Abstract
Sodium valproate and tomato extract have been studied in different experimental models of epilepsy individually. The aim of the present study was to evaluate the effect of lycopene on the antiepileptic effects of sodium valproate against pentylenetetrazol-induced kindling in mice. Swiss albino mice of either sex were randomly divided into 5 groups, with each group containing 8 mice. These groups were treated with pentylenetetrazol (45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14 day, i.p.); sodium valproate (200 mg/kg, p.o.) + pentylenetetrazol; lycopene (2 mg/kg, p.o.) + sodium valproate (200 mg/kg, p.o.) + pentylenetetrazol; and lycopene (4 mg/kg, p.o.) + sodium valproate (200 mg/kg, p.o.) + pentylenetetrazol, for 14 days, respectively. After treatment, the animals were observed for 30 minutes for behavioral analysis. Subsequently, the animals were sacrificed, and their brain was removed for the biochemical estimations of thiobarbituric acid reactive substances, catalase, superoxide dismutase activity, reduced glutathione, and gamma-aminobutyric acid. Significant pentylenetetrazol-induced seizure was characterized by alteration in the seizure score and latency as well as a significant increase in the levels of brain thiobarbituric acid reactive substances and a significant decrease in reduced glutathione, catalase, superoxide dismutase, and gamma-aminobutyric acid levels. Treatment with sodium valproate and lycopene significantly restored the seizure score, latency, thiobarbituric acid reactive substance, reduced glutathione, catalase, superoxide dismutase, and gamma-aminobutyric acid levels near to normal compared to pentylenetetrazol. The present study provides experimental evidence that a combination therapy of lycopene along with sodium valproate attenuated seizure and oxidative stress against pentylenetetrazol-induced kindling in mice.
Keywords: Kindling, Neurologic, Lycopene, Pentylenetetrazol, Epilepsy, Gamma-aminobutyric acid
What’s Known
It is well known that the serious adverse effects of conventional antiepileptic drugs render epilepsy treatment difficult and challenging. Antioxidants such as lycopene have a protective role in the treatment of epilepsy by inhibiting the oxidative mechanism.
What’s New
For the first time, we evaluated lycopene in combination with sodium valproate to treat pentylenetetrazol-induced epilepsy and found significant improvement in animals.
Introduction
Epilepsy is a chronic neurological disorder characterized by recurrent seizures, which are sudden, unprovoked, and transitory, and recurrent episodes of abnormal hypersynchronous neuronal discharge.1 Epilepsy is the most common primary neurological disorder. It is estimated that over 50 million people worldwide are epileptic (1–2% of the world’s population), out of whom 40 million are living in developing countries.2 Epilepsy is the second most common neurological disorder in India.3,4 Regardless of advances in epilepsy research, the pharmacotherapy of epilepsy remains largely pragmatic due to a lack of basic pathology. The use of synthetic anticonvulsants such as phenytoin, carbamazepine, and sodium valproate (SVP) is associated with adverse effects such as negative impacts on learning and memory.5 Furthermore, about 30% of patients with epilepsy have seizures that do not react adequately to conventional antiepileptic drugs (CAEDs).6 These limitations with CAEDs alone highlight the need for exploring drugs that could potentiate the action of CAEDs so as to make the treatment of epilepsy more effective. It might be useful to assess the use of natural remedies possessing antioxidant activity against epileptic seizures. SVP is one of the mostly prescribed antiepileptic drugs in the treatment of many different types of partial and generalized epileptic seizures and also for other neuropsychiatric problems.7
SVP increases the turnover of gamma-aminobutyric acid (GABA) and thereby potentiates GABAergic functions in some specific brain regions thought to be involved in the control of seizure generation and propagation. SVP causes neuronal excitation mediated by the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors for its anticonvulsant effect. It also blocks sodium ion channels.8
Lycopene (LYC), the red pigment in tomatoes, is an unsaturated carotenoid with well-known health benefits.9 Many studies have shown that LYC is involved in the protection of various disorders such as cardiovascular disease, prostate cancer, and respiratory and endothelial cancer.10,11 LYC exerts potent anti-inflammatory effects as an antioxidant and free-radical hunter, which may reduce cellular harm.12,13 LYC stimulates antioxidant enzymes such as superoxide dismutase, glutathione (GSH) peroxidase, and GSH reductase.14 It also inhibits H2O2-induced lipid peroxidation and lipoprotein modification.15
Whereas SVP is used in the treatment of epilepsy, LYC is a potent antioxidant and reduces the generation of reactive oxygen species. Hence, the present study was undertaken to determine whether LYC along with SVP could provide superior seizure control in pentylenetetrazol (PTZ)-induced kindling in mice.
