Abstract
IMPORTANCE
Noninvasive ventilation (NIV) with a face mask is relatively ineffective at preventing endotracheal intubation in patients with acute respiratory distress syndrome (ARDS). Delivery of NIV with a helmet may be a superior strategy for these patients.
OBJECTIVE
To determine whether NIV delivered by helmet improves intubation rate among patients with ARDS.
DESIGN, SETTING, AND PARTICIPANTS
Single-center randomized clinical trial of 83 patients with ARDS requiring NIV delivered by face mask for at least 8 hours while in the medical intensive care unit at the University of Chicago between October 3, 2012, through September 21, 2015.
INTERVENTIONS
Patients were randomly assigned to continue face mask NIV or switch to a helmet for NIV support for a planned enrollment of 206 patients (103 patients per group). The helmet is a transparent hood that covers the entire head of the patient and has a rubber collar neck seal. Early trial termination resulted in 44 patients randomized to the helmet group and 39 to the face mask group.
MAIN OUTCOMES AND MEASURES
The primary outcome was the proportion of patients who required endotracheal intubation. Secondary outcomes included 28-day invasive ventilator–free days (ie, days alive without mechanical ventilation), duration of ICU and hospital length of stay, and hospital and 90-day mortality.
RESULTS
Eighty-three patients (45% women; median age, 59 years; median Acute Physiology and Chronic Health Evaluation [APACHE] II score, 26) were included in the analysis after the trial was stopped early based on predefined criteria for efficacy. The intubation rate was 61.5% (n = 24) for the face mask group and 18.2% (n = 8) for the helmet group (absolute difference, −43.3%; 95% CI, −62.4%to −24.3%; P < .001). The number of ventilator-free days was significantly higher in the helmet group (28 vs 12.5, P < .001). At 90 days, 15 patients (34.1%) in the helmet group died compared with 22 patients (56.4%) in the face mask group (absolute difference, −22.3%; 95% CI, −43.3 to −1.4; P = .02). Adverse events included 3 interface-related skin ulcers for each group (ie, 7.6% in the face mask group had nose ulcers and 6.8% in the helmet group had neck ulcers).
CONCLUSIONS AND RELEVANCE
Among patients with ARDS, treatment with helmet NIV resulted in a significant reduction of intubation rates. There was also a statistically significant reduction in 90-day mortality with helmet NIV. Multicenter studies are needed to replicate these findings.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01680783
Noninvasive ventilation (NIV) by face mask can obviate the need for endotracheal intubation and improve mortality in patients with acute respiratory failure. Complications of endotracheal intubation include pneumonia,1 excessive sedation,2 delirium,3 and intensive care unit (ICU)–acquired weakness.4 Noninvasive ventilation allows patients to remain animated while in the ICU, a strategy now adopted in many ICUs.5 Although benefits of face mask NIV for chronic obstructive pulmonary disease (COPD) exacerbations6 and cardiogenic pulmonary edema7 are compelling, its use in acute hypoxemic respiratory failure (AHRF) remains controversial. Initial reports suggested improved survival in immunocompromised patients with hypoxemic respiratory failure8; however, those findings have not been replicated.9 A study by Frat et al10 showed increased mortality was associated with face mask NIV for AHRF compared with high-flow nasal cannula.
About half of patients with hypoxemia, especially those with acute respiratory distress syndrome (ARDS), are not helped with face mask ventilation.11,12 Often higher levels of positive end-expiratory pressure (PEEP) to improve oxygenation are needed. However, at high PEEP, face mask intolerance and air leak can impede effective oxygenation.13 Therefore, the face mask has limitations that may contribute to reduced efficacy during AHRF.9
An alternative is to deliver NIV via a helmet interface–a transparent hood that covers the entire head of the patient with a soft collar neck seal. This interface confers several advantages over face mask including improved tolerability and less air leak due to the helmet’s lack of contact with the face and improved seal integrity at the neck.14,15 Therefore, the helmet’s design may allow increased titration of positive airway pressures without substantial air leak. This could reduce intubation rates and extend the benefits of NIV to more patients with ARDS.
