Abstract
Continuous passive motion manifests therapeutic effects on inflamed articular joints by an as-yet-unknown mechanism. Here, we show that application of cyclic tensile stress (CTS) in vitro abrogates the catabolic effects of IL-1β on chondrocytes. The effects of CTS are mediated by down-regulation of IL-1β-dependent inducible NO production, and are directly attributed to the inhibition of inducible NO synthase (iNOS) mRNA expression and protein synthesis. The inhibition of iNOS induction by CTS is paralleled by abrogation of IL-1β-induced down-regulation of proteoglycan synthesis. Furthermore, CTS inhibits iNOS expression and up-regulates proteoglycan synthesis at concentrations of IL-1β frequently observed in inflamed arthritic joints, suggesting that the actions of CTS may be clinically relevant in suppressing the sustained effects of pathological levels of IL-1β in vivo. These results are the first to demonstrate that mechanisms of the intracellular actions of CTS in IL-1β-activated chondrocytes are mediated through inhibition of a key molecule in the signal transduction pathway that leads to iNOS expression.
The catabolic effects of proinflammatory cytokines on cartilage are hallmarks of inflammatory joint diseases, such as rheumatoid arthritis (RA)3 and osteoarthritis (OA). Elevated levels of IL-1β present in the synovial fluids of inflamed joints implicate it as a major etiologic agent in both RA and OA (1–5). IL-1β down-regulates extracellular matrix synthesis and up-regulates metalloprotease synthesis via production of NO in chondrocytes (3, 5–8). The increased synthesis of NO by IL-1β-activated chondrocytes is due to increased expression of inducible NO synthase (iNOS) (7–10). Inhibition of iNOS and NO production by N-methyl arginine prevents IL-1β-mediated suppression of proteoglycan and type II collagen synthesis, and increased expression of metalloproteases in chondrocytes (9), demonstrating that NO acts as an endogenous mediator of the catabolic actions of IL-1β (11–15). These results further implicate the significance of NO in the pathophysiology of arthritic diseases.
Continuous passive motion exerts reparative effects on inflamed joints (16–19) by unknown intracellular mechanisms. Since the destructive effects of IL-1β are mediated by NO, we speculated that continuous passive motion may regulate cellular responses to IL-1β via inhibition of NO synthesis. To simulate the stress exerted on chondrocytes during inflammation, an in vitro model was devised to include: 1) IL-1β, a major mediator of inflammation in arthritic joint diseases (1–5); 2) cyclic tensile stress (CTS), which mimics the tensile stress experienced by chondrocytes on the surface of cartilage during movement (20); and 3) chondrocytes in primary cultures, which closely display the phenotypic characteristics of chondrocytes in the cartilage (10, 12, 21, 22). Using this model, we present results to show that CTS suppresses IL-1β-induced NO production. The reduction of NO production by CTS can be attributed to down-regulation of iNOS mRNA expression and of its synthesis. Additionally, manifestation of the effects of CTS on NO production is accompanied by abrogation of IL-1β-induced suppression of proteoglycan synthesis. These studies demonstrate that the intracellular mechanism of CTS action on chondrocytes involves inhibition of IL-1β-mediated signal transduction upstream of iNOS mRNA expression.
Materials and Methods
Preparation of articular chondrocytes
Slices of hyaline articular cartilage were aseptically shaved from the shoulder and knee joints of young adult New Zealand white rabbits (6–7 lbs). Chondrocytes were released by 0.2% trypsin, followed by 0.2% clostridial collagenase (Sigma, St. Louis, MO) digestion in a two-compartment digestion chamber. Chondrocytes were cultured in F12 medium (Life Technologies, Grand Island, NY) supplemented with 10% FCS and penicillin/streptomycin (100 U/100 μg/ml) at 37°C in an atmosphere of 5% CO2 for 7 days (12). Subsequently, chondrocytes (105/well) were transferred to pronectin-coated six-well Flexercell plates (Flexercell International, McKeesport, PA). After an additional 3 days of culture, the cells reached 90% confluence. In these primary cultures, chondrocytes retain their differentiated phenotype and produce chondroitin sulfate proteoglycans and type II collagen (10, 12). These chondrocytes, when grown in cultures for 4–8 wk, exhibit synthesis of a cartilagenous matrix with tensile stiffness similar to that found in vivo. Furthermore, such chondrocytes respond to IL-1β in a manner similar to that of cartilage explants (21, 22).