Materials and Methods
Drugs and Chemicals
SVP was used as a standard drug in the present study. The marketed preparation of SVP (TORVATE Tablet) was purchased from Torrent Pharmaceuticals Ltd., Ahmedabad, India. LYC was procured from Moraceae Pharmaceuticals, Uttarakhand, India. All the other chemicals and solvents used were of AR grade.
Animals
Swiss albino mice of either sex weighing 25–30 g were procured from the Animal House Facility, KIET School of Pharmacy, Ghaziabad (UP), India. The animals were kept in polypropylene cages under standard laboratory conditions. The protocol was approved by the Institutional Animal Ethics Committee (IAEC) of KIET School of Pharmacy (Registration #1099/C/07/CPCSEA and approval #IAEC/KSOP/2013-14/03), Ghaziabad.
Experimental Protocol
The mice were randomly divided into 5 groups and treated as follows: normal control group – treated with normal saline (2 mL/kg on days 8, 10, 12, and 14, i.p.), PTZ control group – treated with PTZ (45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14, i.p.), SVP200 + PTZ – treated with SVP (200 mg/kg, p.o) daily for 14 days and PTZ (45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14, i.p.), LYC2 + SVP200 + PTZ – treated with LYC (2 mg/kg,) and SVP (200 mg/kg) daily for 14 days plus PTZ (45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14, i.p.), LYC4+ SVP200 + PTZ – LYC (4 mg/kg) and SVP (200 mg/kg) daily for 14 days plus PTZ (45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14).
Behavioral Analysis
The kindling score and the latency of kindling were measured according to the method reported by Agarwal et al.16 A subconvulsant dose of PTZ (45 mg/kg) was given intraperitoneally on every second day (i.e., day 8, day 10, and day 12), and then 70 mg/kg was administered on day 14. The PTZ injection was stopped when the animal showed adequate kindling. The convulsive behavior was observed for 30 minutes (i.e., a seizure score of 5 on 4 consecutive injections). After each injection, convulsive behavior was observed as follows:
Stage 0 - No response
Stage 1 - Hyperactivity, restlessness, and vibrissae twitching
Stage 2 - Head nodding, head clonus, and myoclonic jerks
Stage 3- Unilateral or bilateral clonus
Stage 4 - Forelimb clonic seizures
Stage 5 - Generalized clonic seizures with loss of righting reflex.
Biochemical Analysis in the Brain Tissue
The quantitative estimation of lipid peroxidation was done by determining the concentration of thiobarbituric acid reactive substance (TBARS) in the brain using the method of Ohkawa et al.17 Catalase activity was assayed by the method of Clairborne.18 Reduced GSH was estimated according to the method of Ellman.19 Superoxide dismutase activity (SOD) was assayed according to the method of Kono.20
Biochemical Estimation of Gamma-aminobutyric Acid in the Brain Tissue
GABA in the brain tissue was measured according to the method reported by Maynert et al.21
Statistical Analysis
Statistical analysis was carried out using GraphPad Prism 3.0 (GraphPad software; San Diego, CA). The data were expressed as mean±SEM. All the values were analyzed using the one-way analysis of variance, followed by the Dunnett t-test. The results were significant if P<0.05.
Results
Assessment of the Seizure Score
All the mice in each group survived without any complications at the end of the kindling period. In the PTZ group, a repeated administration of a dose of PTZ (45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14, i.p.) resulted in increasing convulsive activity leading to generalized clonic-tonic seizure. Figure 1 shows that a pre-administration of SVP (200 mg/kg) along with LYC (2 and 4 mg/kg, respectively) significantly inhibited the seizure scores compared to PTZ. None of the animals could achieve a score of 5 with 4 injections of PTZ.
Figure 1.
