Abstract
Inflammatory cytokines play a major role in cartilage destruction in diseases such as osteoarthritis and rheumatoid arthritis. Because physical therapies such as continuous passive motion yield beneficial effects on inflamed joints, we examined the intracellular mechanisms of mechanical strain-mediated actions in chondrocytes. By simulating the effects of continuous passive motion with cyclic tensile strain (CTS) on chondrocytes in vitro, we show that CTS is a potent antagonist of IL-1β actions and acts as both an anti-inflammatory and a reparative signal. Low magnitude CTS suppresses IL-1β-induced mRNA expression of multiple proteins involved in catabolic responses, such as inducible NO synthase, cyclo-oxygenase II, and collagenase. CTS also counteracts cartilage degradation by augmenting mRNA expression for tissue inhibitor of metalloproteases and collagen type II that are inhibited by IL-1β. Additionally, CTS augments the reparative process via hyperinduction of aggrecan mRNA expression and abrogation of IL-1β-induced suppression of proteoglycan synthesis. Nonetheless, the presence of an inflammatory signal is a prerequisite for the observed CTS actions, as exposure of chondrocytes to CTS alone has little effect on these parameters. Functional analysis suggests that CTS-mediated anti-inflammatory actions are not mediated by IL-1R down-regulation. Moreover, as an effective antagonist of IL-1β, the actions of CTS may involve disruption/regulation of signal transduction cascade of IL-1β upstream of mRNA transcription. These observations are the first to show that CTS directly acts as an anti-inflammatory signal on chondrocytes and provide a molecular basis for its actions.
Osteoarthritis (OA)3 and rheumatoid arthritis (RA) are diseases of complex etiopathology associated with progressive inflammation and cartilage destruction (1–4). IL-1β plays a pivotal role in cartilage destruction, as evidenced by its presence in the synovial fluids from patients with RA or OA (2, 4, 5), as well as the ameliorative effects of the IL-1R antagonist in animal models of RA and OA and human RA (6). IL-1 induces catabolic responses in chondrocytes by stimulating the expression of inducible NO synthase (iNOS), cyclo-oxygenase II (COX-II), and proteases including stromolysin, collagenase, and tissue plasminogen activator (7–11). IL-1 also suppresses α1(II) procollagen mRNA expression and type II collagen and proteoglycan synthesis via induction of NO (8, 10, 12–14). IL-1 is a potent inhibitor of chondrocyte proliferation induced by serum or by TGF-β (15). Collectively, induction of catabolic enzymes as well as inhibition of matrix synthesis and cell proliferation by IL-1β drive cartilage destruction in chronic inflammatory diseases such as RA or OA (1–14).
Despite the importance of physical therapy in mediating reparative/anabolic effects on diseased or inflamed synovial joints, only limited information is available regarding its mechanism of intracellular actions (15–20). In vivo, continuous passive motion induces rapid recovery of inflamed joints and augments proteoglycan synthesis (19, 20). This has been mainly attributed to increased circulation and dissemination of inflammatory mediators from the inflamed joint (21, 22). Recently, we have shown that in vitro, cyclic tensile strain (CTS) suppresses the actions of IL-1β on chondrocytes by inhibiting the expression of iNOS and NO production (23). However, the intracellular basis for continuous passive motion-induced beneficial effects on inflamed articular joints remains largely unknown.
Because of the pivotal role of IL-1β in the pathogenesis of inflammatory joint diseases, we speculated that the beneficial effects of continuous passive motion may be mediated via direct suppression of IL-1β actions by mechanical strain. By exposing articular chondrocytes to CTS in vitro, we demonstrate that CTS is a potent antagonist of IL-1β actions and exerts its effects via transcriptional regulation of IL-1β response elements. This is evidenced by the fact that in vitro CTS suppresses IL-1-dependent mRNA transcription of multiple genes that are involved in the initiation of catabolic responses in chondrocytes, such as iNOS, COX-II, and collagenase (matrix metalloprotease-1 (MMP-1). On the other hand, CTS suppresses collagen degradation by abrogating IL-1β-induced inhibition of tissue inhibitor of metalloprotease-II (TIMP-II) and collagen type II expression. Additionally, CTS counteracts IL-1β-dependent inhibition of aggrecan mRNA expression through hyperinduction of aggrecan, a prominent component of cartilage proteoglycans. We show that, IL-1β receptor (IL-1R) down-regulation does not play a major role in the anti-inflammatory effects of CTS. However, CTS may act on a key event(s) in the signal transduction cascade of IL-1β upstream of mRNA transcription.
Materials and Methods
Isolation and characterization of rabbit articular chondrocytes
Slices of hyaline articular cartilage were aseptically shaved from the shoulder and knee joints of young adult New Zealand White rabbits (6–7 lb). Chondrocytes were released by 0.2% trypsin, followed by 0.2% clostridial collagenase (Sigma, St. Louis, MO) digestion (8). Cells were washed in TCM (Ham’s F-12 medium (Life Technologies, Grand Island, NY) supplemented with 10% FCS, 100 U/ml penicillin, and 10 μg/ml streptomycin), adjusted to 105 cells/2 ml, transferred to six-well pronectin-coated (24) BioFlex culture plates (Flexcell International, McKeesport, PA), and cultured at 37°C in 5% CO2 for 8 days (23). The cultures reached 90% confluence in 6–8 days. In primary cultures, first-passage chondrocytes retain their differentiated phenotype and synthesize chondroitin sulfate proteoglycans and type II collagen (25, 26) as well as expression of mRNA for aggrecan, biglycan, TGF-β1, and collagen II. Four- to 8-wk cultures of such chondrocytes exhibit synthesis of a cartilaginous matrix with tensile stiffness similar to that found in vivo (25, 26). Because cartilage lacks blood, nerve, and lymphatics, these cultures are highly unlikely to be contaminated by other cell types. Further, such chondrocytes respond to IL-1β in a manner similar to that of cartilage explants (24). Trypan blue exclusion confirmed viability of >99% of cells in culture.
Exposure of chondrocytes to equibiaxial CTS and IL-1β
Confluent primary chondrocytes (6–8 days old) were washed and incubated with serum-free Neuman-Tytell medium overnight. Monolayers of chondrocytes were subjected to equibiaxial CTS (27) at a rate of three cycles per minute (0.05 Hz), i.e., 10 s of a maximum of 6% equibiaxial stress followed by 10 s of relaxation per cycle (180 cycles/h), providing reproducible suppression of IL-1β-induced iNOS mRNA expression and NO production. The strain was calculated as: circumferential strain = 2π(change in radius)/2π(original radius) = (change in radius)/(original radius) = radial strain. In a majority of experiments, chondrocytes were divided into four groups, viz., untreated and unstressed control cells, cells treated with CTS alone, cells treated with IL-1β (1 ng/ml) alone, and cells treated with CTS and IL-1β (1 ng/ml). The cells were subjected to CTS at the time of addition of IL-1β in most of the experiments. The results of studies with various concentrations of rhIL-1β (0.1, 0.5, 1.0, 5.0, and 10.0 ng/ml) as stimulus indicated that 1 ng/ml IL-β induced iNOS mRNA expression optimally within 4 h of incubation (23). Trypan blue exclusion confirmed the viability of >99% of cells in culture following all treatments.
