Abstract
Montelukast and pranlukast are orally active leukotriene receptor antagonists selective for the CysLT1 receptor. Conversely, the hP2Y1,2,4,6,11,12,13,14 receptors represent a large family of GPCRs responding to either adenine or uracil nucleotides, or to sugar-nucleotides. Montelukast and pranlukast were found to inhibit nucleotide-induced calcium mobilization in a human monocyte-macrophage like cell line, DMSO-differentiated U937 (dU937). Montelukast and pranlukast inhibited the effects of UTP with IC50 values of 7.7 and 4.3 μM, respectively, and inhibited the effects of UDP with IC50 values of 4.5 and 1.6 μM, respectively, in an insurmountable manner. Furthermore, ligand binding studies using [3H]LTD4 excluded the possibility of orthosteric nucleotide binding to the CysLT1 receptor. dU937 cells were shown to express P2Y2, P2Y4, P2Y6, P2Y11, P2Y13 and P2Y14 receptors. Therefore, these antagonists were studied functionally in a heterologous expression system for the human P2Y receptors. In 1321N1 astrocytoma cells stably expressing human P2Y1,2,4,6 receptors, CysLT1 antagonists inhibited both the P2Y agonist-induced activation of phospholipase C and intracellular Ca2+ mobilization. IC50 values at P2Y1 and P2Y6 receptors were <1 μM. In control astrocytoma cells expressing an endogenous M3 muscarinic receptor, 10 μM montelukast had no effect on the carbachol-induced rise in intracellular Ca2+. These data demonstrated that CysLT1 receptor antagonists interact functionally with signaling pathways of P2Y receptors, and this should foster the study of possible implications for the clinical use of these compounds in asthma or in other inflammatory conditions.
Keywords: Montelukast, Pranlukast, Purine receptors, Nucleotides, ATP, UDP
1. Introduction
Cysteinyl leukotrienes (CysLTs), LTC4, LTD4 and LTE4, potent inflammatory mediators derived from arachidonic acid [1], are known to be very potent bronchoconstrictors and to play an important role in asthma and allergic rhinitis [2,3]. They have been also implicated in a number of inflammatory conditions including cardiovascular diseases, coronary artery disease, atherosclerosis and stroke [4–6] or in cardiovascular complications of inflammatory processes [7].
CysLTs trigger contractile and inflammatory processes through the specific interaction with cell surface receptors belonging to the rhodopsin family of the G protein-coupled receptor (GPCR) genes. Until now, two receptor subtypes have been cloned, namely CysLT1 and CysLT2 [8]. In particular, when the CysLT1 receptor is expressed in recombinant systems it shows a preferential coupling to Gq/11, whereas when constitutively expressed it has been reported to activate both pertussis toxin (PTX)-sensitive and -insensitive G-proteins [8,9]. This was demonstrated in dimethyl sulfoxide-differentiated U937 (dU937) cells, an immortalized cell line known to constitutively express a high density of CysLT1 receptors upon differentiation to monocytes/macrophages [10,11], CysLT1 receptors respond to LTD4 with a strong increase in cytosolic Ca2+ concentration ([Ca2+]i) partially sensitive to PTX, and with the activation of the Ras-MAPK cascade totally dependent upon Gi/o [12]. These signaling effects were totally inhibited by various specific CysLT1-receptor antagonists, and no CysLT2 receptor mRNA was detected [13,14], thus indicating that in monocyte-macrophage like U937 cells LTD4-induced responses can be totally ascribed to a CysLT1 receptor. The promonocytic leukemia cell U937 was selected because closely related to the inflammatory cells responsible of many CysLT biological actions, and because monocyte/macrophages activation leads to the release of a wide spectrum of cytokines and chemokines that have key roles in all inflammatory diseases.
CysLT receptors belong to the group A subfamily of GPCRs and, in particular, to a cluster of receptors phylogenetically related to the purine P2Y receptors [15]. In addition to a number of orphan receptors this cluster includes receptors that respond to purinergic or pyrimidinergic nucleotides (P2Y receptors, see also below), proteases (F2R) and chemoattractants (FPR) [16,17]. The CysLT receptor nomenclature was originally based on the sensitivity to the antagonists, which include montelukast, zafirlukast, pranlukast, pobilukast and MK571 [9]. Montelukast (Singulair®), a potent CysLT1 receptor antagonist belonging to the chemical class of quinolines [18], is an effective and well-tolerated preventative drug for asthma and allergic rhinitis in adults and children [19]. Pranlukast (Onon®, Azlaire®) belongs to the chemical class of benzopyrans [20] and is indicated for the prophylactic treatment of chronic bronchial asthma in pediatric and adult patients and is currently on the market only in Japan and South America [21]. CysLT1 receptor antagonists have proven effective in various models of induced asthma, as well as in the treatment of chronic asthma. Preclinical and clinical studies have demonstrated that they differ in terms of potency and pharmacokinetics, but have similar pharmacodynamic profiles [22–24].
Conversely, P2Y receptors are GPCRs responsive to both adenine (ATP, ADP) and uracil (UTP, UDP) nucleotides, or to sugar nucleotides (UDP-glucose and UDP-galactose) [25]. Eight P2Y receptor subtypes are currently recognized: the P2Y1,2,4,6,11,12,13,14 receptors (ibidem). These receptors are widely distributed in human tissues, and important patho-physiological roles in brain function, haemostasis, regulation of blood pressure, inflammation and respiratory functions have been suggested [26]. Based on structural, phylogenetic properties and signaling coupling, a subclassification of P2Y receptors into the A and B subclasses has been recently proposed. Receptors in the A subclass (P2Y1,2,4,6,11) are preferentially coupled to Gq or Gs proteins, thus mediating stimulation of phospholipase C and adenylyl cyclase. Receptors in the B subclass (P2Y12,13,14) are instead mainly linked through Gi activation to inhibition of adenylyl cyclase activity (ibidem).
There are several similarities between the CysLT and the P2Y receptor classes. For example, the BLT1 receptor was originally reported as a potential nucleotide receptor based on homology with P2Y receptors but was subsequently found to be insensitive to nucleotides [27]. In a study with chimeric CysLT1/P2Y2 receptors, the chimera essentially behave as the CysLT1 receptor and the activation was antagonized by a CysLT1 receptor antagonist [28]. Crosstalk between purine and leukotriene systems has recently been reported, although the CysLT1 receptor is not a P2Y nucleotide receptor [21]. P2Y1 and CysLT receptors mediate co-release of purines and CysLTs in primary cultures of rat microglia [29].
