Abstract
Background
Nonhuman primate models are critical for understanding mechanisms underlying human psychopathology. We established a non-human primate model of anxious temperament (AT) for studying the early-life risk to develop anxiety and depression. Studies have identified the central nucleus of the amygdala (Ce) as an essential component of AT’s neural substrates. Corticotropin-releasing hormone (CRH) is expressed in the Ce, has a role in stress, and is linked to psychopathology. Here, in young rhesus monkeys, we combined viral vector technology with assessments of anxiety and multimodal neuroimaging to understand the consequences of chronically increased CRH in the Ce-region.
Methods
Using real-time intraoperative MRI-guided convection-enhanced delivery, 5 monkeys received bilateral dorsal amygdala Ce-region infusions of adeno-associated virus serotype 2 (AAV2) containing the CRH construct. Their cage-mates served as unoperated controls. AT, regional brain metabolism, “resting” fMRI, and diffusion tensor imaging (DTI) were assessed before and two months after viral infusions.
Results
Dorsal amygdala CRH overexpression significantly increased AT and metabolism within the dorsal amygdala. Additionally, we observed changes in metabolism in other AT-related regions, as well as in measures of functional and structural connectivity.
Conclusion
This study provides a translational roadmap that is important for understanding human psychopathology by combining molecular manipulations used in rodents with behavioral phenotyping and multimodal neuroimaging measures used in humans. The results indicate that chronic CRH overexpression in primates not only increases AT, but also affects metabolism and connectivity within components of AT’s neural circuitry.
Keywords: central nucleus of the amygdala, AAV2, MRI-guided neurosurgery, FDG-PET, fMRI, DTI
INTRODUCTION
Nonhuman primate models are critical for understanding mechanisms underlying the development and expression of human psychopathology (1, 2). This is supported by the remarkable correspondence between nonhuman and human primates in brain structure and function that underlies their similarities in behavior, rearing methods, psychosocial development, and cognition (2, 3). The imperative to identify new molecular treatment targets to treat psychiatric disorders (4, 5), along with the evolutionary linkage between nonhuman and human primates, provides a compelling rationale to develop techniques in nonhuman primates that can alter the function of candidate genes in a targeted brain-region selective manner. Furthermore, the ability to use multimodal imaging methods to understand the impact of region-specific molecular manipulations in a relevant primate model allows for understanding the mechanisms associated with altered neural circuits in psychiatric illnesses.
To date, our nonhuman primate studies have focused on establishing a model for investigating mechanisms underlying the development of extreme early life anxiety (2, 6–15). First, we developed and standardized the no eye contact (NEC) condition of the human intruder paradigm to assess individual trait-like differences in anxiety-related behavior in response to potential threat (16). This trait-like disposition, termed anxious temperament (AT), is a prominent childhood risk factor for the later development of anxiety disorders, depression, and comorbid substance abuse (17–21). With our nonhuman primate model, we identified the altered neural circuitry that underlies the development of AT and found that it is similar to that observed in humans with anxiety disorders (3, 8, 10, 22). This neural systems level information provides the critical groundwork for directly testing regionally specific molecular hypotheses potentially important in the pathophysiology of AT.
When extreme, AT or its major behavioral component, behavioral inhibition, markedly increase a child’s risk for the later development of stress-related psychopathology (17–19, 23). By combining measures of threat-related behavioral inhibition (increased freezing and decreased vocalizations) and pituitary-adrenal activation (threat-induced cortisol), along with 18fluoro-deoxyglucose positron emission tomography (FDG-PET) in rhesus monkeys, we characterized a nonhuman primate developmental model of AT (6, 14). Although the AT neural circuit is distributed across prefrontal, limbic, and brain-stem regions, neuroimaging studies in monkeys and humans point to the dorsal amygdala, a region containing the central nucleus of the amygdala (Ce), as a fundamental component of the circuit (8, 10, 11) (see Figure 1). The Ce is of particular interest because it is the major outflow region of the amygdala with its downstream projections mediating the hypothalamic and brainstem contributions to the stress response (24, 25). The Ce is causally involved in mediating AT as neurotoxic lesions of the rhesus monkey dorsal amygdala, which encompassed the Ce, decreased the expression of behavioral inhibition and pituitary-adrenal activity (26).
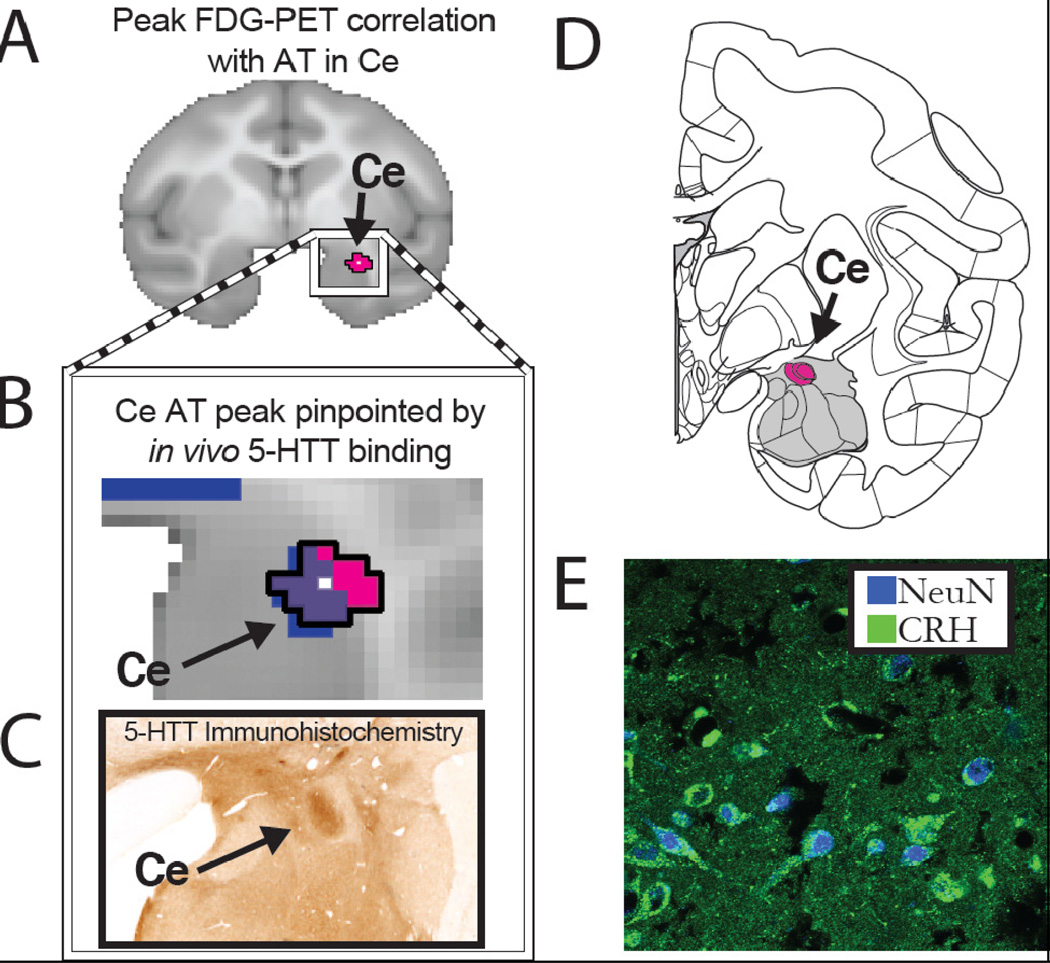
Figure 1. Endogenous CRH expression in the AT-related Ce-region.
