Abstract
Objective
Subjective health is a complex indicator predicting longevity independent of objective health. Few studies examine genetic and environmental mechanisms underlying different facets of subjective health across the life course.
Method
Three subjective health measures were examined in 12,900 twins (Mage = 63.38, range = 25–102) from nine studies in the Interplay of Genes and Environment across Multiple Studies Consortium: self-rated health (SRH), health compared with others (COMP), and health interfering with activities (ACT).
Results
Analyses indicated age and sex differences in mean scores depending on the measure. SRH and ACT showed significant linear and non-linear moderation by age for individual differences in both genetic and environmental variance. Significant sex differences in components of variance were found for SRH and ACT, but not COMP.
Discussion
Subjective health appears to be dependent on frame of reference and reflect different aspects of health. Results suggest different genetic and environmental mechanisms underlie each facet.
Keywords: subjective health, self-rated health, twins, IGEMS, genetic and environmental influences
Introduction
Deceptive in its simplicity, assessment of subjective health has generated hundreds of papers as researchers attempt to uncover the components that contribute to judgments about personal health. The fact that subjective health predicts mortality and a variety of other health outcomes above and beyond objective health supports the importance of subjective health and generates questions about mechanisms and etiologies (Idler & Benyamini, 1997; Latham & Peek, 2013; McFadden et al., 2009). One approach to better understanding the mechanisms and etiologies underlying individual differences in subjective health is through evaluation of genetic and environmental influences, yet few studies have taken this approach. Given the different health trajectories experienced by men and women across adulthood, it is also notable that previous studies have not explored how these influences might interact with sex from early adulthood to old age (Weale, 2009).
Our primary goal was to investigate the extent to which sex and age moderate genetic and environmental influences on different subjective health facets across adulthood through the application of behavior genetic methods to twin data. These methods comprise a powerful tool for understanding sources of individual differences in a population. Even in the era of molecular genetics, twin studies provide a unique means for specification of etiologies and mechanisms underlying behavior (Miller, Deyoung, & McGue, 2012). Our second goal was to evaluate alternative conceptualizations of subjective health through the lens of behavior genetic methods. Conceptualizations about the source of variation in and meaning of subjective health have emerged that produce a variety of predictions about patterns in mean levels of subjective health measures and their association with other health and personality variables (Benyamini, 2011; Jylhä, 2009). For instance, subjective health has been viewed alternatively as (a) a holistic cognitive integration of one’s own physical health, symptoms, and sensations (Bailis, Segall, & Chipperfield, 2003; Benyamini, Blumstein, Lusky, & Modan, 2003; Jylhä, 2009); (b) a representation of cultural concepts of health, expectations about help-seeking, and meanings people give to health problems (Jylhä, 2009); (c) a reflection of personality traits such as control, anxiety, depression, or unrealistic optimism (Bailis et al., 2003; Gerstorf, Rocke, & Lachman, 2011); or (d) context-dependent perceptions that vary depending on the frame of reference (i.e., conditions under which the questions were asked, what questions were used to capture respondents’ perceptions, environmental settings, historic events; Manderbacka, Kåreholt, Martikainen, & Lundberg, 2003; Vuorisalmi, Lintonen, & Jylhä, 2006).
Many studies of subjective health rely on one global self-rated health (SRH) item; for example, “how would you rate your health in general?” or similarly worded questions (Idler & Benyamini, 1997; Pfeiffer, 1975). A second common type of question asks the respondent “how would you rate your health compared to other people your age” (Idler & Benyamini, 1997). A third type of question asks the respondent “do you think that your health status stands in the way of your doing the things you like to do?” that is, whether one’s health interferes with desired activities (Nybo et al., 2001; Pfeiffer, 1975). All of these questions comprise components of subjective health commonly assessed with omnibus scales such as the Older Americans Resources and Services (OARS) Multidimensional Functional Assessment (e.g., Pfeiffer, 1975) or the Medical Outcomes Study Short-Form Health Survey (SF-36; Ware & Sherbourne, 1992). It is unclear, however, if these questions are interchangeable. For instance, in global SRH questions, the frame of reference is ambiguous, whereas in the latter two questions, the frame of reference is more explicit, for example, compared to others your age, compared to things you like to do. In one study, as expected, older adults’ self-evaluation of their global health was poorer than younger adults’ self-evaluation but, when asked to compare their health with that of peers, older adults’ rating of how they were doing was better than younger adults’ (Andersen, Christensen, & Frederiksen, 2007). Some studies report that rating health compared with age peers predicts mortality better than a general health rating (Deeg & Kriegsman, 2003), although other studies disagree (Vuorisalmi, Lintonen, & Jylhä, 2005). It may be that these different phenotypic associations reflect dissimilar etiologies that might be illuminated by examining genetic and environmental influences affecting different subjective health measures.
Typically, twin studies have found that genetic influences account for a modest amount of the variance in measures of subjective health (variously defined) with the heritability of subjective health averaging 25% to 30%, though estimates vary widely (i.e., from 0% to 46%). Most twin studies, however, do not distinguish between different components of subjective health (Christensen, Holm, McGue, Corder, & Vaupel, 1999; Gold, Malmberg, McClearn, Pedersen, & Berg, 2002; Harris, Pedersen, Stacey, McClearn, & Nesselroade, 1992; Leinonen et al., 2005; Lichtenstein, Harris, Pedersen, & McClearn, 1993; Mosing, Zietsch, Shekar, Wright, & Martin, 2009; Romeis et al., 2005; Romeis et al., 2000; Svedberg, Gatz, Lichtenstein, Sandin, & Pedersen, 2005; Svedberg, Lichtenstein, & Pedersen, 2001).
In addition, women consistently report poorer subjective health than men, but investigations of sex differences in the ability of subjective health measures to predict mortality have produced mixed results, with greater explanatory power more often found in men than women (Benyamini et al., 2003; Deeg & Kriegsman, 2003). In the few twin studies to investigate sex differences in heritability of subjective health, results are equivocal (Mosing et al., 2009; Svedberg et al., 2001). Finally, differences in heritability by age have been examined in one sample (Swedish Adoption Twin Study of Aging [SATSA]): cross-sectional and longitudinal analyses indicated generally increasing genetic influences on subjective health with age, using a composite measure (Svedberg et al., 2005; Svedberg et al., 2001).
Prior twin studies cited were limited by both sample size and approach. To date, no twin study has systematically examined differences in heritability of various measures of subjective health. Many studies were limited by reliance on only single-item ratings of current health or by aggregation of different items into a single composite. Theory and previous results suggest that age and sex affect subjective health perceptions, yet few previous twin studies attempted to estimate age or sex influences on heritability of subjective health, and results were often equivocal. Although age and cohort are confounded, as in any cross-sectional study, it is likely that age effects will have a larger impact on components of variance than cohort effects. Finally, we expect that the conceptualization that subjective health taps shared cultural ideas about health should be reflected in estimates of the shared environmental component of variance (C). To date, a few twin studies of subjective health have found evidence for modest C, but detection of shared environmental variance requires more power than the typical twin study possesses (Christensen et al., 1999; Leinonen et al., 2005; Svedberg et al., 2005).
