SUMMARY
In infants with craniofacial disorders, upper airway obstruction is one of the primary causes for morbidity and mortality in the neonatal period. Infants with craniofacial disorders, including Pierre Robin sequence, are at high risk for obstructive sleep apnea syndrome. Because of the complexity of their care, these neonates are usually followed by a multidisciplinary team to ensure timely evaluation and optimal treatment. In addition to history and physical examination, clinical evaluation may include genetic testing, imaging, endoscopy, and polysomnography. There are various treatment options, both surgical and non-surgical, that may be used depending on clinical assessment, underlying condition, and severity of disease. Recent advances have led to better assessment and treatment of these patients, but many questions remain. This review outlines the available literature pertaining to the evaluation and management of upper airway obstruction in the neonate with craniofacial conditions, with a particular focus on Pierre Robin sequence.
Keywords: Obstructive sleep apnea, Apnea–hypopnea index, Craniosynostosis, Pierre Robin sequence, Treacher Collins
1. Introduction
The clinical findings in children with craniofacial disorders are highly variable, but the burden of disease in these patients may be substantial. Along with feeding difficulty, upper airway obstruction is the primary cause for hospitalization in the neonatal period for this population. Because of the complexity of their care, neonates and children with craniofacial disorders are usually followed by multidisciplinary teams that include speech therapists, otolaryngologists, audiologists, plastic surgeons, and orthodontists, among others. Recent advances have led to better assessment and treatment of these patients, but many questions remain. This review outlines the available literature pertaining to the evaluation and management of upper airway obstruction in the neonate with craniofacial conditions, with a particular focus on Pierre Robin sequence (PRS).
2. Types of craniofacial disorder associated with neonatal airway obstruction
2.1. Craniofacial clefts
Craniofacial clefts may include a cleft lip, cleft palate, or both cleft lip and palate (CLP). Whereas >85% of clefts occur in isolation, there are >200 syndromes that include cleft lip and/or palate as a feature [1]. Orofacial clefts are one of the most frequenntly occurring congenital conditions, affecting about one in 700 live births. Cleft palate may be unilateral or bilateral, and may include both the hard and soft palate or the soft palate alone. There are many genetic mutations that cause cleft palate; although some clefts occur as a result of familial inheritance, most are the result of de-novo mutations.
In children with cleft palate, upper airway obstruction is thought to develop from morphologic changes that result in a small midface and retruded mandible as well as a smaller airway. Children with CLP often also have nasal deformities that may further contribute to obstruction. Retrospective studies have shown high rates of obstructive sleep apnea (OSA) in a referred population with CLP, but these studies may be limited by selection bias [2]. A prospective study that included 35 infants with isolated CLP found heavy or loud breathing in two-thirds and 69% who had more than three obstructive events per hour on polysomnography [3]. Whereas normative values for infants have not been well established, an apnea–hypopnea index (AHI) of 2/h is considered elevated in children. Prospective studies of larger cohorts are needed to determine the true prevalence of OSA in this population and to better understand how risk factors differ from the general pediatric population. One of the challenges in understanding the prevalence of and risk factors for OSA in the CLP population is the heterogeneity of the phenotype, with a range from unilateral cleft lip to a cleft that extends through both the soft and hard palate.
2.2. Pierre Robin sequence and conditions with micrognathia
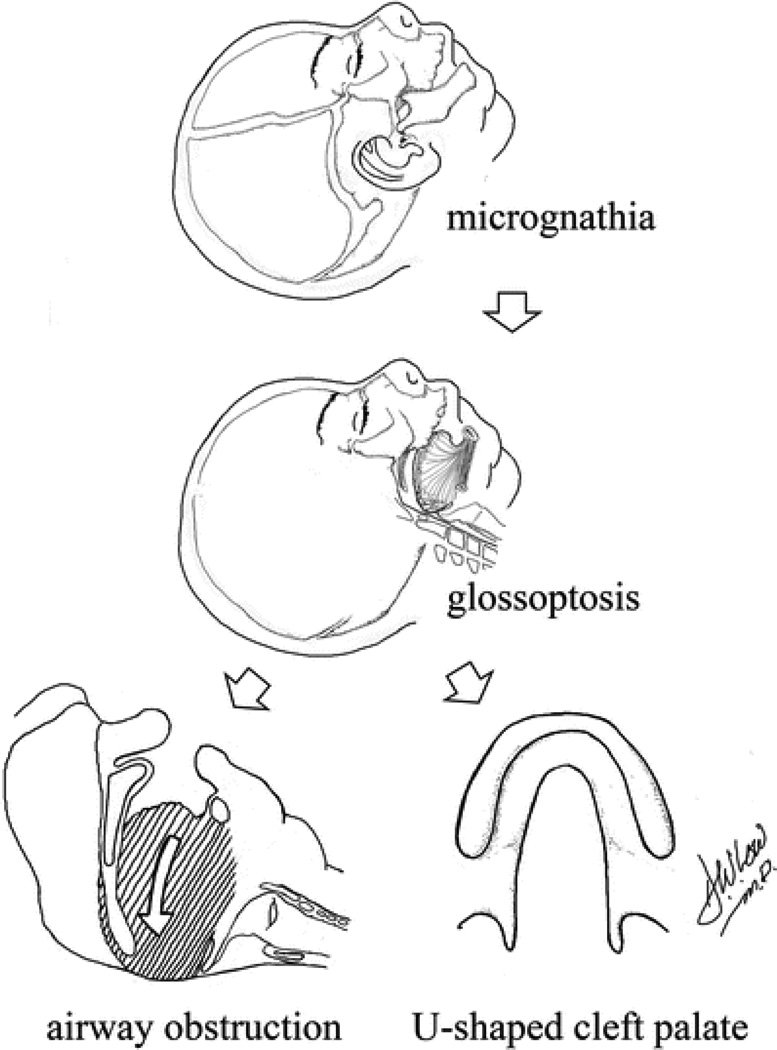
In the neonatal period, the mandible is relatively flat with a short ramus and poorly defined articulation with the base of the skull. During infancy, the mandible is prone to retroposition (retrognathia). This, combined with conditions that include reduced mandibular size in the sagittal direction (micrognathia), may result in a posterior–inferior position of the tongue base, which is anchored to the mandible. These posterior forces lead to displacement of the tongue into the hypopharynx (glossoptosis). The combination of these factors leads to obstruction at the tongue base (Fig. 1). The etiology of the mandibular deformity seen in PRS is unclear but may be the result of the extreme neck flexion in combination with other factors during the sixth to 12th week of gestation [5]. This triad of micrognathia, glossoptosis, and tongue-based airway obstruction was first described in the 1920s by Pierre Robin [6] and may be seen as isolated findings or as part of an underlying syndrome. Pierre Robin sequence is estimated to occur in about one in 8500 live births [7] and half of patients may carry syndromic diagnoses. Frequently observed syndromes associated with PRS include Stickler syndrome, velocardiofacial syndrome, and Treacher Collins syndrome (Fig. 2).
