Abstract
The opioid epidemic in the USA continues to worsen. Medical providers are faced with the challenge of addressing complications from opioid use disorders and associated injection drug use. Unsafe injection practices among people who inject drugs (PWID) can lead to several complications requiring acute care encounters in the emergency department and inpatient hospital. Our objective is to provide a narrative review to help medical providers recognise and address key health issues in PWID, who are being released from the emergency department and inpatient hospital. In the midst of rises in overdose deaths and infections such as hepatitis C, we highlight several health issues for PWID, including overdose and infection prevention. We provide a clinical checklist of actions to help guide providers in the care of these complex patients. The clinical checklist includes strategies also applicable to low-resource settings, which may lack addiction treatment options. Our review and clinical checklist highlight key aspects of optimising the health and safety of PWID.
INTRODUCTION
The transition from acute to outpatient care for people who inject drugs (PWID) after prolonged, complex hospitalisations can be challenging for both patients and providers.1 Our objective is to provide a narrative review to help medical providers recognise and address key health issues in PWID, particularly at the point of follow-up after injection drug-related hospital or emergency department (ED) visits. In this review, we begin with a case presentation to illustrate the opportunities to address these issues and end with clinical recommendations in the form of a checklist.
METHODOLOGY
We searched PubMed through December 2015 for review articles and clinical guidelines pertaining to the care of PWID. Examples of search terms that we used included injection drug users, illicit drug use, people who inject drugs, substance abuse, opioid dependence, mental health and substance abuse and harm reduction. We also manually searched the reference lists from identified articles and focused on those articles, along with other individual manuscripts, that were clinically relevant to this topic. The clinical checklist for this manuscript was generated based on evidence-based guidelines and clinical experience.
CASE PRESENTATION
A male aged 29 years with history of injection drug use had two admissions for methicillin-sensitive Staphylococcus aureus (MSSA) tricuspid valve (TV) endocarditis.
First admission: The patient, who presented with fever and shortness of breath, was found to have MSSA native TV endocarditis, complicated by complete heart block and septic emboli. He was treated with intravenous (IV) antibiotics, dual-chamber pacemaker placement and bioprosthetic TV replacement. While in the hospital he was treated with methadone for opioid use disorder, but this was eventually tapered off. The patient followed up with his primary care physician, who offered buprenorphine treatment and clinic-based counselling. The patient declined, reporting he was aware of the available resources and planned to seek out help ‘if he needed it’.
Second admission: During the year after discharge, the patient relapsed to injecting heroin again. He presented with fever and shortness of breath, and was subsequently treated for MSSA triscupid bioprosthetic valve endocarditis requiring IV antibiotics and pacemaker removal in the setting of persistent bacteremia. His opioid withdrawal was initially treated with methadone, but this was discontinued due to QT prolongation, and not restarted due to multiple episodes of torsade de pointes while off of methadone. After his bacteremia cleared and his pacemaker was replaced, the plan was to discharge the patient on a taper of his opioid medications, with plan for outpatient buprenorphine induction.
In the following narrative review organised as a clinical checklist, we outline the key health issues in PWID, raised by this case. Within the clinical checklist, we provide concrete actions, with an emphasis on overdose (OD) and infection prevention that can be taken to improve health and safety of PWID.
CHECKLIST COMPONENTS ADDICTION HISTORY
Interviewing patients in a non-judgmental manner and approaching addiction like any other medical illness is an important component of taking an addiction history. Both open-ended questions (‘How has heroin use affected your life?’) and quantifiable questions (‘How many times did you use heroin last month?’) should be asked in order to gather more information about patterns of drug use.2 Addiction is a developmental disease that plays out over the life course through recurrent periods of relapse and recovery, where younger age at initiation and higher exposure lead to worse and more chronic outcomes. Therefore, asking about age of first substance use (including tobacco, alcohol, marijuana and other illicit drugs other than opioids) can be useful in understanding the prognosis.3 Understanding the patient’s personal relapse triggers, coping skills, recovery tools and prior experience with treatment are all helpful for creating a strengths-based risk reduction, treatment and relapse prevention plan. An OD history should be collected, as OD is the leading cause of preventable death in PWID.
Street pills/polysubstance use
Polysubstance use is common and should be accounted for in the treatment plan. The use of other drugs such as cocaine and benzodiazepines can complicate management, especially for patients on medication treatment for addiction. Prescriptions with ‘street value’ and misuse potential include but are not limited to opioids, stimulants, benzodiazepines, clonidine, promethazine, gabapentin and quetiapine.4,5 Patients may divert or use these medications to self-medicate underlying symptoms and/or boost ‘the high’ or euphoria from using substances.6–9 Prescribers should educate patients about the risk of tolerance, withdrawal and safety concerns associated with prescription pill misuse. Communication between prescribers is important to reduce the risk of adverse interactions.10,11 These prescription medications should only be prescribed if a demonstrable benefit can be documented and it outweighs the concomitant risk. Urine drug screens can be used to monitor when non-adherence or diversion is suspected; however, not all diverted and misused prescription medications are detectable. A confirmatory method such as gas chromatography-mass spectrometry can be employed to confirm whether or not the drug is actually present in the urine in low levels.12
READINESS FOR TREATMENT
Assessing readiness for change is an integral part of determining what combination of harm reduction services and treatment are appropriate for each patient. This approach involves assessing what stage of motivation for treatment patients are in and tailoring the treatment and safety plan to this motivation stage.13,14 Patients who are highly motivated to seek treatment for addiction may be more appropriate for office-based treatment. However, patients who are more ambivalent about seeking treatment may benefit from a focus on safety planning (also known as harm reduction). A patient-centred approach that accounts for the patients’ preferences and prior experience with specific modalities as well as knowing accessible community resources facilitates formulation and prioritisation of treatment and safety planning.
