Abstract
The successful generation of kidney-like structures from human pluripotent stem cells, although slower to come than other tissue types, brings the hope of new therapies. While the demand for alternative treatments for kidney failure is acute, huge challenges remain to move these exciting but preliminary results towards clinical use.
Chronic kidney disease now affects 10 to 15% of adults across the globe (Coresh et al., 2007). While this apparent epidemic is being fuelled by obesity and diabetes, the treatment options available for patients reaching end stage renal disease have not changed in over half a century. End stage renal disease patients are either provided renal replacement therapy (hemo or peritoneal dialysis) or organ transplantation. With only 1 in 4 patients receiving an organ transplant, and high morbidity and mortality associated with dialysis, the development of new treatment options is critical. With the advent of human pluripotent stem cells (PSCs), the concept of regenerating a kidney became one possible therapeutic approach. However, kidney regeneration is a very challenging task as this is an extremely complex organ. The kidney is comprised of more than 25 different cell types tasked with excretion, secretion, and fluid homeostasis. These allow the kidney to regulate blood pressure, blood volume, pH, red cell count, bone mineral metabolism and toxin removal. The major task for the kidney, filtration of the blood to remove metabolites and nitrogenous wastes and reabsorb water, is carried out by the nephrons, each consisting of an unbranched epithelial tubule that is vascularised at one end (the glomerulus). An adult kidney can repair after injury, but rather than structural regeneration this involves rapid epithelial turnover within the nephrons, either via proliferation of mature cells or the expansion of a residual tubular progenitor. No new nephron formation occurs in adults. Indeed, maladaptive repair responses are seen after chronic injury with this reduced epithelial repair triggering tubulointerstitial fibrosis (Yang et al., 2011).
Understanding kidney development to drive regeneration
The lack of regenerative capacity after birth suggests that the kidney is an ideal organ to recreate using directed differentiation, albeit a challenging one. Progress towards this outcome has been slower than for some organ systems. Embryologically, the kidney is of mesodermal origin, arising from the intermediate mesoderm (Takasato and Little, 2015). While the mesoderm-derived cardiomyocyte was a relatively early outcome of directed differentiation, to date efforts have more strongly focused on neural and endodermal tissues. However, studies into patterning of PSCs to primitive streak and then definitive mesoderm, important for other mesodermal tissues including blood, have proven important for kidney as well. Careful lineage analysis in mouse suggests that the collecting ducts of the kidney arise from anterior intermediate mesoderm whereas the nephrons arise from the posterior intermediate mesoderm (Taguchi et al., 2014). The origin of the vasculogenic endothelial progenitors and the surrounding stromal cells is less defined but is also likely to be intermediate mesoderm, possibly from the posterior end. Importantly, cells present within these anterior and posterior regions are exposed to distinct temporospatial signals (Taguchi et al., 2014, Takasato and Little, 2015). The ureteric bud (arises from anterior IM) is ultimately drawn towards the metanephric mesenchyme (arises from posterior IM) in response to glial-derived neurotrophic factor. Once these distinct populations meet, reciprocal inductive signals between these two populations drive continued organ growth throughout fetal development. Hence, the mesenchyme ensures sufficient collecting duct branching to drain all nephrons and the ureteric bud ensures the formation of sufficient nephrons. Understanding the growth factors involved in these processes as well as the gene expression signatures characteristic of all intermediate steps has been critical for recreating these events in culture.