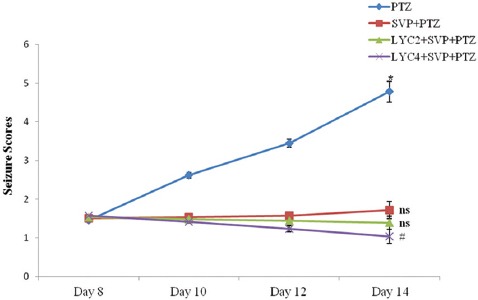
Effects of lycopene and sodium valproate on pentylenetetrazol-induced mean kindling score as assessed on days 8, 10, 12, and 14 of the study are depicted here. PTZ, Pentylenetetrazol; SVP, Sodium valproate (200 mg/kg); LYC, Lycopene (2 and 4 mg/kg). Data are expressed as mean±SEM. *P<0.01 as compared to day 8, #P<0.01 as compared to the PTZ control group. Ns: Non-significant.
Assessment of Latency
With the administration of PTZ, there was a significant decrease in the latency of seizure at the dose of 45 mg/kg on days 8, 10, and 12 and 70 mg/kg on day 14. Pre-treatment with SVP (200 mg/kg) + LYC (2 and 4 mg/kg) significantly (P<0.01 and P<0.001) increased seizure latency compared to PTZ. Maximum increase in seizure latency was observed at 4 mg/kg of LYC along with SVP (figure 2).
Figure 2.
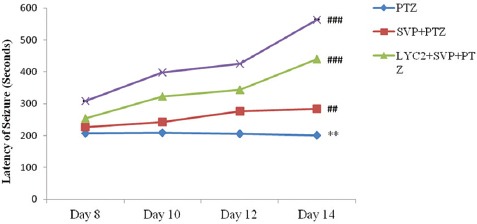
Effects of sodium valproate and lycopene on the latency of seizure are depicted here. Data are expressed as mean ± SEM. **P<0.01 as compared to day 8. ##P<0.01 and ###P<0.001 versus PTZ.
Assessment of Brain Tissue Thiobarbituric Acid Reactive Substance
Table 1 illustrates the brain levels of oxidative stress markers in the kindled and non-kindled mice. PTZ-induced kindling produced a significant (P<0.01) increase in the brain tissue TBARS content, an index of lipid peroxidation, as compared with the normal control group. Pre-treatment with SVP (200 mg/kg) + LYC (2 and 4 mg/kg) significantly (P<0.01) decreased TBARS levels compared to PTZ. LYC decreased TBARS levels in a dose-dependent manner.
Table 1.
Effects of lycopene and sodium valproate on TBARS and reduced glutathione levels in pentylenetetrazol-induced changes in the brain tissue
| S. No. | Groups | TBARS (nmol MDA/mg protein) | Reduced glutathione (nmol/mg of protein) | P value |
|---|---|---|---|---|
| 1 | Normal Control | 0.52±0.22 | 1.012±0.06 | |
| 2 | PTZ Control | 3.49±0.36a | 0.344±0.05a | 0.009 |
| 3 | SVP+PTZ | 2.28±0.32b | 0.899±0.07b | 0.049 |
| 4 | LYC2+SVP+PTZ | 1.32±0.14c | 0.923±0.07c | 0.009 |
| 5 | LYC4+SVP+PTZ | 1.09±0.13c | 0.952±0.07c | 0.008 |
TBARS: Thiobarbituric acid reactive substance; PTZ: Pentylenetetrazol; SVP: Sodium valproate (200 mg/kg); LYC: Lycopene (2 and 4 mg/kg). Data are expressed as mean±SEM.
P<0.01 versus control;
P<0.05;
P<0.01 versus the PTZ control
Assessment of Brain Tissue Reduced Glutathione
Table 1 shows the brain tissue reduced GSH levels. There was a significant (P<0.01) decrease in the GSH level in the PTZ group. Pre-treatment with SVP (200 mg/kg) + LYC (2 and 4 mg/kg, respectively) significantly (P<0.01) increased the GSH levels as compared to PTZ. This effect was more prominent in SVP + LYC (4 mg/kg).
Assessment of Brain Tissue Catalase
Table 2 shows the brain tissue catalase levels. There was a significant (P<0.01) decrease in the catalase level in the PTZ group. Pre-treatment with SVP (200 mg/kg) + LYC (2 and 4 mg/kg) significantly (P<0.05 and P<0.01) increased the catalase levels as compared to PTZ. This effect was observed more prominently in SVP + LYC (4 mg/kg).
Table 2.