To our knowledge, there have been no randomized trials directly comparing face mask to helmet NIV for the prevention of endotracheal intubation in ARDS. We conducted a single-center, randomized clinical trial of patients admitted to the ICU for ARDS requiring NIV to determine whether helmet NIV could reduce the rate of intubation and improve other patient outcomes.
Methods
Consecutive patients admitted to the adult medical ICU at the University of Chicago from September 2012 through September 2015 were screened daily. The institutional review board approved the study. Written informed consent was obtained from participants or from their authorized surrogate decision maker. Patients 18 years or older who required face mask NIV for at least 8 hours for the management of ARDS were eligible for enrollment. Acute respiratory distress syndrome was defined by the Berlin criteria.16
Patients were excluded if they had impending cardiopulmonary arrest, a Glasgow coma scale score lower than 8, absence of airway protective gag reflex, elevated intracranial pressure, tracheostomy, or upper airway obstruction; were pregnant; or had refused endotracheal intubation. Patient demographics such as race were collected by self-report with fixed categories. Race data were collected to reflect the diversity of patients admitted to the medical ICU.
Intervention
After 8 hours of NIV via face mask, patients were approached for consent. They were randomly assigned in a 1:1 ratio to either continue with the face mask (control) or switch to a helmet interface (intervention). A computer-generated, permuted block randomization scheme with varying block sizes ranging from 4 to 8 was used to allocate patients to each group. The block allocation was blinded. Each assignment was designated in a consecutively numbered, sealed, opaque envelope.
Patients randomized to the intervention switched from a face mask (Philips Respironics) to a latex-free helmet (Sea Long). The helmet group received NIV via an ICU ventilator (Engström Carestation, GE Healthcare) in pressure support or continuous positive airway pressure mode. The helmet, made of transparent latex-free polyvinyl chloride, was secured by padded armpit braces attached to 2 hooks on the front and back of a plastic ring connecting the helmet to a latex-free neck seal, thus producing a breathing circuit closed from the outside environment. The patient neck circumference was measured and the neck seal was cut to ensure a tight but comfortable seal. The helmet was connected to the ventilator by conventional respiratory circuitry joining 2 port sites to allow inspiratory and expiratory flow. To avoid carbon dioxide rebreathing, pressure support levels were set to maintain a ventilator inspiratory flow rate of more than 100 L/min.17 To minimize inspiratory effort and optimize patient-ventilator synchrony, the ventilator pressurization time was set to 50 milliseconds and cycling off delay set to 50% of maximal inspiratory flow (Video).18
The face mask group was managed with a single-limb non-invasive ventilator (Philips Respironics V60). The helmet could not be managed with the Philips Respironics V60 ventilator because it requires 2 port sites for inspiratory and expiratory flow. Both groups had titration of NIV by a standard protocol: PEEP was increased in increments of 2 to 3 cm H2O to improve oxygen saturation to more than 90% at an inspired oxygen fraction (FIO2) of 60% or less, if possible. Inspiratory pressure was increased in increments of 2 to 3 cm H2O to obtain a respiratory rate of less than 25/min and disappearance of accessory muscle activity. For NIV weaning, support was reduced progressively in accordance to clinical improvement and discontinued if the patient maintained a respiratory rate of less than 30/min and partial pressure of oxygen (PaO2) of more than 75 mm Hg with FIO2 less than 50% and PEEP of less than 5 cm H2O.
The decision to intubate all patients was based on predetermined criteria similar to those used in previous studies of NIV.10,19 These included neurologic deterioration, persistent or worsening respiratory failure (eg, oxygen saturation <88%, respiratory rate >36/min), intolerance of face mask or helmet, airway bleeding, or copious respiratory secretions. All decisions to intubate were made by the primary care team with no involvement from the research team. Patients who required endotracheal intubation had initial ventilator settings of assist-control mode with tidal volumes of 6 mL/kg of ideal body weight20 and titration of PEEP to achieve oxygen saturation of 88% to 95% at lowest possible FIO2 (goal FIO2 <0.6). Daily interruption of sedation,2 awakening and breathing trials,21 and early mobilization22 were performed per ICU standard care. Adverse events were prespecified to include factors specific to helmet NIV use and included skin ulceration at the neck seal, patient intolerance (ie, claustrophobia), and device complications (ie, helmet deflation).