To address these limitations, subjective health items were identified from the nine twin studies in the Interplay of Genes and Environment Across Multiple Studies (IGEMS) Consortium, creating a sample of 12,900 twins ranging in age from 25 to 102 (Mage = 63.38). The IGEMS sample provides sufficient power to identify even modest age/cohort, sex, and shared environmental effects.
The primary aims of the current analysis were (a) to deepen our understanding of the etiology of subjective health by investigating the extent to which genetic and environmental influences on different subjective health items are moderated by sex and age, and (b) to explore implications of the findings for four different conceptualizations of individual differences in subjective health.
Method
Participants
IGEMS is an international consortium of nine twin studies covering the adult life span (Pedersen et al., 2013). Studies include the SATSA (Finkel & Pedersen, 2004), Origins of Variance in the Oldest-Old (OCTO-Twin; McClearn et al., 1997), Ageing in Women and Men: A Longitudinal Study of Gender Differences in Health Behavior and Health among Elderly (GENDER; Gold et al., 2002), the Twin and Offspring Study in Sweden (TOSS; Neiderhiser & Lichtenstein, 2008), Middle-Aged Danish Twins (MADT; Osler, McGue, Lund, & Christensen, 2008), Longitudinal Study of Aging Danish Twins (LSADT; Christensen, Fredriksen, Vaupel, & McGue, 2003; Christensen et al., 1999), the Minnesota Twin Study of Adult Development and Aging (MTSADA; Finkel & McGue, 1993), the Vietnam Era Twin Study of Aging (VETSA; Kremen et al., 2006), and Midlife in the United States (MIDUS) twin study (South & Krueger, 2012). The studies, sample sizes, and age ranges available from each IGEMS study for the current analyses are presented in Table 1. This study is based on secondary data analysis; all participants in the original studies provided informed consent, and studies were carried out in conformance with the Declaration of Helsinki. All studies were approved by their respective Institutional Review Board committees.
Table 1.
Demographic Characteristics of the Twin Samples.
| Number of twin pairs | Age | ||||||
|---|---|---|---|---|---|---|---|
| Study | N | % female |
MZ (M/F) |
SSDZ (M/F) |
OSDZ | M (SD) | Range |
| GENDER | 498 | 50 | 0/0 | 0/0 | 249 | 74.51 (2.63) | 69.7–80.7 |
| LSADT | 4,731 | 58.93 | 175/276 | 246/415 | 21 | 77.26 (5.65) | 70–102 |
| MADT | 4,314 | 49.05 | 335/329 | 312/183 | 617 | 56.43 (6.33) | 45–68 |
| MIDUS | 1,762 | 55.79 | 158/181 | 108/183 | 218 | 45.01 (12.06) | 25–75 |
| MTSADA | 1,237 | 59.4 | 103/203 | 111/135 | 0 | 58.18 (11.21) | 25.5–92 |
| OCTO-Twin | 702 | 66.67 | 56/93 | 61/141 | 0 | 83.58 (3.17) | 79.4–97.9 |
| SATSA | 1,914 | 58.52 | 116/145 | 177/291 | 0 | 60.12 (13.88) | 26.1–92.9 |
| TOSS | 1,739 | 62.97 | 121/254 | 196/282 | 0 | 44.86 (4.89) | 32–60 |
| VETSA | 1,233 | 0 | 348/0 | 262/0 | 0 | 55.43 (2.49) | 51–60 |
| Total | 18,130 | 52.70 | 1,412/1,481 | 1,473/1,738 | 1,105 | 61.64 (14.67) | 25–102 |
Note. N = number of individual twins; MZ = monozygotic; SSDZ = same-sex dizygotic; OSDZ = opposite-sex dizygotic; GENDER = Aging in Women and Men: A Longitudinal Study of Gender Differences in Health Behavior and Health Among Elderly; LSADT = Longitudinal Study of Aging Danish Twins; MADT = Middle-Aged Danish Twins; MIDUS = Midlife in the United States; MTSADA = Minnesota Twin Study of Adult Development and Aging; OCTO-Twin = origins of variance in the oldest-old; SATSA = Swedish Adoption Twin Study of Aging; TOSS = Twin and Offspring Study in Sweden; VETSA = Vietnam Era Twin Study of Aging.
Measures
Three different types of questions were used to assess facets of subjective health in the different IGEMS studies. Eight of the studies, excluding only MTSADA, asked, “How would you rate your overall health?” In the literature, the label Self-Rated Health is typically used to identify this question. All of the IGEMS studies asked participants to compare their health with others (COMP); two versions were identified: “compared to others your age, how would you rate your overall health?” used by seven of the studies and “I am as healthy as anyone I know” from the SF-36 Version 1 used by TOSS and VETSA. Participants in all studies indicated how their health affected their daily activities (ACT). Six of the studies asked a single question: “Is your health condition preventing you from doing things you like to do?” In three studies (TOSS, VETSA, and MIDUS), participants indicated whether their health affected their physical functioning based on the physical function scale from the SF-36. These comprised 10 activities for TOSS and VETSA participants, nine for MIDUS participants. Responses to activities were averaged to create a single ACT score for these three studies. Of more than 18,000 participants in IGEMS, 12,300 were assessed for SRH and 12,900 for ACT and COMP.
Harmonization
Although the subjective health questions administered across the studies were similar or identical, the response scales varied from dichotomous options to 7-point Likert-type scales. To examine and reconcile differences among these measures, we engaged in a process of harmonization in which new data on all combinations of questions and response scales were collected from an independent sample of 1,065 participants aged 30 to 98 years (Gatz et al., 2015). The harmonization sample allowed us to verify that similarly worded questions correlated significantly, regardless of exact wording or response scales, and to identify the best way to harmonize the questions across studies. Average correlations across different response scales were .77 for SRH, .78 for ACT, and .63 for COMP. Based on the results from the harmonization sample, we adopted the approach of separately standardizing the three subjective health questions within each of the nine studies, then converting to T-scores (M = 50, SD = 10), thereby allowing us to conduct pooled analyses across the nine studies. For all measures, high scores indicate perceptions of better SRH, ACT, or COMP.
Statistical Methods
Phenotypic analyses examining the effects of sex (male/female) and continuous age for each of the three subjective health questions were conducted using the non-linear mixed effects (NLME) package in R (Pinheiro, Bates, DebRoy, Sarkar, & Team, 2012). This procedure allowed for the use of all available data while controlling for the non-independence of the observations inherent with twin data by utilizing a pair-specific ID as a random effect. Significance was determined using the Type III test of fixed effects, indicating the unique association of each element of the model independent of the others.
