Abstract
G protein-coupled receptors (GPCRs) remain a major domain of pharmaceutical discovery. The discovery of GPCR lead compounds and their optimization are now structure-based, thanks to advances in X-ray crystallography, molecular modeling, protein engineering and biophysical techniques. In silico screening provides useful hit molecules. New pharmacological approaches to tuning the pleotropic action of GPCRs include: allosteric modulators, biased ligands, GPCR heterodimer-targeted compounds, manipulation of polypharmacology, receptor antibodies and tailoring of drug molecules to fit GPCR pharmacogenomics. Measurements of kinetics and drug efficacy are factors influencing clinical success. With the exception of inhibitors of GPCR kinases, targeting of intracellular GPCR signaling or receptor cycling for therapeutic purposes remains a futuristic concept. New assay approaches are more efficient and multidimensional: cell-based, label-free, fluorescence-based assays, and biosensors. Tailoring GPCR drugs to a patient’s genetic background is now being considered. Chemoinformatic tools can predict ADME-tox properties. New imaging technology visualizes drug action in vivo. Thus, there is reason to be optimistic that new technology for GPCR ligand discovery will help improve the current narrowing of the pharmaceutical pipeline.
Keywords: Drug discovery, GPCR, X-ray crystallography, structure-based design, signaling, inhibitors
1. Introduction
G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors (GPCRs), also known as 7 transmembrane helical (7TM) receptors, remain a major source of new pharmaceuticals and the focus of extensive research efforts in academia, government and pharma. Recent reviews cover the structural features of the receptors [1,2,17] and the chemical aspects of orthosteric [16,18] and allosteric [88] ligands.
Among the 19 approved drug products with the greatest sales revenues at their peak year in the period up to 2013, 7 are directed toward GPCRs (Table 1) [3]. That is equal to the number of biologic drugs (non-GPCR directed) in the same category of top earners. One of those GPCR drugs, the antithrombotic drug Plavix 1 (Figure 1), the highest in revenues during that period, serves as a prodrug that must be activated in the liver [4]. Other GPCR-related drugs in the blockbuster category, such as selective serotonin reuptake inhibitors (SSRIs), increase the synaptic availability of natural neurotransmitters that act at GPCRs. Since 2013, 15 GPCR-related drugs were approved as new chemical entities (NCEs) in 31 months, with exclusions as specified in Table 2. Among these NCEs, naloxegol 12 is a derivative of a known opioid receptor (OR) antagonist that is covalently linked to a short polyethylene glycol (PEG) chain to prevent its intestinal absorption; thus, it selectively blocks opiate receptors in the gut to prevent side effects of systemic opiates [5]. Several of these new drugs treat sleep conditions: suvorexant 10 blocks two subtypes of the orexin receptor, which is a first drug in that category [6]. Approval of a melatonin receptor agonist, tasimelteon 13 followed several other approved drugs acting at the same GPCR [7].
Table 1.
Top selling pharmaceuticals that act, directly or indirectly, via GPCRs (Peak Sales Year, as of 2013).a
| Drugb (structure class) | Action | Treatment of: | Peak year sales (~ billion $) |
|---|---|---|---|
| clopidogrel 1 (thienopyridine) | P2Y12R antagonist (prodrug) | thrombosis | 9 |
| salmeterol 2 (phenylethanolamine) | β2 adrenergic-R agonist | asthma | 8 |
| aripiprazole 3a (phenylpiperazine) | D2 dopamine-R partial agonist | psychosis | 7 |
| quetiapine 4 (dibenzothiazepine- piperazine) | antagonist, biogenic amine Rs | psychosis | 6 |
| valsartan 5 (tetrazolyl-biphenyl) | AT1R antagonist | high blood pressure, congestive heart failure | 6 |
| montelukast 6 (phenylvinyl- quinoline) | CysLT2R antagonist | asthma, allergies | 6 |
| olanzapine 7 (piperazinyl- benzodiazepine) | 5HT2 serotonin-R and D2 dopamine-R antagonist | psychosis | 5 |
source of sales information: http://pharmamkting.blogspot.com/2013/01/lipitor-plavix-last-of-small-molecule.html
Structures shown in Figure 1.
Figure 1.
The most successful small molecular GPCR ligands (1–7) as of 2013 and the small molecular GPCR ligands that have been approved since 2013 (8–17).
Table 2.
New drugs (New Molecular Entities, NME) acting via GPCRs that were approved by the FDA in recent years (excluding formulations or pure enantiomers of existing drugs and combinations of previously approved drugs).a
| Drugb (year, structureclass) | Action | Treatment of: |
|---|---|---|
| 2015 | ||
| parathyroid hormone (peptide, MW 9400) | PTH-R agonist | hypocalcemia in patients with hypoparathyroidism |
| brexipiprazole 3bc (phenylpiperazine) | D2 dopamine-R partial agonist | psychosis |
| 2014 | ||
| vorapaxarc8 (decahydro-benzo-isobenzofuran) | PAR1 antagonist | reduction of thrombotic cardiovascular events |
| pasireotide 9 (cyclic peptide, MW 1100) | somatostatin-R agonist | acromegaly |
| suvorexantc10 (benzoxazolyl-diazepan) | orexin-R antagonist | insomnia |
| droxidopa 11 (phenyl-serine) | α-adrenergic-R agonist (precursor of norepinephrine) | neurogenic orthostatic hypotension |
| liraglutide (peptide, MW 3700) | GLP-1-R agonist | chronic weight management |
| albiglutide (peptide-fusion protein, MW 73K) | GLP-1-R agonist | type II diabetes |
| dulaglutide (protein, MW 60K) | GLP-1-R agonist | type II diabetes |
| naloxegol 12 (epoxymorphinan-diol) | µ-opioid-R antagonist | opioid-induced constipation |
| tasimelteon 13 (dihydrobenzofuran) | melatonin-R agonist | non-24-hour sleep–wake disorder in totally blind people |
| olodaterol 14 (benzoxazinone) | β2-adrenergic-R agonist (long acting) | chronic obstructive pulmonary disease |
| 2013 | ||
| macitentan 15 (5-phenylpyrimidine) | endothelin-R antagonist | pulmonary arterial hypertension |
| alogliptin 16 (benzyl-uracil) | DPP IV inhibitor (increases GLP-1 and GIP) | type II diabetes |
| vortioxetine 17 (phenylpiperpazine) | serotonin-R modulator and stimulator | Major Depressive Disorder |
a listing of drugs approved by year is at: http://www.centerwatch.com/drug-information/fda-approved-drugs/
Structures of small molecules shown in Figure 1.
The GPCR field is advancing rapidly, and new paradigms for GPCR drug discovery must be considered in the larger context of drug discovery. Drug discovery for GPCR targets has encountered many of the limitations associated with a changing paradigm for drug discovery in general, and there are many commentaries on why the pharmaceutical pipeline has narrowed [84]. Classical approaches to drug discovery have waning productivity; the linear, stepwise and iterative process through which new compounds progressed is now being modified [8]. Traditionally, the target pathway involved a single mechanism, with one-dimensional activity being measured. The classical approaches have not continued to yield new drugs as in past decades, despite the explosion in the number of new chemical substances accessible to researchers. The resource of greater chemical diversity has not yet led to an increase in the number of approved drugs each year, which is either declining or holding steady. Currently, new approaches to the identification of structural leads and their optimization and new technology for characterizing drug action are aiding GPCR drug discovery [12]. GPCR drug action is much more nuanced than previously recognized, and the uncontrolled variation in formerly neglected or unknown pharmacological parameters can likely lead to a lack of efficacy – or undesirable side effects – in later clinical trials [13]. In addition to directly acting GPCR modulators, many approved drugs influence GPCR action indirectly, for example SSRIs and other inhibitors of the transporter family. This commentary does not cover inhibitors of transport or generation of neurotransmitters, which have been reviewed elsewhere [14]. However, modulation of signaling and regulation pathways directly associated with GPCR activation is within the present scope.
2. Structure-based GPCR discovery: Sources of new leads and their rational optimization
2.1. New technology for GPCR structural characterization
The most important new approach in GPCR technology is the structure-based design of agonist and antagonist ligands. New GPCR X-ray structures including both antagonist-bound and agonist-bound complexes provide detailed insight into the structural basis of drug action and guide the design of new ligands [2,15]. Over 100 3D structures of GPCRs with either an agonist or an antagonist bound have been reported [16]. Technological advances that facilitated this revolution in the way we approach GPCRs include the stabilization of GPCR constructs by mutation and new crystallization methodogy [1,17,18]. Fusion proteins, such as T-4 lysozyme (T4L), thermostabilized apocytochrome b562 (BRIL), rubredoxin and other stable protein fragments that have their C and N termini in close proximity, can be inserted, for example, in IL3 of a GPCR construct [19]. Other GPCR constructs for crystallization contain a fusion protein at the N-terminus, as was done in crystallizing a complex of the β2 adrenergic receptor and Gs protein [20]. Strategically-placed mutations to create stabilized receptors (StaRs) allow the crystallization of either antagonist-preferring or agonist-preferring conformations of a given GPCR, and the protein variants consequently can be used like other stable proteins for biophysical studies [21]. For example, purified A2A adenosine StaRs could be reconstituted into high-density lipoprotein (HDL) particles that were immobilized onto a gold sensor chip for Surface Plasmon Resonance (SPR) for determination of ligand affinity and binding kinetics [22]. Alternately, antibody fragments are used to stabilize the crystals; for example, nanobody 80 (Nb80) mimics a Gαs protein to stabilize the β2-adrenergic receptor in an active state. Covalently bound ligands also help to stabilize a GPCR complex for the purpose of crystallization [23]. Crystallization by means of the lipidic cubic phase is used widely for the X-ray structural determination of GPCRs, and novel lipids are also utilized [24]. Novel detergents, such as maltose-neopentyl glycol (MNG) amphiphiles, have been introduced for use in this context [25]. Other techniques such as single-particle negative-stain electron microscopy, NMR, mass spectrometry and molecular modeling contribute important complementary structural information [26]. Hydrogen/deuterium exchange mass spectrometry (HDX-MS) provides a measure of the accessibility or exposure of peptide bond hydrogens to the aqueous medium, which can depend on the conformation or activation state of a GPCR [27].
2.2. Computational approaches
With many new structures available, the computational modeling of the 3D structures of GPCRs has greatly advanced [2]. Previously determined patterns of structure activity relationship (SAR) at some GPCRs have been rationalized with great precision [28], which leads to an ability to test newly proposed small molecules in receptor docking before they are synthesized. Thus, the pharmacology of ligand binding to the same receptor that was crystallized can be extended to chemical classes not present in the crystalline complex. Also, given a closely related structural template, it is possible to model the recognition of other GPCRs that are not yet determined structurally, i.e. the A1 adenosine receptor based on the A2A structure [40]. The selectivity of GPCR ligands can be analyzed structurally by comparing docking at multiple receptor subtypes. Proteochemometric modeling of rat and human adenosine receptors, which represent multiple similar target receptors, has identified novel ligands based on chemical similarity [29]. Molecular dynamics is an important tool, going beyond docking to static structures, in predicting the stable interactions between ligands and their receptors [28,30]. Assessment proved some prior modeling reports to be accurate, while others were far from accurate [31]. For example, rhodopsin was a good template for the modeling of adenosine receptors (α-branch of rhodopsin-like GPCRs) [32], but not P2Y receptors (α-branch of rhodopsin-like GPCRs) [33]. The P2Y12 receptor that is activated by endogenous ADP has an unusual linear and extended TM5 due to the absence of an otherwise conserved proline residue; this linearity was not effectively accommodated in earlier modeling [34]. In general, the use of data from multiple sources and techniques, such as chemogenomics, site directed mutagenesis, SAR analysis and molecular dynamics refinement, is essential for accuracy in modeling a GPCR interaction [35]. Prior to the determination of the 3D structure of the A2A adenosine receptor, an accurate nucleoside docking mode was guided by strategically mutated neoceptors, i.e. engineered GPCRs designed to recognize complementary modifications of an agonist molecule [36]. The neoceptor approach in combination with homology modeling can potentially be used with other GPCRs that have not yet been determined structurally. At least six pairs of the same GPCR with agonist or antagonist bound suggest common conformational changes that occur upon activation, including a contraction of the orthosteric agonist site [17,18,32,34].