Fig. 1.
The “domino effect” of micrognathia and glossoptosis leading to tongue-based airway obstruction in Pierre Robin sequence. (Adapted from Lioy and Sobol [4] with permission.)
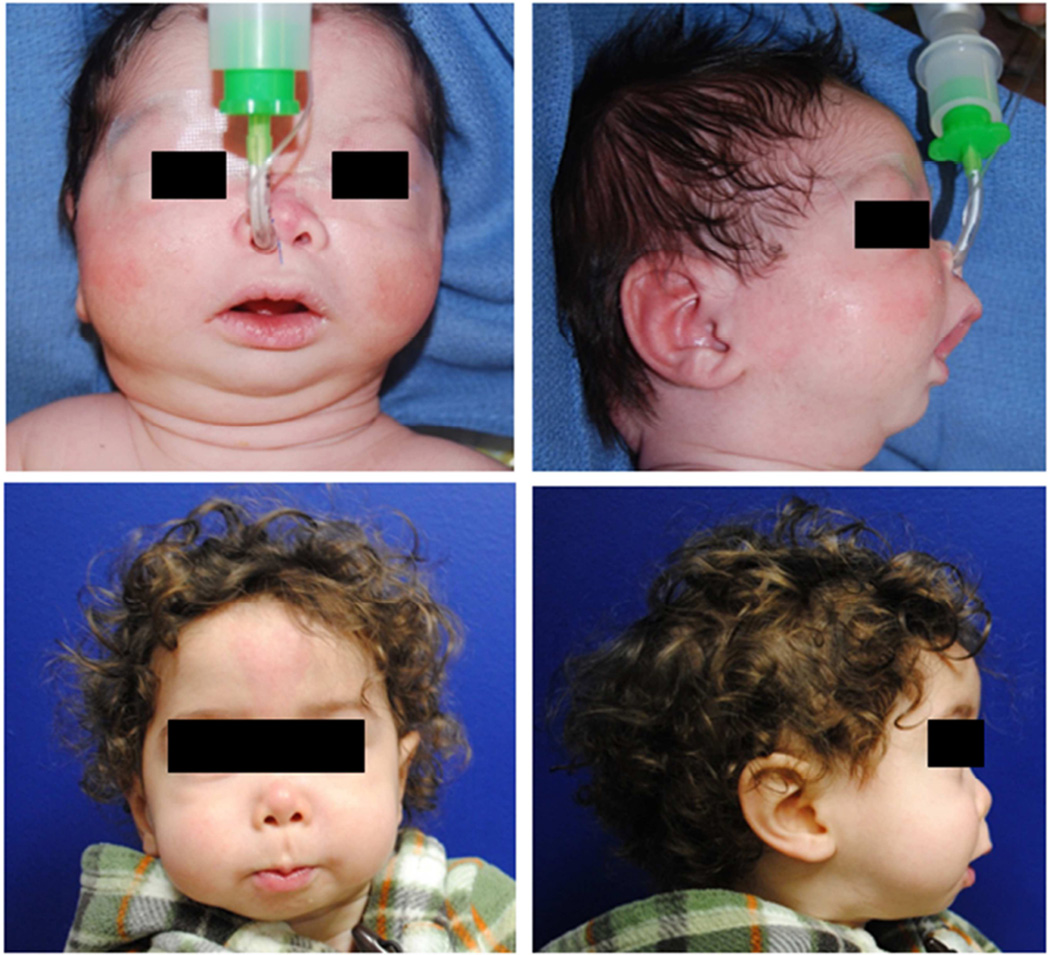
Fig. 2.
Boy with Pierre Robin sequence and severe obstructive sleep apnea who underwent mandibular distraction osteogenesis. In the upper two panels, severe micrognathia can be seen in preoperative images of the patient at 2 weeks. In the lower two panels, the patient is seen at 17 months old. Obstructive sleep apnea resolved after surgery.
Many studies report high rates of OSA in PRS, but most series are limited to patients clinically referred for intervention due to upper airway obstruction [8]. In one recent retrospective study, OSA was identified in 11 of 13 infants with PRS, with a mean AHI of 33.5/h [9]. There was a large amount of variability seen in this series, ranging from 0 to 85.7 obstructions per hour. One prospective study that included nine infants with PRS found that 72% had frequent snoring and that all had significant OSA, with a mean AHI of 45.2/h [3]. The consequences of OSA in these infants are well documented, and include brain injury, failure to thrive, and cor pulmonale [10,11].
Whereas PRS is widely found in isolation, the features may also be seen as part of an identified syndrome (Table 1). Stickler syndrome, Treacher Collins syndrome, and velocardiofacial syndrome are three of the more widely known syndromes that can feature the PRS phenotype with additional characteristic findings discussed below. Hemifacial microsomia also shares some of the same phenotypic features as other conditions with the PRS findings, but the microtia and mandibular hypoplasia are typically unilateral.
Table 1.
Frequency of associated syndromes.a
| Syndrome | Frequency in general population |
Frequency in Pierre Robin syndrome |
|---|---|---|
| Stickler syndrome | 1:8000 | 1:3 |
| Velocardiofacial syndrome | 1:2000 | 1:9 |
| Treacher Collins syndrome | 1:25,000 | 1:20 |
| Undefined | 1:3 |
Adapted from Lioy and Sobol [4] with permission.
2.3. Craniosynostosis
Midface hypoplasia is the main risk factor for OSA in children with syndromic craniosynostosis, but adenotonsillar hypertrophy and choanal atresia may also contribute. Between 40% and 68% of children with syndromic craniosynostosis have OSA but the means of making this diagnosis varies between studies [12,13]. One longitudinal study found that the prevalence of OSA is likely less with advanced age, with children aged <3 years at the highest risk, and that those with midface hypoplasia are more likely to have persistent OSA (Fig. 3) [12].
Fig. 3.
Multiple different types of craniofacial syndromes, all with potential airway anomalies. (Courtesy of Jesse Taylor, MD.)
