Abstract
Background:
Pigment epithelium-derived factor (PEDF) is regarded as a multifunctional protein possessing neurotrophic and neuroprotective properties. PEDF has a very short half-life, and it would require multiple injections to maintain a therapeutically relevant level without a delivery system. However, multiple injections are prone to cause local damage or infection. To overcome this, we chose a cell-based system that provided sustained delivery of PEDF and compared the effect of weekly injections of PEDF and neural stem cell (NSC)-based intraocular administration of PEDF on retinal ganglion cell (RGC) survival and axon regeneration after optic nerve injury.
Methods:
Seventy-two rats were randomly assigned to 3 groups: group with injections of phosphate buffered saline (PBS) (n=24), group with weekly injections of PEDF (n=24), and group with NSC-based administration of PEDF (n=24). Western blot was used to analyze the PEDF protein level 2 weeks after injection. Retinal flat mounts and immunohistochemistry were employed to analyze RGC survival and axon regeneration 2 weeks and 4 weeks after injection. The data were analyzed with one-way ANOVA in SPSS (version 19.0). A P<0.05 was considered significant.
Results:
The PEDF protein level in the group with NSC-based administration of PEDF increased compared with that in the groups with injections of PEDF and PBS (P<0.05). The PEDF-modified NSCs differentiated into GFAP-positive astrocytes andβ-tubulin-III-positive neurons. NSC-based administration of PEDF effectively increased RGC survival and improved the axon regeneration of the optic nerve compared with weekly injections of PEDF.
Conclusion:
Subretinal space transplantation of PEDF-secreting NSCs sustained high concentrations of PEDF, differentiated into neurons and astrocytes, and significantly promoted RGC survival and axon regeneration after optic nerve injury.
Keywords: Pigment epithelium-derived factor, Neural stem cells, Optic nerve
What’s Known
Multiple injections of pigment epithelium-derived factor (PEDF) are prone to cause local damage or infection. Aneural stem cell (NSC)-based system could provide sustained delivery of PEDF and reduce local damage or infection.
What’s New
PEDF-NSCs effectively increased RGC survival and improved axon regeneration in the optic nerve compared with weekly injections of PEDF. Our results provide experimental evidence and form the basis for applying cell-based strategies for the local delivery of PEDF into the retina. Application of cell-based delivery may be extended to other disease conditions beyond optic nerve crush injury.
Introduction
Impaired axonal transport is characterized by a progressive loss of retinal ganglion cells (RGCs) and their axons, resulting in visual field loss and eventually irreversible blindness.1,2 The stimulation of prosurvival signaling pathways by the supplementation of neuroprotective/neuritogenic factors has, therefore, been extensively explored as a strategy to protect RGCs from degeneration and induce axon regeneration.3 Previous studies have identified a number of neuroprotective/neuritogenic factors that are capable of delaying the degeneration of RGCs in various animal models of RGC loss and promoting regeneration.4
Pigment epithelium-derived factor (PEDF), a non-inhibitory member of the serine protease inhibitor superfamily, is a glycoprotein with a molecular weight of 50 kDa identified in various human tissues such as the eye5 and the brain.6 Besides its antioxidant and anti-inflammatory properties, PEDF is characterized as a multifunctional protein possessing neurotrophic and neuroprotective properties.7-9 Given that PEDF has a very short half-life, without a delivery system, it would require multiple injections to maintain a therapeutically relevant level that would exert its neuroprotective/neuritogenic effects. However, multiple injections are likely to cause local damage or infection. To overcome this, we chose a cell-based system that provided sustained delivery of PEDF. Intraocular transplantations of genetically modified cells represent another strategy to continuously deliver neuroprotective factors to the retina.3 Importantly, the use of ex vivo modified cells offers the possibility to adjust the amount of neurotrophic factors administered to the retina before the transplantation. We investigated whether neural stem cells (NSCs) could fulfill one or both paradigms (cell replacement and neuroprotective/neuritogenic effects) in an optic nerve model of RGC injury.