RT-PCR
RNA was extracted with an RNA extraction kit (Qiagen, Santa Clara, CA). A total of 0.5 μg of RNA was mixed with 1 μg of oligo(dT) (12–18 oligomer; Perkin-Elmer, Norwalk, CT) in RT buffer and incubated for 10 min at room temperature. Thereafter, the reaction mixture was cooled on ice and incubated with 200 U of Moloney murine leukemia virus reverse transcriptase for 60 min at 37°C. The cDNA thus obtained was amplified with 0.1 μg of specific primers in a reaction mixture containing 200 μM dNTP and 0.1 U of Taq polymerase in PCR buffer (Perkin-Elmer). PCR was performed in a DNA thermal cycler (Perkin-Elmer) for 30 cycles of 40 s at 94°C, 40 s at 62°C, and 60 s at 72°C. The sequences of sense and antisense rabbit primers used were as follows: GAPDH (293 bp): sense, 5'-TCACCATCTTCCAGGAGCGA-3'; antisense, 5'-CACAATGCCG AAGTGGTCGT-3'; iNOS (243 bp): sense, 5'-CGCCCTTCCGCAGTTTCT-3'; antisense, 5'-TCCAGGAGGACATGCAGCAC-3'; MMP-3 (322 bp): sense, 5'-TCAGTTCGTCCTCACTCCAG-3'; antisense, 5'-TTGGTCCACCT GTCATCTTC-3'; TIMP-I (326 bp): sense, 5'-GCAACTCCGACCTTGT CATC-3'; antisense, 5'-AGCGTAGGTCTTGGTGAAGC-3'; TIMP-II (414 bp): sense, 5'-GTAGTGATCAGGGCCAAG-3'; antisense, 5'-TTCTCTGT GACCCAGTCCAT-3'; biglycan (406 bp): sense, 5'-GATGGCCTGAAGCTCAA-3'; antisense, 5'-GGTTTTTGAAGAGGCTG-3'; versican (310 bp): sense, 5'-GATGTGTATTGTTATGTGGA-3'; antisense, 5'-CATCAAA TCTGCTATCAGGG-3'; COX-2 (282 bp): sense, 5'-TCAGCCACGCAG CAAATCCT-3'; antisense, 5'-GTCATCTGGATGTCAGCACG-3' (28); aggrecan (500 bp): sense, 5'-CTACCTTGGAGGTCGTGGTGA-3'; antisense, 5'-GTGCACGTACACGGTCCTGA-3'; COL type II (526 bp): sense, 5'-TCAACAACCAGATCGAGAGCA-3'; and antisense, 5'-AGGTGAACCT GCTGTTGCCCT-3' (provided by Dr. M. Heidaran, Orquest, Mountain View, CA).
Quantitative RT-PCR
Heterologous competitor DNA for aggrecan or iNOS was constructed by PCR using the BamHI/EcoRI fragment of the v-erbB gene as a template and the PCR MIMIC construction kit (Clontech, Palo Alto, CA) as described previously (23). The equimolar concentrations of the gene products were estimated by densitometric analysis of ethidium bromide-stained DNA in each lane (Optimus software, Media Cybernetics, Silver Spring, MD), and results were expressed as the mean number of mRNA molecules synthesized per microgram of RNA.
PGE2 measurements
PGE2 was measured in the culture supernatants of chondrocytes at various time intervals by RIA (New England Nuclear, Boston, MA).
Western blot analysis
Collagenase synthesis was assessed in 50 μg of protein extracts by Western blot analysis (23), using goat anti-MMP-1 as primary Abs (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-goat-HRP as second Abs, and luminol as a chemiluminescent HRP substrate (New England Nuclear). The luminescence in each band was assessed by exposing the blots to Reflection autoradiographic film (New England Nuclear), followed by densitometric analysis of the luminescent bands using a camera equipped with a computer and Optimus software (Media Cybernetics, Silver Spring, MD).
Proteoglycan synthesis
Total proteoglycan synthesis was measured by incorporation of Na235SO4 in chondroitin sulfate proteoglycans during the last 8 h of the experiment. Subsequently, culture supernatants were extracted with 0.5 M NaOH, and incorporated precursor was separated by size exclusion chromatography 35 using a PD-10 column (Pharmacia, Piscataway, NJ). The 35S incorporation in proteoglycans was measured by scintillation counting (23).
Results
CTS suppresses IL-1β-dependent induction of iNOS
IL-1β initiates cartilage catabolism through transcriptional activation of multiple genes (7–14), such as COX-II, iNOS, and metalloproteases, whereas NO and PGE2 generated by COX-II and iNOS further modulate cellular metabolism. To evaluate the effects of CTS on the actions of IL-1, chondrocytes were first exposed to various intensities of equibiaxial CTS (2.5, 3.75, 5, 6.25, or 7.5% elongation, respectively) in the presence or the absence of IL-1β. In these experiments the IL-1β concentration was kept constant at 1 ng/ml (23). After 24 h, the level of iNOS mRNA expression was analyzed by RT-QCPCR. As shown in Fig. 1A, IL-1β induced significant levels of iNOS mRNA. However, exposure of cells to CTS in the presence of IL-1β consistently resulted in the suppression of iNOS mRNA expression. As little as 5% equibiaxial CTS was sufficient to significantly inhibit IL-1β-induced iNOS mRNA expression, while a maximal response to CTS was achieved between 5.0 and 7.5% equibiaxial CTS. The suppression of iNOS mRNA expression was paralleled by inhibition of NO production (Fig. 1B). Because CTS-mediated abrogation of IL-1-induced expression of iNOS mRNA results in decreased synthesis of iNOS (29), this inhibition in NO synthesis can be attributed to suppression of iNOS synthesis. Under these conditions chondrocytes subjected to CTS exhibited minimal cell deformation compared with unstressed control cells and negligible cell detachment (<0.1%), as assessed by counting nonadherent cells in the wells, or cell death, as assessed by DNA fragmentation (data not shown).
FIGURE 1.
