Abstract
Objective
Prolonged maternal separation (PMS) in the first two weeks of life has been associated with poor growth with lasting effects in the brain structure and function. This study aimed to investigate whether PMS-induced undernutrition could cause systemic inflammation and changes in nutrition-related hormonal levels, affecting hippocampal structure and neurotransmission in C57BL/6J suckling mice.
Methods
This study assessed mouse growth parameters coupled with IGF-1 serum levels. In addition, leptin, adiponectin and corticosterone serum levels were measured following PMS. Hippocampal stereology and the amino acid levels were also assessed. Furthermore, we measured myelin basic protein and synapthophysin (SYN) expression in the overall brain tissue and hippocampal SYN immunolabeling. For behavioral tests, we analyzed the ontogeny of selected neonatal reflexes. PMS was induced by separating half the pups in each litter from their lactating dams for defined periods each day (4h on day 1, 8h on day 2, and 12h thereafter). A total of 67 suckling pups were used in this study.
Results
PMS induced significant slowdown in weight gain and growth impairment. Significant reductions in serum leptin and IGF-1 levels were found following PMS. Total CA3 area and volume were reduced, specifically affecting the pyramidal layer in PMS mice. CA1 pyramidal layer area was also reduced. Overall hippocampal SYN immunolabeling was lower, especially in CA3 field and dentate gyrus. Furthermore, PMS reduces hippocampal aspartate, glutamate, and GABA levels, as compared with unseparated controls.
Conclusion
Altogether, these findings suggest that PMS causes significant growth deficits and alterations in hippocampal morphology and neurotransmission.
Keywords: maternal separation, malnutrition, hippocampus, stereology, leptin, IGF-1, inflammation
1. Introduction
Undernutrition during early post-natal life in children may be associated with a significant slowdown in the rate of central nervous system growth, with reduced brain weight, thinner cerebral cortex, diminished numbers of neurons, deficient myelination, poor neuritic arborization, and several changes in the microscopic features of dendritic spines such as reduction in their width and number, and also cognition impairment [1,2,3,4].
In rodents, undernutrition during early life may result in similar reduced brain weight, reduced total cell number, reduced and delayed myelination, loss of axon terminals in the cerebral cortical neurons, and substantial deficits in the synapse-to-neuron ratio in some brain regions, indicating a reduction in the total synapse number [5]. Furthermore, recent evidence indicates that long-lasting decrements in hippocampal plasticity occur in mice subjected to early environmental stress, such as maternal separation [6,7]. It has also been shown that undernutrition reduces peripheral circulating concentrations of insulin-like growth factor (IGF-1) [8].
Recently, a negative correlation has been found between IGF-1 serum levels and chronic inflammation markers in stunted Zimbabwean infants. However, it remains elusive whether systemic inflammation coupled with low IGF-1 levels, induced by chronic undernutrition, could affect early hippocampal development [9].
In this study, we investigated whether prolonged maternal-offspring separation would cause systemic inflammation and poor growth, and assess its effects on the hippocampus structure and amino acid neurotransmitter levels in two-week old mice, a time in which the hippocampus relies on a strong post-natal plasticity [10]. In order to relate systemic inflammation and nutritional status, we assessed circulating levels of C-reactive protein and nutrition-related hormones in the blood sera following undernutrition.
2. Methods and materials
2.1. Prolonged maternal separation model
C57BL/6J wild-type mice were purchased from Charles River laboratories. Either purchased pregnant mice or breeding pairs were used to obtain the study pups. Detectable pregnant mice (at ~12 day-pregnant) were then caged individually, with free access to standard rodent chow and water, and were monitored daily for delivery, termed Day (D) 0. Newborn litters were adjusted to 6 to 8 pups. Undernutrition was induced by separating half the pups in each litter from their lactating dams for defined periods each day (4h on D4; 8h on D5 and 12h thereafter). Pups were separated 8:00–10:00 AM, daily. At this time of the day, all pups were already breastfed and resting in their cages. In rare cases of encountering breastfeeding, pups were left undisturbed until breastfeeding was finalized. Litters with about the same male:female ratio were selected for the study entry. The separation protocol was not conducted in newborn mice (<4 days old) to guarantee more colostrum intake and to avoid perinatal mortality. A total of 67 pups were used in this study. The maternal separation protocol was adapted from Calikoglu et al [8]. This method has the advantage of providing littermate-control, well-nourished pups to compare with undernourished ones. Weight and tail length were recorded daily until sacrifice immediately before the separation handling. A thermal pad was used to warm the pups during daily measurements (27 ± 2°C). Care was taken to assure the same level of handling for all experimental mice. Protocols from this study were previously approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Virginia and the University of Fortaleza (protocol #15005). All efforts were made to minimize the number of animals used and their suffering.
2.2. Nutritional status
In order to assess changes in growth and weight gain, daily tail length and body weight were analyzed, the former as a measure of linear skeletal growth. Experimental mice were monitored carefully by daily inspection of weight and tail length during the suckling time on days 4–14. Tail length was recorded by means of measuring gently the animal tail from the basis to the tip, using a digital caliber and a card board (to the nearest 0.1 mm). All measurements were conducted before starting the procedures of daily mice separation. Care was taken to keep the same degree of handling during this process for all mice.
2.3. Neonatal reflex ontogeny
We assessed the ontogeny of selected neonatal reflexes along the first two-weeks of life, including cliff avoidance, surface righting, dorsal immobility and swimming behaviors as described elsewhere [11,12]. The cliff avoidance reflex test is used to assess the integration of exteroceptive input (vibrissae) and locomotor output, providing information concerning motor skills as well as sensory function and/or processing [13,14]. The offspring is placed on a platform elevated 10 cm above a table top. The forelimbs and snout of the animals are positioned so that the edge of the platform passes just behind an imaginary line drawn between the eye orbits. Avoidance is scored by reflex latency between being placed on the edge and turning until it is parallel to the edge of the table (0= no response, latency > 60s; 1= response < 10s; 2= <5s). The surface righting reflex is a measure of the capacity to return to a prone position. Pups were placed on their backs on a smooth surface, and the time required to right themselves to a position where the four limbs touches the surface. The time is scored as follows: 2-righting ≤1 sec, 1-righting >1 and ≤2 sec, 0–>2 sec. The swimming behavior test is used to assess navigational and motor development. Pups are placed into a tank with water temperature maintained at 27 ± 1°C and swimming behavior was rated for direction (straight = 3, circling = 2, floating = 1) and head angle (ears out of water = 4, ears half out of water = 3, nose and top of head out of water = 2, and unable to hold head-up = 1). Dorsal immobility (tonus immobility) was measured by suspending the mice by the back up to approximately 10 cm above a flat surface. The procedure is performed with the aid of a modified twissor, covered by rubber on its tip to avoid trauma. The dorsal immobility scores the latency time (in seconds) until the mouse moves to escape the grasping and is related with the ventrolateral mesencephalic periaqueductal gray matter activity [15]. N=8 for PMS and N=6 unseparated mice were used for all behavioral tests done.
2.4. Systemic inflammation marker
In order to find whether prolonged maternal separation could cause systemic inflammation, we measured serum C-reactive protein (N=10 for both PMS and unseparated mice), which is a liver acute phase protein, released during unspecific inflammatory responses. High sensitivity C reactive protein (hs CRP) was assessed using a commercial kit (Labstest® and Bioclin, Belo Horizonte, Brazil).