Study Outcomes
The primary outcome was the proportion of patients who underwent endotracheal intubation based on criteria established a priori.10,19 Secondary outcomes were 28-day invasive ventilator-free days (ie, days alive without mechanical ventilation), duration of ICU and hospital length of stay, hospital and 90-day mortality, and adverse events. Because we have multiple secondary outcomes, and we analyzed them without adjustment for multiple comparisons, we considered them exploratory. Because of the nature of the 2 intervention groups, blinding was not possible for the outcomes of interest.
Statistical Analysis
Assuming an intubation rate of 50% for patients with hypoxemic respiratory failure requiring NIV,10,23,24 we calculated that enrollment of a total of 206 patients would provide 80% power to detect a 20% absolute reduction of the primary outcome, with a 2-sided α level of .05. Because previous work has shown that 50% of patients with ARDS treated with NIV delivered via face mask required intubation,24 we reasoned that a 30% intubation rate (ie, a 20% reduction) would be a clinically significant improvement.
All analyses were performed by an intention-to-treat analysis. Patients who died during the study were assigned scores of 0 for ventilator-free days.25 The χ2 test or Fisher exact test was used as appropriate to compare categorical variables, including the primary outcome. Wilcoxon-Mann-Whitney 2-sample rank sum test or t tests were used to compare continuous variables. The area under the curve was calculated for every measured respiratory rate, oxygen saturation, FIO2, PEEP, and pressure support levels during NIV.26 To evaluate the effect of the intervention on 90-day survival, a time-to-event analysis estimated with the Kaplan-Meier procedure was used. The effect of the intervention was compared between groups using the log-rank test. The cumulative incidence of intubation (with death without intubation as a competing risk) within each randomized group was estimated using a nonparametric estimator and compared using the Fine-Gray test.27
Additional analyses were performed with Cox-regression models that adjusted for Acute Physiology and Chronic Health Evaluation (APACHE) II and the presence of the helmet intervention. Hazard ratios (HRs) together with 95% CIs were estimated using this model. Stata 11.0 (StataCorp LP) software was used for statistical analyses. The study protocol and statistical analysis plan are available in the Supplement.
Safety Monitoring
An independent data and safety monitoring board (DSMB) continuously monitored safety and study conduct. Interim analyses were planned at one-third and two-thirds of enrollment (70 and 140 patients, respectively). Early stopping for efficacy was predetermined at a P value <.001 for rejection of the null hypothesis to declare that the helmet strategy was superior to face mask. At the first interim analysis, the results met criteria for early stoppage of the trial for efficacy; however, the DSMB determined that the trial should continue because the helmet was not available for use outside the trial; therefore, nonstudy patients would not be deprived of the benefit. In addition, the DSMB determined that there were no safety concerns and that the study had not met other secondary end points that (eg, ICU length of stay) could have been reached with further enrollment. Subsequent to this, the DSMB evaluated work by Frat et al10 that reported increased mortality among patients treated with face mask NIV compared with high-flow nasal cannula. The DSMB determined that the face mask group could have been exposed to increased risk of mortality and because the study already had met the preestablished criteria for early stoppage, the DSMB recommended that the study be stopped for both efficacy and safety after the enrollment of 83 patients.
Results
From October 2012 through September 2015, 740 patients were screened, of whom 83 patients were randomized and enrolled (Figure 1). Thirty-nine patients were assigned to conventional face mask and 44 to helmet NIV. No patient was lost to follow-up. The median interval of NIV prior to randomization was not different between face mask and helmet (13.0 vs 10.3 hours, P = .65).
Figure 1. Flow of Participants Through Study.