Here we found that these leukotriene antagonists also inhibit the effects of nucleotides acting at P2Y receptors in dU937 cells, which are known to express a number of nucleotide receptors, such as P2Y2 or P2Y4 [30]. In an effort to characterize the P2 receptor subtypes subject to negative modulation by CysLT1 receptor antagonists we studied the interactions of these compounds in human P2Y1, P2Y2, P2Y4, and P2Y6 receptors stably expressed in 1321N1 astrocytoma cells. The latter have been chosen since these cells are devoid of endogenous P2Y receptors and for this reason have been widely utilized for transfecting and characterizing various P2Y receptor subtypes [31,32].
2. Materials and methods
2.1. Materials
1321N1 astrocytoma cells stably transfected with human P2Y1,2,4,6 receptors were kindly provided by Dr. Robert Nicholas (University of North Carolina, Chapel Hill, NC, USA). myo-[3H]Inositol (20 Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO, USA). Dowex AG 1-X8 resin was purchased from Bio-Rad (Hercules, CA, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Life Technologies, Inc. (Rockville, MD, USA). Phosphate Buffer Saline (PBS), RPMI 1640, bovine serum albumin (BSA), EGTA, penicillin, streptomycin, l-glutamine, dimethyl sulfoxide (DMSO), probenecid, penicillamine, Hepes, UDP, UTP, ATP and hexokinase were from Sigma Chem. Co. (St. Louis, MO, USA). All salts for saline and Tris were from Merck (Darmstadt, Germany). 3H-LTD4 (164–173 Ci/mmol) and Ultima Gold were purchased from Perkin-Elmer Life Sciences (Boston, MA, USA) and LTD4 from Cayman Chemical Co. (Ann Arbor, MI, USA). Pranlukast and montelukast were a kind gift from Menarini (Menarini, Firenze, Italy). Fluo3/AM and pluronic F-127 were purchased from Molecular Probes (Eugene, OR, USA). TRIZOL® Reagent, Superscript II RNA H− Reverse Transcriptase and Platinum Taq DNA Polymerase were from Invitrogen (Milan, Italy). RQ1 RNase-free-DNase was purchased from Promega (Milan, Italy). Random hexamers were from Applied Biosystems (Milan, Italy). All other reagents were purchased from Sigma. Disposable culture flasks were from Corning Glassworks (Corning, NY, USA).
2.2. Cell culture and membrane preparation
1321N1 astrocytoma cells stably transfected with the human (h) P2Y1, P2Y2, P2Y4, or P2Y6 receptor were grown at 37 °C in a humidified incubator with 5% CO2/95% air in Dulbecco's modified Eagle's medium (JRH Biosciences, Inc., Lenexa, KS, USA) and F12 (1:1) supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine. The cells were grown to ~60% confluence for the experiments. For membrane preparation, human astrocytoma cells expressing the human P2Y1 receptors were grown to approximately 80% confluence and then harvested. The cells were homogenized and suspended and centrifuged at 100 × g for 5 min at room temperature. The pellet was resuspended in 50 mM tris(hydroxymethyl) aminomethane (Tris)HCl buffer (pH 7.4). The suspension was homogenized with a polytron homogenizer (Brinkmann) for 10 s and was then recentrifuged at 20,000 × g for 20 min at 4 °C. The resultant pellets were resuspended in Tris buffer (pH 7.4), and the suspension was stored at −80 °C until the binding experiments. The protein concentration was measured with the Bradford assay [33]. Human promonocytic U937 cells (ATCC, Manassas, VA, USA) were routinely cultured in suspension in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C (5% CO2) and differentiated for 96 h with 1.3% DMSO.
2.3. Total RNA isolation and RT-PCR analysis
Cells were collected by centrifugation. Total RNA was extracted from the cell pellet using the TRIZOL® Reagent according to manufacturer's instructions. PCR analysis was performed as previously described [34]. Briefly, after treatment of total RNA with RQ1 RNase-free-DNase, 1 μg of RNA was reverse-transcribed with Superscript II RNA H− Reverse Transcriptase (200 U/sample) in the presence of 100 pmol of random hexamers. Aliquots (15% of the reverse-transcribed cDNA product) were amplified in each PCR assay with Platinum Taq DNA Polymerase (1.25 U/sample) in a 25 μl reaction mixture containing 20 pmol of 5′ and 3′ primers in a standard PCR buffer (50 mM KCl, 1.5 mM MgCl2, 20 mM Tris–HCl, pH 8.4). For cDNA, control samples, which were not subjected to reverse transcription, were processed in parallel with the same experimental protocol to check for contamination of RNA with genomic DNA.
Amplifications were performed in a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) for 40 cycles (typically 95 °C/45 s, 30 s at the annealing temperature ranging from 51 to 60 °C, depending on the specific receptor subtype; 72 °C/45 s) after an initially denaturation at 95 °C for 2 min. The following forward (Fw) and Reverse (Rw) oligonucleotide primers were used (size of PCR product):
| P2Y1 | Fw: 5′-CCTGCGAAGTTATTTCATCTA-3′; Rw: 5′-GTTGAGACTTGCTAGACCTCT-3′ |
| P2Y2 | Fw: 5′-GCAGCATCCTCTTCCTCACCT-3′; Rw: 5′-CATGTTGATGGCGTTGAGGGT-3′ |
| P2Y4 | Fw: 5′-GGCATTGTCAGACACCTTGTA-3′; Rw: 5′-AAGGCACGAAGCAGACAGCAA-3′ |
| P2Y6 | Fw: 5′-CGCTTCCTCTTCTATGCCAA-3′; Rw: 5′-GTAGGCTGTCTTGGTGATGTG-3′ |
| P2Y11 | Fw: 5′-ACTTCCTGTGGCCCATACTG-3′: Rw: 5′-GCTGTCCCCAGACACTTGAT-3′ |
| P2Y12 | Fw: 5′-CCCTCCAGAATCAACAGTTAT-3′; Rw: 5′-CGCTTTGCTTTAACGAGTTC-3′ |
| P2Y13 | Fw: 5′-TGTGTCGTTTTTCTTCGGTG-3′; Rw: 5′-TGCTGCCAAAAAGAGAGTTG-3′ |
| P2Y14 | Fw: 5′-CGCAACATATTCAGCATCGTGT-3′; Rw: 5′-GCTGTAATGAGCTTCGGTCTGAC-3′ |
2.4. Determination of inositol phosphates
The quantity of inositol phosphates was measured by a modification of the method of Gao et al. [35]. Agonists and antagonists were dissolved as stock solutions in PBS buffer (pH 7.4) and stored at −20 °C. The hP2Y1,2,4,6-1321N1 cells were grown to confluence in six-well plates in the presence of myo-[3H]inositol (2 μCi/ml) for 24 h. After an additional incubation of 20 min with 20 mM LiCl at room temperature, cells were treated with agonist or antagonist. Agonists used were as follows: hP2Y1, 2-MeSADP; hP2Y2, UTP; hP2Y4, UTP; hP2Y6, UDP. The reaction was terminated upon aspiration of the medium and addition of cold formic acid (20 mM). After 30 min, supernatants were neutralized with NH4OH, and applied to Bio-Rad Dowex AG 1-X8 anion exchange columns. All of the columns were washed with water followed by a 60 mM sodium formate solution containing 5 mM sodium tetraborate. Total inositol phosphates were eluted with 1 M ammonium formate containing 0.1 M formic acid, and radioactivity was measured using a liquid scintillation counter.