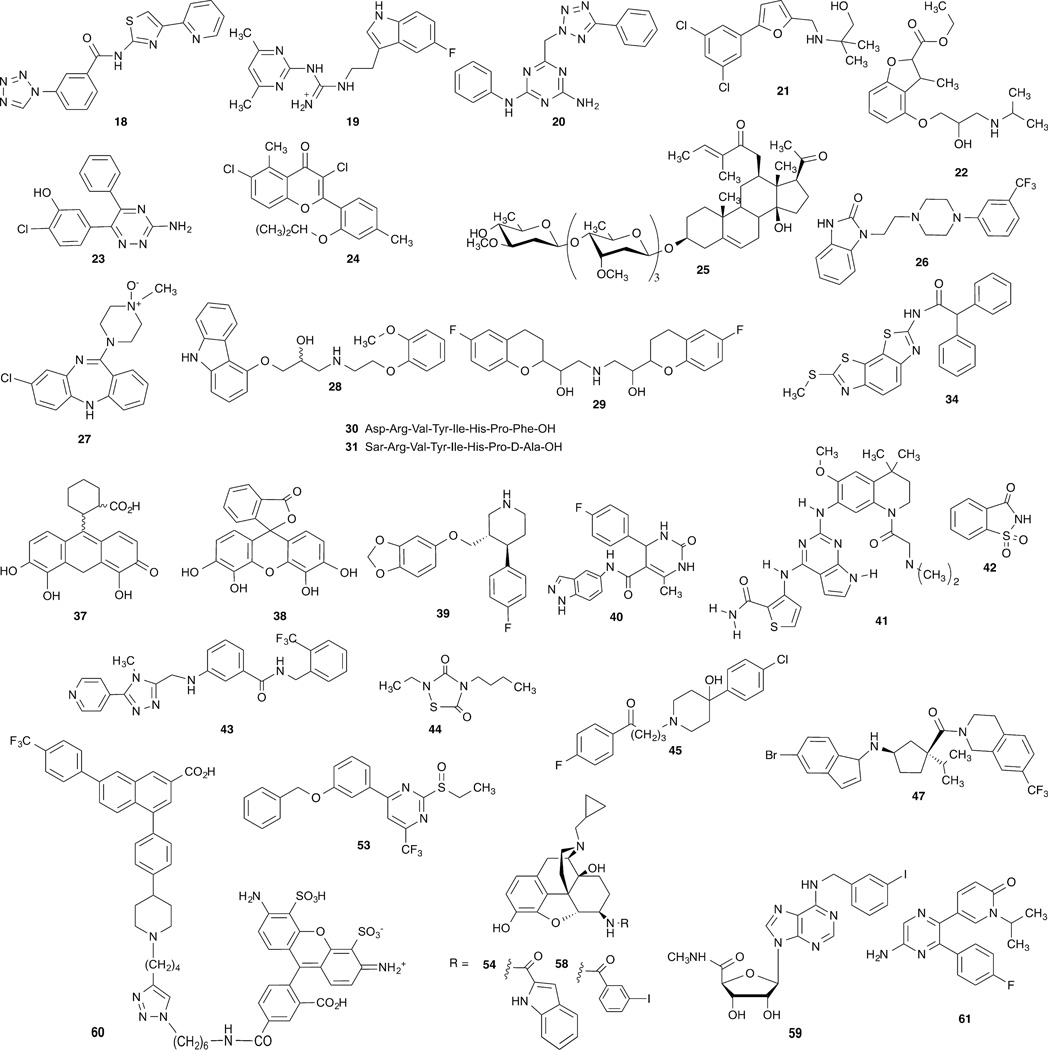
Bioinformatics and chemoinformatics have been applied to GPCRs [35]. Residues occurring at the same relative position on TMs of different GPCRs can have conserved function, especially within the same GPCR family, but the shape and orientation of the ligand binding site can vary widely within the upper portion of the receptor. There are two systems for classifying GPCR sequences: The GRAFS system has five categories: Glutamate; Rhodopsin; Adhesion; Frizzled; Taste2; Secretin, based purely on phylogeny rather that ligand similarity [153]. Alternatively, the IUPHAR system has six categories (A through F), some of which correspond to individual GRAFS categories. The three families most relevant to drug discovery are: A (rhodopsin-like), B (secretin-related) and C (glutamate/GABA-related), and their early evolution has been studied [153]. A database listing >500,000 experimentally-validated GPCR-ligand associations is available on the web [37]. Large scale in silico screening of diverse molecular libraries by receptor docking has been relatively successful in discovering new chemotypes to bind to a given GPCR, especially those related to the α-branch of rhodopsin-like GPCRs [38,39]. For example, antagonist hits with affinities as substantial as the low nM range at A1 18, A2A 19, and A3 20 adenosine receptors, D3 dopamine receptor 21, β2-adrenergic receptor 22 were discovered by this method with screening success rates typically in the range of 30–40% (Figure 2) [113,150,156]. Biophysical mapping of the binding site of the A2A adenosine receptor was used to design new antagonists [22]. Fragment-based screening has also been applied successfully to GPCRs, for example in the discovery of selective A2A antagonist 23 [41,132]. Curiously, in some cases, library screening might provide more successful hits at other subtypes within the same GPCR family than at the subtype used as a docking template [40].
Figure 2.
Other structures that are mentioned in the text, including ligands discovered by in silico screening, through receptor docking of diverse molecular libraries and modulators of signaling pathways.
2.3. Chemically diverse sources of lead molecules
With detailed knowledge of the binding sites of many GPCRs attainable, in silico screening for novel GPCR ligands can be applied to various large datasets with high efficiency in comparison to robotic pharmacological screening. For example, the Zinc database of commercially available compounds now contains >6 million entries [9], which can be used for in silico screening [10]. GPCR-targeted and GPCR-modulating combinatorial chemotype libraries, including those synthesized on chips, are available [11].
Natural products were the oldest source of new medicines, but the use of natural product libraries for drug discovery has been de-emphasized in recent years. This situation might be reversed with recent efforts to discover GPCR ligands. For example, flavonoids, such as A3 adenosine receptor selective antagonist MRS1067 24 (Figure 2) and other phytochemicals, in addition to the alkylxanthines, are a source of atypical adenosine receptor antagonists [42]. Protease-activated receptor (PAR)1 antagonist vorapaxar 8 was discovered following off-target screening of derivatives of the alkaloid himbacine, being sought as muscarinic receptor antagonists [43]. The incidental thrombin inhibitory activity at PAR1 was then optimized leading to this approved antithrombotic agent (Table 2). Recently, Gordonoside F 25, a pregnane steroidal glycoside from an African cactus that was used as an herbal medicine for weight loss, was found to activate G protein-coupled receptor 119 (GPR119) to improve glucose tolerance [44]. Although not a likely source for drug discovery, it is noteworthy that the gut microbiome produces a range of phytochemical metabolites, some of which interact with GPCRs [45]. Curiously, some of the protective effects of nutrient omega-3 polyunsaturated fatty acids might involve interaction with the free fatty acid receptor 4 (FFA4, GPR120) in anti-inflammatory M2 macrophages in adipose tissues and liver and in human prostate cancer cells [46,47].
3. New pharmacological dimensions
3.1. Diversity of GPCRs and their action
7TM proteins are the largest single family of proteins, corresponding to ~4% of those coded by the human genome. Most of the roughly 400 nonolfactory, human GPCRs have not yet been exploited as pharmaceutical targets [141]. There are still ~120 orphan GPCRs for which the endogenous ligand is unknown, but even for hundreds of nonorphan GPCRs there is still untapped potential. Formerly orphan GPCRs that are deorphanized, such as GPR40, GPR119 and GPR120, are proving useful in drug discovery [44,46,51]. Molecular modeling predictions have contributed to the deorphanizing of GPCRs [152]. There are newly explored pharmacological parameters that will contribute to a more selective action of drugs at GPCRs. These dimensions include: biased ligands, allosteric modulators, residence time, polypharmacology, receptor antibodies, and pharmacogenomics of GPCRs. Characterization of these parameters for new compounds promises to be a means of discovering more efficacious GPCR drugs with fewer side effects. Also, it is important to recognize that the effects of native GPCRs may not always be extrapolated from in vitro data; the appropriate selection of model in vivo systems and organisms is important [48]. Furthermore, the expression pattern and physiological roles of a given GPCR can be altered in a disease state [141]. Also, the effects of acute versus chronic drug administration can be differ or even be opposite [49].
3.2. Pleiotropic signaling of GPCRs
The pleiotropic effects of GPCRs and their dynamic signaling through multiple effector pathways are more complex than previously recognized [50]. Receptors that were previously associated with one particular activity in the body have been found to govern seemingly unrelated functions, thus suggesting new therapeutic concepts. For example, examination of the effects of GPR40, a diabetes target also known as FFA1, in the CNS suggests an application in pain control, in addition to its previously established interest in the context of enhancing glucose-stimulated insulin secretion by free fatty acids [51]. An example of renewed interest in a previous GPCR target that had resulted in an approved drug that was later withdrawn is that of the cannabinoid receptors, which are now being explored for neuroprotection, inflammation and osteoporosis [52]. GPCR drugs for improvement of quality of life are also under development, such as mixed serotonin receptor 5HT1A agonist and 5HT2A antagonist flibanserin 26 for treatment of female hypoactive sexual desire disorder [53]. Newly revealed biological actions of previously underexplored GPCRs, such as the hundreds of olfactory receptors [106,145] and a 33-membered family of adhesion GPCRs [54], are providing new avenues for translational development.
Among the many signaling pathways, GPCRs can regulate activity of ion channels and kinases, which affect secretion, proliferation and cell survival. There is also a range of other signaling proteins that are physically associated with GPCRs, such as the nucleotide exchange factor ARNO/cytohesin-2 [89]. Each of the signaling pathways might have its particular kinetics and cellular localization. Under different circumstances, the same receptor might either induce cell death or promote proliferation and protect from cell death [55]. For example, the spectrum of activity of A2B adenosine receptor agonists is a complex mixture that varies for different ligands [56], and the relation to disease treatment is unclear. There are seemingly contradictory reports that either A2B agonists or antagonists might be useful for treating the same condition, such as diabetes or cancer [57]. The in vivo probing of specific effector pathways induced by a given GPCR can be studied using engineered designer receptors exclusively activated by designer drugs (DREADDs), which recognize a synthetic ligand (clozapine-N-oxide 27), but not the native ligand and are coupled selectively to Gi, Gs or Gq [58]. DREADs can be introduced into specific tissues in vivo using viral vectors.
