Abstract
Group Person-Based Cognitive Therapy (PBCT) integrates cognitive therapy and mindfulness to target distinct sources of distress in psychosis. The present study presents data from the first randomised controlled trial investigating group PBCT in people distressed by hearing voices. One-hundred and eight participants were randomised to receive either group PBCT and Treatment As Usual (TAU) or TAU only. While there was no significant effect on the primary outcome, a measure of general psychological distress, results showed significant between-group post-intervention benefits in voice-related distress, perceived controllability of voices and recovery. Participants in the PBCT group reported significantly lower post-treatment levels of depression, with this effect maintained at six-month follow-up. Findings suggest PBCT delivered over 12 weeks effectively impacts key dimensions of the voice hearing experience, supports meaningful behaviour change, and has lasting effects on mood.
Keywords: Schizophrenia, Person-Based Cognitive Therapy, Mindfulness, Group therapy, Distressing voices, Randomised Controlled Trial
1. Introduction
Individual Cognitive Behaviour Therapy for Psychosis (CBTp) is recommended in the US and UK for the treatment of schizophrenia and schizoaffective disorder (e.g. National Collaborating Centre for Mental Health, 2014) – though in the UK, where CBTp is longer established, access to individual CBTp remains poor (The Schizophrenia Commission, 2012) and group CBTp lacks sufficiently robust evidence to be recommended by the National Institute for Clinical Excellence.
Evidence is building for the benefits of group mindfulness-based interventions (MBIs) for psychosis (Chadwick et al., 2005, Khoury et al., 2013, Lopez-Navarro et al., 2015), including for distressing voices (Chadwick et al., 2009). Mindfulness is a state of non-judgemental and accepting awareness of present-moment experiences (such as thoughts, voices and bodily sensations). Qualitative research reveals how learning to respond mindfully to difficult psychotic symptoms can reduce distress and facilitate increased acceptance of both psychosis and the self (Abba et al., 2008). Person-Based Cognitive Therapy (PBCT; Chadwick, 2006) integrates CBTp and mindfulness and was developed specifically for people with distressing psychosis (though has since been found to significantly benefit people with chronic depression: Strauss et al., 2012). It has long been argued that CBTp aims at reducing distress and disturbance, not psychotic symptoms (e.g. Chadwick et al., 1996), and PBCT explicitly targets three distinct sources of distress/disturbance: persecutory delusions and beliefs about voices (‘symptomatic meaning’); self-defeating reactions to psychotic symptoms (e.g. experiential avoidance, fighting with voices, paranoid rumination); and core beliefs (schemata) that define the self as negative and fixed. Therapy combines guided discovery, behavioural experiments, a focus on positive behaviour change, mindfulness practice, and a strong experiential focus.
Auditory hallucinations (voices) are often experienced as highly distressing and disturbing (Birchwood and Chadwick, 1997). Two randomised controlled trials of group CBTp specifically for people with distressing voices have been published. Wykes et al. (2005) reported improvement in social functioning but no effect upon the severity of voices. Penn et al. (2009) compared CBTp with supportive therapy and found CBTp to beneficially impact general psychotic symptoms and supportive therapy to have greater specific impact on auditory hallucinations. The present study is the first randomised controlled trial of group PBCT for distressing voices. Following an earlier uncontrolled study (Dannahy et al., 2011) the primary outcome was a measure of general distress (CORE-OM). We also included outcomes more proximal to the targets of the PBCT therapy including voice distress, voice control, and depression; as well as a recovery-related measure of positive interpersonal behaviour and purpose. The primary hypothesis was that group PBCT plus treatment-as-usual (TAU), in comparison to TAU-only, would lead to reduced distress and disturbance in people distressed by hearing voices with a diagnosis of schizophrenia or schizoaffective disorder. A secondary hypothesis was that these effects would be maintained six months post therapy.
2. Method
2.1. Design
This was a single blind, pragmatic randomised controlled trial (registration number ISRCTN74054823) comparing group PBCT plus TAU (hereafter referred to as PBCT) with TAU alone (hereafter referred to as TAU). Allocation was 1:1. Eligible participants were recruited from two UK mental health services in Sussex and Hampshire. Recruitment began in January 2012 and was completed in September 2013 (recruitment delays required a 6 month extension). Follow-up assessments ran from March 2013 to July 2014. Assessment occurred pre-randomisation, four months post-randomisation (post-treatment) and ten months post-randomisation (follow-up). The Brighton and Sussex Research Ethics Committee (number 11/L0/1330) gave ethical approval.