Materials and Methods
Experimental Animals
Seventy-two male and clean Sprague Dawley rats were selected. They were purchased from Laboratory Animal Center, Zhengzhou University. All the animals (250±15 g) were maintained at standard conditions (temperature of 25 °C, humidity of 60±10%, and a 12-h light/dark cycle). Water and food were free access. The experimental process strictly followed the “Regulations of Experimental Animals”.
Lentivirus Construction and Transduction
PEDF overexpression plasmids were successfully constructed and were then successfully packaged in 293T cells. Before transduction, neurospheres were dissociated into single cells by incubation in 0.1% trypsin-ethylenediaminetetraacetic acid for 2 minutes, followed by centrifugation in 10 mL of Dulbecco’s modified Eagle’s medium-F12 medium. After incubation for 8 hours, the medium was replaced. The cells were observed under a fluorescence microscope for 48 hours to assess the efficiency of transduction.
Optic Nerve Crush and Subretinal Space Injection
Optic nerves were exposed surgically in anaesthetized adult Sprague Dawley rats through a supraorbital approach and crushed using the YASARGIL aneurysm clip, 2 mm behind the posterior eye pole, as described in previous reports.10 Cultured NSCs were transplanted into the subretinal space immediately after the optic nerve crush using a transscleral approach. A 33-gauge blunt needle attached to a 10-μL syringe (Hamilton, Reno, NV) was introduced tangentially through the sclerotomy site into the subretinal region, causing retinal detachment. The retinal detachment was confirmed microscopically. The same procedure was then repeated to slowly inject a suspension of PEDF-modified NSCs (2 μL of 2.0×105 cells). In this study, 72 rats undergoing optic nerve injury were randomly assigned to 3 groups: group with injections of phosphate buffered saline (PBS) (n=24), group with weekly injections of PEDF (n=24), and group with NSC-based administration of PEDF (n=24). Subsequently, 0.67 nM of PEDF dissolved in 5 μL of sterile PBS was injected immediately after optic nerve crush (0 days) and 1week and 2 weeks thereafter. The rats (from each group) were examined at each of the time points post-injection (2 or 4 wk).
Western Blot
Samples were harvested at each time point into a protein extraction buffer at 2 weeks after injection. Equal amounts of protein were denatured for 5 minutes at 95 °C in sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blot analysis was performed using antibodies against PEDF (1:1,000; Millipore, Bedford, MA) diluted in 10% horse serum in TBS-T buffer (0.2 M of NaCl, 25 mM of Tris, pH=7.5, 0.5 mL/liter Tween-20), followed by incubation with a horseradish peroxidase-coupled mouse secondary antibody (1:10,000). The blots were reprobed with b-actin antibody (BD Bioscience, San Jose, CA). Signals were quantified with an image analyzer (UV-Tec). PEDF signals were normalized by comparison with the corresponding β-actin signal of the samples; the data are presented as percentages of the normalized control signal.
Analysis of RGC Survival
The number of the RGCs in the animals that had received an intraorbital crush of the optic nerve was assessed in flat-mounted retinas stained with antibodies to β-tubulin-III, a reliable marker for RGCs.11 The animals were sacrificed 4 weeks after transplantation, and their eyes were fixed for 15 minutes in 4% PA. The retinas were flat-mounted on nitrocellulose membranes, fixed again in 4% PA for 1 hour, blocked in PBS containing 0.1% BSA and 1% Triton X-100, and incubated with polyclonal goat β-tubulin antibodies (Santa Cruz Biotechnology, Inc.) overnight at room temperature. Subsequently, the retinas were incubated with Cy3-conjugated secondary antibodies, stained with DAPI, and mounted onto slides. The retinas were number-coded, and 5 photomicrographs from the center to the periphery of the superior, inferior, nasal, and temporal retinal quadrants were taken, covering a total retinal area of approximately 1.9 mm2. All β-tubulin-positive RGCs visible on these 20 photomicrographs were counted using Adobe Photoshop CS6, and the number of RGCs per mm2 was calculated. Six eyes in each group were analyzed (2 and 4 wk after transplantation). RGC densities were additionally determined in the eyes with injected PBS as a control.
