Abstract
Mitochondrial dysfunction is a common cause of inherited multisystem disease that often involves the nervous system. Despite major advances in our understanding of the pathophysiology of mitochondrial diseases, clinical management of these conditions remains largely supportive. Using a systematic approach, we identified 1,039 publications on treatments for mitochondrial diseases, only 35 of which included observations on more than five patients. Reports of a positive outcome on the basis of a biomarker of unproven clinical significance were more common in nonrandomized and nonblinded studies, suggesting a publication bias toward positive but poorly executed studies. Although trial design is improving, there is a critical need to develop new biomarkers of mitochondrial disease. In this Perspectives article, we make recommendations for the design of future treatment trials in mitochondrial diseases. Patients and physicians should no longer rely on potentially biased data, with the associated costs and risks.
Introduction
Rare diseases affect 7% of the population and, owing to neurological involvement, many individuals with such disorders will present to a neurologist.1 The vast majority of rare diseases are genetically inherited, and most have no available treatment, as identification of effective therapeutic agents for rare diseases is a difficult process. The patient population for rare disorders is usually small and distributed over a wide geographical area, often crossing administrative boundaries. Such factors limit studies of natural history, and can hinder the identification of appropriate, clinically relevant and validated disease end points. Given that the target population in rare disease is small, financial incentives for pharmaceutical companies to develop and test novel treatments are lacking. Thankfully, this lack of incentive is mitigated by legislation and national plans for such diseases,2 which have kindled increasing interest from pharmaceutical corporations in these niche areas. Interest in therapeutic research in rare diseases is also is driven by the hope that medicines for ‘orphan diseases’ might be useful for more-common ailments.
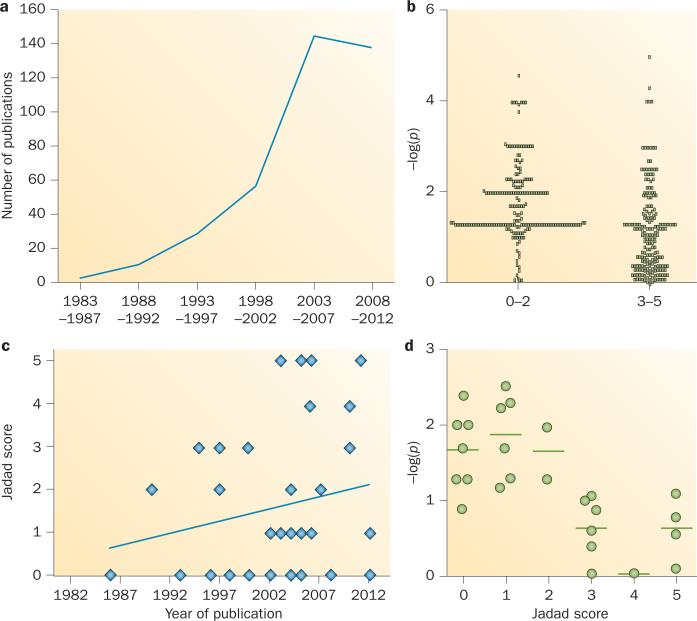
Mitochondrial disorders—a group of rare inherited diseases of energy metabolism—often present with neurological features, and provide an excellent illustration of the problems associated with treatment development for rare diseases. Despite an increase over the past two decades in the number of published studies reporting treatment effects in mitochondrial disease (Figure 1a), a recent systematic review of the literature found no evidence of an effective intervention for any mitochondrial disorder.3 Thanks to advances in molecular diagnostics, however, a growing number of patients with mitochondrial disorders are being identified, and the pressure to find a cure has consequently continued to mount. Mitochondrial disorders are now considered among the most common inherited diseases and, given their relentlessly progressive nature, often worsening over many decades, these disorders cause substantial morbidity. Although mitochondrial disorders can be caused by many different genetic defects of both nuclear and mitochondrial DNA (mtDNA), they share common pathogenic pathways that are potentially amenable to intervention. Here, we critically evaluate proposed treatments for mitochondrial diseases, highlighting the danger of relying on open-label studies, and making recommendations for future trials aimed at developing new therapies for these devastating diseases.
Figure 1.
Trials of treatments for mitochondrial disease. a | Publications listed on MEDLINE in 5-year intervals show that the number of trials has increased over time (the dip at 2008–2012 is probably attributable to ascertainment before the end of 2012 on an exponential curve; see Box 1 for search and methodological details). b | Scatter plot of the negative log10 of all P values listed in included studies (higher numbers indicate a more statistically significant result, –log(p)>1.3 = P <0.05). Lower-quality studies had higher reported statistical significance. c | Scatter plot and trendline show improvement of study quality over time. d | Scatter plot of the negative log10 of all clinically relevant P values in the included studies. Lower-quality studies report greater statistical significance for these end points, which were all nonsignificant in high-quality studies.
Proposed treatments
Mitochondrial disorders are primarily due to a biochemical defect of ATP synthesis. ATP is required for all active cellular processes, and the majority is generated by mitochondrial oxidative phosphorylation (OXPHOS), which facilitates the transfer of electrons between the respiratory chain enzyme complexes. For the most part, early attempts to develop treatments for mitochondrial disorders have focused on enhancing respiratory chain function (Table 1).
Table 1.