TREATMENT OPTIONS
In the USA, the majority of people presenting to care who use injection drugs have an opioid use disorder. There are three medications currently approved by the US Food and Drug Administration (FDA) to treat opioid use disorders: buprenorphine, methadone and naltrexone.13,14 These medications can effectively and legally be initiated during an inpatient hospitalisation by clinical providers.15
Opioid agonist therapy
Buprenorphine is a mu-receptor partial agonist and is coformulated with naloxone, a mu-receptor antagonist, in order to prevent misuse and OD. Buprenorphine/naloxone is an effective treatment option for opioid dependence particularly in the office-based setting. It is safe and well-tolerated, requiring less monitoring than methadone.16–21 Buprenorphine monotherapy can be used in pregnant women.22 Initiating buprenorphine opioid agonist therapy (OAT) for hospitalised patients or patients seen in the ED who are not otherwise engaged in care has also been shown to be effective in linkage to outpatient care.23–25 Methadone is a mu-receptor agonist that is administered daily through federally regulated clinics.18,26 Improved outcomes associated with OAT include reduced opioid use, OD, medical costs and hospital utilisation.27–29 Additional benefits of methadone maintenance therapy (MMT) include reduced criminal activity and reduced HIV transmission.30,31 MMT has been associated with greater retention in care compared with buprenorphine, though evidence is mixed depending on dosing schedules.26
Opioid antagonist therapy
Naltrexone is a mu-receptor antagonist that can be administered orally or in sustained-release form. It is approved for opioid and alcohol use disorders.32–34 Oral naltrexone effectiveness has been limited largely due to adherence issues.17 Long-acting, injectable naltrexone is effective for opioid dependence treatment.35,36 However, the need for patients to be opioid-abstinent for >7 days to avoid precipitated withdrawal can present a substantial barrier to initiating injectable naltrexone.
MENTAL HEALTH
Patients with substance use disorders have a high burden of mental health comorbidities.37 The treatment of addiction problems can unmask underlying mental illness that if untreated, can lead to relapse to substances or a worsening of the underlying mental health problem.38 Individual and/or group counselling, psychopharmacotherapy and supportive services, such as case management, are important components of mental health services for patients with co-occurring mental health and substance use disorders.39 Some studies have found the integration of mental health services with substance use treatment to be equivocal, largely due to adherence issues.40–42 However, with increased emphasis on the patient-centred home, integrating mental healthcare into the addiction treatment and/or primary care setting does has potential benefits,43,44 including mental health treatment retention45 and substance-negative urines.46 Providers should facilitate access to collaborative mental healthcare when patients have mental health problems and care is available.45,47
OVERDOSE PREVENTION
Opioid OD deaths, from both prescription opioids and heroin, have risen rapidly over the past decade among all age groups, genders and almost all racial/ethnic groups.48 Strategies to address OD prevention include OAT (described above), the use of naloxone rescue kits, prescription monitoring programmes (PMPs), safe opioid prescribing, safe storage and disposal and supervised injection facilities (SIFs).49–57
Naloxone rescue kits
The Office of National Drug Control Policy, the US Department of Health and Human Services, the American Medical Association, the Substance Abuse and Mental Health Services Association and the American Society of Addiction Medicine endorse OD prevention education and equipping people with naloxone rescue kits.58–61 Naloxone is cost-effective, rapidly acting, non-addictive opioid antagonist with minimal adverse side effects and is particularly effective when distributed in the hands of PWID and their social networks.62–65 Providers should prescribe naloxone rescue kits to their patients to lower their own risk and to also review how patients will respond to an opioid OD, if they witness one, which includes recognising it, calling for help, rescue breathing, administering naloxone and staying with the person until help arrives.66
Prescription monitoring programmes
States have adopted PMPs in order to address the rising non-medical use of opioids. While the types of drugs monitored may vary depending on state laws, data from these programmes are accessible to those who register. Some PMPs have potential in terms of decreasing ‘doctor shopping’ and the diversion of opioids,67 but they have yet to show an impact of OD on death rates.52 Notably, health provider uptake remains a challenge.68,69
Safe storage and disposal
One strategy to prevent prescription opioid OD and misuse is the use of a medication lock box.58,70 Patients should also be counselled on how to dispose of any unused opioids or chronic pain medications.70 Prescription drug take-back events were developed in order to address opioid diversion, ODs and environmental implications of improper medication disposal. These events are a means of appropriately disposing prescription medications.53,71
Supervised injection facilities
In order to encourage safe drug injection techniques, promote OD prevention and increase access to primary care, SIFs were developed. In these facilities, individuals are provided a sterile environment for injecting and disposal of needles and also have access to harm reduction services. These facilities have been adopted in Canada, Australia and Europe and have been associated with lower levels of drug injections occurring publically, safer syringe disposal and reduced OD death rates.72–74 However, the USA has not yet developed such facilities. While additional outcomes research is needed, SIFs have the potential to reduce OD deaths and infection-related complications associated with drug injection.75
INFECTION PREVENTION
Safer injection techniques
Injection drug use is associated with a range of infectious complications,76–80 which can be reduced through safe injection techniques:
