Abstract
Brucellosis is a widespread zoonotic disease causing considerable economic and public health problems. Despite animal vaccination, brucellosis remains endemic in some areas such as Iran, especially in the western Iranian province of Hamadan. We sought to detect some of the most common virulence-associated genes in Brucella isolated from human blood cultures to determine the prevalence of some virulence genes among Brucella isolates. Fifty-seven isolates were studied from patients with a clinical diagnosis of brucellosis who referred to the Infectious Diseases Ward of Sina Hospital in Hamadan Province, Iran, between April 2013 and July 2014. Blood samples were collected for the diagnosis of brucellosis using the BACTEC blood culture system. All of these isolates were confirmed by the bcsp31 Brucella-specific gene. We detected 11 virulence-associated genes of Brucella, namely cβg, virB, znuA, ure, bvfA, omp25, omp31, wbkA, mviN, manA, and manB, which are important for the pathogenesis of this bacterium in the intracellular environment by multiplex PCR. Totally, 149 patients with a clinical diagnosis of brucellosis were enrolled in this study. Fifty-seven (38.3%) patients had positive blood cultures. On biochemical and molecular testing, all of the isolates were Brucella melitensis. Ten of the virulence genes were detected among all of the 57 isolates, but the bvf gene was detected in 53 (93%) isolates. The high prevalence of virulence-associated genes among the Brucella isolates detected in Hamadan Province, Iran, underscores the pathogenicity of this bacterium in this region.
Keywords: Virulence factors, Brucella melitensis, Brucellin, Iran
What’s Known
We sought to detect some virulence factors in the Brucella genus. Some studies have identified just 2 or 3 virulence factor genes, while others have detected up to 6. We detected 11 virulence factor genes.
What’s New
We studied 50 isolates of Brucella, all of which were Brucellamelitensis.
All the isolates (100%) carried 10 virulence genes, but the bvf gene was detected in 46 (92%) isolates.
Introduction
Brucellosis is a considerable economic and public health problem and a widespread zoonotic disease that infects humans and several animals.1 Brucellosis is well controlled in most developed countries thanks to routine screening and vaccination of animals, but it still remains endemic in some areas such as Hamadan Province, Iran.2 Brucella transmission to humans occurs by the ingestion of infected raw milk and milk products or by exposure to infected animals.3 Among Brucella spp. existing in Iran, Brucella melitensis (B. melitensis) is the most prevalent.4 Therefore, the identification and detection of the most common and important virulence-associated genes of B. melitensis isolates such as the cβg, virB, znuA, ure, bvfA, omp25, omp31, wbkA, mviN, manA, and manB genes, which are essential for the intracellular survival of this bacterium, is of great significance. A thorough understanding of virulence factors and the molecular properties of the different isolates of Brucella in this region can help to control brucellosis.
The cβg gene product (cyclic β-1,2-glucans) interferes with cellular trafficking by acting on lipid rafts found on host cell membranes and preventing the phagosome lysosome fusion cycle, thereby leading to intracellular survival.5 Some pathogenic bacteria such as Bartonella henselae, Legionella pneumophila, and Brucella spp. have a classic type IV secretion system. Type IV secretion system, thought to be responsible for the secretion of macromolecules and proteins across the bacterial cell envelope, is encoded by 12 genes and virB is essential for the intracellular survival and multiplication of Brucella.6
In one study, zinc uptake system protein (znuA) was essential to chelate zinc as nutrient acquisition for Brucella and the znuA mutant failed to replicate in HeLa cells and mouse bone marrow–derived macrophages.7 Brucella evades macrophage killing via the cβg, virB, and znuA virulence factors that lead to intracellular replication.8
The ure gene encodes the urease enzyme, which hydrolyzes urea to form carbonic acid and 2 molecules of ammonia. The ammonia molecules form ammonium, causing the pH to increase. Thus, the degradation of urea facilitates survival in acidic environments.9
Brucella virulence factor A (bvfA) is a small 11 kDa periplasmic protein unique to the genus Brucella. It may play a role in the establishment of the intracellular niche.10 The omp25 protein has been shown to be involved in the virulence of Brucella because this protein inhibits the release of TNF-α from human macrophages.11 The omp31geneis responsible for iron acquisition, binding, and storage in Brucella.12 Also, mviN, is an integral membrane protein similar to the one found in Salmonella and is necessary for virulence potency.13
Mannosyltransferase (wbkA) and phosphomannomutase (manB) are involved in the lipopolysaccharide synthesis. It is worthy of note that the manA and manB mutants are comparatively resistant because they markedly alter the omp topology and also affect phage binding. This suggests that the missing outer core section is part of the receptor.13
In light of the above-mentioned statements, we sought to detect some virulence-associated genes among B. melitensis isolates from human blood cultures in Hamadan Province.