Treatments evaluated in patients with mitochondrial diseases
| Agent | Specific mechanism(s) of action | Highest level of clinical study in humans |
|---|---|---|
| Increase of substrate supply to respiratory chain | ||
| Carnitine | Fatty acid transfer for citric acid cycle intermediates | Case report71 |
| Niacin | Precursor for NADH, which transfers electrons from intermediates to the respiratory chain | Case report72 |
| Thiamine | Enhancement of pyruvate dehydrogenase to decarboxylate pyruvate for oxidation | Case report73 |
| Dichloroacetate | Inhibition of pyruvate dehydrogenase kinase to increase availability of pyruvate for oxidation | Randomized, placebo-controlled crossover trial in MELAS due to m.3243A>G mutation (negative outcome)34 |
| Augmentation of respiratory chain components | ||
| Riboflavin | Precursor for flavin adenine dinucleotide, an electron carrier bound to complexes I and II | Open-label study in complex I deficiency (positive outcome)8 |
| Coenzyme Q10 | Electron carrier from complexes I and II to complex III | Randomized, placebo-controlled crossover trial (negative outcome)32 |
| Idebenone | Analogue of coenzyme Q10 | Randomized, placebo-controlled trial in Leber hereditary optic neuropathy (negative outcome)36 |
| EPI-743 | Analogue of vitamin E | Open-label study in Leigh syndrome and Leber hereditary optic neuropathy (positive outcome)39,47 |
| Bypass of respiratory chain components | ||
| Succinate | Citric acid cycle intermediate which donates electrons directly to complexes I and II, thus partially bypassing complex I | Case report12 |
| Vitamins C and K | Bypass of complex III | Case report13 |
| Energy buffering | ||
| Creatine | ATP storage in muscles via the creatine phosphokinase system | Randomized, placebo-controlled crossover trials in mitochondrial myopathies (negative outcomes in two trials, positive surrogate end points in one trial)35,52,73 |
| Antioxidant activity | ||
| Cysteine | Increases muscle availability of glutathione peroxidase | Randomized, placebo-controlled crossover trial in progressive external ophthalmoplegia (negative outcome)14 |
| Lipoic acid | β-ketoacid dehydrogenase cofactor with antioxidant properties | Case report66; randomized, placebo-controlled crossover trial (with creatine and coenzyme Q10; negative outcomes in various mitochondrial myopathies)46 |
| Dimethylglycine | Antioxidant activity | Randomized, placebo-controlled crossover trial in Saguenay Lac-St-Jean cytochrome c oxidase deficiency (negative outcome)15 |
| Oxidative capacity adaptations | ||
| Aerobic exercise training | Reversal of deconditioning and/or mitochondrial adaptation to improve oxidative capacity | Randomized, non-blinded controlled trial in mitochondrial myopathies (positive outcome)17,51,65,75 |
| Resistance exercise training | Myofibre regeneration and presumed gene shifting | Open-label study (positive outcome)76,77 |
| Nitric oxide metabolism | ||
| Arginine | Substrate for nitric oxide synthase | Open-label placebo-controlled trial in MELAS due to m.3243A>G mutation (positive outcome)18 |
Abbreviation: MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes.
Supplements aimed at increasing respiratory chain substrate availability include carnitine (which facilitates the transfer of fatty acids, thereby increasing the availability of metabolites from the citric acid cycle),4 niacin (the precursor to NADH, which transfers electrons from intermediate metabolites to the respiratory chain),5 and thiamine (which enhances pyruvate dehydrogenase activity and, therefore, the availability of decarboxylate pyruvate for oxidation).6 A synthetic agent, dichloro-acetate—an inhibitor of pyruvate dehydrogenase kinase—has also been used for treatment of mitochondrial disorders on the rationale that this compound increases the availability of pyruvate for oxidation.7
Attempts to enhance electron transfer within the respiratory chain have included supplementation with riboflavin (the precursor for flavin adenine dinucleotide [FAD], an electron carrier bound to complexes I and II),8 and coenzyme Q10 (CoQ10, also known as ubiquinone, which is an electron carrier from complexes I and II to complex III).9 Synthetic agents based on CoQ10 and vitamin E—such as the drugs idebenone and EPI-743—have also been designed to increase the penetration of an electron carrier into mitochondria and/or CNS tissue.10,11
Alternative strategies to treat mitochondrial diseases include biochemical ‘bypass’ of specific respiratory chain complexes, such as with the use of succinate (a citric acid cycle intermediate that donates electrons directly to FAD, thus partially bypassing complex I)12 and a combination of vitamins C and K (in order to bypass complex III).13 Other treatments have focused on the reduction of toxic metabolites through antioxidant activity, and specific agents with this effect include cysteine, vitamins C and E, lipoic acid, and dimethylglycine.14,15 Another approach is ‘energy buffering’; that is, the use of creatine to increase ATP storage through the creatine phosphokinase system.16 Finally, exercise therapy is thought to produce adaptations in mitochondria that improve oxidative capacity and/or reduce muscle deconditioning.17 Exceptions to the above categories include the use of l-arginine in patients with stroke-like episodes (in light of the vasoactive effects of this compound that are mediated through the nitric oxide pathway),18 and corticosteroids.19 Several other experimental treatments are in the preclinical phase of development, and have not been tried in patients to date.20
Although the first case report of a treatment benefit in mitochondrial disease was published in 1981,6 the first trial was not published until 1990,21 and the vast majority of proposed therapies have not been tested in controlled trials. Not surprisingly, both patients and physicians are desperate to find any treatment that helps and, in the absence of hard-core evidence, clinical practice continues to be shaped by studies that involve fewer than five patients—often anecdotal evidence and case reports. Despite lack of proven efficacy, many ‘traditional’ treatments (such as CoQ10, thiamine and carnitine) are used widely,22 in part owing to the low incidence of adverse effects with these therapies. After a prolonged period with no new therapies, however, recent results from open-label studies of new agents have generated interest from patients and patient support groups.23,24 Given the inherent difficulties of conducting randomized clinical trials for rare diseases, should we settle for these open-label data?