2.4. Down syndrome
Infants with Down syndrome are susceptible for OSA in part due to midface and mandibular hypoplasia resulting in narrowing of the hypopharynx and relative macroglossia. One recent study of infants with Down syndrome found that 95% of those referred for polysomnograms had OSA, and that 75% had severe OSA. OSA is highly prevalent in children with Down syndrome, with studies showing that as many as 100% of children have sleep-disordered breathing on polysomnograms [14]. In addition to structural abnormalities, low muscle tone also contributes to OSA in this population, with dynamic magnetic resonance imaging demonstrating dynamic upper airway collapse. Higher rates of lingual tonsilar hypertrophy and obesity also contribute to the high prevalence of OSA [15].
2.5. Achondroplasia
Achondroplasia causes craniofacial hypoplasia, including maxillary hypoplasia and depressed nasal bridge. Snoring occurs frequently in children with achondroplasia and studies suggest that the prevalence of OSA is between 10% and 35% [16]. One large series found that nearly half of children referred for polysomnograms had an abnormal finding, but that hypoxemia was the most common abnormality [17]. Compared with children who have OSA due to adenoidal hypertrophy, children with achondroplasia have radiographic evidence of upper airway narrowing and retrognathia. There are neurologic complications associated with achondroplasia, and these patients are also at risk for central sleep apnea.
3. Evaluation of upper airway obstruction in the neonate
Timely multidisciplinary evaluation, including plastic surgery, neonatology, genetics, pulmonology, otolaryngology, and speech therapy is important in the neonatal period for infants with craniofacial disorders in order to establish a diagnosis and determine a safe plan for treatment of any respiratory, feeding, or other associated problem. Additional specialists may also need to be involved. For example, when Stickler syndrome is suspected, a complete ophthalmologic evaluation is essential to assess for myopia and other associated abnormalities. A complete airway exam is also an important step in evaluation to determine the need for adjunctive airway support, which may include supplemental oxygen, positive airway pressure, or intubation if necessary. Frequently, monitoring in a neonatal intensive care unit is required.
3.1. Clinical presentation
There is a range of presentation of upper airway obstruction in the immediate postnatal period. Patients with severe features of PRS may have clinically apparent signs of respiratory distress, including suprasternal retractions, observable apneic episodes, or even overt respiratory failure. Those less severely affected may present simply with noisy breathing or respiratory distress during feeding. Still others may have no respiratory symptoms. Symptoms are often positional with some improvement in prone position. As resting tone decreases during sleep (particularly during rapid eye movement sleep), the tongue may assume a more posterior posture leading to obstruction.
In the first weeks of life, patients with PRS may present during an initial well-child visit with slow feeding and poor weight gain, even without a history of upper airway obstruction. Difficulty with gaining weight may be due to increased metabolic demand from upper airway obstruction [18], inadequate caloric intake, or both. There are multiple reasons for feeding difficulties in PRS. The small mandible and glossoptosis pose significant physical restrictions on infant suckling. In infants with a palatal cleft, this may be exaggerated. Oropharyngeal dysmotility has been noted in some patients, and those with severe obstructive apnea are more likely to require tube feeding. Additionally, gastroesophageal reflux is especially frequent in those with Robin sequence, with an incidence as high as 85%, and aspiration can be seen in some cases [19].
3.2. Family history
Typically, there is no family history of craniofacial abnormality in patients with PRS. Multiple genetic conditions may result in the phenotypic features found in PRS. In cases of isolated PRS without an underlying syndrome, the most frequent mutation can be mapped upstream to the SOX9 gene in a region of 17q24. In non-syndromic PRS, mutations are usually spontaneous, although it has been reported in some families.
In patients with syndromic forms of PRS, there can be a much higher rate of familial inheritance. Stickler syndrome is genetically heterogeneous but is most frequently associated with mutations in the COL2A1 gene on chromosome 12q13 [20]. Treacher Collins syndrome is associated with autosomal dominant inheritance of the TCOF1 gene on 5q32-33 (Fig. 3).
Velocardiofacial syndrome also has an autosomal dominant inheritance pattern and is associated with the TBX1 gene at the 22q11.21 location and has a prevalence of about 1:4000 births.
3.3. Physical examination
As PRS may be found as part of an underlying syndrome or in isolation, physical exam findings can be highly variable, even within syndromes. Whereas the degree of micrognathia, glossoptosis, and upper airway obstruction all may range in severity, there is not clear evidence that these features are more severe in one syndrome than another.
Although not part of the diagnostic criteria for PRS, cleft palate occurs frequently in this patient population and is often incorrectly included among its essential characteristics. Distinct from other forms of palatal clefting with or without cleft lip, the cleft palate associated with PRS most often is wide and U-shaped, thought to be the result of failure of the palatal shelves to fuse in early gestation due to the presence of an abnormally positioned tongue [21]. There is growing evidence that midface hypoplasia may be an important finding in patients with PRS [22], and is probably more pronounced in some infants than others.
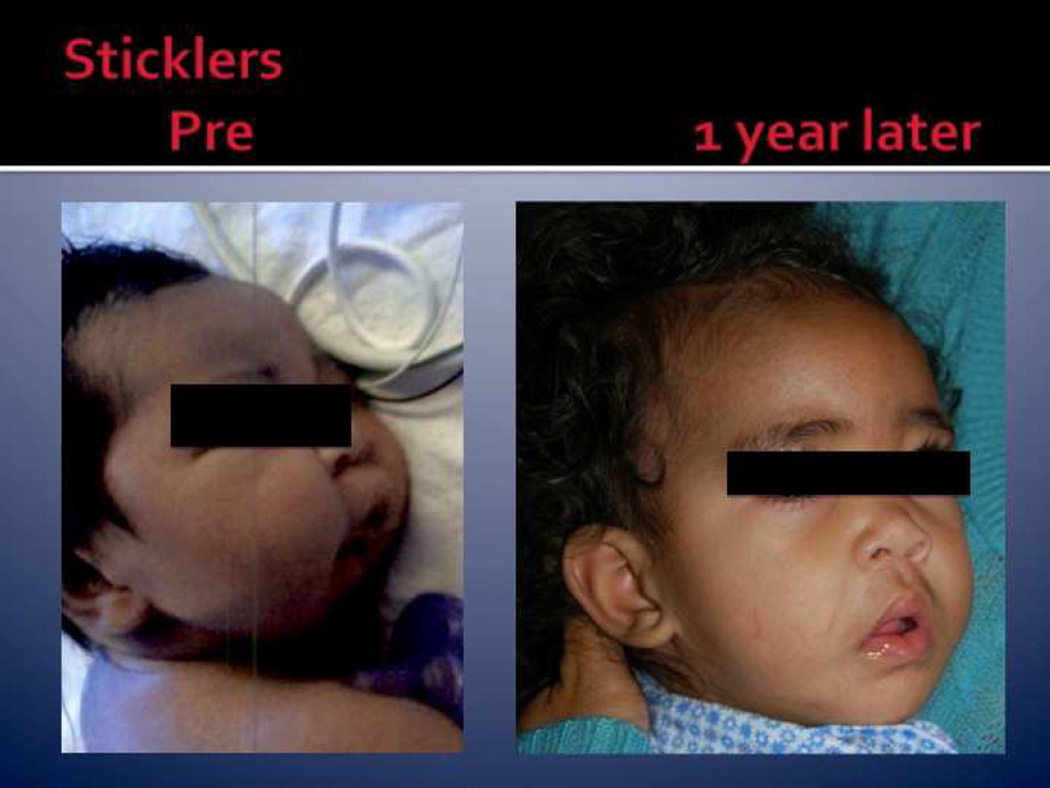
In Stickler syndrome, additional physical exam findings may include myopia that can be progressive and lead to vitreoretinal degeneration, retinal detachment, and cataracts. Sensorineural or conductive hearing loss and/or spondyloepiphyseal dysplasia may also be seen in infancy in addition to midline clefting and the other features common to PRS [20] (Fig. 4).