2.5. Radioligand binding assays
Equilibrium binding studies in intact dU937 cells were performed as previously described [36]. Briefly, cells were incubated at 25 °C for 60 minutes using 0.3 nM 3H-LTD4 and unlabeled compounds at the indicated concentrations. dU937 cells ((5–10) × 106 cells/sample), saline solution pH 7.4, 20 mM CaCl2 and 20 mM penicillamine were added to the incubation mixture to achieve a final volume of 500 μl. Unbound ligand was separated by centrifugation; the pellet was washed once with 1 ml of saline solution at 4 °C and dissolved in 1N NaOH and radioactivity measured in a liquid scintillation counter.
P2Y1 receptor binding experiments were performed as previously described [35,37]. Briefly, membranes (40 μg protein) from astrocytoma cells stably expressing human P2Y1 receptors were incubated with [3H]MRS2279 (8 nM) for 30 min at 4 °C in a total assay volume of 200 μl. The radiolabeled ligand concentration used in all assays approximated the Kd value of 8 nM at the P2Y1 receptor. Binding reactions were terminated by filtration through Whatman GF/B glass–fiber filters under reduced pressure with a MT-24 cell harvester (Brandel, Gaithersburg, MD, USA), and radioactivity was determined with a 1414 liquid scintillation counter (Wallac, Win Spectral, Perkin-Elmer Life Sciences, Downers Grove, IL, USA). Data were expressed as mean ± S.E.
2.6. Calcium mobilization assay
Determination of cytosolic free Ca2+ levels ([Ca2+]i) in dU937 cells was performed as previously described [36]. Briefly, dU937 cells were incubated for 30 min at 30 °C in the dark with 2 μM Fluo3/AM. After loading, Fluo3/AM was removed and cells were further incubated for 30 min at 30 °C to complete the hydrolysis of the fluorescent indicator. When indicated hexokinase was added to UDP stock solution (50 Units/ml) and cells (1 Unit/ml) accordingly to Kumari et al. [38]. Cells were then centrifuged, diluted to the concentration of 106 cells/ml, transferred to the spectrofluorimeter (Perkin-Elmer LS50) and fluorescence was monitored at 37 °C (506 nm excitation, 530 nm emission). Calibration was performed by adding 2 μM ionomycin and 100 μM digitonin (Fmax) and by adding 5 mM EGTA and 60 mM Tris-base (Fmin). [Ca2+]i was calculated according to Tsien et al. [39] with a Kd = 864 [40]. [Ca2+]i elevation was expressed as stimulated over basal (S/B).
Human astrocytoma cells stably expressing human P2Y receptors were cultured in Dulbecco's modified Eagle's medium (DMEM, JRH Biosciences, Inc., Lenexa, KS, USA) and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units penicillin/ml, 100 μg streptomycin/ml, 2 μmol glutamine/ml, and 500 μg geneticin/ml. For the assay mobilization, cells were grown overnight in 100 μl of media in 96 well flat bottom plates at 37 °C at 5% CO2 or until approx. 60–80% confluence. The calcium assay kit (Molecular Devices, Sunnyvale, CA, USA) was used as directed with no washing of cells, and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were loaded upon addition of 50 μl of dye containing probenecid to each well and incubated for 45 min at room temperature prior to addition of agonist. The compound plate was prepared using dilutions of various compounds in Hanks Buffer with 20 mM HEPES, pH 7.2. For antagonist studies. The antagonist was added 20 mM prior to the addition of agonist to the sample plate. Samples were performed in duplicate using a Molecular Devices FlexStation I at room temperature. Cell fluorescence (excitation = 485 nm, emission = 525 nm) was monitored following exposure to compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.7. Statistical analysis
Concentration response-curves were analyzed and parameters calculated using GraphPad Prism software (GraphPad, San Diego, CA, USA). Statistical comparison among different parameters has been performed with GraphPad software utilizing the extra sum of square principle. Data were expressed as mean ± S.E.M.
3. Results
3.1. P2Y receptor expression profile in dU937 cells
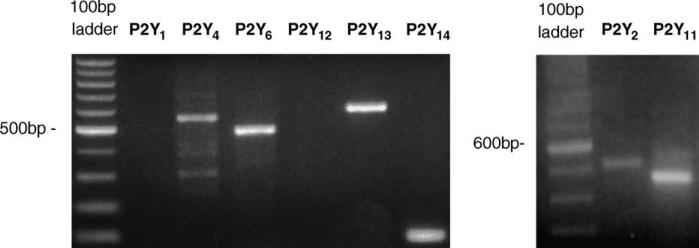
The expression of P2Y receptors in this differentiated macrophage cell line was demonstrated. RT-PCR analysis performed in dU937 cells with primers specifically designed for the various cloned P2Y receptors (see Section 2) revealed bands corresponding to the expected amplification products of P2Y2 (502 bp), P2Y4 (551 bp), P2Y6 (480 bp), P2Y11 (499 bp), P2Y13 (578 bp) and P2Y14 (102 bp) receptors (Fig. 1). These primers did not generate additional bands. No signal was detected in parallel RNA samples that did not undergo retro-transcription demonstrating that the amplified product was not due to contamination of RNA with genomic DNA (data not shown). No amplification products were observed with primers specific for P2Y1 (318 bp) and P2Y12 (1127 bp) receptors, suggesting that these receptors are not expressed in dU937 cells. In a positive control experiment using the same PCR primers for these two P2Y subtypes, amplified products with the expected MW were obtained using cDNA from human brain (data not shown).
Fig. 1.
Expression of P2Y2,4,6,11,13,14 in dU937 cells. Total RNA was subjected to RT and the resulting cDNA was amplified with primers specifically designed for human P2Y1,2,4,6,11,12,13,14 receptors. PCR products were separated on 2% ethidium bromide-stained agarose gel and visualized under UV light. Identification of the PCR products was made on the basis of their predicted sizes (see Section 3).