Application of CTS
The growth medium was replaced with 1 ml/well of serumless Neuman-Tytell medium 24 h before initiating the experiments. Subsequently, the cells were exposed to CTS regimen, comprising of four different treatments: 1) no CTS; 2) recombinant human (rh)IL-1β (1 ng/ml; Genentech, Palo Alto, CA); 3) CTS (0.05 Hz, 20% elongation rate) (23); and 4) CTS and rhIL-1β (1 ng/ml). In some experiments, concentration of IL-1β and/or duration of CTS exposure were varied. Cells were subjected to CTS at a rate of three cycles per min, a cycle being 10 sec of elongation followed by 10 sec of relaxation at a vacuum level equivalent to 5 inches of mercury. This vacuum level creates an empirically-measured deformation on the surface of a Flexercell plate that follows an exponential curve from 0 to 20% elongation along the radius from the center of the well to the edge (23). This regimen of CTS results in negligible (<1%) cell detachment over a period of 24–96 h, and provides reproducible suppression of IL-1β-induced NO production.
Assessment of iNOS mRNA expression
The abundance of mRNA encoding iNOS was assessed by RT-PCR. RNA from chondrocytes subjected to various treatment regimens was extracted using a commercially available kit (Qiagen, Santa Clarita, CA), and a total of 1 μg of RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Perkin-Elmer, Norwalk, CT) for 60 min at 37°C. The cDNA was amplified with 0.1 μg of primers specific for rabbit GAPDH (548 bp; sense 5'-GGTGAAGGTCGGAGTCAACGG-3'; antisense 5'-GGTCATGAGTCCTTCCACGAT-3'), and iNOS (243 bp; sense 5'-GCAGCAGCGACTCCATGACT-3'; antisense 5'-TCCAGGAGGA CATGCAGCAC-3') for 30 cycles (24).
Quantitative analysis of iNOS mRNA expression was conducted by competitive PCR (25). Heterologous competitor DNA was constructed by PCR with a BamHI/EcoRI fragment of v-erbB gene as a template by use of PCR MIMIC construction kit (Clontech Laboratories, Palo Alto, CA). This DNA fragment was amplified with a pair of composite PCR primers: sense 5'-CGCCCTTCCGCAGTTTCTCGCAAGTGAAATCTCCTCCG-3'; antisense 5'-TCCAGGAGGACATGCAGCACTCTGTCAATGCAGTTTGTAG-3', which contained iNOS gene sequences at the 5' end of the v-erbB gene sequences. The DNA within the iNOS gene sequence was then amplified with the iNOS primers to obtain MIMIC DNA. The gene products of target and competitor sequences were thus 243 and 438 bp, respectively. The target cDNA derived from 0.033 μg of cellular RNA was then amplified in the presence of iNOS primers and 1 μl of MIMIC DNA (104 attomoles or its 10-fold serial dilutions). The lanes exhibiting similar concentrations of gene products in target and MIMIC DNA were reanalyzed to derive exact concentrations of target cDNA using 2-fold dilutions of the MIMIC DNA. The bands with equimolar concentration of the gene products were estimated by densitometric analysis of ethidium bromide-stained DNA in each lane. The results were calculated and expressed as the mean number of iNOS mRNA molecules synthesized per μg RNA.
Monitoring of NO and iNOS
NO production was measured as stable nitrite in culture supernatants by a spectrophotometric assay based upon the Griess reaction (26). NO production was expressed as μM of nitrite per 105 cells minus basal nitrite present in the medium. All experiments were done in triplicate, and the level of significance analyzed by ANOVA. A p value of <0.05 was considered significant.
The relative synthesis of iNOS in chondrocytes subjected to the above stress regimens was assessed by Western blot analysis after 8 h of activation, or by indirect immunoperoxidase staining after 24 h of activation. For Western blot analysis (27), proteins were extracted (28) by incubating cells on ice for 15 min in buffer A (10 mM HEPES (pH 7.9), 10 mM KCl, and 0.1 mM EDTA) containing protease inhibitors (0.1 mM EDTA, 1 mM DTT, 0.5 mM p-methyl sulfonic acid, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 0.5 μg/ml benzamidine). Thereafter, deoxycholate was added to a final concentration of 0.8%, and the cells were lysed with 0.16% Nonidet P-40. Lysates were gently transferred to tubes, centrifuged at 13,000 rpm for 30 sec to pellet nuclei, and the supernatant proteins (10 μg/lane)were subjected to SDS-10% PAGE. The proteins were then electrophoretically transferred to Polyscreen polyvinylidene difluoride transfer membrane (Dupont, Wilmington, DE), and the resulting blot was blocked with 5% BSA and 5% nonfat milk in PBS. The blot was then reacted with rabbit anti-iNOS-2 (Transduction Laboratories, Lexington, KY) Abs at 4°C over-night, and washed with PBS containing 0.2% Tween-20. The binding of primary Abs was detected by HRP-conjugated goat anti-rabbit IgG, followed by chemiluminescent substrate Luminol (New England Nuclear, Boston, MA) for 60 s. The blots were exposed to a Reflection NEF-496 film (Dupont) for 30–60 s to visualize iNOS.