Fig. 4.
Stickler’s syndrome: pre-and postmandibular distraction osteogenesis in a neonate. (Adapted from Lioy and Sobol [4] with permission.)
Treacher Collins syndrome features a variety of characteristic features affecting the eyes, ears, and mouth, in addition to the findings found in PRS. Exam findings include microtia and conductive hearing loss, coloboma and antimongoloid slanting of the eyes, as well as hyoplastic zygomatic arches and macrostomia.
Velocardiofacial syndrome less frequently incudes the classic PRS features, and has a highly variable group of additional clinical findings despite its common genetic etiology; whereas frequent features include cleft palate and cardiac abnormalities as well as small optic disks with tortuous vessels, microcephaly, short stature, slender hands and digits, and auricular abnormalities. T-Lymphocyte dysfunction and neonatal hypocalcemia are frequent and developmental disability is highly variable and may be severe in some.
3.4. Imaging
Lateral radiographs may help characterize the degree of micrognathia as well as assess the severity of glossoptosis by allowing for visualization of a patent or occluded airway stripe. In neonates the airway stripe should measure ~4 mm. Computed tomography (CT) is used by some centers in the evaluation of micrognathia, but the assessment is less standardized. CT has the advantage of providing much more data and can be used to assess lengths and volumes of airway and bony structures [23]. MRI has been used to assess other pediatric populations with OSA [24] and may be useful in evaluating the soft tissue structure of infants with micrognathia and other craniofacial conditions. Although radiography may provide insight into the degree of upper airway compromise, it is no substitute for direct visualization of the airway.
3.5. Endoscopy
Bedside fiberoptic evaluation of the upper airway is important in localizing the level of airway obstruction and assessing for other causes of obstruction, such as choanal atresia. It should be performed prior to any surgery. Likewise, micro-laryngo-bronchoscopy performed in the operating room may be useful in the evaluation of subglottic structures and to rule out the presence of laryngomalacia, tracheomalacia, and other pathologies. A jaw-thrust maneuver performed under anesthesia at the time of this procedure may help determine the extent to which mandibular advancement can improve tongue base position. In some cases, the presence of lower airway pathology could alter management decisions. The extent to which the tongue base contributes to upper airway obstruction may be difficult to determine clinically. For this reason, diagnostic airway endoscopy is essential to confirm the presence of glossoptosis and rule out other sources of upper or lower airway obstruction.
3.6. Polysomnography
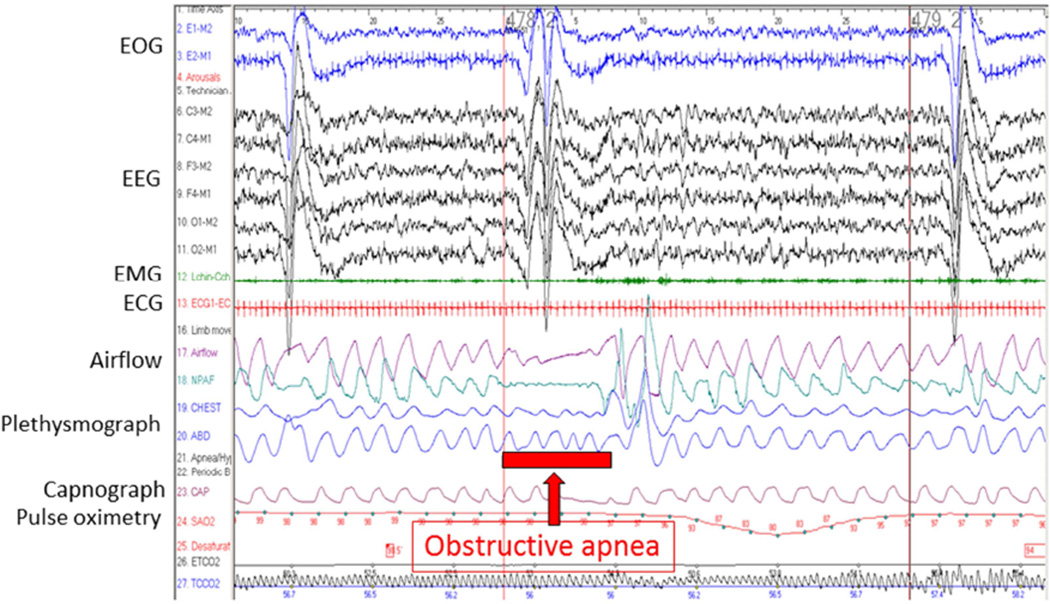
Determining the presence and severity of OSA in infants with micrognathia can be challenging. Relying on pulse oximetry to identify obstructions by episodic desaturation can be problematic because brief desaturations could be missed by long averaging times on hospital monitors and because desaturations could be the result of movement artifact or other unrelated causes. In children, pulse oximetry alone is neither sensitive nor specific for OSA [25] and may be even less so in neonates. Clinical signs such as snoring may also be inadequate to accurately diagnose OSA in infants with PRS [9]. Attended polysomnography is the gold standard for the diagnosis of OSA in children [26]. This modality includes simultaneous electroencephalographic monitoring as well as multiple respiratory and gas exchange parameters, including oxyhemoglobin saturation, end-tidal and/or transcutaneous carbon dioxide. Standard polysomnography assesses both chest and abdominal movement using inductance plethysmography and airflow using a thermistor and nasal pressure changes. This allows for distinction between, and quantification and categorization of, obstructive, mixed, and central apneas and hypopneas. After the study is complete and interpreted by a sleep physician, an apnea–hypopnea index is generated and the quantity and severity of events can be used to determine objectively the severity of apnea. In some pediatric centers, polysomnography may be performed at the infant’s bedside (Fig. 5).