3.2. Characterization of nucleotide-mediated [Ca2+]i transients in dU937 cells
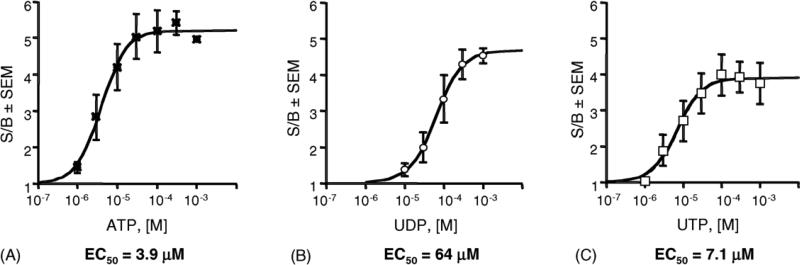
Consistent with the presence of P2Y receptors in the dU937 cells, extracellular nucleotides were found to elicit functional effects at micromolar concentrations. Fig. 2A–C shows that the indicated nucleotides were able to trigger a concentration-dependent increase in [Ca2+]i with an EC50 value of 3.9 ± 0.4 μM reaching a maximum at approximately 100 μM for ATP, EC50 = 64 ± 5 μM, reaching a maximum at approximately 1 mM for UDP and EC50 = 7.1 ± 1.2 μM, reaching a maximum at approximately 100 μM for UTP. The maximal response was 5.2-fold stimulated over basal for ATP, 4.7-fold for UDP and 3.9-fold for UTP. Furthermore, treatment with hexokinase of both UDP stock solution (50 U/ml) and dU937 cells (1 U/ml), to exclude UTP contamination or extracellular conversion of UDP, did not have any effect on UDP potency.
Fig. 2.
Concentration–response curves of the [Ca2+]i transient induced by nucleotides in dU937 cells. Fluo3/AM loaded cells were challenged with increasing concentrations of ATP (A), UDP (B), and UTP (C). When hexokinase was present in the UDP experiment, the response curve was unchanged (EC50 69 μM), indicating that this effect was not the result of contamination by UTP. Results are expressed as the ratio of stimulated over basal (S/B) ± S.E.M. and represent the mean from three independent experiments analyzed simultaneously.
3.3. Effect of montelukast and pranlukast on nucleotide-mediated [Ca2+]i transients in dU937 cells
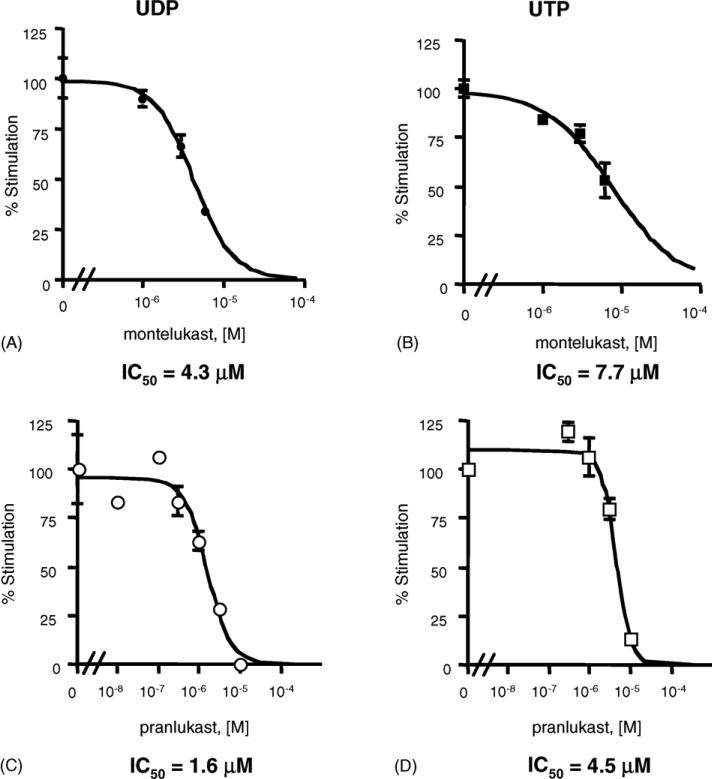
The functional effects of exposure of the dU937 cells to extracellular nucleotides were antagonized by CysLT1 receptor antagonists. Fig. 3 shows that 5 min pretreatment with montelukast or pranlukast were found to inhibit [Ca2+]i transients elicited by UDP and UTP with IC50 values of 4.3 ± 0.3 and 7.7 ± 2.7 μM, respectively, for montelukast (Fig. 3A and B), and with IC50 values of 1.6 ± 0.4 and 4.5 ± 0.8 μM respectively, for pranlukast (Fig. 3C and D).
Fig. 3.
Effect of pranlukast and montelukast on nucleotide-induced [Ca2+]i transients in dU937 cells. Fluo3/AM loaded cells were challenged with 300 μM UDP (A and C) and 30 μM UTP (B and D) in the presence of increasing concentrations of montelukast and pranlukast (5 min pretreatment). Results are expressed as the ratio of stimulated over basal (S/B) ± S.E.M. and represent the mean from at least three independent experiments analyzed simultaneously.
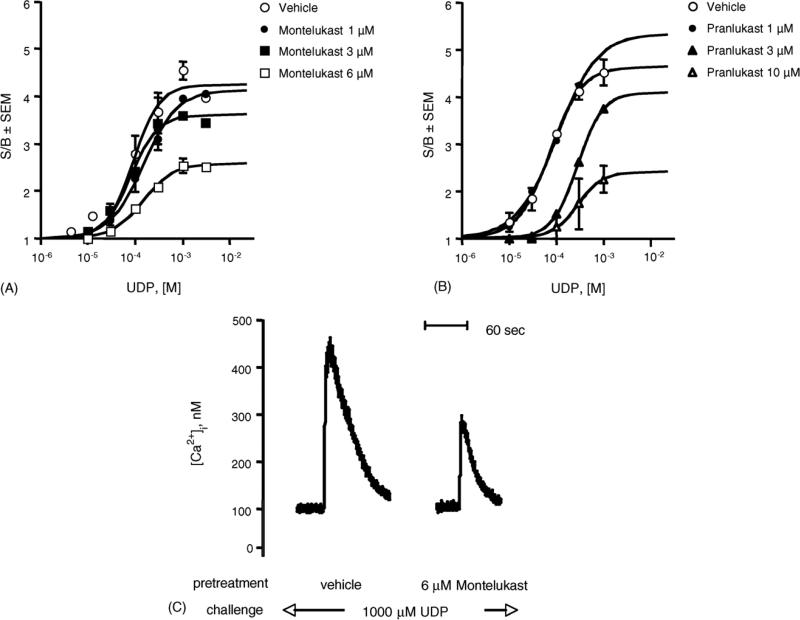
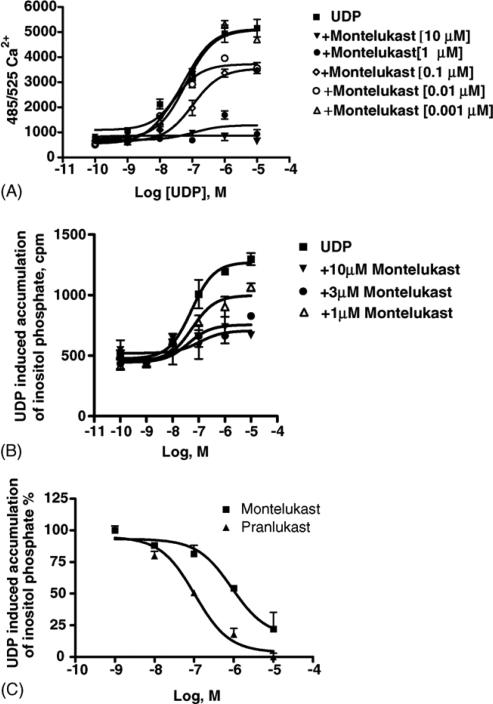
Furthermore, both montelukast and pranlukast have been found to affect the maximal efficacy of UDP-induced [Ca2+]i transients, thus lowering the upper plateau of the concentration–response curves (Fig. 4A and B, p < 0.05), clearly deviating from apparent simple competition, i.e. competitive surmountable antagonism. In addition, pranlukast also affected the apparent UDP potency (Fig. 4B, p < 0.01). These data, therefore, are consistent either with a competitive insurmountable antagonism, characteristic of irreversible compounds, or with a noncompetitive antagonism, characteristic of allosteric compounds, for both antagonists [41]. Similar results were obtained with UTP-induced [Ca2+]i transients (data not shown). In addition, Fig. 4C shows a typical trace of UDP-induced [Ca2+]i before and after treatment with 6 μM montelukast. Treatment with montelukast alone did not modify [Ca2+]i (data not shown).