Filters: Cotton balls or cigarette filters are often used as filters to trap particulate matter when injecting.77 They are subject to skin or oral contamination prior to use and if reused.81 Small, preformed filters, such as micron filters or dental cotton pellets that do not require manipulation are ideal for filtration, and in conjunction with other injection hygiene measures, can reduce bacterial loads.82
Cookers: The process of cooking, or injecting drugs while using heat, can help decrease microbial burden entering the bloodstream. However, patients should avoid reusing or sharing cooking equipment (eg, spoons, bottle caps), as these practices have been linked to infections such as hepatitis B, hepatitis C and HIV.80 If patients share drugs, it is recommended that they cook their drugs separately.83 To avoid contamination with bacteria, including Pseudomonas aeruginosa, sterile water should be used.84
Injection site preparation: PWID should be counselled to practice hand hygiene before and after injecting, use an alcohol pad, gauze pad and bandage at the injection site, clean other surfaces their blood may have touched (such as tourniquets) and to safely dispose of equipment.85 Note that ‘skin popping’ and ‘muscling’ (injection of drugs into subcutaneous or intramuscular sites, respectively), common for people unable to find a vein, can similarly result in soft tissue and endovascular complications from injection drug use.80
Sterile needles and syringes: Ideally, new sterile needles and syringes should be used each time, as bleach and other disinfectants do not sterilise fully.83–85 If available, patients should be directed to syringe exchange programmes and/or pharmacies where they can obtain sterile equipment and potentially receive other preventive services and referrals to substance use treatment.86,87
Acidifiers: When injecting solids such as base heroin or crack cocaine, acidifiers such as vinegar and lemon dose are commonly used to dissolve the drugs.88–91 These acidifiers, however, are caustic, and can cause disseminated infections (eg, candidemia, endophthalmitis). Also, sharing acidifiers increases the risk of transmission of HIV and hepatitis C.92,93 If using acidifiers, patients should be counselled to use single-use vitamin C (ascorbic acid) packets, which are sterile, nontoxic and considered safe acidifiers.91
Sexually transmitted infection evaluation
PWID are at high risk for both acquiring and transmitting sexually transmitted infections (STIs).94 Therefore, asymptomatic PWID should be screened for STIs. This includes at least annual HIV, hepatitis B (if not immune) and C testing as well as syphilis screening, gonococcal and Chlamydia testing, and testing for trichomonas as clinically indicated.93,95 Patients should receive safer sex counselling in conjunction with testing.94
Vaccinations
Hepatitis A and B, Td and Tdap vaccinations should be administered to PWID who are not already immune or who have not received within guideline time windows.94 In susceptible patients at risk for loss to follow-up, the vaccine series for hepatitis B can be initiated immediately after blood is drawn for serologic testing.94 For PWIDs, who smoke or have a concurrent alcohol use disorder, PCV13 and PCV23 vaccines should be administered.96
Screening for tuberculosis
Drug use has been associated with higher risk of tuberculosis and therefore, PWID should be screened as advised by the Centers for Disease Control (CDC) guidelines.94,97,98
Pre-exposure prophylaxis for HIV prevention
PWID are at a higher risk for acquiring HIV infection.99 Pre-exposure prophylaxis (PrEP) involves a high-risk, HIV-negative person taking antiretrovirals in order to prevent acquiring HIV. The CDC recommends daily oral tenofovir/emtricitabine for PrEP in PWID. This recommendation applies to individuals who have injected drugs within the past 6 months, including those who have attended drug treatment centres for injection drug use within the past 6 months. It also applies to PWID who report sharing injection equipment.100 PrEP for HIV prophylaxis is generally both safe and effective when medication adherence is high.100 Regular (every 3 months) monitoring for HIV infection and renal complications from the medication is recommended as part of PrEP.
Hepatitis C treatment
In 2009, over 3.2 million people in the USA were infected with chronic hepatitis C.101 The prevalence of hepatitis C among PWID is high: worldwide, it is estimated that approximately 10 million PWID are infected with hepatitis C.102 In several countries, PWID account for 80% of new hepatitis C cases and 60% of existing hepatitis C cases.103,104 Approximately 75% of individuals who acquire hepatitis C develop persistent infection, which may progress to severe liver disease. The rate of hepatitis C-related deaths, often due to liver-related or drug-related issues, is increasing; deaths from hepatitis C now exceed deaths from HIV in the USA, and a majority of deaths are in relatively young people (aged 45–64 years).103,105,106
Hepatitis C treatment in PWID is highly effective, especially when treatment is integrated with psychiatric or addiction treatment.107–109 Rates of hepatitis C reinfection among PWID have been relatively low; unstable housing and HIV coinfection may be risks for reinfection and ongoing supportive care remains important.109,110
CASE MANAGEMENT FOR CONCRETE NEEDS/BOLSTERING SUPPORTIVE SERVICES
PWID face socioeconomic challenges associated with worse outcomes. For example, unstable housing is associated with higher levels of substance use and other risky injection behaviours, and drug OD is a leading cause of death in the homeless population.111–113 Supportive services provided by addiction counsellors, nurses, case managers and social workers may improve healthcare delivery in PWID.114,115 Homelessness is also a risk factor for increased ED use among PWID; thus, addressing homelessness may reduce unnecessary acute care visits.113 The use of case management to help patients acquire housing, insurance, other benefits and access to education and job training may be effective in linking patients to and retaining them in care.116
DISCUSSION
PWID often present to the hospital or ED when they have serious medical complications. These contacts with the health system provide an opportunity to engage high-risk patients in primary outpatient care, address the addiction, the root cause of these complications and optimise their health and safety.
