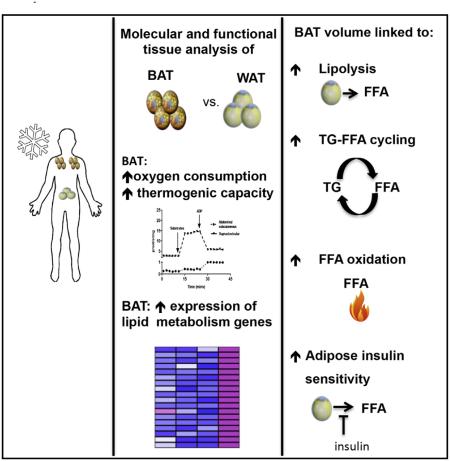
Abstract
Recent studies suggest that brown adipose tissue (BAT) plays a role in energy and glucose metabolism in humans. However, the physiological significance of human BAT in lipid metabolism remains unknown. We studied 16 overweight/obese men during prolonged, non-shivering cold and thermoneutral conditions using stable isotopic tracer methodologies in conjunction with hyperinsulinemic-euglycemic clamps and BAT and white adipose tissue (WAT) biopsies. BAT volume was significantly associated with increased whole-body lipolysis, triglyceride-free fatty acid (FFA) cycling, FFA oxidation, and adipose tissue insulin sensitivity. Functional analysis of BAT and WAT demonstrated the greater thermogenic capacity of BAT compared to WAT, while molecular analysis revealed a cold-induced upregulation of genes involved in lipid metabolism only in BAT. The accelerated mobilization and oxidation of lipids upon BAT activation supports a putative role for BAT in the regulation of lipid metabolism in humans.
Graphical abstract
INTRODUCTION
Mitochondrial proton leaks account for ~20% of energy expenditure (Rolfe and Brown, 1997). Manipulating this component of metabolic rate may be a means of treating obesity and its related metabolic consequences. Brown adipose tissue (BAT), a recently rediscovered tissue in adults (Cypess et al., 2009; Nedergaard et al., 2007; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009), is considered the principal tissue responsible for adaptive thermogenesis during non-shivering cold exposure (CE) (Cannon and Nedergaard, 2004) and thus represents a target for strategies aimed at augmenting metabolic rate. In rodents, BAT can effectively extract significant amounts of triglycerides (TG) from the circulation, lending credence to the notion that BAT may protect from hyperlipidemia (Bartelt et al., 2011; Berbée et al., 2015).
Although rodent studies strongly support a role for BAT in lipid metabolism, the significance of BAT in regulation of lipid metabolism in humans remains unknown. Acute (2 hr) CE stimulates the uptake of plasma FFA into human BAT (Ouellet et al., 2012) (<1% total FFA turnover rate), suggesting a negligible role for BAT in lipid clearance in humans. Moreover, we previously reported that individuals with high amounts of BAT have higher FFA oxidation during cold compared to individuals with no/minimal BAT (Chondronikola et al., 2014). Additionally, BAT activity was recently shown to correlate with cold-induced lipolysis in young, lean adults (Blondin et al., 2015). Although these results imply that human BAT may be involved in lipid homeostasis, evidence directly supporting a role for BAT in lipid mobilization and clearance in humans is currently lacking.
Numerous important questions pertaining to the role of BAT in lipid metabolism in humans remain unanswered. The purpose of this study was to determine if there is a physiologically significant role of BAT activation on whole-body lipid metabolism in humans. We studied overweight/obese men during prolonged, mild CE and thermoneutral (TN) conditions using stable isotopic tracers in conjunction with hyperinsulinemic-euglycemic clamps and adipose tissue biopsies. Our results support the notion that BAT volume is associated with increased whole-body FFA turnover and oxidation, and adipose tissue insulin sensitivity. Functional and molecular analyses of tissue samples further support the role of BAT in lipid mobilization and clearance in humans. Our data support a physiologically significant role for BAT in lipid metabolism in humans.