For immunoperoxidase staining, chondrocytes (105/well) were trypsinized from Flexercell wells, cytocentrifuged onto microscope slides, and fixed for 30 min in ice-cold methanol containing 0.3% hydrogen peroxide to inactivate endogenous peroxidases (29). iNOS was detected by the use of rabbit anti-NOS-2 as primary Abs, and HRP-conjugated goat anti-rabbit IgG as secondary Abs. The chromogen used for HRP detection was diaminobenzidine. The relative intensity of immunoperoxidase staining for iNOS was determined by histomorphometric densitometry using captured images and Optimus software (Media Cybernetics, Silver Spring, MD).
Proteoglycan synthesis
The incorporation of 35SO42− (40 μCi/well) into glycosaminoglycans was allowed during the last 8 h of each experiment, as described previously (27). Glycosaminoglycans were extracted from cells with 0.5 M NaOH and separated on PD-10 size exclusion chromatography columns (Pharmacia, Piscataway, NJ). The incorporation of 35SO42− into proteoglycans was measured by scintillation counting (30).
Results
CTS down-regulates NO production in rhIL-1β-treated chondrocytes
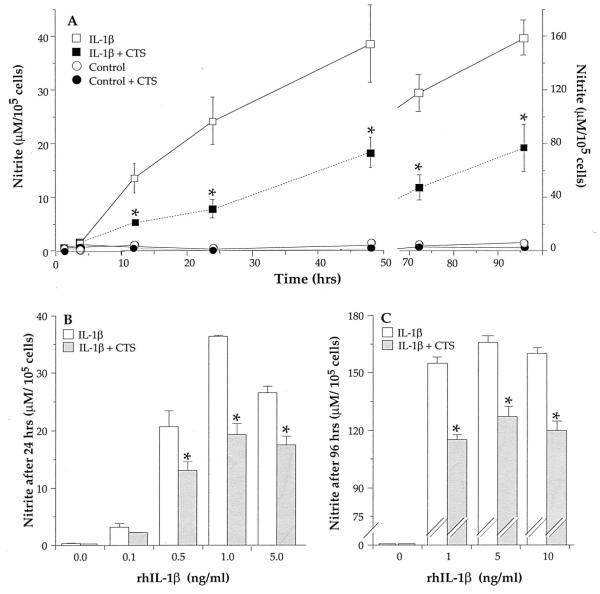
Incubation of chondrocytes with rhIL-1β (1 ng/ml) resulted in the production of high concentrations of NO, as compared with untreated controls (Fig. 1A). The production of NO above basal levels was not observed during the first 4 h of rhIL-1β exposure, but increased within 12 h, and continued to rise in a time-dependent manner during the ensuing 96 h. Coapplication of CTS and rhIL-1β to chondrocytes consistently and significantly ( p < 0.05) suppressed IL-1β-induced NO production at all time points tested (Fig. 1A). The culture supernatants of chondrocytes subjected to CTS in the absence of rhIL-1β and unstressed controls exhibited NO levels similar to that of medium alone (<3 μM), indicating that neither untreated control cells produced measurable NO, nor CTS, per se, stimulated the production of NO (Fig. 1).
FIGURE 1.
CTS suppresses rhIL-1β-dependent NO production in chondrocytes. A, Chondrocytes (5 × 105 cells/well) grown in Flexercell plates were subjected to: medium alone (○), CTS alone (●), rhIL-1β (1 ng/ml) (□), or CTS in the presence of IL-1β (■), for 2, 4, 12, 24, 48, 72, or 96 h. At the end of each incubation period, the culture supernatants were assayed for the presence of nitrite. B and C, Suppression of NO production by CTS at various concentrations of rhIL-1β. Cells were subjected to rhIL-1β (0, 0.1, 0.5, 1, 5, or 10 ng/ml) in the absence (open bars) or presence of CTS (filled bars). NO accumulation in the supernatants was measured after 24 or 96 h of activation. Each point represent means ± SEM of triplicate samples. *, p < 0.05 relative to cells treated with IL-1β alone.
The suppression of NO production by CTS was observed over a wide range of IL-1β concentrations (0.1–10 ng/ml IL-1β; Fig. 1, B and C). More importantly, CTS-suppressed IL-1β induced NO production equally effectively at high as well as low concentrations of IL-1β. The relative suppression of NO induction was significant ( p < 0.05) at all concentrations of IL-1β tested. Furthermore, the inhibition of IL-1β-induced NO production by CTS was also sustained, and could be observed between 24 and 96 h. As observed earlier (9, 10, 26), chondrocytes from different rabbits varied in the extent of NO production in response to IL-1β. Similarly, the degree of CTS-induced inhibition of IL-1β-stimulated NO production also varied in chondrocytes obtained from different rabbits. Nevertheless, in 14 different experiments, each using chondrocytes from a different rabbit, CTS inhibited 44–67% of the IL-1β-induced NO production within 24 h (Fig. 1, A and B). Moreover, in each individual experiment, the IL-1β-induced NO production was significantly suppressed by CTS ( p < 0.05). These variations are attributed to the percentage of IL-1β responsive cells in the culture (31, 32) and to genetic differences among outbred animals.