Materials and Methods
Bacterial Isolates
Fifty-seven isolates of Brucella were obtained from 149 blood samples collected from patients with a clinical diagnosis of brucellosis according to the following criteria: 1) isolation of Brucella spp. in blood, bone marrow, or other body fluids and 2) a clinical symptom such as fever, sweating, chills, headache, and arthralgia and the detection of specific antibody titers in the serum samples of individuals who referred to the Infectious Diseases Ward, Sina Hospital, in Hamadan Province, Iran, between April 2013 and July 2014. Patients without a history of the consumption of any antibiotics were enrolled in the study. The criteria for exclusion were consumption of antibiotics during the previous 2 weeks and refusal to give consent for participation in the study.
The Ethics Committee of Hamadan University of Medical Sciences approved the study protocol (#16.35.453), and a written consent was obtained from all the patients.
The demographic data of each patient, including residence in rural areas, exposure to animals, consumption of unpasteurized dairy products, history of brucellosis, history of antibiotic therapy, occupation, age, and sex were documented.
Antibody titers ≥1:160 of Wright and Coombs Wright tests and titers ≥1:40 of 2ME test were considered as a positive serology test.
The blood samples were cultured in the BACTEC blood culture system (9050 BD Company, U.S.A.), which is a fully automated microbiology growth and detection system designed to detect microbial growth from blood specimens. For 10 rheumatoid arthritis patients out of the 149 patients, synovial fluid and bone marrow aspiration cultures were also performed in addition to blood cultures.
The samples were incubated at 37 °C for 7 to 30 days. Each positive sample was subcultured in the Mueller Hinton Brothplus 5% blood and Brucella agar mediums. Gram stain and differential biochemical tests such as catalase, oxidase, and urease were performed to identify the organisms. As Brucella is an intracellular bacterium and classical virulence factors such as toxins and different enzymes of other bacteria involved in the pathogenesis have not been identified yet, the factors included in the present study constituted the most common virulence factors associated with inside host cell.
Molecular testing was carried out to determine the diagnosis of B. melitensis. The primer sequences and molecular weights of the polymerase chain reaction (PCR) products for B. melitensis detection are described in table 1.
Table 1.
Sequence of the primers and the amplicon sizes of the bcsp31 gene for Brucellamelitensis detection
| Gene | Primer Sequences (5’ 3’) | Amplicon Size | References |
|---|---|---|---|
| bcsp31 | BM: AATCGCGTCCTTGCTGGTCTGA IS711:TGCCGATCACTTAAGGGCCTTCAT |
731 bp | 15 |
DNA Extraction
DNA was extracted from each Brucella isolate on the agar plate suspended in 200 μL of distilled water. The suspension was boiled for 30 minutes, and 50 μL of the supernatant was collected after spinning at 14000 rpm for 10 minutes. The optical density of the boiled extracts was measured at 260nm using a spectrophotometer.
PCR Assay
Following DNA extraction, the PCR assay was performed to identify the Brucella isolates, targeting the bcsp31 gene coding outer protein of Brucella. The sequences of each primer are presented in table 1.
Three multiplex PCR assays targeting a total of 11 Brucella virulence-associated genes were designed as follows: multiplex 1 containing cβg, omp31, wbkA, virB, and znuA; multiplex 2 containing manA, manB, mviN, and omp25; and multiplex 3 containing ure and bvfA primer pairs. Multiplex PCR amplifications were carried out in a final volume of 25 μL containing 12.5 μL of PCR Master Mix (Fermentas, Germany), 1μL of the DNA template, 1μL of each forward and reverse primer (for the ure gene, 2 μL of each primer), and distilled deionized water. All the primer sequences are listed in table 2.
