Abstract
IMPORTANCE
Melanocytic neoplasms with prominent pigment synthesis mimicking equine melanoma represent a rare variant of biologically indeterminate or low-grade malignant melanocytic tumors in which the molecular profile and exact histologic classification are not established. Tumors with these characteristics rarely occur as congenital lesions. We performed genomic analysis of a congenital pigment synthesizing melanocytic neoplasm with indeterminate biological potential.
OBSERVATIONS
The patient was a 5-month-old girl presenting with a 6-cm protuberant scalp mass, which had doubled in size since birth. Histologic examination showed heavily pigmented intradermal proliferation of large, epithelioid melanocytes with mild cytologic atypia, low mitotic activity, focal necrosis, and ulceration. RNA sequencing identified a novel ATPase, Ca2+ transporting, plasma membrane 4 (ATP2B4)–protein kinase C-alpha (PRKCA) fusion transcript. The fusion resulted in an in-frame linkage of the PRKCA catalytic domain with the N-terminal of ATP2B4 and high expression of the PRKCA kinase domain. Break-apart fluorescence in situ hybridization showed PRKCA rearrangement, and reverse transcriptase–polymerase chain reaction confirmed the presence of the fusion transcript. The patient was alive and well, with no evidence of recurrence, at the 1-year follow-up.
CONCLUSIONS AND RELEVANCE
To our knowledge, this is the first report of PRKCA fusions in melanocytic neoplasms. Future studies need to determine the frequency of PRKCA fusions in pigment-synthesizing melanocytic neoplasms.
Melanocytic neoplasms with prominent pigment synthesis mimicking equine melanoma and with histologic features overlapping those of epithelioid blue nevus (EBN) represent a rare variant of melanocytic tumors with low-grade malignant or indeterminate biological potential. Tumors with these characteristics have been variously termed as pigment-synthesizing melanoma, animal-type melanoma, or pigmented epithelioid melanocytoma,1-3 but their exact histologic classification and molecular profile have not been well established.
The lesions are characterized by a predominantly intradermal proliferation of epithelioid and spindle-shaped melanocytes, often with low mitotic activity, associated with abundant fine or coarse melanin pigment, and a prominent population of macrophages.1,3,4 They typically follow an indolent clinical course despite a tendency to recur locally or spread to regional lymph nodes.1,2 Distant metastases, however, may develop in some patients and rare deaths as a result of disease have been documented.1,3,4 The disease can occur at any age,1,4 and congenital forms have also been reported.5,6
There is considerable overlap between the histologic features of pigment-synthesizing melanocytic neoplasms and EBN, a rare variant of blue nevus that commonly occurs in the setting of the Carney complex, a familial lentiginosis and multiple endocrine, multiorgan low-grade neoplasia syndrome.7 Most cases of the Carney complex are caused by inactivating germline mutations in the protein kinase, cAMP-dependent, regulatory, type I, alpha (PRKAR1A) gene (NM_002737).7 Because of the histologic similarities and commonly indolent behavior, a unifying concept for EBN and pigment-synthesizing (animal-type) melanoma under the term pigmented epithelioid melanocytoma has been proposed,2 but whether the 2 entities are related at the genomic level has not been established.
Recently, kinase activation by gene fusions has been identified as a common oncogenic mechanism in spitzoid melanocytic tumors.8 For most EBNs of both Carney complex–associated and sporadic forms, however, loss of expression of the tumor suppressor gene PRKAR1A is postulated to be involved in pathogenesis.9
We performed targeted mutational analysis and whole-transcriptome sequencing in a congenital heavily pigmented melanocytic tumor. We identified a novel protein kinase C-alpha (PRKCA) fusion that has not been previously reported in association with melanocytic neoplasms.