CTS down-regulates iNOS mRNA expression in rhIL-1β-treated chondrocytes
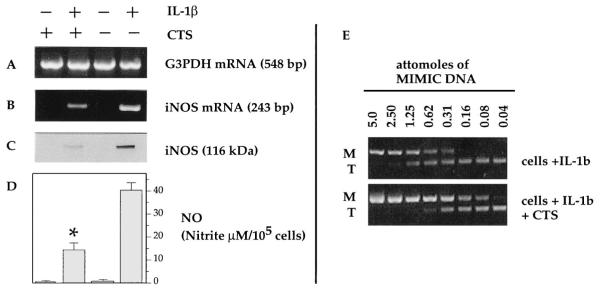
To determine whether the suppression of IL-1β-dependent iNOS mRNA by CTS was due to the inhibition of iNOS mRNA expression, chondrocytes were subjected to CTS regimen for 4 h. The assessment of iNOS mRNA abundance by RT-PCR revealed that CTS alone did not induce expression of iNOS mRNA, whereas rhIL-1β treatment induced significant expression of iNOS mRNA within 4 h (Fig. 2B). The relative expression of iNOS mRNA in chondrocytes subjected to costimulation with rhIL-1β and CTS was markedly suppressed, as compared with chondrocytes treated with rhIL-1β alone (Fig. 2B). Parallel experiments demonstrated that inhibition of rhIL-1β-dependent iNOS mRNA expression by CTS during the first 4 h was followed 4 h later by suppression of iNOS synthesis (Fig. 2C). The sustained suppression of iNOS synthesis by CTS was also confirmed by the observation that CTS inhibited 66% of the total IL-1β-induced NO accumulation in the culture supernatants 24 h later (Fig. 2D).
FIGURE 2.
CTS suppresses rhIL-1β-induced iNOS mRNA expression, iNOS synthesis, and NO production in chondrocytes. Chondrocytes were incubated with rhIL-1β (1 ng/ml) in the absence or presence of CTS. The cells were analyzed after 4 h for iNOS mRNA expression by RT-PCR (B); after 8 h for iNOS protein synthesis by Western blot analysis (C); or after 24 h for NO production by Griess reaction (D) in the same batch of cells. E, Agarose gel electrophoresis of PCR products obtained from quantitative competitive PCR reactions containing iNOS primers, a constant amount of cellular target (T) cDNA (243 bp), and varying amounts (5–0.04 attomoles) of MIMIC DNA (438 bp). cDNA obtained from cells treated with IL-1β alone, and treated with IL-1β and CTS exhibit equimolar PCR products at 0.62 and 0.016 attomoles of MIMIC DNA (M), respectively. Control chondrocytes and cells treated with CTS alone did not exhibit iNOS gene products. Data represent one of three separate experiments with similar results. *, in D, p < 0.05 relative to cells treated with IL-1β alone.
The exact extent of IL-1β-induced iNOS mRNA suppression by CTS was then determined by quantitative competitive PCR. As expected, control untreated cells and cells treated with stress alone did not exhibit the presence of iNOS mRNA. Whereas, mRNA from chondrocytes treated with IL-1β alone and treated with IL-1β and CTS exhibited equimolar gene products in competitive PCR at 0.62 and 0.156 attomoles of MIMIC DNA, respectively (Fig. 2E). Deduced from this data, cells treated with IL-1β alone, and cells treated with IL-1β and CTS, exhibited 112.7 and 28.3 molecules of iNOS mRNA per μg of total RNA, respectively. Thus, CTS suppressed IL-1β-induced iNOS mRNA expression by 75%. In these experiments, total accumulation of NO in the culture supernatants was reduced by 66% in cells exposed to CTS and IL-1β, as compared with cells exposed to IL-1β alone (Fig. 2D).
CTS down-regulates iNOS synthesis in rhIL-1β-treated chondrocytes
To determine whether the reduction in IL-1β-dependent iNOS mRNA expression by CTS was paralleled by a net decrease in the synthesis of iNOS, chondrocytes were subjected to CTS regimen for 8 h in the presence of IL-1β. Thereafter, the cells were harvested, and total iNOS synthesis in cells was assessed by Western blot analysis. As expected, untreated control cells and cells subjected to stress alone did not exhibit iNOS synthesis (Fig. 2C). However, chondrocytes exposed to IL-1β exhibited significantly increased iNOS synthesis, which was suppressed in cells costimulated with IL-1β and CTS. In parallel confirmatory experiments, cells subjected to costimulation with IL-1β, and CTS exhibited a significant reduction in NO production as compared with cells exposed to IL-1β alone (Fig. 2D).