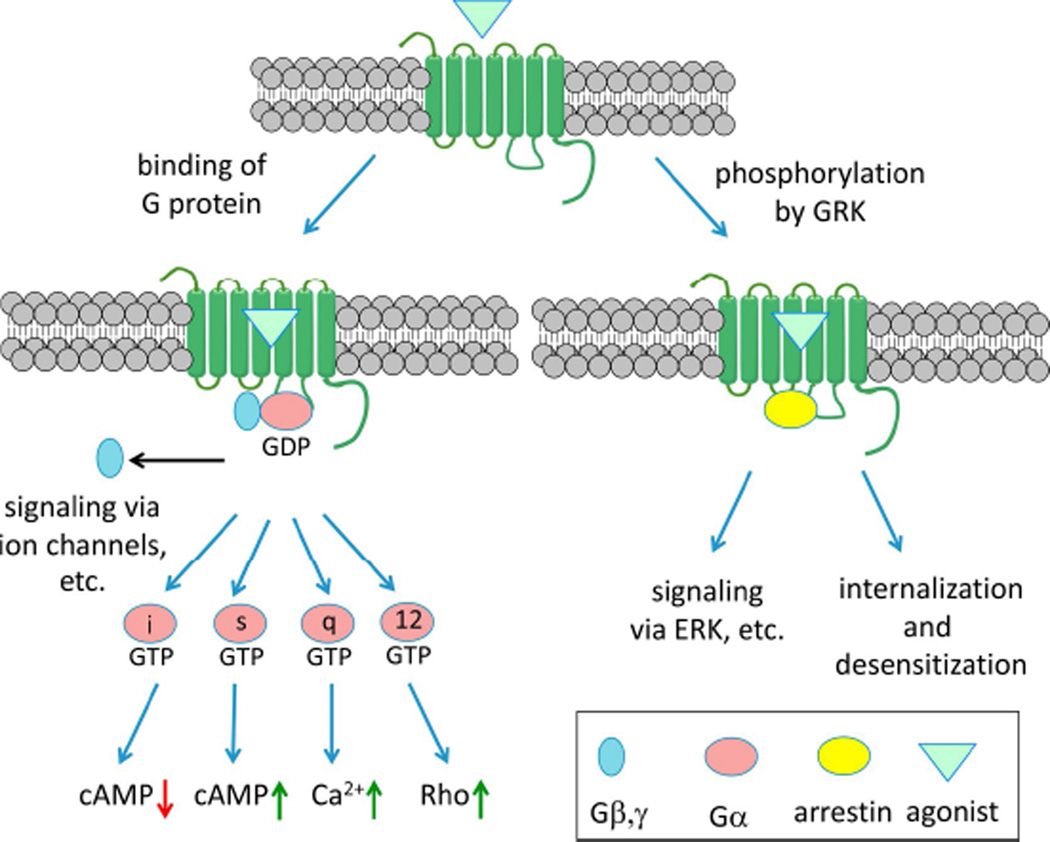
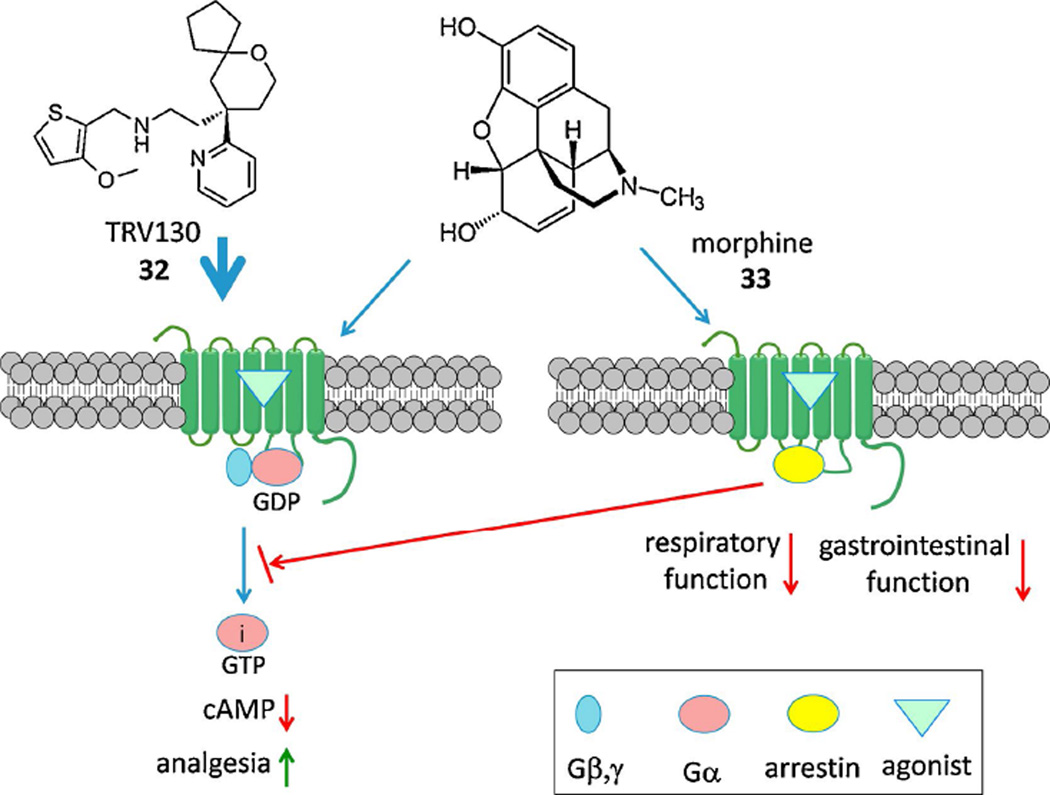
In addition to complex signaling, the regulation of GPCR trafficking and desensitization is also multifactorial. β-Arrestin mobilization is a major non-G protein-dependent signaling pathway of GPCRs as well as inducing desensitization and internalization [158]. The concept of biased ligands was originated with the proposal that G protein-dependent and independent (i.e. β-arrestin-mediated) pathways have different biological sequelae [59] (Figure 3). Therefore, compounds that select a pathway by preference for a given GPCR conformation would be cleaner in their therapeutic actions. The presumption is that the receptor exists as an ensemble of conformations, each of which has specific efficacy and signaling parameters. β-Adrenergic receptor antagonists carvedilol 28 and nebivolol 29 are actually β-arrestin biased agonists; thus, an action independent of Gαs stimulates extracellular signal-regulated kinase (ERK) and NO production [59,60]. Two GPCR biased agonists have already progressed into Phase II clinical trials: a β-arrestin-biased TRV027 31 of the angiotensin II type 1 receptor (AT1R) for cardiac failure [61] and µOR Gi-biased TRV130 32 for postoperative pain [62]. Side effects are avoided by eliminating the other signaling pathway: G protein-dependent signaling through the AT1R is associated with cardiac hypertrophy induced by angiotensin II 30, and β-arrestin-dependent signaling through the µOR is associated with depression of respiratory and intestinal activity induced by unbiased agonist morphine 33 (Figure 4). Another study sensed conformational changes induced by an angiotensin II analogue that was earlier deemed to be G protein-independent [157]. The peptide still had G protein-dependent effects but that unlike those of the native agonist, suggesting that the precise pharmacological signaling profile of ‘biased agonists’ may be assay-dependent. cAMP-biased agonism at the A3 adenosine receptor has been demonstrated [63]. In addition to synthetic agonists engineered for bias, endogenous GPCR ligands may show signaling bias, as demonstrated for several endogenous neuropeptides that activate the µOR [64]. A current research goal is to correlate ligand bias with structural interaction with the GPCR conformation, which has been reported in a few cases, such as 5HT receptors [65]. A "competitive" model for quantitating the degree and direction of bias of GPCR agonists has been introduced [66].
Figure 3.
GPCR agonists may be biased for either G protein-dependent or β-arrestin pathways. Other forms of bias are also possible, such as distinguishing between different G proteins [144].
Figure 4.
A Gi-biased agonist of the µOR compared to morphine, which also acts through the arrestin pathway [62].
The physical association of β-arrestin with the β2 adrenergic receptor following phosphorylation by a GPCR kinase (GRK) has been visualized [26]. However, the roles of individual phosphorylation sites in desensitization may be complex and even dependent on specific agonists. For example, Ala mutation of various Ser or Thr sites on the C-terminal tail of the µOR has differential effects on desensitization induced by morphine or met-enkephalin [69]. Signaling bias in GPCRs can occur within one category of pathways, such as different β-arrestins. An analogue 35 of parathyroid hormone (PTH), in comparison to native PTH 36 (structures not shown), displayed bias between different pathways of arrestin-signaling as detected by multiple informatic analyses of the “in vivo transcriptomic signature” [70]. Bias within the G protein dependent pathway is also possible. A biased, small molecule agonist GUE1654 34 of the Gi/o-coupled lipid receptor for the chemoattractant 5-oxo-6E,8Z,11Z,14Z–eicosatetraenoic acid (5-oxo-ETE), known as OXE-R, activated Gβ,γ- but not Gα-dependent pathways [149].
The phosphorylation of GPCRs, dependent on protein kinase A (PKA), might also switch the preferred G protein coupling, for example from Gs to Gi as a putative feedback mechanism [158]. PKA phosphorylation is also reported to switch the recycling pathway of a GPCR and its partitioning into distinct endosomal domains [159]. The physiological significance of these switching mechanisms is yet to be explored.
Inhibitors of specific GRK isozymes have been studied as regulators of GPCR action and their translational potential demonstrated. GRK2 is up-regulated to alter adrenergic signaling and is a contributor to cell death in patients suffering from heart failure (HF). The β,γ-subunits of the G protein can recruit GRK2 to phosphorylate and downregulate β2-adrenergic receptors, which in the chronic state can lead to HF. Thus, inhibitors of the association of Gβ,γ and GRK2, including M119 37 and galleon 38, are of interest for treating HF [71]. The SSRI paroxetine 39, but not the SSRI fluoxetine, inhibited GRK2 with selectivity over other GRKs [72]. Paroxetine induced greater protection than treatment with a β-adrenergic antagonist in mice following myocardial infarction. GRK2 inhibition in other organs might augment the clinical benefit in HF, for example, GRK2 activity in the adrenal gland can lead to sympathetic hyperactivity, which contributes to HF [73]. Differential scanning fluorimetry was used to screen a collection of diverse, known protein kinase inhibitors that could increase the melting points, and thus form stable complexes with the two most ubiquitously expressed GRK isozymes: GRK2 and GRK5 [74]. Indazole/dihydropyrimidine derivatives, such as GSK180736SA 40, and pyrrolopyrimidine derivatives, such as GSK2163632A 41, were found to inhibit various GRKs with varying selectivity. Amphipathic modulators of sweet and bitter taste, i.e. tastants such as saccharin 42, were found to amplify the Gs-dependent response to β-adrenergic agonist isoproterenol by inhibiting GRK2 [75]. GRK2 and possibly GRK3 promote agonist-induced µOR desensitization [76]. Compound101 43 inhibited agonist-induced µOR phosphorylation at Ser375 and subsequent arrestin translocation and receptor internalization in the brain.
In addition to β-arrestin, a variety of other regulators of GPCR action are known. For example, regulators of G protein signaling (RGS) proteins inhibit G-protein-dependent activity through direct contact with the Gα subunit to enhance its GTPase activity [77]. The degree of regulation of the M3 muscarinic acetylcholine receptor by RGS proteins can be dependent on the GPCR ligand [78]. Selective inhibition of RGS4 by thiadiazolidinone derivatives of nM potency such as CCG-203769 44 shows promise for the treatment of Parkinson’s disease [79]. Inhibition of RGS6 might be a future, multifold treatment of alcohol abuse, through control of dopaminergic signaling in the brain, with associated protection of the liver and heart [80]. R7 regulator RGS-binding protein (R7BP) is an allosteric inhibitor of R7 RGS in the brain, a process that requires palmitoylation of R7BP [81]. Palmitate turnover, which can be inhibited chemically, promotes distribution to the plasma membrane of palmitoylated R7BP, which by removing the associated RGS from the Gα protein, delays closure of G protein-regulated inwardly rectifying K+ (GIRK) channels that are activated by Gi/o. Thus, the manipulation of palmitate turnover to indirectly regulate GPCR signaling might have utility in treating neurological disorders.
3.3. Intracellular signaling
The canonical mode of GPCR signaling arises from activation of a receptor located on the cell surface, i.e. to transmit an extracellular chemical signal to the cytosolic side. However, GPCRs can signal from inside the cell or during the internalization process, and various compartments, including clathrin-coated pits and early and late endosomes, have different signaling preferences [118,119]. Agonist-induced internalization of GPCRs, previously associated with desensitization has to be redefined to include active, intracellular signaling through both G-protein dependent and arrestin-dependent processes (Figure 9). In principle, by changing the physicochemical properties of a ligand, one can alter its distribution on and within the cell. A combination of diffusion-, transporter- or receptor-mediated internalization might occur [120]. It remains to be demonstrated if one can manipulate the rate of internalization and recycling/degradation by pharmacological intervention. Thus, modulation for therapeutic purposes of the processing of GPCRs within the cell and of cycling of GPCRs between endosomes and the cell surface is a yet unfulfilled concept.
Figure 9.
GPCR signaling can occur during the endocytic process [118]. The G protein-dependent pathway is, for example, stimulation of cAMP production, and the arrestin pathway includes stimulation of MAPK. Some internalized GPCR proteins are recycled to the surface to restore signaling.
3.4. Efficacy and residence time as predictive parameters
Pharmacological efficacy of GPCR ligands is measurable in model systems, but the maximal relative efficacy achievable with the same ligand is variable under different conditions. Pharmacological efficacy, as characterized in model cell systems, can influence in vivo activity and eventually clinical efficacy, which if deficient is a major source of failure in clinical trials. Therefore, more predictive studies of efficacy are needed. Partial agonists, i.e. activating a GPCR but not achieving the maximal magnitude of a biological effect known for other ligands, in some cases are preferable over full agonists or antagonists. For example, the antipsychotic drug aripiprazole 3a has fewer side effects than the D2 dopamine receptor inverse agonist haloperidol 45, in part because of the partial agonism of 3a at the D2 receptor [82]. Brexipiprazole 3b is of similar structure and D2 dopamine receptor activity and used for the same indication. Also, A1 adenosine receptor agonists that are partial have fewer side effects than full agonists in the treatment of cardiac arrhythmias [83]. This is ascribed to the differential density of the receptor in different tissues, such that a full effect can be achieved only when spare receptors are present. GPCRs often display constitutive activity (basal activity of the receptor in the absence of an agonist), which is reduced by inverse agonists but not neutral antagonists. Many of the GPCR antagonists reported are actually inverse agonists. Consequently, there can be significant differences between neutral antagonists of GPCRs and inverse agonists, depending on whether there is a basal level of constitutive activation of the receptor [84], although this pharmacological distinction, detected typically in cell systems, has to be validated in vivo.
Residence time, an experimentally measured t1/2 of a drug-target complex, is another intrinsic parameter associated with each ligand-receptor complex that can be predictive of in vivo efficacy [85,103]. This parameter is mathematically related to but actually independent of affinity. Thus, it is informative to probe the structure-kinetics relationship (SKR) of a compound class along with the SAR. Simplified kinetic methods based on two standard time points in radioligand dissociation experiments have been described for measuring residence time. The shape of the radioligand association curve upon co-incubation with unlabeled competitor varies according to the relative dissociation rates of the two ligands, and this can be used to determine the GPCR residence time [85]. Nevertheless, the residence time will be affected by the membrane environment and other assay conditions, and current assays do account for conformational changes induced by ligand binding that can affect residence time [154].
