Abstract
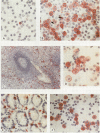
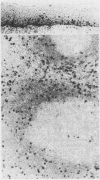
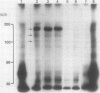
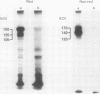
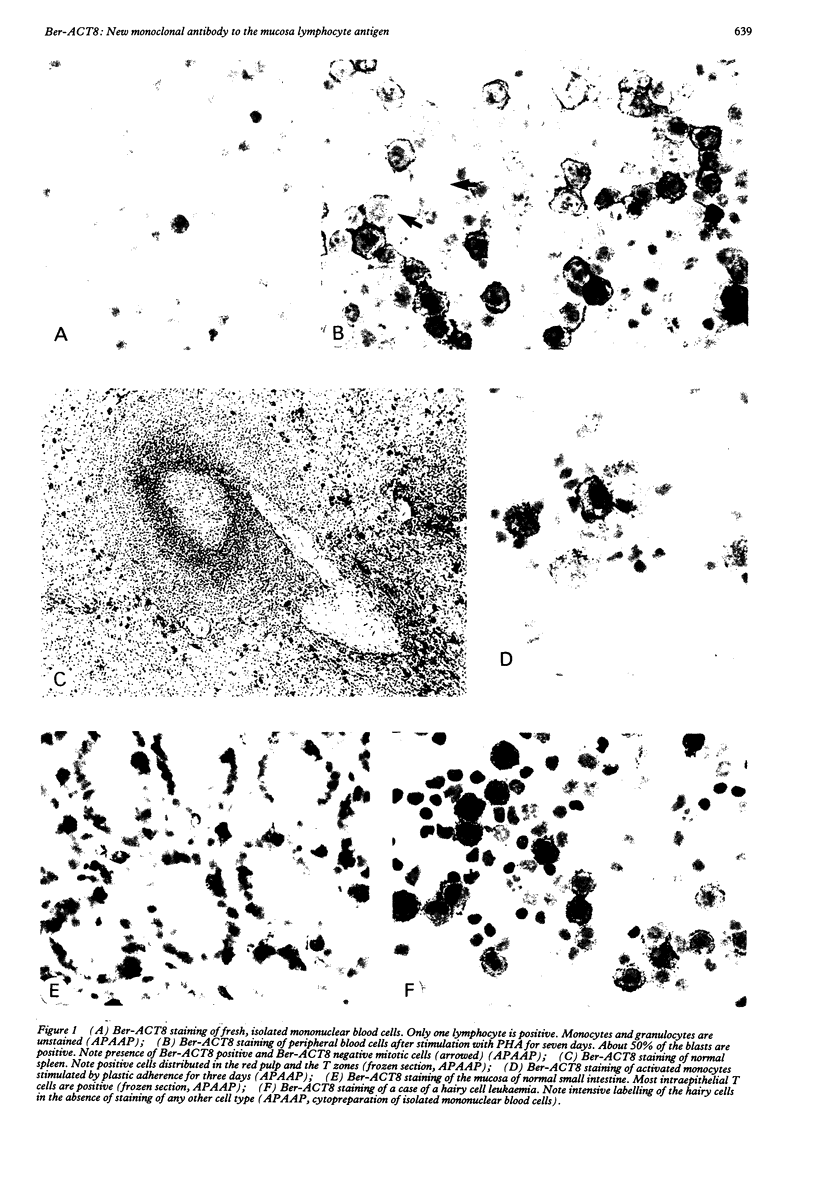
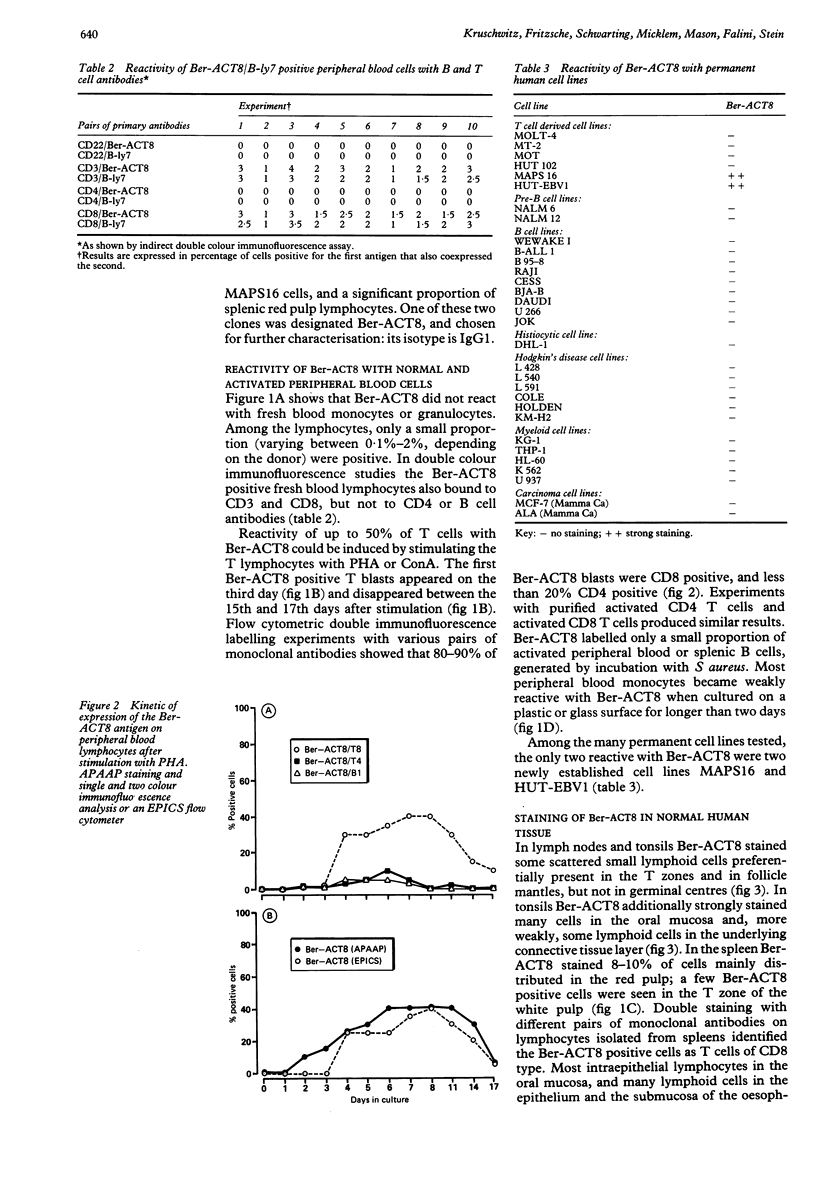
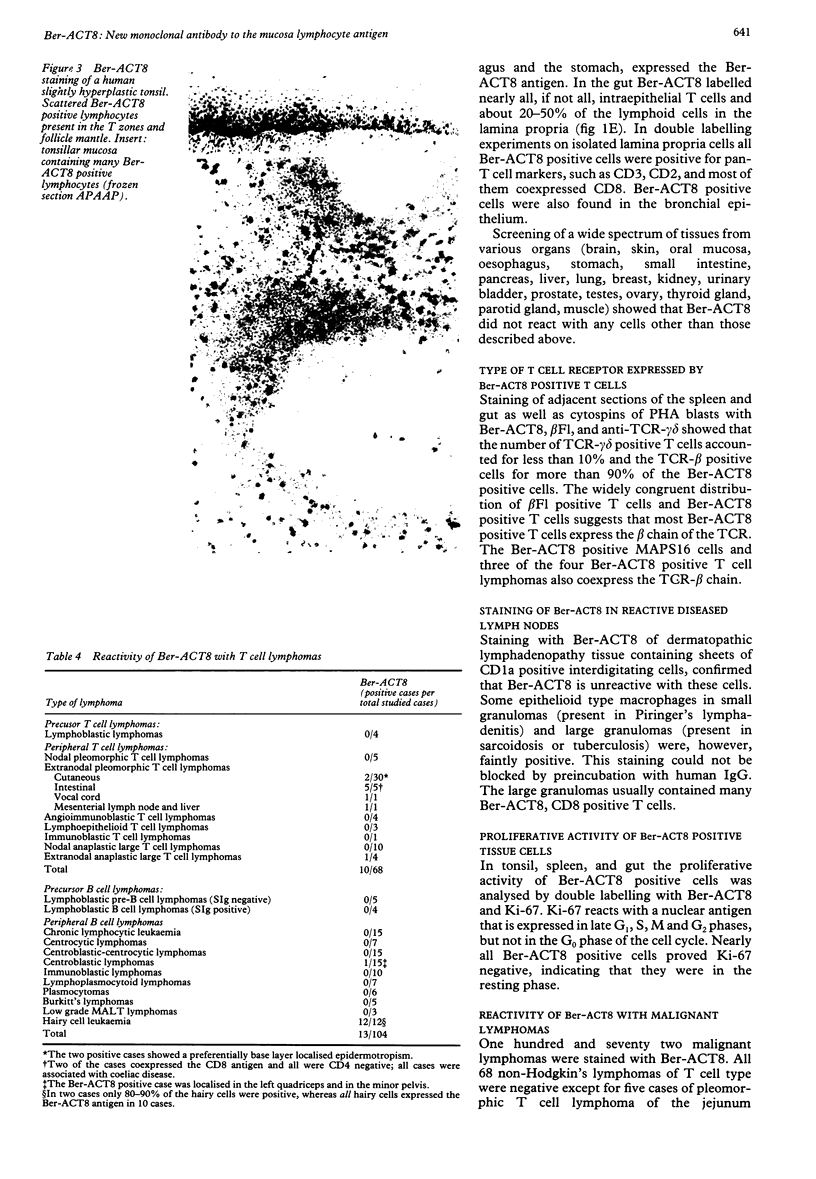
Using a newly established HTLV-1 positive T cell line as an immunogen, a new monoclonal antibody, Ber-ACT8, was produced. It reacts with in vitro activated T cells and a small subset of normal resting T cells, but not with resting B cells or any of the 29 established human permanent cell lines tested. Immunohistological analysis of a wide spectrum of human tissues showed that Ber-ACT8 reactivity is restricted to a few T cells in the peripheral blood, the extrafollicular areas of lymph nodes and tonsils, and splenic red pulp. In the gut Ber-ACT8 labelled most intraepithelial T cells and up to 50% of lamina propria T cells. The antibody also immunostained T cells present in the oral and bronchial mucosa. Double labelling on splenic cells, fresh blood lymphocytes, and in vitro activated T cells showed that most Ber-ACT8 positive cells coexpressed CD8. Ber-ACT8 did not react with any of the 14 Hodgkin's lymphomas nor any of the 172 non-Hodgkin's lymphomas tested, with the exception of 10 cases of T cell lymphomas, five of which were located in the jejunum and associated with coeliac disease, and one B cell lymphoma, and most cases of hairy cell leukaemia tested. Parallel immunostainings with Ber-ACT8, anti-TCR-beta (beta F1), and anti-TCR-delta showed that most Ber-ACT8 positive T cells carry the TCR of alpha beta type. Comparison of Ber-ACT8 with HML-1, B-ly7, and LF61 showed essentially