The planned sample size of 60 participants per treatment arm was based on yielding 80% power for detecting an effect size of d = 0.56 (Chadwick et al., 2009) with a Student's t-test, two-sided 5% alpha and an attrition rate of 18%.
2.2. Recruitment process
Inclusion criteria were: hearing distressing voices for the preceding year; ICD 10 (World Health Organisation, 1992: research criteria) diagnosis of schizophrenia or schizo − affective disorder; aged 18 years and older. Exclusion criteria were: known organic illness; primary diagnosis of substance misuse. Eligible participants identified by Consultant Psychiatrists and Mental Health Practitioners were given information sheets and, if interested, were asked for verbal permission to be contacted by a study research assistant. Research assistants provided further information, obtained written informed consent, and administered baseline measures in line with the assessment protocol (the present article presents data on main outcomes only).
2.3. Randomisation and blinding
Stratified block randomisation was completed by the Clinical Trials Unit at King's College London. When 12–18 participants had been consented and assessed within a centre, recruitment was closed and participants were randomised to receive PBCT or TAU. The Trials Unit then emailed allocation details to the centre Trial Manager and research assistant, who notified participants of their allocation. All post-randomisation assessments were completed by research assistants from a different geographical centre who were blind to participant allocation. When breaks in blinding were reported, assessments were completed by another research assistant who was blind to allocation.
2.4. Intervention
Group PBCT was delivered over 12 one-and-a-half-hour sessions. All groups were supervised by the first author and facilitated by two clinical psychologists experienced in either CBTp, or mindfulness, or both (five therapists in total). The therapy manual is detailed elsewhere (Chadwick, 2006, Strauss and Hayward, 2013). Briefly: All sessions began with mindfulness practice and discussion. Mindfulness practice in PBCT is brief (10 min), with frequent guidance that includes reference to psychotic experience, and combines focussed attention on body and breath with open awareness. Sessions 1–3 socratically drew out participants' voice hearing experiences (onset, impact, meaning, distress and coping) and framed them using the ABC cognitive model. Sessions 4–6 explored personal control, socratically weakening voice omnipotence and enhancing autonomy. Sessions 7–12 added focus on identifying and decentring from negative schemata, and building positive schematic beliefs (including using experiential two chair work) alongside recognition that the self is complex and changing. Participants were encouraged to practice mindfulness daily at home, using a supplied 10 min recording, and each week one further homework was set relating to work on voices or self (e.g. Session 6: keeping a record of times when I chose what to do in spite of the voices).
Therapy adherence was assessed by participants completing checklists indicating adherence to key protocol elements for that session (e.g. Session 3: (i) We did a mindfulness practice, (ii) We talked about what we noticed during the mindfulness practice, (iii) we talked about voices using an ABC table, (iv) we were asked about what we had learned from today's sessions). Ratings were administered by research assistants at 16 randomly selected sessions, covering both sites, and after the therapists had left. 65/67 participant ratings indicated full adherence; at two separate sessions a participant rated one of the four protocol elements as missing. It was not possible to record group therapy sessions due to participant concerns.
All participants received TAU, comprising: 2–3 monthly outpatient appointments with their psychiatrist, antipsychotic medication, and contact with care team members every two weeks, all of which were documented in accordance with the study protocol.
2.5. Measures
2.5.1. Primary outcome. Clinical outcomes in routine evaluation-outcome measure (CORE-OM; Evans et al., 2000)
A 34-item self-report measure of psychological distress with adequate reliability (α 0.75–0.95) developed to evaluate outcome in psychological therapy (Evans et al., 2000). CORE-OM includes subscales of well-being, problems, functioning and risk. Item scores range from 0 to 4 (higher scores indicate more distress).
2.5.2. PSYRATS: Auditory Hallucinations Scale (AHRS; Haddock et al., 1999)
A clinician rated 11-item scale assessing the severity of different dimensions of the voice-hearing experience (Haddock et al., 1999) including frequency, duration, loudness, distress intensity and control. Each item is rated 0 to 4 (higher scores indicate more difficulty). The authors report good psychometric properties and inter-rater reliability (0.78–1.00).