(A) Using FDG-PET imaging, we found that metabolism within the Ce predicted individual differences in AT in young rhesus monkeys (reprinted with permission (8). (B) The Ce region was defined by its overlap (purple) with serotonin transporter ligand binding determined with PET imaging (blue). (C) The serotonin transporter binding characterized by PET mirrors its neuroanatomical distribution observed with immunohistochemical methods (reprinted with permission (98). (D) Within this critical primate Ce region (adapted from (99) we found (E) a moderate amount of endogenous CRH immunoreactivity (green) located in neurons, as defined by the overlap with NeuN expression (blue).
The Ce is predominantly composed of GABAergic neurons that also contain numerous modulatory neuropeptides and receptors (27). Within the Ce there is expression of corticotropin-releasing hormone (CRH; Figure 1), its two receptors, and its binding protein (28–31). CRH containing neurons in the paraventricular nucleus of the hypothalamus (PVN) play a prominent role in mediating the stress-related pituitary-adrenal response. Interestingly, extra-hypothalamic brain CRH neurons, including those in the Ce, can be regulated differently (32) and are important in coordinating the autonomic, emotional, behavioral and cognitive components of the stress response (33, 34). Rodent studies have demonstrated that stress increases the expression of CRH in the PVN and Ce (35), whereas corticosterone administration decreases expression of CRH in the PVN while concomitantly increasing the expression of Ce CRH (32). In addition to its role in modulating adaptive stress responses, overactivity of the CRH system is hypothesized to be an important pathophysiological mediator of symptoms associated with anxiety and depressive disorders (36). Recent reports suggest that structural variation of genes in the CRH family may contribute to the expression and pathophysiology of human depression and anxiety disorders (37–43), as well as to extreme monkey AT and its associated altered brain metabolism (44).
In addition to the translational value of nonhuman primate models, it is important to emphasize that primates significantly differ from rodents in the distribution and organization of brain CRH systems (31). Rodent studies have used transgenic, viral vector and other neuronal modulatory strategies to model CRH molecular alterations hypothesized to underlie stress-related psychopathology (45–50). However, these mechanistic studies have not been translated to primate species. Therefore, using a viral vector gene delivery strategy to chronically increase the expression of CRH in the dorsal amygdala of young rhesus monkeys, we aimed to identify the role of amygdala CRH systems in the expression of primate anxiety.
Here, we demonstrate that regional chronic overexpression of a putative anxiogenic neuropeptide results in increased anxiety-like behaviors along with anxiety-related changes in primate brain function. Using a viral vector infused with convection-enhanced delivery and guided by real-time intraoperative MRI (RT-IMRI), we aimed to chronically overexpress CRH in the Ce-region of the amygdala. This approach was combined with multimodal functional and structural brain imaging to test the hypothesis that increased dorsal amygdala CRH would increase AT, as well as glucose metabolism in the Ce. To allow for new insights into how increased amygdala CRH may influence brain–wide neural alterations underlying stress-related psychopathology, we also examined the impact of chronic dorsal amygdala CRH overexpression on functional and structural connectivity with other relevant brain regions. This study provides a framework for further development of preclinical nonhuman primate strategies to evaluate novel, region-specific molecular targets for the treatment of human psychopathology.
METHODS AND MATERIALS
Overall study design
Behavioral measures of AT and glucose-based measures of brain metabolism (FDG-PET) were assessed during NEC before surgery and again approximately 2 months later in 5 CRH-overexpressing monkeys, and at similar intervals in their 5 matched unoperated controls. Additionally, MRI measures of structural connectivity with diffusion tensor imaging (DTI) and functional intrinsic connectivity with 'resting' fMRI, were acquired before and after surgery. Paired-sample t-tests controlling for age were used to test for group differences in post-pre measures that resulted from CRH overexpression (i.e., CRH group(post-pre) – Control group(post-pre)).
The surgeries were performed using RT-IMRI guidance to localize the target. To estimate the dispersion of AAV2-CRH we infused the viral vector concurrently with the MR visible marker Gadobenate dimeglumine (Gd, MultiHance, Bracco Diagnostics). To characterize the pattern of CRH expression, animals were euthanized approximately one year after surgery and immunohistochemical staining for CRH was performed approximately 10 months after the behavioral assessments.
Note that space limitations do not allow for a complete description of the methods in the body of the paper and much of the methodological detail concerning the surgery and brain imaging analyses (FDG-PET, fMRI, and DTI) can be found in the supplemental information that accompanies this paper.
Subjects
First, two cynomolgus monkeys (Macaca fascicularis) were used in pilot studies to: 1) demonstrate the effectiveness of the AAV2-CRH virus to direct CRH overexpression in vivo and 2) to visual endogenous CRH expression in the Ce (see supplemental information). Next, ten young male rhesus monkeys (Macaca mulatta; 1.76–2.63 years old, 2.77–5.25 kg) were used in the CRH overexpression experiment. These animals were screened with the human intruder paradigm, and were selected to be in the mid-range for freezing responses. Five animals received bilateral infusions (2 infusions per hemisphere) of AAV2-CRH into the dorsal amygdala Ce region. The other 5 non-operated control animals were age-matched and pair-housed with each of their corresponding transfected monkeys. Because of considerations related to performing unnecessary procedures on non-human primates, and because we have used non-operated controls to successfully examine lesion-induced effects on primate anxiety in previous studies (26, 51, 52), we chose to use non-operated cage-mates as control subjects. Animals were housed and cared for at the Harlow Center for Biological Psychology and the Wisconsin National Primate Research Center on a 12-h light/dark cycle, in a temperature and humidity controlled vivarium. For all imaging and surgical procedures, the animals were fasted overnight. The experiments were performed according to the federal guidelines of animal use and care (53) and with approval of UW-Madison IACUC committees.
Characterizing Anxious Temperament (AT) and glucose metabolism during NEC
AT was characterized using the NEC condition of the human intruder paradigm, and was computed as the combination of standardized freezing, cooing and cortisol measures (6, 14). NEC assessment coincided with the administration of FDG followed by µPET scanning (see supplemental information for further detail).
Real-Time Intraoperative MRI (RT-IMRI)
Placement of the MRI-compatible trajectory guide bases followed previously reported methods (see (54, 55) for details) modified for Ce targeting (see Figure 2). The intraoperative targeting was performed using a platform for real-time MR-guided prospective stereotaxy (56) that was initially developed by the University of Wisconsin (57–60). A detailed description of the RT-IMRI methods can be found in the supplemental information. The rhesus Ce is approximately 5 mm long in the A-P plane, and approximately 1–2 mm in the D-V and M-L planes. To cover as much of the Ce as possible while minimizing treatment to surrounding regions, two 12µl infusions were performed per hemisphere (one anterior Ce target, one posterior Ce target), for a total of 24µl per hemisphere. After each infusion the catheter was removed, and after all infusions were complete the animal was transported back to the surgical suite and the craniotomies were closed.
