selected for quality control review of core biometrics (CRL, BPD, HC, AC, FL) using a similar approach as the NICHD Fetal Growth Studies – Singletons.12 The correlation between the expert reviewer and site sonographer was ≥88% for all parameters across visits, with 21 out of 26 measures having a correlation of ≥95%, suggesting excellent reliability.
Statistical Analysis
In the descriptive analysis, baseline and clinical data were compared for participants by study cohort using Chi-square or t-tests for categorical or continuous data, respectively. All serial ultrasound data were used to estimate fetal individual parameters, the HC/AC ratio, and EFW by gestational age. Ultrasonography measurements (BPD, HC, AC, HL, FL), the HC/AC ratio and EFW were log-transformed to stabilize variances across gestational ages and to improve normal approximations for the error structures.
The primary analysis compared fetal growth trajectories for dichorionic twins with 1,737 singleton gestations included in the NICHD Fetal Growth Studies – Singleton standard.8 Briefly, the singleton standard enrolled low-risk women and excluded women with certain pregnancy complications or infants with neonatal conditions such as anomalies, aneuploidy and death. For the twin cohort, all of the data that women contributed to the study were included until they were censored by deactivation, pregnancy loss or delivery. For modeling twin trajectories, we used linear mixed models with a cubic spline mean structure and a random effects structure that included linear, quadratic, and cubic random effects for the twin pair and an intercept term for the individual fetus within twin pair.13 This hierarchical random effect structure incorporates correlation for both twin-pair and fetus-within-twin pair in the modeling. The linear mixed models were also used to test for overall differences (i.e., global tests) between the twin and singleton trajectories (for EFW and other measurements) using likelihood ratio tests of interaction terms between spline mean structure terms and twin-singleton indicator variables. For the cubic spline mean structure, three-knot points (25th, 50th and 75th percentiles) were chosen at gestational ages that evenly split the distributions. For an overall comparison between twin and singleton fetal trajectories, the singleton standards were weighted to have the same distribution of race/ethnicity as in the twin sample. For those ultrasound measurements where there were significant global tests for differences between twins and singletons, we tested for week-specific differences using Wald tests computed at each gestational age. These tests were conducted on the estimated curves with and without adjustments for maternal characteristics: age in years, race/ethnicity, height (cm) and pregravid weight (kg), parity, full-time employment/student status (yes/no), marital status (married/living as married versus not), health insurance (private/managed versus Medicaid/other), income (≤$29,999, $30,000–49,999, $50,000–$74,999, $75,000–$99,999, and ≥$100,000), education (< high school, high school, some college, college undergraduate and postgraduate college) and infant sex (male or female). We used multiple imputation (with 20 imputations) to account for missing covariates when performing covariate-adjusted tests for week-specific twin versus singleton differences in fetal growth curves.14
Since decreased BPD despite similar HC measurements in twins compared with singletons has previously been reported,15 we examined breech position in relation to BPD by including breech position as a time-dependent covariate in the linear mixed model described earlier. This analysis addresses the question of whether the mean BPD measurement changes due to the breech position of the fetus.
Lastly, we evaluated the degree of reclassification in small for gestational age (SGA), defined as below the 10th percentile, that would be introduced if fetal growth estimation for twins was based upon an unweighted singleton non-Hispanic white standard similar to our previous study.8
To assess the robustness of the findings in a low risk cohort of twins, we performed a sensitivity analysis limited to fetuses from women without any preexisting or obstetric diseases (e.g. gestational diabetes, hypertensive disorders) who were not smoking or drinking during pregnancy, and who delivered at or later than 37 weeks of gestation. We also repeated the EFW comparison to the singleton standard limited to dizygotic twin pregnancies, defined at birth as unlike-sex or same-sex pairs with dizygosity confirmed by placental pathology. Finally, we compared EFW trajectories for twin pregnancies conceived spontaneously, by IVF (excluding donor eggs or embryos) or by other medically assisted reproductive techniques.
A post hoc power analysis was performed and demonstrated that we had extremely high power to detect differences. Specifically, using an approximate mean singleton birth weight at 35 weeks of gestation (the average age of delivery of twins) of 2800 g, standard deviation of 440 g16, and an assumed conservative 10% increase in standard deviation for twins, we had 95% power for detecting a 5% difference in weight (as a proxy of trajectories) between the cohort of 1731 singletons in the standard and our cohort of 171 pairs of dichorionic twins, and a 2-tail p-value at <0.05. Further, the above calculations are extremely conservative since they use a single estimate at birth rather than longitudinal trajectories.
All analyses were implemented using SAS (version 9.4, SAS Institute, Inc., Cary, North Carolina) or R (version 3.1.2, http://www.R-project.org). Significance was defined by a two-tail P<.05.
Results
There were 171 women with dichorionic twins recruited into the study, of whom 152 (88.9%) delivered two live born infants (Table 1 and Figure 1). The flow diagram for study participants is presented in Figure 1. Women underwent a median of five ultrasounds. The women with dichorionic twins were primarily non-Hispanic white (54.5%), followed by non-Hispanic black (21.1%), Hispanic (19.3%) and Asian (4.7%) with an average age of 31.6 (±6.1) years and pre-pregnancy BMI of 28.6 (±7.0) kg/m2 (Table 1). The majority of women in the cohort had a college or postgraduate education, a family income of $75,000 or greater, and private or managed health care insurance, and was employed. Conception was spontaneous in 53.2% of the dichorionic twin pregnancies, while 26.9% were conceived by IVF without donor eggs or embryos, 7.6% by IVF with donor eggs or embryos, 5.8% by intrauterine insemination (IUI) and 6.4% by ovulation induction without IVF or IUI. With regard to complications, 5.8% of women had chronic hypertension, 1.8% had pre-gestational diabetes, and 38.0 % had a pre-pregnancy BMI ≥ 30 kg/m2. The mean (SD) age at delivery was 35.1 (4.3) weeks of gestation. There were 15 (8.8%) monozygotic, 133 (77.8%) dizygotic, and 23 (13.5%) unknown zygosity due to the reasons listed in Table 1. Placental pathology information was available for 137 (80.1%) twin pairs and resulted as 134 (97.8%) dichorionic and 3 (2.2%) monochorionic.
Table 1.