RESULTS AND DISCUSSION
Participants and Cold-Induced BAT Activation
16 men (Table 1) were studied during prolonged non-shivering CE (room temperature 19.9°C ± 0.8°C, cooling garment temperature 18.2°C ± 2.1°C) and TN (room temperature 26.2°C ± 1.2°C) conditions. Results from animal studies indicate that activated BAT initially consumes intracellular substrates, but following prolonged activation, BAT relies on circulating substrates for thermogenesis (Cannon and Nedergaard, 2004). Therefore, a prolonged non-shivering (5–8 hr) CE protocol was used to stimulate BAT. CE significantly increased metabolic activity (measured as the mean standardized uptake value [SUV] for 2-Deoxy-2-[18F]fluoroglucose [18F-FDG] using positron emission tomography/computed tomography [PET/CT]) in BAT only (p = 0.003). No significant changes were noted in muscle (m. pectoralis, m. vastus lateralis), subcutaneous and visceral adipose tissue, or liver metabolic activity as measured using the mean SUV for 18F-FDG (Figure 1A). Moreover, CE significantly increased tissue radiodensity, an index of intercellular lipid use, only in BAT (p = 0.01, Figure 1B). These data suggest that CE triggered the use of the BAT intracellular TG for thermogenesis, in agreement with the findings of others (Ouellet et al., 2012).
Table 1.
Subjects’ Characteristics
| Age (years) | 47.8 ± 16.2 |
| BMI (kg/m2) | 30.3 ± 2.1 |
| BSA (m2) | 2.1 ± 0.2 |
| Lean mass (kg) | 62.0 ± 8.5 |
| Body fat (%) | 33.2 ± 8.1 |
| BAT volume (ml) | 40.3 ± 45.1 |
| BAT mean SUV (g/ml) | 1.9 ± 0.8 |
Data presented are mean and SD (n = 16). BMI, body mass index; BSA, body surface area; BAT, brown adipose tissue; SUV, standardized uptake value.
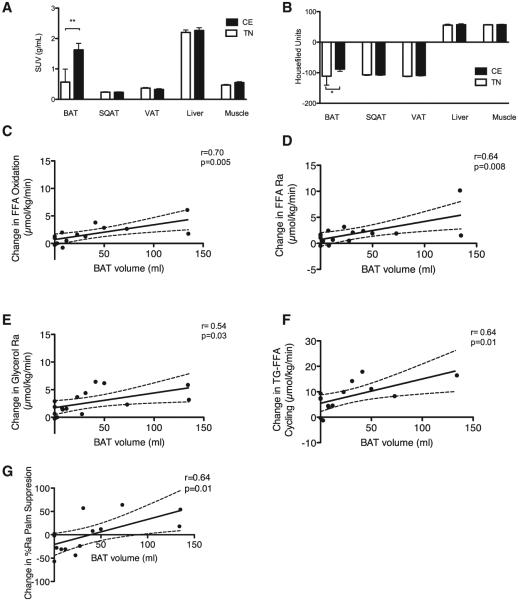
Figure 1. Brown Adipose Tissue Activation and Lipid Kinetics.
(A) Mean standardized disposal value (SUV) for 2-Deoxy-2-[18F]fluoroglucose glucose of various tissues during cold exposure (CE) and thermoneutral (TN) conditions (n = 13–16). SQAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. Data presented are mean and SD. **p = 0.003 using Wilcoxon’s matched pairs signed rank test.
(B) Mean radiodensity of various tissues during CE and TN conditions (n = 13–16). SQAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. Data presented are mean and SD. *p = 0.01 using Wilcoxon’s matched pairs signed rank test.
(C) Correlation of BAT volume with the cold-induced change in whole-body free fatty acid (FFA) oxidation. Indirect calorimetry was completed in 14 participants. The dashed lines represent 95% confidence intervals. p = 0.005 using Pearson’s r.
(D and E) Correlation of BAT volume with the cold-induced change in the whole-body lipolysis rate presented as FFA rate of appearance (Ra) (D) and glycerol Ra (n = 16) (E). The dashed lines represent 95% confidence intervals. p = 0.008 for FFA Ra and p = 0.03 for glycerol Ra using Pearson’s r.
(F) Correlation of BAT volume with the cold-induced change in total triglyceride (TG)-FFA cycling (n = 14). Indirect calorimetry was completed in 14 participants. The dashed lines represent 95% confidence intervals. p = 0.01 using Pearson’s r.
(G) Correlation of BAT volume with the cold-induced change in adipose tissue insulin sensitivity (estimated as percent suppression in Ra [Palm Ra] with insulin). The insulin clamp procedure was completed in 13 participants. The dashed lines represent 95% confidence intervals. p = 0.01 using Pearson’s r.