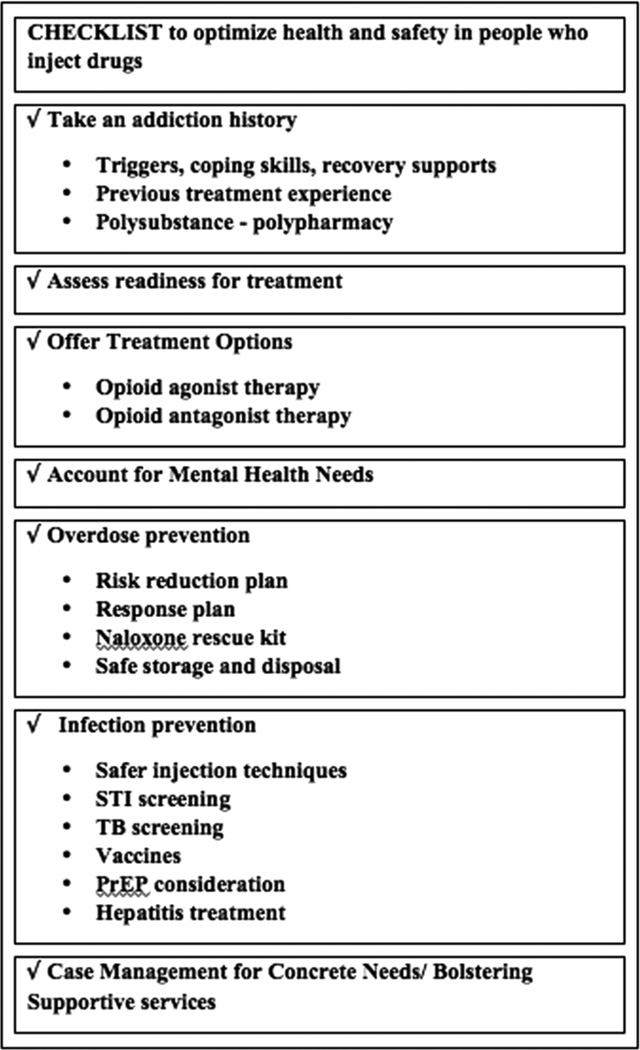
Prioritising which issues to address during an initial evaluation is difficult. As such, we have developed a checklist (figure 1) to serve as a guide for addressing health issues for PWID, with a special emphasis on OD and infection prevention, given the recent increase in rates of drug OD deaths and costly infections such as hepatitis C.117,118 Our checklist includes harm reduction and prevention strategies that can be applied in low-resource settings. Some communities may lack addiction treatment options; therefore, the provider’s approach must be adapted to the patient’s needs, the clinical setting as well as what is accessible in the community. Some components of the checklist can initially be addressed during acute care visits. Initiation of OAT with buprenorphine induction during hospitalisations or ED visits, for example, has been effective in linking patients to outpatient care.23–25 Prescribing naloxone rescue kits upon hospital discharge and bedside counselling on safe injection techniques are other issues that can be initially addressed during acute care visits and further discussed in the outpatient setting. The clinical checklist serves as a summary tool to understand key issues that are important to address during various health encounters with PWID.
Figure 1.
CHECKLIST to optimise health and safety in people who use drugs. PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; TB, tuberculosis.
CONCLUSIONS
Case conclusion—putting the checklist into action
At the patient’s outpatient follow-up appointment, an addiction history revealed that the patient began using substances (alcohol, marijuana, cigarettes) in adolescence. He bought prescription opioid pills illicitly after an injury and used cocaine and benzodiazepine intermittently. He eventually transitioned to injecting heroin. He had tried buprenorphine, methadone and naltrexone implants (not FDA-approved) for addiction, attended multiple residential and detoxification programmes as well as Narcotics Anonymous (NA) and Alcoholics Anonymous meetings. At the follow-up appointment, the patient was started on buprenorphine/naloxone. An OD prevention plan was reviewed with the patient that included the following safety steps in the event of a relapse: not mixing substances, using when others are present and using a small tester amount of drugs. A plan on how he would respond if he witnessed an OD was also developed. He was prescribed a naloxone rescue kit. Use of the kit was reviewed with the patient and his mother, who was also referred to a local support group for parents with children who use opioids. His mother assumed responsibility of monitoring the patient’s prescription medications, which were kept in a locked box. He initiated care with a psychiatrist for his anxiety, who monitored his benzodiazepines. Regularly, the state PMP was checked to confirm the patient was not obtaining pills from unknown providers.
At his follow-up infectious disease appointments, the patient was screened for sexually transmitted diseases and was updated on vaccinations. The concept of PrEP was discussed with the patient, but since he was not actively injecting, sharing drug paraphernalia or engaging in high-risk sexual behaviours, the use of PrEP was deferred.
The patient has been heroin abstinent for >15 months and remains engaged in primary care, cardiology and psychiatry. He continues to attend NA meetings, remains on buprenorphine/naloxone and is making plans to attend veterinary technician school.
The opioid epidemic continues to worsen, and health providers are faced with the challenge of addressing complications from injection drug use, as well as addressing the underlying addiction issues. Our case presentation, review and clinical checklist highlight key aspects of optimising the health and safety of PWID.
Main messages.
-
▸
People who inject drugs (PWID) often present to the hospital or emergency department when they have serious medical complications. These contacts with the health system provide an opportunity to engage high-risk patients in primary outpatient care, address the addiction, the root cause of these complications and optimise their health and safety.
-
▸
Health issues for PWID include: addiction history, readiness for treatment, treatment options, overdose prevention, infection prevention, mental health needs and other concrete needs such as housing.
-
▸
Prioritising health issues is necessary: some communities may lack addiction treatment options, therefore, the provider’s approach must be adapted to the patient’s needs, the clinical setting as well as what is accessible in the community.
Current research questions.
-
▸
What strategies are effective in overdose prevention?
-
▸
What types of medication treatment for opioid use disorders are available in the USA and how do they vary?
-
▸
How should people who inject drugs be counselled on infection prevention?
Key references.
-
▸
Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients; a randomized clinical trial. JAMA Intern Med 2014;174:1369–76.
-
▸
Shanahan CW, Beers D, Alford DP, et al. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med 2010;25:803–808.
-
▸
Marshall BD, Milloy MJ, Wood E, et al. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. Lancet 2011;377:1429–37.
-
▸
Centers for Disease Control. Integrated Prevention Services for HIV Infection, Viral Hepatitis, Sexually Transmitted Diseases and Tuberculosis for Persons Who Use Drugs Illicitly: Summary Guidance From CDC and US Department of Health and Human Services. MMWR, 2012. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6105a1.htm (accessed Jun 2015).
-
▸
D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA 2015;313:1636–44.
Self assessment questions.
Please answer true or false to the below statements.
Patients should never be offered exclusively harm reduction services.
Methadone, buprenorphine and naltrexone can all be prescribed in any outpatient setting in the USA.
Patient outcomes are improved when mental health needs and addiction treatment are addressed concurrently.
Naloxone use is only recommended for medical professionals, as this is a dangerous medication with significant side effects.
Hepatitis C treatment has not been shown to be effective in persons who inject drugs.
Answers.
False—Depending on the patient’s readiness for change, it may be appropriate to offer the patient exclusively harm reduction services until the patient is interested in additional treatments.
False—In the USA, methadone is dispensed through specific federally regulated clinics. Buprenorphine and naltrexone can both be prescribed in any outpatient setting; however, physicians must obtain an additional waiver from the DEA before prescribing buprenorphine.