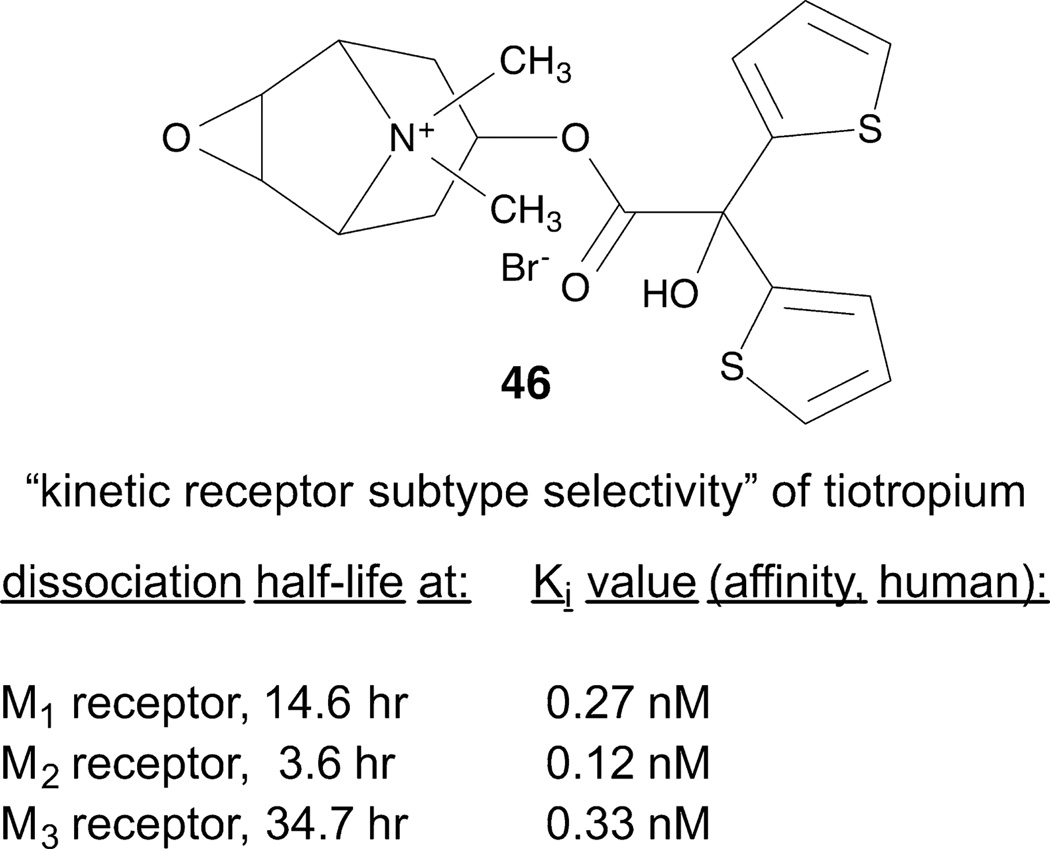
An example of a drug that has a long residence time that might be responsible for its therapeutic success is the bronchodilator tiotropium 46 [85]. This compound dissociates slower from the M3 muscarinic acetylcholine receptor than from other muscarinic receptor subtypes. In binding, tiotropium is not M3-selective in comparison to binding at the M1 and M2 muscarinic acetylcholine receptors, so the kinetic data is the only pharmacological explanation for this in vivo selectivity (Figure 5). The M3 receptor structure shows a protrusion covering the bound tiotropium (“tyrosine lid”) deep in the orthosteric site [30]. The long duration of action of this compound might be related to this physical barrier to dissociation. Characterization of SKR was used to identify antagonists such as 47 (R-isomer) with a long residence time in binding to the chemokine ligand 2 (CCL2) receptor [87].
Figure 5.
GPCR residence time may be measured using radioligand dissociation [85]. An example of this phenomenon, i.e. a long-acting M3 muscarinic acetylcholine receptor agonist 46.
3.5. Allosteric modulation of GPCRs
Allosteric (“different site”) modulators bind to a secondary site on a GPCR, either not involving any of the amino acid residues required to coordinate the endogenous ligand in binding at the orthosteric (“same site”, location of the bound native ligand) binding site, or with limited overlap (Figure 6) [88]. Small molecule allosteric modulators can tune, either positively (as a positive allosteric modulator, PAM) or negatively (as a negative allosteric modulator, NAM), the effect of a native agonist, which might be released in response to stress in an organ. For example, ischemia causes the local release of adenosine that feeds back by activating adenosine receptors to protect the tissue from stress. The presence of a PAM would magnify the effect of the endogenous agonist. In this manner, greater site and event specificity of the desired pharmacological effect is achieved with a PAM in comparison to an orthosteric agonist [89]. The modulation of the effects of an orthosteric agonist could be either with respect to either maximal efficacy or potency, or a combination thereof [86]. It is also possible to achieve allosteric agonism, i.e. to activate the receptor from a different binding site and independently of the endogenous agonist, such as TAK-875 52 at GPR40. Allosteric modulation of GPCRs is probe-dependent, i.e. the same modulator can have entirely different effects on two different agonists of a GPCR [88]. Thus, a given allosteric modulator has to be tested against the endogenous agonist to predict its in vivo effects. Experimental methods for determining allosteric modulation and functional selectivity of GPCR ligands have been described [90].
Figure 6.
Graphical illustration of possible locations and actions of allosteric modulators of GPCRs [88].
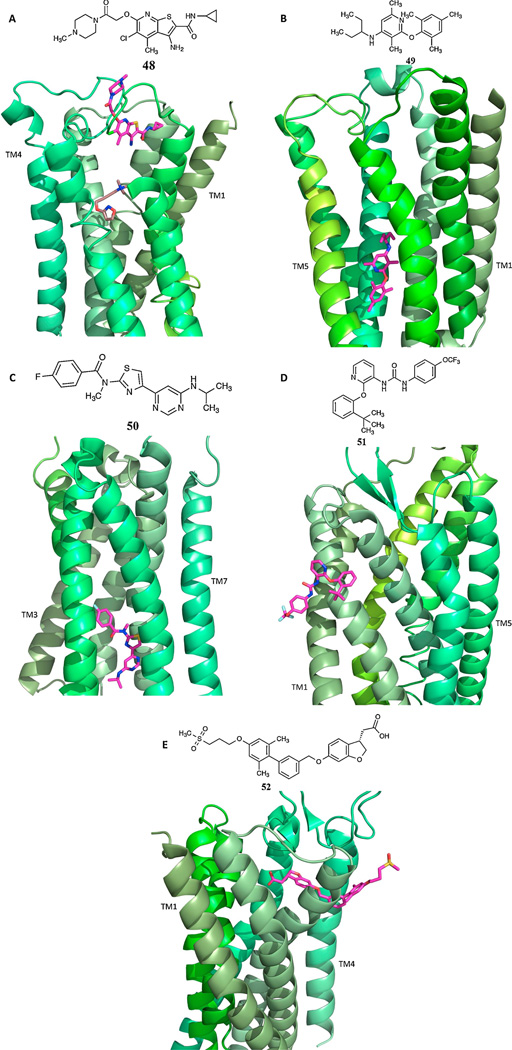
Allostery in GPCRs is recognized as a major direction in GPCR drug discovery, and specific methods have been developed for discovery of small molecule PAMs and NAMs [91]. For example, allosteric modulation of the metabotropic glutamate receptor 5 (mGluR5, PAMs and NAMs [110]) and γ-aminobutyric acid receptor B (GABABR, PAMs [108]) is being explored mainly for CNS disorders, and other allosteric modulators such as GLP-1R PAMs are intended for treating diabetes and other conditions [92–94]. The identification of the first allosteric GPCR modulators was empirical, decades before the receptor structures were elucidated, and in some cases we now understand their structural basis. Five X-ray structures of GPCR allosteric complexes can be compared (Figure 7): muscarinic acetylcholine (Family A), corticotropin releasing hormone 1 (CRF1, Family B), mGlu1 (Family C), P2Y1 receptors (Family A) and GPR40 [92,96], with bound allosteric modulators, LY2119620 (PAM) 48, CP-376395 (NAM) 49, FITM (NAM) 50, BPTU (NAM) 51 and TAK-875 (PAM, allosteric partial agonist) 52, respectively. In each case, the location, relative to the orthosteric site is different. NAMs of mGluR5, GABAB-R and other Family C GPCRs bind at a site deep within the TMs corresponding roughly to the orthorsteric site of family A GPCRs [93], while the orthosteric site in Family C is on an extracellular protrusion with the shape of Venus fly trap. The M3 muscarinic acetylcholine receptor has an outer vestibule that was shown by X-ray crystallography to be a binding site for allosteric modulators such as 48, as confirmed in the structure with two ligands bound [30]. The P2Y1 receptor that is activated by endogenous ADP has an unusual site for allosteric modulation that is located on the outer surface of TMs 2 and 3, in contact with the phospholipid bilayer [33]. For this reason, antagonists of the urea class, including 51, are necessarily hydrophobic, because they approach the binding site from a nonaqueous environment. An X-ray structure of GPR40 shows an allosteric partial agonist 52 bound in the contact region between two helices and protruding into the membrane [97]. BETP 53, a PAM of the GLP-1R6 located on pancreatic β-cells, was found to bind covalently to a Cys residue in the intracellular loop (IL) region (cytosolic end of TM6) where the G protein binds [94]. Small molecular PAMs of the δOR were recently discovered by high throughput screening using a β-arrestin recruitment assay [95]. In silico screening was applied to the identification of NAMs of the GLP1R, which belongs to Family B [148] and dopamine receptors [39].
Figure 7.
X-ray structures of complexes of GPCRs (green ribbon structures, shaded by TM number) and their allosteric modulators (shown in pink) [92,33,97]. PDB accession numbers are: A. M2 with orthosteric agonist iperoxo and PAM LY2119620 48, 4MQT; B. CRF1 with NAM CP-376395 49, 4K5Y; C. mGluR1 with NAM FITM 50, 4OR2; D. P2Y1 with NAM BPTU 51, 4XNV; E. GPR40 with PAM TAK-875 52, 4PHU. Clinical trials of GPR40 agonist fasiglifam (TAK-875) for diabetes were terminated in 2013.
Pepducins, which are peptides corresponding to sequences found on the inside surface of GPCRs, can allosterically stabilize the active state to induce receptor-G protein signaling [96]. One such pepducin for PAR1 corresponds to the H8 helix that is parallel to the plane of the membrane and a “tyrosine propeller” found in TM7. In addition to pure allosteric modulators, normally orthosteric ligands might bind to multiple sites on the same receptor, called metastable or ‘meta-binding’ sites as proposed using earlier modeling methods [13], which might have their own modulatory effects. PAMs of the A3 adenosine receptor were shown to display signaling bias and the ability to restore full activity to an agonist-derived antagonist, i.e. a nucleoside that was reduced in efficacy in stages to become an antagonist [86]. Bitopic ligands, also described as ‘dualsteric’, bridge two different sites on a single GPCR, such as an orthosteric agonist site and an allosteric enhancer site [98,128]. A biotopic antagonist of the M2 muscarinic receptor demonstrated that the orthosteric binding portion directs the allosteric moiety to establish its subtype selectivity [147].
3.6. Oligomerization of GPCRs
The physiological relevance of GPCR dimers, both hetero- and homodimers, versus GPCR monomers, is under debate [99,100]. Family C GPCRs, such as mGluRs are active only in the dimeric state, and the conformation at the interface can transmit allosteric effects [101], and GABAB receptors require a functional heterodimer of subtypes 1 and 2. Other GPCRs such as rhodopsin-like GPCRs can also act either in monomeric or dimeric form. In model cell systems, video imaging on sub-second time scales of single fluorescent-molecules can detect the dynamic equilibrium between GPCR monomers and their homodimers [102]. The dissociation rate constant of GPCR homodimers in mammalian cell membranes is typically 10 sec−1. The presence of dimeric GPCRs has now been demonstrated in living tissue by biophysical and biochemical methods [99]. Dimers themselves may associate to form heteromeric assemblies of four or more GPCR protomers, which may interact functionally [104]. The stoichiometry would require one G protein for every two receptor protomers in association. Moreover, the contact region between GPCR protomers, for example in the intracellular loops [105], conducts allosteric modulation. In some documented examples, the dimeric interface allows a negative cooperativity [100]. Thus, if two ligands bind to the dimeric pair, the activity of the neighboring protomer could be suppressed. It is conceivable that a high concentration of a given GPCR agonist could have a less pronounced biological effect compared to a lower concentration, due to the occupancy of protomers in association. The interplay of these effects could greatly complicate interpretation of pharmacological data and affect the in vitro and in vivo efficacy of a GPCR agonist. Nevertheless, drugs that might favor either GPCR dimers or GPCR monomers are under consideration for development. An effort is underway to develop drugs that block the activity of heterodimers of AT1R and CCR2 receptors for the treatment of chronic kidney disease [107]. Postsynaptic heterodimers of A2A adenosine and D2 dopamine receptors are present in the striatum and are thought to be important in the intended use of A2A adenosine receptor antagonists for Parkinson’s disease treatment. Presynapic heterodimers of A2A and A1 adenosine receptors also are proposed to play a role [104]. Portoghese and colleagues have developed small molecule agonists such as INTA 54 that is reported to selectively activate κOR heteromers (κ-µ and κ-δ) in contrast to monomeric or homodimeric receptors [109]. These compounds are suggested to have reduced addiction risk.