Report of a Case
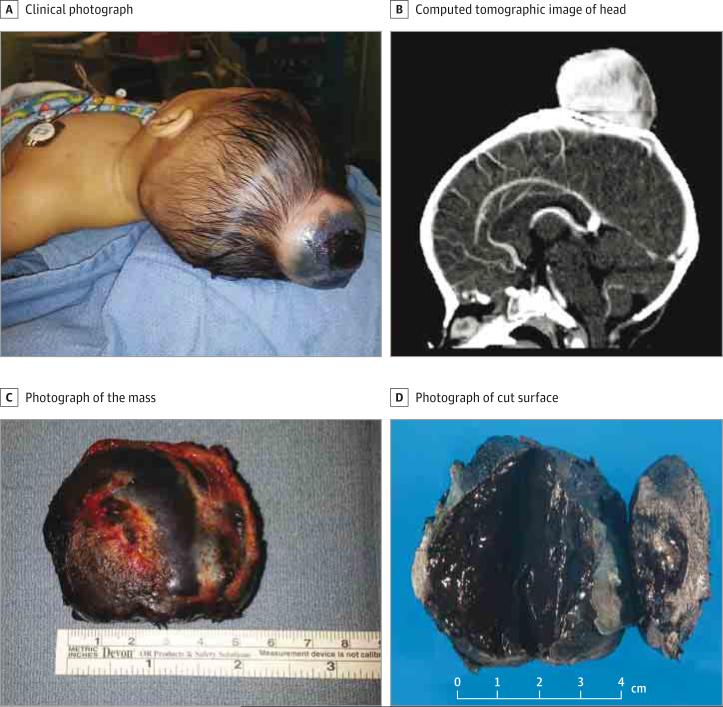
A 5-month-old girl presented with a large scalp mass (Figure 1A). She had been delivered by cesarean section because of failure of descent due to the head mass. Over a period of 5 months, the mass grew rapidly and doubled in size. The family history was negative for melanoma, multiple neoplasia, or early-onset cancers. The mother was healthy and had no suspicious melanocytic lesions. Physical examination revealed a nontender protuberant mass on the vertex of the scalp. A computed tomographic scan of the head revealed a 5.3 × 5.8 × 3.6-cm3 hyperdense, heterogeneous mass with invasion of the underlying calvarium (Figure 1B). The patient underwent complete surgical excision of the mass (Figure 1C). Gross examination revealed a 6.0 ×5.5 × 3.8-cm3 mass with a dark-brown cut surface (Figure 1D).
Figure 1. Clinical and Pathological Findings.
A, Protuberant mass on the scalp of a 5-month-old girl. B, Hyperdense, heterogeneous mass in the scalp invading the underlying calvarium. C, Skin overlying the mass is partially ulcerated and covered with crust over a 2.0 × 2.0-cm2 area. D, Uniform dark-brown cut surface of the mass.
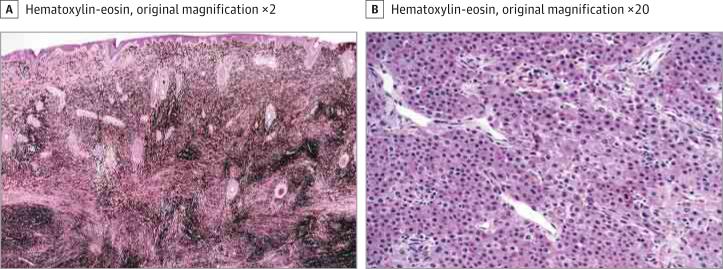
Microscopic examination identified a heavily pigmented dermal infiltration of epithelioid melanocytes arranged in confluent nests and sheets abutting the epidermis (Figure 2A). The melanocytes percolated into the subcutaneous tissue without maturation. Abundant fine and dark-brown coarse deposits of melanin were present. The melanin-bleached preparations revealed the tumor cells with round nuclei, conspicuous nucleoli, and focally mild nuclear pleomorphism (Figure 2B). There was focal necrosis en masse of tumor cells. The mitotic rate was 1 to 2 per mm2. There was no evidence of a preexisting melanocytic nevus. The histologic diagnosis was a severely atypical pigmented epithelioid cell melanocytic proliferation of indeterminate malignant potential. There was no clinical or radiographic evidence of distant metastases. Sentinel lymph node sampling was withheld. Close clinical follow-up revealed no evidence of recurrence 1 year after the diagnosis.
Figure 2. Microscopic Examination.
A, Intradermal atypical melanocytic neoplasm with prominent melanosis. B, Melanin-bleached section showing large polygonal epithelioid cells in confluent nests and sheets within the deep subcutaneous tissue.
Array comparative genomic hybridization (aCGH) analysis was performed on the formalin-fixed paraffin-embedded (FFPE) tissue (see the eMethods in the Supplement). aCGH analysis showed losses in chromosomes 1p36.33-p35.3, 1q32.1-q44, and 17q11.1-24.2 (see the eFigure in the Supplement).
For mutational analysis, genomic DNA was extracted from FFPE tumor tissue according to the manufacturer's protocol by using the Maxwell 16 FFPE Plus LEV DNA Purification Kit (Promega). Tumor DNA was screened for mutational hot spots for BRAF (exon 15), NRAS (exons 1 and 2), GNAQ (exons 4 and 5), GNA11 (exons 4 and 5), PRKAR1A (exons 1A, 1B, 3, 4A, 4B, 5, 6, 8, 9, and 10), and the TERT promoter (HG19 coordinates, chr5: 1295151–1295347), as previously described.10 The tumor was negative for a hot spot mutation in BRAF, NRAS, GNAQ, GNA11, or the TERT promoter. Sequencing of PRKAR1A showed the wild-type gene.