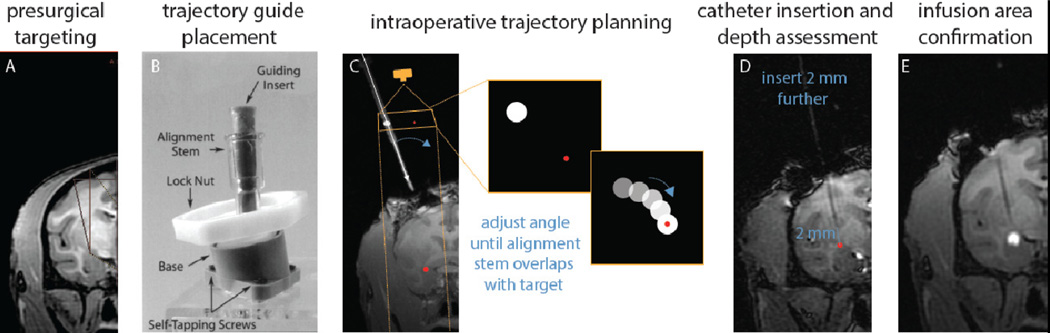
Figure 2. Real-time intraoperative MRI guided targeting and infusion monitoring.
(A) Prior to surgery a structural MRI was obtained to visualize the target and plan the trajectory. (B) A pivot point-based MRI compatible external trajectory guide was mounted on the skull (reprinted with permission (54). (C) Precise targeting was performed by imaging a plane orthogonal to the long axis of the external trajectory guide, as if it were visualized by a camera from above. The inset boxes represent the plane in which the trajectory guide (white dot) is visualized and aligned in relation to the target injection point (red dot). (D) The depth of the catheter is advanced to approximately 2 mm above the target. Another MRI was acquired to make precise measurements between the catheter tip and the target prior to advancing the catheter to its final position. (E) Immediately after the AAV2-CRH / gadolinium infusion was complete, a final MRI was acquired to verify the infusion delivery region.
Assessing CRH overexpression
Approximately 1 year after AAV2 infusions the animals were euthanized. The brains were extracted and fixed overnight in 4% paraformaldehyde, cryoprotected, and then sectioned at 40µm. CRH immunoreactivity was assessed as described in the supplemental information. 1:12 sections through the brain were immunostained for CRH. Adjacent coronal brain sections were processed for acetylcholinesterase (AChE), a cholinergic marker that facilitates anatomical identification of amygdala nuclei and subnuclei (61).
For each animal, the distribution of CRH-positive cells was charted through the rostrocaudal extent of the Ce region using camera lucida techniques. Sections were initially drawn under brightfield illumination at 1.6×, to include labeled cells and landmarks such as blood vessels and fiber tracks. The distribution of labeled cells was then confirmed under 10× brightfield illumination. Adjacent AChE-stained sections were then viewed under darkfield illumination, using landmarks in the charted sections for alignment. This permitted overlay of AChE-determined boundaries of amygdala nuclei and subnuclei for each section. Finally, paper maps were digitized using a drawing tablet in conjunction with the program Adobe Illustrator CS2 (Adobe Systems, San Jose, CA).
To compare the relative distribution of labeled cells resulting from viral-mediated CRH expression and/or endogenous CRH expression across animals, we overlaid individual maps from each animal upon one another in transparent layers, matching rostrocaudal levels as closely as possible. Labeled cells for each pair of animals was color-coded to assist in the analysis.
RESULTS
Characterization of CRH overexpression
Post mortem immunocytochemical analyses demonstrated marked overexpression of CRH in the dorsal amygdala region of the experimental animals compared to the levels of endogenous CRH in the control animals. The RT-IMRI Gd infusion scan from each animal was compared to the animal’s pattern of CRH overexpression defined by immunocytochemistry (Figure 3). As can be seen in Figure 4A, in all experimental animals CRH overexpression was evident in the dorsal amygdala including regions of the lateral and medial divisions of the Ce as well as in surrounding areas such as the dorsal regions of the accessory basal nucleus, the magnocellular region of the basal nucleus, the amygdala striatal transition zone, and portions of the ventral putamen (see Table 1). We found that across all transfected animals the extent and location of the infusions as determined by RT-IMRI matched the areas of CRH overexpression determined with immunocytochemistry (compare Figures 4A and 3E). This demonstrates the ability to use in vivo imaging methods to estimate the extent of infusion as well as AAV2-CRH transfection after one year.
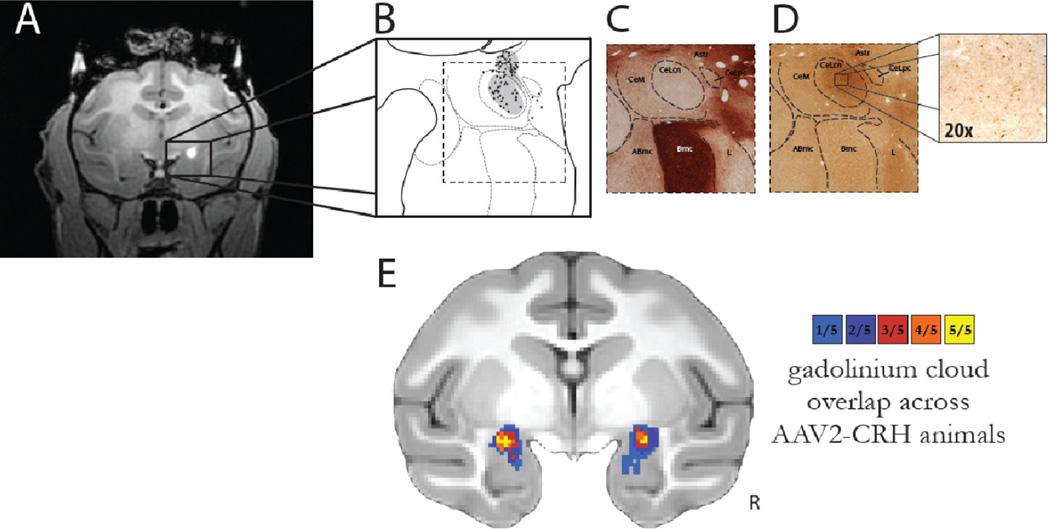
Figure 3. In vivo estimation and post mortem verification of dorsal amygdala CRH overexpression.
(A) The gadolinium clouds in the dorsal amygdala, Ce region, during and immediately following AAV2-CRH delivery provided an estimate of the location and extent of the infusions. (B) Camera lucida drawings of CRH expression from post mortem tissue reflected the extent of viral infusion as estimated from the intraoperative gadolinium signal. Gray regions in B represent neuropil staining and the black dots represent CRH overexpressing cells. (C) Acetylcholinesterase (AChE) staining defined the boundaries of the amygdalar nuclei. Note the relative absence of AChE staining in the CeL. (D) Adjacent sections were used for CRH immunohistochemistry demonstrating marked overexpression in the dorsal amygdala, Ce region. (E) Quantification of gadolinium diffusion extent and cross-subject overlap. Based on the intraoperative gadolinium images, we estimated the infusion extent in standard MRI space. This allows for an estimation of the across-subject overlap of CRH expression, and provides a link to the post mortem CRH expression data (compare to Figure 4A). The overlap of the gadolinium injection clouds across all 5 experimental animals demonstrates the replicability of the MRI-guided targeting procedure. The colors represent the number of animals with gadolinium signal at each voxel. Note the bilateral overlap across all experimental animals within the Ce-region (yellow). R = right.