Reliability of evidence
To address the reliability of current evidence of efficacy for mitochondrial therapies, we objectively evaluated all of the published data on treatments for mitochondrial disease. Our aim was to determine whether less-rigorous studies (that is, nonrandomized, nonblinded studies) can reliably inform clinical decision-making in mitochondrial medicine. A systematic review, performed on 23rd October 2012, yielded 1,039 publications spanning a 47-year period (Box 1). Titles and abstracts were reviewed to include only studies describing treatment effects in mitochondrial diseases in five or more patients, which led to identification of a total of 35 studies.8,14,15,17,18,21,25–52 The methodo-logical quality of each study was independently evaluated by three authors using the Jadad scale (Box 2, Supplementary Table 1 online).53 Studies are awarded a score on this scale on the basis of three factors: randomization (up to 2 points if a valid randomization procedure was specified), blinding (up to 2 points if a valid blinding procedure was specified), and participant-withdrawal characteristics (1 point if withdrawals were correctly documented). The final score ranges from 0–5, with high values denoting good-quality studies and lower scores indicating poor-quality studies.
Study trends
On the basis of our analysis, several trends with regard to the studies on mitochondrial treatment were observed. First, non-randomized and nonblinded studies were substantially more likely to report statistically significant results with lower P values (that is, a higher level of significance) than were randomized and blinded studies (Figure 1b). Notably, a trend towards improved study design has been observed over the past decade (Figure 1c). Second, studies with a low Jadad score were more likely to report a clinically relevant, statistically significant outcome, whereas none of the clinically-relevant primary endpoints (Supplementary Table 2 online) were statistically significant in high-quality studies (Figure 1d). The inevitable subjectivity of many direct clinical measures (such as muscle strength), together with the well-recognized placebo effect, can often account for positive results in clinical trials. These two factors are particularly problematic in open-label (nonblinded) studies, making them more vulnerable to bias. Furthermore, open-label trials involving young children can reveal ‘improvements’ in outcome that are due to normal growth and development, as has been demonstrated in studies of other neuromuscular disorders.54
Publication bias
Although some treatment effects seen in open-label studies could be important, overall our findings strongly suggest a publication bias towards small, nonblinded studies that report positive effects of treatments for mitochondrial disease, despite the fact that the findings are not supported by larger randomized studies. This issue of lack of reproducibility is likely to reflect the ‘winner's curse’, whereby small studies that are carried out without a clearly defined end point, and without a predefined statistical analysis plan, are likely to yield a positive result, particularly if nonblinded and nonrandomized. For example, CoQ10 and carnitine were studied on several occasions and, in both cases, positive open-labelled studies preceded negative randomized controlled trials (most pertinently observed in a single study with both open-label and blinded phases21). The positive outcome in the open-label studies was partly due to the use of nonvalidated surrogate disease markers in the early studies, and was compounded by a lack of blinding and/or randomization. The higher-quality trials showed no treatment effect, despite using much higher doses of the drugs.32
In spite of these negative data, both CoQ10 and carnitine continue to be prescribed in major treatment centres, and these prescriptions are then renewed indefinitely by other practitioners. Consequently, some drugs have been ‘grandfathered’ into prescribing practice on the basis of pre-existing positive open-label data—these drugs seem to be exempt from refutation with high-quality evidence. Paradoxically, the apparent safety of most of these agents has contributed to the problem: the balance of low risk of adverse events with a possible benefit of treatment has motivated continued prescriptions. Hopefully, the CoQ10 issue will be finally resolved, one way or another, following the publication of results from an ongoing randomized double-blind multicentre trial of this treatment.55
Recommendations for future trials
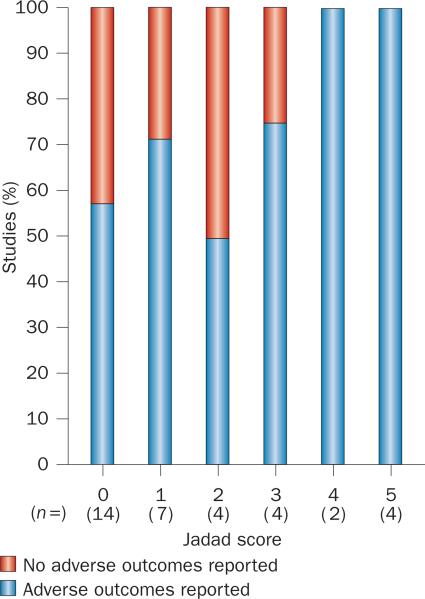
As well as their role in proving efficacy, large randomized trials are of critical importance with regard to patient safety. Dichloroacetate is no longer prescribed in adults with MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) because the drug caused onset or worsening of peripheral neuropathy in 17 of 19 patients, resulting in premature termination of the randomized controlled trial.34 Acute neuropathy had been previously documented following dichloroacetate treatment in individual MELAS cases, but in some patients this effect was attributed to the mitochondrial disorder rather than the drug. Direct linkage of the neuropathic effect to the treatment became possible only when a large number of patients (n = 30) were studied in a trial of high-quality design. Notably, nine of 10 trials with a high-quality design (Jadad score ≥3) included reports on adverse events, whereas these outcomes were reported in only 15 of 25 trials scoring ≤2 on the Jadad scale (Figure 2).
Figure 2.
Adverse outcome reporting improves with study quality. Graph showing proportion of studies in each Jadad-score group that did and did not report adverse outcomes.
Small numbers of participants do not preclude a high-quality result, provided that the randomization is appropriate. Selection bias during recruitment can easily contribute to misleading findings, particularly if the natural history of the disease is poorly understood. A mitochondrial disorder such as Leigh syndrome is particularly vulnerable to such bias as the severity of this disease fluctuates markedly over time in an unpredictable manner. In studies of such cases, regression to the mean during spontaneous recovery of a patient could be misinterpreted as a positive therapeutic response, particularly when numerous end points are used, thereby increasing the chance of a false-positive result. Even for complex multisystem diseases, therefore, a trial should have a simple design, and aim to evaluate a predefined primary end point that is clinically relevant (Box 3). Other end points, such as biomarkers or physiological measurements, remain critically important to confirm that the agent is an effector of its target mechanism, but should not be used to prove clinical efficacy unless the end point is clinically relevant.