Various research groups have introduced tethered ligands that are intended to bridge neighboring GPCR protomers [38]. The tethering approach to simultaneously modulate the activity of dimers and/or gain selectivity for dimeric GPCRs is limited by the relatively high molecular weights needed to achieve efficient binding and by the uncertainty of the spatial relationship of the two binding sites. A different approach to tethering multiple GPCR ligands using a peptide nucleic acid (PNA) scaffold of tunable distance was recently reported [111]. XAC, an amine functionalized congener of xanthine antagonists of the A2A receptor, was spaced at predetermined distances on the rigidified scaffold, and the consequent increases in affinity were suggestive of simultaneous binding to receptor multimers Nanocarriers have been used to target GPCR ligands or to alter their pharmacological profile [112,113].
3.6. Polypharmacology of GPCR ligands
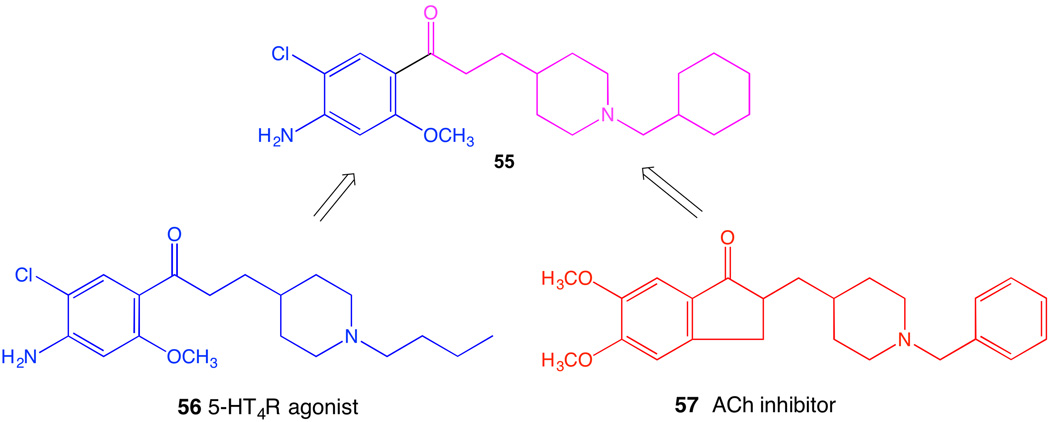
Polypharmacology, i.e. the ability of a ligand to interact with multiple targets in cells, is recognized as both an advantage and an impediment in drug discovery. Recent exhaustive and comprehensive studies of drug molecules and their diverse interactions demonstrated that target diversity is common for approved drugs, and some of the unanticipated pathways detected through modeling and broad screening could affect drug action [114,115]. The discovery of new targets for existing GPCR drugs could lead to drug repurposing, a current area of concentration for NIH [116]. The binding of a series of sterically constrained nucleosides at various GPCRs, as determined empirically, was analyzed showing consistent structural association [35], which can now be used to modify the ligands to augment or diminish polypharmacology. Thus, the rational engineering of polypharmacology of GPCR ligands to allow interaction with multiple receptors (even independently of the concept of dimeric GPCRs) or with additional non-GPCR targets is underway. For example, donecopride 55 is a dual acting molecule discovered for the potential treatment of Alzheimer’s disease (Figure 8) [117]. It is a chimeric small molecule incorporating elements of a known agonist of the 5HT4 serotonin receptor 56 and an inhibitor of the enzyme acetylcholinesterase 57, the simultaneous inhibition of which would be complementary as a treatment approach.
Figure 8.
Design of a dual acting drug 55, possibly of use in treating Alzhemier’s disease [117].
3.8. Biologic ligands
Some of the newly approved parenteral GPCR drugs are large peptides, such as parathyroid hormone. In addition to peptide agonists and antagonists, monoclonal antibodies are under development for modulation of GPCR action. Although it is conceptually and operationally simpler to administer a small molecule GPCR drug, GPCR-directed monoclonal antibodies may present advantages of a narrow mode of interaction with a receptor. Monoclonal antibodies can modulate the activity of GPCRs in either a positive or negative fashion and, in theory, with biased or non-biased signaling (Figure 10) [121]. Thus, such antibodies could be considered allosteric modulators of GPCRs that are biologic, as opposed to small molecules considered above. The theoretical possible modes of application of GPCR antibodies can even include selectivity for GPCR hetero- or homodimers [121]. An example of a negatively acting GPCR antibody is a sphingosine 1-phosphate (S1P3) receptor antagonist that has been shown to slow the growth of breast tumor cells [122]. Although it has been notoriously difficult to obtain specific antibodies against GPCRs for experimental pharmacology, numerous GPCR antibodies are already in clinical development. One such antibody that is directed toward the chemokine receptor CCR4 is already approved for treatment of adult T cell leukemia in Japan [123,124]. A preponderance of GPCR monoclonal antibodies, intended for treatment of cancer, inflammation or viral infection (at viral co-receptors such as CXCR4 and CCR5), work via chemokine receptors. In general, the therapeutic advantages of GPCR antibodies over small molecules remain to be documented.
Figure 10.
Graphical illustration of possible modes of interaction of monoclonal antibodies with GPCRs. Reprinted with permission from Webb et al. [121].
3.9. Pharmacogenomics of GPCRs
Genetic discoveries and the human genome mapping have influenced the development of GPCR ligands. Pharmacogenomics of GPCRs, in which drug substances are tailored for a particular genetic variant of a GPCR or other protein involved in GPCR signaling, would avoid a “one-size fits all” result in the clinic [125]. This might increase efficacy and reduce side effects in cases of pharmacologically important, naturally occurring GPCR sequences or splice variants. Personalized medicine is also designated as an area of focus in NIH. Genetic variation leading to altered pharmacology in GPCRs has been studied. For example, several rare variants of the P2Y12 receptor lead to bleeding diatheses [126]. There are also differences in enzymes that are required to activate prodrugs. For example, roughly one third of the population is genetically impaired in the 2C19 isoform of CYP450s that activates the P2Y12 receptor prodrug Plavix (clopidogrel 1) in the liver [4]. Thus, the effectiveness of a given drug may be highly variable depending on genetic background. Insulin secretion in type 2 diabetes patients was improved by yohimibine (α2A adrenergic receptor antagonist) administered to carriers of a single-nucleotide polymorphism (SNP) of the α2A adrenergic receptor that causes defective β-cell function [127]. Genetic variants of the unsaturated fatty acid receptor GPR120 have differential effects on obesity, both in humans (e.g. obesity-associated p.Arg270His mutation) and dogs, which can have implications for therapeutics and nutrition [48]. The encoding of µOR variants by the gene Oprm1 is subject to extensive alternative splicing, including C-terminal truncations. Curiously, the analgesic activity of 3-iodobenzoyl-6β-naltrexamide (IBNtxA, 58) in models of thermal, inflammatory, and neuropathic pain does not require the full length µOR that contains seven TMs, but rather acts through 6TM-truncated µOR splice variants [129]. This was demonstrated by the rescue of IBNtxA-induced, but not morphine-induced, analgesia in mice lacking all variants of Oprm1 by the expression in the brain of a virally encoded, truncated 6TM construct, mMOR-1G. The level of expression of a GPCR can be used as a biomarker to predict the efficacy of treatment with an agonist of that receptor, as shown during clinical trials of A3 adenosine receptor agonist IB-MECA (piclidenoson) 59 for autoimmune inflammatory disease, including rheumatoid arthritis [130]. Thus, the tailoring of GPCR drugs to a patient’s genetic background is in the early stages of feasibility.
4. Novel screening and assay approaches
4.1. In vitro characterization
Newly developed in vitro and in vivo assay and screening approaches are accelerating the discovery of new leads for GPCR ligands. These approaches include fluorescence-based screens in cells or membranes [131], bioluminescence resonance energy transfer (BRET)-based biosensors [91], and label free drug discovery [133]. For example, the G protein α (energy donor) and γ (energy acceptor) subunits may be labeled with matched fluorescent proteins to achieve a BRET signal when activated by a GPCR [157]. A convenient method for achieving a BRET signal is by using a fluorescent small molecule ligand containing a fluorophore such as BODIPY in cells that express a bioluminescent protein (NanoLuc) fused to the N-terminus of the GPCR [155]. BRET has been used to identity biased effects and/or allosteric and multimeric association of GPCRs [92].
For GPCRs that lack effective radioligands and for some receptors that have them, fluorescent agonists and antagonists have been shown to be useful [131,134–136]. Although the covalent attachment of a fluorophore may work well for peptide GPCRs, for small molecular ligands, such as biogenic amine GPCRs, this requires a much more difficult chemical process. It is necessary to first sample SAR to locate an insensitive site on the molecule and then to determine a suitable tethering chemistry for unambiguous derivatization that maintains or improves upon the affinity of the lead molecule [113]. This process might necessitate a multistep synthetic route, often starting with an early synthetic precursor molecule. Now, molecular modeling of ligand recognition at a given GPCR can simplify the process of locating an attachment site [28,135]. Fluorescent GPCR ligands may be used in membranes or in whole cells with flow cytometric detection and can detect association of a GPCR protein with the other proteins [134]. They can be used as tracers for measuring competitive binding of ligands. For example, a high affinity P2Y14 receptor antagonist 60 (Ki 80 pM) was shown to have very low nonspecific binding in flow cytometry of mammalian cells overexpressing the receptor and could be used to determine the affinity of other ligands [135].
A chemiluminescence complementarity assay for β-arrestin mobilization can be adapted to each GPCR and used for screening agonists, for example at the A3 adenosine receptor [63]. In this engineered PathHunter cell line, a small peptide fragment from the enzyme β-galactosidase is fused to the GPCR of interest (including orphan GPCRs), and a complementing fragment is fused to β-Arrestin2. A fluorescent agonist of a GPCR can be used to follow the internalization of the receptor [136]. Yeast-based fluorescent reporter systems are useful as functional biosensors for GPCR activity, especially with an improved fluorescent protein and engineered Gα receptor, as applied recently to the 5HT1A serotonin receptor [137]. SPR is useful for compound screening when the receptor can be immobilized on a gold surface [22]. Epic dynamic mass redistribution and changes in electrical impedance of a cell monolayer induced by the GPCR activation can be measured [142,143]. Such label-free methods can also be used to detect GPCR heteromerization, as well as for ligand screening. GPCRs that are fused to ion channels and expressed in Xenopus oocytes have provided functional screens of ligands even when an additional fusion protein such as T4L is present in IL3 and would prevent G-protein coupling [138].
4.2. In vivo characterization
New approaches are also useful for vivo characterization of GPCR drugs. Chemoinformatics is useful in the prediction of absorption, distribution, metabolism, and excretion (ADME) and toxicological (Tox) properties of GPCR ligands that are to be used in vivo [139]. Within a family of adenosine derivatives that all display high selectivity at the A3 adenosine receptor, primary phenotypic screening in vivo was used to identify candidate compounds with favorable efficacy in pain models and was predictive of ADME properties [146]. For advanced candidate GPCR drugs in clinical development, imaging by positron emission tomography (PET) is useful to correlate the degree of occupancy of a receptor with the dose needed to elicit a desired biological effect. PET can be used to predict the therapeutic window between the dose that gives minimal receptor occupancy and a toxic dose. An example involves use of a 11C-labeled selective A2A receptor antagonist to image the receptor binding of a mixed A1/A2A receptor antagonist ASP5854 61, a candidate for treatment of Parkinson’s disease [140]. A roughly 85% occupancy of the striatal A2A receptor was found to be needed to produce the desired in vivo anticataleptic effect.
5. Conclusions
In conclusion, there is reason to be optimistic that new approaches, technologies and efficiencies for GPCR ligand discovery will help improve the current narrowing of the pharmaceutical pipeline. The discovery of GPCR lead compounds and their optimization are now structure-based, thanks to advances in X-ray crystallography, protein engineering and biophysical techniques. New pharmacological approaches include: allosteric modulators, biased ligands, GPCR heterodimer-targeted compounds, manipulation of polypharmacology, receptor antibodies and tailoring of drug molecules to fit GPCR pharmacogenomics. Measurements of kinetics and drug efficacy can predict clinical success. With the exception of inhibitors of GRKs, targeting of intracellular GPCR signaling or receptor cycling for therapeutic purposes remains a futuristic concept. Tailoring GPCR drugs to a patient’s genetic background is now being considered. New assay approaches are more efficient and multidimensional: cell-based, label-free fluorescent assays, and biosensors. Chemoinformatic tools can predict ADME-tox properties. New imaging technology visualizes drug action in vivo.
Acknowledgments
Support from the NIH Intramural Research Grant Z01 DK031117 (NIDDK) is acknowledged.
