Iron overload prior to allogeneic hematopoietic stem cell transplantation (allo-HSCT) due to blood transfusions is associated with increased non-relapse mortality (NRM) and with low overall survival.1,2 After allo-HSCT, high iron content in liver biopsies and elevated ferritin concentrations in blood, used as a surrogate parameter for iron overload, are also related to NRM.3–6 The cellular and molecular mechanisms behind this association, however, are yet to be clarified. In particular, available data on the role of ferritin and iron metabolism during the pathophysiology of acute graft-versus-host disease (GvHD), a major contributor to NRM, are scarse. To shed light on the connection between GvHD, ferritin levels, and iron metabolism, we decided to analyse the clinical data of patients undergoing allo-HSCT as well as data from preclinical murine GvHD models.
We generated our working hypothesis after observing concurrent presentations of acute GvHD (aGvHD) and hyperferritinemia in a series of patients undergoing allo-HSCT. In a typical case, a 29 year old female underwent allo-HSCT for severe aplastic anemia. She received four erythrocyte concentrates in total: two prior to allo-HSCT and two during aplasia post allo-HSCT. Around day +200, she suffered from late aGvHD of the liver; stage 3, grade III. At the same time point, a high ferritin level and a high transferrin saturation was measured for the first time in this patient (Online Supplementary Figure S1A). Ferritin levels dropped rapidly after clinical improvement of aGvHD due to administration of high dose steroids. This repeated during a second exacerbation of GvHD. Notably, the transferrin saturation was not affected by the immunosuppression. We found siderosis in bone marrow in this patient (Online Supplementary Figure S1B). During the course of disease, no iron chelation was administered. No mutations were found on the HFE gene. After her second exacerbation, the patient recovered and is still alive. Based on this case, and others similar, we hypothesized that GvHD is associated with hyperferritinemia and organsiderosis, independent of blood transfusions.
We retrospectively analysed allo-HSCT recipients at the Charité-CBF over a six-year period, with a survival of more than 60 days. Patient characteristics and their maximum ferritin levels after allo-HSCT (low/medium vs. high vs. very high) are given in Online Supplementary Table S1. Of 104 patients, 33 developed high ferritin levels (5000μg/l-10000μg/l), and 11 had very high ferritin levels (more than 10000μg/l) after allo-HSCT. There was no significant relationship between the ferritin levels prior to allo-HSCT and the maximum ferritin levels after allo-HSCT. We analysed overall survival of patients with high/very high ferritin levels (5000μg/l or more) vs. patients with low/medium ferritin levels (<5000μg/l) after allo-HSCT. Median follow-up time was 661 days. We found that overall survival in patients with high ferritin levels was significantly lower than in the low/medium ferritin group (Fig. 1A). Of note, none of the patients with Ferritin levels >10.000μg/l had long-term survival. This was due to increased non-relapse mortality (Figure 1B).
Figure 1.
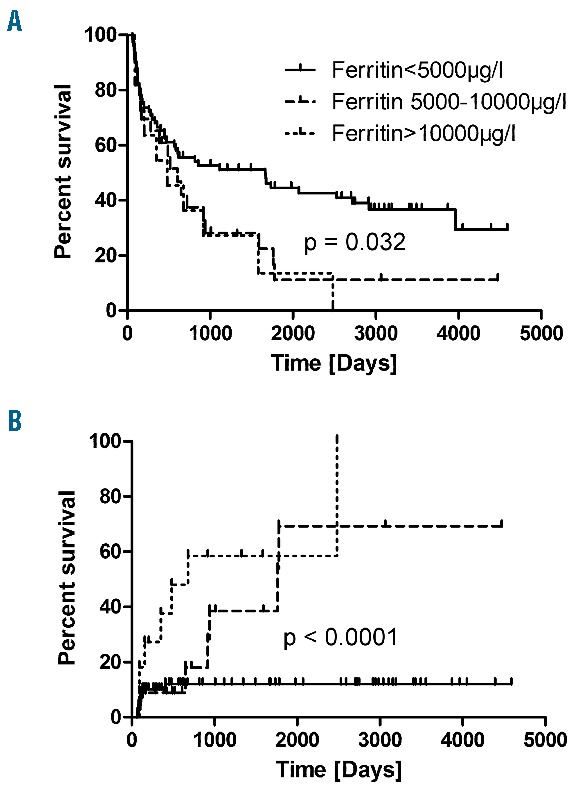
Overall Survival and Non-Relapse Mortality According to Ferritin Levels post Allo-HSCT. Patients with increased ferritin levels after transplantation have a worse overall survival (A) due to increased non-relapse mortality (B).