Table 2.
Sequence of the primers and the amplicon sizes for the detection of 11 virulence-associated Brucellamelitensis
| Genes | Primer Sequences (5’ 3’) | Amplicon Sizes | References |
|---|---|---|---|
| cbg | F: GAATTCGCCAATGAGGAAAA R: ACGATATCGGATGCGAAAAG |
575 bp | 16 |
| mviN | F: GCAGATCAACCTGCTCATCA R: GCCATAGATCGCCAGAATA |
344 bp | 16 |
| manA | F: TCGATCCAGAAACCCAGTTC R: CATACACCACGATCCACTGC |
271 bp | 16 |
| manB | F: GGCTGGTTCGAGAATATCCA R: CAATCGCATACCCTGGTCTT |
228 bp | 16 |
| wbkA | F: GAGCGCTTAGGAATGCTGAT R: CTCCTAGGTTCCAGCCCTTT |
309 bp | 16 |
| omp25 | F: CGTACCTCACGGCTGGTATT R: CGTACCGGCCAGATCATAGT |
188 bp | 16 |
| omp31 | F: GCTGCTCCTGTTGACACCTT R: GCTGAAATCGAACCCGTAAC |
257 bp | 16 |
| znuA | F: CTGGGTCCGAGCATGTTTAT R: AGGCATCGAGTTTTTCTCCA |
465 bp | 16 |
| bvfA | F: CCCTTCGTCGATGTGCTGA R: CCGCGCTGATTTCATCGCTG |
1282 bp | 17 |
| virB | F: CGCTGATCTATAATTAAGGCTA R: TGCGACTGCCTCCTATCGTC |
881 bp | 17 |
| ure | F: GCTTGCCCTTGAATTCCTTTGTGG R: ATCTGCGAATTTGCCGGACTCTAT |
2100 bp | 17 |
The amplification program for all the genes consisted of initial denaturation for 5 minutes at 94 °C, followed by 25 cycles (32 cycles for the ure gene) of denaturation performed for 60 seconds at 94 °C, with annealing at 58 °C (at 65 °C for the ure gene) for 60 seconds and extension at 72 °C for 60seconds. Final extension was performed for 10 minutes at 72 °C in the Bio-Rad thermal cycler (Bio-Rad, U.S.A.).
The PCR products were analyzed by electrophoresis in 1.5% agarose gel. Thereafter, the gels were visualized under UV light and documented using the UVItec System (Vilber Lourmat). A molecular weight Marker with 100-bp increments (100 bp plus ladder, Fermentas, Germany) was used as a DNA standard.
The positive and negative controls are listed in table 3.
Table 3.
Positive and negative controls of the polymerase chain reaction assay
| The controls | Strains | ATCC |
|---|---|---|
| Positive control |
Brucella melitensis Rev1 |
Vaccine strain |
| Negative control |
Streptococcus agalactiae |
12386 |
| Negative control |
Enterococcus faecalis |
29212 |
| Negative control | E coli | 25922 |
| Negative control |
Staphylococcus aureus |
25423 |
| Negative control |
Streptococcus mutans |
Clinical isolate |
| Negative control |
Enterococcus faecum |
Clinical isolate |
| Negative control |
Streptococcus pneumoniae |
Clinical isolate |
| Negative control | Neisseria spp. | Clinical isolate |
Sequencing Analysis
Because there was no positive control for the virulence gene separately to confirm the amplicons, 1 sample of each PCR product (amplicon) of the virulence genes was sequenced using Bioneer Co., Korea, mediated by Takapouzist Co., Iran, and the data were analyzed using the Chromas software. Statistical significance was determined using the χ2 and the Fisher exact tests. A P<0.05 was considered meaningful.