Maternal characteristics at enrollment by number of fetuses and pregnancy characteristics, NICHD Fetal Growth Studies: Twin Gestations (N=171).
| Maternal Characteristic | n (%) |
|---|---|
| Race/ethnicity | |
| White/non-Hispanic | 93 (54.5) |
| Black/non-Hispanic | 36 (21.1) |
| Hispanic | 33 (19.3) |
| Asian | 8 (4.7%) |
| Multiracial | 1 (0.6%) |
| Age, years – Mean (±SD) | 31.6 (±6.1) |
| Gravidity | |
| 1 | 51 (29.8) |
| 2 | 59 (34.5) |
| 3+ | 32 (18.7) |
| Parity | |
| 0 | 96 (56.1) |
| 1 | 54 (31.6) |
| 2+ | 21 (12.3) |
| Maternal height, measured (cm) – Mean (±SD) | 165.1 (±6.4) |
| Prepregnancy weight, self-reported (kg) - Mean (±SD) | 75.4 (±20.2) |
| Prepregnancy BMI (kg/m2) | |
| < 25.0 | 67 (39.2) |
| 25.0 – 29.9 | 39 (22.8) |
| 30.0+ | 65 (38.0) |
| Mean (±SD) | 28.6 (±7.0) |
| Marital status | |
| Never married | 32 (18.7) |
| Married/living as married | 135 (78.9) |
| Divorced/Separated/Widowed | 4 (2.3) |
| Education | |
| < High school | 12 (7.0) |
| High school | 22 (12.9) |
| Some college | 29 (17.0) |
| College undergraduate | 70 (40.9) |
| Postgraduate college | 38 (22.2) |
| Family income: | |
| ≤$29,999 | 35 (20.5) |
| $30,000–49,999 | 9 (5.3) |
| $50,000–$74,999 | 14 (8.2) |
| $75,000–$99,999 | 18 (10.5) |
| ≥$100,000 | 79 (46.2) |
| Health insurance: | |
| Private/managed care | 117 (68.4) |
| Medicaid; other | 40 (23.4) |
| Self-pay | 3 (1.8) |
| Current student: | |
| Yes | 14 (8.2) |
| No | 157 (91.8) |
| Number of current paid jobs: | |
| 0 | 37 (21.6) |
| 1 | 126 (73.7) |
| ≥2 | 8 (4.7) |
| Smoked cigarettes in the past 6 months | |
| Yes | 26 (15.2) |
| No | 144 (84.2) |
| Missing | 1 (0.6) |
| Frequency of alcoholic beverages in the past week | |
| 5 or more times | 0 |
| 2–4 times | 1 (0.6) |
| Once | 3 (1.8) |
| Not at all | 167 (97.7) |
| Conception by ovulation stimulation drugs or assisted reproductive technology | |
| In vitro fertilization (IVF) | 46 (26.9) |
| Intrauterine insemination (IUI) | 10 (5.8) |
| Medications without IVF or IUI | 11 (6.4) |
| Donor eggs, donor embryos | 13 (7.6) |
| None of the above | 91 (53.2) |
| Medical Diseases | |
| Hypothyroid | 11 (6.4) |
| Hyperthyroid | 0 |
| Pregestational Diabetes | 3 (1.8) |
| Asthma | 15 (8.8) |
| Chronic hypertension | 10 (5.8) |
| Cardiovascular disease | 1 (0.6) |
| Anemia | 17 (9.9) |
| Kidney disease | 0 |
| Autoimmune disease | 6 (3.5) |
| Epilepsy | 1 (0.6) |
| HIV | 1 (0.6) |
| Eating disorder | 3 (1.8) |
| Mood disorder, psychiatric disorder, anxiety or depression | 17 (9.9) |
| Other medical condition | 27 (15.8) |
| Pregnancy Outcome (twin/twin) | |
| Live birth ≥ 20 weeks/live birth ≥ 20 weeks | 152 (88.9) |
| Live birth ≥ 20 weeks/fetal death ≥ 20 weeks | 3 (1.8) |
| Fetal death ≥ 20 weeks/fetal death ≥ 20 Weeks | 3 (1.8) |
| Fetal death ≥ 20 weeks/miscarriage < 20 Weeks | 1 (0.6) |
| Fetal death unknown GA/fetal death unknown GA | 1 (0.6) |
| Miscarriage <20 weeks/miscarriage <20 Weeks | 1 (0.6) |
| Miscarriage/unknown outcome | 1 (0.6) |
| Voluntary termination or fetal reduction/same | 1 (0.6) |
| Voluntary termination or fetal reduction/Unknown outcome | 3 (1.8) |
| Unknown outcome/unknown outcome | 5 (2.9) |
| Neonatal sex | |
| Singleton Male or Twins Male/Male | 45 (26.3) |
| Singleton Female or Twins Female/Female | 41 (24.0) |
| Twins Male/Female | 70 (40.9) |
| Twins Male/Unknowna | 1 (0.6) |
| Twins Unknown b | 14 (8.2) |
| Zygosity (same sex twins only) | |
| Monozygotic | 15 (8.8) |
| Dizygoticc | 133 (77.8) |
| Missing with same sex twinsd | 8 (4.7) |
| Missing neonatal sex and zygosity | 15 (8.8) |
Abbreviations: GA, gestational age; SD, standard deviation
Twin A was an antepartum fetal death, and twin B was a miscarriage < 20 weeks.
Included 11 women who deactivated from the study, 2 women with live birth of both twins but neonatal sex not recorded, and 1 pregnancy with no chart review performed so neonatal outcome and sex unknown.
Includes 70 male and female twin pairs and 63 same sex pairs that resulted as dizogotic by zygosity testing.
Zygosity testing not performed due to miscarriage/demise (n=4), participant refusal (n=3), or failed DNA extraction due to inadequate sample (n=1)
Figure 1. Flow diagram for study participants in the NICHD Fetal Growth Studies - Twin Gestations.
All data from women were included in the analysis up until the time that they had an event (e.g. delivery, deactivation, etc.).
aGestational age (range) in weeks in which the visits occurred.
bThe numbers in the parentheses indicate women who missed the visit.
cReasons for deactivation: Voluntary termination of both fetuses (n=1), Voluntary termination of one fetus (n=1), miscarriage of one fetus (n=1), moved (n=1), refusal to continue (n=1).
dReasons for deactivation: Voluntary termination of one fetus (n=2), miscarriage/stillbirth (intrapartum fetal death) of both twins (n=2), antepartum fetal death of both twins (n=1)
eReasons for deactivation: Moved (n=1)
fReasons for deactivation: Antepartum fetal death (n=1), refusal to continue (n=1)
Figure 2 presents the curves for EFW that include the 10th, 50th, and 90th percentiles for dichorionic twins and singletons included in the NICHD Fetal Growth Studies – Singleton standard. All percentiles for dichorionic twin fetal measurements by gestational age are provided in Table 2 along with significance testing for pairwise comparisons in Table 3. Significant differences were observed between the estimated fetal weight curves for dichorionic twins and singletons (Figure 2, global P<.001). The mean EFW for twins deviated from that of the singletons beginning at 32 weeks and continued to diverge through 38 weeks (Table 3). At 32 weeks, the EFW for the 10th, 50th and 90th percentiles were 1518, 1807, and 2151 g for dichorionic twins and 1636, 1912, and 2235 g for singletons in the weighted standard. At 35 weeks of gestation, the average age at delivery for twins, the mean EFW for twins was 191 g smaller than singletons (P<.001), and the 10th percentile was 220 g smaller than singletons (10th, 50th and 90th percentiles were 1960, 2376, and 2879 g for dichorionic twins and 2180, 2567, and 3022 g for singletons in the race/ethnicity weighted standard).
Figure 2. Distribution of estimated fetal weight by number of fetuses and gestation, NICHD Fetal Growth Studies – Twin Gestations.
Estimated 10th, 50th and 90th percentiles for fetal weight for dichorionic twin gestations and singleton gestations included in the standard, as estimated from linear mixed models with log-transformed outcomes and cubic splines. For an overall comparison between twins and singletons fetal trajectories, the singleton standards were weighted to have the same racial/ethnic distribution as observed in the twin cohort.
EFW, estimated fetal weight; GA, gestational age
Table 2.