The presence of iNOS was not detectable in control cells or in cells treated with CTS alone for 24 h, as assessed by immunocytochemistry (Fig. 3, A and B). Interestingly, rhIL-1β activation induced iNOS in only 35 ± 4% of chondrocytes (Fig. 3C). Whereas, chondrocytes costimulated with rhIL-1β and CTS revealed an ~62% suppression of total rhIL-1β-induced iNOS expression, as assessed by the reduction in the intensity of the immunostaining in iNOS-positive cells by histomorphometric analysis (Fig. 3D). Controls, in which nonimmune rabbit serum was used instead of anti-iNOS Abs, did not show any immunoreactivity.
FIGURE 3.
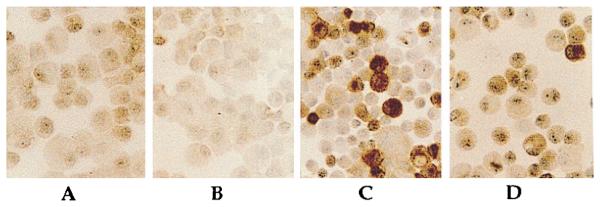
Immunochemical analysis of chondrocytes exhibiting suppression of rhIL-1β-dependent iNOS synthesis by CTS. The iNOS synthesis in chondrocytes was compared in cells subjected to medium alone (A), CTS alone (B), rhIL-1β (C) (1 ng/ml), or costimulation with CTS and IL-1β (D) for 24 h. Histomorphometric analysis of cells showed absence of iNOS in A and B. IL-1β-treated cells (C) show presence of iNOS by intense peroxidase staining in 35 ± 4% of chondrocytes, while cells subjected to simultaneous IL-1β and CTS (D) showed a 62 ± 6% lower staining intensity for iNOS, as compared with iNOS-positive cells in C.
CTS abrogates the rhIL-1β-induced inhibition of proteoglycan synthesis in chondrocytes
Since elevated concentrations of NO in response to rhIL-1β account for the down-regulation of proteoglycan synthesis, we next examined whether the inhibition of proteoglycan synthesis by IL-1β can be abrogated by CTS. In these experiments, chondrocytes were subjected to CTS regimen for 48 or 72 h. Proteoglycan synthesis assessed by incorporation of 35SO42− revealed that CTS alone down-regulated proteoglycan synthesis respectively by 14 and 31% after 48 and 72 h of CTS application. Exposure of cells to rhIL-1β alone inhibited proteoglycan synthesis by 53 and 47.6%, as compared with untreated controls at 48 and 72 h, respectively. However, IL-1β-dependent inhibition of proteoglycan synthesis was significantly abrogated by CTS, so much so that chondrocytes costimulated with IL-1β and CTS exhibited only 2 and 28% ( p < 0.05) down-regulation of proteoglycan synthesis as compared with untreated chondrocytes. When proteoglycan synthesis in chondrocytes costimulated with rhIL-1β and CTS was compared with chondrocytes subjected to CTS alone, no statistically significant differences were observed (Fig. 4, A and B).
FIGURE 4.
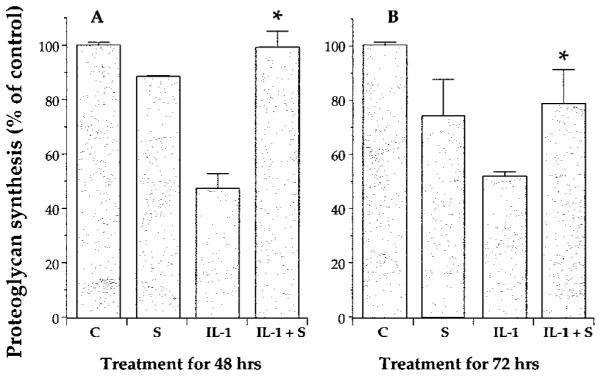
CTS abrogates rhIL-1β-mediated inhibition of proteoglycan synthesis in chondrocytes. Chondrocytes were subjected to medium alone (C), CTS alone (S), rhIL-1β (1 ng/ml) alone (IL-1), or rhIL-1β (1 ng/ml) and CTS (IL-1 + S), for either 48 (A) or 72h (B). The incorporation of 35SO42− into proteoglycans during the last 8 h of experiment shows CTS-induced abrogation of the rhIL-1β-dependent inhibition of proteoglycan synthesis at both time points. Each point represent means ± SEM of triplicate samples. *, p < 0.05 relative to cells treated with IL-1β alone.