To determine whether the genetic and environmental influences of subjective health ratings differ as a function of age, we utilized a modified version of the univariate twin model in which age was included as a moderating variable (Purcell, 2002). The standard univariate twin model leverages the genetic relatedness between monozygotic (MZ) twins and dizygotic (DZ) twins to decompose the variance of any phenotype into the proportion attributed to additive genetic influences (A), common or shared environmental influences (C), and unique environmental influences (E) (Neale & Cardon, 1992). The model used in the present study allows for a linear and quadratic increase or decrease in the A, C, and E parameters, as well as the mean of a phenotype, as a function of a continuous moderator variable, in this case age (Purcell, 2002). The presence of a significant moderator effect on the mean indicates that subjective health is significantly correlated with age. A moderator effect on the genetic and/or environmental variance components would indicate that the relative contributions of these factors to the variance of subjective health vary by age. For the moderator analyses, both members of a twin pair are needed. Preliminary analyses indicated there were very few very young and very old twin pairs (<50 pairs) so ages were truncated to be from age 30 to age 85. Truncation affected 2% of the sample; results in the truncated and original samples were similar.
We systematically fit five models to the data for each of the three measures of subjective health. We began by fitting a model with no age moderation effects (Model 1), and then proceeded to incorporate linear and quadratic moderating effects of age on the mean (Models 2 and 3) and variance components (Models 4 and 5). All models were tested using the structural equation modeling package OpenMx (Boker et al., 2011). The fit of each model was compared against that of its more parsimonious precursor (i.e., a model with linear and quadratic effects on the mean was tested against one with only linear effects). Evaluation of relative model fit was performed using the likelihood-ratio-test (LRT). Significant LRT values (p < .05) indicate that the addition of moderating effects significantly improves the fit of the model. In addition, we used the Akaike Information Criterion (AIC); AIC values represent the balance on the part of the model between goodness-of-fit (LRT) and the number of parameters (Akaike, 1987). A lower AIC value indicates a better fit. All p values are two-tailed.
To account for possible differences in genetic and environmental influences between males and females, as well as sex-specific moderation effects, a sex-limitation approach was used to allow for separate estimates for men and women (Neale & Cardon, 1992). Upon establishing the best-fitting model for the moderating effects of age, we then tested whether effects could be constrained to be equal across men and women. If they cannot be constrained to be equal, that would indicate sex differences. Significance was again determined using the LRT. In this instance, however, the direction of hypothesis testing is reversed from prior model testing; a significant LRT (p < .05) would indicate that equating effects across sexes resulted in a significant deterioration in model fit, suggesting that effects were indeed different between men and women.
Results
Phenotypic Analyses
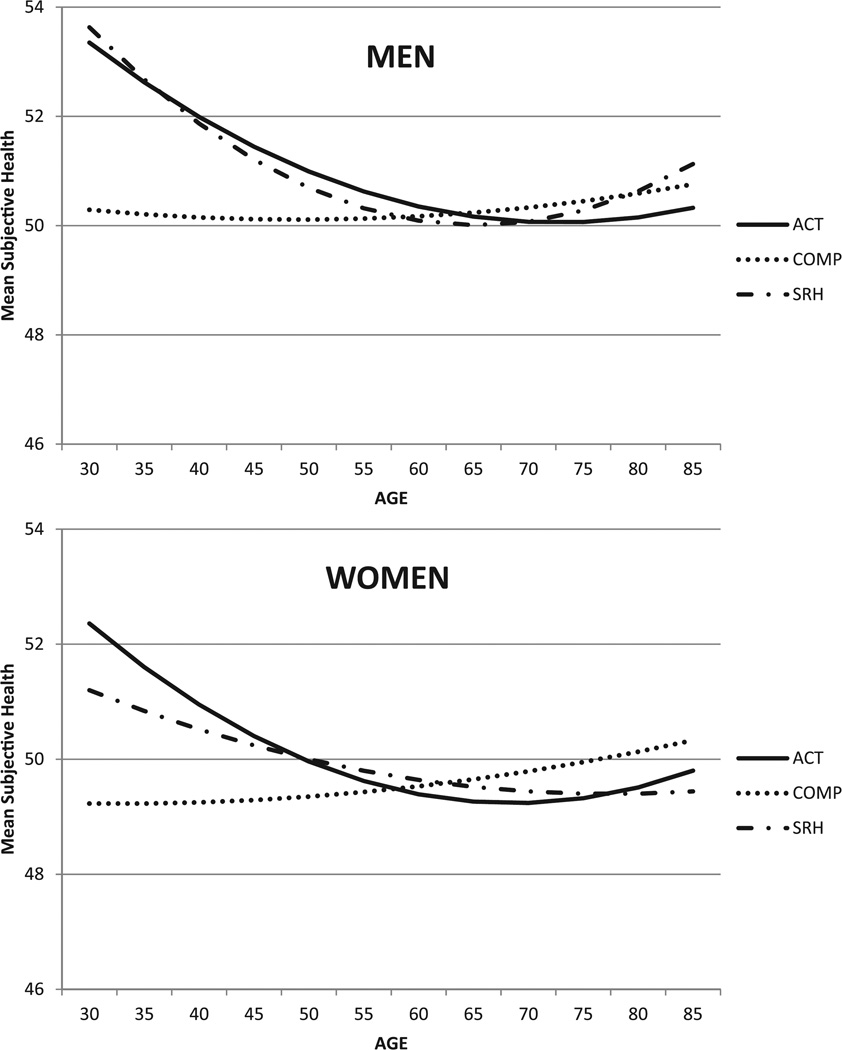
Phenotypic analyses showed significant effects of age and sex that varied depending on the subjective health item (Figure 1; see Table S1 in the supplemental materials for descriptive data). Older adults reported worse SRH and ACT than younger adults. Older adults were more likely than younger adults to report having better health compared to other adults or their age peers (COMP). Overall, women reported worse subjective health than men on all three items.
Figure 1.
Mean responses on three measures of subjective health for men and women across adulthood, converted to T-score metric: health limits activities, self-rated health, and health compared to others.
Note. Higher scores indicate perceptions of better health. ACT = activity; COMP = health compared to others; SRH = self-rated health.
Model fitting results for the tests of age moderation effects on the means of the three subjective health items are presented in Table 2. For each item, inclusion of a linear moderation effect of age on the mean indicated a significant correlation between age and each of the phenotypes (Model 2), as shown by the significant change in model fit. Significant mean-level quadratic effects of age were observed for SRH (p = .0007) and ACT (p = .0006) but not COMP (p = .4966) with greatest differences associated with age occurring before and in middle age and few age differences in older adults (Model 3; Figure 1).
Table 2.