Effect of lycopene and sodium valproate on GABA, SOD, and catalase levels in pentylenetetrazol-induced changes in the brain tissue
| S. No. | Groups | GABA (ng/g of brain tissue) | SOD (IU/mg protein) | Catalase (nmol H2O2 consumed/min/mg protein) | P value |
|---|---|---|---|---|---|
| 1 | Normal Control | 32.25±1.23 | 42.34±1.31 | 12.15±1.11 | |
| 2 | PTZ Control | 8.286±1.46a | 21.57±1.91a | 6.84±0.28a | 0.009 |
| 3 | SVP+PTZ | 28.27±1.16b | 34.12±1.23b | 11.35±0.92b | 0.049 |
| 4 | LYC2+SVP+PTZ | 29.32±1.19c | 39.42±2.17c | 12.13±1.27c | 0.009 |
| 5 | LYC4+SVP+PTZ | 31.21±1.20d | 45.36±2.21c | 14.24±1.63d | 0.008 |
GABA: Gamma-aminobutyric acid; SOD: Superoxide dismutase activity. Data are expressed as mean±SEM.
P<0.01 versus control;
P<0.05;
P<0.01 versus the PTZ control
Assessment of Brain Tissue Superoxide Dismutase
Table 2 depicts the brain tissue superoxide dismutase levels. There was a significant (P<0.01) decrease in the superoxide dismutase level in the PTZ group. Pre-treatment with SVP (200 mg/kg) + LYC (2 and 4 mg/kg) significantly (P<0.01) increased the superoxide dismutase levels as compared to PTZ. This effect was observed more prominently in SVP + LYC (4 mg/kg).
Assessment of Brain Tissue Gamma-Aminobutyric Acid
Table 2 shows the brain tissue GABA levels. There was a significant (P<0.01) decrease in the GABA level in the PTZ group. Pre-treatment with SVP (200 mg/kg + LYC [2 and 4 mg/kg]) significantly (P<0.01) increased the GABA levels as compared to PTZ. This effect was observed more prominently in SVP + LYC (4 mg/kg).
Discussion
It was observed in the present study that an administration of a subconvulsant dose of PTZ on alternate days resulted in a near-to-stage 5 seizure. PTZ-induced kindling in the mice showed an increase in the level of lipid peroxidation and a decrease in the levels of SOD, catalase, GSH, and GABA. PTZ kindling is a standardized animal model for epilepsy and refers to a phenomenon in which repeated injections of a convulsant causes gradual seizure development culminating in generalized tonic-clonic seizures; it is an appropriate model resembling epilepsy in humans.22 In the present study, the PTZ-treated mice showed a significant increase in the score of seizure and latency, which chimes in with the results of a previous study.16
SVP was used in the present study as it is one of the mostly prescribed antiepileptic drugs in the treatment of many different types of partial and generalized epileptic seizures and also for other neuropsychiatric problems such as bipolar disorders, schizoaffective disorder, social phobias, and neuropathic pain as well as for the prophylaxis of migraine headache.7 LYC is potent antioxidant which stimulates antioxidant enzymes such as superoxide dismutase, GSH peroxidase, and GSH reductase.14 It also inhibits H2O2-induced lipid peroxidation and lipoprotein modification.15 In addition, LYC plays an important role in protecting cell membranes from lipid peroxidation, neutralizing hydroxyl radicals, and promoting further protection beyond antioxidant activity.
Treatment with both SVP and LYC significantly decreased the seizure score and increased the latency period. SVP and LYC at a higher dose conferred better protection against PTZ-induced convulsion than that shown by these drugs at a lower dose.