Results
In this study, 57 B. melitensis isolates were obtained from the patients, who were between 11 and 82 years old (mean=41.1±17.2 years). The demographic information of the study population is presented in table 4. Seventeen (11.4%) patients had a history of recent brucellosis with a mean interval of 8.32 months (SD=42.23) and 132 (88.6%) patients were new cases of brucellosis. Moreover, 128 (85.9%) patients had consumed non-pasteurized dairy products, while 21 (14.1%) patients had not consumed any local dairy products. Totally, 6 (4%) patients had no history of contact with animals, consumption of certain local dairy products (the main risk factors for the disease), and any jobs associated with livestock. Additionally, 3 of these patients were negative serologically. The most frequent clinical symptoms were sweating, fever, and arthralgia.
Table 4.
Characteristics of the patients who participated in this study
| Characteristics | Number | Percent |
|---|---|---|
| Sex | ||
| Male | 27 | 47 |
| Female | 23 | 40 |
| Age (mean±SD) | Mean=41.1±17.2 years | |
| Education | ||
| Under diploma | 109 | 73.2 |
| Diploma | 32 | 21.5 |
| Bachelor’s degree | 6 | 4 |
| Master’s degree | 1 | 0.7 |
| Doctorate | 1 | 0.7 |
| Living | ||
| Rural | 83 | 56 |
| Urban | 66 | 44 |
| Occupations | ||
| Farmer | 68 | 45.6 |
| Housewife | 39 | 26.2 |
| Employed | 28 | 18.8 |
| Administrative jobs | 8 | 5.4 |
| Butcher | 4 | 2.7 |
| Veterinarian | 1 | 0.67 |
| Clinical picture | ||
| Fever | 122 | 81.9 |
| Arthralgia | 69 | 46.3 |
| Chills | 240 | 51.2 |
| Headache | 94 | 61.3 |
| Sweating | 112 | 75.2 |
| Anorexia | 110 | 73.8 |
| Low back pain | 102 | 68.5 |
| Weight loss | 112 | 75.2 |
| Myalgia | 132 | 88.6 |
| Arthritis | 17 | 11.4 |
| Hepatomegaly | 7 | 4.7 |
| Orchitis (males) | 20 | 13.4 |
| Meningismus | 1 | 0.7 |
| Splenomegaly | 2 | 1.3 |
| Lymphadenopathy | 4 | 2.7 |
| Spondylitis | 45 | 30.2 |
| Sacroiliitis | 67 | 45 |
We isolated 57 (38.3%) B. melitensis isolates from the blood cultures of 149 patients, who had brucellosis symptoms. The joint fluid cultures were positive in only 2 cases (figure 1).
Figure 1.
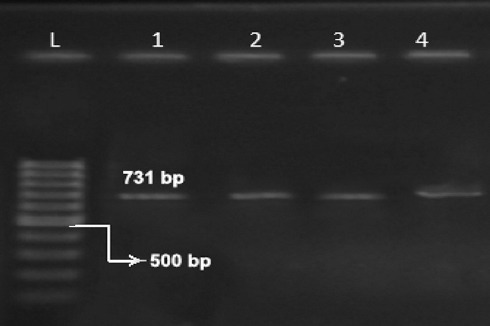
Gel electrophoresis for the results of the polymerase chain reaction (PCR) amplification of the 731-bp fragment using the bcsp31 gene for Brucella melitensis. Lanes L to 4: 100-bp DNA ladder; lane 1: Brucella melitensis Rev1 and vaccine strain (positive control); and lanes 2, 3, and 4: specimens whose results were positive by PCR.
All the Brucella species isolated from the blood cultures were B. melitensis on biological and molecular testing. Ten of the virulence genes, namely cβg, virB, znuA, ure, omp25, omp31, wbkA, mviN, manA, and manB (figures 2 and 3), were detected among all of the 57 isolates, but the bvf gene was detected in 53(93%) isolates (figure 3).
Figure 2.