Fig. 4.
Effect of pranlukast and montelukast on the concentration–response curve for the [Ca2+]i transients induced by UDP in dU937 cells. Fluo3/AM loaded cells were challenged with increasing concentrations of UDP in the absence and presence of the indicated concentrations of montelukast (A) and pranlukast (B) (5 min pretreatment). Results are expressed as the ratio of stimulated over basal (S/B) ± S.E.M. and represent the mean from two independent experiments analyzed simultaneously. (C) Typical trace of UDP-induced [Ca2+]i transients before and after treatment with 6 μM montelukast.
3.4. Effect of nucleotides on [3H]LTD4 binding to the CysLT1receptor
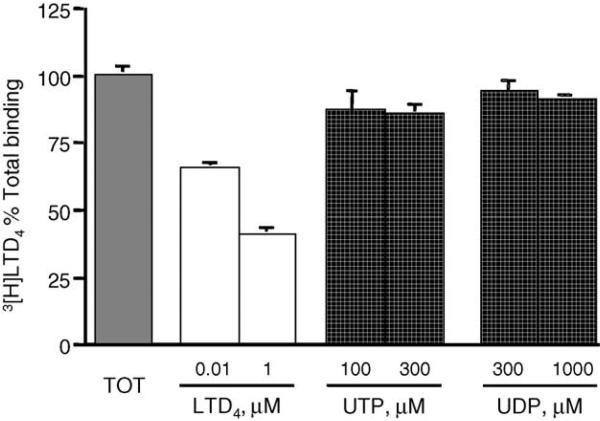
In order to establish whether or not UTP and UDP were indeed able to compete for binding at the LTD4 orthosteric binding site, we performed equilibrium binding studies in intact dU937 cells using [3H]LTD4 as a labeled ligand (Fig. 5). As expected, binding was significantly inhibited by LTD4 in a concentration-dependent manner, but neither nucleotide was able to compete with [3H]LTD4 binding sites.
Fig. 5.
Equilibrium binding of [3H]LTD4 in intact dU937 cells. Data are presented as percent total [3H]LTD4 binding displaced by the indicated concentrations of unlabeled LTD4, UTP or UDP. Data shown are mean ± S.E.M. from two independent experiments performed in triplicates.
3.5. Effect of montelukast and pranlukast on signaling and receptor binding in astrocytoma cells
Inhibition of nucleotide-induced production of inositol phosphates and calcium transients by montelukast and pranlukast was studied in 1321N1 human astrocytes expressing P2Y1,2,4,6 receptors. Effects on signaling at P2Y11, P2Y13 and P2Y14 receptors, although present in the dU937 cells, were not investigated. P2Y1 receptors were included, although they are not expressed in the dU937 cells, because this is one of two P2Y receptor subtypes at which radioligand binding characterization is feasible.
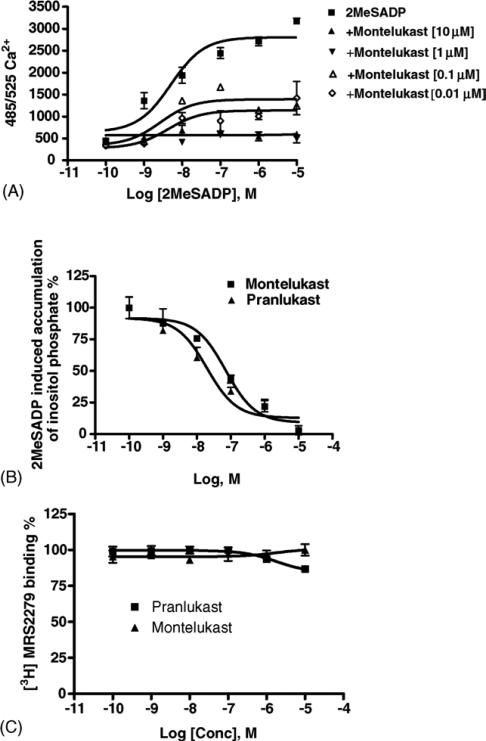
Fig. 6A indicated that montelukast affected the concentration–response curves for intracellular Ca2+ changes induced by 2-MeSADP acting at the P2Y1 receptors expressed in 1321N1 astrocytoma cells. In agreement with data obtained in dU937 cells, montelukast does not behave as a competitive, surmountable antagonist. Full concentration–response curves for the inhibition of the effects of 2-MeSADP at P2Y1 receptors were measured for montelukast and pranlukast (Fig. 6B). Results indicated that the production of inositol phosphates in human P2Y1 receptor-expressing 1321N1 astrocytoma cells in response to 30 nM 2-MeSADP was inhibited in a concentration-dependent manner with IC50 values of 0.122 ± 0.037 and 0.028 ± 0.013 μM, for montelukast and pranlukast, respectively.
Fig. 6.