Percentiles for dichorionic twin fetal anthropometric measurements by gestational age.
| Biparietal Diameter (mm) – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 13.6 | 14.2 | 14.9 | 15.6 | 16.3 |
| 12 | 17.2 | 17.9 | 18.8 | 19.7 | 20.5 |
| 13 | 20.9 | 21.7 | 22.7 | 23.8 | 24.7 |
| 14 | 24.5 | 25.5 | 26.6 | 27.8 | 28.9 |
| 15 | 28.0 | 29.1 | 30.4 | 31.6 | 32.9 |
| 16 | 31.4 | 32.6 | 33.9 | 35.3 | 36.6 |
| 17 | 34.6 | 35.8 | 37.3 | 38.8 | 40.2 |
| 18 | 37.8 | 39.1 | 40.6 | 42.2 | 43.7 |
| 19 | 40.9 | 42.3 | 43.9 | 45.6 | 47.1 |
| 20 | 43.9 | 45.4 | 47.1 | 48.8 | 50.5 |
| 21 | 46.9 | 48.5 | 50.2 | 52.1 | 53.8 |
| 22 | 49.8 | 51.4 | 53.3 | 55.2 | 57 |
| 23 | 52.7 | 54.4 | 56.3 | 58.3 | 60.1 |
| 24 | 55.5 | 57.3 | 59.3 | 61.3 | 63.3 |
| 25 | 58.3 | 60.1 | 62.2 | 64.4 | 66.4 |
| 26 | 61.0 | 62.9 | 65.1 | 67.3 | 69.4 |
| 27 | 63.7 | 65.6 | 67.9 | 70.2 | 72.4 |
| 28 | 66.2 | 68.2 | 70.6 | 73.0 | 75.3 |
| 29 | 68.6 | 70.7 | 73.1 | 75.7 | 78.0 |
| 30 | 70.8 | 73.0 | 75.5 | 78.2 | 80.6 |
| 31 | 72.8 | 75.1 | 77.7 | 80.4 | 82.9 |
| 32 | 74.7 | 77.0 | 79.7 | 82.5 | 85.1 |
| 33 | 76.4 | 78.8 | 81.5 | 84.4 | 87.1 |
| 34 | 77.9 | 80.4 | 83.2 | 86.1 | 88.9 |
| 35 | 79.3 | 81.9 | 84.8 | 87.8 | 90.6 |
| 36 | 80.7 | 83.3 | 86.3 | 89.4 | 92.3 |
| 37 | 82.0 | 84.7 | 87.8 | 91 | 93.9 |
| 38 | 83.3 | 86.1 | 89.3 | 92.6 | 95.7 |
| Head Circumference (mm) – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 52.8 | 55.0 | 57.5 | 60.1 | 62.5 |
| 12 | 65.4 | 68.0 | 70.9 | 74.0 | 76.8 |
| 13 | 78.3 | 81.3 | 84.7 | 88.2 | 91.5 |
| 14 | 91.3 | 94.5 | 98.3 | 102.3 | 105.9 |
| 15 | 103.9 | 107.5 | 111.6 | 115.9 | 119.9 |
| 16 | 116.1 | 120.0 | 124.4 | 129.0 | 133.3 |
| 17 | 128.0 | 132.1 | 136.8 | 141.7 | 146.2 |
| 18 | 139.9 | 144.2 | 149.1 | 154.2 | 158.9 |
| 19 | 151.6 | 156 | 161.2 | 166.5 | 171.4 |
| 20 | 163.1 | 167.7 | 173 | 178.5 | 183.6 |
| 21 | 174.4 | 179.2 | 184.7 | 190.3 | 195.5 |
| 22 | 185.5 | 190.4 | 196.0 | 201.8 | 207.2 |
| 23 | 196.3 | 201.4 | 207.2 | 213.2 | 218.7 |
| 24 | 207.1 | 212.3 | 218.2 | 224.3 | 230 |
| 25 | 217.7 | 223.0 | 229.1 | 235.4 | 241.2 |
| 26 | 228.0 | 233.5 | 239.8 | 246.3 | 252.2 |
| 27 | 238.1 | 243.8 | 250.2 | 256.9 | 263 |
| 28 | 247.7 | 253.6 | 260.3 | 267.1 | 273.4 |
| 29 | 256.9 | 262.9 | 269.8 | 276.9 | 283.4 |
| 30 | 265.4 | 271.7 | 278.8 | 286.1 | 292.9 |
| 31 | 273.3 | 279.7 | 287.1 | 294.7 | 301.6 |
| 32 | 280.4 | 287.1 | 294.7 | 302.6 | 309.8 |
| 33 | 286.9 | 293.8 | 301.7 | 309.8 | 317.3 |
| 34 | 292.7 | 299.9 | 308.1 | 316.4 | 324.2 |
| 35 | 298.0 | 305.4 | 313.8 | 322.5 | 330.5 |
| 36 | 302.8 | 310.4 | 319.1 | 328.1 | 336.4 |
| 37 | 307.0 | 315.0 | 324.0 | 333.3 | 341.9 |
| 38 | 310.9 | 319.1 | 328.5 | 338.3 | 347.2 |
| Abdominal Circumference (mm) – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 38.9 | 41.2 | 43.9 | 46.8 | 49.6 |
| 12 | 48.7 | 51.5 | 54.8 | 58.3 | 61.6 |
| 13 | 59.1 | 62.4 | 66.3 | 70.4 | 74.3 |
| 14 | 70.0 | 73.8 | 78.2 | 82.9 | 87.4 |
| 15 | 81.0 | 85.2 | 90.2 | 95.6 | 100.6 |
| 16 | 92.0 | 96.7 | 102.3 | 108.1 | 113.7 |
| 17 | 103.0 | 108.2 | 114.2 | 120.6 | 126.7 |
| 18 | 114.0 | 119.6 | 126.2 | 133.1 | 139.6 |
| 19 | 125.0 | 131.0 | 138 | 145.4 | 152.4 |
| 20 | 135.9 | 142.3 | 149.8 | 157.6 | 165 |
| 21 | 146.5 | 153.3 | 161.2 | 169.6 | 177.4 |
| 22 | 157.0 | 164.1 | 172.5 | 181.2 | 189.5 |
| 23 | 167.2 | 174.7 | 183.5 | 192.7 | 201.4 |
| 24 | 177.2 | 185.1 | 194.3 | 204.0 | 213.1 |
| 25 | 187.2 | 195.4 | 205.1 | 215.2 | 224.7 |
| 26 | 197.0 | 205.6 | 215.7 | 226.2 | 236.2 |
| 27 | 206.6 | 215.6 | 226.1 | 237.1 | 247.5 |
| 28 | 216.0 | 225.4 | 236.4 | 247.9 | 258.7 |