Immunocytochemistry
The specimens were fixed for 30 minutes at room temperature with 4% paraformaldehyde (Sigma, St. Louis, MO) in 0.1 M of phosphate buffer (pH=7.4) and then washed 3times with 0.01M of PBS (PBS; pH=7.4). Next, the specimens were permeabilized by incubation in 0.5% Triton X-100 for 30 minutes at room temperature and subsequently washed 3times with 0.01 M of PBS. Nonspecific binding was blocked with 5% normal goat serum, and then the plates were incubated overnight at 4 °C with mouse primary antibodies. The specimens were washed with PBS, and secondary antibody was applied for 2 hours at room temperature in the dark. The following antibodies were used: β-tubulin (1:100; Millipore) for neurons and GFAP (1:100; Millipore) for astrocytes, GAP-43 (1:500; Abcam), and IgG-Cy3-linked goal antimouse secondary antibody (1:500; Beyotime). The specimens were washed again in PBS and then mounted in a ProLong mounting medium (Invitrogen, Carlsbad, CA) that contained DAPI. The preparations were photographed under a fluorescence microscope (Leica, Wetzlar, Germany), and the captured images were prepared in Adobe Photoshop.
Quantification of Axon Regeneration
Regenerating axons in the optic nerve were assessed according to previously described methods.12 Briefly, an observer blinded to the identity of the slides counted the number of axons extending beyond a vertical line at 500 μm distal to the lesion site in 8 longitudinal sections through each nerve (n=6 rats/12 optic nerves). Optic nerve diameter was recorded using AxioVision (Zeiss) and the total number of axons per nerve extending distance d (Σad), in an optic nerve of radius r calculated by summing over all sections with a thickness (t) of 15 μm according to the following formula:
∑ad=πr2×(average axons mm−1)/t
Statistical Analysis
All data are presented as mean±SD. The data were evaluated using one-way ANOVA. Significant differences were analyzed in SPSS (version 19.0, SPSS Inc., Chicago, USA). The data were entered into the GraphPad Prism. A P<0.05 was regarded as statistically significant.
Results
Subretinal Space Injection of PEDF-Secreting NSCs Enhanced the PEDF Protein Level
A comparison of the PEDF protein level via Western blot analysis between the group with weekly injections of PEDF and the group with NSC-based administration of PEDF showed that the relative expression level of PEDF protein was higher in the latter group (3.26±0.47) than in the group with weekly injections of PEDF (2.41±0.28) and the PBS group (1±0.10) (P<0.05) (table 1, figure 1).
Table 1.
Relative expression level of PEDF protein in the retina
| Group | Expression level | P value |
|---|---|---|
| PBS | 1±0.10 | |
| PEDF | 2.41±0.28 | 0.008 |
| PDEFsecreting NSCs | 3.26±0.47 | <0.01 |
Figure 1.
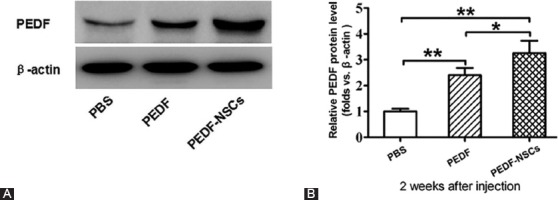
Pigment epithelium-derived factor (PEDF) level in retinas at 2 weeks after the injection of PEDF-secreting neural stem cells (NSCs) is depicted. Western blot analysis of the PEDF protein levels in the retinas (A). Quantification of relative protein density after normalization with β-actin (B). Values were normalized to β-actin, used as a loading control. *P<0.05; **P<0.01.
Injected PEDF-Secreting NSCs Exerted Neural Differentiation
Immunostaining of the retinas revealed that the PEDF-modified NSCs were differentiated into GFAP-positive astrocytes and β-tubulin-III-positive neurons (figure 2). Evidence for the formation of tumors by the injected cells was not observed (data not shown).
Figure 2.