Model Fitting Results for Tests of Linear and Quadratic Age Moderation on Means and Variance Components.
| Model | −2LL | df | AIC | LRT | Δdf | p |
|---|---|---|---|---|---|---|
| Self-rated health | ||||||
| 1. No moderation | 89,177.75 | 12,044 | 65,089.75 | — | — | — |
| 2. Model 1 + Linear Means Moderation |
89,158.16 | 12,042 | 65,074.16 | 19.59 | 2 | .0001 |
| 3. Model 2 + Quadratic Means Moderation |
89,143.56 | 12,040 | 65,063.56 | 14.60 | 2 | .0007 |
| 4. Model 3 + Linear Variance Component Moderation |
89,131.95 | 12,034 | 65,063.95 | 11.61 | 6 | .0713 |
|
5. Model 4 + Quadratic Variance Component Moderation |
89,118.11 | 12,028 | 65,062.11 | 13.84 | 6 | .0314 |
| Extent to which health limits activities | ||||||
| 1. No moderation | 94,069.24 | 12,710 | 68,649.24 | — | — | — |
| 2. Model 1 + Linear Means Moderation |
94,046.76 | 12,708 | 68,630.76 | 22.48 | 2 | <.0001 |
| 3. Model 2 + Quadratic Means Moderation |
94,032.01 | 12,706 | 68,620.01 | 14.75 | 2 | .0006 |
| 4. Model 3 + Linear Variance Component Moderation |
93,983.60 | 12,700 | 68,583.60 | 48.41 | 6 | <.0001 |
|
5. Model 4 + Quadratic Variance Component Moderation |
93,935.89 | 12,694 | 68,547.89 | 47.71 | 6 | <.0001 |
| Health compared to others | ||||||
| 1. No moderation | 94,209.79 | 12,681 | 68,847.79 | — | — | — |
| 2. Model 1 + Linear Means Moderation |
94,197.59 | 12,679 | 68,839.59 | 12.20 | 2 | .0022 |
| 3. Model 2 + Quadratic Means Moderation |
94,196.19 | 12,677 | 68,842.19 | 1.40 | 2 | .4966 |
|
4. Model 2 + Linear Variance Component Moderation |
94,178.79 | 12,673 | 68,832.79 | 17.40 | 6 | .0079 |
| 5. Model 4 + Quadratic Variance Component Moderation |
94,172.19 | 12,667 | 68,838.19 | 6.60 | 6 | .3594 |
Note. Best-fitting variance component moderation model for each measure is bolded. LL = log-likelihood; AIC = Akaike information criterion; LRT = Likelihood ratio chi-square test; Δdf = change in degrees of freedom between the model in question and the comparison model; p = significance of LRT.
Behavior Genetic Analyses
Heritability of subjective health items
We found significant genetic influences on mean level of SRH (centered at age 65) for both men and women, for ACT in men, and for COMP in women. Heritability estimates for men and women, respectively, were 27% and 32% (SRH), 24% and 2% (ACT), and 12% and 21% (COMP). Shared environmental factors were modest, in the range of 0% to 20%. Unique environmental influences, reflecting experiences specific to an individual and measurement error, accounted for the remaining variance.
Age moderation on variance components of subjective health
Model fitting results for the tests of age moderation effects on the variance components of the three subjective health items are presented in Table 2 (Models 4 and 5). A significant linear moderation effect on the variance components was observed for ACT and COMP (Model 4). For SRH, the change in model fit (p = .0713) following the addition of this moderation effect did not reach our threshold for significance; however, examination of the parameter estimates revealed significant moderation effects for men and nearly no effects for women (see Figure 2). The addition of quadratic effects (Model 5) on the variance components resulted in significant changes in model fit for SRH (p = .0314) and ACT (p < .0001) but did not significantly change the fit for COMP (p = .3594). Examination of the AIC statistic supported this conclusion: The model providing the best fit to the data (highlighted in bold in Table 2) was Model 5 for SRH and ACT and Model 4 for COMP. Thus, at the variance component levels, there were significant linear and non-linear moderation effects of age on two of the subjective health items (SRH and ACT), although the model-fitting results were less definitive for SRH than for ACT. Only linear effects of age were observed for COMP. Thus, genetic and environmental influences on different subjective health measures vary by age.
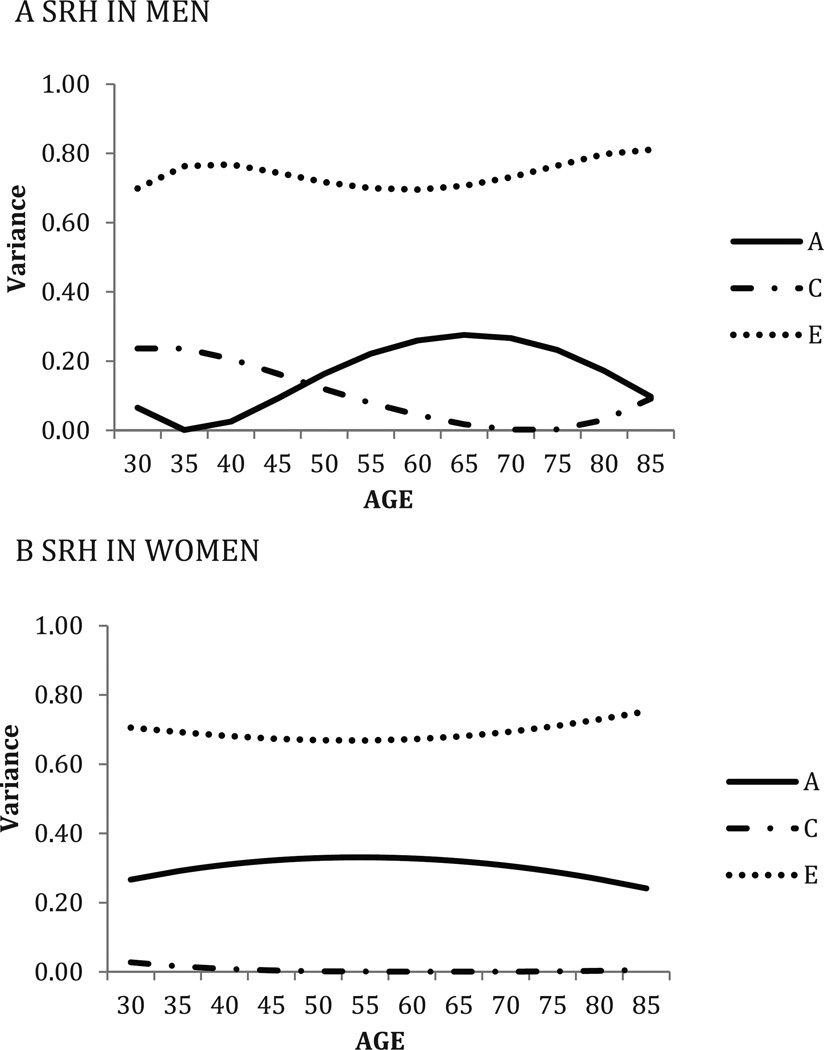
Figure 2.
Self-rated health: Standardized genetic and environmental variance components over time, by sex; best-fitting model showing linear and quadratic means moderation, linear and quadratic variance moderation, no equating sexes.
Note. “A” represents genetic variance, “C” represents shared environmental variance, and “E” represents non-shared environmental variance. Age is shown on the x axis, and standardized variance is shown on the y axis. SRH = self-rated health.