| 29 | 225.2 | 235.0 | 246.5 | 258.4 | 269.7 |
| 30 | 234.3 | 244.5 | 256.4 | 268.8 | 280.6 |
| 31 | 243.1 | 253.8 | 266.1 | 279.1 | 291.3 |
| 32 | 251.8 | 262.9 | 275.7 | 289.2 | 301.9 |
| 33 | 260.4 | 271.8 | 285.2 | 299.2 | 312.3 |
| 34 | 268.7 | 280.6 | 294.4 | 309.0 | 322.6 |
| 35 | 276.9 | 289.2 | 303.6 | 318.6 | 332.8 |
| 36 | 284.9 | 297.7 | 312.5 | 328.2 | 342.9 |
| 37 | 292.7 | 306.0 | 321.4 | 337.6 | 352.9 |
| 38 | 300.2 | 314.0 | 330.1 | 347.0 | 363.0 |
| Femur Length (mm) – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 3.5 | 3.8 | 4.1 | 4.4 | 4.8 |
| 12 | 5.6 | 6.0 | 6.5 | 7.0 | 7.5 |
| 13 | 8.3 | 8.8 | 9.5 | 10.2 | 10.9 |
| 14 | 11.2 | 11.9 | 12.8 | 13.7 | 14.5 |
| 15 | 14.3 | 15.2 | 16.1 | 17.2 | 18.2 |
| 16 | 17.4 | 18.3 | 19.4 | 20.6 | 21.7 |
| 17 | 20.3 | 21.4 | 22.6 | 23.9 | 25.1 |
| 18 | 23.3 | 24.4 | 25.7 | 27.1 | 28.4 |
| 19 | 26.2 | 27.4 | 28.8 | 30.3 | 31.6 |
| 20 | 29.0 | 30.3 | 31.8 | 33.3 | 34.8 |
| 21 | 31.7 | 33.0 | 34.6 | 36.2 | 37.8 |
| 22 | 34.2 | 35.6 | 37.3 | 39.0 | 40.6 |
| 23 | 36.6 | 38.1 | 39.8 | 41.7 | 43.4 |
| 24 | 38.9 | 40.5 | 42.3 | 44.3 | 46.1 |
| 25 | 41.1 | 42.8 | 44.7 | 46.8 | 48.7 |
| 26 | 43.2 | 45.0 | 47.1 | 49.3 | 51.3 |
| 27 | 45.3 | 47.2 | 49.4 | 51.7 | 53.9 |
| 28 | 47.3 | 49.3 | 51.6 | 54 | 56.3 |
| 29 | 49.2 | 51.3 | 53.7 | 56.3 | 58.7 |
| 30 | 51.1 | 53.3 | 55.8 | 58.5 | 61.0 |
| 31 | 52.9 | 55.2 | 57.8 | 60.6 | 63.2 |
| 32 | 54.7 | 57.1 | 59.8 | 62.6 | 65.3 |
| 33 | 56.5 | 58.9 | 61.6 | 64.6 | 67.3 |
| 34 | 58.2 | 60.6 | 63.5 | 66.4 | 69.3 |
| 35 | 59.8 | 62.3 | 65.2 | 68.3 | 71.1 |
| 36 | 61.3 | 63.9 | 66.9 | 70.0 | 73.0 |
| 37 | 62.8 | 65.4 | 68.5 | 71.8 | 74.8 |
| 38 | 64.0 | 66.8 | 70.1 | 73.5 | 76.7 |
| Humerus Length (mm) – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 3.5 | 3.8 | 4.1 | 4.5 | 4.8 |
| 12 | 5.9 | 6.3 | 6.8 | 7.3 | 7.8 |
| 13 | 8.7 | 9.3 | 10.0 | 10.7 | 11.4 |
| 14 | 11.8 | 12.5 | 13.4 | 14.4 | 15.3 |
| 15 | 14.9 | 15.8 | 16.8 | 17.9 | 18.9 |
| 16 | 17.8 | 18.8 | 19.9 | 21.1 | 22.3 |
| 17 | 20.5 | 21.6 | 22.8 | 24.1 | 25.4 |
| 18 | 23.2 | 24.3 | 25.6 | 27.0 | 28.3 |
| 19 | 25.8 | 26.9 | 28.3 | 29.7 | 31.1 |
| 20 | 28.2 | 29.4 | 30.9 | 32.4 | 33.7 |
| 21 | 30.5 | 31.8 | 33.3 | 34.8 | 36.3 |
| 22 | 32.7 | 34.0 | 35.5 | 37.1 | 38.6 |
| 23 | 34.7 | 36.1 | 37.7 | 39.3 | 40.8 |
| 24 | 36.7 | 38.1 | 39.7 | 41.4 | 43.0 |
| 25 | 38.5 | 40.0 | 41.7 | 43.5 | 45.1 |
| 26 | 40.2 | 41.8 | 43.6 | 45.4 | 47.2 |
| 27 | 41.9 | 43.5 | 45.4 | 47.4 | 49.2 |
| 28 | 43.5 | 45.2 | 47.1 | 49.2 | 51.1 |
| 29 | 44.9 | 46.7 | 48.8 | 51.0 | 53.0 |
| 30 | 46.4 | 48.2 | 50.4 | 52.7 | 54.8 |
| 31 | 47.7 | 49.7 | 52.0 | 54.3 | 56.6 |
| 32 | 49.1 | 51.1 | 53.5 | 55.9 | 58.2 |
| 33 | 50.3 | 52.4 | 54.9 | 57.4 | 59.9 |
| 34 | 51.5 | 53.7 | 56.2 | 58.9 | 61.4 |
| 35 | 52.7 | 54.9 | 57.6 | 60.3 | 62.8 |
| 36 | 53.8 | 56.1 | 58.8 | 61.6 | 64.2 |
| 37 | 54.8 | 57.2 | 60.0 | 62.9 | 65.6 |
| 38 | 55.8 | 58.2 | 61.1 | 64.1 | 66.9 |
| Estimated Fetal Weight (grams) – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 15 | 97.0 | 103.7 | 111.7 | 120.3 | 128.6 |
| 16 | 122.6 | 131.1 | 141.2 | 152.1 | 162.6 |
| 17 | 153.7 | 164.4 | 177.1 | 190.7 | 204.0 |
| 18 | 190.6 | 203.9 | 219.7 | 236.7 | 253.2 |
| 19 | 233.8 | 250.2 | 269.7 | 290.7 | 311.0 |
| 20 | 283.9 | 303.8 | 327.6 | 353.2 | 378.0 |
| 21 | 341.2 | 365.4 | 394.1 | 425.2 | 455.2 |
| 22 | 406.4 | 435.4 | 469.9 | 507.3 | 543.4 |
| 23 | 479.8 | 514.3 | 555.6 | 600.1 | 643.3 |
| 24 | 561.9 | 602.8 | 651.7 | 704.5 | 755.8 |
| 25 | 653.0 | 701.1 | 758.7 | 821.1 | 881.6 |
| 26 | 753.0 | 809.3 | 876.9 | 950.1 | 1021.2 |
| 27 | 861.8 | 927.5 | 1006.2 | 1091.7 | 1174.8 |