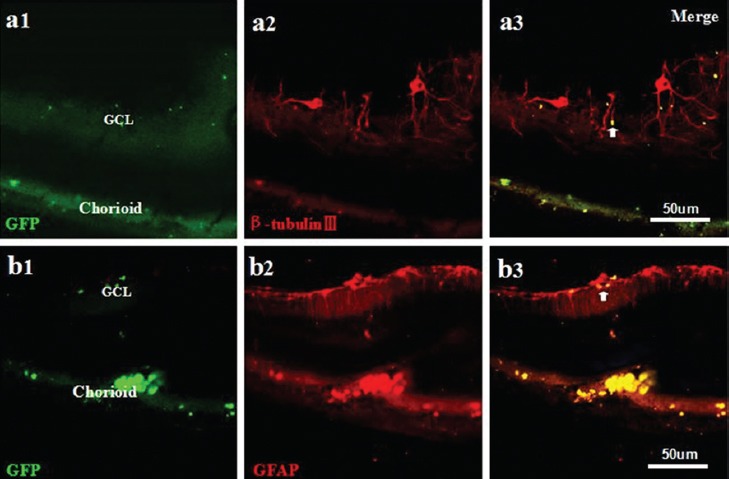
Neural differentiation after the injection of PEDF-secreting NSCs. The PEDF-modified NSCs were differentiated into β-tubulin-III-positive neurons (a1-a3) and GFAP-positive astrocytes (b1-b3). a1-b3: Scale bar=50 μm.
Injected PEDF-Secreting NSCs Enhanced RGC Survival in Vivo
The number of surviving RGCs was analyzed at 4 weeks in the flat-mounted retinas stained with antibodies to β-tubulin-III. We found that the number of surviving RGCs (1629±185 RGCs/mm2) in the group with NSC-based administration of PEDF increased compared with that of the PBS group (726±83 RGCs/mm2) and the group with weekly injections of PEDF (1184±123 RGCs/mm2) (table 2, figure 3).
Table 2.
Comparison of the number of surviving RGCs
| Group | RGCs/mm2 | P value |
|---|---|---|
| PBS | 726±83 | |
| PEDF | 1184±123 | 0.031 |
| PDEFsecreting NSCs | 1629±185 | 0.006 |
Figure 3.
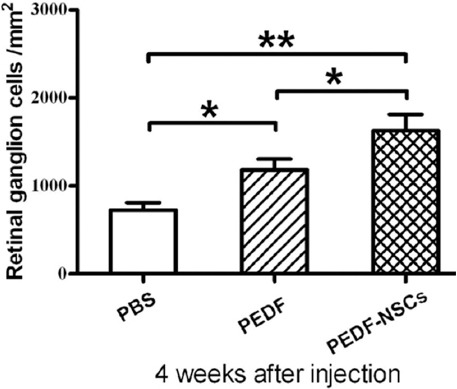
Quantitative analysis of the neuroprotective effect of the injected PEDF-secreting NSCs on RGCs is illustrated (n=6). The number of β-tubulin-III-positive RGCs was compared between the eyes injected with PEDF-secreting NSCs and those receiving weekly injections of PEDF 4 weeks after optic nerve crush. *P<0.05; **P<0.01.
Injected PEDF-Secreting NSCs Promoted RGC Axon Regeneration
Staining of GAP-43 was performed to assess whether the injection of PEDF-modified NSCs induced regrowth of the injured RGC axons. To analyze the extent of axonal regrowth, we measured the distance between the distal margin of the lesion site and the tip of the longest regrown axon in longitudinally sectioned optic nerves from the animals with the injection of PBS and PEDF-secreting NSCs at 2 weeks and 4 weeks, respectively.
GAP-43-positive axons were observed after injection in the proximal optic nerve stump of the control eyes, treated with PBS, and only 35±3 μm and 74±5 μm at 2 weeks and 4 weeks, respectively, beyond the lesion site and in the distal stump (figure 4). In the animals treated with PEDF-secreting NSCs, in comparison, RGC axons regrew for up to 1273±115 and 3293±346 μm across the lesion site into the distal optic nerve stump compared with 2026±265 and 2369±247 μm in the rats injected with PEDF at 2 weeks and 4 weeks, respectively (P<0.01) (table 3, figure 4).
Figure 4.