Abbreviations
- cAMP
3′,5′-cyclic adenosine monophosphate
- ADP
adenosine 5′-diphosphate
- GPCR
G protein-coupled receptor
- TM
transmembrane helix
- ERK
extracellular signal-regulated kinase
- T4L
T-4 lysozyme
- BRIL
thermostabilized apocytochrome b562
- StaRs
stabilized receptors
- GRK
GPCR kinase
- DREADD
designer receptor exclusively activated by designer drugs
- NMR
nuclear magnetic resonance
- RGS
regulator of G protein signaling
- 5HT
5-hydroxytrypamine (serotonin)
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- GIP
glucose-dependent insulinotropic peptide
- GLP
glucagon like peptide
- GLP-R
glucagon-like peptide receptor
- CRF-R
corticotropin-releasing factor receptor
- DPP IV
dipeptidyl peptidase-4
- GABA-R
γ-aminobutyric acid receptor
- mGluR
metabotropic glutamate receptor
- HF
heart failure
- IL
intracellular loop
- AT1R
angiotensin receptor type 1
- CCR2
chemokine receptor type 2
- PEG
polyethylene glycol
- PET
positron emission tomography
- PNA
peptide nucleic acid
- SKR
structure-kinetics relationship
- SSRI
selective serotonin reuptake inhibitor
- NIH
National Institutes of Health (United States)
- OR
opioid receptor
- BRET
bioluminescence resonance energy transfer
- ADME
absorption, distribution, metabolism, and excretion
- Tox
toxicology
- CYP450
cytochrome-P450
- SAR
structure activity relationship
- SNP
single-nucleotide polymorphism
- SPR
Surface Plasmon Resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Costanzi S. New insights for drug design from the X-ray crystallographic structures of GPCRs. Mol Pharmacol. 2012;82:361–371. doi: 10.1124/mol.112.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. http://pharmamkting.blogspot.com/2013/01/lipitor-plavix-last-of-small-molecule.html and http://www.forbes.com/sites/simonking/2013/07/15/the-best-selling-drugs-since-1996-why-abbvies-humira-is-set-to-eclipse-pfizers-lipitor/
- 4.Beitelshees AL, McLeod HL. Clopidogrel pharmacogenetics promising steps towards patient care? Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1681–1683. doi: 10.1161/01.ATV.0000232583.51472.73. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Wang H, Ma Z, Wu B. Effects of pharmaceutical PEGylation on drug metabolism and its clinical concerns. Expert Opin Drug Metab Toxicol. 2014;10:1691–1702. doi: 10.1517/17425255.2014.967679. [DOI] [PubMed] [Google Scholar]
- 6.Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 7.Vachharajani NN, Yeleswaram K, Boulton DW. Preclinical pharmacokinetics and metabolism of BMS-214778, a novel melatonin receptor agonist. J Pharm Sci. 2003;92(4):760–772. doi: 10.1002/jps.10348. [DOI] [PubMed] [Google Scholar]
- 8.MacCoss M, Baillie TA. Organic chemistry in drug discovery. Science. 2004;303:1810–1813. doi: 10.1126/science.1096800. [DOI] [PubMed] [Google Scholar]
- 9.Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Ryan G, Coleman RG. ZINC: A Free Tool to Discover Chemistry for Biology. J Chem Inf Model. 2012;52:1757–1768. doi: 10.1021/ci3001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez D, Gao ZG, Moss SM, Jacobson KA, Carlsson J. Molecular docking screening using agonist-bound GPCR structures: Probing the A2A adenosine receptor. J Chem Inf Model. 2015;55:550–563. doi: 10.1021/ci500639g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reutlinger M, Rodrigues T, Schneider P, Schneider G. Combining on-chip synthesis of a focused combinatorial library with computational target prediction reveals imidazopyridine GPCR ligands. Angew Chem Int Ed. 2014;53:582–585. doi: 10.1002/anie.201307786. [DOI] [PubMed] [Google Scholar]
- 12.Fuoco D. Hypothesis for changing models: Current pharmaceutical paradigms, trends and approaches in drug discovery. PeerJ. 2015 Feb;3 [Google Scholar]
- 13.Moro S, Hoffmann C, Jacobson KA. Role of the extracellular loops of G protein-coupled receptors in ligand recognition: A molecular modeling study of the human P2Y1 receptor. Biochemistry. 1999;38:3498–3507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres G, Gainetdinov R, Caron M. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 15.Michino M, Beuming T, Donthamsetti P, Newman AH, Javitch JA, Shi L. What Can Crystal Structures of Aminergic Receptors Tell Us about Designing Subtype-Selective Ligands? Pharmacol Rev. 2015;67(1):198–213. doi: 10.1124/pr.114.009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shonberg J, Kling RC, Gmeiner P, Löber S. GPCR Crystal structures: Medicinal chemistry in the pocket. Bioorg Med Chem. 2014 doi: 10.1016/j.bmc.2014.12.034. doi: http://dx.doi.org/10.1016/j.bmc.2014.12.034. [DOI] [PubMed]
- 17.Kobilka B. The Structural Basis of G-Protein-Coupled Receptor Signaling (Nobel Lecture) Angew Chem Int Ed. 2013;52:6380–6388. doi: 10.1002/anie.201302116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews SP, Brown GA, Christopher JA. Structure-based and fragment-based GPCR drug discovery. ChemMedChem. 2014;9:256–275. doi: 10.1002/cmdc.201300382. [DOI] [PubMed] [Google Scholar]
- 19.Chun E, Thompson AA, Liu W, Roth CB, Griffith MT, Katritch V, et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure. 2012;20(6):967–976. doi: 10.1016/j.str.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhukov A, Andrews SP, Errey JC, et al. Biophysical mapping of the adenosine A2a receptor. J Med Chem. 2011;54(13):4312–4323. doi: 10.1021/jm2003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segala E, Errey JC, Fiez-Vandal C, Zhukov A, Cooke RM. Biosensor-based affinities and binding kinetics of small molecule antagonists to the adenosine A2areceptor reconstituted in HDL like particles. FEBS Lett. 2015;589:1399–1405. doi: 10.1016/j.febslet.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Weichert D, Gmeiner P. Covalent Molecular Probes for Class A G Protein-Coupled Receptors: Advances and Applications. ACS Chem Biol. 2015;10:1376–1386. doi: 10.1021/acschembio.5b00070. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh E, Kumari P, Jaiman D, Shukla AK. Methodological advances: the unsung heroes of the GPCR structural revolution. Nature Rev Mol Cell Biol. 2015;16:69–81. doi: 10.1038/nrm3933. [DOI] [PubMed] [Google Scholar]
- 25.Milić D, Veprintsev DB. Large-scale production and protein engineering of G protein-coupled receptors for structural studies. Front Pharmacol. 2015;6:66. doi: 10.3389/fphar.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512:218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Lee SY, Chung KY. Conformational analysis of G protein-coupled receptor signaling by hydrogen/deuterium exchange mass spectrometry. Meth Enzymol. 2015;557:261–278. doi: 10.1016/bs.mie.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo K, Paoletta S, Kiselev E, Jacobson KA. Molecular modeling of the human P2Y14 receptor: A template for structure-based design of selective agonist ligands. Bioorg Med Chem. 2015;23:4056–4064. doi: 10.1016/j.bmc.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Westen GJ, van den OO, van der Hoven Pijl R, Mulder-Krieger T, de Vries H, Wegner JK, et al. Identifying novel adenosine receptor ligands by simultaneous proteochemometric modeling of rat and human bioactivity data. J Med Chem. 2012;55(16):7010–7020. doi: 10.1021/jm3003069. [DOI] [PubMed] [Google Scholar]
- 30.Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, et al. Structure and Dynamics of the M3 Muscarinic Acetylcholine Receptor. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kufareva I, Katritch V Participants of GPCR Dock 2013. Stevens RC, Abagyan R. Advances in GPCR modeling evaluated by the GPCR Dock 2013 assessment: meeting new challenges. Structure. 2014;22(8):1120–1139. doi: 10.1016/j.str.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, et al. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Gao ZG, Zhang K, Kiselev E, Crane S, Wang J, et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature. 2015;520:317–321. doi: 10.1038/nature14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, Zhang J, Gao ZG, Paoletta S, Zhang D, Han GW, et al. Agonist-bound structure of the human P2Y12R receptor. Nature. 2014;509:119–122. doi: 10.1038/nature13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson KA, Costanzi S, Paoletta S. Computational studies to predict or explain GPCR polypharmacology. Trends Pharmacol Sci. 2014;35:658–663. doi: 10.1016/j.tips.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson KA, Gao ZG, Liang BT. Neoceptors: Reengineering GPCRs to recognize tailored ligands. Trends Pharmacol Sci. 2007;28:111–116. doi: 10.1016/j.tips.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan WK, Zhang H, Yang J, Brender JR, Hur J, Özgür A, et al. GLASS: a comprehensive database for experimentally-validated GPCR-ligand associations. Bioinformatics. 2015 doi: 10.1093/bioinformatics/btv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brogi S, Nebigil-Désaubry CG, Tafi A, Désaubry LG. Discovery of GPCR ligands for probing signal transduction pathways. Front Pharmacol. 2014;5:255. doi: 10.3389/fphar.2014.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane JR, Chubukov P, Liu W, Canals M, Cherezov V, Abagyan R, et al. Structure-based ligand discovery targeting orthosteric and allosteric pockets of dopamine receptors. Mol Pharmacol. 2013;84:794–807. doi: 10.1124/mol.113.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolb P, Phan K, Gao ZG, Marko AC, Sali A, Jacobson KA. Limits of ligand selectivity from docking to models: In silico screening for A1 adenosine receptor antagonists. PLoS ONE. 2012;7:e49910. doi: 10.1371/journal.pone.0049910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christopher JA, Brown J, Doré AS, Errey JC, Koglin M, Marshall FH, et al. Biophysical fragment screening of the β1-adrenergic receptor: identification of high affinity arylpiperazine leads using structure-based drug design. J Med Chem. 2013;56:3446–3455. doi: 10.1021/jm400140q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji XD, Melman N, Jacobson KA. Interactions of flavonoids and other phytochemicals with adenosine receptors. J Med Chem. 1996;39:781–788. doi: 10.1021/jm950661k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chackalamannil S, Wang Y, Greenlee WJ, Hu Z, Xia Y, Ahn HS, et al. Discovery of a Novel, Orally Active Himbacine-Based Thrombin Receptor Antagonist (SCH 530348) with Potent Antiplatelet Activity. J Med Chem. 2008;51:3061–3064. doi: 10.1021/jm800180e. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Ma Y, Li J, Ma J, Yu B, Xie X. Molecular matchmaking between the popular weight-loss herb Hoodia gordonii and GPR119, a potential drug target for metabolic disorder. Proc Natl Acad. Sci USA. 2014;111:14571–14576. doi: 10.1073/pnas.1324130111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nature Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 46.Im DS. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.03.094. in press. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Hopkins MM, Zhang Z, Quisenberry CB, Fix LC, Galvan BM, et al. Omega-3 fatty acids and other FFA4 agonists inhibit growth factor signaling in human prostate cancer cells. J Pharmacol Exp Ther. 2015;352:380–394. doi: 10.1124/jpet.114.218974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langenhan T, Barr MM, Bruchas MR, Ewer J, Griffith LC, Maiellaro I, et al. Model Organisms in GPCR Research. Mol Pharmacol. 2015 doi: 10.1124/mol.115.098764. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson KA, von Lubitz DKJE, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute and chronic treatment. Trends Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bock A, Kostenis E, Tränkle C, Lohse M, Mohr K. Pilot the pulse: controlling the multiplicity of receptor dynamics. Trends Pharmacol Sci. 2014;35:620–638. doi: 10.1016/j.tips.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Karki P, Kurihara T, Nakamachi T, Watanabe J, Asada T, Oyoshi T, et al. Attenuation of inflammatory and neuropathic pain behaviors in mice through activation of free fatty acid receptor GPR40. Mol Pain. 2015;11:6. doi: 10.1186/s12990-015-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picone RP, Kendall DA. Minireview: From the Bench, Toward the Clinic: Therapeutic Opportunities for Cannabinoid Receptor Modulation. Mol Endocrinol. 2015;29:801–813. doi: 10.1210/me.2015-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uphouse L. Pharmacology of serotonin and female sexual behavior. Pharmacol Biochem Behav. 2014;121:31–42. doi: 10.1016/j.pbb.