See also Tables S1–S3 and Figure S1.
Cold-Induced BAT Activation Is Associated with Increased Lipid Mobilization and Oxidative Disposal
FFA constitute the primary substrate for BAT (Cannon and Nedergaard, 2004), while the regulatory role of BAT in systemic lipid clearance has been established in rodents (Bartelt et al., 2011; Berbée et al., 2015). To assess the effect of BAT activation on lipid kinetics in humans, we infused stable isotope tracers during non-shivering CE and TN conditions (Figure S1). BAT volume was significantly correlated with the cold-induced change in whole-body FFA oxidation (r = 0.70, p = 0.005; Figure 1C), whole-body lipolysis (expressed as glycerol rate of appearance [Ra] r = 0.54, p = 0.03 and FFA Ra r = 0.64, p = 0.008; Figures 1D and 1E), and TG-FFA cycling (r = 0.64, p = 0.01; Figure 1F), suggesting a potential role for BAT in whole-body lipid metabolism. No association was observed between the aforementioned outcomes with the mean SUV in muscle (Table S1), which others propose as a proxy of muscle shivering activity (Blondin et al., 2014).
BAT activity has been found to be inversely associated with aging (Cypess et al., 2009; Yoneshiro et al., 2011) and adiposity (Saito et al., 2009; van Marken Lichtenbelt et al., 2009). To account for potential confounding, we performed multiple linear regression analysis adjusting for age and adiposity (Table 2). After adjustment for age and adiposity, BAT volume was significantly associated with an increase in whole-body FFA oxidation (p = 0.0036), adipose tissue lipolysis (p = 0.033), and TG-FFA cycling (p = 0.006). Collectively, these results indicate that cold-stimulated BAT may directly or indirectly trigger lipid mobilization from adipose tissue to provide adequate substrate for BAT mitochondria. Our results suggest that 1 ml of detectable BAT is associated with a 0.03 μmol/kg/min increase in FFA oxidation. During periods of negative energy balance (e.g., cold-induced BAT activation), increased sympathetic tone promotes lipolysis, thereby increasing FFA availability (Bartness et al., 2010). Lipolysis can stimulate thermogenesis in BAT since FFA activates uncoupling protein 1 [UCP1]) (Cannon and Nedergaard, 2004) while providing BAT mitochondria with fuel (Ma and Foster, 1986). In addition, increased TG-FFA cycling can further enhance the net flux of FFA (i.e., increased FFA availability) to meet the increased requirements of thermogenesis (Wolfe, 1990).
Table 2.
Multivariate Linear Regression Analysis
| Independent Predictors | Univariate Analysis |
Multivariate Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Beta Coefficient | Standard Error | St. Beta | p Value | Beta Coefficient | Standard Error | St. Beta | p Value | |
| Dependent Variable: Cold-Induced Change in Whole-Body FFA Oxidation | ||||||||
|
| ||||||||
| BAT volume (ml) | 0.027 | 0.0008 | 0.703 | 0.005 | 0.028 | 0.011 | 0.727 | 0.036 |
|
| ||||||||
| Age (years) | −0.032 | 0.0029 | −3.04 | 0.290 | −0.031 | 0.034 | 0.323 | 0.301 |
|
| ||||||||
| Body fat (%) | −0.121 | 0.055 | −0.539 | 0.047 | −0.060 | 0.070 | −0.265 | 0.415 |
|
| ||||||||
| Dependent Variable: Cold-Induced Change in Whole-Body Lipolysisa | ||||||||
|
| ||||||||
| BAT volume (ml) | 0.039 | 0.013 | 0.643 | 0.010 | 0.050 | 0.020 | 0.820 | 0.033 |
|
| ||||||||
| Age (years) | −0.038 | 0.038 | −0.266 | 0.338 | 0.004 | 0.041 | 0.028 | 0.925 |
|
| ||||||||
| Body fat (%) | −0.103 | 0.073 | −0.365 | 0.181 | 0.063 | 0.103 | 0.222 | 0.554 |
|
| ||||||||
| Dependent Variable: Cold-Induced Change in Total TG-FFA Cycling | ||||||||
|
| ||||||||
| BAT volume (ml) | 0.095 | 0.033 | 0.630 | 0.014 | 0.159 | 0.045 | 1.062 | 0.006 |
|
| ||||||||
| Age (years) | −0.106 | 0.098 | −0.297 | 0.302 | −0.055 | 0.092 | −0.594 | 0.565 |
|
| ||||||||
| Body fat (%) | −0.133 | 0.198 | −0.191 | 0.512 | 0.479 | 0.226 | 0.686 | 0.061 |
|
| ||||||||
| Dependent Variable: Cold-Induced Change in Adipose Tissue Insulin Sensitivityb | ||||||||
|
| ||||||||
| BAT volume (ml) | 0.523 | 0.240 | 0.550 | 0.052 | 0.258 | 0.364 | 0.271 | 0.496 |
|
| ||||||||
| Age (years) | −1.105 | 0.681 | −0.439 | 0.134 | −0.068 | 0.929 | −0.027 | 0.944 |
|
| ||||||||
| Body fat (%) | −2.656 | 1.124 | −0.580 | 0.038 | −1.686 | 1.955 | −0.368 | 0.411 |
FFA, free fatty acid; BAT, brown adipose tissue; TG, triglyceride.