2.5.3. Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith, 1983)
A 14-item self-report measure of anxiety (7 items) and depression (7 items). Items are scored 0–3, with higher scores indicating greater distress. The HADS has well established psychometric properties (Zigmond and Snaith, 1983) and reliability (α 0.83 for anxiety and 0.82 for depression; Bjelland et al., 2002).
2.5.4. Choice of outcome in CBT for psychoses (CHOICE; Greenwood et al., 2010)
A 24-item self-report questionnaire developed with patients to assess goals for CBTp related to recovery (e.g. self-confidence, positive ways of relating to people, and a positive purpose and direction in life) that is reliable (α 0.83 for Severity and 0.88 for Satisfaction) and valid (Greenwood et al., 2010). Each item is rated on a 10-point scale for (1) severity and (2) satisfaction (higher scores indicate better functioning).
2.6. Statistical analyses
All outcomes (excluding distress intensity and control) were evaluated using Analysis of Covariance (ANCOVA) at post treatment (4 months) and at follow-up (10 months) separately using the intention-to-treat (ITT) principle where all participants were analysed as per their randomisation allocation. Corresponding baseline scores were used as covariates in the ANCOVA and centre (Sussex or Hampshire) and treatment group (PBCT + TAU or TAU only) were treated as fixed factors. All analyses were carried out in STATA (version 13); treatment effects were estimated using regress. Due to the ordinal nature of the distress intensity and control outcomes, non-parametric methodology was used to conduct a between-group comparison of the change in scores from baseline to the post treatment and follow-up, separately. The change scores for each group were compared using a Mann-Whitney test for unpaired groups (using STATA ranksum) and then the Cohen's d standardised treatment effect size was calculated from the Z-scores (Lenhard and Lenhard, 2015).
Missing follow-up data is commonly experienced in longitudinal trials and it is often assumed that the data are missing at random (MAR). We looked at the predictability of missingness from other non-missing variables and the MAR assumption was deemed appropriate. To address the impact of drop-outs, adjustment rates were calculated using inverse probability weights that could be used in the regression models to compensate for the missing values (Little and Rubin, 1987). For each individual, the probability of providing follow-up data at 4 and 10 months separately was estimated using treatment compliance (attended at least 8 sessions), centre, treatment group and the baseline outcome value as predictors in an unweighted logistic regression (using STATA logit). The adjustment weight was calculated as the reciprocal of this probability which was then entered in the regression model used in the main analyses. The unstandardised treatment effect was taken directly from the coefficient for the treatment group. The standardised effect (Cohen's d) was calculated from the treatment group coefficient divided by the corresponding outcome pooled SD at baseline. Estimated unadjusted (complete case analysis) and adjusted (for drop-out) treatment effects are reported with their standard error, 95% confidence interval, significance levels and Cohen's d. No adjustments were made for the distress intensity and control outcomes. An exploratory per-protocol analysis was also carried out where the outcomes of participants who were treatment compliant (i.e. attended 8 or more therapy sessions) were compared with the TAU group.
3. Results
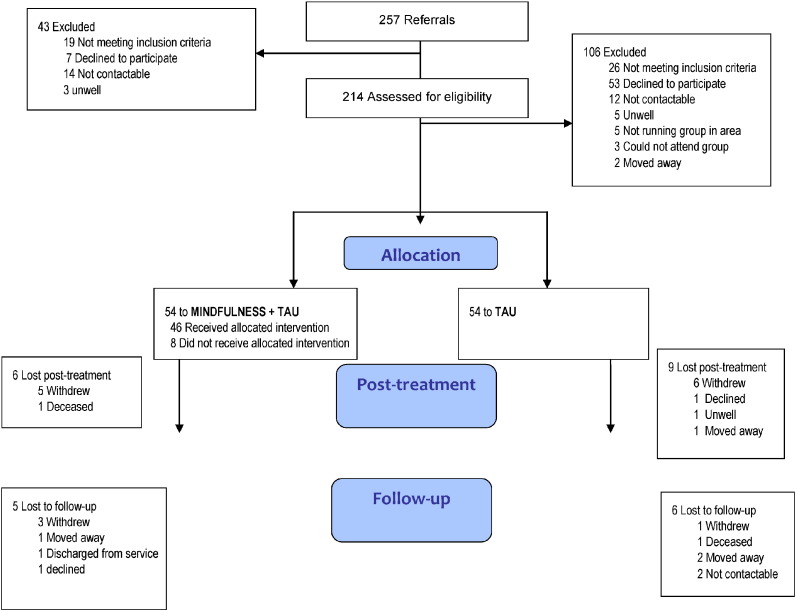
The CONSORT diagram shows the trial profile (see Fig. 1). Sixty-three participants were recruited in the Sussex centre and forty-five participants in Hampshire. Three PBCT groups were run in Hampshire and four in Sussex.