Directed differentiation to nephron-forming cell types
While slower off the mark than other tissues, several groups have now applied stepwise protocols drawing on this understanding of embryogenesis to generate nephrons from hPSCs in vitro (Freedman et al., 2015, Morizane et al., 2015, Sharmin et al., 2015, Taguchi et al., 2014, Takasato et al., 2015). These studies all report evidence of critical intermediate mesodermal differentiation steps and draw on a similar set of growth factor signalling events to reach a kidney endpoint. A variety of culture formats and media have been used yet they require the activation of a relatively small number of growth factor signalling pathways, with all protocols exploiting canonical WNT and FGF signalling. Indeed, the initial mesodermal patterning events all involve canonical Wnt signalling with or without addition of activin A and BMP4. With the exception of Freedman et al (2015), all protocols induce intermediate mesoderm via the addition of FGF2 or FGF9. These studies also report the formation of spontaneously patterning and segmenting nephrons with proximal and distal tubular segments as well as Bowman’s capsules containing podocytes (Freedman et al., 2015, Morizane et al., 2015, Sharmin et al., 2015, Taguchi et al., 2014, Takasato et al., 2015). The identity of the specific kidney cell types within these cultures has relied upon our understanding of nephron patterning in mouse development, with clear evidence that the elongation, patterning and differentiation of specific cell types known to exist in each nephron is occurring in the dish as has been described in development. More importantly, in a number of cases evidence supports a functional identity for component cell types within these differentiation cultures, including the demonstration of appropriate uptake of albumin and response to nephrotoxins by the proximal tubular segments (Freedman et al., 2015, Morizane et al., 2015, Takasato et al., 2015). Transplantation of mouse iPSC-derived nephrons under the renal capsule showed evidence of glomerular vascularisation resulting in the formation of a urinary filtrate (Sharmin et al., 2015). Finally, the generation of nephrons from CRISPR-edited human iPSCs containing mutations in PKD1 or PKD2 suggested evidence for disease-associated cyst formation (Freedman et al., 2015). Taken together, this suggests that it is feasible to recreate a kidney nephron from PSCs and have these structures reach sufficient maturity for potential application in disease modelling and drug screening.
Kidney organoids from stem cells
Generating nephron-like structure from stem cells is a major advance. While these epithelial structures are key for blood filtration, reabsorption and concentration of the urinary filtrate, in the kidney they are linked to a collecting duct network, vascularised by blood vessels and present in a specific alignment within a surrounding interstitium. Recreation of a kidney will require all of these additional elements. Our own work (Takasato et al., 2015) has shown that, after aggregation of cell cultures into a 3D format, a complex organoid structure arises. Within these kidney organoids, there is evidence for the generation of not only nephrons but accompanying collecting ducts with these tubular elements present within an interstitium also containing vasculature and perivascular cells (Takasato et al., 2015) (Figure 1). These kidney organoids, therefore, contain elements arising from along the length of the intermediate mesoderm, comprising more than 8 distinct cell types. Expression profiling of total kidney organoids in comparison to human fetal tissue confirmed their identity showed that they are most similar to trimester 1 human kidney. While early evidence of vascularisation of the glomeruli can be seen in these organoids, the nephrons are at a ‘capillary loop’ stage requiring significant additional maturation. Further experiments are needed to determine whether kidney organoids derived using this approach are capable of drawing in a vasculature when transplanted under the kidney capsule of a recipient animal.
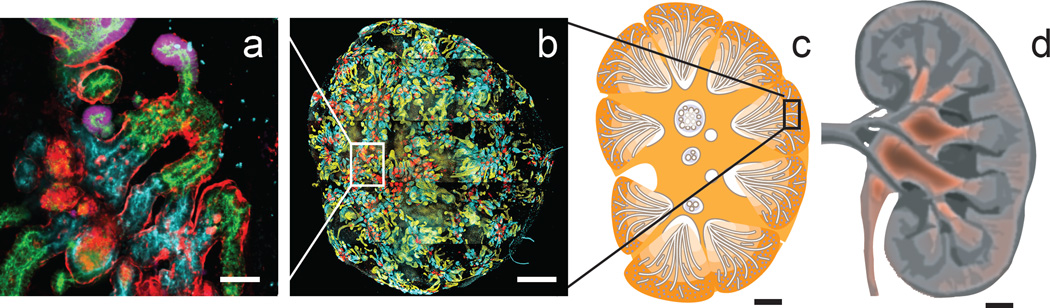
Figure 1.
The scale challenge when generating kidney from human pluripotent stem cells. a. Group of segmenting nephrons within a human iPSC-derived kidney organoid showing collecting duct (GATA3, pink), distal tubule (CDH1, green), proximal tubule (LTL, blue) and glomeruli (NPHS1, red) b. Whole organoid containing approximately 100 nephrons. Inset shows relative size of panel a. c. Diagram of a developing human kidney showing relative size of organoid. d. Diagram of adult kidney. Scale bars are 70µm (a), 1mm (b), 3mm (c), 1.4cm (d). Panels a and b adapted from Takasato et al, 2015.