Expressed as glycerol rate of appearance.
Adipose tissue insulin sensitivity was calculated as the percent suppression in palmitate rate of appearance with insulin.
Plasma Lipid and Lipoprotein Concentrations in Cold and Thermoneutral Conditions
The changes noted in lipid kinetics were also reflected in the concentration of plasma lipids. 5 hr of exposure to cold increased plasma FFA (0.2 μmol/ml, 95% confidence interval [CI]: 0.1 to 0.3, p = 0.005) and glycerol concentration (0.024 μmol/ml, 95% CI: 0.005 to 0.043, p = 0.01) compared to the TN conditions (Table S2). No differences were noted in the plasma concentration of TG or the various cholesterol subclasses and lipoprotein particles. Interestingly, the day after the CE study, participants demonstrated decreased plasma concentrations of fasting TG (−28 mg/dl, 95% CI: −49 to −8, p = 0.01) and very low-density lipoprotein cholesterol (−5.6 mg/dl, 95% CI: −9.6 to −1.6, p = 0.01) compared to TN conditions (Table S3), indicating that, similar to exercise (Tsekouras et al., 2008), CE results in long-term alterations in lipid metabolism.
Cold-Induced BAT Activation and Adipose Tissue Insulin Sensitivity
BAT activation has been linked to improved whole-body insulin sensitivity in humans (Chondronikola et al., 2014; Lee et al., 2014). Insulin controls numerous processes in different tissues; therefore, changes in insulin sensitivity represent altered responsiveness to the action of insulin in various metabolic pathways (Kahn, 1978). In rodents, BAT transplantation increases WAT insulin sensitivity (Stanford et al., 2013), while cold acclimation has been reported to increase BAT activity and decrease fasting plasma FFA concentrations (an indirect measure of insulin sensitivity) in humans (Hanssen et al., 2015). These results indicated a potential link between BAT and adipose tissue insulin sensitivity.
To assess the relation of cold-induced BAT activation with adipose tissue insulin sensitivity, we performed infusion of U-13C palmitate in conjunction with a hyperinsulinemic-euglycemic clamp. Adipose tissue insulin sensitivity was measured as the insulin-mediated suppression of lipolysis (Conte et al., 2012; Fabbrini et al., 2012). According to our results, adipose tissue insulin sensitivity was significantly associated with BAT volume (p = 0.05; Figure 1G and Table 2). After adjustment for age and adiposity, the relationship of BAT volume with adipose tissue insulin sensitivity did not remain significant. Nevertheless, the cold-induced change in free triodothyronine (r = 0.69, p = 0.03) and leptin (r = 0.67, p = 0.03) levels correlated positively with the change in adipose tissue insulin sensitivity. Thyroid hormones and leptin can improve insulin sensitivity (Lin et al., 2002; Lin and Sun, 2011; Petersen et al., 2002; Sivitz et al., 1997), and they have been linked with increased BAT activity (Chondronikola et al., 2014; Collins et al., 1996). These results suggest that BAT activation may (directly or indirectly) abolish the negative effect of cold-induced increase in catecholamines in adipose tissue insulin sensitivity (Mullins et al., 2014).