Fig. 1.
Flow of participants through the study.
39 participants (72%) randomised to PBCT attended at least 8 sessions (‘completers’). Eight participants randomised to PBCT attended no sessions (three dropped out of the study, and five completed at least one post-randomisation assessment); of those who attended at least one session, 85% completed therapy. Study retention was 93/108 (86%) at 4-months and 82/108 (76%) at 10-months assessments (the two serious adverse events, both deaths, were reviewed by the Trial Steering Committee and deemed highly unlikely to be related to the study). Data were missing from 14% of participants post treatment and 24% of participants at follow-up.
3.1. Sample characteristics
In the study sample, 53 (50%) were male; the median age of the participants was 42 years (range 18–65); the median onset age of hearing voices was 21 years (range 5–55); 98 (91%) were White British; 82 (76%) were unemployed, 16 (15%) were employed and 10 (9%) had another status e.g. retired. In terms of highest level of education received, 20 (19%) had primary education, 41 (38%) had lower secondary education, 16 (15%) had upper secondary education, 14 (13%) had post-secondary non-tertiary, 6 (6%) had short-cycle tertiary education and 11 (10%) had bachelor degrees or equivalent. In terms of clinical diagnosis, all participants had a diagnosis of Schizophrenia or Schizoaffective disorder. Baseline characteristics between the two study arms were similar (Table 1).
Table 1.
Socioeconomic profiles and outcome measures at baseline.
| PBCT + TAU (n = 54) | TAU (n = 54) | |
|---|---|---|
| Gender | ||
| Male | 27 (50) | 26 (48) |
| Female | 27 (50) | 27 (50) |
| Age median years (range) | 42 (18–65) | 42 (19–59) |
| Ethnicity | ||
| White British | 49 (91) | 49 (91) |
| Black & minority ethnicity | 5 (9) | 5 (9) |
| Site | ||
| Sussex | 31 (58) | 32 (59) |
| Hampshire | 23 (43) | 22 (41) |
| Employment status | ||
| Employed | 9 (17) | 7 (13) |
| Unemployed | 39 (72) | 43 (80) |
| Other | 6 (11) | 4 (8) |
| Education Level (ISCE) | ||
| Primary | 9 (17) | 11 (20) |
| Lower secondary | 23 (43) | 18 (33) |
| Upper secondary | 11 (20) | 5 (9) |
| Post-secondary non-tertiary education | 5 (9) | 9 (17) |
| Short-cycle tertiary education | 1 (2) | 5 (9) |
| Bachelor or equivalent | 5 (9) | 6 (11) |
| Age of hearing voices median years (range) | 22 (7–50) | 20 (5–55) |
| CORE-OM mean | 1.81 (0.70) | 1.91 (0.66) |
| HADS depression total | 9.70 (4.91) | 10.26 (4.45) |
| HADS anxiety total | 13.26 (3.65) | 13.57 (3.69) |
| CHOICE severity mean | 4.41 (1.92) | 4.03 (1.62) |
| CHOICE satisfaction mean | 3.77 (2.20) | 3.48 (1.98) |
| PSYRATS total | 30.35 (5.55) | 30.20 (7.11) |
| PSYRATS distress intensity score* | 3 (0–4) | 3 (0–4) |
| PSYRATS control score* | 4 (0–4) | 3 (0–4) |
Values are numbers (percentages) or Mean (SD), unless otherwise indicated; * indicates variables summarised using median (range) because the scale item is ordinal and the data distribution is skewed. ISCE = 2011 International Standard Classification of Education, CORE-OM = clinical outcomes in routine evaluation Outcome Measure, HADS = Hospital Anxiety & Depression Scale, PSYRATS = psychotic symptom rating scales, CHOICE = self-report assessment of recovery.