Fig. 5.
Polysomnogram demonstrating severe obstructive sleep apnea syndrome in an infant with Pierre Robin sequence. (Adapted from Lioy and Sobol [4] with permission.)
3.7. GILLS criteria
A variety of classification schemes have been developed to stratify disease severity in infants with PRS. Whereas an association with identified syndromes may in some cases portend a worse diagnosis, there is a great deal of variability in presentation within syndromes. The only validated classification system for patients with PRS is the GILLS criteria (Gastroesophageal reflux disease, preoperative Intubation, Late surgical intervention, Low birth weight, and Syndromic diagnosis), which was developed to determine which patients would benefit from tongue–lip adhesion (Table 2). In one study, all patients assessed using the GILLS criteria that were found to have a score of ≤2 had successful surgery, whereas there was a failure rate close to 50% in those with a score of ≥3 [28].
Table 2.
GILLS scoring system for tongue-based airway obstruction.a
| 1 | Presence of gastroesophageal reflux |
| 2 | Preoperative intubation |
| 3 | Late presentation (aged >2 weeks) |
| 4 | Low birth weight (<2500 g) |
| 5 | Syndromic diagnosis |
4. Management of upper airway obstruction
4.1. Challenges
Although there are numerous published algorithms for the management of PRS [29,30], prospective comparative studies are lacking. A systematic review of the literature highlights the dearth of high quality evidence related to the management of this challenging patient population [31]. In 126 peer-reviewed articles published between 1980 and 2010, the authors found few studies utilizing standardized diagnostic criteria, therapeutic algorithms, or outcome measures, thus making side-by-side comparison difficult.
For mild upper airway obstruction and in cases where surgical intervention is not possible, there are several conservative approaches in the management of upper airway obstruction.
Surgical intervention, including tongue–lip adhesion (TLA), mandibular distraction osteogenesis (MDO), and tracheostomy, should be considered for persistent or severe airway obstruction that has failed or is not amenable to conservative treatment alone.
4.2. Non-surgical management
4.2.1. Growth
In children with craniofacial conditions including those with micrognathia, it is unclear how growth affects the degree of upper airway obstruction as the child develops during fetal life into childhood.
The natural history of upper airway obstruction in infants with craniofacial conditions remains unknown, as there have been few non-surgical longitudinal studies in this population. OSA may resolve in up to 50% of older children without intervention, including a smaller percentage with severe OSA [32]. Longitudinal cephalometric studies of infants with PRS show that whereas during infancy they had a shorter tongue and mandibular length when compared to infants with isolated cleft palate and healthy controls, these differences became less apparent over the first several years of life [33]. Infants with PRS have been managed successfully using conservative therapies [34], suggesting that upper airway obstruction may improve with growth. However, a recent study of 45 patients with PRS referred for polysomnogram found no reduction in degree of OSA found during the first year of life [35]. There are reports of spontaneous resolution of OSA with growth in children with Pierre Robin sequence [36] but the long-term effect of growth on upper airway obstruction both on those who have had surgery and those who have not remains unknown. In patients with craniosynostosis, there is some evidence that as children grow, there is reduction in AHI [12]. However, especially in cases of severe OSA, watchful waiting could result in significant morbidity.
4.2.2. Positioning
When OSA is only present in the supine position, sleeping in an alternate position could provide effective therapy. Lateral or prone positioning in infants with micrognathia or glossoptosis has been used since the days of Pierre Robin and has been reported to be successful in treating upper airway obstruction [37], but polysomnographic evidence of success has not been demonstrated. Studies that include polysomnographic recording in multiple positions are needed to evaluate the efficacy of positional therapy in this population. This therapy is difficult to maintain once an infant is able to roll over.
4.2.3. Nasal airway
A nasopharyngeal tube that can be inserted through the naris extending to the oropharynx can act as a stent of the upper airway, preventing collapse. One study of young children with syndromic craniosynostosis found that using a nasopharyngeal airway for long-term management of upper airway obstruction successfully treated OSA and improved quality of life [38]. A series including 12 infants with PRS found that nasopharyngeal tubes reduced hospital stay and did not result in complications when used at home [39]. More studies are needed to evaluate the long-term safety and efficacy of this modality. Frequent suctioning or replacement of the stents may be needed to maintain patency.
4.2.4. Positive airway pressure
Positive airway pressure is first-line therapy for most adults with OSA and for many children where surgery is not curative. Continuous positive airway pressure (CPAP) uses an air compressor to deliver pressurized air through a nasal mask, creating a pneumatic stent that prevents upper airway obstruction. CPAP may be challenging to use in infants with craniofacial conditions for several reasons. There is a lack of available interfaces to fit children with normal craniofacial anatomy, and infants with craniofacial syndromes are even harder to accommodate. Additionally, infants sleep much more than older children and adults who use CPAP, and its use is somewhat burdensome for parents and caregivers. There is some concern for midface hypoplasia developing from prolonged CPAP use in children, and infants might be susceptible to this consequence. CPAP has been used successfully in patients with micrognathia but has not systematically been studied or compared to other therapies in infants [40]. More research is needed to understand which patients would most benefit from CPAP and how long it can safely be used.
4.3. Surgical management
4.3.1. Mandibular distraction osteogenesis
Mandibular distraction osteogenesis is a surgical procedure that lengthens the body of the mandible. During the procedure, the mandible is accessed bilaterally, either through intraoral or submandibular incisions, and mandibular osteotomies are made through the mandibular body. Internal or external distraction devices are then applied. After a short latency period, the devices are activated, slowly separating the mandibular segments at a rate of 1 mm/day. Once the desired advancement has been achieved, the devices are left in position for 6–8 weeks until the new space has reossified, at which time the devices are removed. Mandibular lengthening with MDO causes advancement of the tongue base at the site of the attachment of the genioglossus to the mandible, increasing the size of the upper airway [23].