Sex-limitation effects
Tests of sex-limitation effects (i.e., whether moderation effects could be equated for men and women) found that when parameters were constrained to be equal across men and women, a significant change in model fit was observed for SRH (−2LL = 89,157.95, LRT = 39.84, df = 12,042, p = .0003) and ACT (−2LL = 94,024.21, LRT = 12,708, df = 12,708; p < .0001). A significant effect here demonstrates that the effects of age on components of variance for these subjective health items are different for men and women. In contrast, for COMP, the effects of age were equivalent for men and women; the equating of parameters resulted in a non-significant change in model fit for COMP (−2LL = 94196.47, LRT = 17.68, df = 12683, p = .06).
Figures 2, 3, and 4 present the results of the best-fitting model for age moderation of variance components results for each subjective health item. Results are presented in terms of the standardized variance components; thus genetic, shared, and unique environmental variance components at any age add up to 100%. Results for the SRH and ACT best-fitting model (Model 5) are presented separately for men and women because of the significant sex effects. Table S2 in supplemental materials presents the parameter estimates for each best-fitting models.
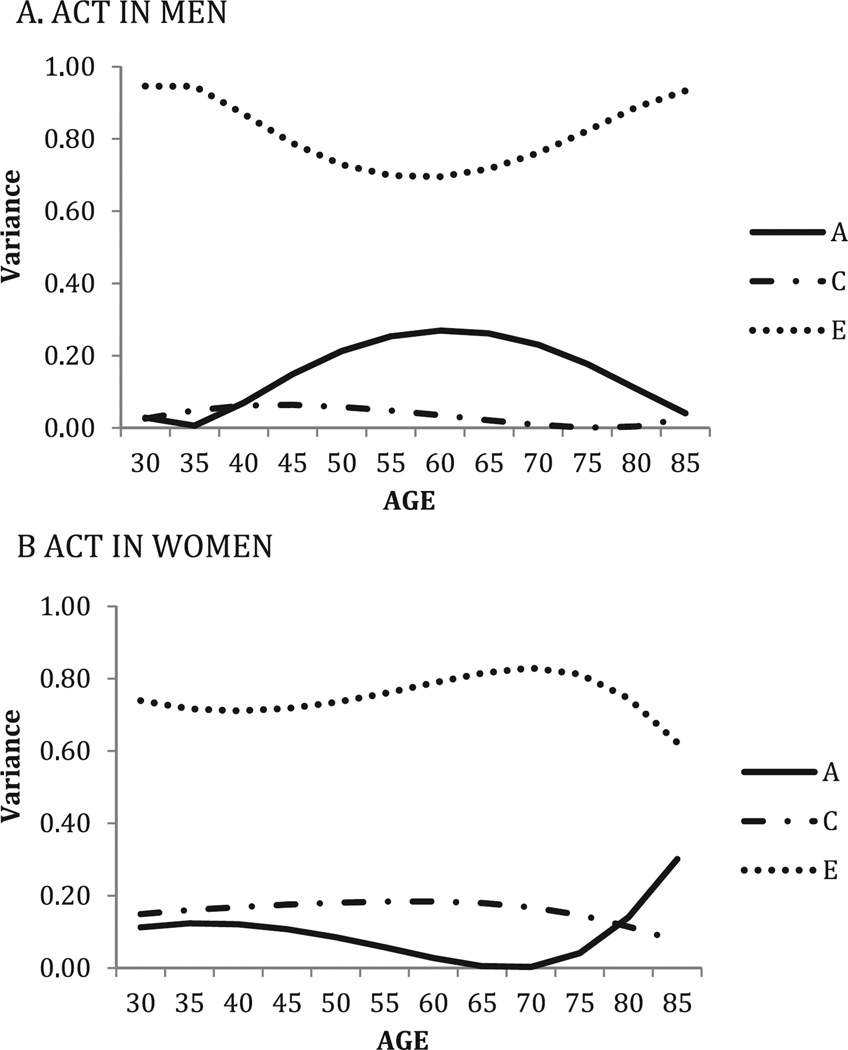
Figure 3.
Health limits activities: Standardized genetic and environmental variance components over time, by sex; best-fitting model showing linear and quadratic means moderation, linear and quadratic variance moderation, no equating sexes.
Note. “A” represents genetic variance, “C” represents shared environmental variance, and “E” represents non-shared environmental variance. Age is shown on the x axis, and standardized variance is shown on the y axis. ACT = activity.
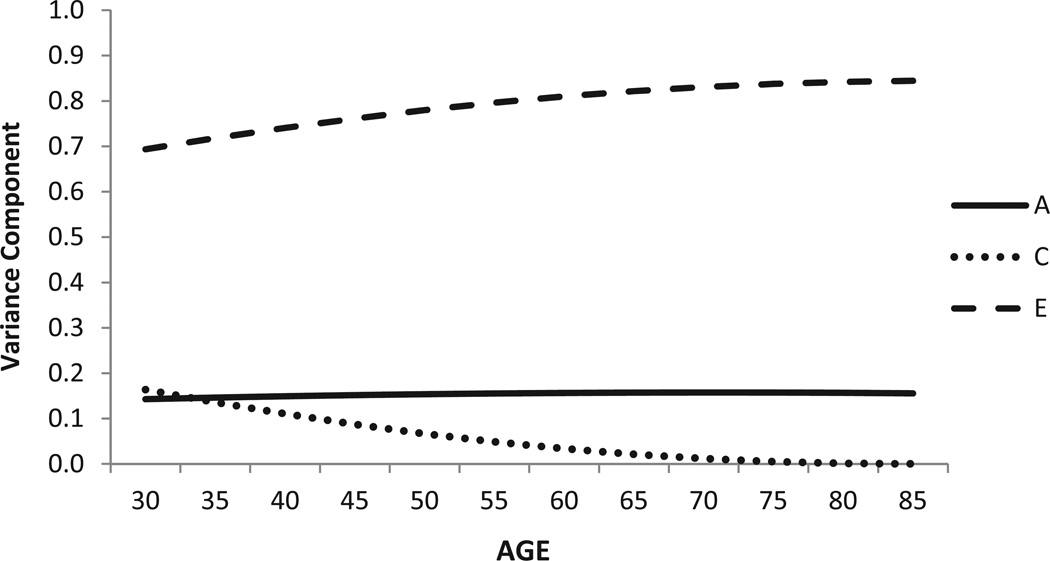
Figure 4.
Health compared to others: Standardized genetic and environmental variance components over time.
Note. Best-fitting model showing linear means moderation, linear variance moderation, sexes equated. “A” represents genetic variance, “C” represents shared environmental variance, and “E” represents non-shared environmental variance. Age is shown on the x axis, and standardized variance is shown on the y axis.
In men, heritability (A) of SRH is lower in younger men and the oldest men, with the highest genetic influences (27%) found around age 65 (Figure 2). In contrast, variance attributable to shared environment (C) is greatest early in adulthood (24%), lowest at age 65, and then slightly higher again after age of 80. Unique environmental variance (E) was more or less stable. In comparison, for SRH in women, the heritability (30%) and unique environmental variance (70%) differed little above the age range of the study. No evidence for shared environmental influences was found for women.