Functional effects of leukotriene antagonists on 2-MeSADP-induced signaling in stably transfected astrocytoma cells. (A) Effect of montelukast on concentration–response curves for intracellular Ca2+ changes induced by 2-MeSADP acting at P2Y1 receptors expressed in 1321N1 astrocytoma cells. The cells were pre-treated for 20 min at room temperature with antagonist before application of the agonist 2-MeSADP. The fluorescence emission at 525 nm with excitation at 485 nm is proportional to [Ca2+]i. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure. (B) Concentration-dependence of inhibition by montelukast and pranlukast of inositol phosphate production induced by 30 nM 2-MeSADP in human P2Y1 transfected 1321N1 human astrocytoma cells. (C) Effect of montelukast and pranlukast on the binding of [3H]MRS2279 to membranes from astrocytoma cells stably expressing the human P2Y1 receptor. Data shown are mean ± S.E.M. from three independent experiments performed in duplicate.
Since the leukotriene antagonists displayed functional antagonism of P2Y1 receptor-elicited effects, we examined the ability to interfere with radioligand binding at that subtype. The specific P2Y1 receptor antagonist [3H]MRS2279 was used as a high affinity radioligand in binding to membranes from astrocytoma cells stably expressing human P2Y1 receptors [37]. This binding of this radioligand is competitive with other nucleotide ligands of the P2Y1 receptor, both agonists and antagonists. Specific binding of [3H]MRS2279 was defined in these experiments using the antagonist MRS2179 (10 μM). Essentially no inhibition of binding of [3H]MRS2279 was detected in the presence of 10 μM montelukast and pranlukast (Fig. 6C), both of which antagonized the P2Y1 receptor-mediated effects of 2-MeSADP. Binding was displaced by the P2Y1 antagonist MRS2179, as expected (not shown).
CysLT receptors have been detected in astrocytes [45], and PCR analysis of 1321N1 astrocytoma cells indicated a low level of expression of both CysLT1 and CysLT2 receptors (data not shown). Nevertheless, attempts to detect [3H]LTD4 binding in membranes from 1321N1 astrocytoma cells stably expressing human P2Y1 receptors indicated only non-specific binding levels (data not shown).
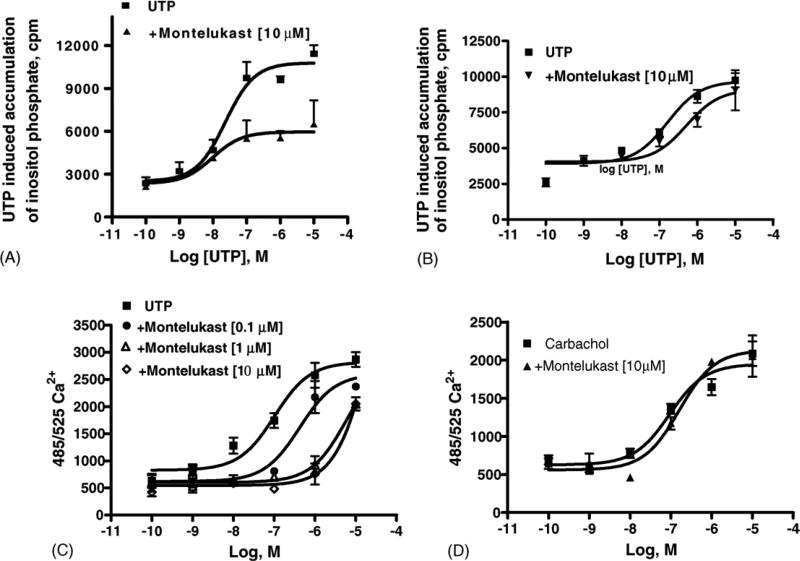
Montelukast inhibited the functional effects of UDP acting as agonist at hP2Y6 receptors (Fig. 7). In good agreement with data obtained in dU937 cells, once again montelukast behaved as an insurmountable antagonist. The production of inositol phosphates in human P2Y6 receptor-expressing 1321N1 astrocytoma cells in response to 300 nM UDP was inhibited in a concentration-dependent manner with IC50 values of 0.859 ± 0.053 and 0.150 ± 0.065 μM, for montelukast and pranlukast, respectively. Less pronounced effects of the CysLT1 antagonists were seen on the functional responses to UTP acting at P2Y2 and P2Y4 receptors. Montelukast (10 μM) also blocked the human P2Y2 receptor signaling through PLC induced by the agonist UTP (Fig. 8A), while pranlukast (10 μM) had no significant effects on UTP-induced human P2Y2 receptor activation (data not shown). Montelukast (10 μM) also had a slight effect on the human P2Y4 receptor signaling (activation of PLC, Fig. 8B), which was more evident in the assay of [Ca2+]i mobilization (Fig. 8C). Control 1321N1 astrocytoma cells did not have a significant response of either PLC or [Ca2+]i mobilization when exposed to ADP, ATP, UDP, or UTP (data not shown). Control experiments using carbachol to stimulate [Ca2+]i mobilization via an endogenous m3 muscarinic acetylcholine receptor in the control astrocytoma cells demonstrated a lack of significant inhibition by montelukast (Fig. 8D).
Fig. 7.
Functional effects of leukotriene antagonists on UDP-induced signaling in stably transfected P2Y6 receptor-expressing astrocytoma cells. (A) Effect of montelukast on concentration–response curves for intracellular Ca2+ changes induced by UDP acting at P2Y6 receptors expressed in 1321N1 astrocytoma cells. The cells were pre-treated for 20 min at room temperature with antagonist before application of the agonist UDP. The fluorescence emission at 525 nm with excitation at 485 nm is proportional to [Ca2+]i. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure. Data shown are mean ± S.E.M. from three independent experiments performed in duplicate. (B) Inositol phosphate production in human P2Y6 transfected 1321N1 human astrocytes. After labeling with myo-[3H]inositol (1 μCi/106 cells) for 24 h, the cells were treated for 20 min at 37 °C with montelukast (10, 3 and 1 μM concentration) in the presence of LiCl, followed by addition of the agonist, UDP, for another 30 min. The quantity of inositol phosphates was analyzed after the extraction though Dowex AG 1-X8 columns (see Section 2). Data shown are mean ± S.E.M. from two independent experiments performed in duplicate. (C) Concentration dependence of inhibition by montelukast and pranlukast of inositol phosphate production induced by 300 nM UDP in human P2Y6 transfected 1321N1 human astrocytes. Data shown are mean ± S.E.M. from three independent experiments performed in duplicate.
Fig. 8.