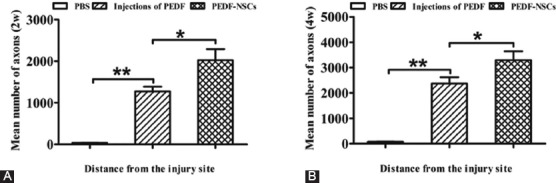
Injection of PEDF-secreting NSCs promoted RGC axon regeneration at 2 weeks (A) and 4 weeks (B) after optic nerve crush (n=6). Comparison of the distance of GAP-43-positiveaxon growth in the distal optic nerve stump from the lesion center in the optic nerve after treatment with PBS, weekly injections of PEDF, and injection of PEDF-secreting NSCs. *P<0.05; **P<0.01.
Table 3.
Comparison of RGC axon regeneration
| Group | Two weeks | Four weeks | ||
|---|---|---|---|---|
| RGCs axons (μm) | P value | RGCs axons (μm) | P value | |
| PBS | 35±3 | 74±5 | ||
| PEDF | 1273±115 | <0.01 | 2369±247 | <0.01 |
| PDEF secreting NSCs | 2026±265 | <0.01 | 3293±346 | <0.01 |
Discussion
In the present study, we demonstrated that PEDF-modified NSCs sustained high concentrations of PEDF and differentiated into neurons and astrocytes. PEDF-secreting NSCs therapy is more effective in promoting RGC survival and axon regeneration than weekly injections.
Currently, NSC-based therapy is deemed a promising modality following optic nerve injury.13 Indeed, it has been reported that it is feasible to replace dysfunctional or degenerated RGCs via NSC transplantation.14 However, for retinal disorders induced by the loss of RGCs, cell-replacement therapy is complicated because the transplanted cells not only have to integrate as functional RGCs into the host retinas but also have to regrow their axons over long distances to project in a topographically appropriate manner to the visual centers of the brain. Therefore, current therapeutic strategies after optic nerve injury primarily seek to protect endogenous RGCs from loss rather than replace the lost RGCs.15 Neurotrophic factor deprivation induces the loss of RGCs after impaired axonal transport,16 while neurotrophic factor supplementation reduces the loss of RGCs after optic nerve injury.17
PEDF is constitutively expressed by pigmented, ciliary, and corneal epithelial cells, Müller glia, retinal astrocytes and RGCs, and the levels of PEDF are generally low in normal retina tissues.12 Therefore, exogenous PEDF delivery, especially by maintaining high levels of PEDF through PEDF-secreting NSC therapy, is a therapeutically advantageous strategy to promote RGC survival and axon regeneration. Secreted retinal glia-derived PEDF may supplement endogenous titers of PEDF.18,19 Retinal glia-derived PEDF does not contribute to enhanced retinal neuron/RGC neuroprotection and axogenesis.20 Neurotrophic factors usually have a short half-life, and robust and long-lasting neuroprotective effects may depend on a sustained delivery of these factors. However, neurotrophic factors applied to the vitreous rarely penetrate the ocular surface or enter into the retina.21 In addition, sustained direct injections of drugs increase the risk of significant complications, including cataracts, vitreous hemorrhage, and retinal detachment.22,23 Maintaining high levels of PEDF in the retina is challenging.
Recently, a supply of neurotrophic factors has been achieved by implantations of slow-release devices and viral or nonviral gene transfer to retina cells. Transplantation of cells that have been genetically modified to overexpress neurotrophic factors represents an alternative strategy for the sustained delivery of these factors to retinas and has been successfully used in preclinical studies.