In the pathogenesis of seizure, free radicals have an important role. Accordingly, the protective effects of antioxidants have been studied against different types of seizures.23-25 Some research findings suggest that free radicals are produced during a PTZ-induced kindling model in rats and may contribute to the biochemical sequelae of events leading to seizure-induced cell death.26,27 As a result, LYC was used in the present study as it exerts potent anti-inflammatory effects through its action as an antioxidant and free-radical scavenger by reducing cellular damage.12,13
In the present study, PTZ treatment in the mice significantly increased the levels of TBARS, indicating that oxidative stress occurred as a consequence of seizures and thus contributed to seizure-induced brain damage. The results of the present study are similar to those of a previous study.28 Treatment of the mice with PTZ along with SVP and LYC significantly prevented PTZ-induced elevations in lipid peroxidation. It is also known that convulsions followed by an increase in lipid peroxidation in the brain tissue decrease the levels of antioxidant enzymes.29 Furthermore, free radicals are normal products of cellular aerobic metabolism involved in the development of seizures.30 Free-radical-scavenging enzymes such GSH, CAT, and SOD are the first-line cellular defense against oxidative stress, eliminating reactive oxidative stress such as GSH, catalase, superoxide, and hydrogen peroxide and preventing the formation of more reactive hydroxyl radicals. In the present study, the GSH, SOD, catalase, and GABA levels were significantly decreased in the PTZ-treated mice, while these levels were significantly increased in the mice treated with SVP and LYC. Treatment with SVP in the PTZ-treated mice elevated the GABA levels. The results of the present study are concordant with those of a previous study by Akbas et al.31 SVP has antiepileptic effects and acts as an antioxidant according to Ozerol et al.32 LYC is a potent antioxidant insofar as the administration of petroleum ether and alcoholic tomato extracts significantly increased the tissue GSH and decreased the malondialdehyde and protein level in the brain.33 The findings of the present study are consistent with those of a previous study where SVP with N-acetyl cysteine at the fixed dose ratios was found to be synergistic.34 These findings indicate that LYC potentiates the antiepileptic effects of SVP against PTZ-induced kindling in mice and that these effects may be attributed to its cellular antioxidative defense mechanism and the synergistic antioxidant effects of LYC and SVP.
There was no funding source for the present study. Our research facilities were too limited to carry out the research work at molecular level in the present study.
Conclusion
Treatment with SVP and LYC significantly restored behavioral and biochemical alterations. Thus, LYC potentiates the antiepileptic effect of SVP by attenuating the seizure score and oxidative stress in mice.
Acknowledgement
The authors are grateful to Moraceae Pharmaceuticals, Uttarakhand, India, for providing lycopene as a gift sample.
Conflict of Interest: None declared.
References
- 1.Brahmane RI, Wanmali VV, Pathak SS, Salwe KJ. Role of cinnarizine and nifedipine on anticonvulsant effect of sodium valproate and carbamazepine in maximal electroshock and pentylenetetrazole model of seizures in mice. J Pharmacol Pharmacother. 2010;1:78–81. doi: 10.4103/0976-500X.72348. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satish S, Abdul Kareem M. [Internet]. Seizure information: Characteristics of seizures. [Cited 2014 April 4]. Available from: http://www.medihelp.co.za/Libraries/Health_Research/Epilepsy.sflb.ashx .
- 3.Bharucha NE. Epidemiology of epilepsy in India. Epilepsia. 2003;44:9–11. doi: 10.1046/j.1528-1157.44.s.1.5.x. [DOI] [PubMed] [Google Scholar]
- 4.Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna DK. Prevalence of neurological disorders in Bangalore, India: a community-based study with a comparison between urban and rural areas. Neuroepidemiology. 2004;23:261–8. doi: 10.1159/000080090. [DOI] [PubMed] [Google Scholar]
- 5.Sudha S, Lakshmana MK, Pradhan N. Chronic phenytoin induced impairment of learning and memory with associated changes in brain acetylcholine esterase activity and monoamine levels. Pharmacol Biochem Behav. 1995;52:119–24. doi: 10.1016/0091-3057(95)00059-6. [DOI] [PubMed] [Google Scholar]
- 6.Reddy DS. Pharmacotherapy of catamenial epilepsy. Indian J Pharmacol. 2005;37:288–93. doi: 10.4103/0253-7613.16851.. [DOI] [Google Scholar]
- 7.Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003;9:199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–94. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 9.Pennathur S, Maitra D, Byun J, Sliskovic I, Abdulhamid I, Saed GM, et al. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic Biol Med. 2010;49:205–13. doi: 10.1016/j.freeradbiomed.2010.04.003. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahan K, Fennal M, Kumar NB. Lycopene in the prevention of prostate cancer. J Soc Integr Oncol. 2008;6:29–36. [PubMed] [Google Scholar]