| 28 | 979.1 | 1055.1 | 1146.6 | 1245.9 | 1342.7 |
| 29 | 1104.2 | 1191.9 | 1297.5 | 1412.4 | 1524.6 |
| 30 | 1236.4 | 1337.0 | 1458.4 | 1590.8 | 1720.2 |
| 31 | 1374.7 | 1489.6 | 1628.5 | 1780.4 | 1929.2 |
| 32 | 1517.8 | 1648.4 | 1806.7 | 1980.2 | 2150.6 |
| 33 | 1664.2 | 1812.0 | 1991.6 | 2189.1 | 2383.5 |
| 34 | 1812.3 | 1978.8 | 2181.8 | 2405.7 | 2626.7 |
| 35 | 1960.3 | 2147.1 | 2375.5 | 2628.3 | 2878.6 |
| 36 | 2106.4 | 2314.9 | 2570.9 | 2855.1 | 3137.8 |
| 37 | 2248.1 | 2480 | 2765.9 | 3084.7 | 3402.9 |
| 38 | 2382.5 | 2639.8 | 2958.4 | 3315.5 | 3673.5 |
| Head Circumference / Abdominal Circumference – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 1.187 | 1.242 | 1.306 | 1.373 | 1.437 |
| 12 | 1.18 | 1.233 | 1.294 | 1.359 | 1.419 |
| 13 | 1.167 | 1.218 | 1.277 | 1.34 | 1.398 |
| 14 | 1.152 | 1.201 | 1.258 | 1.318 | 1.374 |
| 15 | 1.135 | 1.182 | 1.237 | 1.295 | 1.349 |
| 16 | 1.118 | 1.163 | 1.217 | 1.272 | 1.324 |
| 17 | 1.102 | 1.146 | 1.197 | 1.251 | 1.301 |
| 18 | 1.088 | 1.131 | 1.181 | 1.233 | 1.282 |
| 19 | 1.076 | 1.118 | 1.167 | 1.217 | 1.265 |
| 20 | 1.065 | 1.107 | 1.155 | 1.204 | 1.251 |
| 21 | 1.057 | 1.097 | 1.144 | 1.193 | 1.239 |
| 22 | 1.049 | 1.089 | 1.136 | 1.184 | 1.23 |
| 23 | 1.042 | 1.082 | 1.128 | 1.177 | 1.222 |
| 24 | 1.036 | 1.076 | 1.122 | 1.17 | 1.215 |
| 25 | 1.031 | 1.071 | 1.116 | 1.164 | 1.209 |
| 26 | 1.026 | 1.066 | 1.111 | 1.159 | 1.203 |
| 27 | 1.021 | 1.06 | 1.106 | 1.153 | 1.198 |
| 28 | 1.015 | 1.055 | 1.100 | 1.148 | 1.192 |
| 29 | 1.009 | 1.048 | 1.094 | 1.141 | 1.186 |
| 30 | 1.002 | 1.041 | 1.086 | 1.134 | 1.178 |
| 31 | 0.993 | 1.033 | 1.078 | 1.125 | 1.169 |
| 32 | 0.984 | 1.023 | 1.068 | 1.115 | 1.159 |
| 33 | 0.973 | 1.012 | 1.057 | 1.103 | 1.147 |
| 34 | 0.962 | 1.001 | 1.045 | 1.092 | 1.135 |
| 35 | 0.951 | 0.989 | 1.033 | 1.079 | 1.122 |
| 36 | 0.939 | 0.977 | 1.021 | 1.067 | 1.11 |
| 37 | 0.928 | 0.966 | 1.009 | 1.055 | 1.098 |
| 38 | 0.916 | 0.954 | 0.998 | 1.044 | 1.087 |
| Femoral Length / Abdominal Circumference – Dichorionic Twin | |||||
|---|---|---|---|---|---|
| Gestational age, weeks | 10th | 25th | 50th | 75th | 90th |
| 11 | 0.079 | 0.086 | 0.093 | 0.102 | 0.11 |
| 12 | 0.102 | 0.11 | 0.119 | 0.129 | 0.139 |
| 13 | 0.124 | 0.133 | 0.143 | 0.154 | 0.165 |
| 14 | 0.142 | 0.152 | 0.163 | 0.175 | 0.187 |
| 15 | 0.157 | 0.167 | 0.179 | 0.191 | 0.203 |
| 16 | 0.168 | 0.178 | 0.190 | 0.202 | 0.214 |
| 17 | 0.176 | 0.186 | 0.197 | 0.210 | 0.222 |
| 18 | 0.182 | 0.192 | 0.204 | 0.216 | 0.228 |
| 19 | 0.187 | 0.197 | 0.208 | 0.221 | 0.232 |
| 20 | 0.190 | 0.200 | 0.212 | 0.224 | 0.236 |
| 21 | 0.193 | 0.203 | 0.214 | 0.226 | 0.238 |
| 22 | 0.195 | 0.204 | 0.216 | 0.228 | 0.239 |
| 23 | 0.196 | 0.205 | 0.217 | 0.229 | 0.240 |
| 24 | 0.196 | 0.206 | 0.217 | 0.229 | 0.241 |
| 25 | 0.196 | 0.206 | 0.218 | 0.23 | 0.241 |
| 26 | 0.196 | 0.206 | 0.218 | 0.23 | 0.242 |
| 27 | 0.196 | 0.206 | 0.218 | 0.23 | 0.242 |
| 28 | 0.196 | 0.206 | 0.218 | 0.23 | 0.242 |
| 29 | 0.196 | 0.206 | 0.218 | 0.23 | 0.242 |
| 30 | 0.195 | 0.205 | 0.217 | 0.23 | 0.242 |
| 31 | 0.195 | 0.205 | 0.217 | 0.229 | 0.241 |
| 32 | 0.194 | 0.204 | 0.216 | 0.229 | 0.241 |
| 33 | 0.194 | 0.204 | 0.216 | 0.228 | 0.240 |
| 34 | 0.193 | 0.203 | 0.215 | 0.227 | 0.239 |
| 35 | 0.193 | 0.203 | 0.214 | 0.227 | 0.238 |
| 36 | 0.192 | 0.202 | 0.213 | 0.226 | 0.238 |
| 37 | 0.191 | 0.201 | 0.213 | 0.225 | 0.237 |
| 38 | 0.190 | 0.200 | 0.212 | 0.225 | 0.236 |
Table 3.
Statistical significance (p-values) for dichorionic twin comparisons to the singleton standard of fetal anthropometric measurements by gestational age.