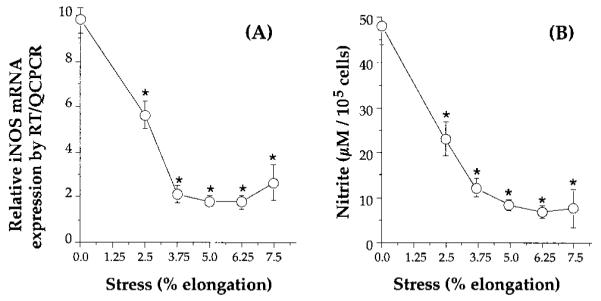
Effects of various intensities of equibiaxial CTS on rhIL-1β-induced iNOS mRNA expression and NO production. A, Inhibition of rhIL-1β-induced iNOS mRNA expression in chondrocytes subjected to 2.5, 3.75, 5, 6.25, or 7.5% equibiaxial CTS at a rate of 0.05 Hz, for 24 h. The inhibition of mRNA expression was assessed by RT-QCPCR. B, Inhibition of rhIL-1β-dependent NO production in chondrocytes following the treatment described in A. NO production was assessed in the culture supernatants of the chondrocytes as total nitrite by Griess reaction (8). Control unstressed chondrocytes or chondrocytes treated with CTS alone did not exhibit iNOS mRNA expression or NO production. Each point in both figures represents the mean ± SEM of triplicate values. *, p ≤ 0.05 compared with cells treated with IL-1β alone.
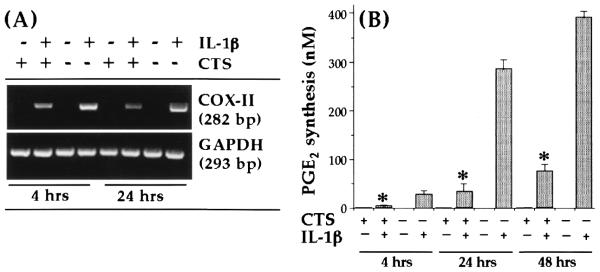
CTS suppresses IL-1β-dependent induction of COX-II
Another important proinflammatory molecule that is enhanced markedly following IL-1β-dependent activation of chondrocytes is PGE2, which is synthesized by COX-II (9, 11). To determine whether the physiologic consequences of CTS action involve inhibition of IL-1β-dependent COX-II induction and its parallel reduction by PGE2 production, chondrocytes were subjected to IL-1β and CTS simultaneously or individually for either 4 or 24 h. As is apparent from Fig. 2A, CTS significantly suppressed ( p < 0.01) IL-1β-induced COX-II mRNA expression. The measurement of the PCR products in each band by semiquantitative densitometric analysis revealed 86 and 92% inhibition of COX-II mRNA expression within the first 4 h and the ensuing 20 h, respectively. This significant ( p < 0.05) inhibition of COX-II mRNA induction by CTS was paralleled by 82 and 81% inhibition of IL-1β-dependent PGE2 production after 24 and 48 h, respectively (Fig. 2B), suggesting that CTS substantially contributes to the inhibition of proinflammatory actions of IL-1β under these conditions. Unactivated chondrocytes or those exposed to CTS alone did not express COX-II mRNA. This was not surprising, as mechanical strain at moderate intensities has not been reported to exhibit proinflammatory effects (29–31).
FIGURE 2.
Inhibition of rhIL-1β-dependent COX-II mRNA expression and PGE2 synthesis by CTS in articular chondrocytes. A, COX-II mRNA expression in chondrocytes either untreated or subjected to rhIL-1β, CTS, or rhIL-1β and CTS for 4 or 24 h. B, PGE2 synthesis by chondrocytes subjected to the treatment regimens described in A for 4, 24, or 48 h. PGE2 synthesis was measured in the culture supernatants of chondrocytes by RIA. The data in A represent one of three separate experiments. The data in B represent the mean and SEM of triplicate values. *, p < 0.05 compared with cells treated with IL-1β alone.
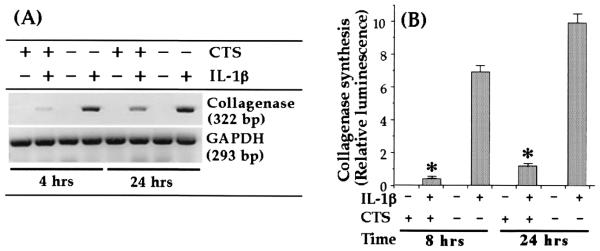
CTS suppresses IL-1β-dependent induction of collagenase (MMP-1)
Previous studies have demonstrated that besides other MMPs, induction of MMP-1 in response to IL-1β plays a critical role during cartilage degradation in inflammatory joint disease (7, 12, 29–31). If so, for effective control of IL-1β-induced catabolism, CTS actions must include suppression of collagenase production. Although chondrocytes exposed to IL-1β expressed significant amounts of MMP-1, cells subjected to IL-1β and CTS exhibited a marked (98% during the first 4 h and 83% after 24 h) inhibition of MMP-1 mRNA expression (Fig. 3A). More importantly, the reduction in MMP-1 mRNA expression was reflected in the inhibition of MMP-1 synthesis, as assessed by Western blot analysis, showing a 92% inhibition during the first 8 h and 87% after 24 h (Fig. 3B). Untreated control cells did not express MMP-1 mRNA constitutively. CTS alone did not induce mRNA expression or synthesis of MMP-1, demonstrating that the actions of CTS on chondrocytes are totally dependent on the presence of an inflammatory signal, such as IL-1β.
FIGURE 3.
Inhibition of rhIL-1β-dependent collagenase mRNA expression and synthesis by CTS in articular chondrocytes. A, Expression of collagenase mRNA in chondrocytes either untreated or subjected to rhIL-1β, CTS, or rhIL-1β and CTS for 4 or 24 h. B, Collagenase synthesis by chondrocytes subjected to the treatment regimens described in A. Collagenase synthesis was measured as the relative intensity of each band in Western blot analysis. The data in A are representative of one of three separate experiments. The data in B represent the mean and SEM of triplicate values. *, p < 0.05 compared with cells treated with IL-1β alone.
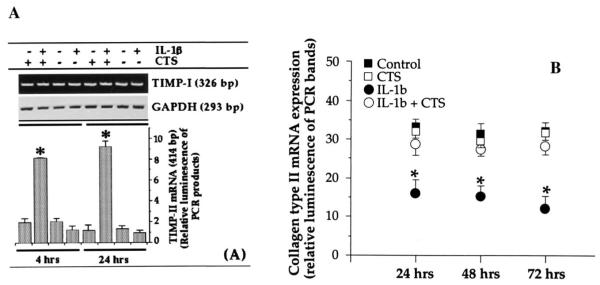
CTS abrogates IL-1β-dependent inhibition of TIMP expression
A synergistic mechanism for enhanced cartilage degradation during proinflammatory actions of IL-1β involves not only activation of metalloproteases but also inhibition of TIMP production (32–34). Inhibition of TIMPs prolongs the enzymatic activity of metalloproteases (32–34). To examine whether the actions of CTS also include abrogation of IL-1β-induced suppression of gene transcription for TIMPs, both mRNA expression and protein synthesis of TIMP-I and TIMP-II were assessed in chondrocytes exposed to IL-1β in the presence or the absence of CTS. The densitometric analysis of the PCR products for TIMP-I revealed that exposure to IL-1β does not inhibit TIMP-I mRNA expression significantly after either 4 or 24 h of exposure (Fig. 4A). Furthermore, CTS alone or in the presence of IL-1β did not affect TIMP-I mRNA expression (Fig. 4A).