the same reactivity and an identical molecular target. The molecular structure recognised seems to be a trimeric molecule with components of 150, 125 and 105 kilodaltons, with the Ber-ACT8 epitope localised on the 150 kilodalton chain. The 150 kilodalton molecule contains an 0-linked carbohydrate moiety of about 10 kilodaltons. Because of its very selective distribution, the trimeric antigen is a powerful reagent for the diagnosis of gut T cell-derived T cell lymphomas and other extranodal T cell lymphomas, as well as hairy cell leukaemia.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blue M. L., Daley J. F., Levine H., Schlossman S. F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985 Apr;134(4):2281–2286. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Corte G., Moretta L., Damiani G., Mingari M. C., Bargellesi A. Surface antigens specifically expressed by activated T cells in humans. Eur J Immunol. 1981 Feb;11(2):162–164. doi: 10.1002/eji.1830110220. [DOI] [PubMed] [Google Scholar]
- Flenghi L., Spinozzi F., Stein H., Krushwitz M., Pileri S., Falini B. LF61: a new monoclonal antibody directed against a trimeric molecule (150 kDa, 125 kDa, 105 kDa) associated with hairy cell leukaemia. Br J Haematol. 1990 Dec;76(4):451–459. doi: 10.1111/j.1365-2141.1990.tb07900.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Brenner M. B., Mann D., Strominger J. L. VLA-1: a T cell surface antigen which defines a novel late stage of human T cell activation. Eur J Immunol. 1985 May;15(5):502–508. doi: 10.1002/eji.1830150515. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., O'Connor N. T., Spencer J., Bevan D. H., Connolly C. E., Kirkham N., Pollock D. J., Wainscoat J. S., Stein H., Mason D. Y. Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet. 1985 Sep 28;2(8457):688–691. doi: 10.1016/s0140-6736(85)92930-7. [DOI] [PubMed] [Google Scholar]