| Biparietal Diameter | Head Circumference | Abdominal Circumference | Femur Length | Humerus Length | Estimated Fetal Weight | Head Circumference / Abdominal Circumference | Femoral Length / Abdominal Circumference | |
|---|---|---|---|---|---|---|---|---|
| Gestational age, weeks | P-value | P-value | P-value | P-value | P-value | P-value | P-value | P-value |
| 11 | 0.008 | 0.085 | 0.017 | 0.883 | 0.016 | - | 0.683 | 0.052 |
| 12 | 0.284 | 0.609 | 0.101 | 0.162 | 0.726 | - | 0.342 | 0.001 |
| 13 | 0.630 | 0.635 | 0.486 | 0.050 | 0.036 | - | 0.289 | 0.002 |
| 14 | 0.172 | 0.367 | 0.844 | 0.136 | 0.029 | - | 0.354 | 0.058 |
| 15 | 0.064 | 0.313 | 0.964 | 0.555 | 0.178 | 0.469 | 0.427 | 0.460 |
| 16 | 0.028 | 0.312 | 0.944 | 0.668 | 0.876 | 0.340 | 0.499 | 0.736 |
| 17 | 0.010 | 0.260 | 0.908 | 0.300 | 0.537 | 0.317 | 0.537 | 0.343 |
| 18 | <0.001 | 0.134 | 0.951 | 0.446 | 0.709 | 0.413 | 0.499 | 0.456 |
| 19 | <0.001 | 0.070 | 0.996 | 0.861 | 0.813 | 0.582 | 0.461 | 0.799 |
| 20 | <0.001 | 0.069 | 0.987 | 0.943 | 0.624 | 0.738 | 0.488 | 0.981 |
| 21 | <0.001 | 0.118 | 0.939 | 0.966 | 0.717 | 0.857 | 0.580 | 0.948 |
| 22 | <0.001 | 0.248 | 0.876 | 0.693 | 0.984 | 0.936 | 0.725 | 0.776 |
| 23 | 0.004 | 0.474 | 0.819 | 0.409 | 0.620 | 0.979 | 0.895 | 0.574 |
| 24 | 0.015 | 0.710 | 0.788 | 0.244 | 0.371 | 0.994 | 0.947 | 0.427 |
| 25 | 0.039 | 0.866 | 0.784 | 0.181 | 0.254 | 1.000 | 0.825 | 0.353 |
| 26 | 0.080 | 0.944 | 0.778 | 0.170 | 0.207 | 0.972 | 0.763 | 0.339 |
| 27 | 0.152 | 0.971 | 0.736 | 0.187 | 0.198 | 0.891 | 0.784 | 0.384 |
| 28 | 0.279 | 0.982 | 0.630 | 0.209 | 0.205 | 0.731 | 0.912 | 0.494 |
| 29 | 0.504 | 0.992 | 0.450 | 0.204 | 0.205 | 0.493 | 0.836 | 0.671 |
| 30 | 0.862 | 0.931 | 0.252 | 0.166 | 0.190 | 0.252 | 0.519 | 0.911 |
| 31 | 0.681 | 0.829 | 0.110 | 0.121 | 0.169 | 0.096 | 0.257 | 0.794 |
| 32 | 0.302 | 0.711 | 0.041 | 0.090 | 0.154 | 0.030 | 0.106 | 0.507 |
| 33 | 0.113 | 0.625 | 0.015 | 0.076 | 0.143 | 0.009 | 0.040 | 0.305 |
| 34 | 0.048 | 0.603 | 0.007 | 0.068 | 0.124 | 0.004 | 0.014 | 0.187 |
| 35 | 0.031 | 0.675 | 0.003 | 0.059 | 0.092 | 0.002 | 0.004 | 0.119 |
| 36 | 0.043 | 0.872 | 0.003 | 0.067 | 0.075 | 0.002 | <0.001 | 0.100 |
| 37 | 0.133 | 0.840 | 0.008 | 0.165 | 0.132 | 0.004 | 0.003 | 0.184 |
| 38 | 0.459 | 0.621 | 0.054 | 0.421 | 0.343 | 0.022 | 0.028 | 0.400 |
P-values obtained by the Wald test from the linear mixed model with adjustment for maternal age, height, weight, parity, race, job, marital status, insurance, income, education and infant sex. Note: Estimated fetal weight not calculated less than 15 weeks of gestation.
The curves for the individual fetal measurements also were statistically significantly different between dichorionic twins and singletons (Figure 3, global P<.001 for all). The BPD was smaller in twins compared with singletons at 11 weeks, though larger than singletons at 16 through 25 weeks; most differences were 1 mm or less. The BPD was also smaller than in singletons beginning at 34 weeks through 36 weeks with differences of 2 mm or less. Breech twin fetuses had slightly larger BPD measurements than non-breech twins, regardless of whether the fetuses were assessed at 11 to 27 weeks (P=.002) or 28 to 38 weeks (P=.004). The AC was smaller in twins compared with singletons at 11 weeks with a difference of 1.4 mm. The AC also became statistically progressively smaller in twins beginning at 32 weeks of gestation and this effect continued through 37 weeks, reaching a mean difference of 12.1 mm at 37 weeks of gestation. Comparisons at each gestational age did not identify any statistically significant differences in HC or FL for twins compared with singletons. The HL in twins was also slightly shorter at 11 weeks and slightly longer from 13 to 14 weeks with differences of 0.3 mm or less. The mean HC/AC ratio declined for both twins and singletons over gestation, but was larger for twins beginning at 33 and continuing through 38 weeks of gestation suggesting a more asymmetrical pattern of growth compared with singletons. At 35 weeks of gestation, the mean HC/AC ratio in twins compared with singletons was 1.033 versus 1.005, respectively (P=.004). Male fetuses had an EFW that was higher than female fetuses; however, this differential was similar for twins and singletons.
Figure 3. Distribution of fetal anthropometric measurements by number of fetuses and gestation, NICHD Fetal Growth Studies – Twin Gestations.
Estimated 10th, 50th and 90th percentiles for the fetal anthropometric parameters for dichorionic twin gestations and singleton gestations included in the standard (a–e), as estimated from linear mixed models with log-transformed outcomes and cubic splines.
GA, gestational age
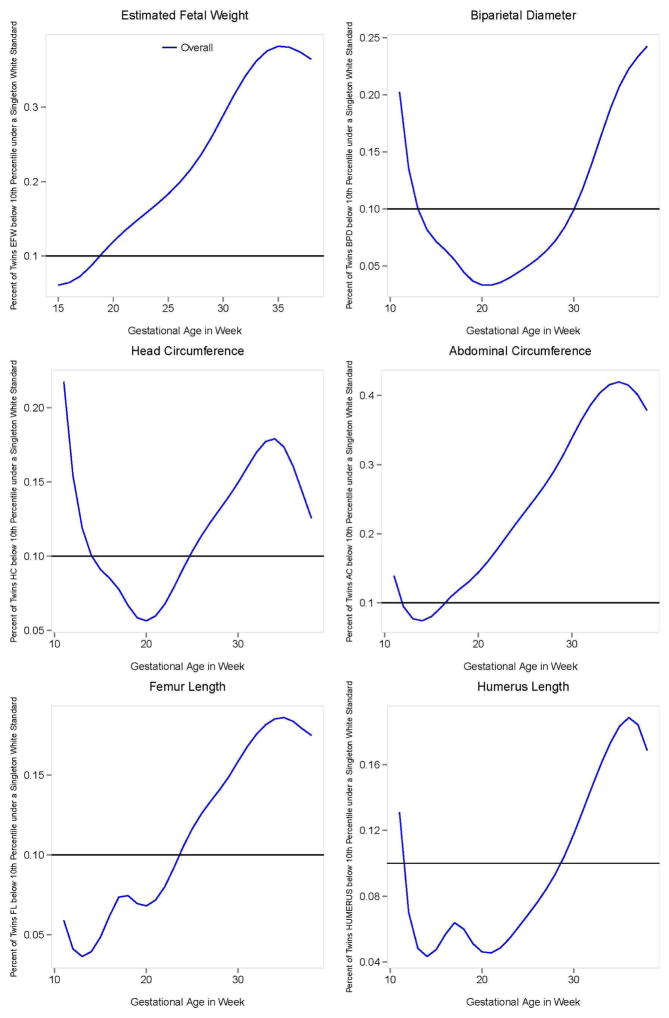
We evaluated the percentage of dichorionic twins who would be classified below the 10th percentile using the study-generated singleton non-Hispanic white standard (Figure 4). Beginning at 19 weeks of gestation, the percentage of dichorionic twins with an EFW classified below the 10th percentile exceeded 10%. For example, at 32 weeks of gestation, the time when the mean EFW for the twin average became smaller than the singletons, 34% of twins would be classified as below the 10th percentile. At 35 weeks of gestation, 38% of dichorionic twins would be classified as below the 10th percentile.