FIGURE 4.
Effect of CTS on rhIL-1β-dependent inhibition of mRNA expression for TIMPs and type II collagen. Expression of mRNA for TIMP-I and TIMP-II (A) and type II collagen (B) in chondrocytes either untreated or exposed to rhIL-1β, CTS, or rhIL-1β and CTS. The mRNA expression was assessed by RT-PCR using 1 μg of RNA from cells in each group. Amplification of GAPDH mRNA was used to assure equal input in all lanes. The data represent one of three separate experiments. Bars represent the mean and SEM of triplicate values. *, p < 0.05 compared with cells treated with IL-1β alone.
In parallel experiments (Fig. 4A), IL-1β treatment of chondrocytes resulted in a consistent inhibition of the constitutive expression of TIMP-II mRNA. More importantly, as shown in Fig. 4, coexposure of chondrocytes to IL-1β and CTS resulted in hyper induction of TIMP-II mRNA, i.e., a 4 ± 0.62-fold increase in TIMP-II mRNA was observed during first 4 h, and a 7.4 ± 1.1-fold increase was observed after 24 h in comparison of untreated control cells ( p < 0.05). CTS alone did not affect TIMP-II mRNA expression, further demonstrating that CTS acts on chondrocytes solely in an IL-1β-dependent manner.
CTS abrogates IL-1β-dependent inhibition of type II collagen synthesis
In addition to activation of proteolytic enzymes, IL-1-mediated cartilage destruction involves inhibition of type II collagen synthesis (35–38). Because collagen type II inhibition by IL-1β is regulated via inhibition of its mRNA expression (36), we examined whether CTS also suppresses IL-1β-mediated induction of mRNA for collagen type II. As expected, application of CTS in the presence of IL-1β significantly abrogated the IL-1-induced inhibition of collagen type II mRNA expression ( p ≤ 0.05) for a sustained period of time (Fig. 4B). Under the same conditions, we did not observe induction of collagen type II mRNA expression by CTS alone, providing further evidence of the IL-1β dependence of CTS actions.
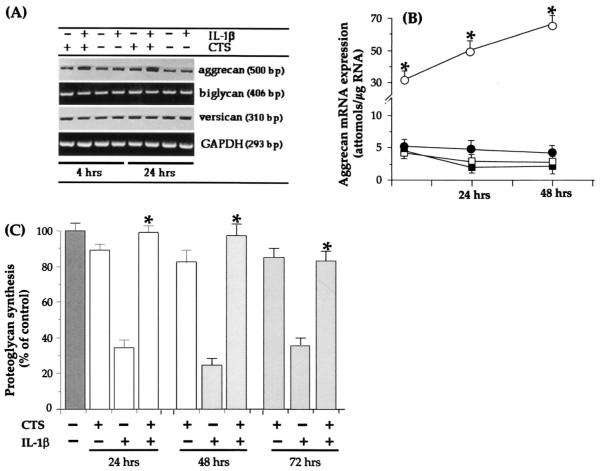
CTS abrogates IL-1β-dependent inhibition of proteoglycan synthesis
A marked inhibition of proteoglycan synthesis observed in joints afflicted with RA is attributed to the actions of IL-1β. Therefore, we examined whether CTS actions also involve abrogation of the effects of IL-1β on proteoglycan synthesis. IL-1β-dependent down-regulation of proteoglycan synthesis can possibly occur at two levels, i.e., via direct inhibition of the synthesis of individual components of proteoglycans such as core proteins and GAG chains (7, 8) and via inhibition of sulfation of proteoglycans, which is controlled by a second messenger, inducible NO (8, 14, 39, 40). Chondrocytes subjected to IL-1β did not reveal appreciable reduction in mRNA induction for versican or biglycan, whereas a consistent inhibition of aggrecan mRNA synthesis was observed following 4 and 24 h of IL-1β exposure. Yet in the presence of CTS and IL-1β, a sustained and significant hyperinduction of aggrecan, but not of versican or of biglycan, was apparent (Fig. 5A). Quantitative assessment of aggrecan mRNA hyperinduction by RT-QCPCR showed that IL-1β inhibited ~12–14% aggrecan mRNA expression, whereas CTS in the presence of IL-1β induced 2.6-, 4.1-, and 5.8-fold increases in aggrecan mRNA induction after 4, 24, and 48 h of treatment, respectively (Fig. 5B). In these experiments aggrecan synthesis was compared with that in untreated control chondrocytes. Interestingly, cells treated with CTS alone also exhibited a 9 ± 4 to 11 ± 4% down-regulation of aggrecan synthesis after 24, 48, and 72 h compared with untreated controls.
FIGURE 5.
Effect of CTS on rhIL-1β-dependent inhibition of proteoglycan synthesis. A, Expression of mRNA for aggrecan, biglycan, and versican in chondrocytes either untreated or exposed to rhIL-1β, CTS, or rhIL-1β and CTS for 4 or 24 h. The mRNA expression was assessed by RT-PCR. Amplification of GAPDH mRNA in 1 μg of total RNA was used as a standard. B, Quantitative assessment of aggrecan mRNA expression by RT-QCPCR in chondrocytes either untreated (■) or exposed to CTS (□), rhIL-1β (●), or CTS and rhIL-1β (○). C, Total chondroitin sulfate proteoglycans in the cell culture supernatants of chondrocytes shown in B. Proteoglycans were assessed by incorporation of Na235SO4 during the last 8 h of incubation. The data represent one of three separate experiments. Data in B and C are the mean ± SEM of triplicate values. *, p ≤ 0.05 compared with cells treated with rhIL-1B alone.
We next determined whether CTS-mediated abrogation of IL-1β-dependent inhibition of aggrecan mRNA expression was paralleled by proteoglycan synthesis. In parallel experiments chondrocytes exposed to IL-1β, CTS, or IL-1β and CTS for 24, 48, or 72 h in the above experiments were washed and pulsed with TCM containing Na235SO4 during the last 8 h of incubation. The incorporation of 35SO4 into proteoglycans was used as an indicator of IL-1β and/or CTS responses. Fig. 5C shows that CTS alone down-regulated proteoglycan synthesis by 15 ± 3%, 18 ± 3%, and 14 ± 3% at 24, 48, and 72 h, respectively. Likewise, exposure of cells to rhIL-1β alone resulted in 62 ± 5%, 67 ± 4%, and 61% reductions in 35SO4 incorporation in proteoglycans at 24, 48, and 72 h, respectively. Application of CTS almost completely abrogated rhIL-1β-dependent inhibition of proteoglycan synthesis at all the time points tested.