Complications from MDO include infection, jaw malocclusion, hypertrophic scarring, and injury to the surrounding nerves or teeth in as many as 23% [41]. The effect on mandibular growth plates is not entirely known. In infants with micrognathia and severe OSA, MDO can be an effective alternative to tracheostomy. A meta-analysis found that 84% of children who were tracheostomy dependent were able to be decannulated following MDO [42]. Early studies of MDO in infants reported high rates of clinical resolution of symptoms [42] and subsequent studies involving polysomnography have shown significant improvement in OSA in the majority of patients [43]. There have been no large prospective studies of MDO in children with micrognathia.
4.3.2. Tongue–lip adhesion
Tongue–lip adhesion (TLA) is a surgical procedure that involves securing the tongue to the mucosa and muscle of the lower lip to correct the glossoptosis that causes upper airway obstruction in children with PRS and other similar conditions. Unlike MDO, TLA does not address any skeletal malformations. TLA was first proposed in 1946 and for many years was the only surgical alternative to tracheostomy for severe upper airway obstruction. The impact of tongue–lip adhesion on OSA in children with infants has not been carefully studied, but surgical case series suggest that it improves OSA although resulting in incomplete resolution in most patients [44,45]. Recent studies comparing surgical outcomes of TLA with MDO suggest that TLA results in a shorter hospital stay but that MDO is better at treating OSA and earlier return to oral feeding [46], at least in non-syndromic infants with micrognathia. Infants undergoing MDO may be less likely to require a second surgery to correct persistent upper airway obstruction [43].
4.3.3. Tracheostomy
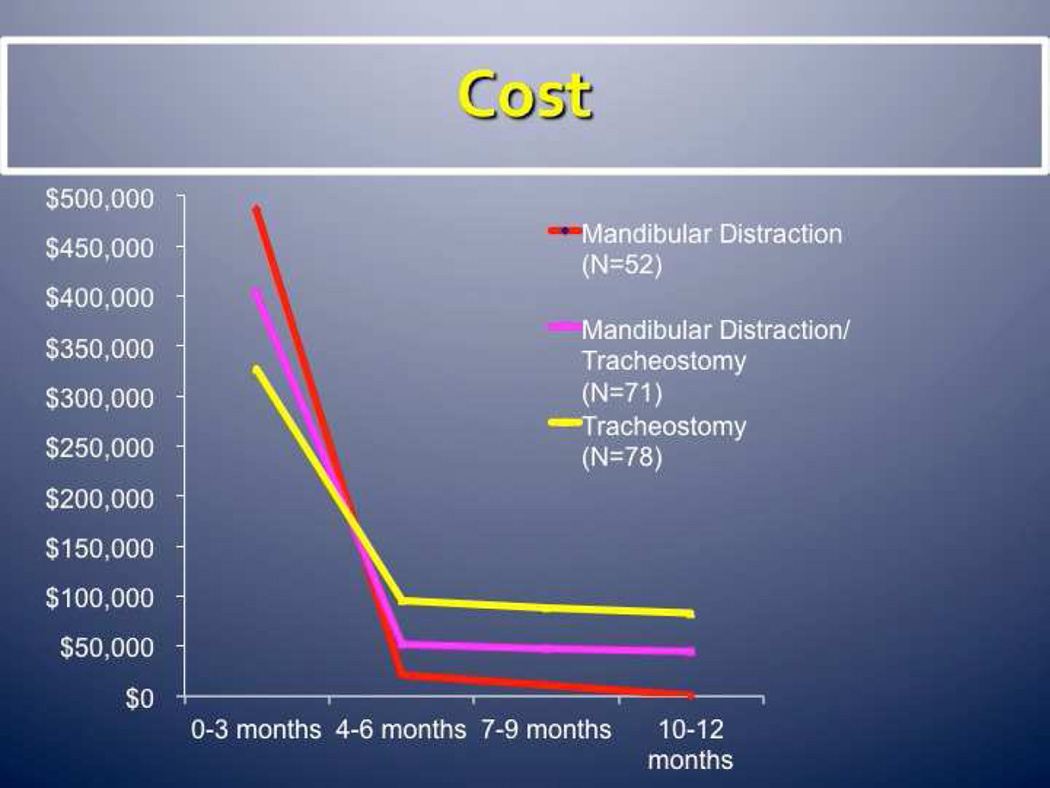
By bypassing the site of obstruction completely, tracheostomy is a highly effective means of treating severe upper airway obstruction in infants with craniofacial conditions. One study of children with PRS found that those undergoing tracheostomy tended to have a lower birth weight and an underlying syndrome, and spent more time in the ICU and hospitalized than those undergoing other procedures [47]. Having a tracheostomy requires extensive training for parents; infants usually require constant monitoring, and there are risks of significant complications, including airway obstruction, that may result in death. One study found that infants with PRS treated with tracheostomy had four times the complications and at least three times the cost compared to those treated with MDO [48]. In infants who have failed other surgical and non-surgical options, tracheostomy may be a good option. More research is needed to determine which patients are the best candidates for the different surgical techniques, including tracheostomy. Over the first year of life, cost analysis has shown that MDO can save upwards of US$100,000 (J. Taylor, personal communication) (Fig. 6).
Fig. 6.
Cost analysis of mandibular distraction osteogenesis versus tracheostomy. (Courtesy of Jesse Taylor, MD.)
4.3.4. Adenoidectomy
Adenotonsillectomy is first-line therapy for many otherwise healthy children with OSA but has not been well-studied as a therapy in the craniofacial population. Adenoidectomy is one of the most widely performed procedures for children aged <2 years with cleft palate and PRS with OSA [49]. Studies have shown adenoidectomy or adenotonsillectomy to be modestly effective in children with a variety of craniofacial conditions, including cleft palate, Down syndrome, achondroplasia, and craniosynostosis [15,50,51]; the efficacy in infant populations is likely limited.
4.4. Conclusions
Infants with PRS and other craniofacial conditions are at increased risk for OSA. These patients should be carefully evaluated by a multidisciplinary group, including neonatologists, plastic surgeons, geneticists, otolaryngologists, pulmonologists, and speech therapists. Physical exam findings may be variable, but in PRS will include glossoptosis, micrognathia, and upper airway obstruction. Polysomnography should be used to determine the severity of OSA in this population, as clinical assessment may be inadequate. Various treatment options, both surgical and conservative, exist for OSA in this population.
Research directions.