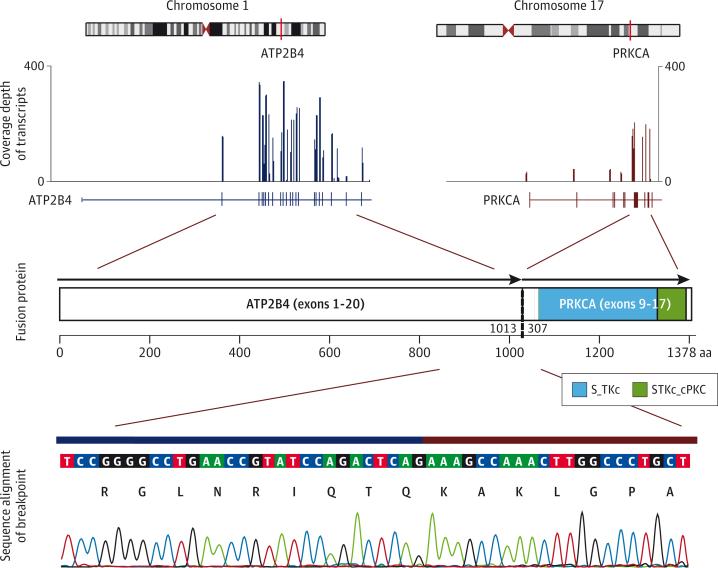
For RNA sequencing (RNA-seq) analysis, RNA from FFPE tumor tissue was extracted with the Maxwell 16 LEV RNA FFPE Kit (Promega) and quantitated by fluorescence dye by using the Quant-iT RNA assay (Life Technology). The RNA quality was evaluated by a 2100 Bioanalyzer (Agilent Technologies) with a Nano RNA 6000 Chip. RNA-seq libraries enriched for coding regions were prepared by using the Truseq RNA Access Library Prep Kit (Illumina) following the manufacturers protocol. Sequencing was performed on a HiSeq2000 to generate 100 base-paired end reads. A novel assembly-based method (CICERO) was used to detect structural variations. Details of the RNA-seq analysis are provided in the eMethods in the Supplement. RNA-seq analysis revealed a single fusion transcript that was predicted to encode the intact catalytic domain of the serine/threonine PRKCA fused in frame with the N-terminal of ATP2B4 (Figure 3). When compared with samples of pediatric melanocytic tumors for which RNA-sequencing data were available (data not shown), this tumor had the highest PRKCA expression.
Figure 3. Schematic of the ATP2B4-PRKCA Fusion.
RNA-seq data showing chromosomal loci for the 2 genes participating in the translocation: ATP2B4 at 1q32.1 and PRKCA at 17q22-q23.2 (top lane). Exon 20 of ATP2B4 (NM_001684) was fused to exon 9 of PRKCA (NM_002737), yielding a 1378-amino acid chimeric product containing the intact kinase domain of PRKCA (middle lane). Sequence alignment of the ATP2B4-PRKCA breakpoint region by Sanger sequencing (bottom lane).
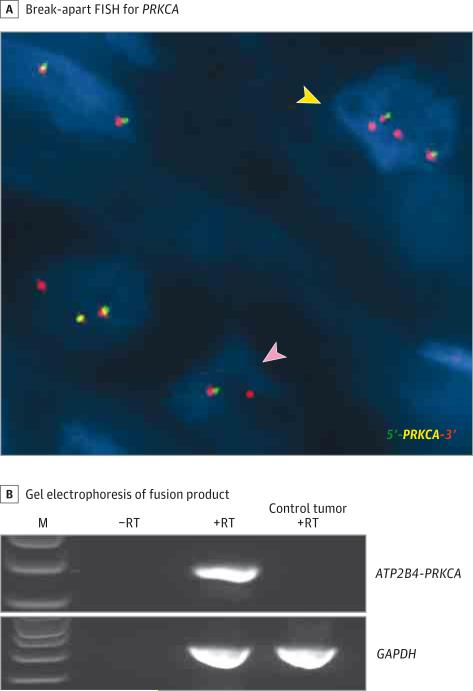
For reverse transcriptase–polymerase chain reaction (RT-PCR), total RNA was isolated from FFPE sections of the tumor and reverse-transcribed using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) as per manufacturer's protocol. The RT-PCR analysis was performed by using GoTaq Long PCR Master Mix (Promega) to amplify the ATP2B4-PRKCA fusion by using the following primers for 35 cycles: (forward: 5′-AGGAGATCACCAAGGATGCC-3′, reverse: 5′- TCCAAAACTCCCCTTTCCCA-3′). The RT-PCR products were successfully amplified and processed for Sanger sequencing. RT-PCR and direct sequencing detected the fusion product (Figure 3 and Figure 4B).