2.5. Nutrition and stress-related hormone serum levels
In order to find whether prolonged maternal separation could cause changes in nutrition and stress-related hormones (leptin, adiponectin, and corticosterone) serum levels were assessed. On day 14, blood was drawn from some mice following decapitation (N=10 per group). We also assessed IGF-1 serum levels as IGF-1 deficiency has been associated with stunting. These hormones were measured in serum samples using an enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
2.6. Hippocampal structure and amino acid levels
2.6.1. Hippocampal stereology
A total of 14 male C57BL/6J mice were used in this experiment. Mice were divided into the following four groups: 1-unseparated mice (n=7), 2- prolonged maternal separated mice (n=7). Hippocampal tissue was collected on day 14 (the end point), following a transcardiac perfusion-fixation with Palay’s solution (containing 1% formaldehyde and 1% glutaraldehyde in 0.12M phosphate buffer at pH 7.2) [16] and immediately immersed in the same solution for stereological analyses.
After euthanasia the hippocampus was dissected out from the brain and halved. Each hemi-hippocampus was systematically, uniformly and randomly selected (SURS) [17,18] between left and right and weighed. Subsequently, the selected hemi-hippocampus (left or right) was manually straightened along its septotemporal axis to diminish the anatomical organ curvature.
Subsequently, the straightened hippocampus was embedded in a 10% agar solution and exhaustively cut into a thin section (100μm-thick) followed by a thick section (1 mm-thick), alternately and using a vibratome (VT 1000 S (Leica®) [19]. Sections were orthogonal to hippocampus’ long axis and a fraction (10−1) of those paired sections (thin and thick sections) was SUR selected. The average interval (K) between the section pairs was 400μm. Thin sections were collected onto glass slides, stained with 1% alcoholic toluidine blue solution, dehydrated in progressive ethanol concentrations, mounted under a cover slip with DPX (Fluka®) and used not only to record the exact position of the studied layer in the hippocampus, i.e. mapping sections [19], but also for the estimation of post-embedding hippocampus and hippocampal layer volumes using Cavalieri’s principle.
Thick sections were used to produce vertical, uniform and random sections (VUR sections) [20]. First of all thick sections were positioned onto a transparent plate of Silgard® in the centre of a circle with 36 (360°) equidistant divisions along the perimeter. Next step, a random number between 0 and 36 was generated using a random number table (SURS) [19].
Subsequently, a transparent cutting guide containing lines was placed onto the thick section at the same selected angle. Finally, a razor blade was used to produce bars from the sections guided by the lines in the cutting guide.
Each bar containing hippocampus was rotated by 90° around the vertical axis to the sections and allowed for VUR sections parallel to the vertical axis. The bars from each section were then re-embedded in a 10% agar solution and exhaustively sectioned at 50μm-thick using a vibratome.
Thick sections (from bars) were collected onto glass slides, stained with an 1% alcoholic toluidine blue solution, dehydrated in progressive ethanol concentrations, mounted under a cover slip with DPX (Fluka®) and they were therefore used to simultaneously estimate number of hippocampal neurons.
Section images were acquired using a Leica® DMR Microscope coupled with a Digital Camera PLA622 (Pixellink®) and a stereological software New Cast Visiopharm® (version 2.16.1.0). The area of the unbiased counting frame used was 5,000 μm2 [21]. Before starting the counting procedure, a z-axis distribution (calibration) was performed to know the neuron distribution throughout section thickness and establish the dissector height, which was 20μm. Section thickness was measured in every second field of view using the central point on the unbiased counting frame. The neuron nucleus was defined as the counting unit.
In this study the whole hippocampal structure including the granular cell layer of dentate gyrus (DG) and the CA1 and CA3 pyramidal cell layers were defined at all levels of sectioning according to the stereotaxic coordinates published elsewhere [22].
Hippocampus Volume: V (HIP)
Hemi-hippocampus fresh weight was converted into volume to estimate tissue shrinkage. The formula used was: V(volume) = m(mass)/d(density). The specific density (d(density)) was 1.04 g · cm−3 [19]. In the results we have reported the bilateral volume which was elicited multiplying V (HIP) by 2 since the left or right hemi-hippocampus was SUR sampled [18].
Volume of CA1, CA3 and Dentate Gyrus layers: V and V(HIPpost-emb)
The volume of the examined CA layer and the post-embedding volume of the selected hemi-hippocampus were estimated using Cavalieri’s principle on the thin sections (100μm-thick). Then, V or V(HIPpost-emb):= ΣP · ap · BA · K, where P is the number of test points hitting the tissue (region or the whole hippocampus) (we have used on average 100 (hippocampal region) and 200 (HIP) points per animal), ap is the area associated with each test point, BA is the mean block advance (or mean section thickness= 100 μm) and K is the distance between the sections sampled.
The error variance of Cavalieri’s estimator (CE) was estimated according to Nyengaard [23]. Therefore, the error variance of Cavalieri’s estimate was 0.04 for group 1, 0.04 for group 2, 0.03 for group 3, 0.03 for group 4, and 0.04 for group 5.
The volume shrinkage was then calculated as:
Volume shrinkage = 1 − volume after : volume before, according to Hosseini-Sharifabad M, Nyengaard [19].
Numerical density CA1 and CA3 pyramidal cells and DG granule cells: NV
The optical disector was used to estimate the numerical density of CA1 neurons in a given hemi-hippocampus. The formula for NV estimation is: where ΣQ− is the total number of particles counted by dissectors, a is the counting frame area, p is the number of reference points per counting frame and ΣP is the total number of reference points in each counting frame, which hit the reference volume, the studied hippocampal layer, t̄q− is the Q−-weighted mean section thickness measured and BA is the mean block advance in the vibratome.
Total Number of CA1, CA3 pyramidal cells and DG granule cells: N
The total number of neurons was estimated by multiplying the numerical density of the studied hippocampal layer neurons by the volume of the respective layer. Therefore:
The bilateral number was obtained by simply multiplying N (neuron estimate.2) since the left or right hemi-hippocampus was SUR sampled [18].
The error variance of total neuron number (CE(N)) was estimated as shown in Gundersen et al. [24] and Nyengaard [23].
2.6.2. Hippocampal amino acids levels
Since the hippocampal neurotransmission activity has been found disturbed by early maternal separation challenge [25], we assessed hippocampal amino acid levels following PMS. To avoid neurochemical changes due to anesthesia, a subset of 19 mice (N=10 for PMS and N=9 for unseparated mice) were euthanized by decapitation to assess hippocampal amino acid levels. Dissected hippocampi were obtained from 14 day-old experimental pups, and stored at −80°C until analyzed. Analyses of amino acids (aspartate, glutamate, taurine, and gamma-aminobutyric acid, GABA) were carried out from dissected hippocampus using a high-performance liquid chromatography apparatus (HPLC, Shimadzu, Japan), and a fluorimetric detection method. Briefly, frozen tissue specimens were homogenized in 0.1M perchloric acid, and sonicated for 30 s at 25°C. After sonication, samples were centrifuged at 15,000 rpm, for 15min at 4°C. Supernatants were removed and filtered through a membrane (Millipore, 0.22 μm), and the amino acids were derivatized with mercaptoethanol and O-phthaldialdehyde. O-phthaldialdehyde derivatives were then separated on a C18 column (150mm × 4.6mm; from Shimadzu, Japan) and after derivatization, amino acids were separated, using a mobile phase, consisting of sodium phosphate buffer (50mM, pH 5.5) and 20% methanol. The area of each peak was determined with a Shimadzu software, and compared with the peak area of the corresponding external standard. Amino acid concentrations were expressed as μmol/g of wet tissue.