Figure 4. Percentage of dichorionic twin fetuses below the 10th percentile of the Non-Hispanic White singleton standard.
Percentage of twin fetuses below the 10th percentile of the Non-Hispanic White singleton standard by gestational age. The difference between the twin-specific curves and the 0.10 line reflect the amount of classification attributed to using the Non-Hispanic White singleton standard.
In a sensitivity analysis limited to 36 low-risk women with uncomplicated dichorionic twin pregnancies that delivered at 37 weeks of gestation or later, the pattern of increasing disparity in the mean EFW of twins compared with singletons persisted, beginning at 32 weeks and continuing through term (P=.002). When comparing EFW trajectories for 133 certain dizyogotic twins with the singleton standard, the results were similar to the main analysis (P<.001). Also, no statistically significant differences were observed in twin EFW growth trajectories based on method of conception (spontaneous, IVF or other assisted reproductive techniques).
Comment
In our prospective cohort study of dichorionic twin gestations, the EFW and AC differed from those of singletons beginning at 32 weeks of gestation through delivery. The BPD was slightly larger in twins earlier in mid-pregnancy but was smaller from 34 weeks through 36 weeks, while the HC remained similar to singletons. The femur length was not different in twins. The pattern of growth was more asymmetric in twins as characterized by the larger HC/AC ratio compared with singletons at beyond 32 weeks.
Our finding that the mean EFW deviated from that of singletons around 32 weeks is similar to studies that have used clinical ultrasound data and restricted to dichorionic twins. Min et al. found that the mean EFW for dichorionic twins became smaller than singletons around 30 weeks, although the mean EFWs from 30 to 38 weeks were lower than in our study, probably due to their inclusion of more minority women.17 Shivkumar et al. studied a Canadian population from a single hospital and found the mean EFW became smaller for dichorionic twins compared to a published singleton chart from Hadlock et al. starting at 32 weeks which is similar to our sensitivity analysis of low risk dichorionic twins.18, 19 The mean twin EFWs in our study were generally lower than another U.S. prospective study of 35 twins published in 1987.5 These dissimilar results may be at least partly due to differences in study populations (as ours focused on a more contemporary population), accuracy of measurements including shorter measurements with newer ultrasound machines due to a narrower beam width20 or other potential confounders. In the present study, a standardized ultrasound protocol was implemented to ensure high quality measurements and confirmed by our quality control analysis. Importantly, we directly compare dichorionic twin fetuses to singletons from healthy pregnancies using novel methods for the estimation of growth and EFW.
Compared with singletons, dichorionic twins had a smaller AC and EFW beginning at 32 weeks of gestation but similar HC and long bones. These findings are consistent with neonatal anthropometric data where HC and length of twins were found to be comparable to singletons at birth.21 Compared with appropriate for gestational age singletons, birth weights for concordant twins have been found to be lower but the HC and body length to be similar.22 Our findings are also similar to a prospective ultrasound study at a single U.S. center of 103 concordant twin pairs (albeit chorionicity and maternal race were not reported) that were compared to published singleton references.23 It has previously been reported that dolicocephalic breech twins have smaller BPDs but similar HC, a phenomenon attributed to a compression effect from fetal crowding.15,18 This theory is supported by the observation of dolichocephaly being associated with both breech presentation and oligohydramnios.24, 25 Interestingly, in our study, twin fetuses in the breech presentation had larger BPD’s compared with non-breech twins, indicating that breech presentation did not explain the differences between the smaller BPD in twins compared to singletons beginning at 32 weeks of gestation.
The HC/AC ratio has been described as a way to distinguish early-onset, symmetric growth restriction, associated with factors such as aneuploidy and intrauterine infections, from later onset, asymmetric cases, associated with placental insufficiency.26, 27 Our finding that the HC/AC ratio in twins became larger than singletons starting at 33 weeks is consistent with a constrained and comparatively asymmetric growth pattern in twins.
The progressively asymmetrical pattern of slower growth in dichorionic twins is consistent with the concept that the intrauterine environment is less capable of sustaining adequate growth in twin fetuses as the pregnancy progresses.28 Maternal constraint is a known phenomenon whereby the genetic potential of fetal growth is not fully achieved, such as that associated with short stature and nulliparity.29 In one study of twin growth estimated at 28 weeks of gestation, fetuses with a HC, AC or FL below the 10th percentile were shorter in childhood at age three years and those with AC or FL below the 10th percentile had evidence for stunting at age three years as indicated by lower weight for age percentiles and z-scores compared with normally grown twin fetuses.30 Reassuringly, there were no differences in mental or motor development. Long-term surveillance is needed to determine whether twin neonates that are born smaller than gestational age-matched singletons but within a normal reference range of birthweight, experience adverse future health sequelae.
The sporadic minimal twin-singleton differences indicate that the differences in the mean between the two groups are highly statistically significant; however, these small differences may not be detectable on an individual level or have clinical meaning. We acknowledge the differences could be due to unmeasured confounding, but may support a biological effect. Blickstein reviewed birth weight data and concluded that since the combined birth weight of the pair of fetuses in twins exceeds that of the average singleton, that the entire twin pregnancy represents more of a growth promotion.31 Perhaps the maternal body is primed early on to recruit additional resources for multiple fetuses, which may explain the larger measurements of some parameters in twins compared with singletons earlier in gestation.
Our rate of 97.8% confirmation of dichorionic twins when placental pathology was available is consistent with what has been reported in the literature, 95.6% at a single site and 77.3–100% in other studies.32 The few monochorionic twins in the series did not account for the differences found between the singletons and twins. The major strengths of our study were its prospective design with longitudinal measurement of fetal size, systematic attention to twin designation, and implementation of a standardized ultrasound protocol to ensure high quality measurements (as reflected in our quality control analysis). We also used ante hoc credentialing of sonographers and carefully considered relevant covariates in our analyses.
Given the high percentage of twins that are classified as SGA using a singleton non-Hispanic white standard, it could be argued that our findings indicate the need for an ultrasound reference that is specific for twins. However, the clinical challenge is to differentiate SGA associated with the normal adaptive process in multiple gestations from fetal growth restriction that is associated with increased morbidity and mortality.28 Future studies with long term follow up are needed to determine whether dichorionic twin fetuses in otherwise uncomplicated pregnancies that are classified as SGA using a singleton standard are at increased risk for short or long term morbidity. In the short term, careful consideration should be given prior to intervening for a small EFW percentile based on a singleton standard in the setting of normal testing such as umbilical artery Doppler, amniotic fluid volume, non-stress testing and biophysical profile.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract Numbers: HHSN275200800013C; HHSN275200800002I; HHSN27500006; HHSN275200800003IC; HHSN275200800014C; HHSN275200800012C; HHSN275200800028C; HHSN275201000009C)
The authors acknowledge Drs. Jun Zhang and Roberto Romero for their earlier efforts in helping to develop the study protocol, Dr. Karin Fuchs for her assistance with the credentialing of sonographers and the research teams at all participating clinical centers, including Christina Care Health Systems, Columbia University, Northwestern University, University of Alabama at Birmingham, University of California, Irvine, Medical University of South Carolina, and Women and Infants Hospital of Rhode Island. The authors also acknowledge C-TASC and The EMMES Corporations in providing data and imaging support for this multi-site study, Hanyun Li for her programming support and Dr. Stefanie Hinkle for her comments on earlier versions of this work. This work would not have been possible without the assistance of GE Healthcare Women’s Health Ultrasound for their support and training on the Voluson and Viewpoint products over the course of this study.