- Monoclonal antibody (HML-1) labelling of T-cell lymphomas. Lancet. 1989 Jan 28;1(8631):223–224. [PubMed] [Google Scholar]
- Mulligan S. P., Travade P., Matutes E., Dearden C., Visser L., Poppema S., Catovsky D. B-ly-7, a monoclonal antibody reactive with hairy cell leukemia, also defines an activation antigen on normal CD8+ T cells. Blood. 1990 Sep 1;76(5):959–964. [PubMed] [Google Scholar]
- Pfisterer M., Burkart F., Jockers G., Meyer B., Regenass S., Burckhardt D., Schmitt H. E., Müller-Brand J., Skarvan K., Stulz P. Trial of low-dose aspirin plus dipyridamole versus anticoagulants for prevention of aortocoronary vein graft occlusion. Lancet. 1989 Jul 1;2(8653):1–7. doi: 10.1016/s0140-6736(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Michie S. A., Rott L. S., Butcher E. C. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990 May;136(5):1053–1068. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Terstappen L. W., Rott L. S., Streeter P. R., Stein H., Butcher E. C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990 Nov 15;145(10):3247–3255. [PubMed] [Google Scholar]
- Schieferdecker H. L., Ullrich R., Weiss-Breckwoldt A. N., Schwarting R., Stein H., Riecken E. O., Zeitz M. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990 Apr 1;144(7):2541–2549. [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Schwarting R., Dienemann D., Kruschwitz M., Fritsche G., Stein H. Specificities of monoclonal antibodies B-ly7 and HML-1 are identical. Blood. 1990 Jan 1;75(1):320–321. [PubMed] [Google Scholar]