Discussion
Although it is well accepted that continuous passive motion exerts reparative effects on inflamed joints (16–19), the mechanism of its intracellular actions are not understood. The results presented here are the first to provide evidence that iNOS serves as a focal point for the actions of CTS on IL-1β-activated chondrocytes in vitro. In these studies, human arthritic disease was closely mimicked in vitro. For example, we used primary chondrocytes in their first passage, which are phenotypically similar to those in native cartilage in their ability to: 1) synthesize chondroitin sulfate proteoglycan and collagen II within 1 week of culture (10, 12), 2) synthesize a mature in vivo-like cartilagenous matrix within 4–8 wk of culture under identical conditions (21, 22), and 3) respond to IL-1β in a manner similar to that observed in cartilage explants (22). IL-1β is shown to be a major etiologic agent in human arthritic diseases (1–5), hence, the chondrocytes were activated with IL-1β. Additionally, continuous passive motion was simulated by cyclic tensile stress, due to the fact that the tangential layers of cartilage undergo tensile stress during movement (20), and it is the cells in the superficial layers of cartilage that are most responsive to IL-1β (10, 31, 32).
Earlier studies show that NO is a potent mediator of catabolic actions of IL-1β, and its sustained production mediates IL-1β-induced proteoglycan degradation in arthritic diseases (5–11). Hence, it is not surprising that the counteracting effects of CTS on IL-1β actions involve iNOS. Our results show that CTS down-regulates IL-1β-induced NO production in chondrocytes in a time-dependent manner. Further, the reduction in IL-1β-induced NO production by CTS is directly attributable to a net decrease of nearly 75% iNOS mRNA abundance and iNOS synthesis. This indicates that CTS inhibits IL-1β-induced cellular responses upstream to iNOS mRNA expression, which consequently results in the down-regulation of NO production and suppression of the catabolic effects of IL-1β. Interestingly, CTS alone did not induce NO production, further suggesting that effects of CTS are geared toward inhibition of the inflammatory effects of IL-1β.
Previous studies have shown that IL-1β induces sustained NO production in chondrocytes (9, 14), which, in turn, down-regulates matrix synthesis while up-regulating matrix-degrading enzymes. Our results demonstrate that CTS inhibits IL-1β-dependent NO production in a sustained manner, which is paralleled by prolonged abrogation of IL-1β-dependent inhibition of proteoglycan synthesis. CTS inhibits IL-1β-dependent NO production over a wide range of IL-1β concentrations, which are similar to those observed in synovial fluids of arthritic joints, and to those associated with cartilage degradation in vivo and inhibition of proteoglycan synthesis in vitro (10, 12, 13, 30). Together, these results suggest that actions of CTS may be clinically relevant in suppressing sustained effects of pathologic levels of IL-1β. More importantly, the fact that CTS alone neither induces NO production nor proteoglycan synthesis indicates that the CTS may act primarily as an antiinflammatory agent and exert its effects only on the inflamed chondrocytes.
It has been reported that chondrocytes in articular cartilage exhibit metabolic heterogeneity with respect to IL-1β responsiveness and iNOS production (10, 31, 32). Specifically, chondrocytes in the tangential layers of cartilage are most responsive to IL-1β and synthesize high levels of iNOS as compared with chondrocytes in the deeper layers (10, 31, 32). In view of the fact that in our studies immunochemical staining for iNOS also revealed that only 35% of the chondrocytes were most responsive to IL-1β, it is likely that this iNOS positive cell population represents the cells derived from the tangential layer of articular cartilage. This population of cells may also be the select population of chondrocytes that are responsive to CTS.
To simulate the effects of continuous passive motion on IL-1β-treated chondroytes in the superficial layers of cartilage, we subjected chondrocytes to CTS in vitro (20). Interestingly, subjecting chondrocytes to CTS alone down-regulated proteoglycan synthesis by 16–30%. These observations are similar to those observed in bovine articular chondrocytes, where CTS at 17% elongation rate has been shown to result in the inhibition of proteoglycan synthesis (33). In these experiments, CTS alone did not induce NO synthesis or expression of mRNA for iNOS, indicating that the inhibition of proteoglycan synthesis by CTS alone is mediated by a pathway that does not involve iNOS. Recently, compressive stress has been shown to down-regulate constitutive NO synthase (cNOS) without affecting proteoglycan synthesis (34). In our experiments, involvement of cNOS was not evident, as total nitrite production in cells subjected to CTS alone and untreated control cells was identical to that of background. The effects of CTS also differ from those of fluid-induced shear stress, which has been shown to enhance chondrocytic glycosaminoglycan synthesis, despite NO induction (35). Clearly, the intracellular actions of tensile, compressive, and shear stresses use diseparate pathways in chondrocytes.