2.6.3. Brain plasticity markers
In order to evaluate brain plasticity following PMS, we assessed the protein expression of synaptophysyn (SYN), a synaptic vesicle protein and therefore a maker of synaptic activity and myelin basic protein (MBP), a constitutive protein associated with the myelin sheet, in the overall brain tissue. In a subset of mice the entire brain was carefully dissected and immediately frozen in liquid nitrogen. Thawed specimens were pulverized with an electric homogenizer (ultra-Turrax homogenizer, Sigma, Saint Louis, MO), containing lysis buffer and then transferred to test tubes with protease inhibitor cocktail and centrifuged at 14 000 rpm. Supernatants were assayed using the bicinchoninic acid method, BCA Protein Assay Kit (Pierce, Rockford, IL) to standardize 50μg of protein product. Samples were loaded into 12.5% denaturizing polyacryamide mini gels (Bio-Rad, Hercules, CA), and gels were transferred overnight and then blotted onto nitrocellulose membranes. Membranes were incubated with either rabbit synaptophysin (SYN) or myelin basic protein (MBP) antibodies (at dilution of 1:2000 and 1:500 respectively) for 1 hour and then rinsed 3 times in rinsing buffer then incubated in a secondary antibody (1:5000) and rinsed as described above. Each membrane was washed and exposed to Kodak X-Omat AR film (Kodak, Rochester, NY). Stripped blots were later incubated with GAPDH antibodies as an internal control. In addition, we evaluated SYN immunolabeling in paraffin-embedded hippocampi of the experimental mice, as described elsewhere [26]. At least, five hippocampus samples per group were used for both western blot and immunohistochemistry.
2.7. Statistical analysis
Unpaired Student t test was used to assess mean differences between groups. P < 0.05 was considered statistically significant. Data are represented as mean ± standard error (SEM). Statistical analyses were performed using Graph Pad Prism 6.0 (La Jolla, CA, USA).
3. Results
3.1. Nutritional status
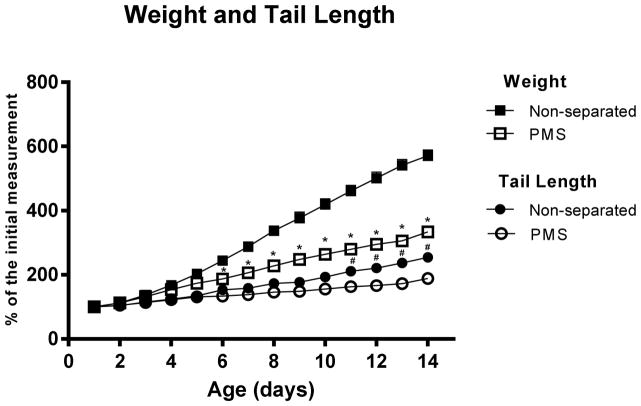
In this model, we have found significant slower linear growth and poor weight gain, following prolonged maternal separation, during suckling, as measured by daily tail length and weight gain, as compared to their respective nourished, non-separated controls, p<0.05 (Fig. 1). As expected, linear growth deficits appeared later on PND 11, one-week following the onset of the maternal separation protocol, indicating a chronic under nutrition. Significant weight faltering was found earlier on PND 6, two-days following the onset of the maternal separation protocol. On day 14, at the end point, nourished mice displayed an average with 1.6-fold higher body weight gain vs. challenged mice (Fig. 1). In addition, PMS-mice are 3-SD below the mean tail length of the unseparated nourished controls, characterizing a poor linear length growth, analogous to children stunting.
Fig. 1.
Relative weight and tail length gain from experimental mice during timed-prolonged maternal separation (PMS) (N=16) and respective non-separated controls (N=10). Curves are presented as percentage of initial weight at post-natal day 1. Results are expressed in mean ± SEM and were analyzed by unpaired Student t test. * P <0.05 vs non-separated controls.
Results are expressed in mean ± SEM and were analyzed by unpaired Student t test. * P <0.05 vs non-separated controls.
3.2 Neonatal reflex ontogeny
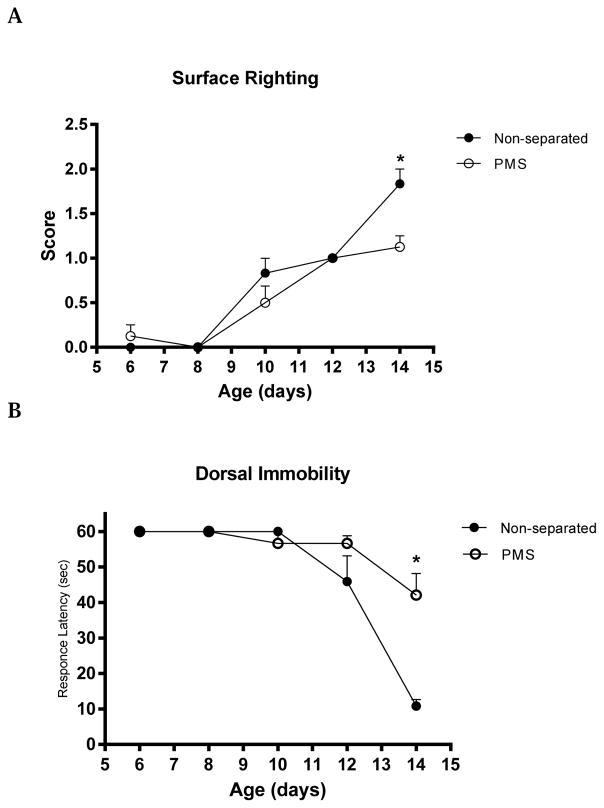
Prolonged maternal separation induced deficits in surface righting and dorsal immobility reflexes, which were seen in the end of the second week of life (on day 14) (p< 0.05) (Fig 2). No significant differences were found regarding swimming behaviors and cliff avoidance over the first two weeks.
Fig. 2.
Behavioral tests (A: surface righting) and (B: dorsal immobility) conducted in the first two weeks of life in the non-separated (N=6) and prolonged maternal separated (PMS) (N=8) groups. For the surface righting testing, results are shown in scores. The performed time was scored as follows: 2-righting ≤1 sec, 1-righting >1 and ≤2 sec, 0–>2 sec. For the dorsal immobility test, data are shown as latency mean time in seconds. The results are shown as mean ± SEM and were analyzed by unpaired Student t test. * P <0.05 vs non-separated controls.
3.3. C-reactive protein (CRP) serum levels
Prolonged maternal separation caused significant increase in CRP serum levels (6.6 ± 1.8 mg/L, n=10) when compared with the well-nourished counterparts (4.9±1.0 mg/L, n=10), p=0.01, (Fig. 3).
Fig. 3.