In the present study, we used NSCs as cellular vectors to continuously deliver PEDF to adult rat retinas after optic nerve injury. We found that the PEDF-secreting NSC therapy sustained high concentrations of PEDF in the retina compared with weekly injections of PEDF, indicating that PEDF-modified NSC therapy enhanced the levels of PEDF. Thus, PEDF-secreting NSC therapy is more RGC neuroprotective and axogenic than weekly PEDF injections. Subretinal space injections of NSCs lentivirally modified to secrete PEDF contributed to the significant attenuation of RGC loss in a rat model of optic nerve crush. Notably, sustained expression of PEDF in retinas with PEDF-secreting NSCs correlated with a significant attenuation of RGC loss, as shown by increased numbers of surviving RGCs in the retinas treated with PEDF-secreting NSC by comparison with the retinas treated with weekly injections of PEDF at 4 weeks after optic nerve injury. The survival of RGCs exceeds the survival of a maximum of 60% of RGCs supported by most neuroprotective strategies at 2 weeks after injury.24,25 The possible reason is that the severity of the injury induced with the YASARGIL aneurysm clip in the present study is different from that caused with other methods used by other investigators.26
In addition to promoting RGC survival, PEDF has been shown to promote the regrowth of injured RGC axons. We found that some regrown axons in the distal nerve stump in the eyes treated with PEDF-secreting NSCs were much longer than those in the eyes treated with weekly injections of PEDF after optic nerve injury. Furthermore, glial cell-line derived neurotrophic factor, brain-derived neurotrophic factor, and nerve growth factor secreted by the transplantation of transfected NSCs have been shown to synergistically promote the survival and axonal regrowth of axotomized RGCs in adult rats.4,27 These data further suggest that the effect of the continuous delivery of PEDF from PEDF-modified NSCs to the adult rat retina outweighs that of the weekly injections of PEDF. The grafted PEDF-secreting NSCs were differentiated into neurons after transplantation.
The underlying mechanism of the effect of PEDF on enhanced retinal RGC survival and axon regeneration has yet to be fully elucidated. PEDF might downregulate the apoptotic genes caspase-2, calpain, and mitogen-activated protein kinase-1 (MAPK1) after binding to 1 or more high-affinity PEDF receptors, stimulating downstream phospholipase A2 enzymatic activity.8,12,28 Additionally, PEDF might mediate retinal neuron/RGC neuroprotection via the activation of NFκB and ERK1/2 pathways.29,30 PEDF might attenuate the neuroprotective/neuritogenic effects by inhibiting both nuclear kappa-light-chain-enhancer of activated B cell and extracellular signal-related kinase-1/2 pathways in cultured retinal neurons.27,31,32 PEDF might activate the Ras/Raf/MAPK and the phosphoinositide-3 kinase/protein kinase-B signaling pathways, both of which are axogenic and regulate axon sprouting and elongation.33 A recent report revealed that PEDF might upregulate glutamine synthetase and l-glutamate/l-aspartate transporter expression and decrease glutamate levels by suppressing the role of IL-1βas an anti-inflammatory factor under hypoxia, and these functions may underlie the neuroprotective effects of PEDF.34
Intravitreal injections and subretinal injections constitute the main approach of cell-based therapies in the eye. Intravitreal injection is an easy delivery approach, but the barrier of the inner limiting membrane reduces the integration of the transplanted cells into the host retinas, with few cells in the host retinas.15 When NSCs were grafted into the subretinal space subjected to optic nerve crush, 2 weeks post transplantation, there was widespread incorporation of the grafted cells into the host retinas, with few cells remaining in the subretinal space,10 indicating that subretinal injections might be a better delivery approach for grafted NSCs. In the present study, we employed subretinal injection as an approach to deliver NSCs.
Conclusion
In the current study, the injected PEDF-secreting NSCs differentiated into astrocytes and neurons in the retinas after injury. For first time, we revealed that the injected PEDF-secreting NSCs protected RGCs from loss and promoted the regeneration of RGC axons. Our findings indicated that genetically modified NSCs might serve as a useful strategy for preclinical studies aimed at evaluating the therapeutic potential of a sustained NSC-based subretinal space administration of neurotrophic and neuroprotective factors. Although genetically modified NSC therapy is a promising strategy, the presence of several obstacles, including ethical issues, dissimilarities in anatomy, and differences in underlying pathophysiological processes in humans, has precluded human studies of PEDF-secreting NSCs. Therefore, the transition from animal to human clinical trials with a view to treating patients with retinal injury is an extremely lengthy process.
Acknowledgement
This work was supported by the Natural Science Fund of Henan Province (# HN2013ZA042).
Conflict of Interest: None declared.