Footnotes
Disclosure: The authors report no conflict of interest.
This research was presented as a platform presentation at the Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Denver, CO, June 16, 2015.
Disclaimer: K.L. Grantz, J. Grewal, P.S. Albert, S. Kim, C. Zhang, and G.M. Buck Louis are U.S. federal government investigators; please see accompanying cover sheet.
Reprints will not be available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2014. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64:1–64. [PubMed] [Google Scholar]
- 2.Luke B, Brown MB. The changing risk of infant mortality by gestation, plurality, and race: 1989–1991 versus 1999–2001. Pediatrics. 2006;118:2488–97. doi: 10.1542/peds.2006-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petterson B, Nelson KB, Watson L, Stanley F. Twins, triplets, and cerebral palsy in births in Western Australia in the 1980s. BMJ. 1993;307:1239–43. doi: 10.1136/bmj.307.6914.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander GR, Kogan M, Martin J, Papiernik E. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clinical obstetrics and gynecology. 1998;41:114–25. doi: 10.1097/00003081-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Yarkoni S, Reece EA, Holford T, O’Connor TZ, Hobbins JC. Estimated fetal weight in the evaluation of growth in twin gestations: a prospective longitudinal study. Obstet Gynecol. 1987;69:636–9. [PubMed] [Google Scholar]
- 6.Liao AW, de Brizot ML, Kang HJ, Assuncao RA, Zugaib M. Longitudinal reference ranges for fetal ultrasound biometry in twin pregnancies. Clinics (Sao Paulo) 2012;67:451–5. doi: 10.6061/clinics/2012(05)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. The New England journal of medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449 e1–49 e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahado-Singh RO, Wapner R, Thom E, Zachary J, Platt L, Mahoney MJ, et al. Elevated first-trimester nuchal translucency increases the risk of congenital heart defects. Am J Obstet Gynecol. 2005;192:1357–61. doi: 10.1016/j.ajog.2004.12.086. [DOI] [PubMed] [Google Scholar]
- 10.Kalish RB, Gupta M, Perni SC, Berman S, Chasen ST. Clinical significance of first trimester crown-rump length disparity in dichorionic twin gestations. Am J Obstet Gynecol. 2004;191:1437–40. doi: 10.1016/j.ajog.2004.06.101. [DOI] [PubMed] [Google Scholar]
- 11.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151:333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 12.Hediger ML, Fuchs K, Grantz KL, Grewal J, Kim S, Gore-Langton RE, et al. Ultrasound Quality Assurance (QA) for Singletons in the NICHD Fetal Growth Studies. Journal of Ultrasound in Medicine. 2016 doi: 10.7863/ultra.15.09087. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro JC, Bates DM. Mixed-effects models in S and S-plus. New York: Springer Science - Business Media New York; Number of pages. [Google Scholar]
- 14.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reece EA, Yarkoni S, Abdalla M, Gabrielli S, Holford T, O’Connor TZ, et al. A prospective longitudinal study of growth in twin gestations compared with growth in singleton pregnancies. I. The fetal head. J Ultrasound Med. 1991;10:439–43. doi: 10.7863/jum.1991.10.8.439. [DOI] [PubMed] [Google Scholar]
- 16.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 17.Min SJ, Luke B, Gillespie B, Min L, Newman RB, Mauldin JG, et al. Birth weight references for twins. Am J Obstet Gynecol. 2000;182:1250–7. doi: 10.1067/mob.2000.104923. [DOI] [PubMed] [Google Scholar]
- 18.Shivkumar S, Himes KP, Hutcheon JA, Platt RW. An ultrasound-based fetal weight reference for twins. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 20.Okland I, Bjastad TG, Johansen TF, Gjessing HK, Grottum P, Eik-Nes SH. Narrowed beam width in newer ultrasound machines shortens measurements in the lateral direction: fetal measurement charts may be obsolete. Ultrasound Obstet Gynecol. 2011;38:82–7. doi: 10.1002/uog.8954. [DOI] [PubMed] [Google Scholar]
- 21.Socol ML, Tamura RK, Sabbagha RE, Chen T, Vaisrub N. Diminished biparietal diameter and abdominal circumference growth in twins. Obstet Gynecol. 1984;64:235–8. [PubMed] [Google Scholar]
- 22.Crane JP, Tomich PG, Kopta M. Ultrasonic growth patterns in normal and discordant twins. Obstet Gynecol. 1980;55:678–83. [PubMed] [Google Scholar]
- 23.Grumbach K, Coleman BG, Arger PH, Mintz MC, Gabbe SV, Mennuti MT. Twin and singleton growth patterns compared using US. Radiology. 1986;158:237–41. doi: 10.1148/radiology.158.1.3510021. [DOI] [PubMed] [Google Scholar]
- 24.Kasby CB, Poll V. The breech head and its ultrasound significance. British journal of obstetrics and gynaecology. 1982;89:106–10. doi: 10.1111/j.1471-0528.1982.tb04674.x. [DOI] [PubMed] [Google Scholar]
- 25.Levine D, Kilpatrick S, Damato N, Callen PW. Dolichocephaly and oligohydramnios in preterm premature rupture of the membranes. J Ultrasound Med. 1996;15:375–9. doi: 10.7863/jum.1996.15.5.375. [DOI] [PubMed] [Google Scholar]
- 26.Crane JP, Kopta MM. Prediction of intrauterine growth retardation via ultrasonically measured head/abdominal circumference ratios. Obstet Gynecol. 1979;54:597–601. [PubMed] [Google Scholar]
- 27.Rosso P, Winick M. Intrauterine growth retardation. A new systematic approach based on the clinical and biochemical characteristics of this condition. J Perinat Med. 1974;2:147–60. doi: 10.1515/jpme.1974.2.3.147. [DOI] [PubMed] [Google Scholar]
- 28.Blickstein I. Is it normal for multiples to be smaller than singletons? Best practice & research Clinical obstetrics & gynaecology. 2004;18:613–23. doi: 10.1016/j.bpobgyn.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiological reviews. 2014;94:1027–76. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luke B, Brown MB, Hediger ML, Nugent C, Misiunas RB, Anderson E. Fetal phenotypes and neonatal and early childhood outcomes in twins. Am J Obstet Gynecol. 2004;191:1270–6. doi: 10.1016/j.ajog.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Blickstein I. Normal and abnormal growth of multiples. Seminars in neonatology : SN. 2002;7:177–85. doi: 10.1053/siny.2002.0105. [DOI] [PubMed] [Google Scholar]
- 32.Lee YM, Cleary-Goldman J, Thaker HM, Simpson LL. Antenatal sonographic prediction of twin chorionicity. Am J Obstet Gynecol. 2006;195:863–7. doi: 10.1016/j.ajog.2006.06.039. [DOI] [PubMed] [Google Scholar]