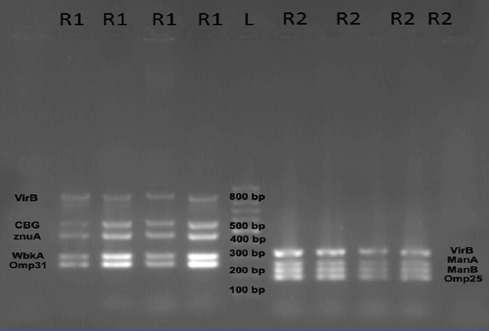
Gel electrophoresis for the results of the polymerase chain reaction amplification of the specimens whose results were positive for virB (881bp), cbg (575bp), znuA (465bp), wbkA (309bp), mviN (344bp), manA (271bp), manB (228bp), and omp25(188bp). Lane R1 (multiplex 1) contains virB (881bp), cbg (575bp), znuA (465bp), wbkA (309bp), omp31 (257bp), and fragment using the primer pair 16S rRNA. Lane L: 100-bp DNA ladder. Lane R2 (multiplex 2) contains mviN (344bp), manA (271bp), manB (228bp), and omp25 (188bp).
Figure 3.
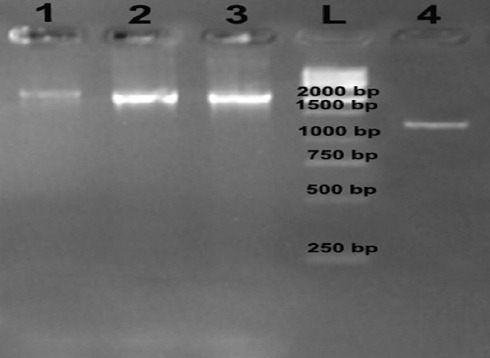
Gel electrophoresis for the results of the polymerase chain reaction amplification of the ure and bvfA genes. Lanes 1 to 3: specimens whose results were positive for the ure gene (2100 bp); lane L: 1-kb DNA ladder; and lane 4: specimens whose results were positive for the bvfA gene (1282 bp).
We followed up the epidemiological features and the clinical manifestations of adult cases of brucellosis from 2013 to 2014.
Results of Gene Sequencing
The analysis of all our sequenced genes with related PCR products using Chromas software and blast in the NCBI (National Center for Biotechnology Information) site showed the same DNA sequences; therefore, all the PCR assay results were confirmed.
Discussion
Brucella transmits to humans by the ingestion of raw milk and milk products or by exposure to infected animals and can cause subsequent complications. In our study, 74 (49.6%) patients had encounters with animals and were at high risk in comparison to the other patients, who had different occupations, which indicated that the consumption of unsafe dairy products could be the main route of infection. Our data showed that most of the patients were male (27 [47%]). The age range of 21–40 years was the most frequent among the brucellosis cases, which is consistent with other studies from the northeast of Iran, where the majority of the patients were male and were most frequently at the age range of 20–40 years old.14 The highest incidence of brucellosis in the Middle East is in spring and early summer.15
In this investigation, we found that the most prevalent species in our adult cases of brucellosis was B. melitensis, which is similar to some other studies16,17 and dissimilar to some others.18 However, according to other studies, the predominant Brucella species in the different areas of Iran is B. melitensis. Most intracellular pathogens have the ability to replicate within professional phagocytic cells. The pathogenicity of Brucella is due to its ability to adapt to environmental encounters in its intracellular replicative niche, and it is subsequently equipped with a large number of virulence factors; it, however, does not have the classical ones such as toxins and capsules. One of the most important virulence factors is virB. The virB gene encodes one of the central virulence traits required for the intracellular lifestyle of the bacterium and uses it for the translocation of the virulence factors into the host cell.19 Based on our findings, 100% of the isolates had the virB and ure genes, which are essential for intracellular survival. In a study in the Iranian city of Shiraz, the frequencies of the bvfA, virB, and ure genes among 42 B. melitensis strains isolated from aborted fetuses of sheep and goats were reported to be 78.5%, 73.8%, and 88.09%, respectively, by Derakhshandeh et al.20 (2013). The insignificant discrepancy between the results obtained from the virB and ure genes may be explained by the variety of the bacteria species and the different types of bacteria in the different areas, although the genome of Brucella spp. is very similar.