Functional and Molecular Characterization of BAT
While the nature of human studies limits our ability to perform in-depth mechanistic experiments, we performed molecular and ex vivo functional characterization of supraclavicular and abdominal subcutaneous adipose tissues collected from a subset of the study participants.
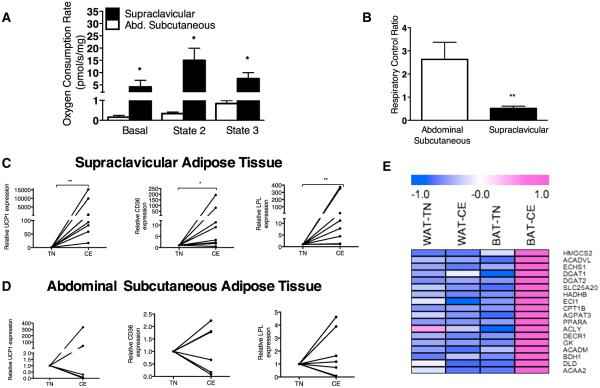
Supraclavicular BAT had a 45-fold higher leak respiration rate compared with the abdominal WAT during basal conditions (p = 0.03) (Figure 2A). These results demonstrate that, per unit of tissue, human BAT has a significantly higher capacity for heat production compared to WAT, a finding that is likely explained by greater mitochondrial volume density in BAT. Purine nucleotides (i.e., GDP, GTP, ADP, and ATP) can inhibit the thermogenic function of UCP1 (Matthias et al., 2000). Therefore, we calculated the respiratory control ratio (RCR) for ADP in WAT and BAT samples as an index of the presence of uncoupled mitochondria with functional UCP1. RCR was significantly lower in supraclavicular BAT compared to WAT samples (p = 0.001), indicating qualitative differences in WAT and BAT mitochondria. Indeed, the RCR in BAT was below 1, indicating that ADP inhibited respiration, providing evidence of functional UCP1 (Figure 2B).
Figure 2. Functional and Molecular Analysis of Supraclavicular and Abdominal Subcutaneous Adipose Tissue Samples.
(A) Oxygen consumption rates in supraclavicular brown adipose tissue (BAT) (n = 4) and subcutaneous abdominal (n = 4) adipose tissue samples from the same participants, collected during cold exposure (CE). Supraclavicular samples with significant amounts of uncoupling protein 1 (UCP1)-positive adipocytes were determined by the suppression of leak respiration upon addition of purine nucleotides. Leak respiration (basal) with sample alone is reported followed by leak respiration with complex I substrates but no ADP (State 2), followed by phosphorylating respiration with complex I substrates and saturating (5 mM) ADP (State 3). Data presented are means and SD; *p < 0.05 using Student’s t test.
(B) Respiratory control ratio (State 3 to State 2 respiration) was calculated as an index of uncoupled mitochondria in supraclavicular BAT (n = 4) and abdominal subcutaneous (n = 4) adipose tissue samples. Data are means and SD. **p = 0.001 using Student’s t test.
(C and D) Relative mRNA expression in supraclavicular (n = 8) (C) and subcutaneous abdominal adipose tissue (n = 8) (D) of uncoupling protein 1 (UCP1) and genes regulating lipid metabolism in BAT lipoprotein lipase (LPL), and cluster of differentiation 36 (CD36) in CE and thermoneutral (TN) conditions. *p = 0.02 and **p = 0.008 by Wilcoxon rank test.
(E) Expression profiles of genes involved in fatty acid/lipid metabolism in the BAT and WAT depots from Subject 2 during CE and TN conditions. The color scale shows z-scored fragments per kilobase of transcript per million mapped reads representing the mRNA level of each gene in blue-white-red scheme (blue, low expression; red, high expression). HMGCS2, 3-hydroxy-3-methylglutaryl-CoA synthase 2; ACADVL1, acyl-CoA dehydrogenase very long chain; ECHS1, enoyl CoA hydratase short chain 1; DGAT1/2, diacylglycerol acyltransferase 1 and 2; SLC25A20, solute carrier family 25 carnitine/acylcarnitine translocase member 20; HADHB, hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/rnoyl-CoA hydratase beta subunit; ECI1, enoyl-CoA delta isomerase 1; CPT1B, carnitine-palmitoyltransferase 1B; AGPAT3, 1-acylglycerol-3-phosphate O-acyltransferase 3; PPARA, peroxisome proliferator-activated receptor alpha; ACLY, ATP citrate lyase; DECR1, 2,4-dienoyl CoA reductase 1; GK, glycerol kinase; ACADM, acyl-CoA dehydrogenase C-4 to C-12 straight chain; BDH1, 3-hydroxybutyrate dehydrogenase type 1; DLD, dihydrolipoamide dehydrogenase; ACAA2, acetyl-CoA acyltransferase 2.