Functional effects of leukotriene antagonists on signaling in astrocytoma cells (either in control cells or in cells stably expressing P2Y receptors). (A) Inositol phosphate production in human P2Y2-receptor transfected 1321N1 human astrocytes. After labeling with myo-[3H]inositol (1 μCi/106 cells) for 24 h, the cells were treated for 30 min at 37 °C with montelukast (10 μM concentration) in the presence of LiCl, followed by addition of the agonist, UTP, for another 30 min. The quantity of inositol phosphates was analyzed after the extraction though Dowex AG 1-X8 columns (see Section 2). Data shown are mean ± S.E.M. from three independent experiments performed in duplicate. (B) Inositol phosphate production induced by UTP in human P2Y4-receptor transfected 1321N1 human astrocytes. (C) Effect of montelukast on concentration–response curves for intracellular Ca2+ changes induced by UTP acting at P2Y4 receptors stably expressed in 1321N1 astrocytoma cells. The fluorescence emission at 525 nm with excitation at 485 nm is proportional to [Ca2+]i. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure. (D) Lack of effect of montelukast on concentration–response curves for intracellular Ca2+ changes induced by carbachol acting at endogenous M3 muscarinic acetylcholine receptors in control 1321N1 astrocytoma cells. The cells were pre-treated for 20 min at room temperature with antagonist before application of the agonist carbachol. Data shown are mean ± S.E.M. from two independent experiments performed in triplicate.
4. Discussion
A relationship between UDP and the CysLT1 receptor has already been suggested, with the hypothesis that UDP may act directly on the receptor [42,43].
Nevertheless, the concentrations of CysLT1 antagonists required to functionally antagonize the effects of UDP in this study are not comparable to the nM concentrations at which the compounds act at the CysLT1 receptor. Although it does not establish the molecular basis for interaction, this study expands the range of possible interactions to other uracil and adenine nucleotides.
In this study, montelukast and pranlukast were found to inhibit nucleotide-induced calcium mobilization in a human monocyte-macrophage like cell line, DMSO-differentiated U937 (dU937) in an insurmountable manner. dU937 cells were shown to express P2Y2, P2Y4, P2Y6, P2Y11, P2Y13 and P2Y14 receptors. Therefore, these antagonists were studied functionally in a heterologous expression system for the human P2Y receptors. In 1321N1 astrocytoma cells stably expressing hP2Y1,2,4,6 receptors, CysLT1 antagonists inhibited both the P2Y agonist-induced activation of phospholipase C and intracellular Ca2+ mobilization. The inhibition was concentration-dependent but in an insurmountable manner. In control astrocytoma cells expressing an endogenous M3 muscarinic receptor, 10 μM montelukast had no effect on the carbachol-induced rise in intracellular Ca2+. Thus, these data demonstrated that CysLT1 receptor antagonists interact functionally with signaling pathways of P2Y receptors, and independent of another PLC-coupled receptor.
Furthermore, ligand binding studies using [3H]LTD4 excluded the possibility of competitive nucleotide binding to the CysLT1 receptor in dU937 cells, but not eliminating the possibility of an allosteric interaction at the CysLT1 receptor level. Another possible explanation for these effects is that the leukotriene antagonists are interacting directly with the P2Y receptors, to which they are related in the phylogenetic dendrogram of GPCRs [15]. However, in the P2Y1 receptor-expressing astrocytoma cells the binding of a specific antagonist (nucleotide) radioligand of the P2Y1 receptor, [3H]MRS2279, was not displaced by the leukotriene antagonists. Since MRS2279 is a competitive ligand, if the interaction of the leukotriene antagonist is directly with this receptor, it would necessarily be through an allosteric interaction as already suggested by data obtained in dU937 cells (see Figs. 4 and 5). Other examples of allosteric modulation of P2Y receptors have been reported [35]. Another possibility is that the antagonism occurs at a yet unidentified signaling step subsequent to activation of P2Y, but not M3 muscarinic, receptors. Thus, the functional inhibition of the nucleotide-elicited effects was either through direct allosteric interaction with the receptor or through a signaling pathway common to the P2Y receptors but not characteristic of cholinergic signaling.
Furthermore, we have recently shown that activation of P2Y receptors with extracellular nucleotides induced heterologous desensitization of the CysLT1 receptor in dU937 cells, but not receptor trafficking or internalization. Conversely, LTD4-induced CysLT1 receptor activation had no effect on P2Y receptor responses, suggesting that the latter have a hierarchy in producing desensitizing signals [14]. Thus, while a putative heterodimerization between CysLT1 and P2Y receptors might as well explain our results, it is unlikely to occur in our system, considering the lack of cross-desensitization, the different mechanism of agonist- or nucleotides-induced CysLT1 desensitization, and the different trafficking induced by LTD4 or by extracellular nucleotides signals [14].
In conclusion, our data demonstrated that montelukast and pranlukast non-competitively blocked P2Y signaling in several cell systems, but in a relatively nonsubtype-specific manner. The functional antagonism was especially evident at heterologously expressed P2Y1 and P2Y6 receptors, at which IC50 values for inhibition of calcium mobilization were <1 μM. Since the inhibition of P2Y1 receptor-elicited effects occurred without affecting specific nucleotide binding, if there is a direct interaction, it may be allosteric. The implications of these data for the clinical use of leukotriene antagonists is yet to be explored, with the question if some of their effects at therapeutic doses [44] may be related to their ability to inhibit the signaling pathways of P2Y receptors for extracellular nucleotides. These observations should foster the study of possible implications for the clinical use of these compounds in asthma or in other inflammatory conditions.