Effect of CTS pre-exposure on IL-1β-induced down-regulation of iNOS induction
Because CTS-dependent suppression of IL-1β responses may be due to down-regulation of IL-1R on chondrocytes, we subjected cells to CTS for 1 h before addition of IL-1β (1 ng/ml). Subsequently, the expression of iNOS mRNA and NO production were compared with those in cells that were not exposed to CTS. Chondrocytes pretreated with CTS exhibited a 14 ± 11% reduction in the expression of mRNA for iNOS compared with cells treated with IL-1β alone, as assessed by RT-QCPCR (Fig. 6A). Similarly, NO production in cells pretreated with CTS was only 11 ± 6% less than that in cells treated with IL-1β alone (Fig. 6B). Additionally, cells coincubated with IL-1β and CTS exhibited an 81 ± 4.3% inhibition of iNOS mRNA expression and an 84.8 ± 4% inhibition of NO production. In view of the fact that functional analysis provides a better estimation of IL-1R on cell surface than direct measurements of receptor numbers (40, 41), these results suggest that CTS-mediated inhibition of IL-1 actions may not involve significant IL-1R down-regulation.
FIGURE 6.
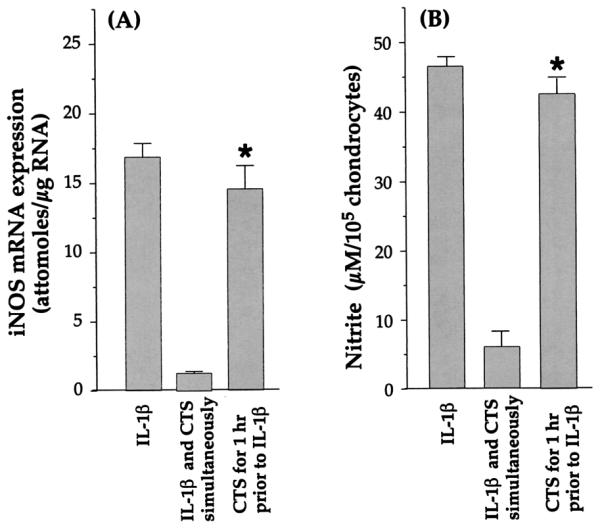
Suppression of rhIL-1β-dependent iNOS mRNA expression and NO production in chondrocytes exposed to CTS 1 h before or simultaneously with the addition of rhIL-1β. A, Expression of iNOS mRNA in chondrocytes exposed to CTS either 1 h before addition of rhIL-1β (-1 h) or simultaneously with rhIL-1β. mRNA expression was assessed by RT-PCR, 24 h after the addition of IL-1β. The data represent densitometric analysis of RT-PCR products for iNOS mRNA in one of three separate experiments. B, Analysis of NO production in the supernatants of the same chondrocytes exposed to the treatments shown in A, The data represent the mean and SEM of triplicate values. *, p ≤ 0.05 compared with cells treated with rhIL-1β and CTS.
Effect of CTS post-treatment on IL-1β-induced down-regulation of iNOS induction
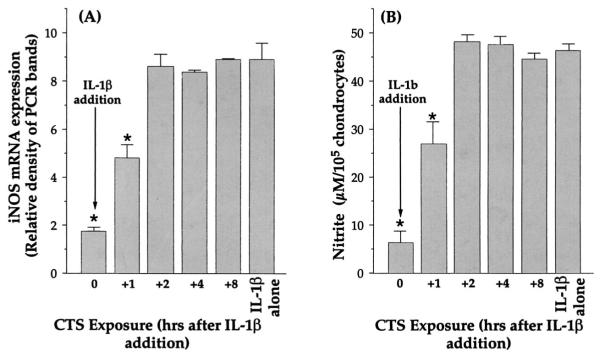
With regard to putative intracellular actions of CTS, we next focused on the time interval when application of CTS was essential to inhibit IL-1β actions. Inhibition of iNOS mRNA expression and NO production was used as an index to measure CTS actions. In these experiments chondrocytes were treated with 1 ng/ml IL-1β at 0 h, while CTS was applied simultaneously (0 h) or after addition of IL-1β (+1, 2, 4, or 8 h). The assessment of mRNA for iNOS or the total NO production 24 h after addition of IL-1β showed that CTS maximally inhibited (82 ± 3.5%) iNOS mRNA expression only when CTS was applied to chondrocytes simultaneously with IL-1β (0 h). Only a partial (40%) inhibition of iNOS mRNA expression was observed if CTS was applied to cells 1 h after addition of IL-1β (Fig. 6), whereas application of CTS was ineffective when applied more than 2 h after the addition of IL-1β (Fig. 7).
FIGURE 7.
Suppression of rhIL-1β-dependent iNOS mRNA expression and NO production in chondrocytes exposed to CTS at various time intervals following addition of rhIL-1β. A, Expression of iNOS mRNA in chondrocytes exposed to CTS either simultaneously with (0 h) or 1, 2, 4, or 8 h after the addition of rhIL-1β. iNOS mRNA expression was assessed by RT-PCR, 24 h after the addition of IL-1β. The data represent densitometric analysis of RT-PCR products for iNOS mRNA in one of three separate experiments. B, Analysis of NO production in the supernatants of chondrocytes subjected to the treatments shown in A. The data represent the mean and SEM of triplicate values. *, p ≤ 0.05 compared with cells treated with rhIL-1β alone.
Discussion
The data presented here represent the first evidence that CTS acts as a powerful antagonist of IL-1β actions, a major inflammatory agent implicated in the etiology of arthritic diseases (1–14). This correlates with the well-known effects of continuous passive motion in the augmentation of speedier and more physiologically sound recovery in orthopedic patients (16–19, 22). We have employed equibiaxial CTS to closely simulate the effects of continuous passive motion because articular cartilage exposed to continuous passive motion is subjected to tensile strain on the superficial layers (42). Furthermore, chondrocytes residing in the superficial layers of cartilage are the cells that are exposed to inflammatory mediators in inflamed joints. These chondrocytes are also the most responsive cells to IL-1β that take part in cartilage destruction (43). Thus, this system, while closely mimicking the in vivo effects of continuous passive motion on inflamed or rehabilitating joints, also provided defined parameters to examine the CTS-transduced signals that are converted into biochemical responses in chondrocytes.