See also Figure S2.
To gain further insight regarding the mechanisms of accelerated lipid metabolism in relation to the cold-activated BAT, we performed molecular characterization of supraclavicular and subcutaneous abdominal adipose tissue samples collected during CE and TN conditions. As predicted by animal studies on bona fide BAT (Bartelt et al., 2011), CE increased the expression of UCP1 (p = 0.02), cluster differentiation factor 36 (CD36, p = 0.02), and lipoprotein lipase (LPL, p = 0.008) (Figure 2C) in supraclavicular adipose tissue samples. No significant changes were noted in the expression of the same genes in subcutaneous abdominal adipose tissue samples after CE (Figure 2D). These results further support the notion that acute CE induces transcriptional events in BAT to facilitate lipoprotein breakdown, fatty acid uptake, and oxidation to accommodate the high oxidative substrate demand for UCP1 thermogenesis.
To further analyze the molecular changes in the BAT in response to CE, we analyzed gene expression profiles of the BAT and subcutaneous WAT of Subjects 1 and 2 during TN and CE. Consistent with the above observations, expression of a large number of genes involved in fatty acid/lipid metabolism, including carnitine-palmitoyltransferase 1B (CPT1B), diacylglycerol acyltransferase 1 and 2 (DGAT1/2), and solute carrier family 25 carnitine-acylcarnitine translocase member 20 (SLC25A20), were significantly upregulated in the BAT from Subject 2 during CE. The upregulation in the lipid metabolism genes during CE was selective to BAT, since no significant difference was found when compared to WAT (Figure 2E). In the BAT and WAT of Subject 1, we observed no statistically significant changes in these genes in response to cold (data not shown) due to the fact that the supraclavicular biopsy sample from the Subject 1 contained largely white adipocytes. In fact, we did not observe any statistically significant enrichment in the expression of UCP1 and cell death activator (CIDEA) between the BAT and WAT of Subject 1.
Collectively, the functional and molecular analyses of BAT and WAT samples further support the significance of human BAT in lipid metabolism due to the high thermogenic capacity of BAT mitochondria and the acute upregulation of genes involved in lipid metabolism following CE.
Conclusions
This study suggests that BAT plays a significant role in systemic lipid metabolism in humans. Although performing mechanistic studies in human is not trivial, the use of state-of-the-art metabolic techniques in vivo along with molecular and functional analyses of supraclavicular BAT and WAT samples allow us to provide physiologically relevant evidence supporting a role for BAT in lipid metabolism in humans. BAT activation appears to promote increased lipid mobilization from peripheral stores and oxidative disposal, presumably to accommodate the increased fuel needs of thermogenic UCP1-positive BAT mitochondria. Further research is needed to establish the effect of BAT in TG and lipoprotein metabolism and the therapeutic potential of the “browning” of the more abundant WAT on lipid metabolism in humans.
EXPERIMENTAL PROCEDURES
Subjects
16 overweight/obese males participated in this study. For a subset of the participants, the results on the relation of BAT with glucose and energy metabolism have been previously published (Chondronikola et al., 2014). Informed written consent was obtained prior to inclusion in the study from all participants in accordance with the Declaration of Helsinki. The Institutional Review Board and the Institute for Translational Science-Clinical Research Center (ITS-CRC) Scientific Review Committee at the University of Texas Medical Branch (UTMB) approved the experimental protocol.
Experimental Protocol
For the CE trial, subjects followed an individualized non-shivering CE protocol. The two trials were performed ~2 weeks apart. The CE trial was performed first to determine the anatomical location of BAT. After 5 hr of CE or TN conditions, 185 MBq of 18F-FDG was administered to the subjects as a bolus. 1 hr later, a PET/CT (General Electric Medical Systems) scan was performed to assess BAT volume and activity. For three participants, PET/CT imaging was conducted only in CE conditions.