Open trial results should be considered preliminary at best and, if positive, be followed up with a randomized study. Such a trial could be targeted to a specific phenotypic or genetic group on the basis of data from a pilot open-label study. On the other hand, negative results from potentially underpowered studies in patients with fluctuating phenotypes and genetically heterogeneous diseases may mask minor therapeutic benefits. Small treatment effects are important when no other treatments are available (Box 3).
A novel approach
One approach to addressing the difficulties in obtaining high-quality evidence for rare disorders is exemplified by Health Canada's conditional approval to prescribe idebenone in Friedreich ataxia. Approval was provided under the condition that enhanced post-marketing surveillance took place under the Notice of Compliance with Conditions policy.56 The original approval for this trial was based on evidence of treatment benefit from a single randomized controlled trial.57 When no efficacy was demonstrated in the subsequent randomized trial for the primary or secondary end points,58 Health Canada issued an open letter to physicians on 20th January 2010 to draw attention to the negative results. When further trials of idebenone in Freidrich ataxia were also negative59 and systematic reviews concluded that there was no evidence of efficacy,60 Santhera Pharmaceuticals announced on 27th February 2013 that the agent would be withdrawn from the market after consultation with Health Canada.61 This system of drug approval enabled a balanced approach to the problem: a potentially valuable drug was made available at the earliest opportunity on the basis of high-quality evidence, with the possibility of later withdrawal if further trial data were not positive.
Critical issues for the future
For the reasons described above, drug development in mitochondrial disorders has been highly problematic. Tightening of safety and efficacy standards is well-recognized to have led to an increase in the costs of developing novel agents for these disorders. Consequently, despite increasing investment in research and development, the number of drugs successfully brought to market each year continues to decrease.62 To address this issue, the FDA introduced the Critical Path Initiative, which provides recommendations to help reconcile soci ety's high safety expectations for novel drugs with the pharmaceutical industry's limited capacity to produce these treatments given the increasing costs.62 A key component of these recommendations is multidisciplinary collaboration to identify biomarkers that correspond with clinical benefit and/or adverse reactions, in order to identify suitable or unsuitable compounds at an early phase of testing.
Mitochondrial disorders have no shortage of potential biomarkers, ranging from biochemical measurements (such as lactate, pyruvate, alanine, citrulline, creatine kinase, organic acid quantification and antioxidant levels), physiological measurements (including cardiac dimensions and/ or output, visual parameters, and various measurements of aerobic or anaerobic exercise capacity or muscle power), genetic measurements (mtDNA deletion/mutation burden or copy number), and imaging (magnetic resonance spectroscopy [MRS] of brain or muscle). To date, none of the biomarkers that have been altered by treatments in high-quality studies have been shown to correlate closely with a clinical outcome (namely, lactate,23,30,32,46,49 pyruvate,29 alanine,29 antioxidant levels,14,46 and MRS findings in the brain29).
Future studies would be greatly aided by the discovery of a clinically valid biomarker or outcome measure.63,64 These biomarkers may not be specific for mitochondrial disease per se; for example, a measure of cardiac, visual or auditory function could be useful in patients with an m.3243A>G mutation in the MTTL1 gene—one of the most common mutations associated with MELAS. Agreement on an accepted bio-marker would enable its use for ‘screening’ of new treatments in small exploratory experimental medicine studies (phase Ib or phase II), potentially revealing major adverse effects, or showing that an agent is ineffective at an early stage. Notably, a positive result from such early studies should only be used to inform planning for a randomized placebo-controlled trial (phase III), and would not provide evidence of clinical efficacy. A major pitfall of this approach is the risk that a new treatment could be rejected prematurely because it did not influence a selected biomarker (type II error, false negative). Again, this risk could be mitigated with the use of an accepted, sensitive, well-characterized biomarker that correlates with disease severity.
Finally, efficacy (phase III) studies are not without their challenges. Without detailed natural history data, it may not be possible to identify a sensitive and reliable primary trial end point that is directly related to disability. The end point may be different for each mitochondrial sub-phenotype, and the aim of the study will also be critical: will the treatment prevent progression (such as in Leber hereditary optic neuropathy34), reduce the frequency of relapses (for example, with l-arginine in MELAS18), or reverse a functional deficit (as in exercise studies17,51,65)? Ultimately, the aim of these studies will be to improve quality of life (QoL), but demonstrating a significant change with crude QoL questionnaires will be challenging in a study with perhaps a few hundred patients at most.
Practically speaking, recent work has shown that the most common mitochondrial syndromes are sufficiently prevalent to allow multicentre trials to achieve adequate enrolment,66 and prior trials have demonstrated effective multi centre collaboration across multiple national jurisdictions.36,49 Nevertheless, to study all treatments using this approach will not be possible. For some subgroups of mitochondrial disease, small studies will be the only way forward. We believe that such studies can be highly valuable, provided that a high-quality study design is employed (Box 3). In short, the ‘big pharma’ model (specifically, phase III trials) may not be possible, so other approaches should be employed if we are to make headway. The past should not be forgotten, however, and new treatments should be compared with both placebo and current best standard of care. Inevitably, this approach could involve incorporation of drugs into the trial that are already grandfathered into clinical practice, even if their adoption has a weak evidence basis.
Conclusions
The increase in publications of trials of mitochondrial treatments over the past decade has been mirrored by a trend towards improved study design (Figure 1). These methodological advances have been underpinned by disease registries and multi centre collaborations (such as the North American and European Mitochondrial Diseases Networks67–69), which provide proof of principle that rigorous testing of mitochondrial medicines is possible, even for this heterogeneous group of rare disorders. In general, rare dis orders with incidence above five per 100,000 individuals are more likely to have orphan drugs approved for their treatment.70 Cause for optimism exists, therefore, that novel treatments will continue to be trialled for mitochondrial disease.