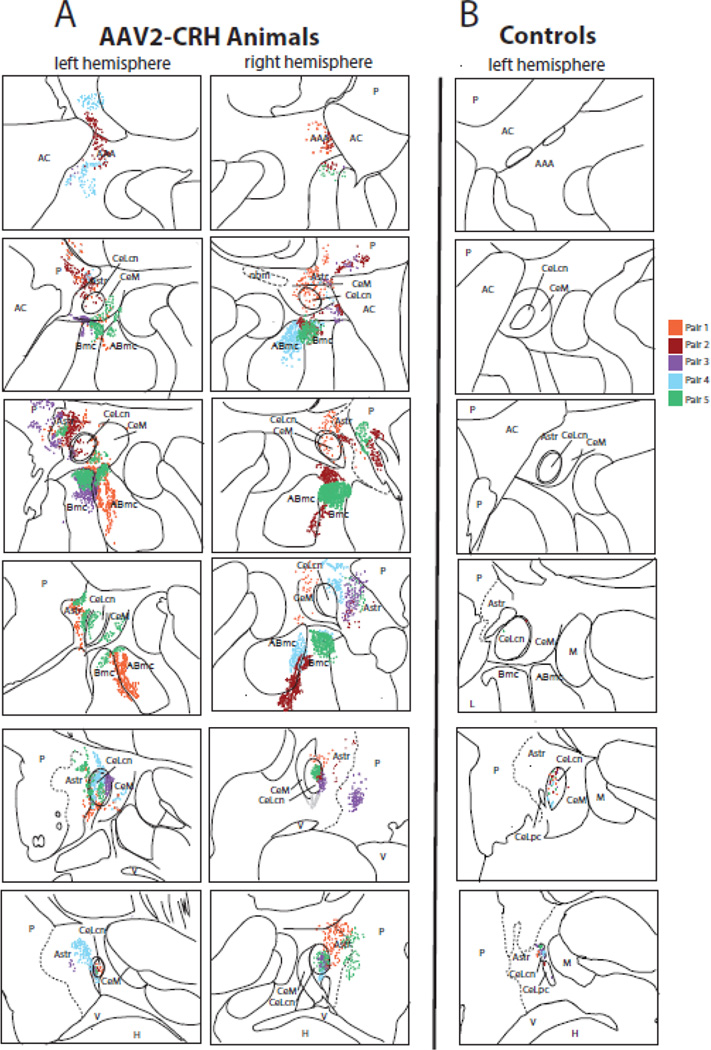
Figure 4. Quantification of dorsal amygdala CRH expression.
(A) Post mortem analyses demonstrated overexpression of CRH in the dorsal amygdala and surrounding regions in the experimental animals, compared to (B) the levels of endogenous CRH observed in the cage-mate control animals (top = anterior, bottom = posterior). Each pair of animals is represented by a different color in the composite image, and each dot represents a CRH expressing cell body. Note that endogenous CRH expression levels in controls were found in the most posterior regions of the Ce (only the left hemisphere is presented), and were substantially lower than that induced by AAV2-CRH transfection. Abbreviations: AAA, anterior amygdaloid area; ABmc, accessory basal nucleus, magnocellular subdivision; AC, anterior commissure; Astr, amygdalostriatal transition zone; Bmc, basal nucleus, magnocellular subdivision; CeLcn, central nucleus, lateral central subdivision; CeLpc, central nucleus, lateral paracapsular subdivision; CeM, central nucleus, medial subdivision; H, hippocampus; L, lateral nucleus; M, medial nucleus; nbm, nucleus basalis of Meynert; P, putamen; V, ventricle.
Table 1.
Relative density of CRH-labeled cells in both hemispheres, summing across all rostrocaudal levels.
| CASE | CeLcn/pc | CeM | Bmc | ABmc | Astr | ventral putamen |
|---|---|---|---|---|---|---|
| CRH-1 | ++ | ++ | ++ | +++++ | +++++ | +++ |
| CRH-2 | ++ | + | +++++ | ++++ | +++++ | +++++ |
| CRH-3 | +++ | + | +++++ | + | +++++ | +++++ |
| CRH-4 | +++ | ++ | + | +++++ | +++++ | + |
| CRH-5 | +++ | +++ | +++++ | +++++ | ++++ | +++++ |
| CON-1 | + | |||||
| CON-2 | + | |||||
| CON-3 | + | |||||
| CON-4 | + | |||||
| CON-5 | + |
Abbreviations: ABmc, accessory basal nucleus, magnocellular subdivision; Astr, amygdalostriatal transition zone; Bmc, basal nucleus, magnocellular subdivision; CeLcn/pc, central nucleus, lateral central subdivision/paracapsular subdivision; CeM, central nucleus, medial subdivision.
Effects of CRH overexpression on AT and brain metabolism
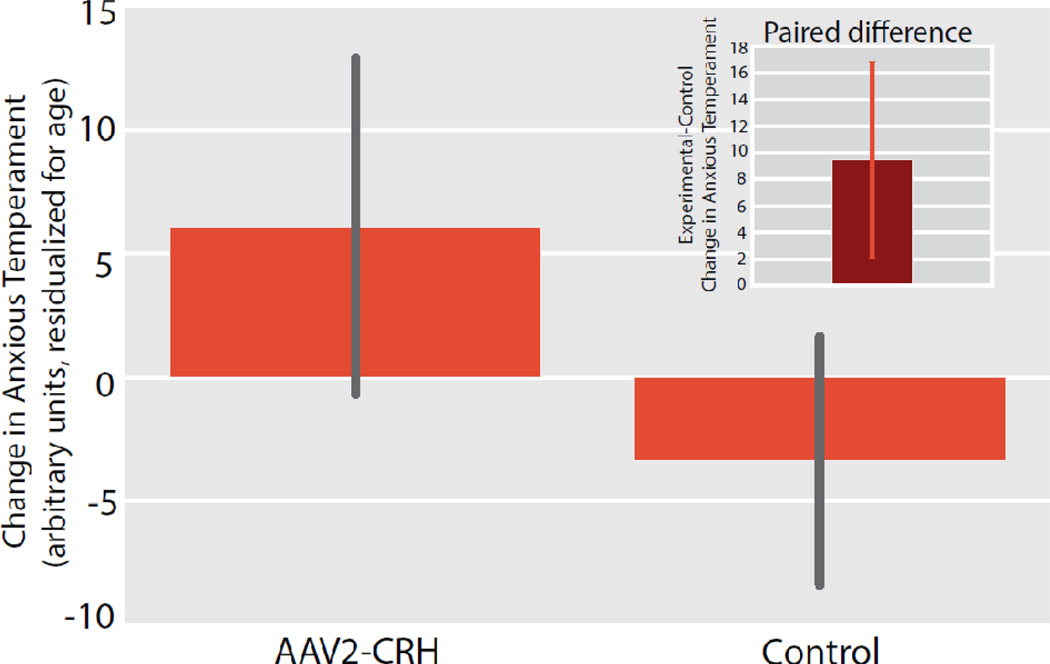
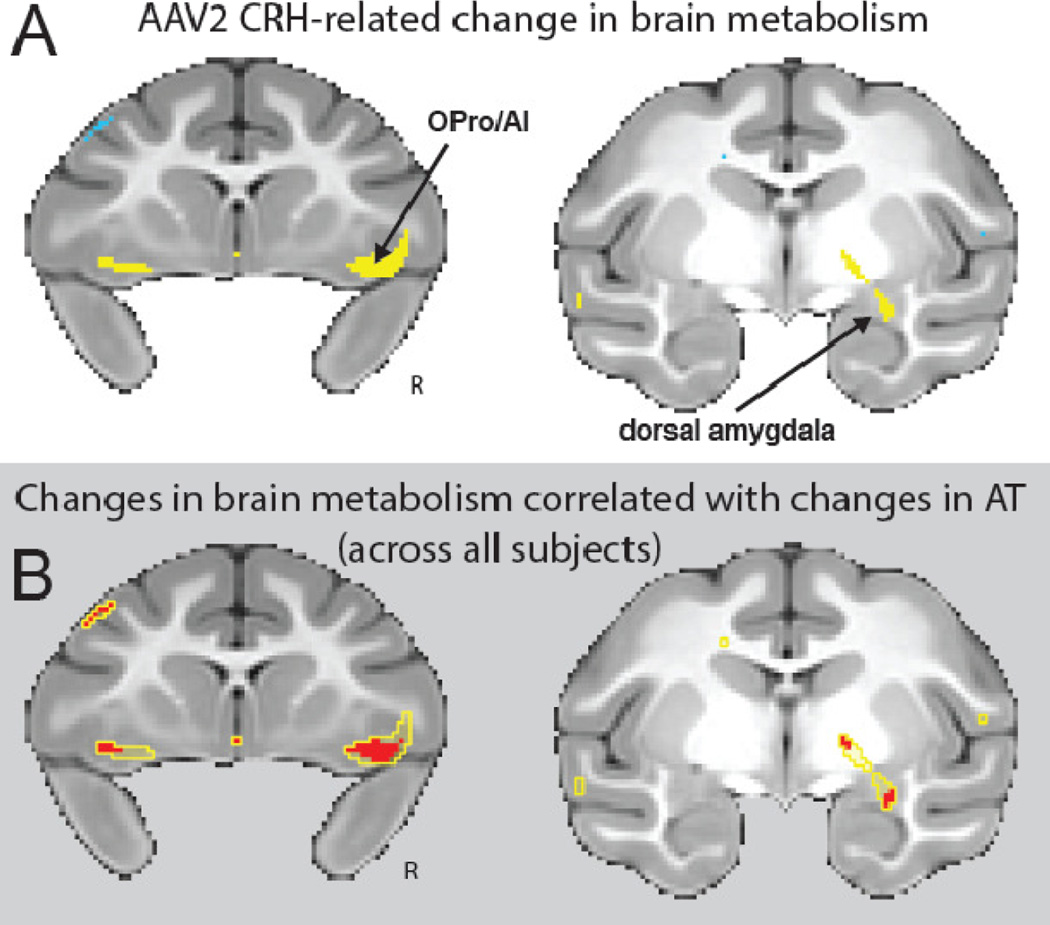
As predicted, compared to controls the CRH overexpressing animals demonstrated a significant increase in AT [CRH group(post-pre) – Control group(post-pre)] (p<.05, one-tailed, Figure 5). A complementary unpaired between-groups analysis (controlling for age as in the paired analysis) revealed significant group differences in the AT phenotype (t = 2.040, p = 0.0405, one-tailed; see supplemental Figure s3). It is noteworthy that CRH concentrations in cerebrospinal fluid (CSF) did not significantly differ between experimental and control animals (see supplemental information for methods). Corresponding analyses of FDG–PET data revealed that CRH overexpression also resulted in significant increases in dorsal amygdala metabolism (p<.01, uncorrected, Figure 6A). Additional whole-brain voxelwise analyses revealed that the CRH overexpression animals had significantly greater increases in metabolism in orbitofrontal/anterior insular cortices (OPro/AI), and hippocampus (p<.01, two-tailed, uncorrected; Figure 6A and supplemental Table s1). It is important to underscore that these regions have been implicated as part of the AT network as well as in human anxiety disorders. To identify regions across both control and experimental animals in which post-pre changes in AT were predicted by changes in metabolism, we looked within brain regions that demonstrated an effect of CRH overexpression Figure 6B, yellow outline). This analysis revealed that, regardless of treatment condition, changes in AT from the pre- to post-surgical assessment were predicted by individual differences in metabolic increases in regions of the dorsal amygdala, OPro/AI, and hippocampus (red, p<.05, two-tailed, uncorrected, Figure 6B and supplemental Table s2). These findings suggest that the effects of dorsal amygdala CRH overexpression on increasing AT involve activation of this distributed neural circuit.