Subjects followed a weight-maintaining diet and refrained from physical activity and alcohol or caffeine consumption for 3 days before each study. The evening before the study, subjects were admitted to the ITS-CRC, where they were fed a standardized evening meal prior to an overnight fast. During CE and TN studies, subjects remained fasted and rested in bed. Subjects wore standardized clothing (T-shirt and shorts).
At the third hour of the study, we collected a blood and a breath sample to determine background enrichment and administered the following tracers (Cambridge Isotope Laboratories) through a catheter in a forearm vein: (a) a constant 2 hr infusion of potassium [U-13C16] palmitate (0.02 μmol/kg/min) to assess FFA kinetics, (b) a primed (18 μmol/kg), constant (0.12 μmol/kg/min) 2 hr infusion of [1,1,2,3,3-2H5] glycerol to assess whole-body lipolysis, and (c) a bolus of 13C sodium bicarbonate (55 μmol/kg dissolved in 0.9% NaCl solution) to prime the bicarbonate pool, thereby achieving a steady state in breath CO2 enrichment within 2 hr. Arterialized blood samples were obtained at 1 hr 50 min, 1 hr 55 min, and 2 hr of the infusion period to determine isotopic enrichments of glycerol and palmitate, as well as to measure plasma substrate concentrations. Additional breath samples were collected at 1 hr 30 min, 1 hr 45 min, 1 hr 50 min, 1 hr 55 min, and 2 hr to measure breath CO2 enrichment. The same stable isotope infusion regimen including only infusion of [U-13C16] palmitate was repeated during a hyperinsulinemic-euglycemic clamp (insulin infusion rate of 20 mU/m2/min) during the last 2 hr of each trial (performed after the PET/CT scan). For three subjects, the insulin clamp procedure was not completed due to failure of the retrograde catheter.
The day after each metabolic study (~14 hr following completion of the study), the 13 participants returned to the ITS-CRC for a fasting blood draw. Participants were instructed to keep their food intake constant the evening after the end of each metabolic study.
Statistical Analysis
All results are presented as means ± SD. Differences between CE and TN trials were evaluated using paired t tests for normally distributed data, while differences for non-normally distributed data were evaluated using Wilcoxon’s matched pairs signed rank test. Differences in tissue oxygen consumption rates were evaluated using Student’s t test. Pearson’s r was used to evaluate the correlation between the different outcomes of interest. Multiple regression analysis was performed to assess the relation of BAT volume with the metabolic outcomes of interest after adjusting for age and adiposity. Statistical analyses were performed using GraphPad v.5 for Mac OS X and SPSS for Mac (IBM). All statistical tests assumed a 95% level of confidence.
Additional Methods
Sections describing the tissue sampling, cooling protocol, indirect calorimetry, analyses of blood samples to measure FFA and glycerol enrichments and concentrations of metabolites and hormones, calculations to determine lipid kinetics, body composition analysis, mitochondrial respirometry, and gene expression and transcriptomics analyses can be found in the Supplemental Information.
Supplementary Material
Highlights.
BAT activation is associated with accelerated lipid metabolism
Cold induced the expression of genes involved in lipid metabolism in BAT
BAT mitochondrial thermogenesis is 45-fold greater than that ofWAT
Cold leads to a delayed decrease in TG and VLDL levels
In Brief.
Chondronikola et al. explore the role of BAT in lipid metabolism in humans and show that cold-induced BAT activation is associated with increased whole-body lipolysis, triglyceride-free fatty acid (FFA) cycling, FFA oxidation, and adipose tissue insulin sensitivity. Cold upregulates the expression of genes involved in lipid metabolism specifically in BAT.
ACKNOWLEDGMENTS
The authors want to thank the study participants and the nursing and administrative personnel at the Institute of Translational Sciences Clinical Research Center at the University of Texas Medical Branch. We thank Sarah Toombs Smith of the Sealy Center on Aging, University of Texas Medical Branch for critically editing the manuscript; Clark R. Andersen of the Institute of Translational Sciences for assisting with the statistical analysis of the results; Rajesh Kumar of the Department of Nuclear Medicine, University of Texas Medical Branch for performing the PET/CT scans; Cynthia Locklin, Carrie Barone, and Aikaterini Illiadou of the Metabolism Unit, Shriners Hospital for Children for their administrative and technical support.