In infants with craniofacial conditions, especially PRS, there are substantial gaps and a need for clinical studies that are larger and that include longer-term follow-up with polysomnography.
More mechanistic studies are needed to better understand the structural and neuromotor risk factors associated with OSA in this population and the benefits of interventions, including surgery.
Because of selection bias, the true prevalence of OSA in infants with micrognathia is difficult to estimate. The use of PSG in clinical trials of all infants with OSA with or without surgical correction is needed to determine best practice.
Improved reporting with collaborative database including multicenter studies or multi-institutional databases is essential in pooling data, especially for rare syndromes.
There is a need for long-term studies by craniofacial centers on all subgroups of micrognathia with or without genetic syndromes, following these children after surgery, tracking outcomes such as feeding, cognition, and growth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christensen K, Mitchell LE. Familial recurrence-pattern analysis of nonsyndromic isolated cleft palate – a Danish Registry study. Am J Hum Genet. 1996;58:182–190. [PMC free article] [PubMed] [Google Scholar]
- 2.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008;118:348–353. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 3.Maclean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Archs Dis Childh. 2012;97:1058–1063. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 4.Lioy J, Sobol S. Disorders of the neonatal airway: fundamentals for practice. Berlin: Springer; 2015. [Google Scholar]
- 5.Diewert VM. Craniofacial growth during human secondary palate formation and potential relevance of experimental cleft palate observations. J Craniofac Genet Dev Biol Suppl. 1986;2:267–276. [PubMed] [Google Scholar]
- 6.Robin P. A fall of the base of the tongue considered as a new cause of nasopharyngeal respiratory impairment: Pierre Robin sequence, a translation. 1923. Plast Reconstr Surg. 1994;93:1301–1303. [PubMed] [Google Scholar]
- 7.Bush PG, Williams AJ. Incidence of the Robin Anomalad (Pierre Robin syndrome) Br J Plast Surg. 1983;36:434–437. doi: 10.1016/0007-1226(83)90123-6. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AC, Moore DJ, Moore MH, Martin AJ, Staugas RE, Kennedy JD. Late presentation of upper airway obstruction in Pierre Robin sequence. Archs Dis Childh. 2000;83:435–438. doi: 10.1136/adc.83.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson IC, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48:614–618. doi: 10.1597/10-100. [DOI] [PubMed] [Google Scholar]
- 10.Cozzi F, Pierro A. Glossoptosis–apnea syndrome in infancy. Pediatrics. 1985;75:836–843. [PubMed] [Google Scholar]
- 11.Dykes EH, Raine PA, Arthur DS, Drainer IK, Young DG. Pierre Robin syndrome and pulmonary hypertension. J Pediatr Surg. 1985;20:49–52. doi: 10.1016/s0022-3468(85)80391-2. [DOI] [PubMed] [Google Scholar]
- 12.Driessen C, Joosten KF, Bannink N, et al. How does obstructive sleep apnoea evolve in syndromic craniosynostosis? A prospective cohort study. Archs Dis Childh. 2013;98:538–543. doi: 10.1136/archdischild-2012-302745. [DOI] [PubMed] [Google Scholar]
- 13.Lo LJ, Chen YR. Airway obstruction in severe syndromic craniosynostosis. Ann Plast Surg. 1999;43:258–264. doi: 10.1097/00000637-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Goffinski A, Stanley MA, Shepherd N, et al. Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic. Am J Med Genet A. 2015;167A:324–330. doi: 10.1002/ajmg.a.36903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen D. Management of obstructive sleep apnea associated with Down syndrome and other craniofacial dysmorphologies. Curr Opin Pulm Med. 2011;17:431–436. doi: 10.1097/MCP.0b013e32834ba9c0. [DOI] [PubMed] [Google Scholar]
- 16.Afsharpaiman S, Saburi A, Waters KA. Respiratory difficulties and breathing disorders in achondroplasia. Paediatr Respir Rev. 2013 doi: 10.1016/j.prrv.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Mogayzel PJ, Jr, Carroll JL, Loughlin GM, Hurko O, Francomano CA, Marcus CL. Sleep-disordered breathing in children with achondroplasia. J Pediatr. 1998;132:667–671. doi: 10.1016/s0022-3476(98)70358-0. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Carroll JL, Koerner CB, Hamer A, Lutz J, Loughlin GM. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr. 1994;125:556–562. doi: 10.1016/s0022-3476(94)70007-9. [DOI] [PubMed] [Google Scholar]
- 19.Monasterio FO, Molina F, Berlanga F, et al. Swallowing disorders in Pierre Robin sequence: its correction by distraction. J Craniofac Surg. 2004;15:934–941. doi: 10.1097/00001665-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Snead MP, Yates JR. Clinical and molecular genetics of Stickler syndrome. J Med Genet. 1999;36:353–359. [PMC free article] [PubMed] [Google Scholar]
- 21.Parada C, Han D, Grimaldi A, et al. Disruption of the ERK/MAPK pathway in neural crest cells as a potential cause of Pierre Robin sequence. Development. 2015;142:3734–3745. doi: 10.1242/dev.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krimmel M, Kluba S, Breidt M, et al. Three-dimensional assessment of facial development in children with Pierre Robin sequence. J Craniofac Surg. 2009;20:2055–2060. doi: 10.1097/SCS.0b013e3181be87db. [DOI] [PubMed] [Google Scholar]
- 23.Roy S, Munson PD, Zhao L, Holinger LD, Patel PK. CT analysis after distraction osteogenesis in Pierre Robin Sequence. Laryngoscope. 2009;119:380–386. doi: 10.1002/lary.20011. [DOI] [PubMed] [Google Scholar]
- 24.Schwab RJ, Kim C, Bagchi S, et al. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med. 2015;191:1295–1309. doi: 10.1164/rccm.201501-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk VG, Bohn SG, Flemons WW, Remmers JE. Comparison of home oximetry monitoring with laboratory polysomnography in children. Chest. 2003;124:1702–1708. doi: 10.1378/chest.124.5.1702. [DOI] [PubMed] [Google Scholar]