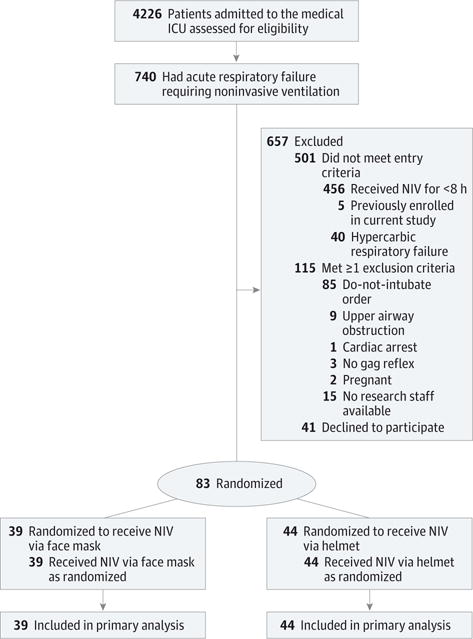
ICU indicates intensive care unit; NIV, noninvasive ventilation.
Characteristics at Inclusion
There was no statistically significant difference between baseline characteristics of patients in both groups. Sixty patients (72%) had a PaO2/FIO2 ratio of less than 200. Both groups had a high severity of illness as indicated by APACHE II scores. About half of the patients in each group were immunocompromised by virtue of cancer or transplant, and about one-third in each group had an immunocompromised pneumonia (Table 1).
Table 1.
Characteristics of Patients at Baseline
| Characteristic | No. (%) of Patients Receiving Noninvasive Ventilation | |
|---|---|---|
| Face Mask (n = 39) |
Helmet (n = 44) |
|
| Age, median (IQR), y | 60.9 (56.4–71.1) | 58 (49.8–67.8) |
| Women | 18 (46) | 20 (45) |
| Black | 22 (56) | 28 (64) |
| White, non-Hispanic | 13 (33) | 11 (25) |
| White, Hispanic | 3 (8) | 3 (7) |
| Asian | 1 (3) | 2 (5) |
| Body mass index, median (IQR) | 28 (23–35) | 27 (24–36) |
| APACHE IIa, median (IQR) | 26 (23–30) | 25 (20–28) |
| Medical History | ||
| Solid cancer | 10 (26) | 5 (11) |
| Hematologic cancer | 6 (15) | 7 (16) |
| Solid organ transplant | 3 (8) | 5 (11) |
| Stem cell transplant | 1 (3) | 5 (11) |
| Reason for Acute Respiratory Failure | ||
| Pneumonia | 14 (36) | 23 (52) |
| Aspiration | 5 (13) | 3 (7) |
| Extrapulmonary ARDS | 6 (15) | 3 (7) |
| Pneumonia due to immunosuppressionb | 14 (36) | 15 (34) |
| Respiratory and Hemodynamic Parameter, Median (IQR) | ||
| Duration of NIV before randomization, median, h | 13 (8–19.7) | 10.3 (8.3–13.4) |
| Inspiratory positive airway pressure, cm H2O | 10 (10–15) | 12 (10–14.5) |
| Expiratory positive airway pressure, cm H2O | 5 (5–8) | 5 (5–8) |
| SpO2, % | 95 (91–99) | 97 (95–99) |
| FIO2,% | 60 (50–80) | 60 (40–90) |
| PaO2:FIO2 | 144 (90–223) | 118 (93–170) |
| Shock, No. (%) | 12 (31) | 9 (20) |
| Medications | ||
| Pressor requirement | 4 (10) | 1 (2) |
| Steroid use | 15 (38) | 23 (52) |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation, FIO2; fraction of inspired oxygen; IQR, interquartile range; PaO2, partial pressure of oxygen; SpO2, peripheral oxygen saturation by pulse oximeter.
Scores on APACHE II range from 0 to 71, with higher scores indicating increased risk of death.
Immunosuppression was defined as hematologic malignancy or solid tumor (active or in remission <5 y), solid organ transplant, long-term (>30 d) steroid use of more than 20 mg/d, or use of any immunosuppressive drug for more than 30 days.