Our results demonstrate that CTS significantly and consistently suppresses IL-1β-dependent mRNA induction for multiple proteins responsible for the initiation of cartilage degradation. By reducing the mRNA abundance of two pivotal proinflammatory enzymes, iNOS and COX-II, CTS also suppresses their secondary inflammatory products, NO and PGE2. Both NO and PGE2 have been shown to mediate IL-1β-induced reduction of proteoglycan synthesis (10, 44). Recently, we reported that CTS inhibits the actions of IL-1β over a wide range of concentrations, encompassing those frequently found in synovial fluids (23). Here we further show that a wide range of low magnitude CTS can inhibit IL-1β-dependent responses in chondrocytes, thus emphasizing the potential of CTS in antagonizing the effects of IL-1β. These findings are of considerable clinical relevance in the treatment of inflamed joints. Interestingly, the presence of an inflammatory signal was a prerequisite for the observed CTS actions, because CTS alone failed to induce a response. This verifies earlier reports showing that a low magnitude of CTS alone is not sufficient to induce the synthesis of proinflammatory mediators (45). Furthermore, these studies emphasize the importance of the magnitude of CTS in its anti-inflammatory actions, because a high magnitude of CTS has been shown to induce NO production and COX-II expression in chondrocytes and bone cells (45, 46). A high magnitude of CTS is also associated with proteoglycan degradation, which, in turn, is regulated by intracellular levels of both NO and PGE2 (7, 8, 10, 44, 46), as well as via induction of IL-1 synthesis (45).
Two important prerequisites for cartilage breakdown in arthritic diseases are breakdown of extracellular matrix and inhibition of its synthesis. During inflammation of joints chondrocytes exhibit both chronic collagenase production as well as inhibition of type II collagen synthesis (13, 36, 44–46). Therefore, effective control of cartilage catabolism requires prolonged suppression of collagenase production and a concurrent increase in collagen synthesis. We show that CTS exposure results in a significant and prolonged suppression of IL-1β-dependent collagenase synthesis via inhibition of its mRNA expression. As observed previously, a low magnitude of CTS alone was not sufficient to induce collagenase synthesis in these studies (45).
It is known that IL-1β down-regulates the production of TIMPs to augment collagen breakdown in chondrocytes (31–34), while application of TIMP-I and TIMP-II inhibits IL-1β-induced collagen degradation in cartilage (32–34). Consequently, we examined the effects of CTS exposure on the IL-1β-mediated inhibition of TIMP-I and TIMP-II in chondrocytes. We show that concomitant to the inhibition of collagenase production, CTS reverses the effects of IL-1β on TIMP-II production. Moreover, in the presence of IL-1β, CTS induces striking hyperinduction of TIMP-II, amounting to 4- and 7.3-fold increases in 4 and 24 h, respectively. Whether all the TIMP-II induced by CTS is in its active form is as yet not clear. TIMP-II has been shown to block collagenase activity effectively at less than an equimolar ratio, suggesting that the observed hyperinduction of TIMP-II in its active form by CTS for prolonged periods may be an effective mechanism to reduce metalloprotease-mediated extracellular matrix degradation. As observed with the other proinflammatory mediators, the hyperinduction of TIMP-II transcription by CTS takes place exclusively in the presence of an inflammatory signal such as IL-1β, while exposure of chondrocytes to CTS alone is not sufficient for the induction of TIMP-II. Unlike previous reports (32–34), we did not observe significant down-regulation of TIMP-I by IL-1β. Furthermore, CTS did not affect TIMP-I mRNA expression, re-emphasizing the IL-1β dependence of CTS actions.
In human chondrocytes, along with collagen degradation IL-1β-induces inhibition of collagen type II synthesis (34–38). CTS is effective in revoking IL-1-dependent inhibition of collagen type II synthesis. Thus, these results collectively provide evidence that CTS not only antagonizes IL-1β-induced matrix degradation via inhibition of collagenase production and neutralization of its activity through hyperinduction of TIMP-II, but also via augmentation of collagen synthesis. Interestingly, while continuous passive motion has been widely used for postoperative rehabilitation of patients, a number of reports have shown that its application is not always beneficial (18). Given that a high magnitude of CTS initiates collagen degradation via induction of collagenase synthesis (42–45), we can speculate that the magnitude of mechanical strain may play a critical role in the success or failure of continuous passive motion therapy. For instance, failure of continuous passive motion therapy could be ascribed to the high magnitude of mechanical load, whereas its beneficial effects may be associated with low magnitudes of mechanical strain in vivo, which appears to be reparative in nature.
The synthesis of the major component of the cartilaginous extracellular matrix, chondroitin sulfate proteoglycan, is known to be dramatically reduced following exposure of chondrocytes to IL-1β (1, 8, 12, 40). As observed previously (32, 39), our data demonstrate that IL-1β down-regulates aggrecan mRNA expression within the first 4 h of IL-1β actions and is sustained for the next 48 h. CTS antagonizes IL-1β-dependent down-regulation of aggrecan synthesis. In fact, application of CTS in the presence of IL-1β results in the hyperinduction of aggrecan mRNA. Interestingly, while IL-1β induces only a 10–15% inhibition of aggrecan mRNA abundance, in the presence of IL-1β CTS up-regulates aggrecan mRNA expression 4- to 8-fold higher than in untreated cells. This hyperinduction of aggrecan by CTS is likely to be critical in de novo proteoglycan synthesis during inflammation, where aggrecan breakdown is a common occurrence (8, 10, 13, 39). This is also supported by the evidence that CTS almost entirely abrogates the IL-1β-dependent inhibition of proteoglycan synthesis. We have recently shown that CTS antagonizes IL-1β actions by abrogating IL-1β-mediated inhibition of proteoglycan synthesis in parallel to inhibition of NO production (23). Because NO inhibits sulfation of proteoglycans (39), it is also possible that CTS negates the effects of IL-1β-dependent suppression of proteoglycan synthesis via both up-regulation of aggrecan synthesis as well as inhibition of NO production, which, in turn, results in increased chondroitin sulfate proteoglycan synthesis. Versican and biglycan are small, nonaggregating proteoglycans found in cartilage. IL-1β did not inhibit versican and biglycan mRNA expression, nor did CTS induce their up-regulation significantly. It has been reported that biglycan synthesis is down-regulated by IL-1β (38); our data suggest that it occurs translationally or post-translationally.
It is commonly accepted that IL-1R down-regulation is an important mechanism that allows cells to calibrate their responses to exogenous signals (40, 41). Therefore, the possibility existed that chondrocytes down-regulate their IL-1Rs in response to CTS exposure, which, in turn, results in the suppression of the IL-1β responsiveness of cells. The functional analysis demonstrates that pre-exposure of chondrocytes to CTS for 1 h results in only a minor reduction in their responsiveness to IL-1β, as assessed by the induction of iNOS mRNA expression. We verified these results by measuring the total NO production 24 h after application of IL-1β, and our data show that prior exposure of chondrocytes to CTS failed to significantly inhibit the IL-1β responsiveness of cells. These results thus provide evidence that CTS may not downregulate IL-1R, but its actions may involve inhibition or modulation of one or more steps in the IL-1β signal transduction cascade upstream of mRNA transcription. This assumption is supported further by our observations that inhibition of responsiveness toIL-1β requires cells to be exposed to CTS during the early actions of IL-1β. For example, CTS antagonizes IL-1β actions only when applied before, simultaneously with, or within the first hour of IL-1β application, whereas exposure of chondrocytes to CTS >2 h after IL-1β application fails to block IL-1β actions.