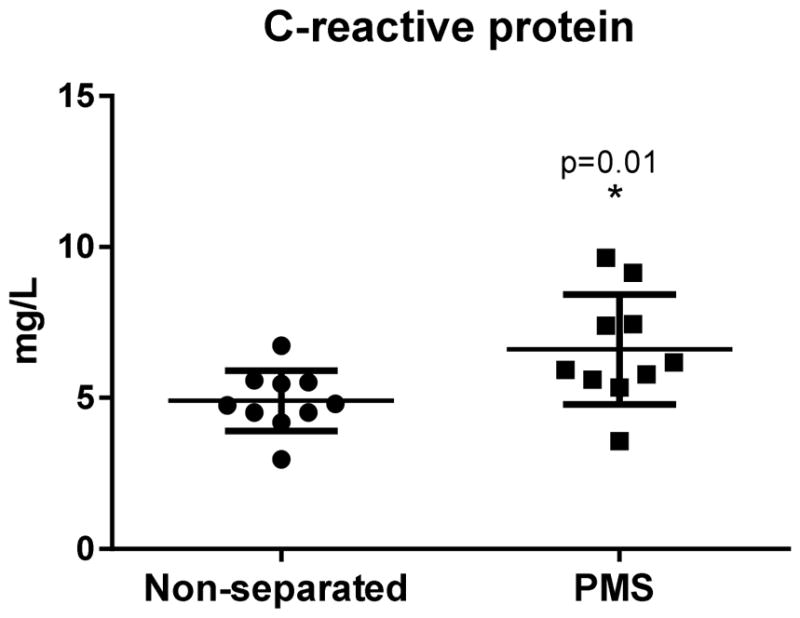
C-reactive protein serum levels of experimental mice following prolonged maternal separation (PMS) (N=10) and the respective unseparated-controls (N=10) (on day 14). Data are expressed in mg/L. Results are shown as mean ± SEM and were analyzed by unpaired Student t test. * P <0.05 vs non-separated controls.
3.4. Nutrition and stress-related hormones
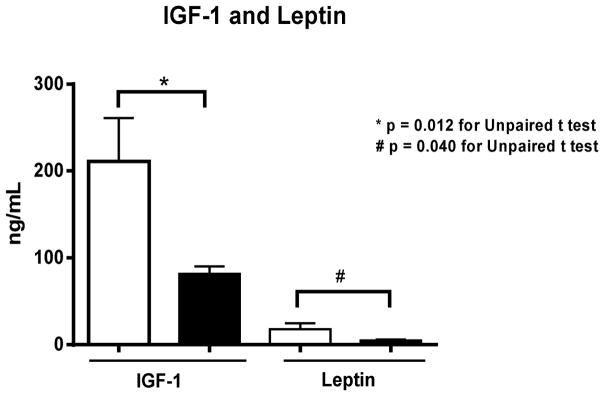
We found significant reductions in leptin and IGF-1 serum levels following prolonged maternal separation when compared with the non-separated controls (Fig. 4). No significant differences were found with corticosterone (79.7±35.9 ng/L, n=5 vs 152.3±85.5, n=8, p=0.1) and adiponectin (8176.2±0.7 ng/L, n=5 vs 8202.7±1996.8, n=8, p=0.9) for the non-separated and PMS-groups, respectively.
Fig. 4.
IGF-1 and leptin serum levels of experimental mice following prolonged maternal separation (PMS) and the respective unseparated-controls (on day 14). At least five mice were used for these analyses. Data are expressed in ng/mL. Results are shown as mean ± SEM and were analyzed by unpaired Student t test. * P <0.05 vs non-separated controls.
3.5. Hippocampal stereology
The hemi-hippocampus shrinkage volume (%) was estimated to be (mean ± SD): 4.75 ± 1.22 (group 1); 5.56 ± 1.52 (group 2); 4.16 ± 1.21 (group 3); 5.05 ± 1.17 (group 4), and 5.75 ± 1.12 (group 5). No correction for global shrinkage was performed since inter-group differences were not statistically significant (p= 0.13). The malnutrition challenge reduced the total volume and area of the CA3 hippocampal field and CA3 pyramidal layer volume. In addition, we found reduced CA1 pyramidal layer area. No other significant changes were found regarding the hippocampal CA1 and CA3 fields and DG between experimental groups (Table 1).
Table 1.
Hippocampal stereological estimations.
| Hippocampal fields | Non-separated | PMS | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N° cells (104) | Volume (108) | Area (105) | N° cells/Area | N° cells (104) | Volume (108) | Area (105) | N° cells/Area | ||
|
|
|
||||||||
| DG | Gr | 7.7 ± 1.6 | 1.3 ± 0.2 | 8.0 ± 1.5 | 0.1 ± 0.02 | 8.0 ± 1.0 | 1.2 ± 0.1 | 5.7 ± 0.4 | 0.1 ± 0.01 |
| Mol | - | 2.1 ± 0.5 | 12.7 ± 3.1 | - | - | 1.8 ± 0.1 | 9.9 ± 0.9 | - | |
| Pol | - | 1.3 ± 0.3 | 7.2 ± 1.8 | - | - | 0.7 ± 0.1 | 3.6 ± 0.7 | - | |
| Total | - | 4.7 ± 0.6 | 27.9 ± 5.9 | - | - | 3.7 ± 0.4 | 19.2 ± 2.0 | - | |
| CA1 | Pyr | 4.4 ± 0.5 | 1.0 ± 0.08 | 7.3 ± 0.5 | 0.06 ± 0.008 | 4.5 ± 0.4 | 1.1 ± 0.06 | 5.7 ± 0.3* | 0.07 ± 0.009 |
| Or | - | 1.9 ± 0.1 | 10.2 ± 0.9 | - | - | 1.8 ± 0.1 | 10.2 ± 0.8 | - | |
| Rad | - | 1.8 ± 0.1 | 10.1 ± 1.0 | - | - | 1.7 ± 0.1 | 9.9 ± 0.7 | - | |
| Total | - | 4.7 ± 0.2 | 27.6 ± 2.3 | - | - | 4.6 ± 0.2 | 25.8 ± 1.7 | - | |
| CA3 | Pyr | 4.4 ± 0.4 | 1.6 ± 0.1 | 10.4 ± 1.3 | 0.04 ± 0.005 | 4.6 ± 0.5 | 1.2 ± 0.1* | 7.2 ± 0.7 | 0.06 ± 0.007 |
| Or | - | 2.3 ± 0.3 | 12.6 ± 1.9 | - | - | 1.5 ± 0.1 | 8.5 ± 0.7 | - | |
| Rad | - | 1.5 ± 0.1 | 8.8 ± 1.0 | - | - | 1.2 ± 0.1 | 6.5 ± 0.8 | - | |
| Total | - | 5.4 ± 0.4 | 31.8 ± 3.8 | - | - | 3.9 ± 0.3* | 22.2 ± 1.9* | - | |
PMS= prolonged maternal separated mice
DG= Dentate gyrus; CA1= Cornus Ammonis 1; CA3=Cornus Ammonis 3
Gr=granular cells and Pyr=pyramidal cell. Mol=molecular layer; Pol=polymorphic layer; Or=oriens layer; Rad=stratum radiatum layer. Total=sum of each of the hyppocampal field layers.
p<0.05 versus the non-separated group by Unpaired Student t –test. N=7 for non-separated and PMS groups. Data are expressed as mean ± standard error of the mean (SEM).