- 26.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 27.Rogers GF, Murthy AS, Mulliken JB. The GILLS score: part I. Patient selection for tongue-lip adhesion in Robin sequence. Plast Reconstr Surg. 2011;128:243–251. doi: 10.1097/PRS.0b013e318217420d. [DOI] [PubMed] [Google Scholar]
- 28.Abramowicz S, Bacic JD, Mulliken JB, Rogers GF. Validation of the GILLS score for tongue–lip adhesion in Robin sequence patients. J Craniofac Surg. 2012;23:382–386. doi: 10.1097/SCS.0b013e318240fc7b. [DOI] [PubMed] [Google Scholar]
- 29.Paes EC, van Nunen DP, Speleman L, et al. A pragmatic approach to infants with Robin sequence: a retrospective cohort study and presence of a treatment algorithm. Clin Oral Invest. 2015;19:2101–2114. doi: 10.1007/s00784-015-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer RB, Stadler JA, 3rd, Gosain AK. To distract or not to distract: an algorithm for airway management in isolated Pierre Robin sequence. Plast Reconstr Surg. 2004;113:1113–1125. doi: 10.1097/01.prs.0000110323.50084.21. [DOI] [PubMed] [Google Scholar]
- 31.Bookman LB, Melton KR, Pan BS, et al. Neonates with tongue-based airway obstruction: a systematic review. Otolaryngol Head Neck Surg. 2012;146:8–18. doi: 10.1177/0194599811421598. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figueroa AA, Glupker TJ, Fitz MG, BeGole EA. Mandible, tongue, and airway in Pierre Robin sequence: a longitudinal cephalometric study. Cleft Palate Craniofac J. 1991;28:425–434. doi: 10.1597/1545-1569_1991_028_0425_mtaaip_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 34.Li HY, Lo LJ, Chen KS, Wong KS, Chang KP. Robin sequence: review of treatment modalities for airway obstruction in 110 cases. Int J Pediatr Otorhinolaryngol. 2002;65:45–51. doi: 10.1016/s0165-5876(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee JJ, Thottam PJ, Ford MD, Jabbour N. Characteristics of sleep apnea in infants with Pierre–Robin sequence: is there improvement with advancing age? Int J Pediatr Otorhinolaryngol. 2015;79:2059–2067. doi: 10.1016/j.ijporl.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Kiely JL, Deegan PC, McNicholas WT. Resolution of obstructive sleep apnoea with growth in the Robin sequence. Eur Respir J. 1998;12:499–501. doi: 10.1183/09031936.98.12020499. [DOI] [PubMed] [Google Scholar]
- 37.Meyer AC, Lidsky ME, Sampson DE, Lander TA, Liu M, Sidman JD. Airway interventions in children with Pierre Robin Sequence. Otolaryngol Head Neck Surg. 2008;138:782–787. doi: 10.1016/j.otohns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Randhawa PS, Ahmed J, Nouraei SR, Wyatt ME. Impact of long-term nasopharyngeal airway on health-related quality of life of children with obstructive sleep apnea caused by syndromic craniosynostosis. J Craniofac Surg. 2011;22:125–128. doi: 10.1097/SCS.0b013e3181f6f82c. [DOI] [PubMed] [Google Scholar]
- 39.Anderson KD, Cole A, Chuo CB, Slator R. Home management of upper airway obstruction in Pierre Robin sequence using a nasopharyngeal airway. Cleft Palate Craniofac J. 2007;44:269–273. doi: 10.1597/06-020. [DOI] [PubMed] [Google Scholar]
- 40.Miller SD, Glynn SF, Kiely JL, McNicholas WT. The role of nasal CPAP in obstructive sleep apnoea syndrome due to mandibular hypoplasia. Respirology. 2010;15:377–379. doi: 10.1111/j.1440-1843.2009.01681.x. [DOI] [PubMed] [Google Scholar]
- 41.Tahiri Y, Viezel-Mathieu A, Aldekhayel S, Lee J, Gilardino M. The effectiveness of mandibular distraction in improving airway obstruction in the pediatric population. Plast Reconstr Surg. 2014;133:352e–359e. doi: 10.1097/01.prs.0000438049.29258.a8. [DOI] [PubMed] [Google Scholar]
- 42.Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001;108:302–311. doi: 10.1097/00006534-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Flores RL, Tholpady SS, Sati S, et al. The surgical correction of Pierre Robin sequence: mandibular distraction osteogenesis versus tongue–lip adhesion. Plast Reconstr Surg. 2014;133:1433–1439. doi: 10.1097/PRS.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 44.Sedaghat AR, Anderson IC, McGinley BM, Rossberg MI, Redett RJ, Ishman SL. Characterization of obstructive sleep apnea before and after tongue–lip adhesion in children with micrognathia. Cleft Palate Craniofac J. 2012;49:21–26. doi: 10.1597/10-240. [DOI] [PubMed] [Google Scholar]
- 45.Resnick CM, Dentino K, Katz E, Mulliken JB, Padwa BL. Effectiveness of tongue–lip adhesion for obstructive sleep apnea in infants with Robin sequence measured by polysomnography. Cleft Palate Craniofac J. 2015 Jul 8; doi: 10.1597/15-058. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Papoff P, Guelfi G, Cicchetti R, et al. Outcomes after tongue–lip adhesion or mandibular distraction osteogenesis in infants with Pierre Robin sequence and severe airway obstruction. Int J Oral Maxillofac Surg. 2013;42:1418–1423. doi: 10.1016/j.ijom.2013.07.747. [DOI] [PubMed] [Google Scholar]
- 47.Kam K, McKay M, MacLean J, Witmans M, Spier S, Mitchell I. Surgical versus nonsurgical interventions to relieve upper airway obstruction in children with Pierre Robin sequence. Can Respir J. 2015;22:171–175. doi: 10.1155/2015/798076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paes EC, Fouche JJ, Muradin MS, Speleman L, Kon M, Breugem CC. Tracheostomy versus mandibular distraction osteogenesis in infants with Robin sequence: a comparative cost analysis. Br J Oral Maxillofac Surg. 2014;52:223–229. doi: 10.1016/j.bjoms.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Robison JG, Otteson TD. Increased prevalence of obstructive sleep apnea in patients with cleft palate. Arch Otolaryngol Head Neck Surg. 2011;137:269–274. doi: 10.1001/archoto.2011.8. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Aziz M. The effectiveness of tonsillectomy and partial adenoidectomy on obstructive sleep apnea in cleft palate patients. Laryngoscope. 2012;122:2563–2567. doi: 10.1002/lary.23507. [DOI] [PubMed] [Google Scholar]
- 51.Sisk EA, Heatley DG, Borowski BJ, Leverson GE, Pauli RM. Obstructive sleep apnea in children with achondroplasia: surgical and anesthetic considerations. Otolaryngol Head Neck Surg. 1999;120:248–254. doi: 10.1016/S0194-5998(99)70414-6. [DOI] [PubMed] [Google Scholar]