Age moderation effects on ACT were the most pronounced (Figure 3). Similar to results for SRH, in men, genetic influences were lower at the youngest and oldest ages, with the highest heritability (24%) found at about age 65. This pattern was paired with a complementary and opposite trend in the unique environment variance across the age range. In women, heritability was minimal in most of adulthood but significantly greater (25%) after the age of 75. Variance attributable to shared rearing environment was more substantial in women (17%) than in men but was somewhat less after age 70.
Moderation effects for COMP were less dramatic and were limited to the environmental variance components (Figure 4). Across the age range, heritability was constant at 16%. Although the shared environmental variance was highest at age 30 (18%) and then lower at later ages, the unique environmental variance was highest in the oldest ages.
Discussion
In this sample of more than 12,000 adult twins ranging in age from 30 to 85 years, we identified different patterns of age and sex moderation on means and individual differences in subjective heath depending on what aspect was analyzed. Consistent with previous phenotypic studies, women rated all aspects of their subjective health more poorly than men. Older adults, more often than younger adults, reported their health was poorer and that health problems limited their activities (Eriksson, Unden, & Elofsson, 2001; McCullough & Laurenceau, 2004). In contrast, older men and women were more likely than younger adults to rate their health as better compared with that of other people. The finding that perceptions of one’s own health compared with others improved with age is consistent with reference group theory, which proposes that the peer reference group for older adults may be in worse health (e.g., dead or disabled) than the peer reference group for younger adults (Andersen et al., 2007; Jylhä, 2009), thereby creating a different context for older and younger adults’ health perceptions.
Our primary goal was to investigate the extent to which sex and age moderated individual differences in genetic and environmental influences on subjective health across adulthood through the use of twin data and behavior genetic methods and to use what was found better to understand those three facets of subjective health. The total variance in all three subjective health components was higher in older age groups. These age differences indicate greater individual differences in later adulthood, perhaps due to the accumulation of health issues with age for some adults. However, it could be that cohort effects explain some of the age differences as the groups varied by the factors such as health care available to them as they aged. There was additional moderation by sex. Across the adult age range, unique environmental variance was the most important source of individual differences. However, genetic and environmental variance components differed significantly by subjective health item, age, and sex. For men on SRH and ACT and for women on SRH, heritability tended to be less in late adulthood than earlier. This pattern of lower heritability may reflect stochastic processes associated with physical aging, thereby affecting perceptions of health. For women on ACT, heritability peaked in older age; it is possible that higher genetic variance for self-report of functional limitations (ACT) after age 75 reflects the greater increase with age in prevalence of disabling chronic illnesses in women than in men and their impact on mobility (Sainio et al., 2006).
Our second goal was to use behavior genetic methods to explore and illuminate four common theoretical perspectives on subjective health. Overall, the most striking result is the marked difference in patterns of moderation for each measure of subjective health. This outcome is most consistent with the conceptualization that perception of subjective health is dependent on frame of reference. It is also notable that the two items (COMP, ACT) overtly providing a frame of reference had quite different age and moderation effects. Comparing one’s own health to that of others (COMP) had the most stable (though low) heritability across age, no sex effects, and only linear age moderation. The questions about whether health limits activities (ACT) specifically situate participants in their specific personal environment/context; interestingly, responses to these limitation questions showed the most pronounced moderation effects. Contextual aspects of one’s environment that affect health limitations could include elements as basic as walkability of a neighborhood, having stairs in one’s home, or living in urban versus rural settings.
These analyses provided only modest support for the conceptualization that subjective health measures reflect shared cultural concepts of health (Jylhä, 2009). One test of this conceptualization would be through findings of shared environmental influences on subjective health (i.e., through growing up or living in similar cultural contexts). However, only small shared environmental variance was found. When detected, higher levels of shared environmental variance were more likely to be found in younger adults than in middle-aged and older adults. Previous studies had insufficient power to adequately estimate shared environmental variance; the sample size included in the current analyses allowed us to identify significant but modest effects. Shared environmental effects could reflect the lingering effects of the rearing environment; rearing environmental effects on many characteristics tend to decline as soon as individuals leave the childhood home (Plomin, DeFries, Knopik, & Neiderhiser, 2013). Some studies find that younger adult cohorts are more aware of their personal responsibility for health and health behaviors than older cohorts (Chen, Cohen, & Kasen, 2007), which may result in cohort or age differences. Overall, however, there was little evidence for shared environmental effects.
These results provided the least support for the conceptualization that subjective health perceptions are trait-like or reflect underlying traits. Based on the evidence that trait-like characteristics are likely to be stable across adulthood and are moderately heritable, we would have anticipated moderately high heritability (>40%) at all ages. Instead, we found significant variation in heritability across ages (SRH and ACT) or only low heritability (COMP).
The conceptualization that subjective health is an indicator of holistic cognitive integration of one’s physical health, symptoms, and sensations received the strongest support from the SRH item. Based on this conceptualization, genetic and environmental contributions to subjective health would be predicted to mirror their contributions to objective measures of physical health, as well as any age and sex differences. A recent review of genetic influences on health concluded that most measures of objective health in adulthood demonstrate moderate heritability in the range of 35% to 40% and little evidence for shared environmental effects, although heritability estimates vary widely across health domains (Finkel, Gerritsen, Reynolds, Dahl, & Pedersen, 2014). Genetic influences were the strongest for SRH, though somewhat weaker compared with that typically found for physical health measures. Further research is needed, however, to explicitly examine associations between objective and subjective health. In summary, these results provide the most support for the conceptualization that subjective health perceptions are context-dependent and tap into individual differences in health and health-related functioning.
This study has several limitations. First, despite the very large sample created by aggregating data from nine studies, there were too few very old adult twins (above age 85) to extend the analyses into very late old age. In addition, the studies were all of twins from either the United States or Scandinavia, thus limiting generalizability of these results to other countries. About one third of the current IGEMS subjects were possibly included in one or another of the studies cited in the introduction; however, typically only one subjective health item (SRH) or a composite had been analyzed in those studies. Moreover, previous studies rarely investigated age and sex effects on heritability. As with any cross-sectional study, age and cohort were confounded; thus, it is possible that age differences in genetic and environmental contributions to subjective health also reflect cohort differences. Finally, any study of aging is influenced by survivor effects. Research participants are those adults who are still alive and well enough to participate at any particular time point. Thus, older adults in this sample likely represent adults with somewhat better objective and perhaps better subjective health than the general population. Longitudinal designs will ultimately help to address possible cohort effects within and across the different studies, and can help mitigate some of these concerns as they provide insight into attrition patterns over time.
Our results have wide-ranging implications for further research and health policy; here, we highlight three. First, there is a strong legacy in subjective health research that perceptions have major consequences for morbidity and mortality; yet, to date, little research has elucidated the different etiologies and mechanisms underlying these perceptions. By identifying which measures show the strongest genetic influences (and under what circumstances), these results can help inform molecular research on the neurobiology of individual differences in subjective health. Second, considerable resources are invested into surveys of health and attitudes by organizations such as the World Health Organization or Gallup Poll to better understand gaps in health policy. Remarkably little attention has been paid, however, to the nature of individual differences in subjective health. Our findings of different genetic and environmental etiologies as well as different patterns of moderation for the different measures can guide the development of optimized measures of subjective health, and improve analysis of predictors and outcomes of different types of subjective health. Finally, the results highlight the strong influences of individual specific environmental influences on perceptions of subjective health. Although further study is needed to identify what types of environmental events most strongly affect different aspects of subjective health, it is clear that identification of environmental influences is important and could potentially lead to development of interventions involving more healthy conditions (e.g., from modifying community structures, to use of technologies, to provision of mental health services—to name a few) that may then be instrumental in improving health outcomes.