True—as mental health can strongly impact the success of addiction treatment, it is best practice to approach both diseases concurrently.
False—Many organisations support broad community distribution of naloxone.
False—People who inject drugs can have their hepatitis C successfully treated, especially with treatment integrated into their psychiatric and addiction treatment.
Acknowledgments
FundingThis work was primarily supported by T32 A1052074-10 from the National Institute of Health/National Institute of Allergy and Infectious Diseases. The project was also supported by the Fellow Immersion Training Program under the CARE grant, R25DA013582.
Footnotes
Contributors Conceived and designed the study: KT and AYW. Writing and revision of manuscript: KT, ZMW and AYW.
Competing interests KT reports grants from NIH during the conduct of the study.
Patient consent Obtained.
Ethics approval Prior to the submission of this manuscript, written and verbal consent was obtained from the patient in our case study, whose identity is anonymous.
REFERENCES
- 1.Campbell BK, Tillotson CJ, Choi D, et al. Predicting outpatient treatment entry following detoxification for injection drug use: the impact of patient and program factors. J Subst Abuse Treat. 2010;38(Suppl 1):S87–S96. doi: 10.1016/j.jsat.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series, No. 40. Center for Substance Abuse Treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 2004. [PubMed] [Google Scholar]
- 3.Dennis M, Babor TF, Roebuck MC, et al. Changing the focus: the case for recognizing and treating cannabis use disorders. Addiction. 2002;97(Suppl 1):4–15. doi: 10.1046/j.1360-0443.97.s01.10.x. [DOI] [PubMed] [Google Scholar]
- 4.Bardhi F, Sifaneck SJ, Johnson BD, et al. Pills, Thrills and Bellyaches: Case Studies of Prescription Pill Use and Misuse among Marijuana/Blunt Smoking Middle Class Young Women. Contemp Drug Probl. 2007;34:53–101. doi: 10.1177/009145090703400104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt SA, Taverna EC, Hallock RM. A survey of nonmedical use of tranquilizers, stimulants, and pain relievers among college students: patterns of use among users and factors related to abstinence in non-users. Drug Alcohol Depend. 2014;143:272–276. doi: 10.1016/j.drugalcdep.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Green TC, Bowman SE, Ray M, et al. Collaboration or coercion? Partnering to divert prescription opioid medications. J Urban Health. 2013;90:758–767. doi: 10.1007/s11524-012-9784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis WR, Johnson BD. Prescription opioid use, misuse, and diversion among street drug users in New York City. Drug Alcohol Depend. 2008;92:267–276. doi: 10.1016/j.drugalcdep.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi JA, Surratt HL, Kurtz SP, et al. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8:171–183. doi: 10.1111/j.1526-4637.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inciardi JA, Surratt HL, Cicero TJ, et al. The “black box” of prescription drug diversion. J Addict Dis. 2009;28:332–347. doi: 10.1080/10550880903182986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertholf RL, Johannsen LM, Reisfield GM. Sensitivity of an opiate immunoassay for detecting hydrocodone and hydromorphone in urine from a clinical population: analysis of subthreshold results. J Anal Toxicol. 2015;39:24–28. doi: 10.1093/jat/bku109. [DOI] [PubMed] [Google Scholar]
- 11.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164:1–9. doi: 10.7326/M15-0038. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. HHS Publication No. (SMA) PEP15-FEDGUIDEOTP. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. [accessed Apr 2015]. http://store.samhsa.gov/shin/content/PEP15-FEDGUIDEOTP/PEP15-FEDGUIDEOTP.pdf. [Google Scholar]
- 14.Center for Substance Abuse Treatment. Substance abuse treatment: group therapy. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 2005. [accessed Apr 2015]. Treatment Improvement Protocol (TIP) Series, No. 41.) 5 Stages of Treatment. http://www.ncbi.nlm.nih.gov/books/NBK64208/ [PubMed] [Google Scholar]
- 15.Tai B, Saxon AJ, Ling W. Medication-assisted therapy for opioid addiction. J Food Drug Anal. 2013;21:S13–S15. doi: 10.1016/j.jfda.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23:63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 17.Noska A, Mohan A, Wakeman S, et al. Managing opioid use disorder during and after acute hospitalization: a case-based review clarifying methadone regulation for acute care settings. J Addict Behav Ther Rehabil. 2015;4:1000138. doi: 10.4172/2324-9005.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonhomme J, Shim RS, Gooden R, et al. Opioid addiction and abuse in primary care practice: a comparison of methadone and buprenorphine as treatment options. J Natl Med Assoc. 2012;104:342–350. doi: 10.1016/s0027-9684(15)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor PG, Oliveto AH, Shi JM, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med. 1998;105:100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan LE, Fiellin DA. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med. 2008;148:662–670. doi: 10.7326/0003-4819-148-9-200805060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hard B. Management of opioid painkiller dependence in primary care: ongoing recovery with buprenorphine/naloxone. BMJ Case Rep. 2014 Nov 28; doi: 10.1136/bcr-2014-207308. Published Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones HE, Finnegan LP, Kaltenbach K. Methadone and buprenorphine for the management of opioid dependence in pregnancy. Drugs. 2012;72:747–757. doi: 10.2165/11632820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174:1369–1376. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanahan CW, Beers D, Alford DP, et al. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25:803–808. doi: 10.1007/s11606-010-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636–1644. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattick RP, Kimber J, Breen C, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(Suppl 8):S235–S248. [PubMed] [Google Scholar]
- 28.Schwartz RP, Gryczynski J, O’Grady KE, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am J Public Health. 2013;103:917–922. doi: 10.2105/AJPH.2012.301049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fingerhood MI, King VL, Brooner RK, et al. A comparison of characteristics and outcomes of opioid-dependent patients initiating office-based buprenorphine or methadone maintenance treatment. Subst Abuse. 2014;35:122–126. doi: 10.1080/08897077.2013.819828. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz RP, Jaffe JH, O’Grady KE, et al. Interim methadone treatment: impact on arrests. Drug Alcohol Depend. 2009;103:148–154. doi: 10.1016/j.drugalcdep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gowing LR, Hickman M, Degenhardt L. Mitigating the risk of HIV infection with opioid substitution treatment. Bull World Health Organ. 2013;91:148–149. doi: 10.2471/BLT.12.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kranzler HR, Wesson DR, Billot L Drug Abuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- 33.Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 34.Bryson WC, McConnell J, Korthuis PT, et al. Extended-release naltrexone for alcohol dependence: persistence and healthcare costs and utilization. Am J Manag Care. 2011;17(Suppl 8):S222–S234. [PMC free article] [PubMed] [Google Scholar]
- 35.Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 36.Kunøe N, Lobmaier P, Ngo H, et al. Injectable and implantable sustained release naltrexone in the treatment of opioid addiction. Br J Clin Pharmacol. 2014;77:264–271. doi: 10.1111/bcp.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGovern MP, Xie H, Segal SR, et al. Addiction treatment services and co-occurring disorders: prevalence estimates, treatment practices, and barriers. J Subst Abuse Treat. 2006;31:267–275. doi: 10.1016/j.jsat.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Integrated Treatment for Co-occurring disorders evidence-based practices (EBP) Kit. U.S Department of Health and Human Services. Substance Abuse and Mental Health Association. [accessed Aug 2015]; http://store.samhsa.gov/shin/content//SMA08-4367/TheEvidence-ITC.pdf.