Figure 5. CRH overexpressing animals demonstrated a significant increase in Anxious Temperament (AT).
Compared to their matched controls, the CRH overexpressing animals demonstrated increased post-surgical levels of AT (mean ± S.E.M). Significance was determined using a paired-samples t-test comparing dorsal amygdala CRH animals and their cage-mate controls [CRH group(post-pre) – Control group(post-pre)] (p<.05, one-tailed; see inset and methods for details).
Figure 6. CRH overexpressing animals demonstrated significant increases in brain metabolism.
(A) Compared to their matched controls, the CRH overexpressing animals demonstrated increased post-pre change in metabolism within the dorsal amygdala, orbitofrontal/insular cortex (OPro/AI), and hippocampus (yellow, p < 0.01, two-tailed uncorrected; and see Supplementary Table s1). All of these regions have been implicated in Anxious Temperament (AT). (B) Within regions that were affected by CRH overexpression (yellow outline), we identified areas in which the post-pre change in metabolism correlated with the post-pre change in AT (red; p < 0.05, one-tailed uncorrected). Across all 10 animals, changes in AT were associated with changes in brain metabolism in the dorsal amygdala and in bilateral regions of the posterior orbitofrontal/insular cortex (OPro/AI). R = right.
Effects of CRH overexpression on functional connectivity and white matter integrity
The fMRI data demonstrated that chronic CRH overexpression altered resting functional connectivity. The dorsal amygdala seed region for the functional connectivity analyses was determined by the overlap of the infusion area detected with Gd (see yellow region in Figure 3E) with that of the metabolic region that was affected by CRH overexpression (see yellow region in Figure 6A, right). Results demonstrated that CRH overexpression altered functional connectivity such that connectivity with the right dorsal amygdala seed in various regions differed between groups (p<.01, two-tailed, uncorrected, see supplemental Table s3). Of note, increased connectivity was found between right dorsal amygdala and regions encompassing bilateral portions of OPro/AI.
Analyses of the DTI data were performed to examine effects of long-term CRH overexpression on white matter microstructure. Voxelwise analyses were performed on measures of diffusivity and fractional anisotropy (FA). Investigation of mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) demonstrated significant alterations in various regions (see supplemental Tables s4–s6). Of particular interest, CRH overexpression in the dorsal amygdala was associated with significant increases in MD, AD and RD in a region of the brain that overlaps with the extended amygdala/bed nucleus of the stria terminalis (p < 0.005, two-tailed, uncorrected). Such an increase in MD, AD and RD can be indicative of decreased density of microstructure, but can also be explained by increased levels of CSF. Since this region is proximal to the ventricles this possibility should be considered.