Figure 4. Identification of PRKCA Fusion by Fluorescence In Situ Hybridization (FISH) and Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR).
A, Break-apart FISH for PRKCA shows gene rearrangement with loss of the green signal (pink arrowhead), suggestive of an unbalanced translocation. A subset of cells (yellow arrowhead) shows 1 or 2 extra red signals. B, Gel electrophoresis of ATP2B4-PRKCA RT-PCR fusion product. Amplicon spanning the location of the fusion was observed in the tumor (245 base pairs), but not in the control reactions for which RT was omitted. The amplicon was also absent in a control tumor sample (brain) predicted to be negative for the fusion. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers were included as an internal positive control. The identity of the fusion was confirmed by amplicon sequencing. M indicates 1-kb ladder.
For fluorescence in situ hybridization (FISH), bacterial artificial chromosome clones (BACPAC Resources) were used to develop break-apart probes for PRKCA (CH17-384F08 and CH17-209D17) and ATP2B4 (RP11-813P3 and CH17-72A07). Dual-color FISH was performed as previously described.10 In addition, PRKCA FISH was performed on 8 atypical spitzoid melanocytic tumors, known from a previous analysis10 to be negative for the known recurrent kinase fusions in spitzoid neoplasms.8
Break-apart FISH showed PRKCA rearrangement with loss of one 5′ green signal, which suggested an unbalanced translocation. In addition, a subset of cells had 1 or 2 extra 3′ red signals (Figure 4A). Break-apart FISH for ATP2B4 was not successful even after multiple attempts because of background interference from heavy melanin pigmentation. FISH was negative for PRKCA rearrangement in the 8 kinase fusion-negative spitzoid tumors tested, suggesting that PRKCA fusions are not likely to occur frequently in spitzoid tumors.
To investigate whether the lesion was related to those EBNs described as being deficient for R1α (the protein product of PRKAR1A),9 we analyzed PRKAR1A expression in the patient sample. PRKAR1A was expressed at levels similar to those in control melanoma samples. Also, immunohistochemical analysis revealed that R1α expression was retained in tumor cells (see the Supplement).
Discussion
In this study, we show a novel PRKCA fusion with a potential pathogenic role in a pigment-producing melanocytic neoplasm. The fusion event likely leads to constitutive activation of the kinase domain of PRKCA by removing its autoinhibitory domain or by the promoter activity of a ubiquitously expressed gene, such as ATPase, Ca2+ transporting, plasma membrane 4 (ATP2B4).11 PRKCA, a serine-threonine kinase, is an isoform of protein kinase C that is implicated in various cellular functions such as proliferation, apoptosis, differentiation, cell transformation, and adhesion.12 Note that the conserved PRKCA kinase domain induces phosphorylation of RAF-1, which in turn activates the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) cascade, a key signaling pathway in melanocyte growth and survival.13 Furthermore, PRKCA fusions have been identified as putative pathogenic events in other tumors, such as papillary glioneuronal tumor and lung squamous cell carcinoma.14,15
In immunohistochemical studies, Zembowicz et al9 showed that most sporadic and Carney complex–associated EBNs lose expression of the PRKAR1A-encoded R1α protein. Tumors (including EBNs) in patients with the Carney complex commonly show loss of heterozygosity at the gene locus on chromosome 17q24.2, suggesting a double-hit model of tumorigenesis.7,9 In contrast, the exact molecular mechanism for the apparent loss of gene function for sporadic forms of EBN is unknown.9 Because aCGH analysis of the patient sample showed a loss at 17q11.1-24.2, which encompassed loci of both PRKCA and PRKAR1A, we further investigated the status of PRKAR1A and its protein in the patient's sample. PRKAR1A was expressed at normal levels, and immunohistochemical analysis revealed that its expression was retained in tumor cells. In addition, the mutational analysis showed no evidence of PRKAR1A mutations. Therefore, our case does not seem to be associated with the R1α (PRKAR1A)–deficient EBN type. Given the proximity of PRKAR1A and PRKCA, it might be useful to analyze PRKCA as potential candidate for families with the Carney complex who are negative for inactivating germline PRKAR1A mutations.