Despite its limitations, this study provided new and innovative findings about subjective health perceptions across 55 years of the life course from age 30 to age 85. These differences among subjective health items at a genetic and environmental level may have important implications for predicting health outcomes and may help to clarify inconsistent findings in the literature. Other studies demonstrate that some of the genetic variance for subjective health is shared with genetic variance for associated phenotypes such as optimism and good mental health (Mosing et al., 2009), depression (Mosing, Pedersen, Martin, & Wright, 2010), or disease severity (Leinonen et al., 2005), yet these studies seldom distinguish among types of questions about subjective health. Attempts to identify genetic and environmental contributions to health and longevity may need to consider components of subjective health independently in addition to other contextual components such as sex and age. Finally, more research needs to be conducted on identifying specific types of environmental influences that, if modifiable, could affect perceptions of subjective health and potentially improve health and longevity. Measures of physical health, cognitive function, emotional health, and environmental factors available from the studies in the IGEMS consortium will allow us to deepen our understanding of the nature of genetic and environmental contributions to subjective health.
Supplementary Material
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Interplay of Genes and Environment Across Multiple Studies (IGEMS) is supported by the National Institutes of Health Grant R01 AG037985. SATSA was supported by Grants R01 AG04563 and R01 AG10175, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS; 97:0147:1B, 2009-0795), and Swedish Research Council (825-2007-7460, 825-2009-6141). OCTO-Twin was supported by Grant R01 AG08861. Gender was supported by the MacArthur Foundation Research Network on Successful Aging, The Axel and Margaret Ax:son Johnson’s Foundation, The Swedish Council for Social Research, and the Swedish Foundation for Health Care Sciences and Allergy Research. TOSS was supported by Grant R01 MH54610 from the U.S. National Institutes of Health. The Danish Twin Registry is supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the U.S. National Institutes of Health (P01 AG08761). The Minnesota Twin Study of Adult Development and Aging was supported by NIA Grant R01 AG 06886. VETSA was supported by National Institutes of Health Grants NIA R01 AG018384, R01 AG018386, R01 AG022381, R03 AG046413, and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. This MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by National Institute on Aging Grant R01 AG20166.
Appendix
Members of the consortium on Interplay of Genes and Environment Across Multiple Studies (IGEMS) include Nancy L. Pedersen (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, and Department of Psychology, University of Southern California, Los Angeles, California), Kaare Christensen (Department of Epidemiology, University of Southern Denmark, Odense, Denmark), Anna Dahl (Institute of Gerontology, School of Health Sciences, Jönköping University, Jönköping, Sweden), Deborah Finkel (Department of Psychology, Indiana University Southeast, New Albany, Indiana), Carol E. Franz (Department of Psychiatry, University of California, San Diego, La Jolla, California), Margaret Gatz (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, and Department of Psychology, University of Southern California, Los Angeles, California), Briana N. Horwitz (Department of Psychology, California State University, Fullerton, California), Boo Johansson (Department of Psychology, University of Gothenburg, Gothenburg, Sweden), Wendy Johnson (Department of Psychology and Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, UK), Jaakko Kaprio (Department of Public Health, University of Helsinki, Helsinki, Finland), William S. Kremen (Department of Psychiatry, University of California, San Diego, and VA Center of Excellence for Stress and Mental Health, La Jolla, CA), Robert Krueger (Department of Psychology, University of Minnesota, Minneapolis, MN), Michael J. Lyons (Department of Psychological and Brain Sciences, Boston University, Boston, MA), Matt McGue (Department of Psychology, University of Minnesota, Minneapolis, Minnesota), Jenae M. Neiderhiser (Department of Psychology, The Pennsylvania State University, University Park, Pennsylvania), Matthew S. Panizzon (Department of Psychiatry, University of California, San Diego, La Jolla, California), Inge Petersen (Department of Epidemiology, University of Southern Denmark, Odense, Denmark), and Chandra A. Reynolds (Department of Psychology, University of California-Riverside, Riverside, California). Carol E. Franz and Deborah Finkel are joint senior authors. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, the VA, or other funders.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Andersen FK, Christensen K, Frederiksen H. Self-rated health and age: A cross-sectional and longitudinal study of 11,000 Danes aged 45–102. Scandinavian Journal of Public Health. 2007;35:164–171. doi: 10.1080/14034940600975674. [DOI] [PubMed] [Google Scholar]
- Bailis DS, Segall A, Chipperfield JG. Two views of self-rated general health status. Social Science & Medicine. 2003;56:203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Benyamini Y. Why does self-rated health predict mortality? An update on current knowledge and a research agenda for psychologists. Psychology and Health. 2011;26:1407–1413. doi: 10.1080/08870446.2011.621703. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in self-rated health-mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? The Gerontologist. 2003;43:396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, Kasen S. Cohort differences in self-rated health: Evidence from a three-decade, community-based, longitudinal study of women. American Journal of Epidemiology. 2007;166:439–446. doi: 10.1093/aje/kwm100. [DOI] [PubMed] [Google Scholar]
- Christensen K, Frederiksen H, Vaupel JW, McGue M. Age trajectories of genetic variance in physical functioning: A longitudinal study of Danish twins aged 70 years and older. Behavior Genetics. 2003;33:125–136. doi: 10.1023/a:1022501817781. [DOI] [PubMed] [Google Scholar]
- Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. Journal of Aging and Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- Deeg DJH, Kriegsman DMW. Concepts of self-rated health: Specifying the gender difference in mortality risk. The Gerontologist. 2003;43:376–386. doi: 10.1093/geront/43.3.376. [DOI] [PubMed] [Google Scholar]
- Eriksson I, Unden AL, Elofsson S. Self-rated health. Comparisons between three different measures. Results from a population study. International Journal of Epidemiology. 2001;30:326–333. doi: 10.1093/ije/30.2.326. [DOI] [PubMed] [Google Scholar]
- Finkel D, Gerritsen L, Reynolds CA, Dahl AK, Pedersen NL. Etiology of individual differences in human health and longevity. In: Sprott RL, editor. Annual review of gerontology and geriatrics—Genetics. New York, NY: Springer; 2014. pp. 189–227. [Google Scholar]
- Finkel D, McGue M. The origins of individual differences in memory among the elderly: A behavior genetic analysis. Psychology and Aging. 1993;8:527–537. doi: 10.1037//0882-7974.8.4.527. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish adoption/Twin Study of aging. Aging, Neuropsychology, and Cognition. 2004;11:325–345. [Google Scholar]