Generating a complete organ
The directed differentiation of human pluripotent stem cells to kidney tissue is likely to facilitate the development of more accurate drug screening for nephrotoxicity as well as disease modelling for heritable forms of kidney disease. While such applications are exciting and promising, for those on the transplant list, the prospect of a bioengineered tailor-matched replacement organ would be the more important outcome. Our own data showing the generation of kidney organoids was ambitiously described in the media as a ‘kidney in a dish’ (Takasato et al., 2015). While this and other recent reports are significant advances for stem cell biology, from here there is a very long way to go to creating a complete organ suitable for treatment. We will discuss here four major and very obvious challenges: scale, structure, function and integration.
Generating sufficient scale
A functional human kidney contains 200,000 to 2.5 million nephrons. This wide variation is determined prior to birth and it is assumed that kidneys on the lower end of this broad spectrum continue to deliver normal renal function by having each nephron take more of the load. While we know that humans show significant variability in nephron number, and that effective glomerular filtration rate (GFR) reduces with age and disease, a patient will not require dialysis to substitute renal function until their GFR drops to 10–15% of normal. By comparison, human iPSC-derived kidney organoids of around 5–7 mm in diameter contain approximately 100 nephrons (Takasato et al., 2015), falling well below that required to provide any meaningful glomerular filtration level (Figure 1). While many protocols for generating kidney cells pass through a nephron progenitor stage, this population does not appear to persist long term after nephron formation is induced. For this reason, it is not yet possible to continually increase the nephron number. In addition, none of the current culture protocols have been adapted for longer term survival in culture. This would be critical for scaling up to the size of organ required. In addition, it is not yet clear how long such a culture could be maintained without providing circulation.
Recreating appropriate structure
As highlighted, the cellular complexity and functional diversity of the kidney provides a substantial barrier to regeneration. In addition, many physiological functions are achieved as a result of the actual architecture of the tissue. For example, the capacity for regulation of fluid balance relies upon a parallel alignment of the loops of Henle and a partitioning of tubular segments between a cortex and medulla. While recent studies have shown a capacity to generate segmenting nephrons, this formation of cortex and medulla with nephrons aligned with respect to each other is not evident. Similarly, the fetal organ is derived from an initial ureteric bud which dichotomously branches to form the collecting duct network through which the urinary filtrate can exit the organ (Takasato and Little, 2015). A regenerated organ must contain such a common exit path for urinary filtrate or risk the destruction of the nephrons as they fill with urine. Hence, the challenge remains providing an exit path for the urine.
Another obvious challenge is creating a blood supply. Recently reported kidney organoids contain vascular progenitors and collecting duct epithelium, however the glomeruli are poorly vascularised and not attached to a circulatory system, hence they will not generate urinary filtrate (Takasato et al., 2015). Human iPSC-derived nephrons can vascularise when transplanted under the renal capsule of a recipient animal (Sharmin et al., 2015). Similarly, iPSC-derived nephron-forming mesenchyme has been co-cultured with HUVEC cells and mesenchymal stem cells to provide vasculature and interstitium respectively. The resulting ‘organ buds’ have also been shown to vascularise if transplanted retro-orbitally (Takebe et al., 2015). To create a replacement organ, this challenge of tissue vascularisation, and indeed connecting this to the recipient, must be addressed.
Functional maturity of component cells
In none of the directed differentiation protocols published to date is there comprehensive evidence of functional maturation. Based upon directed differentiation of pluripotent stem cells to other tissue endpoints, reaching the level of functional maturation observed in an adult cell type has proven challenging. At best, the resulting tissues represent early embryonic structures. Hence, facilitating functional maturation is a major challenge. A baseline for any studies of kidney cell maturation will first require a thorough analysis of the cell types that can be identified in cultures of iPSC-derived kidney tissues. As most are likely to represent embryonic stages of differentiation, the optimal comparator would be human fetal tissue. However, expression profiling of individual human cell types during kidney development is not readily available. There is some analysis of maturation of proximal tubules in several studies. However, while proximal tubule cells derived from iPSCs have been shown to respond apparently appropriately to cisplatin (Freedman et al., 2015, Morizane et al., 2015, Takasato et al., 2015), different nephrotoxicants act on distinct basolateral or apical solute channels, many of which may not be expressed yet. It may be possible to isolate specific cell types and encourage further maturation in vitro. However, maturation may require onward differentiation in association with surrounding cell types or exposure to physiological conditions that do not exist or occur in isolated culture. Indeed, it is not clear if flow of urinary filtrate assists in proximal tubular maturation. If it does, this will require association of iPSC-derived nephrons with a vasculature. The issue of glomerular vascularisation, which we discussed above, may also not occur without angiogenic ingrowth from a viable circulation. There is much work to do here.