- Spencer J., Cerf-Bensussan N., Jarry A., Brousse N., Guy-Grand D., Krajewski A. S., Isaacson P. G. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1988 Jul;132(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Sperling M., Kaudewitz P., Braun-Falco O., Stein H. Reactivity of T cells in mycosis fungoides exhibiting marked epidermotropism with the monoclonal antibody HML-1 that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1989 May;134(5):955–960. [PMC free article] [PubMed] [Google Scholar]
- Stein H., Dienemann D., Sperling M., Zeitz M., Riecken E. O. Identification of a T cell lymphoma category derived from intestinal-mucosa-associated T cells. Lancet. 1988 Nov 5;2(8619):1053–1054. doi: 10.1016/S0140-6736(88)90068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Gatter K., Asbahr H., Mason D. Y. Use of freeze-dried paraffin-embedded sections for immunohistologic staining with monoclonal antibodies. Lab Invest. 1985 Jun;52(6):676–683. [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Visser L., Shaw A., Slupsky J., Vos H., Poppema S. Monoclonal antibodies reactive with hairy cell leukemia. Blood. 1989 Jul;74(1):320–325. [PubMed] [Google Scholar]
- Wano Y., Uchiyama T., Fukui K., Maeda M., Uchino H., Yodoi J. Characterization of human interleukin 2 receptor (Tac antigen) in normal and leukemic T cells: co-expression of normal and aberrant receptors on Hut-102 cells. J Immunol. 1984 Jun;132(6):3005–3010. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Vitetta E. S. Biosynthetic, surface labeling, and isolation of membrane immunoglobulin. Methods Enzymol. 1984;108:426–433. doi: 10.1016/s0076-6879(84)08109-x. [DOI] [PubMed] [Google Scholar]