In the present study, we found that most of the Brucella isolates exhibited potent urease activity, which has been hypothesized to play a role in the pathogenesis of the disease, to play a role in the hydrolysis of urea into carbonic acid and 2 molecules of ammonia, and to help bacteria to survive in acidic environments.21
wbkA and manB are involved in the lipopolysaccharide synthesis and were found in all the isolates. Lipopolysaccharide is deemed one of the main virulence factors of Brucella, and it confers intracellular survival and protects the bacterium from the host’s defense reticuloendothelial system.22,23
Crawford et al.24 (1996) reported that the zinc ATP-binding cassette transporter periplasmic zinc-binding protein (znuA and mannose-6-phosphateisomerase [manA]) was correlated with the virulence of Brucella and showed that these genes were more common in B. melitensis than were in B. abortus. This may be due to the fact that B. melitensis is more virulent than is B. abortus.
The cβg gene is important for bacterial infection within the host cell and creates successful and persistent infections and prevents the phagosome-lysosome fusion cycle. Its mutants are unable to establish successful pathogenic or symbiotic associations with their hosts.5 Our study showed that all of the isolates had the cβg and manA genes, in contrast to the results of another study.16
We found that the frequencies of the omp25, omp31, mviN, and bvfA genes were 100%, 100%, 100%, and 80.7% in the isolates, correspondingly, which chimes in with the results of other studies. The high frequency of these genes shows the pathogenicity of the Brucella strains in this region. In a study in Babylon Province, Iraq, the frequencies of the cβg, omp25, manA, wbkA, manB, omp31, mviN, and znuA genes among 6 B. melitensis strains and also 2 B. abortus strains isolated from human blood samples were reported to be 75%, 87.5%, 87.5%, 100%, 100%, 100%, 100%, and 100%, respectively, by Razzaq et al.13 (2014). As the results showed in the 2 studies, most of the virulence gens had the same pattern; however, in 2 of them (i.e., omp25, and manA), there were some differences because the researchers reported that 2 of the 8 species isolated belonged to B. abortus and these genes were not found in 2 isolates. In our study, there were no B. abortus isolates. Other discrepancies between the studies may be in consequence of the number of the isolates. In our study, the total number of the isolates was 57, in comparison to 8 isolates reported by the previous investigations.
Brucellosis remains endemic in some provinces of Iran, not least in Hamadan, and creates economic and public health concerns. To our knowledge, the current study is possibly the first of its kind to investigate the most common virulence factors among Brucella spp. originated from clinical samples in Iran. Nonetheless, further research on the expression of virulence genes and molecular typing methods covering wider geographical areas of Iran is needed to determine the predominant strains of Brucella with gene expression properties with a view to developing an effective vaccine. This may constitute a limitation of our study.
Conclusion
The results of the present study demonstrated that B. melitensis isolated from patients in Hamadan Province, Iran, had most of the virulence-associated genes in their genomes. As these genes affect the virulence of Brucella, this paper could offer a fresh insight into the pathogenicity of these isolates. Be that as it may, given that many factors are involved in the expression of these genes, confirmation of these predictions requires future investigations into the expression of these genes.
Acknowledgement
The authors wish to acknowledge the Vice Chancellor of Hamadan University of Medical Sciences for funding the current study.
Conflict of Interest: None declared.