3.2. Outcomes
Table 2 displays the summary statistics for the outcome measures. The CORE-OM post-treatment mean score, the primary endpoint, was lower in the PBCT condition compared with the TAU condition. This is shown in the estimates of treatment effects from the cross-sectional analyses (Table 3). The estimated between-group effect size (unstandardized) and corresponding 95% Confidence Interval (CI) post treatment was − 0.14 (95% CI − 0.337 to 0.007; p = 0.188) which equates to a standardised effect size (Cohen's d) of − 0.20. The corresponding adjusted between-group effect size was − 0.157 (95% CI − 0.35 to 0.04) and adjusted Cohen's d, d* = − 0.23. The Bayes factor for a sample difference between PBCT and TAU (of 0.135, SE = 0.102) was 0.71 which implies that the data are not sensitive enough to conclude whether or not there is a difference between the two groups (Dienes, 2008).
Table 2.
Primary and secondary outcomes at 4 months and 10 months.
| 4 months |
10 months |
|||
|---|---|---|---|---|
| PBCT + TAU (n = 54) | TAU (n = 54) | PBCT + TAU (n = 54) | TAU (n = 54) | |
| CORE-OM mean | 1.58 (0.77); n = 48 | 1.71 (0.55); n = 45 | 1.57 (0.72); n = 43 | 1.68 (0.62); n = 39 |
| HADS depression total | 7.96 (5.20); n = 48 | 9.71 (3.80); n = 45 | 7.62 (4.95); n = 42 | 9.21 (3.71); n = 38 |
| HADS anxiety total | 11.73 (4.32); n = 48 | 12.44 (3.62); n = 45 | 11.71 (4.44); n = 42 | 12.50 (3.56); n = 38 |
| CHOICE severity mean | 5.00 (2.01); n = 48 | 4.39 (1.48); n = 44 | 5.17 (1.77); n = 42 | 4.65 (1.56); n = 38 |
| CHOICE satisfaction mean | 4.62 (2.28); n = 48 | 3.83 (1.85); n = 44 | 4.56 (1.98); n = 42 | 4.22 (2.18); n = 38 |
| PSYRATS total | 26.92 (7.81); n = 48 | 27.43 (8.35); n = 44 | 28 (7.62); n = 42 | 24.94 (10.35); n = 38 |
| PSYRATS distress intensity* | 3 (0–4); n = 48 | 3 (0–4); n = 44 | 2.5 (0–4); n = 42 | 2.5 (0–4); n = 36 |
| PSYRATS control* | 3 (0–4); n = 48 | 4 (1–4); n = 43 | 3 (0–4); n = 41 | 3 (0–4); n = 36 |
Values are mean (SD) or *indicates variables summarised using median (range) because the scale item is ordinal and the data distribution is skewed. Some scales have missing data and numbers (n) are provided to indicate samples with complete data. CORE-OM = clinical outcomes in routine evaluation; HADS = Hospital Anxiety & Depression Scale; PSYRATS = psychotic symptom rating scales; CHOICE = self-report assessment of recovery.
Table 3.
Estimates of unadjusted and adjusted treatment effect at each time point.
| Unadjusted |
Adjusted |
|||||||
|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | 95% CI | P value | Standardised treatment effecta | Estimate (SE) | 95% CI | P value | Standardised treatment effect | |
| CORE-OM mean | ||||||||
| 4 months | − 0.135 (0.102) | − 0.337 to 0.067 | 0.188 | − 0.198 | − 0.157 (0.099) | − 0.353 to 0.039 | 0.114 | − 0.231 |
| 10 months | − 0.108 (0.134) | − 0.373 to 0.158 | 0.423 | − 0.158 | − 0.154 (0.131) | − 0.416 to 0.107 | 0.243 | − 0.227 |
| HADS anxiety total | ||||||||
| 4 months | − 0.648 (0.705) | − 2.049 to 0.754 | 0.361 | − 0.177 | − 0.649 (0.674) | − 1.989 to 0.69 | 0.338 | − 0.178 |
| 10 months | − 0.865 (0.874) | − 2.605 to 0.875 | 0.325 | − 0.236 | − 0.726 (0.809) | − 2.339 to 0.886 | 0.372 | − 0.199 |
| HADS depression total | ||||||||
| 4 months | − 1.743 (0.712) | − 3.157 to − 0.328 | 0.016 | − 0.373 | − 1.67 (0.719) | − 3.1 to − 0.241 | 0.023 | − 0.358 |
| 10 months | − 1.489 (0.74) | − 2.962 to − 0.016 | 0.048 | − 0.319 | − 1.456 (0.685) | − 2.821 to − 0.092 | 0.037 | − 0.312 |
| Choice severity mean | ||||||||