3.6. Hippocampal amino acid levels
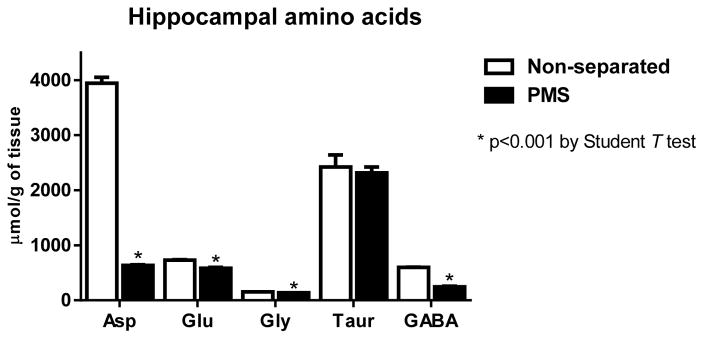
The amino acid assessment in the hippocampus using the HPLC method showed that PMS induced significant decreased concentrations (μmol/g tissue) of aspartate (3944.6±317.1, n=9 vs 636.5±33.3, n=10), glutamate (730.2±16.6, n=9 vs 584.1±47.1, n=10), glycine (155.5±3.9, n=9 vs 135.9±4.7, n=10), and GABA (598.9±10.9, n=9 vs 246.7±27.9, n=10) (p<0.05), compared to the unseparated control group (Fig. 5).
Fig 5.
Hippocampal amino acid levels of experimental mice following prolonged maternal separation (PMS) (N=10) and the respective unseparated-controls (N=9), detect by high-performance liquid chromatography on day 14. Data are expressed in μmol/g of tissue. Results are shown as mean ± SEM and were analyzed by unpaired Student t test. * P <0.05 vs non-separated controls.
3.7. Synaptophysin (SYN) and Myelin basic protein (MBP)
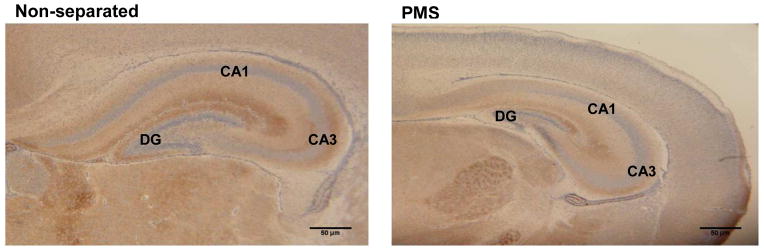
We could not find significant different expression of either SYN or MBP in the overall brain tissue from experimental mice. Qualitatively there was a conspicuous and stronger SYN immunolabeling in overall hippocampus, but particularly in the DG and CA3 regions (Fig. 6).
Fig. 6.
Representative hippocampal synaptophysin (SYN) immunohistochemistry from experimental groups, 100X. N=5 for each group. Scale bar: 50 μm.
4. Discussion
In our study, we have used a prolonged maternal separation (PMS) model characterized by repetitive and long-term maternal-offspring separation in the first two-weeks of life to induce moderate-to-severe under nutrition. The first two weeks of life in mice is a key time-window of brain development, especially in the cerebellum, cerebral cortex, and the hippocampus [10]. In this model, PMS mice were found to have a poor linear growth (analogous to human stunting) and were profoundly under-weight (below 2SD of the control mean). Indeed, neonatal PMS induced a chronic malnutrition status.
In addition, surface righting and dorsal immobility were affected by maternal separation only in the end of experimental protocol (day 14), which may indicate more cumulative adverse effects of maternal separation. Delays in the development of some neonatal reflexes were found by Ladd et al. (2010) in a study of malnutrition using larger litters in the second week of postnatal life. In the same study, the authors also reported a beneficial effect of zinc supplementation (500mg/L in the drinking water of animals) in improving some of the parameters [26].
The surface righting reflex involves the vestibular-cerebellar neural pathways [27], suggesting that the SMP affects negatively the cerebellum development, which has a strong postnatal development component. Interestingly, a transient increase in some markers of cerebellar myelination and neurotrophic factors was found after 3h-maternal separation compared to unseparated controls, which may indicate a compensatory response to rescue normal cerebellar development [28]. Our current study using a chronic maternal deprivation model has not assessed morphological changes in the cerebellum that may have contributed to delays in the acquisition of neonatal reflexes. In support of our findings, Zhang and colleagues also found deficits in surface righting in the mouse offspring of food-restricted mothers [27].
The dorsal immobility response (or tonic immobility) involves the periaqueductal gray matter (PGM) of the midbrain. This structure has a role in pain modulation and defensive behavior and the fine motor output for basic survival [29]. The PGM keeps anatomical and functional connections with structures such as the thalamus, hypothalamus and amygdala [30,31]. However, impairment of PGM with involvement of hippocampus due to chronic malnutrition has not yet been reported.
Barros and colleagues adapted a low protein and high carbohydrate diet to mimic the dietary characteristics of endemic malnutrition regions of Brazil. They found delays in the maturation of most of the locomotion-related neonatal reflexes tested in the first three weeks of life in offspring breastfed by undernourished dams [32].
Another important finding of this study was a noticeable reduction in hippocampal synaptophysin (SYN) immunostaining of PMS-challenged mice, especially in the CA3 and DG regions. However, we could not find differences in SYN and MBP in the total brain tissue. These findings suggest that the hippocampus is highly sensitive to the PMS-induced effects. Wistar rats separated from dams for 4 hours daily for 18 days in the first two weeks of life did not show hippocampal CA1 and CA3 remarkable increase in SYN expression on days 25 to 60 days compared to the unseparated controls and did not show the same degree of needed pruning later on post-natal day 100 in the same hippocampal fields [33]. The hippocampal layers are the following: alveus, stratum oriens, stratum pyramidale (with pyramidal cell bodies), stratum radiatum, stratum lacunosum-moleculare in the hippocampal proper (in the cornus amnonis (CA) fields); in the dentate gyrus area: stratum moleculare, stratum granulosum (granular cell bodies), and the hilus of the fascia dentata [34].
The hippocampus is a brain region that seems to be particularly vulnerable to the effects of stress due to a high density of glucocorticoid receptors and susceptibility to epigenetic modulation [35]. Early exposure to stress or corticosteroids can cause hippocampus remodeling (or atrophy) [36], effects which may be associated with a reduction of the dendritic arborization, increase vulnerability to a subsequent insult, an neurogenesis disorders in adulthood [37].
Albeit not reaching statistical significance corticosterone serum levels were higher after PMS compared to controls. Corticosterone serum levels were measured on post-natal day 14, ten days after the onset of maternal separation. At the end of the second postnatal week, mice usually have access to mixed feeding and are not so dependent of breast milk. Perhaps during early maternal separation, when the mice are highly dependent on maternal care, corticosterone levels would be more affected. Thus, we cannot rule out the possibility that early changes in corticosterone levels have influenced hippocampal alterations seen in our study. Hsu et al after performing handling and maternal separation in Sprague–Dawley rats on post-natal days 9 (for 30 min) and 10 (for 6h) did not find difference in corticosterone levels compared to unseparated pups on post-natal day 10. Nonetheless, these authors identified later alterations in GABA neurotransmission into adulthood [38].