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monk KR, Hamann J, Langenhan T, Nijmeijer S, Schöneberg T, Liebscher I. Adhesion GPCRs: From In Vitro Pharmacology to In Vivo Mechanisms. Mol Pharmacol. 2015 doi: 10.1124/mol.115.098749. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor (A3AR) agonists. Drug Disc Today. 2012;17:359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao ZG, Balasubramanian R, Kiselev E, Wei Q, Jacobson KA. Probing biased/partial agonism at the G protein-coupled A2B adenosine receptor. Biochem Pharmacol. 2014;90:297–306. doi: 10.1016/j.bcp.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eisenstein A, Patterson S, Ravid K. The many faces of the A2b adenosine receptor in cardiovascular and metabolic diseases. J Cell Physiol. 2015 doi: 10.1002/jcp.25043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giguere PM, Kroeze WK, Roth BL. Tuning up the right signal: chemical and genetic approaches to study GPCR functions. Curr Opin Cell Biol. 2014;27:51–55. doi: 10.1016/j.ceb.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefkowitz R. A Brief History of G-Protein Coupled Receptors (Nobel Lecture) Angew Chem Int Ed. 2013;52:6366–6378. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- 60.Erickson CE, Gul R, Blessing CP, Nguyen J, Liu T, Pulakat L, et al. The β-blocker nebivolol is a GRK/β-arrestin biased agonist. PLoS ONE. 2013;8(8):e71980. doi: 10.1371/journal.pone.0071980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, et al. The β-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol. 2013;305:H856–H866. doi: 10.1152/ajpheart.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci. 2014;35:308–316. doi: 10.1016/j.tips.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Gao ZG, Jacobson KA. Translocation of arrestin induced by human A3 adenosine receptor ligands in an engineered cell line: Comparison with G protein-dependent pathways. Pharmacol Res. 2008;57:303–311. doi: 10.1016/j.phrs.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson GL, Lane JR, Courdat T, Sexton PM, Christopoulos A, Canals M. Biased Agonism of Endogenous Opioid Peptides at the Mu-Opioid Receptor. Mol Pharmacol. 2015;88:335–346. doi: 10.1124/mol.115.098848. [DOI] [PubMed] [Google Scholar]
- 65.Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, et al. Structural Features for Functional Selectivity at Serotonin Receptors. Science. 2013;340:615–619. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stahl EL, Zhou L, Ehlert FJ, Bohn LM. A novel method for analyzing extremely biased agonism at G protein-coupled receptors. Mol Pharmacol. 2015;87(5):866–877. doi: 10.1124/mol.114.096503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Srinivasan Y, Arlow DH, Fung JJ, Palmer D, Zheng Y, et al. High-resolution crystal structure of human protease-activated receptor 1. Nature. 2102;492:387–392. doi: 10.1038/nature11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin J, Mobarec JC, Kolb P, Rosenbaum DM. Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature. 2015;519(7542):247–250. doi: 10.1038/nature14035. [DOI] [PubMed] [Google Scholar]
- 69.Miyabe M, Gin A, Onozawa E, Daimon M, Yamada H, Oda H, et al. Genetic variants of the unsaturated fatty acid receptor GPR120 relating to obesity in dogs. J Vet Med Sci. 2015 doi: 10.1292/jvms.15-0031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maudsley S, Martin B, Gesty-Palmer D, Cheung H, Johnson C, Patel S, et al. Delineation of a Conserved Arrestin-Biased Signaling Repertoire In Vivo. Mol. Pharmacol. 2015;87:706–717. doi: 10.1124/mol.114.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, et al. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107(4):532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7(277):277ra31. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lymperopoulos A, Rengo G, Koch WJ. GRK2 inhibition in heart failure: something old, something new. Curr Pharm Des. 2012;18(2):186–191. doi: 10.2174/138161212799040510. [DOI] [PubMed] [Google Scholar]
- 74.Homan KT, Larimore KM, Elkins JM, Szklarz M, Knapp S, Tesmer JJ. Identification and structure-function analysis of subfamily selective G protein-coupled receptor kinase inhibitors. ACS Chem Biol. 2015;10(1):310–319. doi: 10.1021/cb5006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malach E, Shaul ME, Peri I, Huang L, Spielman AI, Seger R, et al. Membrane-permeable tastants amplify β2-adrenergic receptor signaling and delay receptor desensitization via intracellular inhibition of GRK2's kinase activity. Biochim Biophys Acta. 2015;1850(7):1375–1388. doi: 10.1016/j.bbagen.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 76.Lowe JD, Sanderson HS, Cooke AE, Ostovar M, Tsisanova E, Withey SL, et al. Role of G Protein-Coupled Receptor Kinases 2 and 3 in µ-Opioid Receptor Desensitization and Internalization. Mol Pharmacol. 2015;88:347–356. doi: 10.1124/mol.115.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woodard GE, Jardín I, Berna-Erro A, Salido GM, Rosado JA. Regulators of G-protein-signaling proteins: negative modulators of G-protein-coupled receptor signaling. Int Rev Cell Mol Biol. 2015;317:97–183. doi: 10.1016/bs.ircmb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Karpinsky-Semper D, Volmar CH, Brothers SP, Slepak VZ. Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor–induced Ca2+ influx and release. Mol Pharmacol. 2014;85:758–768. doi: 10.1124/mol.114.091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blazer LL, Storaska AJ, Jutkiewicz EM, Turner EM, Calcagno M, Wade SM, et al. Selectivity and Anti-Parkinson's Potential of Thiadiazolidinone RGS4 Inhibitors. ACS Chem Neurosci. 2015;6:911–919. doi: 10.1021/acschemneuro.5b00063. [DOI] [PubMed] [Google Scholar]
- 80.Stewart A, Maity B, Anderegg SP, Allamargot C, Yang J, Fisher RA. Regulator of G protein signaling 6 is a critical mediator of both reward-related behavioral and pathological responses to alcohol. Proc Natl Acad Sci USA. 2015;112:E786–E795. doi: 10.1073/pnas.1418795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jia L, Chisari M, Maktabi MH, Sobieski C, Zhou H, Konopko AM, et al. A mechanism regulating G protein-coupled receptor signaling that requires cycles of protein palmitoylation and depalmitoylation. J Biol Chem. 2014;289(9):6249–6257. doi: 10.1074/jbc.M113.531475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urs NM, Nicholls PJ, Caron MG. Integrated approaches to understanding antipsychotic drug action at GPCRs. Curr Opin Cell Biol. 2014;27:56–62. doi: 10.1016/j.ceb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szentmiklosi AJ, Galajda Z, Cseppento A, Gesztelyi R, Susan Z, Hegyi B, et al. The Janus face of adenosine: antiarrhythmic and proarrhythmic actions. Curr Pharmaceut Des. 2015;21:965–976. doi: 10.2174/1381612820666141029100346. [DOI] [PubMed] [Google Scholar]
- 84.Winquist RJ, Mullane K, Williams K. The fall and rise of pharmacology – (Re-)defining the discipline? Biochem Pharmacol. 2014;87:4–24. doi: 10.1016/j.bcp.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Guo D, Hillger JM, IJzerman AP, Heitman LH. Drug-target residence time-a case for G protein-coupled receptors. Med Res Rev. 2014;34:856–892. doi: 10.1002/med.21307. [DOI] [PubMed] [Google Scholar]
- 86.Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, IJzerman AP, et al. Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol. 2011;82:658–668. doi: 10.1016/j.bcp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vilums M, Zweemer AJ, Barmare F, van der Gracht AM, Bleeker DC, Yu Z, et al. When structure-affinity relationships meet structure-kinetics relationships: 3-((Inden-1-yl)amino)-1-isopropyl-cyclopentane-1-carboxamides as CCR2 antagonists. Eur J Med Chem. 2015;93:121–134. doi: 10.1016/j.ejmech.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 88.Wooten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nature Rev Drug Disc. 2013;12:630–644. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 89.Gsandtner I, Charalambous C, Stefan E, Ogris E, Freissmuth M, Zezula J. Heterotrimeric G protein-independent signaling of a G protein-coupled receptor: direct binding of arno/cytohesin-2 to the carboxyl terminus of the A2A adenosine receptor is necessary for sustained activation of the ERK/MAP kinase pathway. J Biol Chem. 2005;280:31898–31905. doi: 10.1074/jbc.M506515200. [DOI] [PubMed] [Google Scholar]
- 90.Gao ZG, Jacobson KA. Allosteric modulation functional selectivity of G protein-coupled receptors Drug Disc. Today: Technologies. 2013;10:e237–e242. doi: 10.1016/j.ddtec.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schann S, Neuville P, Bouvier M. Chapter Eighteen - Novel Screening Paradigms for the Identification of Allosteric Modulators and/or Biased Ligands for Challenging G-Protein-Coupled Receptors. In: Desai Manoj C, editor. Annual Rep Med Chem. Vol. 49. 2014. pp. 285–300. [Google Scholar]
- 92.Conn PJ, Lindsley CW, Meiler J, Niswender CM. Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nature Rev Drug Disc. 2014;13:692–708. doi: 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rondard J, Pin JP. Dynamics and modulation of metabotropic glutamate receptors. Curr Opin Pharmacol. 2015;20:95–101. doi: 10.1016/j.coph.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Nolte WM, Fortin JP, Stevens BD, Aspnes GE, Griffith DA, Hoth LR, Ruggeri RB, et al. A potentiator of orthosteric ligand activity at GLP-1R acts via covalent modification. Nat Chem Biol. 2014;10(8):629–631. doi: 10.1038/nchembio.1581. [DOI] [PubMed] [Google Scholar]
- 95.Burford NT, Livingston KE, Canals M, Ryan MR, Budenholzer LM, Han Y, et al. Discovery, Synthesis, and Molecular Pharmacology of Selective Positive Allosteric Modulators of the δ-Opioid Receptor. J Med Chem. 2015;58(10):4220–4229. doi: 10.1021/acs.jmedchem.5b00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang P, Leger AJ, Baleja JD, Rana R, Corlin T, Nguyen N, et al. Allosteric activation of a G protein coupled receptor with cell-penetrating receptor mimetics. J Biol Chem. 2015 May 1; doi: 10.1074/jbc.M115.636316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014;513:124–127. doi: 10.1038/nature13494. [DOI] [PubMed] [Google Scholar]
- 98.Narlawar R, Lane JR, Doddareddy M, Lin J, Brussee J, IJzerman AP. Hybrid Ortho/Allosteric Ligands for the Adenosine A1 Receptor. J Med Chem. 2010;53:3028–3037. doi: 10.1021/jm901252a. [DOI] [PubMed] [Google Scholar]
- 99.Guidolin D, Agnati LF, Marcoli M, Borroto-Escuela DO, Fuxe K. G-protein-coupled receptor type A heteromers as an emerging therapeutic target. Expert Opin Ther Targets. 2015;19(2):265–283. doi: 10.1517/14728222.2014.981155. [DOI] [PubMed] [Google Scholar]
- 100.Lambert NA, Javitch JA. CrossTalk opposing view: weighing the evidence for class A GPCR dimers, the jury is still out. J Physiol. 2014;592:2443–2445. doi: 10.1113/jphysiol.2014.272997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue L, Rovira X, Scholler P, Zhao H, Liu J, Pin JP, et al. Major ligand-induced rearrangement of the heptahelical domain interface in a GPCR dimer. Nature Chem Biol. 2015;11:134–140. doi: 10.1038/nchembio.1711. [DOI] [PubMed] [Google Scholar]
- 102.Kasai RS, Kusumi A. Single-molecule imaging revealed dynamic GPCR dimerization. Curr Opin Cell Biol. 2014;27:78–86. doi: 10.1016/j.ceb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 103.Tummino PJ, Copeland RA. Residence time of receptor-ligand complexes and its effect on biological function. Biochemistry. 2008;47(20):5481–5492. doi: 10.1021/bi8002023. [DOI] [PubMed] [Google Scholar]
- 104.Ferré S. The GPCR heterotetramer: challenging classical pharmacology. Trends Pharmacol Sci. 2015;36:145–152. doi: 10.1016/j.tips.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernández-Dueñas V, Gómez-Soler M, Jacobson KA, Kumar TS, Fuxe K, Borroto-Escuela DO, et al. Molecular determinants of the adenosine A2A R-dopamine D2 receptor-receptor allosterism: Role of the intracellular loop 3 of the dopamine D2 receptor. J Neurochem. 2012;123:373–384. doi: 10.1111/j.1471-4159.2012.07956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spehr M, Munger SD. Olfactory receptors: G protein-coupled receptors and beyond. J Neurochem. 2009 Jun;109(6):1570–1583. doi: 10.1111/j.1471-4159.2009.06085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ayoub MA, Zhang Y, Kelly RS, See HB, Johnstone EKM, McCall EA, et al. Functional Interaction between AT1R and CCR2 receptor with Implications for Chronic Kidney Disease. PLOS ONE. 2015;10(3):e0119803. doi: 10.1371/journal.pone.0119803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mugnaini C, Pedani V, Casu A, Lobina C, Casti A, Maccioni P, et al. Synthesis and Pharmacological Characterization of 2-(Acylamino)thiophene Derivatives as Metabolically Stable, Orally Effective, Positive Allosteric Modulators of the GABAB Receptor. J Med Chem. 2013;56(9):3620–3635. doi: 10.1021/jm400144w. [DOI] [PubMed] [Google Scholar]