Notably, however, premature use of a new treatment can have far-reaching consequences that are quite separate from the high cost and potential adverse effects. Ineffective medicines undermine patient confidence in both medical practitioners and the medical research community, who may be accused of exploiting patients and their families for commercial gain. Such lack of trust will blunt enthusiasm for future clinical trials. We therefore urge judicious use of off-licence medicines on a named-patient basis (also known as ‘expanded access’ or ‘special access’ programmes in North America). The hope of short-term benefit must be counterbalanced by the chance of causing longer-term damage, in part through the ‘opportunity cost’ of offlicence prescribing for a specific patient, which delays the more rigorous evaluation of newer treatments. The root cause of the problem is likely to be multifaceted, with academics motivated by publication-linked career advancement, industry being driven by financial incentives, and patients and families driven by their immediate needs for disease improvement and health.
Resolution of these potentially conflicting issues will not be easy, but all stakeholders must work together to ensure efficient progress. Critical issues involve the identification of disease biomarkers that correspond to the clinical outcome of the patient, the use of multicentre collaborations to include adequate patient populations for study, and multidisciplinary collaborations to identify novel agents with novel mechanisms, with innovative and accurate study design using clinically relevant primary end points. Leading mitochondrial physicians should set an example, avoiding overemphasis on the theoretical benefits of unproven treatments; patient groups can better educate their members to engage in high-quality research and controlled trials; and both should work collaboratively with industry in well-powered, multicentre randomized controlled trials. Only by doing this will we make headway in developing treatments for these currently incurable diseases.
Supplementary Material
Box 1 | Systematic review methods.
We identified studies of English-language publications on MEDLINE using OvidSP via the following searches: “mitochondrial disease OR mitochondrial disorder OR mitochondrial myopathy OR Leber optic neuropathy OR Leber optic neuropathy OR Leigh syndrome OR Leigh syndrome OR congenital lactic acidosis OR progressive external ophthalmoplegia OR Kearns Sayre syndrome OR mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS)” and “treatment OR therapy OR coenzyme Q10 OR idebenone OR EPI-743 OR creatine OR carnitine OR vitamin OR exercise OR arginine OR dichloroacetate”. Our search, performed on 23rd October 2012, yielded 1,039 results. Titles and abstracts were reviewed, and 49 original research studies in humans that tested a therapeutic agent in patients with mitochondrial disorders were identified. After exclusion of studies involving fewer than five patients, a total of 35 studies remained; the full-length articles for these articles were reviewed in detail. For trials with a single primary end point, these end points were selected as the relevant end point: only three trials met this criterion.15,34,36 For all other trials, which had multiple end points, we selected end points that involved clinically important measures: in the case of multisystem disorders, these end points included quality of life measurements, combined scores such as the GATE or Newcastle scores, or measurements that clearly indicated relevance to patient symptomatology. In the case of mitochondrial myopathies, we included global measures of muscle strength (Medical Research Council scales), functional muscle tests (walking tests) or other standardized neurological examination results as applicable. In the case of Leber hereditary optic neuropathy, we included improvement in visual acuity as the relevant end point. In the case of one study,26 the P value was not provided in the manuscript for treatment effect, and this was calculated ourselves using a grouped two-tailed Student t-test.
Box 2 | Jadad scoring.
The Jadad scores (minimum score 0, maximum score 5) for identified studies were determined by three independent reviewers. When authors disagreed the studies were re-reviewed and discussed until the most appropriate scoring was agreed upon. Points were allocated as follows:
Randomization
■ +1 if study described as randomized
■ −1 if an inappropriate method of randomization was described
■ +1 if an appropriate method of randomization was described
Blinding
■ +1 if the study was described as double-blind
■ −1 if an inappropriate method of blinding was described
■ +1 if an appropriate method of double-blinding was described
Withdrawals
■ +1 if participant withdrawals were accounted for
Box 3 | Recommendations for treatment trials in mitochondrial diseases.
■ Mitochondrial disorders are heterogeneous, and often have a complex multisystem phenotype that fluctuates over time in an unpredictable manner, which presents a major challenge for the design and interpretation of clinical trial data
■ Small studies should focus on patients with a similar genotype and phenotype, and ideally those at a similar stage of the disease; for rare mitochondrial disease this usually requires international collaboration
■ Simple trial designs are often the best, using validated, clinically meaningful and prespecified primary end points. End points should be chosen that are most relevant to the genotype or phenotype in question. A key issue is the identification of biomarkers that are indicative of clinically relevant outcomes, which will require multidisciplinary collaboration and patient involvement
■ Open-label trials are prone to bias through unanticipated placebo effects and subjective clinical measurements. These studies are important as a first step in evaluating treatments, but they must be considered preliminary, and should not shape routine clinical practice
■ Open-label studies should be published only if they have a small number of defined prespecified end points and a clear predefined statistical analysis plan, and are publically registered on ClinicalTrials.gov before recruitment commences. The results of these studies should not be considered as preliminary evidence for the benefit and safety of an intervention, but merely serve as a signal to proceed with further evaluation in appropriately controlled trials
■ Large multicentre randomized controlled trials have been carried out for mitochondrial disease, and several others are in progress. These trials establish proof of principle that data of the highest quality can be produced to underpin mitochondrial medicine, facilitated by international consortia
■ Off-licence prescription of medicines or food supplements could have value in a compassionate context, but the lack of objective efficacy should be made clear to patients and families, who should be advised that prescribing may stop if a high-quality negative trial is published
Acknowledgements
G. Pfeffer is the recipient of a Bisby Fellowship from the Canadian Institutes of Health Research. A. Suomalainen is supported by the Sigrid Juselius Foundation and the Jane and Atos Erkko Foundation. R. McFarland is an honorary consultant paediatric neurologist at Newcastle upon Tyne Foundation Hospitals NHS Trust and a DoH/HEFCE-funded Clinical Senior Lecturer. J. Smeitink receives additional support from the Eurostars Programme (ESTAR 11205), ZonMW PM Rare and the Dutch Science Organisation (NWO) CSBR Program (853.00.130). P. F. Chinnery is an Honorary Consultant Neurologist at Newcastle upon Tyne Foundation Hospitals NHS Trust, is a Wellcome Trust Senior Fellow in Clinical Science (084980/Z/08/Z), and a UK National Institute for Health Research (NIHR) Senior Investigator. P. F. Chinnery receives additional support from the Wellcome Trust Centre for Mitochondrial Research (096919Z/11/Z), the Medical Research Council (UK) Centre for Translational Research in Neuromuscular Diseases, EU FP7 TIRCON, and the NIHR Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The support of the Marriott Foundation is gratefully acknowledged. The views expressed are those of the authors and not necessarily those of the NHS, MF, ZonMW, NOW, the NIHR or the Department of Health. M. Zeviani (GPP10005) and V. Carelli are supported by Telethon Italy. For this study, T. Klopstock acknowledges funding from the German Federal Ministry of Education and Research (BMBF, grant number 01GM1113A) for the German Network for Mitochondrial Disorders (mitoNET). T. Klopstock receives additional support from the BMBF (German Centre for Vertigo and Balance Disorders, grant number 01EO0901) and from the European Commission Seventh Framework Programme (FP7/2007-2013, HEALTH-F2-2011, grant agreement No. 277984, TIRCON).