The large intra-group variation in corticosterone levels in our study among separated suckling mice may be a result of a greater competition for breast-milk compared with the nourished counterparts. We speculate that among the undernourished-separated mice, the dominant pups would have slightly more access to the breastmilk and therefore less stressed while returned to the dams, as opposed to the non-dominant siblings, effect which may partly explain these variations.
Early neonatal stress may induce lasting hippocampal changes that may go into adulthood. Significant reductions in hippocampal volume (71%) and size of pyramidal (62%) and granular (60%) cell layers of the adult hippocampus have been found following early-life maternal deprivation [39].
Oreland and colleagues did not find significant differences in the volumes of CA1, CA2, CA3 fields and DG of the hippocampus in 3 week-old rats separated either by 15 min or 6 hours daily during the first 21 days of post-natal life. However, they found reduction in numerical density of neurons in DG and CA3 in the 6h-separation group [40]. Studies using other models of protein deprivation both prenatally [41] and postnatally [42] did find decreases in the number of CA1 pyramidal cells and irreversible loss of hippocampal cholinergic and GABAergic interneurons, respectively. In an earlier study from our group, breastmilk deprived-suckling pups raised in large litters did not show differences in CA1 numerical neuronal density, area and volume. However, an increase in the volume of CA1 pyramidal neurons was found in the undernourished group, suggesting cell degeneration [26].
Despite no changes in the total cell number in the hippocampal regions, this study found reductions in the CA1 pyramidal cell layer area and CA3 area and volume. CA1-CA3 synaptic neuron connections are very important for learning as a key piece of the trisynaptic circuitry of the hippocampus [43]. Presumably, factors such as loss of neuronal and glial extensions may have contributed to these volume/area changes, regardless of cell numbers.
The amino acids glutamate, γ-aminobutyric acid, glycine, aspartate, and taurine are neurotransmitters and neuromodulators in the CNS [44]. Glutamate is critical for hippocampal circuitry development and synapses, as well as an important mediator of emotional and cognitive behaviors [45]. GABA has been described as an excitatory neurotransmitter in the neonatal brain, playing a role in synaptic plasticity in the hippocampus circuits [46], and may have a protective role to hippocampal pyramidal neurons against hypoxia-ischemia injury in neonatal mice [47].
Pickering et al have found diminished hippocampal RNA expression of NMDA NR2B, AMPA GluR1 and GluR2 25 weeks after 6h-daily maternal separation during 1–21 post-natal days [48]. In our study, we found decreased hippocampal levels of glutamate in the PMS group compared to the controls at 14 days of age.
Similarly to our findings, Ladd et at found lower hippocampal levels of aspartate and glycine in the undernourished mice [26], however these effects were not reversed by glutamine and zinc treatment. In addition, Rotta et al. after inducing protein malnutrition to young rats by restricting protein content of the mother’s diet during pregnancy and lactation, have found reduced Na+-independent glutamate binding in the cell membranes of the cerebral cortex and low vesicular uptake [49].
PMS diminished leptin and IGF-1 serum levels in 14 day-old mice, similar to what was found in Zimbabwean stunted children, an effect also associated with systemic inflammation [9]. Reduced circulating leptin and increased corticosterone levels were found after neonatal maternal deprivation on post-natal days 9 and 10 (critical period for hypothalamus development) by 24h separation [50].
Viveros et al have found increased hypothalamic leptin receptor mRNA levels 24 h after maternal separation (on day 9) in both genders, which were normalized in females 12 h later but not in males. Hypothalamic IGF-1 mRNA levels were found significantly lower after 24h of maternal separation [50]. In another study by the same group, 24h-maternal separated rats (on day 9) and euthanized rats on days 13, 35 and 75 showed significant reduced leptin plasma levels only in day 75. Adiponectin levels were also reduced by day 75 in females. They did not find changes in hypothalamic levels of IGF-1 and leptin receptor mRNAs [51]. Interestingly, in the hypothalamus, a saturable leptin-transporter takes leptin more rapidly in lower serum leptin levels. Conversely, the hippocampus takes up leptin more rapidly at higher serum leptin levels [52], effects which may explain why no difference in hypothalamic leptin mRNA levels was found by Viveros (2010) and may in part explain the structural and functional alterations seen in the hippocampus in our study due to lower PMS-induced serum leptin levels. Leptin has been recognized to be important for hippocampal synaptic function [53]. Noteworthy, leptin modulates GABAergic transmission in developing CA3 pyramidal cells of newborn rats [54], a field of the hippocampus where we found poor synaptophysin immunolabeling in PMS-challenged mice.
Peak postnatal leptin occurs around the tenth day of life. Thus, episodes of maternal separation break this development signal [55]. Postnatal leptin has emerged as a critical factor affecting the maturation of the pituitary-hypothalamic axis and possibly other brain regions [25,56]. In addition, leptin may influence inflammatory responses [57].
In our study, increased CRP serum levels (a marker of systemic inflammation [58] following PMS suggest chronic inflammation, which may be related to the intestinal barrier disruption and continuous (and low grade) gut-to-blood bacterial translocation due to neonatal stress [59]. Undernutrition in the first two weeks of post-natal life has been associated with intestinal inflammation in mice [26] and may predispose and potentiate early-life enteric infections [60,61]. Parental separation may induce a lasting inflammation going into adulthood [62]. Recently, Preidis and colleagues inducing undernutrition in mice with a similar model of timed maternal separation found urinary metabolites related with chronic inflammation and liver dysfunction [63].
Low leptin, IGF-1 levels and body weight have been associated with marasmic children [64]. Prendergast et al. (2014) found that Zimbabwean stunted children (6 weeks to 12 months of age) show increased CRP and AGP and reduced IGF-1 and IGFBP3 plasma levels. In addition, stunted children (aged 3–12 months) showed high levels of intestinal fatty acid-binding protein (FABP-I, a marker of intestinal cell shedding and barrier disruption) in children, supporting this relationship of intestinal barrier breakdown, inflammation and poor IGF-1 responses. One recent clinical trial has documented improvements in linear growth and IGF-1 levels with reduced CRP circulating levels in children living in an endemic area of enteric infections following vitamin A and zinc supplementation [65], suggesting an important role of systemic inflammation regulating IGF-1-growth effects and potential benefits with nutritional interventions.
The prolonged undernutrition period, with restriction of breastfeeding (without access to food and water) to the pups, especially during the 12h-maternal separation protocol (Day 6 to Day 14), may have caused transitory severe starvation and therefore a strong driver of nutrient deficiency to the developing hippocampus. Caution is needed though to extrapolate our findings of this mouse model to humans, whom are exposed to a very much complex environmental and maternal influences.
5. Conclusions
To the best of our knowledge, this is the first study associating maternal separation-induced undernutrition, which has led to systemic inflammation, deficits in IGF-1 levels and growth, and hippocampal alterations. This model may be helpful to further study the gut-brain axis associated with environmental enteropathy in areas of endemic malnutrition and enteric infections in the developing world and the potential use of nutritional interventions to ameliorate these effects. Interventional studies with key brain trophic micronutrients (such as zinc supplementation) are now being planned by our laboratory to redirect the trajectory of normal hippocampal development in relationship with the intestinal microbiota (a recognized factor influencing hippocampal neurotransmission [66]) to maternal separated mice. Our group has demonstrated the benefits of zinc supplementation to ameliorate cognitive outcomes in children at risk for malnutrition and enteric diseases [65, 66].