Conclusions
We show the presence of a PRKCA fusion in a congenital melanocytic neoplasm with indeterminate biological potential with prominent pigment synthesis. The frequency of this genetic alteration in heavily pigmented melanocytic neoplasms or other melanocytic tumors needs to be determined in future studies. The association of PRKCA fusion–positive melanocytic neoplasms with EBNs of both Carney complex–associated and sporadic types also remains to be determined.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported in part by the National Cancer Institute (NCI) of the National Institutes of Health under Award Number P30CA021765 and by ALSAC.
Role of the Funder/Sponsor: The NCI had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Bahrami and Barnhill had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bahrami, Barnhill.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bahrami, Lee, Rahvar, Zhang.
Critical revision of the manuscript for important intellectual content: Bahrami, Lee, Wu, Kerstetter, Rahvar, Li, Easton, Barnhill.
Statistical analysis: Lee, Zhang.
Obtained funding: Bahrami.
Administrative, technical, or material support: Rahvar, Li, Easton, Barnhill.
Study supervision: Bahrami, Zhang.
Additional Contributions: We thank the patient for granting permission to publish this information.
Previous Presentation: This study was presented at the 2015 American Society for Clinical Investigation and the Association of American Physicians Joint Meeting; April 24-26, 2015; Chicago, Illinois.
Conflict of Interest Disclosures: None reported.
Supplemental content at jamadermatology.com
REFERENCES
- 1.Antony FC, Sanclemente G, Shaikh H, Trelles AS, Calonje E. Pigment synthesizing melanoma (so-called animal type melanoma): a clinicopathological study of 14 cases of a poorly known distinctive variant of melanoma. Histopathology. 2006;48(6):754–762. doi: 10.1111/j.1365-2559.2006.02411.x. [DOI] [PubMed] [Google Scholar]
- 2.Zembowicz A, Carney JA, Mihm MC. Pigmented epithelioid melanocytoma: a low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am J Surg Pathol. 2004;28(1):31–40. doi: 10.1097/00000478-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Crowson AN, Magro CM, Mihm MC., Jr Malignant melanoma with prominent pigment synthesis: “animal type” melanoma: a clinical and histological study of six cases with a consideration of other melanocytic neoplasms with prominent pigment synthesis. Hum Pathol. 1999;30(5):543–550. doi: 10.1016/s0046-8177(99)90199-5. [DOI] [PubMed] [Google Scholar]
- 4.Ludgate MW, Fullen DR, Lee J, et al. Animal-type melanoma: a clinical and histopathological study of 22 cases from a single institution. Br J Dermatol. 2010;162(1):129–136. doi: 10.1111/j.1365-2133.2009.09271.x. [DOI] [PubMed] [Google Scholar]
- 5.Battistella M, Prochazkova-Carlotti M, Berrebi D, et al. Two congenital cases of pigmented epithelioid melanocytoma studied by fluorescent in situ hybridization for melanocytic tumors: case reports and review of these recent topics. Dermatology. 2010;221(2):97–106. doi: 10.1159/000314160. [DOI] [PubMed] [Google Scholar]
- 6.Richardson SK, Tannous ZS, Mihm MC., Jr Congenital and infantile melanoma: review of the literature and report of an uncommon variant, pigment-synthesizing melanoma. J Am Acad Dermatol. 2002;47(1):77–90. doi: 10.1067/mjd.2002.120602. [DOI] [PubMed] [Google Scholar]
- 7.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1):89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 8.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zembowicz A, Knoepp SM, Bei T, et al. Loss of expression of protein kinase a regulatory subunit 1alpha in pigmented epithelioid melanocytoma but not in melanoma or other melanocytic lesions. Am J Surg Pathol. 2007;31(11):1764–1775. doi: 10.1097/PAS.0b013e318057faa7. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Barnhill RL, Dummer R, et al. TERT promoter mutations are predictive of aggressive clinical behavior in patients with spitzoid melanocytic neoplasms. Sci Rep. 2015;5:11200. doi: 10.1038/srep11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81(1):21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima S. Protein kinase C alpha (PKC alpha): regulation and biological function. J Biochem. 2002;132(5):669–675. doi: 10.1093/oxfordjournals.jbchem.a003272. [DOI] [PubMed] [Google Scholar]
- 13.Kolch W, Heidecker G, Kochs G, et al. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 14.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridge JA, Liu XQ, Sumegi J, et al. Identification of a novel, recurrent SLC44A1-PRKCA fusion in papillary glioneuronal tumor. Brain Pathol. 2013;23(2):121–128. doi: 10.1111/j.1750-3639.2012.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.