Footnotes
Author contributions
G. Pfeffer, R. Horvath, and P. F. Chinnery performed data analysis. G. Pfeffer and P. F. Chinnery wrote the article. All authors provided substantial contribution to discussion of content, and to the review and/or editing of the manuscript before submission.
Supplementary information is linked to the online version of the paper at www.nature.com/nrneurol.
Competing interests
T. Klopstock declares associations with the following companies: Actelion Pharmaceuticals, Boehringer Ingelheim Pharma, Eisai, FinTech Global Capital, Gerson Lehrman Group, GlaxoSmithKline, H. Lundbeck A/S, Santhera Pharmaceuticals. V. K. Mootha declares an association with the following company: Ember Therapeutics. V. Carelli declares associations with the following companies: Edison Pharmaceuticals, Sigma-tau. J. Smeitink declares an association with the following company: Khondrion. P. F. Chinnery declares an association with the following company: Santhera Pharmaceuticals. See the article online for full details of the relationships. The other authors declare no competing interests.
References
- 1.Murphy SM, Puwanant A, Griggs RC. Unintended effects of orphan product designation for rare neurological diseases. Ann. Neurol. 2012;72:481–490. doi: 10.1002/ana.23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesselheim AS. Ethical considerations in orphan drug approval and use. Clin. Pharmacol. Ther. 2012;92:153–155. doi: 10.1038/clpt.2012.92. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database of Systematic Reviews. (4) doi: 10.1002/14651858.CD004426.pub3. Art. No.: CD004426. http://dx.doi.org/10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed]
- 4.Pons R, De Vivo DC. Primary and secondary carnitine deficiency syndromes. J. Child. Neurol. 1995;10(Suppl. 2):S8–S24. [PubMed] [Google Scholar]
- 5.Majamaa K, Rusanen H, Remes AM, Pyhtinen J, Hassinen IE. Increase of blood NAD+ and attenuation of lactacidemia during nicotinamide treatment of a patient with the MELAS syndrome. Life Sci. 1996;58:691–699. doi: 10.1016/s0024-3205(96)80008-7. [DOI] [PubMed] [Google Scholar]
- 6.Lou HC. Correction of increased plasma pyruvate and plasma lactate levels using large doses of thiamine in patients with Kearns–Sayre syndrome. Arch. Neurol. 1981;38:469. doi: 10.1001/archneur.1981.00510070103027. [DOI] [PubMed] [Google Scholar]
- 7.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 8.Bernsen PL, Gabreëls FJ, Ruitenbeek W, Hamburger HL. Treatment of complex I deficiency with riboflavin. J. Neurol. Sci. 1993;118:181–187. doi: 10.1016/0022-510x(93)90108-b. [DOI] [PubMed] [Google Scholar]
- 9.Rauchová H, Drahota Z, Lenaz G. Function of coenzyme Q in the cell: some biochemical and physiological properties. Physiol. Res. 1995;44:209–216. [PubMed] [Google Scholar]
- 10.Orsucci D, Mancuso M, Lenco EC, LoGerfo A, Siciliano G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr. Med. Chem. 2011;18:4053–4064. doi: 10.2174/092986711796957257. [DOI] [PubMed] [Google Scholar]
- 11.Shrader WD, et al. α-Tocotrienol quinone modulates oxidative stress response and the biochemistry of aging. Bioorg. Med. Chem. Lett. 2011;21:3693–3698. doi: 10.1016/j.bmcl.2011.04.085. [DOI] [PubMed] [Google Scholar]
- 12.Shoffner JM, et al. Spontaneous Kearns– Sayre/chronic external ophthalmoplegia plus syndromes associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc. Natl Acad. Sci. USA. 1989;86:7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eleff S, et al. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc. Natl Acad. Sci. USA. 1984;81:3529–3533. doi: 10.1073/pnas.81.11.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso M, et al. Oxidative stress biomarkers in mitochondrial myopathies, basally and after cysteine donor supplementation. J. Neurol. 2010;257:774–781. doi: 10.1007/s00415-009-5409-7. [DOI] [PubMed] [Google Scholar]
- 15.Liet JM, et al. The effect of short-term dimethylglycine treatment on oxygen consumption in cytochrome oxidase deficiency: a double-blind randomized crossover clinical trial. J. Pediatr. 2003;142:62–66. doi: 10.1067/mpd.2003.mpd0333. [DOI] [PubMed] [Google Scholar]
- 16.Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cejudo P, et al. Exercise training in mitochondrial myopathy: a randomized controlled trial. Muscle Nerve. 2005;32:342–350. doi: 10.1002/mus.20368. [DOI] [PubMed] [Google Scholar]
- 18.Koga Y, et al. L-Arginine improves the symptoms of strokelike episodes in MELAS. Neurology. 2005;64:710–712. doi: 10.1212/01.WNL.0000151976.60624.01. [DOI] [PubMed] [Google Scholar]
- 19.Walcott BP, et al. Steroid responsive A3243G mutation MELAS: clinical and radiographic evidence for regional hyperperfusion leading to neuronal loss. Neurologist. 2012;18:159–170. doi: 10.1097/NRL.0b013e318247bcd8. [DOI] [PubMed] [Google Scholar]
- 20.Hassani A, Horvath R, Chinnery PF. Mitochondrial myopathies: developments in treatment. Curr. Opin. Neurol. 2010;23:459–465. doi: 10.1097/WCO.0b013e32833d1096. [DOI] [PubMed] [Google Scholar]
- 21.Bresolin N, et al. Ubidecarenone in the treatment of mitochondrial myopathies: a multi-center double-blind trial. J. Neurol. Sci. 1990;100:70–78. doi: 10.1016/0022-510x(90)90015-f. [DOI] [PubMed] [Google Scholar]
- 22.Kerr DS. Treatment of mitochondrial electron transport chain disorders: a review of clinical trials over the past decade. Mol. Genet. Metab. 2010;99:246–255. doi: 10.1016/j.ymgme.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 23.CureMito [online] 2012 http://curemito.org/
- 24.United Mitochondrial Disease Foundation [online] 2013 www.umdf.org.