Highlights.
Prolonged maternal separation induces systemic inflammation
Prolonged maternal separation affects IGF-1 serum levels and growth
Prolonged maternal separation delays neonatal reflex development
Prolonged maternal separation affects hippocampal morphology and amino acid levels
Acknowledgments
This work was supported in part by NIH research grant 5RO1HD053131, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Dietary Supplements, and the Brazilian grants from CNPq and CAPES. The authors would like to thank Dr. Patricia Foley for veterinarian technical support and Dr. José Paulo Andrade for the excellent comments and suggestions to improve this manuscript.
Footnotes
NS contributed with the stereological studies.
ILF and RBO contributed with the behavioral studies
ILF, RBO and RLG contributed with the study design, study analysis and manuscript preparation
GAM and PBF contributed with neurochemical brain analyses.
JIAL and GMA contributed with hormonal and CRP serum analyses
DGC, KMC and RSR contributed with animal experimentation and data collection
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollitt E. Developmental sequel from early nutritional deficiencies: conclusive and probability judgements. J Nutr. 2000;130:350S–353S. doi: 10.1093/jn/130.2.350S. [DOI] [PubMed] [Google Scholar]
- 2.Ivanovic DM, Leiva BP, Perez HT, Almagia AF, Toro TD, Urrutia M, et al. Nutritional status, brain development and scholastic achievement of Chilean high-school graduates from high and low intellectual quotient and socio-economic status. Br J Nutr. 2002;87:81–92. doi: 10.1079/bjn2001485. [DOI] [PubMed] [Google Scholar]
- 3.Ivanovic DM, Leiva BP, Perez HT, Inzunza NB, Almagia AF, Toro TD, et al. Long-term effects of severe undernutrition during the first year of life on brain development and learning in Chilean high-school graduates. Nutrition. 2000;16:1056–1063. doi: 10.1016/s0899-9007(00)00431-7. [DOI] [PubMed] [Google Scholar]
- 4.Benitez-Bribiesca L, De lR-A I, Mansilla-Olivares A. Dendritic spine pathology in infants with severe protein-calorie malnutrition. Pediatrics. 1999;104:e21. doi: 10.1542/peds.104.2.e21. [DOI] [PubMed] [Google Scholar]
- 5.Beas-Zarate C, Ortuno-Sahagun D, Angel Meza AR, Feria-Velasco A. Effect of a corn diet during development on [3H]-spiperone binding in the brain of rats at the perinatal stage. Comp Biochem Physiol A Physiol. 1995;112:161–166. doi: 10.1016/0300-9629(95)00073-g. [DOI] [PubMed] [Google Scholar]
- 6.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 7.Nishi M, Horii-Hayashi N, Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci. 2014;8:166. doi: 10.3389/fnins.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calikoglu A, Karayal A, D’Ercole A. Nutritional regulation of IGF-I expression during brain development in mice. Pediatr Res. 2001;49:197–202. doi: 10.1203/00006450-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, et al. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One. 2014;9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu DX, Marchetto MC, Gage FH. How to make a hippocampal dentate gyrus granule neuron. Development. 2014;141:2366–2375. doi: 10.1242/dev.096776. [DOI] [PubMed] [Google Scholar]
- 11.Rotstein M, Bassan H, Kariv N, Speiser Z, Harel S, Gozes I. NAP enhances neurodevelopment of newborn apolipoprotein E-deficient mice subjected to hypoxia. J Pharmacol Exp Ther. 2006;319:332–339. doi: 10.1124/jpet.106.106898. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T. Effects of litter size on behavioral development in mice. Reprod Toxicol. 1998;12:613–617. doi: 10.1016/s0890-6238(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleoni GC, Fanini D, Sponta AM, Palumbo G, Giorgi R, Adams PM. Effects of maternal exposure to polychlorobiphenyls (PCBs) on F1 generation behavior in the rat. Fundam Appl Toxicol. 1988;11:440–449. doi: 10.1016/0272-0590(88)90108-x. [DOI] [PubMed] [Google Scholar]
- 14.Palanza P, Parmigiani S, vom Saal FS. Effects of prenatal exposure to low doses of diethylstilbestrol, o,p’DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm Behav. 2001;40:252–265. doi: 10.1006/hbeh.2001.1697. [DOI] [PubMed] [Google Scholar]
- 15.Monassi CR, Leite-Panissi CR, Menescal-de-Oliveira L. Ventrolateral periaqueductal gray matter and the control of tonic immobility. Brain Res Bull. 1999;50:201–208. doi: 10.1016/s0361-9230(99)00192-6. [DOI] [PubMed] [Google Scholar]
- 16.Andrade JP, Madeira MD, Paula-Barbosa MM. Effects of long-term malnutrition and rehabilitation on the hippocampal formation of the adult rat. A morphometric study. J Anat. 1995;187(Pt 2):379–393. [PMC free article] [PubMed] [Google Scholar]
- 17.Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Madsen TM, Wegener G, Nyengaard JR. Changes in rat hippocampal CA1 synapses following imipramine treatment. Hippocampus. 2008;18:631–639. doi: 10.1002/hipo.20423. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini-Sharifabad M, Nyengaard JR. Design-based estimation of neuronal number and individual neuronal volume in the rat hippocampus. J Neurosci Methods. 2007;162:206–214. doi: 10.1016/j.jneumeth.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 21.Gundersen HJ. Estimators of the number of objects per area unbiased by edge effects. Microsc Acta. 1978;81:107–117. [PubMed] [Google Scholar]
- 22.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Elsevier; 2013. [Google Scholar]
- 23.Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999;10:1100–1123. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- 24.Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 25.Marco EM, Llorente R, Lopez-Gallardo M, Mela V, Llorente-Berzal A, Prada C, et al. The maternal deprivation animal model revisited. Neurosci Biobehav Rev. 2015;51:151–163. doi: 10.1016/j.neubiorev.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Ladd FV, Ladd AA, Ribeiro AA, Costa SB, Coutinho BP, Feitosa GA, et al. Zinc and glutamine improve brain development in suckling mice subjected to early postnatal malnutrition. Nutrition. 2010;26:662–670. doi: 10.1016/j.nut.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Li N, Yang J, Zhang T, Yang Z. Effects of maternal food restriction on physical growth and neurobehavior in newborn Wistar rats. Brain Res Bull. 2010;83:1–8. doi: 10.1016/j.brainresbull.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Miki T, Yokoyama T, Kusaka T, Suzuki S, Ohta K, Warita K, et al. Early postnatal repeated maternal deprivation causes a transient increase in OMpg and BDNF in rat cerebellum suggesting precocious myelination. J Neurol Sci. 2014;336:62–67. doi: 10.1016/j.jns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian HH, Holstege G. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog Brain Res. 2014;212:351–384. doi: 10.1016/B978-0-444-63488-7.00017-3. [DOI] [PubMed] [Google Scholar]
- 30.Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36:557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- 31.Monassi CR, Hoffmann A, Menescal-de-Oliveira L. Involvement of the cholinergic system and periaqueductal gray matter in the modulation of tonic immobility in the guinea pig. Physiol Behav. 1997;62:53–59. doi: 10.1016/s0031-9384(97)00134-0. [DOI] [PubMed] [Google Scholar]