Treatments
Patients in both groups had similar postrandomization durations of NIV treatment. Patients in the helmet group had a median sustained PEEP of 8.0 H2O vs 5.1 cm H2O in the face mask group (absolute between-group difference, 1.7 (95% CI, 0.6–2.9; P = .006). The 2 groups had statistically similar oxygen saturations. The helmet group had an FIO2 of 50% vs 60% in the face mask group (absolute difference, −7.5; 95% CI, −14.2 to −0.8; P = .02). Titration of PEEP to higher levels per protocol in the face mask group was limited because of patient intolerance and excess air leak. There was no significant change in respiratory rate in patients receiving NIV via face mask at the time of randomization (baseline, 28.3/min; to after randomization, 29.1/min; absolute difference, −0.8/min; 95% CI, −4.9/min to 3.3/min; P = .21). In contrast, the transition from face mask to helmet resulted in a significant reduction in tachypnea from 27.7/min at baseline to 24.5/min after randomization (absolute difference, 3.2/min; 95% CI, 0.2/min to 6.1/min; P = <.001).
Primary and Secondary Outcomes
The intubation rate was 61.5% in the face mask group and 18.2% in the helmet group (absolute difference, −43.3%; 95% CI, −62.4% to −24.3%; P < .001, Table 2. In a competing risk analysis,27 the unadjusted subhazard ratio for the helmet group for the primary outcome of endotracheal intubation was 0.22 (95% CI, 0.11–0.47; P < .001). After adjusting for the APACHE II score and the intervention, the subhazard score for the helmet remained significant (HR, 0.24; 95% CI, 0.11–0.50; P < .001). The most common reason for intubation among patients in the face mask group was respiratory failure—ie, tachypnea and hypoxemia despite protocolized adjustment of NIV settings (83.3% for face mask vs 37.5% for helmet; absolute difference, −45.3; 95% CI, −82.5 to −9.1; P = .01). In contrast, neurologic failure (ie, altered mental status, loss of airway protective reflex) was the most common reason for intubation in the helmet group (62.5% for helmet vs 4.2% for face mask; absolute difference, 58.3; 95% CI, 24.8–92.8; P = .001).
Table 2.
Primary and Secondary Outcomes and Adverse Events
| Face Mask (n = 39) |
Helmet (n = 44) |
Absolute Difference (95% CI) |
P Value | |
|---|---|---|---|---|
| Primary outcome, No. (%) | ||||
| Endotracheal intubation | 24 (61.5) | 8 (18.2) | −43.3 (−62.4 to −24.3) | <.001 |
| Reason for intubation | ||||
| Respiratory failure | 20 (83.3) | 3 (37.5) | −45.3 (−82.5 to −9.1) | .01 |
| Circulatory failure | 3 (12.5) | 0 (0) | −12.5 (−25.7 to 0.7) | .55 |
| Neurologic failure | 1 (4.2) | 5 (62.5) | 58.3 (24.8 to 92.8) | .001 |
| Secondary outcomes, median (IQR), d | ||||
| Ventilator-free days | 12.5 (0.49–28) | 28 (13.7–28) | 8.4 (13.4 to 3.4) | <.001 |
| ICU length of stay | 7.8 (3.9–13.8) | 4.7 (2.5–8.7) | −2.76 (−6.07 to 0.54) | .04 |
| Hospital length of stay | 15.2 (7.8–19.7) | 10.1 (6.5–15.9) | −2.92 (−8.47 to 2.63) | .16 |
| Mortality, No. (%) | ||||
| Hospital | 19 (48.7) | 12 (27.3) | −21.4 (−41.9 to −1.0) | .04 |
| 90 da | 22 (56.4) | 15 (34.1) | −22.3 (−43.3 to −1.4) | .02 |
| Adverse events | ||||
| Mask deflation | 0 (0) | 2 (4.5) | ||
| Skin ulceration | 3 (7.6) | 3 (6.8) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
90-d Mortality includes hospital mortality.
In the exploratory secondary analyses, the helmet group had more ventilator-free days than the face mask group (28vs 12.5; absolute difference, 8.4; 95% CI, 13.4 to 3.4; P < .001). The helmet group spent 4.7 days in the ICU vs 7.8 days for the face mask group (absolute difference, −2.76; 95% CI, −6.07 to 0.54; P = .04) but did not spend statistically significant less time in the hospital (10.1 days for the helmet group vs 15.2 days for the face mask group; absolute difference, −2.92 days; 95% CI, −8.47 to 2.63 days; P = .16).