References
- 1.Wang J, Hamm RJ, Povlishock JT. Traumatic axonal injury in the optic nerve: evidence for axonal swelling, disconnection, dieback, and reorganization. J Neurotrauma. 2011;28:1185–98. doi: 10.1089/neu.2011.1756. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy ET, Chrysostomou V, Crowston JG. Mini-Review: Impaired Axonal Transport and Glaucoma. Curr Eye Res. 2016;41:273–83. doi: 10.3109/02713683.2015.1037924. [DOI] [PubMed] [Google Scholar]
- 3.Dahlmann-Noor A, Vijay S, Jayaram H, Limb A, Khaw PT. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol. 2010;45:333–41. doi: 10.3129/i10-077. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson TA, Son YJ. Extrinsic and intrinsic determinants of nerve regeneration. J Tissue Eng. 2011;2:2041731411418392. doi: 10.1177/2041731411418392. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ, Duncan DS, Echevarria FD, McLaughlin WM, Hatcher JB, Sappington RM. Pressure-Induced Alterations in PEDF and PEDF-R Expression: Implications for Neuroprotective Signaling in Glaucoma. J Clin Exp Ophthalmol. 2015:6. doi: 10.4172/2155-9570.1000491. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzi MY, Casalis P, Lang V, Zille M, Brundl E, Storr EM, et al. Effects of pigment epithelium-derived factor on traumatic brain injury. Restor Neurol Neurosci. 2015;33:81–93. doi: 10.3233/RNN-140417. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi S, Matsui T, Nakamura K. Atheroprotective properties of pigment epithelium-derived factor (PEDF) in cardiometabolic disorders. Curr Pharm Des. 2009;15:1027–33. doi: 10.2174/138161209787581940. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Subramanian P, Shen D, Tuo J, Becerra SP, Chan CC. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro. 2013;5:e00126. doi: 10.1042/AN20130028. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truong A, Wong TY, Khachigian LM. Emerging therapeutic approaches in the management of retinal angiogenesis and edema. J Mol Med (Berl) 2011;89:343–61. doi: 10.1007/s00109-010-0709-z. [DOI] [PubMed] [Google Scholar]
- 10.Yang XT, Bi YY, Chen ET, Feng DF. Overexpression of Wnt3a facilitates the proliferation and neural differentiation of neural stem cells in vitro and after transplantation into an injured rat retina. J Neurosci Res. 2014;92:148–61. doi: 10.1002/jnr.23314. [DOI] [PubMed] [Google Scholar]
- 11.Jiang SM, Zeng LP, Zeng JH, Tang L, Chen XM, Wei X. beta-III-Tubulin: a reliable marker for retinal ganglion cell labeling in experimental models of glaucoma. Int J Ophthalmol. 2015;8:643–52. doi: 10.3980/j.issn.2222-3959.2015.04.01. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang IH, Zeng H, Fleenor DL, Clark AF. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007;8:11. doi: 10.1186/1471-2202-8-11. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015;14:243–57. doi: 10.1016/j.scr.2015.02.003. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Saloupis P, Shaw SJ, Rickman DW. Engraftment of adult neural progenitor cells transplanted to rat retina injured by transient ischemia. Invest Ophthalmol Vis Sci. 2003;44:3194–201. doi: 10.1167/iovs.02-0875. [DOI] [PubMed] [Google Scholar]
- 15.Flachsbarth K, Kruszewski K, Jung G, Jankowiak W, Riecken K, Wagenfeld L, et al. Neural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult mice. Invest Ophthalmol Vis Sci. 2014;55:7029–39. doi: 10.1167/iovs.14-15266. [DOI] [PubMed] [Google Scholar]
- 16.Li HY, Ruan YW, Ren CR, Cui Q, So KF. Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen Res. 2014;9:565–74. doi: 10.4103/1673-5374.130093. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Migallon MC, Valiente-Soriano FJ, Nadal-Nicolas FM, Vidal-Sanz M, Agudo-Barriuso M. Apoptotic Retinal Ganglion Cell Death After Optic Nerve Transection or Crush in Mice: Delayed RGC Loss With BDNF or a Caspase 3 Inhibitor. Invest Ophthalmol Vis Sci. 2016;57:81–93. doi: 10.1167/iovs.15-17841. [DOI] [PubMed] [Google Scholar]