| 4 months | 0.653 (0.298) | 0.06 to 1.245 | 0.031 | 0.367 | 0.581 (0.3) | − 0.015 to 1.177 | 0.056 | 0.327 |
| 10 months | 0.401 (0.318) | − 0.233 to 1.035 | 0.212 | 0.225 | 0.35 (0.319) | − 0.286 to 0.986 | 0.277 | 0.197 |
| Choice satisfaction mean | ||||||||
| 4 months | 0.881 (0.383) | 0.119 to 1.643 | 0.024 | 0.422 | 0.879 (0.372) | 0.141 to 1.618 | 0.020 | 0.421 |
| 10 months | 0.363 (0.433) | − 0.499 to 1.226 | 0.404 | 0.174 | 0.387 (0.416) | − 0.442 to 1.216 | 0.356 | 0.185 |
| PSYRATS total | ||||||||
| 4 months | − 1.198 (1.509) | − 4.196 to 1.801 | 0.430 | − 0.189 | − 1.273 (1.488) | − 4.23 to 1.685 | 0.395 | − 0.201 |
| 10 months | 2.331 (2.007) | − 1.667 to 6.329 | 0.249 | 0.367 | 2.449 (2.082) | − 1.696 to 6.595 | 0.243 | 0.386 |
CORE-OM = clinical outcomes in routine evaluation; HADS = Hospital Anxiety & Depression Scale; PSYRATS = psychotic symptom rating scales; CHOICE = self-report assessment of recovery.
Treatment effect divided by the baseline variance.
The estimated unadjusted and adjusted treatment effects for distress and disturbance at follow-up (CORE-OM) and specific measures of distress (PSYRATS distress intensity and HADS depression and anxiety) and disturbance (PSYRATS control, CHOICE Severity and CHOICE Satisfaction) shown in Table 3, Table 4 demonstrate favourable changes in favour of PBCT, although not all were statistically significant. At post-treatment there were significant between-group effects on measures of distress (HADS depression and PSYRATS distress intensity) and disturbance (PSYRATS control, CHOICE Severity and CHOICE Satisfaction), but significant effects were maintained at follow-up only for HADS depression. In addition, the statistical significance for the post-treatment effect of CHOICE Severity became non-significant when it was adjusted. The PBCT intervention did not have demonstrable effects on the CORE-OM at follow-up or on HADS Anxiety at post-treatment or follow up. Exploratory per-protocol analysis findings were in line with, and did not alter, the ITT analysis findings.
Table 4.
Estimate of standardised treatment effect at each time point: Mann-Whitney test results.
| Number of observations | Z | p value | Standardised treatment effect Cohen's d | |
|---|---|---|---|---|
| PSYRATS distress intensity | ||||
| 4 months | 91 | − 2.315 | 0.021 | − 0.500 |
| 10 months | 77 | − 0.758 | 0.449 | − 0.173 |
| PSYRATS control | ||||
| 4 months | 89 | − 2.003 | 0.045 | − 0.435 |
| 10 months | 76 | − 1.909 | 0.056 | − 0.449 |
Note: Treatment effects estimated using the Lenhard and Lenhard (2015) effect size conversion calculator.
4. Discussion
The study examined effectiveness of group PBCT on distress and disturbance in people distressed by hearing voices with an ICD-10 diagnosis of schizophrenia or schizoaffective disorder. Between-group effect sizes on general psychological distress (CORE-OM) at post-intervention and follow-up were small (in favour of PBCT) and non-significant. Intensity of voice distress was improved post-treatment - the first RCT of group CBTp for voices to find this. PBCT also led to significant improvement in depression: CBTp trials focussing on voices have typically not found effects on depression (Birchwood et al., 2014, Penn et al., 2009, Trower et al., 2004). The study also found post-intervention reduction in feeling controlled by voices – easing voice omnipotence is a vital early goal in psychosocial interventions for distressing voices (Chadwick et al., 2000). Furthermore, there was a significant effect on CHOICE, a patient generated measure of behavioural disturbance and positive action.