Hospital and 90-day mortality were significantly lower in the helmet group than in the face mask group (Table 2). The unadjusted HR for death at 90 days was 0.47 (95% CI, 0.24 to 0.91 days; P = .03) in the helmet group. The APACHE II score was also independently associated with death at 90 days (HR, 1.08; 95% CI, 1.01 to 1.15; P = .02). The risk of death at 90 days remained significantly lower in the helmet NIV group after adjustment for APACHE II score ratio (HR, 0.51; 95% CI, 0.23 to 0.99; P = .047; Figure 2).
Figure 2. Probability of Survival From Randomization to Day 90.
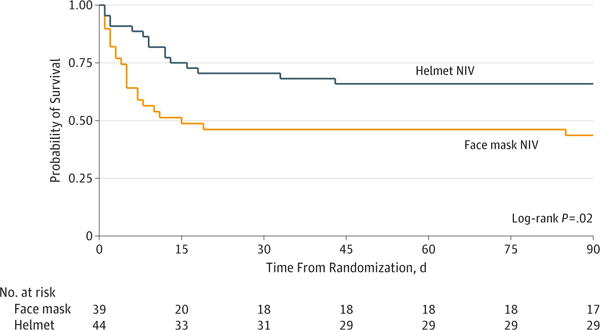
NIV indicates noninvasive ventilation.
Adverse Events
Overall, the incidence of adverse events was low. There were 2 instances when the helmet was deflated, which was quickly corrected and did not result in endotracheal intubation. There was no statistical difference in the rate of mask-related skin ulceration between groups with 3 patients (7.6%) in the face mask group with a nose ulcer and 3 patients (6.8%) in the helmet group with a neck ulcer.
Discussion
In this single-center, randomized clinical trial, NIV delivered by helmet significantly reduced the intubation rate among patients with ARDS compared with the patients receiving NIV by face mask. The helmet also was associated with improved ventilator-free days and significantly reduced ICU length of stay as well as 90-day mortality.
Avoiding intubation is critical for patients with acute respiratory failure because endotracheal intubation is associated with numerous infectious1 neurologic,28 respiratory, and musculoskeletal complications.29 Such complications can have long-standing consequences, particularly among patients with ARDS.30 The 8-hour period of face mask NIV was chosen a priori as a study entry criterion to avoid patients needing NIV for only a short time; this ensured that only those with high illness acuity and a substantial chance of requiring endotracheal intubation were enrolled. The significant reduction in the intubation rate may be explained in part by the effective delivery of higher levels of PEEP. We hypothesized that the helmet’s neck seal would allow for delivery of higher airway pressures without substantial air leak. In the exploratory secondary analyses, patients randomized to the helmet group had substantially higher levels of PEEP, which were sustained throughout NIV. This corresponded with a significant reduction in the respiratory rate and similar oxygen saturation levels on a lower FIO2 than achieved with face mask. These higher sustained PEEP levels appear to have maintained acceptable gas exchange, thereby reducing the need for intubation. In addition to the PEEP effects, high ventilator fresh gas flow with the helmet interface was noted, typically between 100 to 200 L/min. High fresh gas flow rates reduce the risk of CO2 rebreathing in the helmet.17 Thus, the PEEP and fresh gas flow effects of helmet NIV appear to have improved oxygenation and work of breathing so that failures of helmet NIV were rarely due to respiratory failure, but instead usually due to mental status changes and loss of the airway protective reflex.