- 25.Barshop BA, et al. Chronic treatment of mitochondrial disease patients with dichloroacetate. Mol. Genet. Metab. 2004;83:138–149. doi: 10.1016/j.ymgme.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Blankenberg FG, et al. Brain uptake of Tc99m-HMPAO correlates with clinical response to the novel redox modulating agent EPI-743 in patients with mitochondrial disease. Mol. Genet. Metab. 2012;107:690–699. doi: 10.1016/j.ymgme.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Campos Y, et al. Plasma carnitine insufficiency and effectiveness of L-carnitine therapy in patients with mitochondrial myopathy. Muscle Nerve. 1993;16:150–153. doi: 10.1002/mus.880160205. [DOI] [PubMed] [Google Scholar]
- 28.Chen RS, Huang CC, Chu NS. Coenzyme Q10 treatment in mitochondrial encephalomyopathies. Short-term double-blind, crossover study. Eur. Neurol. 1997;37:212–218. doi: 10.1159/000117445. [DOI] [PubMed] [Google Scholar]
- 29.De Stefano N, et al. Short-term dichloracetate treatment improves indices of cerebral metabolism in patients with mitochondrial disorders. Neurology. 1995;45:1193–1198. doi: 10.1212/wnl.45.6.1193. [DOI] [PubMed] [Google Scholar]
- 30.Duncan GE, Perkins LA, Theriaque DW, Neiberger RE, Stacpoole PW. Dichloroacetate therapy attenuates the blood lactate response to submaximal exercise in patients with defects in mitochondrial energy metabolism. J. Clin. Endocrinol. Metab. 2004;89:1733–1738. doi: 10.1210/jc.2003-031684. [DOI] [PubMed] [Google Scholar]
- 31.Enns GM, et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol. Genet. Metab. 2012;105:91–102. doi: 10.1016/j.ymgme.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Glover EI, et al. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve. 2010;42:739–748. doi: 10.1002/mus.21758. [DOI] [PubMed] [Google Scholar]
- 33.Gold R, et al. Phosphorus magnetic resonance spectroscopy in the evaluation of mitochondrial myopathies: results of a 6-month therapy study with coenzyme Q. Eur. Neurol. 1996;36:191–196. doi: 10.1159/000117246. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann P, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–330. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- 35.Klopstock T, et al. A placebo-controlled crossover trial of creatine in mitochondrial diseases. Neurology. 2000;55:1748–1751. doi: 10.1212/wnl.55.11.1748. [DOI] [PubMed] [Google Scholar]
- 36.Klopstock T, et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134:2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koga Y, et al. Effects of L-arginine on the acute phase of strokes in three patients with MELAS. Neurology. 2002;58:827–828. doi: 10.1212/wnl.58.5.827. [DOI] [PubMed] [Google Scholar]
- 38.Komura K, Hobbiebrunken E, Wilichowski EK, Hanefeld FA. Effectiveness of creatine monohydrate in mitochondrial encephalomyopathies. Pediatr. Neurol. 2003;28:53–58. doi: 10.1016/s0887-8994(02)00469-1. [DOI] [PubMed] [Google Scholar]
- 39.Martinelli D, et al. EPI-743 reverses the progression of the pediatric mitochondrial disease--genetically defined Leigh Syndrome. Mol. Genet. Metab. 2012;107:383–388. doi: 10.1016/j.ymgme.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Mashima Y, Kigasawa K, Wakakura M, Oguchi Y. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? J. Neuroophthalmol. 2000;20:166–170. doi: 10.1097/00041327-200020030-00006. [DOI] [PubMed] [Google Scholar]
- 41.Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology. 1993;43:2484–2490. doi: 10.1212/wnl.43.12.2484. [DOI] [PubMed] [Google Scholar]
- 42.Mori M, Yamagata T, Goto T, Saito S, Momoi MY. Dichloroacetate treatment for mitochondrial cytopathy: long-term effects in MELAS. Brain Dev. 2004;26:453–458. doi: 10.1016/j.braindev.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa T, et al. Multi-center trial on the early effects of silodosin on lower urinary tract symptoms associated with benign prostatic hyperplasia [Japanese]. Hinyokika Kiyo. 2008;54:757–764. [PubMed] [Google Scholar]
- 44.Panetta J, Smith LJ, Boneh A. Effect of high-dose vitamins, coenzyme Q and high-fat diet in paediatric patients with mitochondrial diseases. J. Inherit. Metab. Dis. 2004;27:487–498. doi: 10.1023/B:BOLI.0000037354.66587.38. [DOI] [PubMed] [Google Scholar]
- 45.Remes AM, et al. Ubiquinone and nicotinamide treatment of patients with the 3243A-->G mtDNA mutation. Neurology. 2002;59:1275–1277. doi: 10.1212/wnl.59.8.1275. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez MC, et al. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 47.Sadun AA, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch. Neurol. 2012;69:331–338. doi: 10.1001/archneurol.2011.2972. [DOI] [PubMed] [Google Scholar]
- 48.Stacpoole PW, et al. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223–e1228. doi: 10.1542/peds.2007-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stacpoole PW, et al. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki S, et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA 3243 (A to G) mutation. Diabetologia. 1998;41:584–588. doi: 10.1007/s001250050950. [DOI] [PubMed] [Google Scholar]
- 51.Taivassalo T, et al. Endurance training and detraining in mitochondrial myopathies due to single large-scale mtDNA deletions. Brain. 2006;129:3391–3401. doi: 10.1093/brain/awl282. [DOI] [PubMed] [Google Scholar]
- 52.Tarnopolsky MA, Roy BD, MacDonald JR. Randomised control trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve. 1997;20:1502–1509. doi: 10.1002/(sici)1097-4598(199712)20:12<1502::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 53.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 54.Mazzone E, et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011;77:250–256. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 55.US National Library of Medicine ClinicalTrials. gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT00432744?term=NCT00432744&rank=1.