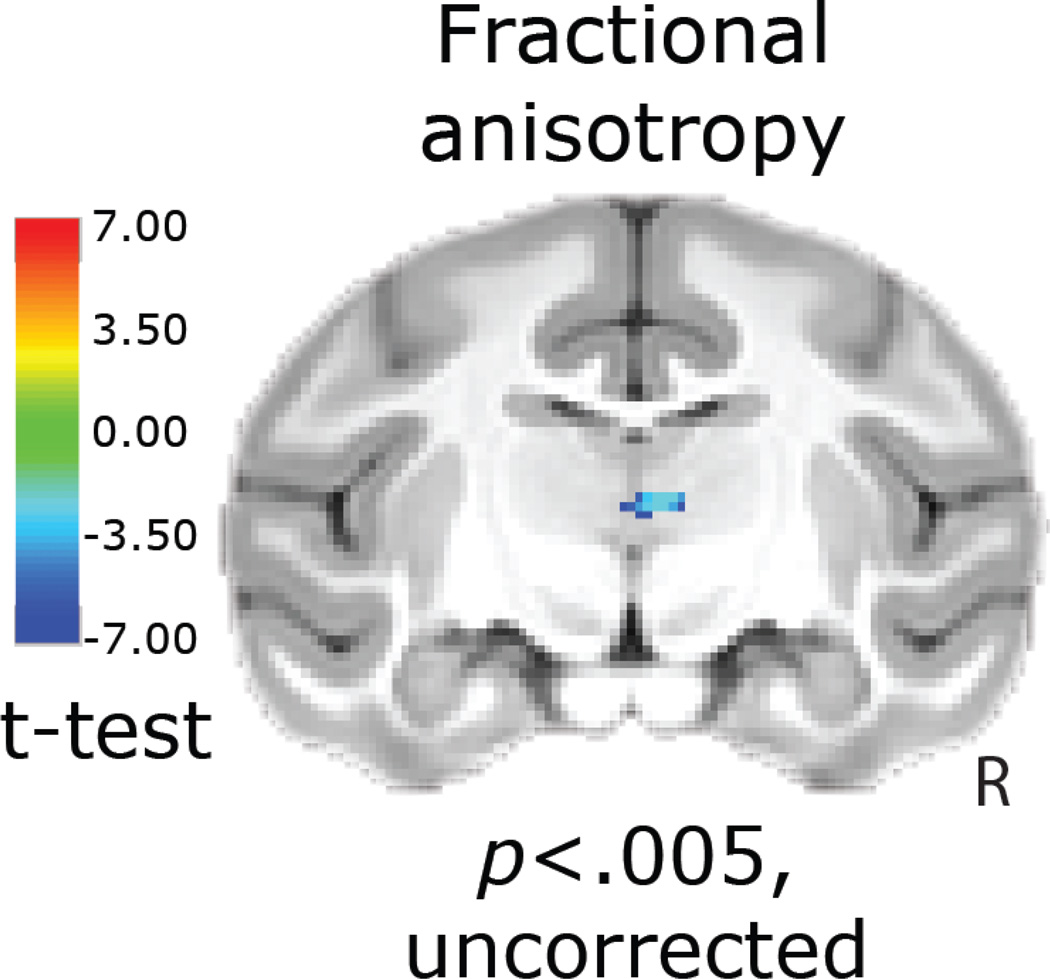
Analysis of FA, an overall indicator of white matter integrity, demonstrated that dorsal amygdala-CRH overexpression resulted in decreased FA in various regions including portions of the medial/midline thalamus (p < 0.005, two-tailed, uncorrected, Figure 7 and Supplemental Table s7). This region of medial thalamus encompasses the ventral edge of the medial dorsal thalamus, portions of the central medial and paracentral thalamic nuclei, as well as the magnocellular division of the ventral anterior thalamic nucleus. Fiber tractography enabled an investigation of the connectivity of this region with the rest of the brain (see supplementary information and supplemental Figure s4).
Figure 7. Diffusion tensor imaging demonstrated altered thalamic structural integrity as assessed with FA.
Whole-brain voxelwise analyses indicated CRH-induced reductions in FA in a region overlapping with the medial dorsal/midline thalamus [CRH group(post-pre) – Control group(post-pre)] (p < 0.005, uncorrected). R = right. Deterministic tractography demonstrates that this thalamic region is connected to the prefrontal cortex, and medial temporal lobe (see supplementary Figure s4).
Discussion
In this study, we validated methods combining RT-IMRI with convection-enhanced delivery to reliably locate and accurately infuse AAV2-CRH into the primate Ce region. This demonstrates the feasibility of translating rodent mechanistic studies that directly manipulate gene function in the brain to primates, and implicates overactive brain CRH systems in the pathophysiology of excessive primate anxiety. These findings further point to the dorsal amygdala, Ce region, as a key site involved in determining individual differences in dispositional anxiety and the phenotype that represents the risk to develop stress-related psychopathology. This study is the first to use viral vector strategies in non-human primates to directly manipulate CRH molecular systems hypothesized to be involved in human psychiatric disorders. Our primate model provides a unique opportunity to assess the effects of gene manipulation on primate behavior in conjunction with the same in vivo measures of brain function and structure that are used to assess human neuropsychiatric patients.
While much of the mechanistic work focused on the role of CRH in anxiety and fear has been performed in rodents, a few studies have been done in primates. Because of the similarities in brain function and structure, behavior, and social functioning between non-human primates and humans, rhesus monkeys provide an important and valuable model for studying human psychopathology. The marked difference in distribution of brain CRH receptors between rodents and primates further supports the use of primates for studies of stress-related psychopathology. For example, primates have both CRH R1 and CRH R2 in the Ce whereas the rodent Ce is only populated with CRH R1 (31, 62). Early rodent studies site-specifically administering CRH or CRH antagonists established a key role for the amygdala, including the Ce, as being important in mediating the effects of CRH on anxiety and fear responses (63–69). Other studies suggest that within the rodent Ce, it is likely that CRH acts via activation of CRH R1 (70–72). Additionally, mouse transgenic and knockout studies manipulating expression of CRH or CRHR1 demonstrated an important role for CRH systems in mediating adaptive and maladaptive behavioral and physiological responses to stress (for review see (73)). Because of the presence of CRH R2 receptors in the primate Ce, it is possible that the anxiogenic effects of CRH are mediated by activation of CRH R2 receptors.
Our early work in rhesus monkeys is consistent with results from the rodent studies. For example, we demonstrated that intraventricularly administered CRH increased anxiety when administered at low doses and at higher doses resulted in depressive-like behaviors (74). Because of the wide distribution of CRH receptors throughout the brain (31, 62, 75, 76), it is likely that these effects were mediated by activation of CRH receptors in diverse brain regions – a finding that is supported by brain-wide metabolic brain changes seen following very high doses of intraventricular CRH (77). In another study, we also reported a relation between CSF levels of CRH and threat-induced behavioral inhibition (78), however this finding has not been replicated (unpublished data). It is important to note that some, but not all, human studies demonstrate increased CSF CRH concentrations associated with depression and/or suicide (79) and post mortem analyses of suicide victims have revealed increased activity in brain CRH systems (80–84). Neurotoxic lesion studies in primates demonstrated that Ce lesions not only reduced anxiety but also decreased concentrations of CSF CRH (26). Thus, in the Ce lesion study, the reduction in anxiety could be accounted for by the global reduction in CSF CRH. In the current study, in which we use a viral vector strategy to overexpress CRH in the dorsal amygdala region, anxiety and brain function were affected in the absence of a detectable increase in CSF CRH levels. Taken together, these findings suggest that while CRH can have profound impacts throughout the brain, the Ce is a site that is critically involved in mediating these effects.
Recent viral vector studies in rodents have implicated the chronic overexpression of Ce CRH in inducing anxiety- and depression-related behaviors (46, 48, 49, 85, 86). In general, but not always, overexpression of Ce CRH is reported to affect physiological parameters such as the startle response and HPA activity (48, 49, 85, 86). Our current findings in nonhuman primates are consistent as we demonstrate that overexpression of CRH increases AT.