The observed intubation and mortality rates among patients in the face mask group were higher than some recently reported studies of AHRF.9,10,31 However, our patients had very high APACHE II scores, with predicted mortality rates in the 50% range.32 A study by Frat et al10 recently reported increased mortality among patients with AHRF randomized to face mask NIV compared with patients randomized to high-flow nasal cannula, although there were no differences in overall intubation rates. The patients in this trial had much lower severity of illness than patients in our trial, as measured by average Simplified Acute Physiology Scores (SAPS II) of between 24 and 27. These scores predict a hospital mortality of between 5.8% and 7.9%.33 Lemiale et al9 noted no difference inintubationor28-day mortality in immunocompromised patients randomized to receive mask NIV or oxygen therapy alone. Despite being immunocompromised, the median admission Sequential Organ Failure Assessment (SOFA) score was 5 in both groups,9 compared with a SOFA score of 7 in the current study. In contrast, Antonelli et al24 previously reported that patients with ARDS treated with face mask NIV had a 51% intubation rate and a 64% hospital mortality, similar to the face mask NIV group.
The helmet interface is a relatively novel approach to NIV and this study has several cautions and limitations. First, the large internal volume of the helmet and its high compliance may lead to CO2 rebreathing17,34 and patient-ventilator dyssynchrony.35 Also recruitment maneuvers cannot be applied with noninvasive ventilation.36 The study findings suggest that patients whose ARDS was managed with helmet NIV should have pressure support levels set to ensure high inspiratory flow levels (ie, greater than 100 L/min—this was always easily achievable with modest pressure support settings; Table 3),17 as well as periodic arterial blood sampling during helmet use.34
Table 3.
Level of Respiratory Support and Physiologic Parameters During Noninvasive Ventilation
| Noninvasive Ventilation, Median (IQR) | P Value | ||
|---|---|---|---|
| Face Mask (n = 39) |
Helmet (n = 44) |
||
| Respiratory support with NIVa | |||
| Duration of NIV, h | 26.4 (7.0–60.0) | 19.8 (8.4–45.6) | .68 |
| PEEP, cm H2O | 5.1 (5.0–8.0) | 8 (5.0–10.0) | .006 |
| Pressure support, cm H2O | 11.2 (10.0–14.5) | 8 (5.6–10.0) | <.001 |
| FIO2,% | 60 (50.0–68.6) | 50 (40.0–60.0) | .02 |
| SpO2, % | 95.3 (92.3–96.7) | 96.2 (94.8–98.4) | .13 |
| Respiratory rate, breaths/min | |||
| Baseline | 28.3 (22.1–34.4)b | 27.7 (21.5–34.6)b | |
| After randomization | 29.1 (22.1–37.6) | 24.5 (20.4–30.5) | |
Abbreviations: FIO2, fraction of inspired oxygen; NIV, noninvasive ventilation; PEEP, positive end-expiratory pressure; SpO2, peripheral oxygen saturation by pulse oximeter.
Median area under the curve of respiratory support.
Comparison of baseline and after randomization respiratory rates within groups: for the face mask group, the absolute difference was 0.8/min (95% CI, −4.9/min to 3.3/min; P = .21); for the helmet group, the absolute difference was 3.2/min (95% CI, 0.2/min to 6.1/min; P<.001).
Second, like any new tool or technology, there is likely to be a learning curve as clinicians gain familiarity. Careful training of all physicians and staff will be needed, just as was the case 20 years ago when face mask NIV was first introduced. Physicians, nurses, and respiratory therapists involved in this study quickly became familiar and comfortable with helmet NIV during the course of the trial.
Third, the nature of this trial intervention made blinding impossible. Accordingly, we followed predetermined criteria for endotracheal intubation to decrease bias. Fourth, as a single-center trial, our results may not have external validity. Fifth, although this study was stopped early for efficacy based on predetermined criteria, the significance of the effect size of the primary outcome suggests that the probability of type I error is very low. However, early stoppage of trials tends to exaggerate the magnitude of the effect size and future studies replicating this trial may report lower efficacy of helmet NIV.
The physiologic effects observed with helmet NIV suggest biologic plausibility for the prevention of endotracheal intubation by enhanced PEEP effect. These findings also affirm the far-reaching benefits of spontaneous yet highly supported ventilation in an awake, animated patient over invasive mechanical ventilation via endotracheal tube. These findings warrant further investigation of helmet NIV for patients with ARDS and other types of AHRF, particularly with attention to long-term outcomes.30
Conclusions
For patients with ARDS, treatment with helmet NIV was associated with a significant reduction of intubation rates compared with delivery by face mask. There was also a statistically significant reduction in 90-day mortality with helmet NIV. Multicenter studies are needed to replicate these findings.