Providing cellular integration
Given the enormity of the challenge to grow an entire organ, another approach is to generate specific kidney cell types from stem cells for delivery back into the existing kidney of the patient. This cellular therapy approach should, in theory, require fewer cells and potentially only critical cell types, such as podocytes or proximal tubular epithelium. It should also have less architectural challenges since the original organ provides the structure. Here, the hope would be to deliver functionally mature, or even kidney progenitor, cell types into a patient with successful integration of these cells improving organ function. As with most other organs, the major challenge is delivery. Injection into the parenchyma brings a risk of bleeding and no proof that the cells injected will insert into existing structures. No patient would receive such therapy without existing renal damage, much of which is accompanied by tubular damage, interstitial fibrosis and inflammation. Such tissue damage would challenge integration of any introduced cell type.
The evidence in mice for substantive integration of any cell type into the damaged kidney is weak and there is little prospect of a clinical trial in humans, even post pre-clinical trial, prior to substantive functional decline. Another approach is the recellularisation of a decellularised adult organ scaffold using human iPSC-derived kidney cells. Again the complexity of the organ itself provides major challenges. This approach would exploit the intact basement membrane as an instructive environment for reintroduced cells. With this method, the only logical access point for nephron cell types is back up the collecting duct tree, which represents a very long and very thin access route, particularly for the podocytes of the glomeruli. However, some recent data suggests that sustained delivery of epithelial cells via the artery can deliver such cell back into tubular compartments (Caralt et al. 2015). These advantages notwithstanding ,there is a long way to go to recellualrise an entire scaffold with sufficient cells, and the capacity for this scaffold to instruct cell fate still remains undetermined.
Other approaches for the future
This discussion has focused on the large gap between the generation of kidney cell types and the generation of a replacement kidney, but there are interim options, such as drug screening, disease modelling and partial functional replacement, which are also useful to consider. Different cell types and structures provide various elements of renal function. Specific functions may replicated by a subset of hPSC-derived kidney cells analogous to how haemodialysis replaces the filtration and excretory role of the kidney without effective fluid reabsorption or other functions. For example, the provision of iPSC- derived cell types that produce erythropoietin may ameliorate the requirement for pharmacological provision of such compounds. Indeed, it may be possible to deliver other endocrine regulators in this way. The seeding of proximal tubular epithelium within extracorporeal devices may also supersede or improve dialysis. As intermediate products, these are likely to be more achievable but will also assist in the optimisation of scale-up protocols for the generation of functional kidney cell types. Ultimately, from a clinical perspective, the target needs to be functional improvement over current dialytic approaches. With all these issues in mind, and with time, the challenge may become more realistic.
Acknowledgments
ML is a National Health and Medical Research Council Senior Principal Research Fellow. Her work is supported by the NHMRC, Australian Research Council, Royal Childrens Hospital Foundation, Kidney Health Australia and the National Institutes of Health, USA. MCRI is supported by the Victorian Government's Operational Infrastructure Support Program. We acknowledge Kylie Georgas for assistance with graphics.
References
- Caralt M, Uzarski JS, Iacob S, Obergfell KP, Berg N, Bijonowski BM, Kiefer KM, Ward HH, Wandinger-Ness A, Miller WM, et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am J Transplant. 2015;15:64–75. doi: 10.1111/ajt.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nature Communications. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature Biotechnology. 2015;33:1193–1200. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R. Human Induced Pluripotent Stem Cell-Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015010096. (EPub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- Takasato M, Little MH. The origin of the mammalian kidney: implications for recreating the kidney in vitro. Development. 2015;142:1937–1947. doi: 10.1242/dev.104802. [DOI] [PubMed] [Google Scholar]
- Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contributions to Nephrology. 2011;174:149–155. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]