In summary, we have demonstrated that CTS is an effective antagonist of IL-1β actions on chondrocytes. Intracellular actions of CTS are mediated through transcriptional regulation of multiple genes activated by IL-1β. Furthermore, CTS actions involve disruption/regulation of a critical step(s) in the signal transduction cascade of IL-1β. By down-regulating the induction of catabolic proteins as well as up-regulating the induction of extracellular matrix proteins, CTS not only acts as an anti-inflammatory signal but also as a reparative signal in IL-1β-treated chondrocytes. Nevertheless, the presence of IL-1β is a prerequisite for these actions of CTS. As a potent antagonist of IL-1β, the actions of CTS on chondrocytes appear to be remarkably similar to those of current therapeutic agents that are being implemented to minimize cartilage degradation, such as anti-IL-1 Igs, IL-1R antagonist, or metalloprotease inhibitors (47–53). In this regard, while these therapeutics are expected to reduce the catabolic actions of IL-1, CTS appears to initiate anabolic activity in chondrocytes as well. Whether CTS actions are specifically IL-1β dependent, or it counteracts the actions of other inflammatory mediators such as TNF-a or microbial LPS found in inflamed synovial joints (3, 4, 6) has yet to be determined. Our results thus not only provide molecular evidence for the biochemical signals generated by CTS, but also provide crucial leads to further unveil the pathways regulated by mechanical strain to fully understand the mechanisms of continuous passive motion-mediated reparative actions on inflamed joints.
Acknowledgments
We are indebted to Dr. Mohammed Heidaran (Orquest, Mountain View, CA) for providing sequences to rabbit primers for aggrecan and collagen type II, and to Drs. Jian Ying Zhang and Robert Gassner for their critical comments on the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants RO1AR42025 and R15DE12976 and an Oral and Maxillofacial Surgery Foundation research grant.
- OA
- osteoarthritis
- RA
- rheumatoid arthritis
- CTS
- cyclic tensile strain
- iNOS
- inducible NO synthase
- COX-II
- cyclo-oxygenase II
- TIMP
- tissue inhibitor of metalloproteases
- MMP-I
- matrix metalloprotease I
- rh
- recombinant human
- QCPCR
- quantitative competitive PCR
References
- 1.Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin. Arthritis Rheum. 1998;27:392. doi: 10.1016/s0049-0172(98)80019-x. [DOI] [PubMed] [Google Scholar]
- 2.Bandara G, Georgescu HI, Lin CW, Evans CH. Synovial activation of chondrocytes: evidence for complex cytokine interactions. Agents Actions. 1991;34:285. doi: 10.1007/BF01993304. [DOI] [PubMed] [Google Scholar]
- 3.Flugge LA, Miller-Deist LA, Petillo PA. Towards a molecular understanding of arthritis. Chem. Biol. 1999;6:R157. doi: 10.1016/S1074-5521(99)80043-X. [DOI] [PubMed] [Google Scholar]
- 4.van Den Berg WB. Lessons for joint destruction from animal models. Curr. Opin. Rheumatol. 1997;9:221. doi: 10.1097/00002281-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Firstein GS, Boyle DL, Yu C, Paine MM, Whisenand TD, Svaifler NJ, Aredn WP. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 1994;37:644. doi: 10.1002/art.1780370507. [DOI] [PubMed] [Google Scholar]
- 6.Evans CH, Ghivizzani SC, Robbins PD. Blocking cytokines with genes. J. Leukocyte Biol. 1998;64:55. doi: 10.1002/jlb.64.1.55. [DOI] [PubMed] [Google Scholar]
- 7.Evans CH. The role of proteinases in cartilage destruction. Agents Actions. 1991;32:135. doi: 10.1007/978-3-0348-7405-2_19. [DOI] [PubMed] [Google Scholar]
- 8.Evans CH, Watkins SC, Stefanovic-Racic M. Nitric oxide and cartilage metabolism. Methods Enzymol. 1996;269:75. doi: 10.1016/s0076-6879(96)69011-9. [DOI] [PubMed] [Google Scholar]
- 9.Manfield L, Jang D, Murrell AC. Nitric oxide enhances cyclooxygenase activity in articular cartilage. Inflamm. Res. 1996;45:254. doi: 10.1007/BF02259612. [DOI] [PubMed] [Google Scholar]
- 10.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans CH. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by IL-1. Biochem. Biophys. Res. Commun. 1994;200:142. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 11.Morisset S, Patry C, Lora M, de Brum-Fernandes AJ. Regulation of cyclooxygenase-2 expression in bovine chondrocytes in culture by interleukin1a, tumor necrosis factor-α, glucocorticoids, and 17β-estradiol. J. Rheumatol. 1998;25:1146. [PubMed] [Google Scholar]
- 12.Lotz M, Blanco FJ, Von Kempis J, Dudler J, Maier R, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J. Rheumatol. 1995;22:104. [PubMed] [Google Scholar]
- 13.Murrell GAC, Jang D, Williams RJ. Nitric oxide activates metalloprotease in articular cartilage. Biochem. Biophys. Res. Commun. 1995;206:15. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 14.Stefanovic-Racic M, Mollers MO, Miller LA, Evans CH. Nitric oxide and proteoglycan turnover in rabbit articular cartilage. J. Orthopaed. Res. 1997;15:442. doi: 10.1002/jor.1100150318. [DOI] [PubMed] [Google Scholar]
- 15.Colwell CW, Jr., Morris BA. The influence of continuous passive motion on the results of total knee arthroplasty. Clin. Orthopaed. Rel. Res. 1992;276:225. [PubMed] [Google Scholar]
- 16.McCarty WL, Darnell MW. Rehabilitation of the temporomandibular joint through the application of motion. J. Craniomand. Pract. 1993;11:298. doi: 10.1080/08869634.1993.11677982. [DOI] [PubMed] [Google Scholar]
- 17.Kim HK, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J. Rheumatol. 1995;22:1714. [PubMed] [Google Scholar]
- 18.Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clinics. 1994;10:211. [PubMed] [Google Scholar]
- 19.Koob TJ, Clark PE, Hernandez DA, Thurmond FA, Vogel KG. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch. Biochem. Biophys. 1992;298:303. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- 20.Williams JM, Moran M, Thonar E, Salter RB. Continuous passive motion stimulates repair of rabbit knee articular cartilage after matrix proteoglycan loss. Clin. Orthop. Rel. Res. 1994;304:252. [PubMed] [Google Scholar]
- 21.Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clinics. 1994;10:211. [PubMed] [Google Scholar]
- 22.Von Schroeder HP, Coutts RD, Billings E, Jr., Mai MT, Aratow M. The changes in intramuscular pressure and femoral vein flow with continuous passive motion, pneumatic compressive stockings, and leg manipulations. Clin. Orthop. 1991;266:218. [PubMed] [Google Scholar]