Supplementary Material
Acknowledgments
Funding/Support: The helmets were purchased using funds from an unrestricted internal grant from the Daniel J. Edelman family. Fellow salary and support for Drs Patel and Wolfe was provided by grant T32 HL007605 from the NIH/NHLBI. All helmet devices were obtained commercially from Sea Long.
Role of the Funder/Sponsor: Edelman family had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Video at jama.com
Supplemental content at jama.com
Author Contributions: Drs Patel and Kress had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Patel, Hall, Kress.
Acquisition, analysis, or interpretation of data: Patel, Wolfe, Pohlman, Kress.
Drafting of the manuscript: Patel, Wolfe, Hall, Kress.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Patel, Wolfe, Kress.
Administrative, technical, or material support: Patel, Pohlman, Kress.
Study supervision: Hall, Kress.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Additional Contributions: We thank respiratory therapy, physical and occupational therapists, and nursing staff for their support of this project.
References
- 1.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 4.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363(12):1176–1180. doi: 10.1056/NEJMe1007136. [DOI] [PubMed] [Google Scholar]
- 6.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 7.Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013(5):CD005351. doi: 10.1002/14651858.CD005351.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283(2):235–241. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- 9.Lemiale V, Mokart D, Resche-Rigon M, et al. Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH) Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA. 2015;314(16):1711–1719. doi: 10.1001/jama.2015.12402. [DOI] [PubMed] [Google Scholar]
- 10.Frat JP, Thille AW, Mercat A, et al. FLORALI Study Group; REVA Network High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 11.Thille AW, Contou D, Fragnoli C, Córdoba-Izquierdo A, Boissier F, Brun-Buisson C. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care. 2013;17(6):R269. doi: 10.1186/cc13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AR, Kacmarek RM, Hess DR. Factors affecting oxygen delivery with bi-level positive airway pressure. Respir Care. 2004;49(3):270–275. [PubMed] [Google Scholar]
- 14.Squadrone V, Coha M, Cerutti E, et al. Piedmont Intensive Care Units Network (PICUN) Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293(5):589–595. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 15.Principi T, Pantanetti S, Catani F, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med. 2004;30(1):147–150. doi: 10.1007/s00134-003-2056-9. [DOI] [PubMed] [Google Scholar]
- 16.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Taccone P, Hess D, Caironi P, Bigatello LM. Continuous positive airway pressure delivered with a “helmet”: effects on carbon dioxide rebreathing. Crit Care Med. 2004;32(10):2090–2096. doi: 10.1097/01.ccm.0000142577.63316.c0. [DOI] [PubMed] [Google Scholar]
- 18.Vargas F, Thille A, Lyazidi A, Campo FR, Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37(6):1921–1928. doi: 10.1097/CCM.0b013e31819fff93. [DOI] [PubMed] [Google Scholar]
- 19.Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339(7):429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 20.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 22.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 24.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27(11):1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld DA, Bernard GR, ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandharipande PP, Girard TD, Jackson JC, et al. BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermans G, Van Mechelen H, Clerck B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 30.Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 31.Bellani G, Laffey JG, Pham T, et al. LUNG SAFE Investigators; ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 32.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 33.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 34.Hill NS. Noninvasive interfaces: should we go to helmets? Crit Care Med. 2004;32(10):2162–2163. doi: 10.1097/01.ccm.0000142945.20310.e4. [DOI] [PubMed] [Google Scholar]
- 35.Nava S, Navalesi P. Helmet to deliver noninvasive ventilation: “handle with care”. Crit Care Med. 2009;37(6):2111–2113. doi: 10.1097/CCM.0b013e3181a5e6b5. [DOI] [PubMed] [Google Scholar]
- 36.Kacmarek RM, Villar J. Management of refractory hypoxemia in ARDS. Minerva Anestesiol. 2013;79(10):1173–1179. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