- Gatz M, Reynolds CA, Finkel D, Hahn C, Zhou Y, Zavala C. Data harmonization in aging research: Not so fast. Experimental Aging Research. 2015;41:475–495. doi: 10.1080/0361073X.2015.1085748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Rocke C, Lachman ME. Antecedent-consequent relations of perceived control to health and social support: Longitudinal evidence for between-domain associations across adulthood. Journals of Gerontology Series B. Psychological Sciences and Social Sciences. 2011;66(1):61–71. doi: 10.1093/geronb/gbq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: A study of older unlike-sex twins. The Journals of Gerontology, Series B. Psychological Sciences & Social Sciences. 2002;57(3):S168–S176. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- Harris JR, Pedersen NL, Stacey C, McClearn GE, Nesselroade JR. Age differences in the etiology of the relationship between life satisfaction and self-rated health. International Journal of Aging and Health. 1992;4:187–195. [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science & Medicine. 2009;69:307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung Y-MJ, Grant MD, Franz CE, Eisen SA, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Latham K, Peek CW. Self-rated health and morbidity onset among late midlife U.S adults. The Journals of Gerontology, Series B. Psychological Sciences & Social Sciences. 2013;68:p107–p116. doi: 10.1093/geronb/gbs104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen R, Kaprio J, Jylhä M, Tolvanen A, Koskenvuo M, Heikkinen E, Rantanen T. Genetic influences underlying self-rated health in older female twins. Journal of the American Geriatric Society. 2005;53:1002–1007. doi: 10.1111/j.1532-5415.2005.53319.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Harris JR, Pedersen NL, McClearn GE. Socioeconomic status and physical health, how are they related? An empirical study based on twins reared apart and twins reared together. Social Science & Medicine. 1993;36:441–450. doi: 10.1016/0277-9536(93)90406-t. [DOI] [PubMed] [Google Scholar]
- Manderbacka K, Kåreholt I, Martikainen P, Lundberg O. The effect of point of reference on the association between self-rated health and mortality. Social Science & Medicine. 2003;56:1447–1452. doi: 10.1016/s0277-9536(02)00141-7. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influences on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1523. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Laurenceau JP. Gender and the natural history of self-rated health: A 59-year longitudinal study. Health Psychology. 2004;23:651–655. doi: 10.1037/0278-6133.23.6.651. [DOI] [PubMed] [Google Scholar]
- McFadden E, Luben R, Bingham S, Wareham N, Kinmonth A-L, Khaw K-T. Does the association between self-rated health and mortality vary by social class? Social Science & Medicine. 2009;68:p275–p280. doi: 10.1016/j.socscimed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Miller MB, Deyoung CG, McGue M. Assumptions in studies of heritability and genotype-phenotype association. Behavioral & Brain Sciences. 2012;35:372–373. doi: 10.1017/S0140525X12001380. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Pedersen NL, Martin NG, Wright MJ. Sex differences in the genetic architecture of optimism and health and their interrelation: A study of Australian and Swedish twins. Twin Research and Human Genetics. 2010;13:322–329. doi: 10.1375/twin.13.4.322. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Zietsch BP, Shekar SN, Wright MJ, Martin NG. Genetic and environmental influences on optimism and its relationship to mental and self-rated health: A study of aging twins. Behavior Genetics. 2009;39:597–604. doi: 10.1007/s10519-009-9287-7. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 1992. [Google Scholar]
- Neiderhiser JM, Lichtenstein P. The Twin and Offspring Study in Sweden: Advancing our understanding of genotype-environment interplay by studying twins and their families. Acta Psychologica Sinica. 2008;40:1116–1123. [Google Scholar]
- Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K. Functional status and self-rated health in 2,262 nonagenarians: The Danish 1905 Cohort Survey. Journal of the American Geriatrics Society. 2001;49:601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- Osler M, McGue M, Lund R, Christensen K. Marital status and twins’ health and behavior: An analysis of middle-aged Danish twins. Psychosomatic Medicine. 2008;70:482–487. doi: 10.1097/PSY.0b013e31816f857b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Christensen K, Dahl AK, Finkel D, Franz CE, Gatz M, Reynolds CA. IGEMS: The consortium on interplay of genes and environment across multiple studies. Twin Research and Human Genetics. 2013;16:481–489. doi: 10.1017/thg.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E. In: Multidimensional functional assessment: The OARS methodology— A manual. Center for the Study of Aging and Human Development, editor. Durham, NC: Duke University; 1975. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar DR R Development Core Team. nlme: Linear and nonlinear mixed effects models. R package version 3. 2012:1–103. [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Behavioral genetics. 6th. New York, NY: Worth; 2013. [Google Scholar]
- Purcell S. Variance component models for gene-environment interaction in twin analyses. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Romeis JC, Heath AC, Xian H, Eisen SA, Scherrer JF, Pedersen NL, True WR. Heritability of SF-36 among middle-age, middle-class, male-male twins. Medical Care. 2005;43:1147–1154. doi: 10.1097/01.mlr.0000183217.11811.bd. [DOI] [PubMed] [Google Scholar]
- Romeis JC, Scherrer JF, Xian H, Eisen SA, Bucholz K, Heath AC, True WR. Heritability of self-reported health. Health Services Research. 2000;35:995–1010. [PMC free article] [PubMed] [Google Scholar]
- Sainio P, Koskinen S, Heliovaara M, Martelin T, Harkanen T, Hurri H, Aromaa A. Self-reported and test-based mobility limitations in a representative sample of Finns aged 30+ Scandinavian Journal of Public Health. 2006;34:378–386. doi: 10.1080/14034940500489859. [DOI] [PubMed] [Google Scholar]
- South SC, Krueger RF. Genetic strategies for probing conscientiousness and its relationship to aging. Developmental Psychology. 2012;50:1362–1376. doi: 10.1037/a0030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedberg P, Gatz M, Lichtenstein P, Sandin S, Pedersen NL. Self-rated health in a longitudinal perspective: A 9-year follow-up twin study. The Journals of Gerontology, Series B. Psychological Sciences & Social Sciences. 2005;60(6):S331–S340. doi: 10.1093/geronb/60.6.s331. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Lichtenstein P, Pedersen NL. Age and sex differences in genetic and environmental factors for self-rated health: A twin study. The Journals of Gerontology, Series B. Psychological Sciences & Social Sciences. 2001;56(3):S171–S178. doi: 10.1093/geronb/56.3.s171. [DOI] [PubMed] [Google Scholar]
- Vuorisalmi M, Lintonen T, Jylhä M. Global self-rated health data from a longitudinal study predicted mortality better than comparative self-rated health in old age. Journal of Clinical Epidemiology. 2005;58:680–687. doi: 10.1016/j.jclinepi.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Vuorisalmi M, Lintonen T, Jylhä M. Comparative vs global selfrated health: Associations with age and functional ability. Aging Clinical and Experimental Research. 2006;18:211–217. doi: 10.1007/BF03324651. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Weale R. Biomarkers by gender. Archives of Gerontology and Geriatrics. 2009;49:208–211. doi: 10.1016/j.archger.2008.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.