- 56.Notice of compliance with conditions—NOC/c (therapeutic products). Health Canada [online] 2013 http://www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/fs-fi/noccfs_accfd-eng.php.
- 57.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich's ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 2007;6:878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- 58.Lynch DR, Perlman SL, Meier T. A phase 3, double-blind, placebo-controlled trial of idebenone in friedreich ataxia. Arch. Neurol. 2010;67:941–947. doi: 10.1001/archneurol.2010.168. [DOI] [PubMed] [Google Scholar]
- 59.Lagedrost SJ, et al. Idebenone in Friedreich ataxia cardiomyopathy-results from a 6-month phase III study (IONIA). Am. Heart J. 2011;161:639, e1–645, e1. doi: 10.1016/j.ahj.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 60.Kearney M, Orrell RW, Fahey M, Pandolfo M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database of Systematic Reviews. (4) doi: 10.1002/14651858.CD007791.pub3. Art. No.: CD007791. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007791.pub3/abstract. [DOI] [PubMed]
- 61.CATENA® (idebenone)—voluntary withdrawal of CATENA® from the Canadian market. Health Canada [online] 2013 http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/23509a-eng.php.
- 62.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu. Rev. Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 63.Koene S, et al. Towards the harmonization of outcome measures in children with mitochondrial disorders. Dev. Med. Child. Neurol. doi: 10.1111/dmcn.12119. http://dx.doi.org/10.1111/dmcn.12119. [DOI] [PubMed]
- 64.Koene S, et al. Developing outcome measures for pediatric mitochondrial disorders: which complaints and limitations are most burdensome to patients and their parents? Mitochondrion. 2013;13:15–24. doi: 10.1016/j.mito.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Jeppesen TD, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129:3402–3412. doi: 10.1093/brain/awl149. [DOI] [PubMed] [Google Scholar]
- 66.Joppi R, Bertele V, Garattini S. Orphan drug development is not taking off. Br. J. Clin. Pharmacol. 2009;67:494–502. doi: 10.1111/j.1365-2125.2009.03369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.North American Mitochondrial Disease Consortium [online] 2013 http://rarediseasesnetwork.epi.usf.edu/NAMDC/
- 68.The Children's Mitochondrial Disease Network [online] 2013 http://www.emdn-mitonet.co.uk/
- 69.mitoNET—German Network for Mitochondrial Disorders [online] 2013 http://mitonet.org/
- 70.Heemstra HE, van Weely S, Büller HA, Leufkens HG, de Vrueh RL. Translation of rare disease research into orphan drug development: disease matters. Drug Discov. Today. 2009;14:1166–1173. doi: 10.1016/j.drudis.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Hsu CC, et al. CPEO and carnitine deficiency overlapping in MELAS syndrome. Acta Neurol. Scand. 1995;92:252–255. doi: 10.1111/j.1600-0404.1995.tb01697.x. [DOI] [PubMed] [Google Scholar]
- 72.Majamaa K, Rusanen H, Remes A, Hassinen IE. Metabolic interventions against complex I deficiency in MELAS syndrome. Mol. Cell Biochem. 1997;174:291–296. [PubMed] [Google Scholar]
- 73.Kornblum C, et al. Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial DNA deletions: a placebo-controlled, double-blind 31P-MRS crossover study. Eur. J. Neurol. 2005;12:300–309. doi: 10.1111/j.1468-1331.2004.00970.x. [DOI] [PubMed] [Google Scholar]
- 74.Barbiroli B, et al. Lipoic (thioctic) acid increases brain energy availability and skeletal muscle performance as shown by in vivo 31P MRS in a patient with mitochondrial cytopathy. J. Neurol. 1995;242:472–477. doi: 10.1007/BF00873552. [DOI] [PubMed] [Google Scholar]
- 75.Taivassalo T, et al. Aerobic training benefits patients with mitochondrial myopathies more than other chronic myopathies. Neurology. 1997;48:A214. [Google Scholar]
- 76.Fu K, et al. A novel heteroplasmic tRNAleu(UUR) mtDNA point mutation in a sporadic patient with mitochondrial encephalomyopathy segregates rapidly in muscle and suggests an approach to therapy. Hum. Mol. Genet. 1996;5:1835–1840. doi: 10.1093/hmg/5.11.1835. [DOI] [PubMed] [Google Scholar]
- 77.Clark K, et al. Correction of a mitochondrial DNA defect in human skeletal muscle. Nat. Genet. 1997;16:222–224. doi: 10.1038/ng0797-222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.