The present study has limitations. First, the study did not meet its recruitment target (there was no suggestion this was due to the specific therapy) and was unable to provide a definitive test on the primary outcome measure (CORE-OM); recruitment delays, particularly in one site, may have contributed to 8 participants randomised to PBCT not attending a single session. It is encouraging that 85% of participants who attended at least one session went on to complete therapy. Relatedly, whilst recognition is growing that primary outcome in CBTp should relate to distress and disturbance, there is no agreed outcome measure for assessing this with voices (see Thomas et al., 2014). The CORE-OM was developed to assess global psychological distress in broad psychotherapy research and may not be the best outcome measure in CBTp research. Future PBCT research should prioritise measures that assess change in variables directly targeted by the intervention (e.g. voice distress, depression, behaviour change). Second, the lack of an active control group, while appropriate for an initial assessment of PBCT effectiveness, leaves open the possibility of either placebo effects or effects due to nonspecific group factors (our research indicates the importance of universality in mindfulness groups for psychosis: Chadwick et al., 2009, p. 410). Third, the study did not assess completion of homework. Finally, whilst the effect on depression was maintained at 6-month follow-up, other positive effects were not. PBCT targets change in a number of domains – delusional beliefs; relationship with positive psychotic symptoms; mindfulness; positive behaviour change; and a more balanced, flexible and accepting self-concept. Future research should examine a longer intervention phase to consolidate the important post-treatment gains seen - supplementing face-to-face sessions with e-Health and m-Health mindfulness platforms might consolidate gains without reducing accessibility. Future research might also assess use of antipsychotic medication and mental health services, including admission to hospital; and whilst protocol adherence was assessed in the present study, future research could also use independent raters to assess competence of delivery.
In sum, the findings of post-group effects on depression, voice distress, voice controllability and recovery provide a mandate for future research. Overall when delivered over 12 weeks, group PBCT for distressing voices shows promise as an intervention to reduce the distress and disturbance associated with voice hearing experiences in the context of psychosis.
Role of funding source
The research was funded by a grant from NIHR (PB-PG-0110-21239). The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Author contributions
All authors contributed to the design of the study, supported the trial and contributed to writing the present article. Chadwick was Chief Investigator on the grant and supervised all therapy groups. Hayward, Strauss, Kingdon and Dannahy were co-applicants on the grant. Hayward, Strauss, Ellett and Dannahy ran the therapy groups and contributed to the write up. Jones was the study statistician.
Conflict of interest
The authors declare there are no conflicts of interest.
Acknowledgement
We would like to express our gratitude to Ruth Chandler, Wendy Turton, Tanya Telling and Graham Dunn for their support of the present study, and to the participants for their openness and commitment.
References
- Abba N., Chadwick P., Stevenson C. Responding mindfully to distressing psychosis: a grounded theory analysis. Psychother. Res. 2008;18(1):77–87. doi: 10.1080/10503300701367992. [DOI] [PubMed] [Google Scholar]
- Birchwood M., Michail M., Meaden A., Tarrier N., Lewis S., Wykes T., Davies L., Dunn G., Peters E. Cognitive behaviour therapy to prevent harmful compliance with command hallucinations (COMMAND): a randomised controlled trial. Lancet Psychiatry. 2014;1(1):23–33. doi: 10.1016/S2215-0366(14)70247-0. [DOI] [PubMed] [Google Scholar]
- Birchwood M.J., Chadwick P. The omnipotence of voices: testing the validity of a cognitive model. Psychol. Med. 1997;27:1345–1353. doi: 10.1017/s0033291797005552. [DOI] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Chadwick P. John Wiley & Sons; England: 2006. Person-based Cognitive Therapy for Distressing Psychosis. [Google Scholar]
- Chadwick P., Birchwood M., Trower P. John Wiley & Sons; England: 1996. Cognitive Therapy for Delusions, Voices and Paranoia. [Google Scholar]