- 11.Rao AV. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Biol Med (Maywood) 2002;227:908–13. doi: 10.1177/153537020222701011. [DOI] [PubMed] [Google Scholar]
- 12.Yaping Z, Wenli Y, Weile H, Ying Y. Anti-inflammatory and anticoagulant activities of lycopene in mice. Nutrition Research. 2003;23:1591–5. doi: 10.1016/S0271-5317(03)00177-5.. [DOI] [Google Scholar]
- 13.Saedisomeolia A, Wood LG, Garg ML, Gibson PG, Wark PA. Lycopene enrichment of cultured airway epithelial cells decreases the inflammation induced by rhinovirus infection and lipopolysaccharide. J Nutr Biochem. 2009;20:577–85. doi: 10.1016/j.jnutbio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Subhash K, Bose C, Agrawal BK. Effect of short term supplementation of tomatoes on antioxidant enzymes and lipid peroxidation in type-II diabetes. Indian J Clin Biochem. 2007;22:95–8. doi: 10.1007/BF02912889. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X, Yang X, Peng Y, Lin J. Protective effects of lycopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc Drugs Ther. 2009;23:439–48. doi: 10.1007/s10557-009-6206-3. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal NB, Jain S, Agarwal NK, Mediratta PK, Sharma KK. Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine. 2011;18:756–9. doi: 10.1016/j.phymed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Clairborne A. Assay of catalase. In: Greenwald RA, editor. Handbook of Methods of Oxygen Free Radical Research. Boca Raton: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 21.Maynert EW, Klingman GI, Kaji HK. Tolerance to morphine II. Lack of effects on brain 5-hydroxytryptamine and gamma-aminobutyric acid. J Pharmacol Exp Ther. 1962;135:296–9. [PubMed] [Google Scholar]
- 22.Becker A, Grecksch G, Ruthrich HL, Pohle W, Marx B, Matthies H. Kindling and its consequences on learning in rats. Behav Neural Biol. 1992;57:37–43. doi: 10.1016/0163-1047(92)90735-M. [DOI] [PubMed] [Google Scholar]
- 23.Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic Biol Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- 24.Kabuto H, Yokoi I, Ogawa N. Melatonin inhibits iron-induced epileptic discharges in rats by suppressing peroxidation. Epilepsia. 1998;39:237–43. doi: 10.1111/j.1528-1157.1998.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogunmekan AO, Hwang PA. A randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopheryl acetate (vitamin E), as add-on therapy, for epilepsy in children. Epilepsia. 1989;30:84–9. doi: 10.1111/j.1528-1157.1989.tb05287.x. [DOI] [PubMed] [Google Scholar]
- 26.Rauca C, Zerbe R, Jantze H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999;847:347–51. doi: 10.1016/S0006-8993(99)02084-3. [DOI] [PubMed] [Google Scholar]
- 27.Frantseva MV, Perez Velazquez JL, Tsoraklidis G, Mendonca AJ, Adamchik Y, Mills LR, et al. Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience. 2000;97:431–5. doi: 10.1016/S0306-4522(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 28.Celik I, Suzek H. Effects of subacute exposure of dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicol Environ Saf. 2009;72:905–8. doi: 10.1016/j.ecoenv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Silva MI, Silva MA, de gAquino Neto MR, Moura BA, de Sousa HL, de Lavor EP, et al. Effects of isopulegol on pentylenetetrazol-induced convulsions in mice: possible involvement of GABAergic system and antioxidant activity. Fitoterapia. 2009;80:506–13. doi: 10.1016/j.fitote.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Sejima H, Ito M, Kishi K, Tsuda H, Shiraishi H. Regional excitatory and inhibitory amino acid concentrations in pentylenetetrazol kindling and kindled rat brain. Brain Dev. 1997;19:171–5. doi: 10.1016/S0387-7604(96)00492-5. [DOI] [PubMed] [Google Scholar]
- 31.Akbas SH, Yegin A, Ozben T. Effect of pentylenetetrazol-induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem. 2005;38:1009–14. doi: 10.1016/j.clinbiochem.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Ozerol E, Aslan M, Cakmak EA, Gulec M, Yakinci C, Akyol O. The effect of long-term therapy with sodium valproate on oxidant/antioxidant status in epileptic children. Neurosci Res Commun. 2003;32:115–22. [Google Scholar]
- 33.Azharuddin M, Imran P, Ayaz S. Anticonvulsant Activity of Lycopersicon esculentum (Tomato) in Maximum Electroshock Induced Seizures in Mice. Inventi Rapid: Ethnopharmacology. 2013;4:1–4. [Google Scholar]
- 34.Devi PU, Saraogi P, Manocha A, Vohora D. Pharmacological and biochemical analysis of interactions between N-acetylcysteine and some antiepileptic drugs on experimental seizures in mice. CNS Neurosci Ther. 2012;18:406–13. doi: 10.1111/j.1755-5949.2011.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