- 40.Donald M, Dower J, Kavanagh D. Integrated versus non-integrated management and care for clients with co-occurring mental health and substance use disorders: a qualitative systematic review of randomised controlled trials. Soc Sci Med. 2005;60:1371–1383. doi: 10.1016/j.socscimed.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 41.Kidorf M, Brooner RK, Gandotra N, et al. Reinforcing integrated psychiatric service attendance in an opioid-agonist program: a randomized and controlled trial. Drug Alcohol Depend. 2013;133:30–36. doi: 10.1016/j.drugalcdep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kidorf M, King VL, Brooner RK. Counseling and psychosocial services. In: Strain EC, Stitzer ML, editors. The treatment of opioid dependence. Baltimore: Johns Hopkins University Press; 2006. pp. 119–150. [Google Scholar]
- 43.Mauer BJ. Behavioral Health/Primary Care Integration Models, Competencies, and Infrastructure. Substance Abuse and Mental Health Services Association. [accessed 11 Mar 2015]; http://www.integration.samhsa.gov/about-us/Mauers_Behav_Health_Models_Competencies_Infra.pdf.
- 44.Klostermann K, O’Farrell TJ. Treating substance abuse: partner and family approaches. Soc Work Public Health. 2013;28:234–247. doi: 10.1080/19371918.2013.759014. [DOI] [PubMed] [Google Scholar]
- 45.Brooner RK, Kidorf MS, King VL, et al. Managing psychiatric comorbidity within versus outside of methadone treatment settings: a randomized and controlled evaluation. Addiction. 2013;108:1942–1951. doi: 10.1111/add.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neufeld K, Kidorf M, King V, et al. Using enhanced and integrated services to improve response to standard methadone treatment: changing the clinical infrastructure of treatment networks. J Subst Abuse Treat. 2010;38:170–177. doi: 10.1016/j.jsat.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 48.Improving the Quality of Health Care for Mental and Substance-Use Conditions: Quality Chasm Series. Washington DC: National Academies Press (US); 2006. Institute of Medicine (US) Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. 5, Coordinating Care for Better Mental, Substance-Use, and General Health. [PubMed] [Google Scholar]
- 49.Centers for Disease Control. Increases in Heroin Overdose Deaths. [accessed Apr 2015];MMWR. 2014 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6339a1.htm.
- 50.Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA. 2015;313:891–892. doi: 10.1001/jama.2014.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clausen T, Waal H, Thoresen M, et al. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- 52.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12:747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 53.Gray JA, Hagemeier NE. Prescription drug abuse and DEA-sanctioned drug take-back events: characteristics and outcomes in rural Appalachia. JAMA Intern Med. 2012;172:1186–1887. doi: 10.1001/archinternmed.2012.2374. [DOI] [PubMed] [Google Scholar]
- 54.Albert S, Brason FW, II, Sanford CK, et al. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 2011;12(Suppl 2):S77–S85. doi: 10.1111/j.1526-4637.2011.01128.x. [DOI] [PubMed] [Google Scholar]
- 55.Center for Substance Abuse Treatment. Emerging Issues in the Use of Methadone. HHS Publication No. (SMA) 09-4368. Substance Abuse Treatment Advisory. 2009;8 [Google Scholar]
- 56.Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462–468. doi: 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- 58.Use of Naloxone for the Prevention of Drug Overdose Deaths. American Society of Addiction Medicine American Medical Association, Substance Abuse and Mental Health Services Association and the American Society of Addiction Medicine. [accessed 3 Jun 2015]; http://www.asam.org/advocacy/find-a-policy-statement/view-policy-statement/public-policy-statements/2014/08/28/use-of-naloxone-for-the-preventionof-drug-overdose-deaths. [Google Scholar]
- 59.SAMHSA Overdose Toolkit. Substance Abuse and Mental Health Services Association. [accessed 3 Jun 2015]; https://store.samhsa.gov/shin/content/SMA13-4742/Overdose_Toolkit_2014_Jan.pdf.