- Chadwick P., Sambrooke S., Rasch S., Davies E. Challenging the omnipotence of voices: group cognitive behavior therapy for voices. Behav. Res. Ther. 2000;38(10):993–1003. doi: 10.1016/s0005-7967(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Chadwick P., Newman-Taylor K.N., Abba N. Mindfulness groups for people with psychosis. Behav. Cogn. Psychother. 2005;33(03):351–359. [Google Scholar]
- Chadwick P., Hughes S., Russell D., Russell I., Dagnan D. Mindfulness groups for distressing voices and paranoia: a replication and randomized feasibility trial. Behav. Cogn. Psychother. 2009;37(4):403–412. doi: 10.1017/S1352465809990166. [DOI] [PubMed] [Google Scholar]
- Dannahy L., Hayward M., Strauss C., Turton W., Harding E., Chadwick P. Group person-based cognitive therapy for distressing voices: pilot data from nine groups. J. Behav. Ther. Exp. Psychiatry. 2011;42(1):111–116. doi: 10.1016/j.jbtep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Dienes Z. Macmillan; Palgrave: 2008. Understanding Psychology as a Science: An Introduction to Scientific and Statistical Inference. [Google Scholar]
- Evans C., Mellor-Clark J., Margison F., Barkham M., Audion K., Connell J., McGrath G. CORE: clinical outcomes in routine evaluation. J. Ment. Health. 2000;9(3):247–255. [Google Scholar]
- Greenwood K.E., Sweeney A., Williams S., Garety P., Kuipers E., Scott J., Peters E. CHoice of outcome in Cbt for psychoses (CHOICE): the development of a new service user-led outcome measure of CBT for psychosis. Schizophr. Bull. 2010;36(1):126–135. doi: 10.1093/schbul/sbp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock G., McCarron J., Tarrier N., Faragher E.B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol. Med. 1999;29(4):879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- Khoury B., Lecomte T., Gaudiano B.A., Paquin K. Mindfulness interventions for psychosis: a meta-analysis. Schizophr. Res. 2013;150(1):176–184. doi: 10.1016/j.schres.2013.07.055. [DOI] [PubMed] [Google Scholar]
- Lenhard W., Lenhard A. Psychometrica; Bibergau (Germany): 2015. Calculation of Effect Sizes. Available: http://www.psychometrica.de/effect_size.html. (Accessed on 16/11/2015) [Google Scholar]
- Little R., Rubin D. Wiley; New York: 1987. Statistical Analysis with Missing Data. [Google Scholar]
- Lopez-Navarro E., Del Canto C., Belber M., Mayol A., Fernandez-Alonso O., Lluis J., Munrar E., Chadwick P. Mindfulness improves psychological quality of life in community-based patients with severe mental health problems: a pilot randomized clinical trial. Schizophr. Res. 2015;168:530–536. doi: 10.1016/j.schres.2015.08.016. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health . Nice, Feb 54 Clinical Guidelines 178. 2014. Psychosis and schizophrenia in adults: treatment and management. [Google Scholar]
- Penn D.L., Meyer P.S., Evans E., Wirth R.J., Cai K., Burchinal M. A randomized controlled trial of group cognitive-behavioral therapy vs. enhanced supportive therapy for auditory hallucinations. Schizophr. Res. 2009;109(1–3):52–59. doi: 10.1016/j.schres.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Strauss C., Hayward M. Group person-based cognitive therapy for distressing psychosis. In: Johns L., Morris E., Oliver J., editors. ACT and Mindfulness for Psychosis. Wiley-Blackwell; London: 2013. p. 240. [Google Scholar]
- Strauss C., Hayward M., Chadwick P. Group person-based cognitive therapy for chronic depression: a pilot randomized controlled trial. Br. J. Clin. Psychol. 2012;51(3):345–350. doi: 10.1111/j.2044-8260.2012.02036.x. [DOI] [PubMed] [Google Scholar]
- The Schizophrenia Commission . Rethink mental Illness; London: 2012. The abandoned illness: a report from the Schizophrenia Commission. [Google Scholar]
- Thomas N., Hayward M., Peters E., van der Gaag M., Bentall R.P., Jenner J. Psychological therapies for auditory hallucinations (voices): current status and key directions for future research. Schizophr. Bull. 2014;40(Suppl. 4):S202–S212. doi: 10.1093/schbul/sbu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trower P., Birchwood M., Meaden A., Byrne S., Nelson A., Ross K. Cognitive therapy for command hallucinations: randomised controlled trial. Br. J. Psychiatry. 2004;184(4):312–320. doi: 10.1192/bjp.184.4.312. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 1992. The ICD-10 Classification of Mental and Behavioural Disorders. [Google Scholar]
- Wykes T., Hayward P., Thomas N., Green N., Surguladze S., Fannon D., Landau S. What are the effects of group cognitive behaviour therapy for voices? A randomised control trial. Schizophr. Res. 2005;77(2–3):201–210. doi: 10.1016/j.schres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale (HADS) Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]