With the functional and structural brain imaging measures used in our study, we were able to extend the rodent studies by examining the impact of chronically increased dorsal amygdala CRH on brain metabolism, as well as functional and structural connectivity. These analyses provide potential insights into mechanisms underlying the neural circuit alterations associated with human anxiety and other stress-related psychopathologies. Our data are the first to demonstrate that an overactive amygdala CRH system has local effects on brain metabolic activity as well as on other components of the neural circuit associated with anxiety and AT. Specifically, we found that CRH overexpression resulted in increased metabolism in posterior regions of the orbitofrontal cortex (OPro), anterior insula (AI) regions, and hippocampus. Since the areas of overexpression encompassed Ce, other dorsal regions of the amygdala, and neighboring structures (e.g., putamen), it is possible that CRH expression in these different regions contributed to the observed effects. Additionally, we found evidence that in some cases the virus was anterogradely and/or retrogradely transported to other brain regions that are monosynaptically connected to the site of infusion.
We also found that dorsal amygdala CRH overexpression increased functional connectivity between this area and the OPro/AI region of posterior orbital cortex. The findings regarding the OPro/AI region may be particularly relevant as this region of the prefrontal cortex is highly connected with the amygdala (87). We recently demonstrated in a sample of 592 young rhesus monkeys that NEC-related glucose metabolism in the OPro/AI region (along with the bed nucleus of the stria terminalis (BST) and the periaqueductal gray (PAG)) correlated with AT and was heritable (10). Importantly, brain metabolism in these regions was also genetically correlated with AT, which implies the involvement of similar genes in mediating AT and altered brain function in OPro/AI, BST, and PAG.
Perhaps even more interesting are the structural brain changes that were associated with long-term increased CRH overexpression. Using measures of white matter integrity we found evidence for decreases in FA in the medial thalamus encompassing portions of the central medial thalamic nucleus, paracentral thalamic nucleus, and the magnocellular division of the ventral anterior thalamic nucleus. Primate studies demonstrate that these regions contain CRH-immunoreactive cell bodies and fibers as well as relatively high densities of CRH R1 (31, 88, 89). It is therefore possible that overexpression of CRH in the dorsal amygdala could lead to increased activation of medial thalamic CRH R1. Tractography methods demonstrated that white matter fibers link the thalamic region of significant FA change with other components of the neural network important in the expression and regulation of anxiety (see supplemental Figure s4). This is consistent with ex vivo tract tracing studies in macaques that demonstrate projections from the dorsal amygdala to midline thalamic nuclei (90, 91). Also, medial thalamic nuclei are reciprocally connected to posterior orbitofrontal cortex regions that include the OPro/AI (92). Thus, the medial/midline thalamus may link dorsal amygdala CRH to metabolic changes in the OPro/AI.
Early excitement related to developing new treatments for human anxiety and depression resulted from numerous mechanistic studies, mostly performed in rodents, directly manipulating brain CRH systems (for reviews see (93, 94)). Although findings from these studies failed to be translated to positive outcomes in human CRH R1 antagonist clinical trials (95–97), our current findings suggest that continued pursuit of mechanisms directed at altering Ce CRH function in primates might be useful for providing insights into optimizing CRH-altering treatments for human disorders. For example, it may be worth performing human studies targeting the CRH R2 receptor because of the possibility that CRH R2 could mediate the effects we observed. Our study also underscores the potential for gene delivery in primate models to elucidate the mechanisms of regional gene-expression on distributed brain function, as well as to explore novel treatment strategies for refractory psychiatric illnesses. Taken together these results indicate that chronically increased dorsal amygdala CRH expression influences AT, metabolic activity within AT’s neural substrates, as well as long-range functional connectivity and white-matter microstructure. This work, aimed at understanding the effects of increased CRH in the dorsal amygdala, will help motivate the design of novel interventions to prevent the development of anxiety disorders and other stress-related psychopathology.
Supplementary Material
Acknowledgments
We thank the personnel of the Harlow Center for Biological Psychology, the Health Emotions Research Institute, the Waisman Laboratory for Brain Imaging and Behavior, the Wisconsin National Primate Research Center, the Wisconsin Institutes for Medical Research, K. Brunner, A. Shackman, M. Jesson, L. Williams, D. Hsu, S. Shelton, H. Van Valkenberg, and N. Alcock. This work was supported by the National Institutes of Health grants R01-MH046729 to NHK, R01-MH63291 to JLF, R24-OD019803 to MEE, R43-CA177205 to ALA and WFB, and P51-OD011106 (Wisconsin National Primate Research Center, University of Wisconsin-Madison).
Drs. Alexander, Block and Brodsky are part owners of inseRT MRI, Inc., which provided technology that was used for navigation and monitoring of the infusion experiments. Dr. Kalin has received honoraria from CME Outfitters, Elsevier, and the Pritzker Neuropsychiatric Disorders Research Consortium. He is on the Advisory Boards for Corcept Therapeutics and Skyland Trail - George West Mental Health Foundation. Dr. Kalin is a Stockholder in Corcept Therapeutics, and owns several patents including: promoter sequences for corticotropin-releasing factor alpha (U.S. Patent #7071323, issued on 07-04-06); a method of identifying agents that alter the activity of the promoter sequences (U.S. Patent #7531356 issued on 05-12-09); promoter sequences for urocortin II and the use thereof (U.S. Patent #7087385 issued on 08-08-06); and promoter sequences for corticotropin-releasing factor binding protein and use thereof (U.S. Patent #7122650, issued on 10-17-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary versions of this work were presented in poster form at the 2014 Society for Neuroscience meeting in Washington, D.C., the 2014 American College of Neuropsychopharmacology meeting in Phoenix, Arizona, and at the 2015 Society of Biological Psychiatry meeting in Toronto, Ontario.