- 32.Barros KM, Manhaes-De-Castro R, Lopes-De-Souza S, Matos RJ, Deiro TC, Cabral-Filho JE, et al. A regional model (Northeastern Brazil) of induced mal-nutrition delays ontogeny of reflexes and locomotor activity in rats. Nutr Neurosci. 2006;9:99–104. doi: 10.1080/10284150600772148. [DOI] [PubMed] [Google Scholar]
- 33.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 34.Lopes da Silva FH, Arnolds DE. Physiology of the hippocampus and related structures. Annu Rev Physiol. 1978;40:185–216. doi: 10.1146/annurev.ph.40.030178.001153. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala and Prefrontal Cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 37.Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 38.Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aksic M, Radonjic NV, Aleksic D, Jevtic G, Markovic B, Petronijevic N, et al. Long-term effects of the maternal deprivation on the volume and number of neurons in the rat neocortex and hippocampus. Acta Neurobiol Exp (Wars ) 2013;73:394–403. doi: 10.55782/ane-2013-1946. [DOI] [PubMed] [Google Scholar]
- 40.Oreland S, Nylander I, Pickering C. Prolonged maternal separation decreases granule cell number in the dentate gyrus of 3-week-old male rats. Int J Dev Neurosci. 2010;28:139–144. doi: 10.1016/j.ijdevneu.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Lister JP, Tonkiss J, Blatt GJ, Kemper TL, DeBassio WA, Galler JR, et al. Asymmetry of neuron numbers in the hippocampal formation of prenatally malnourished and normally nourished rats: a stereological investigation. Hippocampus. 2006;16:946–958. doi: 10.1002/hipo.20221. [DOI] [PubMed] [Google Scholar]
- 42.Andrade JP, Paula-Barbosa MM. Protein malnutrition alters the cholinergic and GABAergic systems of the hippocampal formation of the adult rat: an immunocytochemical study. Neurosci Lett. 1996;211:211–215. doi: 10.1016/0304-3940(96)12734-8. [DOI] [PubMed] [Google Scholar]
- 43.Gruart A, Delgado-Garcia JM. Activity-dependent changes of the hippocampal CA3-CA1 synapse during the acquisition of associative learning in conscious mice. Genes Brain Behav. 2007;6(Suppl 1):24–31. doi: 10.1111/j.1601-183X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 44.Perry M, Li Q, Kennedy RT. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal Chim Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molnar E, Pickard L, Duckworth JK. Developmental changes in ionotropic glutamate receptors: lessons from hippocampal synapses. Neuroscientist. 2002;8:143–153. doi: 10.1177/107385840200800210. [DOI] [PubMed] [Google Scholar]
- 46.Gaiarsa JL. Plasticity of GABAergic synapses in the neonatal rat hippocampus. J Cell Mol Med. 2004;8:31–37. doi: 10.1111/j.1582-4934.2004.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azimi-Zonooz A, Shuttleworth CW, Connor JA. GABAergic protection of hippocampal pyramidal neurons against glutamate insult: deficit in young animals compared to adults. J Neurophysiol. 2006;96:299–308. doi: 10.1152/jn.01082.2005. [DOI] [PubMed] [Google Scholar]
- 48.Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- 49.Rotta LN, Leszczinski DN, Brusque AM, Pereira P, Brum LF, Nogueira CW, et al. Effects of undernutrition on glutamatergic parameters in the cerebral cortex of young rats. Physiol Behav. 2008;94:580–585. doi: 10.1016/j.physbeh.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Viveros MP, Diaz F, Mateos B, Rodriguez N, Chowen JA. Maternal deprivation induces a rapid decline in circulating leptin levels and sexually dimorphic modifications in hypothalamic trophic factors and cell turnover. Horm Behav. 2010;57:405–414. doi: 10.1016/j.yhbeh.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Viveros MP, Llorente R, Diaz F, Romero-Zerbo SY, Bermudez-Silva FJ, Rodriguez de FF, et al. Maternal deprivation has sexually dimorphic long-term effects on hypothalamic cell-turnover, body weight and circulating hormone levels. Horm Behav. 2010;58:808–819. doi: 10.1016/j.yhbeh.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav. 2006;89:472–476. doi: 10.1016/j.physbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Irving AJ, Harvey J. Leptin regulation of hippocampal synaptic function in health and disease. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130155. doi: 10.1098/rstb.2013.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guimond D, Diabira D, Porcher C, Bader F, Ferrand N, Zhu M, et al. Leptin potentiates GABAergic synaptic transmission in the developing rodent hippocampus. Front Cell Neurosci. 2014;8:235. doi: 10.3389/fncel.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cottrell EC, Mercer JG, Ozanne SE. Postnatal development of hypothalamic leptin receptors. Vitam Horm. 2010;82:201–217. doi: 10.1016/S0083-6729(10)82011-4. [DOI] [PubMed] [Google Scholar]
- 56.Bouret SG, Simerly RB. Development of leptin-sensitive circuits. J Neuroendocrinol. 2007;19:575–582. doi: 10.1111/j.1365-2826.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 57.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 58.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 59.de Queiroz CA, Fonseca SG, Frota PB, Figueiredo IL, Aragao KS, Magalhaes CE, et al. Zinc treatment ameliorates diarrhea and intestinal inflammation in undernourished rats. BMC Gastroenterol. 2014;14:136. doi: 10.1186/1471-230X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coutinho BP, Oria RB, Vieira CM, Sevilleja JE, Warren CA, Maciel JG, et al. Cryptosporidium infection causes undernutrition and, conversely, weanling undernutrition intensifies infection. J Parasitol. 2008;94:1225–1232. doi: 10.1645/GE-1411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castro IC, Oliveira BB, Slowikowski JJ, Coutinho BP, Siqueira FJ, Costa LB, et al. Arginine decreases Cryptosporidium parvum infection in undernourished suckling mice involving nitric oxide synthase and arginase. Nutrition. 2012;28:678–685. doi: 10.1016/j.nut.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacey RE, Kumari M, McMunn A. Parental separation in childhood and adult inflammation: the importance of material and psychosocial pathways. Psychoneuroendocrinology. 2013;38:2476–2484. doi: 10.1016/j.psyneuen.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Preidis GA, Keaton MA, Campeau PM, Bessard BC, Conner ME, Hotez PJ. The undernourished neonatal mouse metabolome reveals evidence of liver and biliary dysfunction, inflammation, and oxidative stress. J Nutr. 2014;144:273–281. doi: 10.3945/jn.113.183731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haspolat K, Ece A, Gurkan F, Atamer Y, Tutanc M, Yolbas I. Relationships between leptin, insulin, IGF-1 and IGFBP-3 in children with energy malnutrition. Clin Biochem. 2007;40:201–205. doi: 10.1016/j.clinbiochem.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 65.Adriani M, Wirjatmadi B. The effect of adding zinc to vitamin A on IGF-1, bone age and linear growth in stunted children. J Trace Elem Med Biol. 2014;28:431–435. doi: 10.1016/j.jtemb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]