Acknowledgements
The present work was partially supported by the Italian Ministry of Education and Research (Program of national interest on: Extracellular nucleotides as signaling and differentiating molecules of human immune and nervous system cells), by a FIRB project on “The multiple roles of extracellular ATP in physiological and pathological processes” to MPA and by a CARIPLO Foundation grant 2004 to GER and MPA. LM is grateful for financial support from Gilead Sciences, Foster City, CA. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- IP3
inositol trisphosphate
- CysLT
cysteinyl leukotriene
- GPCR
G protein-coupled receptor
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2279
(1′R,2′S,4′S,5′S)-4-(2-chloro-6-methylamino-purin-9-yl)-1-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane
- PLC
phospholipase C
REFERENCES
- 1.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–75. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 2.Dahlen SE, Hedqvist P, Hammarstrom S, Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–6. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- 3.Nicosia S, Capra V, Rovati GE. Leukotrienes as mediators of asthma. Pulm Pharmacol Ther. 2001;14:3–19. doi: 10.1006/pupt.2000.0262. [DOI] [PubMed] [Google Scholar]
- 4.Porreca E, Di Febbo C, Di Sciullo A, Angelucci D, Nasuti M, Vitullo P, et al. Cysteinyl leukotriene D4 induced vascular smooth muscle cell proliferation: a possible role in myointimal hyperplasia. Thromb Haemost. 1996;76:99–104. [PubMed] [Google Scholar]
- 5.Lotzer K, Spanbroek R, Hildner M, Urbach A, Heller R, Bretschneider E, et al. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arterioscl Throm Vas Biol. 2003;23:E32–6. doi: 10.1161/01.ATV.0000082690.23131.CB. [DOI] [PubMed] [Google Scholar]
- 6.Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol. 2001;133:1323–9. doi: 10.1038/sj.bjp.0704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folco G, Rossoni G, Buccellati C, Berti F, Maclouf J, Sala A. Leukotrienes in cardiovascular diseases. Am J Respir Crit Care Med. 2000;161:S112–6. doi: 10.1164/ajrccm.161.supplement_1.ltta-22. [DOI] [PubMed] [Google Scholar]
- 8.Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for Leukotriene and Lipoxin Receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Capra V. Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharm Res. 2004;50:1–11. doi: 10.1016/j.phrs.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Frey EA, Nicholson DW, Metters KM. Characterization of the leukotriene D4 receptor in dimethylsulphoxide-differentiated U937 cells: comparison with the leukotriene D4 receptor in human lung and guinea-pig lung. Eur J Pharmacol. 1993;244:239–50. doi: 10.1016/0922-4106(93)90149-4. [DOI] [PubMed] [Google Scholar]
- 11.Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol. 1999;56:657–63. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- 12.Capra V, Ravasi S, Accomazzo MR, Parenti M, Rovati GE. CysLT1 signal transduction in differentiated U937 cells involves the activation of the small GTP-binding protein Ras. Biochem Pharmacol. 2004;67:1569–77. doi: 10.1016/j.bcp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Nothacker H-P, Wang Z, Zhu Y, Reinscheid RK, Lin SHS, Civelli O. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol Pharmacol. 2000;58:1601–8. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- 14.Capra V, Ravasi S, Citro S, Accomazzo MR, Grimoldi M, Abbracchio MP, et al. CysLT1 Receptor is a target for extracellular nucleotides-induced heterologous desensitization: a possible feed-back mechanism in inflammation. J Cell Sci. doi: 10.1242/jcs.02668. in press. [DOI] [PubMed] [Google Scholar]
- 15.Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: Structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 17.Kroeze WK, Sheffler DJ, Roth BL. G protein-coupled receptors at a glance. J Cell Sci. 2003;116:4867–9. doi: 10.1242/jcs.00902. [DOI] [PubMed] [Google Scholar]
- 18.Jones TR, Zamboni R, Belley M, Champion E, Charette L, Ford-Hutchinson AW, et al. Pharmacology of L-660,711 (MK-571): a novel potent and selective leukotriene D4 receptor antagonist. Can J Physiol Pharmacol. 1989;67:17–28. doi: 10.1139/y89-004. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis B, Markham A. Montelukast: a review of its therapeutic potential in persistent asthma. Drugs. 2000;59:891–928. doi: 10.2165/00003495-200059040-00015. [DOI] [PubMed] [Google Scholar]
- 20.Obata T, Katsube N, Miyamoto T, Toda M, Okegawa T, Nakai H, et al. New antagonists of leukotrienes: ONO-RS-411 and ONO-RS-347. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:229–31. [PubMed] [Google Scholar]
- 21.Keam SJ, Lyseng-Williamson KA, Goa KL. Pranlukast: a review of its use in the management of asthma. Drugs. 2003;63:991–1019. doi: 10.2165/00003495-200363100-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dahlen SE. Lipid mediator pathways in the lung: leukotrienes as a new target for the treatment of asthma. Clin Exp Aller. 1998;28(Suppl 5):141–6. doi: 10.1046/j.1365-2222.1998.028s5141.x. [discussion 171–3] [DOI] [PubMed] [Google Scholar]
- 23.Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 24.Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–46. doi: 10.1378/chest.119.5.1533. [DOI] [PubMed] [Google Scholar]
- 25.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–5. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology. Update and subclassification of the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2005 doi: 10.1124/pr.58.3.3. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold CL, Li Q, Schachter JB, Harden TK, Nicholas RA. Lack of nucleotide-promoted second messenger signaling responses in 1321N1 cells expressing the proposed P2Y receptor, p2y7. Biochem Biophys Res Commun. 1997;235:717–21. doi: 10.1006/bbrc.1997.6884. [DOI] [PubMed] [Google Scholar]
- 28.Gearing KL, Barnes A, Barnett J, Brown A, Cousens D, Dowell S, et al. Complex chimeras to map ligand binding sites of GPCRs. Protein Eng. 2003;16:365–72. doi: 10.1093/protein/gzg045. [DOI] [PubMed] [Google Scholar]
- 29.Ballerini P, Di Iorio P, Ciccarelli R, Caciagli F, Poli A, Beraudi A, et al. P2Y1 and cysteinyl leukotriene receptors mediate purine and cysteinyl leukotriene co-release in primary cultures of rat microglia. Int J Immunopathol Pharmacol. 2005;18:255–68. doi: 10.1177/039463200501800208. [DOI] [PubMed] [Google Scholar]
- 30.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123:789–94. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, et al. Identification of a novel human ADP receptor coupled to Gi. J Biol Chem. 2001;276:41479–85. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 32.Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochem Pharmacol. 2004;68:113–24. doi: 10.1016/j.bcp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Fumagali M, Brambilla R, D'Ambrosi N, Volonté C, Matteoli M, Verderio C, et al. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43:218–30. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 35.Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol. 2004;68:231–7. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capra V, Accomazzo MR, Ravasi S, Parenti M, Macchia M, Nicosia S, et al. Involvement of prenylated proteins in calcium signaling induced by LTD4 in differentiated U937 cells. Prostaglandins Other Lipid Mediat. 2003;71:235–51. doi: 10.1016/s1098-8823(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 37.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji X-d, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–57. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumari R, Goh G, Ng LL, Boarder MR. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br J Pharmacol. 2003;140:1169–76. doi: 10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsien RY, Pozzan T, Rink TJ. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982;94:325–34. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merritt JE, McCarthy SA, Davies MP, Moores KE. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Loading cells with the dye, calibration of traces, measurements in the presence of plasma, and buffering of cytosolic Ca2+. Biochem J. 1990;269:513–9. doi: 10.1042/bj2690513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neubig RR, Spedding M, Kenakin T, Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 42.Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195:583–92. doi: 10.1084/jem.20020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellor EA, Maekawa A, Austen KF, Boyce JA. Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci USA. 2001;98:7964–9. doi: 10.1073/pnas.141221498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Migoya E, Kearns GL, Hartford A, Zhao J, Adelsberg J, Carol A, et al. Pharmacokinetics of montelukast in asthmatic patients 6–24 months old. J Clin Pharmacol. 2004;44:487–94. doi: 10.1177/0091270004264970. [DOI] [PubMed] [Google Scholar]
- 45.Ciccarelli R, D'Alimonte I, Santavenere C, D'Auro M, Ballerini P, Nargi E, et al. Cysteinyl-leukotrienes are released from astrocytes and increase astrocyte proliferation and glial fibrillary acidic protein via Cys-LT1 receptors and mitogen-activated protein kinase pathway. Eur J Neurosci. 2004;20:1514–24. doi: 10.1111/j.1460-9568.2004.03613.x. [DOI] [PubMed] [Google Scholar]