- 23.Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanvich-Racic M, Piesco NP, Evans CH, Agarwal S. Cyclic tensile stress exerts anti-inflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J. Immunol. 1999;163:2187. [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto-Iwamoto M, Iwamoto M, Nakashima K, Mukudai Y, Boettiger D, Pacifici M, Kurisu K, Suzuki F. Involvement of α5β1 integrins in matrix interactions and proliferation of chondrocytes. J. Bone Miner. Res. 1997;12:1124. doi: 10.1359/jbmr.1997.12.7.1124. [DOI] [PubMed] [Google Scholar]
- 25.Fedewa MM, Oegema TR, Jr., Schwartz MH, MacLeod A, Lewis JL. Chondrocytes in cellular culture produce a mechanically functional tissue. J. Orthopaed. Res. 1998;16:227. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Grynpas M, Kandel RL. Composition of cartilaginous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997;18:1425. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD. An equibiaxial strain system for cultured cells. Am. J. Physiol. 1996;271:C14000. doi: 10.1152/ajpcell.1996.271.4.C1400. [DOI] [PubMed] [Google Scholar]
- 28.Hart DA, Boykiw R, Sciore P, Reno C. Complex alterations in gene expression occur in the knee ligaments of the skeletally mature multiparous rabbit during pregnancy. Biochim. Biophys. Acta. 1998;1397:331. doi: 10.1016/s0167-4781(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 29.Saito S, Katoh M, Masumoto M, Matsumoto S, Masuho Y. Involvement of MMP-1 and MMP-3 in collagen degradation induced by IL-1 in rabbit cartilage explant culture. Life Sci. 1998;62:359. doi: 10.1016/s0024-3205(98)00181-7. [DOI] [PubMed] [Google Scholar]
- 30.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J. Biol. Chem. 1996;271:23577. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- 31.Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- 32.Cawston T, Billington C, Cleaver C, Elliott S, Hui W, Koshy P, Shingleton B, Rowan A. The regulation of MMPs and TIMPs in cartilage turnover. Ann. NY Acad. Sci. 1999;878:120. doi: 10.1111/j.1749-6632.1999.tb07678.x. [DOI] [PubMed] [Google Scholar]
- 33.Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y. The effects of cytokines on metalloproteinase inhibitors TIMP and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin. Exp. Immunol. 1993;94:145. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis AJ, Curry VA, Powell EK, Cawston TE. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem. Biophys. Res. Commun. 1994;201:94. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekhar S, Harvey AK, Higginbotham JD, Horton WE. Interleukin-1-induced suppression of type II collagen gene transcription involves DNA regulatory elements. Exp. Cell Res. 1990;191:105. doi: 10.1016/0014-4827(90)90042-9. [DOI] [PubMed] [Google Scholar]
- 36.Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-γ of type II collagen gene expression in human chondrocytes. J. Cell Biochem. 1994;54:85. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- 37.Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, Arbiser JL, Apperley JF. Interleukin-1β-modulated gene expression in immortalized human chondrocytes. J. Clin. Invest. 1994;94:2307. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Den Hoff H, de Koning M, van Kampen J, van der Korst J. Interleukin-1 reversibly inhibits the synthesis of biglycan and decorin in intact articular cartilage in culture. J. Rheumatol. 1995;22:1520. [PubMed] [Google Scholar]
- 39.Hickery MS, Baylis MT. Interleukin-1 induced nitric oxide inhibits sulphation of glycosaminoglycan chains in human articular chondrocytes. Biochim. Biophys. Acta. 1998;1425:282. doi: 10.1016/s0304-4165(98)00080-4. [DOI] [PubMed] [Google Scholar]
- 40.Martel-Pelletier J, Mccollum R, Dibattista J. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992;35:530. doi: 10.1002/art.1780350507. [DOI] [PubMed] [Google Scholar]
- 41.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095. [PubMed] [Google Scholar]
- 42.Kelly PA, O’Connor JJ. Transmission of rapidly applied loads through articular cartilage. I. Uncracked cartilage. J. Engin. Med. 1996;210:27. doi: 10.1243/PIME_PROC_1996_210_388_02. [DOI] [PubMed] [Google Scholar]
- 43.Häuselman HJ, Flechtenmacher J, Michal M, Thonar EJ-MA, Shinmei M, Kuettner KE, Aydelotte ME. The superficial layer of human articular cartilage is more susceptible to interleukin-1-induced damage than the deeper layers. Arthritis Rheum. 1996;39:478. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda K, Ohtani K, Dan H, Tanaka S. Interleukin-1 inhibits keratan sulfate production by rabbit chondrocytes: possible role of prostaglandin E2. Inflamm. Res. 1995;44:178. doi: 10.1007/BF01782816. [DOI] [PubMed] [Google Scholar]
- 45.Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J. Biochem. 1999;125:966. doi: 10.1093/oxfordjournals.jbchem.a022376. [DOI] [PubMed] [Google Scholar]
- 46.Pitsillides AA, Rawlinson SC, Suswillo RF, Bourrin S, Zaman G, Lanyon LE. Mechanical strain-induced NO production by bone cells: possible role in adaptive bone remodeling. FASEB J. 1995;9:1614. doi: 10.1096/fasebj.9.15.8529841. [DOI] [PubMed] [Google Scholar]
- 47.Mueller-Ladner U, Roberts CR, Franklin BN, Gray RE, Robbins PD, Evans DH, Gay S. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J. Immunol. 1997;158:3492. [PubMed] [Google Scholar]
- 48.Evans CH, Robbins PD. The interleukin-1 receptor antagonist and its delivery by gene transfer. Receptor. 1994;4:9. [PubMed] [Google Scholar]
- 49.Van De Loo FA, Arntz OJ, Otterness IG, van den Berg WB. Protection against cartilage proteoglycan synthesis inhibition by anti-interleukin-1 antibodies in experimental arthritis. J. Rheumatol. 1992;19:348. [PubMed] [Google Scholar]
- 50.Lane NE, Williams RJ, Schurman DJ, Smith RL. Inhibition of interleukin 1 induced chondrocyte protease activity by a corticosteroid and a nonsteroidal anti-inflammatory drug. J. Rheumatol. 1992;19:135. [PubMed] [Google Scholar]
- 51.Ku G, Faust T, Lauffer LL, Livingston DJ, Harding MW. Interleukin-1β converting enzyme inhibition blocks progression of type II collagen-induced arthritis in mice. Cytokine. 1996;8:377. doi: 10.1006/cyto.1996.0052. [DOI] [PubMed] [Google Scholar]
- 52.Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: a comparative study using anti-TNFa, anti-IL-1α/β, and IL-1Ra. Arthritis Rheum. 1996;39:797. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 53.Spirito S, Doughty J, O’Byrne E, Ganu V, Goldberg RL. Metalloprotease inhibitors halt collagen breakdown in IL-1 induced bovine nasal cartilage cultures. Inflamm. Res. 1995;44:S131. doi: 10.1007/BF01778297. [DOI] [PubMed] [Google Scholar]