Financial Disclosures
Dr. Fox, Ms. Kovner, Ms. Riedel, Dr. Fekete, Dr. Roseboom, Ms. Tromp, Mr. Grabow, Mr. Olsen, Dr. McFarlin, Dr. Emborg, Dr. Fudge and Dr. Oler reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Nelson EE, Winslow JT. Non-human primates: model animals for developmental psychopathology. Neuropsychopharmacology. 2009;34:90–105. doi: 10.1038/npp.2008.150. [DOI] [PubMed] [Google Scholar]
- 2.Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- 3.Fox AS, Kalin NH. A Translational Neuroscience Approach to Understanding the Development of Social Anxiety Disorder and Its Pathophysiology. The American journal of psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel TR. Next-generation treatments for mental disorders. Sci Transl Med. 2012;4:155ps119. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- 5.Hyman SE. The unconscionable gap between what we know and what we do. Sci Transl Med. 2014;6:253cm259. doi: 10.1126/scitranslmed.3010312. [DOI] [PubMed] [Google Scholar]
- 6.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alisch RS, Chopra P, Fox AS, Chen K, White AT, Roseboom PH, et al. Differentially methylated plasticity genes in the amygdala of young primates are linked to anxious temperament, an at risk phenotype for anxiety and depressive disorders. J Neurosci. 2014;34:15548–15556. doi: 10.1523/JNEUROSCI.3338-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, et al. Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci U S A. 2015;112:9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci U S A. 2012;109:18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, et al. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29:9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roseboom PH, Nanda SA, Fox AS, Oler JA, Shackman AJ, Shelton SE, et al. Neuropeptide Y receptor gene expression in the primate amygdala predicts anxious temperament and brain metabolism. Biol Psychiatry. 2014;76:850–857. doi: 10.1016/j.biopsych.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox AS, Oakes TR, Shelton SE, Converse AK, Davidson RJ, Kalin NH. Calling for help is independently modulated by brain systems underlying goal-directed behavior and threat perception. Proc Natl Acad Sci U S A. 2005;102:4176–4179. doi: 10.1073/pnas.0409470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 17.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual review of psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 18.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. e1061. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladstone GL, Parker GB. Is behavioral inhibition a risk factor for depression? J Affect Disord. 2006;95:85–94. doi: 10.1016/j.jad.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- 22.Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. The American journal of psychiatry. 2010;167:40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 25.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 28.Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 32.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 33.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–472. [PubMed] [Google Scholar]
- 34.Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 35.Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 36.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 37.Hsu DT, Mickey BJ, Langenecker SA, Heitzeg MM, Love TM, Wang H, et al. Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene influences fMRI signal responses during emotional stimulus processing. J Neurosci. 2012;32:3253–3260. doi: 10.1523/JNEUROSCI.5533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Archives of general psychiatry. 2010;67:369–379. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- 40.Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- 41.Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, et al. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, Kraemer FB, et al. HPA axis genetic variation, cortisol and psychosis in major depression. Mol Psychiatry. 2014;19:220–227. doi: 10.1038/mp.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, et al. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18:700–707. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li XF, Hu MH, Li SY, Geach C, Hikima A, Rose S, et al. Overexpression of corticotropin releasing factor in the central nucleus of the amygdala advances puberty and disrupts reproductive cycles in female rats. Endocrinology. 2014;155:3934–3944. doi: 10.1210/en.2014-1339. [DOI] [PubMed] [Google Scholar]
- 47.Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, et al. Forebrain-specific CRF overproduction during development is sufficient to induce enduring anxiety and startle abnormalities in adult mice. Neuropsychopharmacology. 2014;39:1409–1419. doi: 10.1038/npp.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 49.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 54.Emborg ME, Joers V, Fisher R, Brunner K, Carter V, Ross C, et al. Intraoperative intracerebral MRI-guided navigation for accurate targeting in nonhuman primates. Cell transplantation. 2010;19:1587–1597. doi: 10.3727/096368910X514323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emborg ME, Hurley SA, Joers V, Tromp do PM, Swanson CR, Ohshima-Hosoyama S, et al. Titer and product affect the distribution of gene expression after intraputaminal convection-enhanced delivery. Stereotact Funct Neurosurg. 2014;92:182–194. doi: 10.1159/000360584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Truwit CL, Liu H. Prospective stereotaxy: a novel method of trajectory alignment using real-time image guidance. Journal of magnetic resonance imaging : JMRI. 2001;13:452–457. doi: 10.1002/jmri.1065. [DOI] [PubMed] [Google Scholar]
- 57.Brodsky EK, Block WF, Alexander AL, Emborg ME, Ross CD, Sillay KA. Intraoperative device targeting using real-time MRI; Biomedical Sciences and Engineering Conference (BSEC), 2011; 2011. pp. 1–4. [Google Scholar]
- 58.Grabow B, Block W, Alexander AL, Hurley S, CD R, Sillay K, et al. ISMRM Nineteenth Annual Scientific Meeting and Exhibition, poster presentation #1585. Melbourne, Australia: 2012. Extensible realtime MRI platform for intraoperative targeting and monitoring. [Google Scholar]
- 59.Grabow BP, Oler JA, Riedel M, Fekete EM, Kovner R, brodsky EK, et al. ISMRM Twenty-First Annual Scientific Meeting and Exhibition, oral presentation #672. Milan, Italy: 2014. Alteration of Molecular Neurochemistry: MRI-guided Delivery of Viral Vectors to the Primate Amygdala. [Google Scholar]
- 60.Brady ML, Raghavan R, Block W, Grabow B, Ross C, Kubota K, et al. The Relation between Catheter Occlusion and Backflow during Intraparenchymal Cerebral Infusions. Stereotact Funct Neurosurg. 2015;93:102–109. doi: 10.1159/000367665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: An immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- 62.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 63.Liang KC, Lee EHY. Intra-Amygdala Injections of Corticotropin Releasing Factor Facilitate Inhibitory Avoidance Learning and Reduce Exploratory Behavior in Rats. Psychopharmacology. 1988;96:232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- 64.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 65.Tazi A, Dantzer R, LeMoal M, Rivier J, Vale W, Koob GF. Corticotropin-Releasing factor Antagonist Blocks Stress-Induced Fighting in Rats. Regulatory peptides. 1987;18:37–42. doi: 10.1016/0167-0115(87)90048-6. [DOI] [PubMed] [Google Scholar]
- 66.Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-Releasing Factor Antagonist Reduces Emotionality in Socially Defeated Rats Via Direct Neurotropin Action. Brain Research. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- 67.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sajdyk TJ, Gehlert DR. Astressin, a corticotropin releasing factor antagonist, reverses the anxiogenic effects of urocortin when administered into the basolateral amygdala. Brain Res. 2000;877:226–234. doi: 10.1016/s0006-8993(00)02638-x. [DOI] [PubMed] [Google Scholar]
- 69.Jochman KA, Newman SM, Kalin NH, Bakshi VP. Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci. 2005;119:1448–1458. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- 70.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 71.Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 73.Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry. 2000;48:1175–1198. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- 74.Kalin NH, Shelton SE, Kraemer GW, McKinney WT. Corticotropin-releasing factor administered intraventricularly to rhesus monkeys. Peptides. 1983;4:217–220. doi: 10.1016/0196-9781(83)90117-1. [DOI] [PubMed] [Google Scholar]
- 75.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 1998;286:459–468. [PubMed] [Google Scholar]
- 77.Strome EM, Wheler GH, Higley JD, Loriaux DL, Suomi SJ, Doudet DJ. Intracerebroventricular corticotropin-releasing factor increases limbic glucose metabolism and has social context-dependent behavioral effects in nonhuman primates. Proc Natl Acad Sci U S A. 2002;99:15749–15754. doi: 10.1073/pnas.232480899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol Psychiatry. 2000;47:579–585. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- 79.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 80.Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 81.Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 82.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. The American journal of psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 85.Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 87.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kostich WA, Grzanna R, Lu NZ, Largent BL. Immunohistochemical visualization of corticotropin-releasing factor type 1 (CRF1) receptors in monkey brain. J Comp Neurol. 2004;478:111–125. doi: 10.1002/cne.20271. [DOI] [PubMed] [Google Scholar]
- 89.Foote SL, Cha CI. Distribution of corticotropin-releasing-factor-like immunoreactivity in brainstem of two monkey species (Saimiri sciureus and Macaca fascicularis): an immunohistochemical study. J Comp Neurol. 1988;276:239–264. doi: 10.1002/cne.902760208. [DOI] [PubMed] [Google Scholar]
- 90.Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggelton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and mental Dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- 91.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993;337:1–31. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- 93.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 94.Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37:308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- 96.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 97.Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. The American journal of psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- 98.O'Rourke H, Fudge JL. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paxinos G, Huang X, Petrides M, Toga A. The rhesus monkey brain in stereotaxic coordinates. 2nd. San Diego: Academic Press; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.