- 109.Le Naour M, Lunzer MM, Powers MD, Kalyuzhny AE, Benneyworth MA, Thomas MJ, et al. Putative kappa opioid heteromers as targets for developing analgesics free of adverse effects. J Med Chem. 2014;57:6383–6392. doi: 10.1021/jm500159d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Conde-Ceide S, Martínez-Viturro CM, Alcázar J, Garcia-Barrantes PM, Lavreysen H, Mackie C, et al. Discovery of VU0409551/JNJ-46778212: An mGlu5 Positive Allosteric Modulator Clinical Candidate Targeting Schizophrenia. ACS Med Chem Lett. 2015;6:716–720. doi: 10.1021/acsmedchemlett.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dix AV, Moss SM, Phan K, Hoppe T, Paoletta S, Kozma E, et al. Programmable nanoscaffolds that control ligand display to a G protein coupled-receptor in membranes allow dissection of multivalent effects. J Am Chem Soc. 2014;136:12296–12303. doi: 10.1021/ja504288s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez C, El Hajj Diab D, Connord V, Clerc P, Meunier E, Pipy B, et al. Targeting a G-Protein-Coupled Receptor Overexpressed in Endocrine Tumors by Magnetic Nanoparticles To Induce Cell Death. ACS Nano. 2014;8(2):1350–1363. doi: 10.1021/nn404954s. [DOI] [PubMed] [Google Scholar]
- 113.Jacobson KA. Philip S, editor. Structure-based approaches to ligands for G protein-coupled adenosine and P2Y receptors, from small molecules to nanoconjugates. 2012 Philip S. Portoghese Medicinal Chemistry Lectureship. J Med Chem. 2013;56:3749–3767. doi: 10.1021/jm400422s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492(7428):215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Y, Palczewski K. Systems Pharmacology Links GPCRs with Retinal Degenerative Disorders. Ann Rev Pharmacol Toxicol. 2015;56:1.1–1.26. doi: 10.1146/annurev-pharmtox-010715-103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rochais C, Lecoutey C, Gaven F, Giannoni P, Hamidouche K, Hedou D, et al. Novel multitarget-directed ligands (MTDLs) with acetylcholinesterase (AChE) inhibitory and serotonergic subtype 4 receptor (5-HT4R) agonist activities as potential agents against Alzheimer’s disease: The design of donecopride. J Med Chem. 2015;58:3172–3187. doi: 10.1021/acs.jmedchem.5b00115. [DOI] [PubMed] [Google Scholar]
- 118.Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsvetanova NG, Irannejad R, von Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J Biol Chem. 2015;290(11):6689–6696. doi: 10.1074/jbc.R114.617951. Epub 2015 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsson P, Fenu LA, Lundquist P, Wiśniewski JR, Kansy M, Artursson P. Quantifying the impact of transporters on cellular drug permeability. Trends Pharmacol Sci. 2015;36:255–262. doi: 10.1016/j.tips.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 121.Webb DR, Handel TM, Kretz-Rommel A, Stevens RC. Opportunities for functional selectivity in GPCR antibodies. Biochem Pharmacol. 2013;85(2):147–152. doi: 10.1016/j.bcp.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harris GL, Creason MB, Brulte GB, Herr DR. In Vitro and In Vivo Antagonism of a G Protein-Coupled Receptor (S1P3) with a Novel Blocking Monoclonal Antibody. PLOS One. 2012;7:e35129. doi: 10.1371/journal.pone.0035129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mujic-Delic A, de Wit RH, Verkaar F, Smit MJ. GPCR-targeting nanobodies: attractive research tools, diagnostics, and therapeutics. Trends Pharmacol Sci. 2014;35:247–255. doi: 10.1016/j.tips.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Koglin M, Hutchings CJ. BioProcess international. 2014 http://www.bioprocessintl.com/upstream-processing/expression-platforms/targeting-g-proteincoupled-receptors-with-biologics-for-therapeutic-use-part-1/
- 125.Thompson MD, Cole DEC, Capra V, Siminovitch KA, Rovati GE, Burnham WM, et al. Pharmacogenetics of the G Protein-Coupled Receptors. In: Yan Qing, editor. Pharmacogenomics in Drug Discovery and Development, Methods in Molecular Biology. Vol. 1175. 2014. pp. 189–242. [DOI] [PubMed] [Google Scholar]
- 126.Lecchi A, Razzari C, Paoletta S, Dupuis A, Nakamura L, Ohlmann P, et al. Identification of a new dysfunctional platelet P2Y12 receptor variant, associated with bleeding diathesis. Blood. 2015;125:1006–1013. doi: 10.1182/blood-2013-07-517896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang Y, Axelssen AS, Spégel P, Andersson LE, Mulder H, Groop LC, et al. Genotype-based treatment of type 2 diabetes with an α2A–adrenergic receptor antagonist. Sci Transl Med. 2014;6:257ra139. doi: 10.1126/scitranslmed.3009934. [DOI] [PubMed] [Google Scholar]
- 128.Jo E, Bhhatarai B, Repetto E, Guerrero M, Riley S, Brown SJ, et al. Novel Selective Allosteric and Bitopic Ligands for the S1P3 Receptor. ACS Chem Biol. 2012;7(12):1975–1983. doi: 10.1021/cb300392z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu Z, Xu J, Rossi GC, Majumdar S, Pasternak GW, Pan YX. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest. 2015 May 26; doi: 10.1172/JCI81070. Epub 2015 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol. 2008;35(1):41–48. [PubMed] [Google Scholar]
- 131.Stoddart LA, Kilpatrick LE, Briddon SJ, Hill SJ. Probing the pharmacology of G protein-coupled receptors with fluorescent ligands. Neuropharmacol. doi: 10.1016/j.neuropharm.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 132.Chen D, Errey JC, Heitman LH, Marshall FH, IJzerman AP, Siegal G. Fragment Screening of GPCRs Using Biophysical Methods: Identification of Ligands of the Adenosine A2A Receptor with Novel Biological Activity. ACS Chem Biol. 2012;7(12):2064–2073. doi: 10.1021/cb300436c. [DOI] [PubMed] [Google Scholar]
- 133.Fang Y. Label-free drug discovery. Front Pharmacol. 2014;5:52. doi: 10.3389/fphar.2014.00052. http://dx.doi.org/10.3389/fphar.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ciruela F, Jacobson KA, Fernández-Dueñas V. Portraying G protein-coupled receptors with fluorescent ligands. ACS Chem Biol. 2014;9:1918–1928. doi: 10.1021/cb5004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kiselev E, Barrett M, Katritch V, Paoletta S, Weitzer CD, Hammes E, et al. Exploring a 2-naphthoic acid template for the structure-based design of P2Y14 receptor antagonist molecular probes. ACS Chem Biol. 2014;9:2833–2842. doi: 10.1021/cb500614p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brand F, Klutz A, Jacobson KA, Fredholm BB, Schulte G. Adenosine A2areceptor dynamics studied with the novel fluorescent agonist Alexa488-APEC. Eur J Pharmacol. 2008;590:36–42. doi: 10.1016/j.ejphar.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakamura Y, Ishii J, Kondo A. Applications of yeast-based signaling sensor for characterization of antagonist and analysis of site-directed mutants of the human serotonin 1A receptor. Biotechnol Bioeng. 2015 doi: 10.1002/bit.25597. in press. [DOI] [PubMed] [Google Scholar]
- 138.Moreau CJ, Niescierowicz K, Caro LN, Revilloud J, Vivaudou M. Ion Channel Reporter for Monitoring the Activity of Engineered GPCRs. Meth Enzymol. 2015;556:425–454. doi: 10.1016/bs.mie.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 139.Zhang T, Heimbach T, Lin W, Zhang J, He H. Prospective Predictions of Human Pharmacokinetics for Eighteen Compounds. J Pharm Sci Article first published online. 2015 Feb 17; doi: 10.1002/jps.24373. [DOI] [PubMed] [Google Scholar]
- 140.Mihara T, Noda A, Arai H, Mihara K, Iwashita A, Murakami Y, et al. Brain adenosine A2Areceptor occupancy by a novel A1/A2A receptor antagonist, ASP5854, in rhesus monkeys: relationship to anticataleptic effect. J Nucl Med. 2008;49:1183–1188. doi: 10.2967/jnumed.108.051474. [DOI] [PubMed] [Google Scholar]
- 141.Insel PA, Wilderman A, Zambon AC, Snead AN, Murray F, Aroonsakool N, et al. GPCR expression in native cells: “Novel” endoGPCRs as physiologic regulators and therapeutic targets. Mol Pharmacol. 2015 doi: 10.1124/mol.115.098129. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balenga NA, Martínez-Pinilla E, Kargl J, Schröder R, Peinhaupt M, Platzer W, et al. Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signaling. Br J Pharmacol. 2014;171:5387–5406. doi: 10.1111/bph.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miyano K, Sudo Y, Yokoyama A, Hisaoka-Nakashima K, Morioka N, Takebayashi M, et al. History of the G Protein-Coupled Receptor (GPCR) Assays From Traditional to a State-of-the-Art Biosensor Assay. J Pharmacol Sci. 2014;126:302–309. doi: 10.1254/jphs.14R13CP. [DOI] [PubMed] [Google Scholar]
- 144.Watari K, Nakaya M, Kurose H. Multiple functions of G protein-coupled receptor kinases. J Mol Signal. 2014;9:1. doi: 10.1186/1750-2187-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim SY, Mammen A, Yoo SJ, Cho B, Kim EK, Park JI, et al. Phosphoinositide and Erk signaling pathways mediate activity-driven rodent olfactory sensory neuronal survival and stress mitigation. J Neurochem Article first published online. 2015 Jun 8; doi: 10.1111/jnc.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tosh DK, Finley A, Paoletta S, Moss SM, Gao ZG, Gizewski E, et al. In vivo phenotypic screening for treating chronic neuropathic pain: Modification of C2-arylethynyl group of conformationally constrained A3 adenosine receptor agonists. J Med Chem. 2014;57:9901–9914. doi: 10.1021/jm501021n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schmitz J, van der Mey D, Bermudez M, Kloeckner J, Schrage R, Kostenis E, et al. Dualsteric Muscarinic Antagonists - Orthosteric Binding Pose Controls Allosteric Subtype Selectivity. J Med Chem. 2014;57:6739–6750. doi: 10.1021/jm500790x. [DOI] [PubMed] [Google Scholar]
- 148.Graaf C, Rein C, Piwnica D, Giordanetto F, Rognan D. Structure-based discovery of allosteric modulators of two related class B G-protein coupled receptors. ChemMedChem. 2011;6(12):2159–2169. doi: 10.1002/cmdc.201100317. [DOI] [PubMed] [Google Scholar]
- 149.Blättermann S, Peters L, Ottersbach PA, Bock A, Konya V, Weaver CD, et al. A biased ligand for OXE-R uncouples Gα and Gβγ signaling within a heterotrimer. Nature Chem Biol. 2012;8:631–638. doi: 10.1038/nchembio.962. [DOI] [PubMed] [Google Scholar]
- 150.Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H, Schlessinger A, et al. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nature Chem Biol. 2011;7(11):769–778. doi: 10.1038/nchembio.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kolb P, Rosenbaum DM, Irwin JJ, Fung JJ, Kobilka BK, Shoichet BK. Structure-based discovery of beta2-adrenergic receptor ligands. Proc Natl Acad Sci USA. 2009;106:6843–6848. doi: 10.1073/pnas.0812657106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: A Promiscuous L-α-Amino Acid Receptor with Preference for Basic Amino Acids. Mol Pharmacol. 2005;67(3):589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- 153.Krishnan A, Schiöth HB. The role of G protein-coupled receptors in the early evolution of neurotransmission and the nervous system. J Exp Biol. 2015;218:562–571. doi: 10.1242/jeb.110312. [DOI] [PubMed] [Google Scholar]
- 154.Hoffmann C, Castro M, Rinken A, Leurs R, Hill SJ, Vischer HF. Ligand residence time at GPCRs – why we should take our time to study it. Mol Pharmacol. 2015 doi: 10.1124/mol.115.099671. in press. [DOI] [PubMed] [Google Scholar]
- 155.Stoddart LA, Johnstone EKM, Wheal AJ, Goulding J, Robers MB, Machleidt T, et al. Application of BRET to monitor ligand binding to GPCRs. Nature Meth. 2015 doi: 10.1038/nmeth.3398. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodríguez D, Ranganathan A, Carlsson J. Discovery of GPCR Ligands by Molecular Docking Screening: Novel Opportunities Provided by Crystal Structures. Curr Topics Med Chem. 2015 doi: 10.2174/1568026615666150701112853. in press. [DOI] [PubMed] [Google Scholar]
- 157.Sauliere A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, et al. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 158.Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- 159.Vistein R, Puthenveedu MA. Reprogramming of G protein- coupled receptor recycling and signaling by a kinase switch. Proc Natl Acad Sci USA. 2013;110:15289–15294. doi: 10.1073/pnas.1306340110. [DOI] [PMC free article] [PubMed] [Google Scholar]