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch and supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health, the American Diabetes Association (1-14-TS-35 to L.S.S.), Shriners Hospitals for Children grants (84090 and 85310 to L.S.S.), the John Sealy Memorial Endowment Fund for Biomedical Research (66992 to L.S.S.), the Claude Pepper Older Americans Independence Center (P30 AG024832 to E.V.), the Sealy Center on Aging (grant to L.S.S.), and the NIH DK97441 to S.K. M.C. was funded by the Onassis Foundation. S.M.L. was funded by a Canadian Institute of Health Research postdoctoral fellowship. C.P. was supported in part by a National Institute of Disability and Rehabilitation Research Postdoctoral Training Grant (H133P110012).
Footnotes
ACCESSION NUMBERS
RNA sequencing data have been deposited in ArrayExpress: E-MTAB-4031.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, two figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.cmet.2016.04.029.
AUTHOR CONTRIBUTIONS
L.S.S. designed the study in conjunction with E.V., E.B., M.C., and C.Y. M.C., T.C., C.Y., and N.M.H. performed the clinical trials. M.C. performed the stable isotope analyses and statistical analyses. M.K.S. analyzed the hormone measurements of blood samples. S.M.L. performed the PET/CT scan quantification analyses. C.P. and T.C. performed the mitochondrial respiration analysis. P.A. performed the adipose tissue biopsies. E.V. and P.A. were responsible for the medical coverage of the studies. F.C. performed and supervised the PET/CT scans. M.C. and M.K.S. designed and performed the gene expression experiments. D.W., K.S., and S.K. performed the transcriptomics analysis of the brown and white adipose tissue samples. M.C. wrote the initial draft of the manuscript. All authors critically reviewed the manuscript.
REFERENCES
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbée JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin DP, Labbé SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Carpentier AC, Richard D, Haman F. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J. Clin. Endocrinol. Metab. 2014;99:E438–E446. doi: 10.1210/jc.2013-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J. Physiol. 2015;593:701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 2012;35:1316–1321. doi: 10.2337/dc11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Magkos F, Conte C, Mittendorfer B, Patterson BW, Okunade AL, Klein S. Validation of a novel index to assess insulin resistance of adipose tissue lipolytic activity in obese subjects. J. Lipid Res. 2012;53:321–324. doi: 10.1194/jlr.D020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jörgensen JA, Boekschoten MV, Hesselink MK, Havekes B, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015;21:863–865. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978;27(Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. 12. [DOI] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Sun Z. Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. Br. J. Pharmacol. 2011;162:597–610. doi: 10.1111/j.1476-5381.2010.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2002;282:E1084–E1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- Ma SW, Foster DO. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can. J. Physiol. Pharmacol. 1986;64:609–614. doi: 10.1139/y86-101. [DOI] [PubMed] [Google Scholar]
- Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J. Biol. Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- Mullins GR, Wang L, Raje V, Sherwood SG, Grande RC, Boroda S, Eaton JM, Blancquaert S, Roger PP, Leitinger N, Harris TE. Catecholamine-induced lipolysis causes mTOR complex dissociation and inhibits glucose uptake in adipocytes. Proc. Natl. Acad. Sci. USA. 2014;111:17450–17455. doi: 10.1073/pnas.1410530111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz WI, Walsh SA, Morgan DA, Thomas MJ, Haynes WG. Effects of leptin on insulin sensitivity in normal rats. Endocrinology. 1997;138:3395–3401. doi: 10.1210/endo.138.8.5327. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsekouras YE, Magkos F, Kellas Y, Basioukas KN, Kavouras SA, Sidossis LS. High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. Am. J. Physiol. Endocrinol. Metab. 2008;295:E851–E858. doi: 10.1152/ajpendo.90545.2008. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. The role of triglyceride-fatty acid cycling and glucose cycling in thermogenesis and amplification of net substrate flux in human subjects. In: Muller MJ, Danrforth E, Burger AG, editors. Hormones and nutrition in obesity and cachexia. Springer; New York: 1990. [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Obesity. Vol. 19. Silver Spring; 2011. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans; pp. 1755–1760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.