References
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–9. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.B Lopes L, Nicolino R, PA Haddad J. Brucellosis-risk factors and prevalence: A review. Open Vet Sci J. 2010;4:72–84. doi: 10.2174/1874318801004010072.. [DOI] [Google Scholar]
- 3.Hashemi SH, Keramat F, Ranjbar M, Mamani M, Farzam A, Jamal-Omidi S. Osteoarticular complications of brucellosis in Hamedan, an endemic area in the west of Iran. Int J Infect Dis. 2007;11:496–500. doi: 10.1016/j.ijid.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Zowghi E, Ebadi A, Yarahmadi M. Isolation and identification of Brucella organisms in Iran. Archives of Clinical Infectious Diseases. 2009;3:185–8. [Google Scholar]
- 5.Arellano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, et al. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol. 2005;6:618–25. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 6.Cascales E, Christie PJ. The versatile bacterial type IV secretion systems. Nat Rev Microbiol. 2003;1:137–49. doi: 10.1038/nrmicro753. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Watanabe K, Shirahata T, Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J Vet Med Sci. 2004;66:1059–63. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- 8.Briones G, Inon de Iannino N, Roset M, Vigliocco A, Paulo PS, Ugalde RA. Brucella abortus cyclic beta-1,2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect Immun. 2001;69:4528–35. doi: 10.1128/IAI.69.7.4528-4535.2001. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Ramos H, Glaser P, Wray LV, Jr, Fisher SH. The Bacillus subtilis ureABC operon. J Bacteriol. 1997;179:3371–3. doi: 10.1128/jb.179.10.3371-3373.1997. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavigne JP, Patey G, Sangari FJ, Bourg G, Ramuz M, O’Callaghan D, et al. Identification of a new virulence factor, BvfA, in Brucella suis. Infect Immun. 2005;73:5524–9. doi: 10.1128/IAI.73.9.5524-5529.2005. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmonds MD, Cloeckaert A, Elzer PH. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet Microbiol. 2002;88:205–21. doi: 10.1016/S0378-1135(02)00110-4. [DOI] [PubMed] [Google Scholar]
- 12.Delpino MV, Cassataro J, Fossati CA, Goldbaum FA, Baldi PC. Brucella outer membrane protein Omp31 is a haemin-binding protein. Microbes Infect. 2006;8:1203–8. doi: 10.1016/j.micinf.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Razzaq MSA, AlSaadi MA, Al-Yassari AKS. Molecular Study of Virulence Genes of Brucella Isolated from Human Clinical cases in Babylon Province. Journal of Babylon University. 2014;22:1531–44. [Google Scholar]
- 14.Bokaie S, Sharifi L, Alizadeh H. Epidemiological survey of brucellosis in human and animals in Birjand, east of Iran. J Anim Vet Adv. 2008;7:460–3. [Google Scholar]
- 15.Refai M. Incidence and control of brucellosis in the Near East region. Vet Microbiol. 2002;90:81–110. doi: 10.1016/S0378-1135(02)00248-1. [DOI] [PubMed] [Google Scholar]
- 16.Khosravi AD, Abasi E, Alavi SM. Isolation of Brucella melitensis and Brucella abortus from brucellosis patients by conventional culture method and polymerase chain reaction technique. Pak J Med Sci. 2006;22:396–400. [Google Scholar]
- 17.Hajia M, Fallah F, Angoti G, Karimi A, Rahbar M, Gachkar L, et al. Comparison of methods for diagnosing brucellosis. Laboratory Medicine. 2013;44:29–33. doi: 10.1309/LM4J9MWOBIPA6RBN.. [DOI] [Google Scholar]
- 18.Garshasbi M, Ramazani A, Sorouri R, Javani S, Moradi S. Molecular detection of Brucella species in patients suspicious of Brucellosis from Zanjan, Iran. Braz J Microbiol. 2014;45:533–8. doi: 10.1590/S1517-83822014005000048. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–56. doi: 10.1084/jem.20030088. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derakhshandeh A, Firouzi R, Goudarztalejerd A. Detection of virulence genes (bvfA, virB and ure) in Brucella melitensis isolated from aborted fetuses of sheep and goats. Iran J Microbiol. 2013;5:402–5. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler S, Foulongne V, Ouahrani-Bettache S, Bourg G, Teyssier J, Ramuz M, et al. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc Natl Acad Sci U S A. 2002;99:15711–6. doi: 10.1073/pnas.232454299. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapaque N, Moriyon I, Moreno E, Gorvel JP. Brucella lipopolysaccharide acts as a virulence factor. Curr Opin Microbiol. 2005;8:60–6. doi: 10.1016/j.mib.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Cloeckaert A, Grayon M, Grepinet O, Boumedine KS. Classification of Brucella strains isolated from marine mammals by infrequent restriction site-PCR and development of specific PCR identification tests. Microbes Infect. 2003;5:593–602. doi: 10.1016/s1286-4579(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 24.Crawford RM, Van De Verg L, Yuan L, Hadfield TL, Warren RL, Drazek ES, et al. Deletion of purE attenuates Brucella melitensis infection in mice. Infect Immun. 1996;64:2188–92. doi: 10.1128/iai.64.6.2188-2192.1996. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