The inhibition of proteoglycan synthesis during cartilage degradation in RA and OA has been well documented (1–8). In vitro studies have implicated IL-1β as the major mediator of cartilage degradation, which inhibits proteoglycan synthesis via up-regulation of NO production (5–8, 10, 13). Examination of the effects of CTS on chondrocytes in the presence of IL-1β shows that CTS abrogates IL-1β-induced inhibition of proteoglycan synthesis. These effects of CTS are paralleled by inhibition of IL-1β-dependent NO production. The fact that CTS completely abrogates the actions of IL-1β on proteoglycan synthesis during the first 48 h indicates that CTS suppresses the catabolic effects of IL-β by inhibiting one of the key steps in the signal transduction cascade of IL-1β. This results in the inhibition of NO production, as well as its ensuing second messenger functions involved in the catabolic actions of IL-1β.
In conclusion, these results strongly suggest that CTS functions as a potent effector mechanism for suppressing pathologic effects of IL-1β in vitro through inhibition of inducible NO production. It is likely that in vivo continuous passive motion may exert its effects on atrthritic joints in a similar manner by inhibiting the inflammatory effects induced by IL-1β via interruption of iNOS production. In turn, this would limit the production of NO and its catabolic effects on cartilage. Our results thus reveal an intracellular mechanism that may mediate the therapeutic actions of continuous passive motion in the treatment of inflammatory joint diseases. Further identification of the specific stress-sensitive molecules in the IL-1β signal transduction cascade that lead to the inhibition of iNOS mRNA induction will lead to the understanding of key mechanisms involved in the intracellular actions of CTS.
Footnotes
This work was supported by National Institutes of Health Grants RO1 AR42025 (to C.H.E.) and R15 DE12976 (to M.J.B) and the Oral and Maxillofacial Surgery Foundation (to M.J.B. and S.A.).
- RA
- rheumatoid arthritis
- OA
- osteoarthritis
- CTS
- cyclic tensile stress
- iNOS
- inducible NO synthase
- rh
- recombinant human
References
- 1.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095. [PubMed] [Google Scholar]
- 2.Pettipher ER, Higgs GA, Henderson B. Interleukin-1 induces leukocyte infiltration and cartilage degradation in the synovial joints. Proc. Natl. Acad. Sci. USA. 1986;83:8749. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubbard JR, Steinberg JJ, Bednar MS, Sledge CB. Effect of purified human interleukin-1 on cartilage degradation. J. Orthop. Res. 1988;6:180. doi: 10.1002/jor.1100060204. [DOI] [PubMed] [Google Scholar]
- 4.Firestein GS, Boyel DL, Yu C, Paine MM, Whisenand TD, Zvaifler NJ, Arend WP. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 1994;37:644. doi: 10.1002/art.1780370507. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum. Dis. Clin. N. Am. 1993;19:545. [PubMed] [Google Scholar]
- 6.Woessner JF, Gunja-Smith Z. Role of metalloproteases in human arthritis. J. Rheumatol. 1991;18(Suppl. 27):99. [PubMed] [Google Scholar]
- 7.Stichtenoth DO, Frolich JC. Nitric oxide and inflammatory joint diseases. Br. J. Rheumatol. 1998;37:246. doi: 10.1093/rheumatology/37.3.246. [DOI] [PubMed] [Google Scholar]
- 8.Jang D, Murrell GAC. Nitric oxide in arthritis. Biol. Med. 1998;24:1511. doi: 10.1016/s0891-5849(97)00459-0. [DOI] [PubMed] [Google Scholar]
- 9.Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, Simmons RL, Evans CH. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J. Immunol. 1991;147:3915. [PubMed] [Google Scholar]
- 10.Haäuselmann HJ, Stefanovic-Racic M, Michel BA, Evans CH. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J. Immunol. 1998;160:1444. [PubMed] [Google Scholar]
- 11.Murrell GAC, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem. Biophys. Res. Commun. 1995;206:15. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 12.Lotz M, Blanco FJ, vonKempis J, Dudler J, Maier M, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J. Rheumatol. 1995;22(Suppl. 43):104. [PubMed] [Google Scholar]
- 13.Stefanovic-Racic M, Morales TI, Taskiran D, McIntyre LA, Evans CH. The role of nitric oxide in proteoglycan turnover by bovine articular cartilage organ cultures. J. Immunol. 1996;156:1213. [PubMed] [Google Scholar]