- 18.Vigneswara V, Berry M, Logan A, Ahmed Z. Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PLoS One. 2012;7:e53473. doi: 10.1371/journal.pone.0053473. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi DS, Van Hoffelen SJ, Theusch E, Parker E, Orasky J, Harper MM, et al. Transplantation of neural progenitor cells into the developing retina of the Brazilian opossum: an in vivo system for studying stem/progenitor cell plasticity. Dev Neurosci. 2004;26:336–45. doi: 10.1159/000082275. [DOI] [PubMed] [Google Scholar]
- 20.Vigneswara V, Esmaeili M, Deer L, Berry M, Logan A, Ahmed Z. Eye drop delivery of pigment epithelium-derived factor-34 promotes retinal ganglion cell neuroprotection and axon regeneration. Mol Cell Neurosci. 2015;68:212–21. doi: 10.1016/j.mcn.2015.08.001. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gvritishvili AG, Leung KW, Tombran-Tink J. Codon preference optimization increases heterologous PEDF expression. PLoS One. 2010;5:e15056. doi: 10.1371/journal.pone.0015056. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung A, Chin EK, Almeida DR. Recurrent Vitreous Hemorrhage Despite Pars Plana Vitrectomy, Laser, and Injections. JAMA Ophthalmol. 2016;134:231–2. doi: 10.1001/jamaophthalmol.2015.2136. [DOI] [PubMed] [Google Scholar]
- 23.Rayess N, Rahimy E, Shah CP, Wolfe JD, Chen E, DeCroos FC, et al. Incidence and clinical features of post-injection endophthalmitis according to diagnosis. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2015-307707. [DOI] [PubMed] [Google Scholar]
- 24.Monnier PP, D’Onofrio PM, Magharious M, Hollander AC, Tassew N, Szydlowska K, et al. Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J Neurosci. 2011;31:10494–505. doi: 10.1523/JNEUROSCI.0148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Perez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009;89:32–41. doi: 10.1016/j.exer.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Gao H, Zhang HL, Shou J, Chen L, Shen Y, Tang Q, et al. Towards retinal ganglion cell regeneration. Regen Med. 2012;7:865–75. doi: 10.2217/rme.12.97. [DOI] [PubMed] [Google Scholar]
- 27.Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: new insights into phylogeny, function, and signaling. Front Biosci. 2005;10:2131–49. doi: 10.2741/1686. [DOI] [PubMed] [Google Scholar]
- 28.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–36. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 29.Yabe T, Wilson D, Schwartz JP. NFkappaB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J Biol Chem. 2001;276:43313–9. doi: 10.1074/jbc.M107831200. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez A, Tripathy D, Yin X, Luo J, Martinez J, Grammas P. Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of Bcl-2. Neurosci Res. 2012;72:1–8. doi: 10.1016/j.neures.2011.09.003. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unterlauft JD, Claudepierre T, Schmidt M, Muller K, Yafai Y, Wiedemann P, et al. Enhanced survival of retinal ganglion cells is mediated by Muller glial cell-derived PEDF. Exp Eye Res. 2014;127:206–14. doi: 10.1016/j.exer.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Vigneswara V, Berry M, Logan A, Ahmed Z. Pigment epithelium-derived factor is retinal ganglion cell neuroprotective and axogenic after optic nerve crush injury. Invest Ophthalmol Vis Sci. 2013;54:2624–33. doi: 10.1167/iovs.13-11803. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanimoto S, Kanamoto T, Mizukami M, Aoyama H, Kiuchi Y. Pigment epithelium-derived factor promotes neurite outgrowth of retinal cells. Hiroshima J Med Sci. 2006;55:109–16. [PubMed] [Google Scholar]
- 34.Wang Y, Lu Q, Gao S, Zhu Y, Gao Y, Xie B, et al. Pigment epithelium-derived factor regulates glutamine synthetase and l-glutamate/l-aspartate transporter in retinas with oxygen-induced retinopathy. Curr Eye Res. 2015;40:1232–44. doi: 10.3109/02713683.2014.990639. [DOI] [PubMed] [Google Scholar]