- 60.Promoting Prevention of Fatal Opioid Overdose. American Medical Association. [accessed 3 Jun 2015]; http://www.ama-assn.org/ama/pub/news/news/2012-06-19-ama-adopts-new-policies.page. [Google Scholar]
- 61.Public Policy Statement on the Use of Naloxone for Prevention of Drug Overdose Deaths. American Society of Addiction Medicine. [accessed 3 Jun 2015]; http://www.asam.org/docs/default-source/publicy-policy-statements/1naloxone-rev-8-14.pdf?sfvrsn=0. [Google Scholar]
- 62.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal in Russian cities. J Med Econ. 2013;16:1051–1060. doi: 10.3111/13696998.2013.811080. [DOI] [PubMed] [Google Scholar]
- 63.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158:1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- 64.Walley AY, Doe-Simkins M, Quinn E, et al. Opioid overdose prevention with intranasal naloxone among people who take methadone. J Subst Abuse Treat. 2013;44:241–247. doi: 10.1016/j.jsat.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patient Education Videos. Prescribe to Prevent. [accessed Apr 2015]; http://prescribetoprevent.org. [Google Scholar]
- 67.Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33:319–328. doi: 10.3109/01612840.2011.654046. [DOI] [PubMed] [Google Scholar]
- 68.Rutkow L, Turner L, Lucas E, et al. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood) 2015;34:484–492. doi: 10.1377/hlthaff.2014.1085. [DOI] [PubMed] [Google Scholar]
- 69.Griggs CA, Weiner SG, Feldman JA. Prescription drug monitoring programs: examining limitations and future Approaches. West J Emerg Med. 2015;16:67–70. doi: 10.5811/westjem.2014.10.24197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hahn KL. Strategies to prevent opioid misuse, abuse, and diversion that May also reduce the associated costs. Am Health Drug Benefits. 2011;4:107–114. [PMC free article] [PubMed] [Google Scholar]
- 71.US Department of Justice, Drug Enforcement Administration, Office of Diversion Control Web site. [accessed 11 Mar 2015]; http://www.deadiversion.usdoj.gov/drug_disposal/takeback/National take-back initiative.
- 72.Potier C, Laprévote V, Dubois-Arber F, et al. Supervised injection services: what has been demonstrated? A systematic literature review. Drug and Alcohol Depend. 2014;145:48–68. doi: 10.1016/j.drugalcdep.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Kinnard EN, Howe CJ, Kerr T, et al. Self-reported changes in drug use behaviors and syringe disposal methods following the opening of a supervised injecting facility in Copenhagen, Denmark. Harm Reduct J. 2014;11:29. doi: 10.1186/1477-7517-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall BD, Milloy MJ, Wood E, et al. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. Lancet. 2011;377:1429–1437. doi: 10.1016/S0140-6736(10)62353-7. [DOI] [PubMed] [Google Scholar]
- 75.Semaan S, Fleming P, Worrell C, et al. Potential role of safer injection facilities in reducing HIV and Hepatitis C infections and overdose mortality in the United States. Drug Alcohol Depend. 2011;118:100–110. doi: 10.1016/j.drugalcdep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Chiang W, Goldfrank L. The medical complications of drug abuse. Med J Aust. 1990;152:83–88. doi: 10.5694/j.1326-5377.1990.tb124463.x. [DOI] [PubMed] [Google Scholar]
- 77.Cherubin CE, Sapira JD. The medical complications of drug addiction and the medical assessment of the intravenous drug user: 25 years later. Ann Intern Med. 1993;119:1017–1028. doi: 10.7326/0003-4819-119-10-199311150-00009. [DOI] [PubMed] [Google Scholar]
- 78.Murphy EL, DeVita D, Liu H, et al. Risk factors for skin and soft-tissue abscesses among injection drug users: a case-control study. Clin Infect Dis. 2001;33:35–40. doi: 10.1086/320879. [DOI] [PubMed] [Google Scholar]
- 79.Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med. 2005;353:1945–1954. doi: 10.1056/NEJMra042823. [DOI] [PubMed] [Google Scholar]
- 80.Kaushik KS, Kapila K, Praharaj AK. Shooting up: the interface of microbial infections and drug abuse. J Med Microbiol. 2011;60(Pt 4):408–422. doi: 10.1099/jmm.0.027540-0. [DOI] [PubMed] [Google Scholar]
- 81.Human Rights Watch. Injecting Reason. [accessed Apr 2015]; http://www.hrw.org/reports/2003/09/08/injecting-reason. [Google Scholar]
- 82.Ng H, Patel RP, Bruno R, et al. Filtration of crushed tablet suspensions has potential to reduce infection incidence in people who inject drugs. Drug Alcohol Rev. 2015;34:67–73. doi: 10.1111/dar.12196. [DOI] [PubMed] [Google Scholar]
- 83.Harm Reduction Coalition. A Safety Manual for Injection Drug Users. [accessed Jun 2015]; http://harmreduction.org/wp-content/uploads/2011/12/getting-off-right.pdf. [Google Scholar]
- 84.Reyes MP, Ali A, Mendes RE, et al. Resurgence of pseudomonas endocarditis in Detroit, 2006–2008. Medicine (Baltimore) 2009;88:294–301. doi: 10.1097/MD.0b013e3181b8bedc. [DOI] [PubMed] [Google Scholar]
- 85.Centers for Disease Control. Syringe Disinfection for Injection Drug Users. [accessed Jun 2015]; http://www.cdc.gov/idu/facts/disinfection.pdf.
- 86.Centers for Disease Control. Syringe Exchange Programs—United States, MMWR. [accessed Jun 2015];2008 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5945a4.htm/Syringe-Exchange-Programs-United-States-2008.