- 14.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans CH. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem. Biophys. Res. Commun. 1994;200:142. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 15.Blanco FJ, Geng Y, Lotz M. Differentiation-dependent effects of IL-1 and TGF-β on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J. Immunol. 1995;154:4018. [PubMed] [Google Scholar]
- 16.Kim HKW, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J. Rheumatol. 1995;22:1714. [PubMed] [Google Scholar]
- 17.Kreder HJ, Moran M, Keeley FW, Salter RB. Biologic resurfacing of a major joint defect with cryoreserved allogeneic periosteum under the influence of continuous passive motion in a rabbit model. Clin. Orthop. Relat. Res. 1994;300:288. [PubMed] [Google Scholar]
- 18.Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand. Clinics. 1994;10:211. [PubMed] [Google Scholar]
- 19.Moran ME, Kim HKW, Salter RB. Biological resurfacing of full-thickness defects in patellar articular cartilage of the rabbit. J. Bone Jt. Surg. 1992;74:659. doi: 10.1302/0301-620X.74B5.1527109. [DOI] [PubMed] [Google Scholar]
- 20.Kelly PA, O’Connor JJ. Transmission of rapidly applied loads through articular cartilage. I. Uncracked cartilage. J. Engin. Med. 1996;210:27. doi: 10.1243/PIME_PROC_1996_210_388_02. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Grynpas M, Kandel RA. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997;18:1425. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 22.Fedewa MM, Oegema TR, Schwartz MH, MacLeod A, Lewis JL. Chondrocytes in culture produce a mechanically functional tissue. J. Orthop. Res. 1998;16:227. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda K, Asada S, Kumano F, Saitoh M, Otani K, Tanaka S. Cyclic tensile stretch on bovine articular chondrocytes inhibits protein kinase C activity. J. Lab. Clin. Med. 1997;130:209. doi: 10.1016/s0022-2143(97)90098-6. [DOI] [PubMed] [Google Scholar]
- 24.Hart DA, Sciore P, Boykiw R, Reno C. Pregnancy induces complex changes in the pattern of mRNA expression in knee ligaments of the adolescent rabbit. Matrix Biol. 1998;17:21. doi: 10.1016/s0945-053x(98)90122-6. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell J, Goode T, Shanahan F. Quantitative measurement of mRNA expression by competitive RT-PCR. Methods Mol. Biol. 1998;92:183. doi: 10.1385/0-89603-497-6:183. [DOI] [PubMed] [Google Scholar]
- 26.Evans CH, Watkins SC, Stefanovic-Racic M. Nitric oxide in cartilage metabolism. Methods Enzymol. 1996;269:75. doi: 10.1016/s0076-6879(96)69011-9. [DOI] [PubMed] [Google Scholar]
- 27.Liu RH, Jacob J, Tennant B. Chemiluminescent detection of protein molecular weight markers in western blot techniques. Biotechniques. 1997;22:594. doi: 10.2144/97224bm01. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Aggarwal B. Protein tyrosine phosphatase inhibitors block tumor necrosis factor-dependent activation of the nuclear transcription factor NF-κB. J. Biol. Chem. 1995;270:10631. doi: 10.1074/jbc.270.18.10631. [DOI] [PubMed] [Google Scholar]
- 29.Forstermann U, Dun N. Immunohistochemical localization of nitric oxide synthases. Methods Enzymol. 1996;268:510. doi: 10.1016/s0076-6879(96)68053-7. [DOI] [PubMed] [Google Scholar]
- 30.Stefanovic-Racic M, Moäller MO, Miller LA, Evans CH. Nitric oxide and proteoglycan turnover in rabbit articular chondrocytes. J. Orthop. Res. 1997;15:442. doi: 10.1002/jor.1100150318. [DOI] [PubMed] [Google Scholar]
- 31.Haäuselmann HJ, Flechtenmacher J, Michal L, Thonar EJ, Shinmei M, Kuettner KE, Aydelotte MB. The superficial layer of human articular cartilage is more susceptible to interleukin-1-induced damage than the deeper layers. Arthritis Rheum. 1996;39:478. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Etsuko A, Yamate T, Taguchi Y, Jasin HE. Nitric oxide production by superficial and deep articular chondrocytes. Arthritis Rheum. 1997;40:261. doi: 10.1002/art.1780400210. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in ostearthritis: a review. Semin. Arthritis Rheum. 1998;27:392. doi: 10.1016/s0049-0172(98)80019-x. [DOI] [PubMed] [Google Scholar]
- 34.Lee DA, Frean SP, Lees P, Bader DL. Dynamic mechanical compression influences nitric oxide production by articular chondrocytes seeded in agarose. Biochem. Biophys. Res. Commun. 1998;251:580. doi: 10.1006/bbrc.1998.9520. [DOI] [PubMed] [Google Scholar]
- 35.Das P, Schurman DJ, Smith RL. Nitric oxide and G proteins mediate the response of bovine articular chondrocytes to fluid-induced shear. J. Orthop. Res. 1997;15:87. doi: 10.1002/jor.1100150113. [DOI] [PubMed] [Google Scholar]