- 87. AIDS.gov. [accessed Jun 2015];Substance Abuse/Use. https://www.aids.gov/hiv-aids-basics/prevention/reduce-your-risk/substance-abuse-use/
- 88.Scott J, Winfield A, Kennedy E, et al. Laboratory study of the effects of citric and ascorbic acids on injections prepared with brown heroin. Int J Drug Policy. 2000;11:417–422. doi: 10.1016/s0955-3959(00)00068-2. [DOI] [PubMed] [Google Scholar]
- 89.Garden J, Roberts K, Taylor A, et al. Evaluation of the provision of single use citric acid sachets to injecting drugs users. [accessed Jun 2015];Effective Interventions Unit. 2003 http://www.drugmisuse.isdscotland.org/eiu/pdfs/citric_acid_full.pdf. [Google Scholar]
- 90.British Columbia Centre for Disease Control, Harm Reduction Strategies and Services. Acidifier (Vitamin C—Ascorbic Acid) and Injection Drug Use: Questions and Answers. [accessed Jun 2015]; http://www.bccdc.ca/NR/rdonlyres/95C6F0E7-27E0-430AB825-C5A09C7260C3/0/VitaminCQandAJanuary2011.pdf. [Google Scholar]
- 91.Strike C, Leonard L, Millson M, et al. Toronto: Ontario Needle Exchange Coordinating Committee; 2006. [accessed Jun 2015]. Ontario needle exchange programs: best practice recommendations. http://www.health.gov.on.ca/english/providers/pub/aids/reports/ontario_needle_exchange_programs_best_practices_report.pdf. [Google Scholar]
- 92.Buchanan D, Tooze JA, Shaw S, et al. Demographic, HIV risk behavior, and health status characteristics of “crack” cocaine injectors compared to other injection drug users in three New England cities. Drug Alcohol Depend. 2006;81:221–229. doi: 10.1016/j.drugalcdep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Scott J. Safety, risks and outcomes from the use of injection paraphernalia. Scotland: Scottish Government Social Research; 2008. [accessed Jun 2015]. http://www.gov.scot/Resource/Doc/127313/0057758.pdf. [Google Scholar]
- 94.Centers for Disease Control. Integrated Prevention Services for HIV Infection, Viral Hepatitis, Sexually Transmitted Diseases and Tuberculosis for Persons Who Use Drugs Illicitly: Summary Guidance From CDC and US Department of Health and Human Services. [accessed Jun 2015];MMWR. 2012 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6105a1.htm. [PubMed]
- 95.Kanno MB, Zenilman J. Sexually transmitted diseases in injection drug users. Infect Dis Clin North Am. 2002;16:771–780. doi: 10.1016/s0891-5520(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 96.Centers for Disease Control. Immunization Schedules. [accessed Jul 2015]; http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
- 97.Deiss RG, Rodwell TC, Garfein RS. Tuberculosis and illicit drug use: review and update. Clin Infect Dis. 2009;48:72–82. doi: 10.1086/594126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 updated by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1–e34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 99.Centers for Disease Control. HIV Surveillance Report, 2012 (Volume 24): diagnoses of HIV infection in the United States and dependent areas. [accessed Jun 2015];2012 http://www.cdc.gov/hiv/pdf/statistics_2012_HIV_Surveillance_Report_vol_24.pdf.
- 100.Centers for Disease Control. Preexposure Prophylaxis for the Prevention of HIV in the United States—2014: A Clinical Practice Guideline. [accessed Aug 2015]; http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf.
- 101.Centers for Disease Control. Hepatitis C Information for the Public. [accessed Aug 2015]; http://www.cdc.gov/hepatitis/hcv/cfaq.htm.
- 102.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grebely J, Matthews GV, Lloyd AR, et al. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clin Infect Dis. 2013;57:1014–1020. doi: 10.1093/cid/cit377. [DOI] [PubMed] [Google Scholar]
- 104.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 105.Centers for Disease Control. CDC National Health Report: Leading Causes of Morbidity and Mortality and Associated Behavior Risk and Protective Factors—United States, 2005–2013. [accessed May 2015];MMWR. http://www.cdc.gov/mmwr/preview/mmwrhtml/su6304a2.htm.
- 106.Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral Hepatitis in the United States between 1999 and 2007. Annals. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 107.Ho SB, Bräu N, Cheung R, et al. Integrated care increases treatment and improves outcomes of patients with chronic Hepatitis C Virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol. 2015;13:2005–2014. e1–e3. doi: 10.1016/j.cgh.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 108.Alavi M, Grebely J, Micallef M, et al. Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS Study. Clin Infect Dis. 2013;57(Suppl 2):S62–S69. doi: 10.1093/cid/cit305. [DOI] [PubMed] [Google Scholar]
- 109.Machouf N, Trottier B, Galanakis C, et al. Low incidence of reinfection with hepatitis C virus after successful treatment in montreal. J Hepatology. 2015;62:1250. [Google Scholar]
- 110.Midgard H, Bjøro B, Dalgard O. Incidence of hepatitis C reinfection following sustained virologic response—a seven year follow-up of Scandinavian patients infected through injecting drug use; Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver, Vienna, abstract O061. [Google Scholar]
- 111.Des Jarlais DC, Braine N, Friedmann P. Unstable housing as a factor for increased injection risk behavior at US syringe exchange programs. AIDS Behav. 2007;11(6 Suppl):78–84. doi: 10.1007/s10461-007-9227-6. [DOI] [PubMed] [Google Scholar]
- 112.Baggett TP, Hwang SW, O’Connell JJ, et al. Mortality among homeless adults: shifts in causes of death over a 15-year period. JAMA Intern Med. 2013;173:189–195. doi: 10.1001/jamainternmed.2013.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thakarar K, Morgan JR, Gaeta JM, et al. Predictors of frequent emergency room visits among a homeless population. PLoS ONE. 2015;10:e0124552. doi: 10.1371/journal.pone.0124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drainoni ML, Farrell C, Sorensen-Alawad A, et al. Patient perspectives of an integrated program of medical care and substance use treatment. AIDS Patient Care STDS. 2014;28:71–81. doi: 10.1089/apc.2013.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thompson RG, Wall MM, Greenstein E, et al. Substance-use disorders and poverty as prospective predictors of first-time homelessness in the United States. Am J Public Health. 2013;103(Suppl 2):S282–S288. doi: 10.2105/AJPH.2013.301302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rapp RC, Van Den Noortgate W, Broekaert E, et al. The efficacy of case management with persons who have substance abuse problems: a three-level meta-analysis of outcomes. J Consult Clin Psychol. 2014;82:605–618. doi: 10.1037/a0036750. [DOI] [PubMed] [Google Scholar]
- 117.Centers for Disease Control. Increases in Drug and Opioid Overdose Deaths. [accessed Decem 2015];MMWR. 2015 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm64e1218a1.htm.
- 118.Centers for Disease Control. Increases in Hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR. 2015 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6417a2.htm. [PMC free article] [PubMed]