Next, we were interested to find out if high ferritin levels after allo-HSCT were associated with acute GvHD. For analyses we created three cohorts of allo-HSCT recipients according to their clinical aGvHD grade: 1) No aGvHD, 2) Grade I–II and 3) Grade III–IV. We then picked the maximum serum ferritin level of each patient after allo-HSCT. Figure 2 shows the distribution of ferritin levels between the cohorts. We found that maximum ferritin levels after allo-HSCT were significantly higher in patients during aGvHD than in patients without aGvHD (Figure 2A). Of note, very high ferritin levels (>10000μg/l) occurred exclusively in patients during aGvHD; they were not observed in the absence of aGvHD (Figure 2A). Interestingly, we did not observe an association between ferritin levels and chronic GvHD (Figure 2B).
Figure 2.
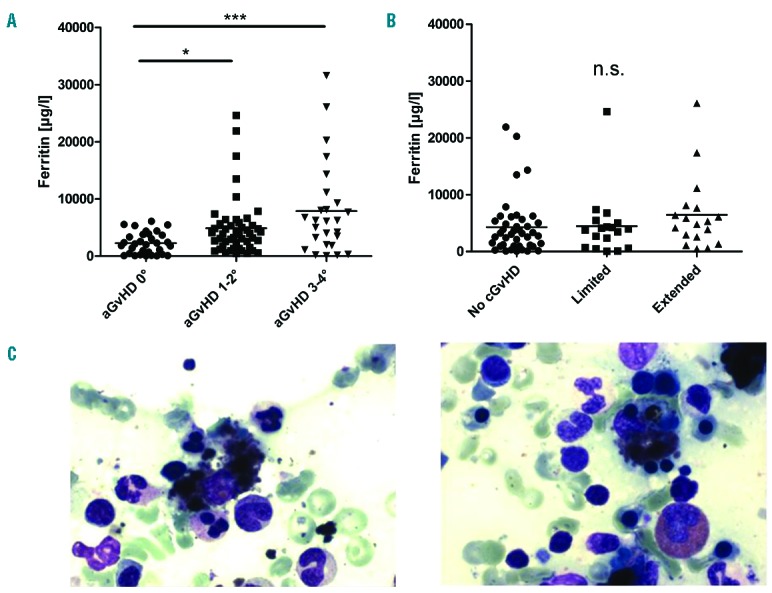
Elevated Ferritin levels in patients with GvHD are associated with hemophagocytosis. Very high ferritin levels (more than 10000 μg/l) after HSCT were seen exclusively in patients with acute GvHD (A); (unpaired two tailored t-test; *P=0.007; ***P=0.003). There was no association between ferritin levels and chronic GvHD (B). In 8/9 bone marrow aspirates from patients with ferritin >10000μg/l, heophagocytosis was observed (C); shown are two representative examples.
Importantly, mean ferritin levels taken prior to allo-HSCT did not differ significantly between allo-HSCT recipients who later developed no aGvHD (1194 SEM ± 268.2), aGvHD Grade I–II (1295 SEM ± 174.7) or aGvHD Grade III+IV (1194 ± 268.2) (P=0.61).
We were then interested to establish if we were able to detect hemophagocytes in patients with hyperferritninaemia after allo-HSCT. The rationale is that hyperferritinemic syndromes are usually characterized by macrophage activation and by hemophagocytosis. The standard technique to visualize hemophagocytosis is assessment of BM aspirates. BM aspirates were available in 9 out of 11 allo-HSCT recipients who developed ferritin levels above 10000μg/l during aGvHD. In one of these specimens, many siderophages were found; however, there was no clear evidence of hemophagocytosis. The remaining 8 specimens showed clear hemophagocytosis in the BM (Figure 2C). We conclude that very high ferritin levels after allo-HSCT are associated with hemophagocytosis. To investigate if aGvHD is associated with organsiderosis, we used a preclinical murine allo-BMT model (Balb/c into C57BL/,6 conditioning with treosulfan and cyclophosphamide), which has been described previously.7 At day +9, we sacrificed allo-BMT and syn-BMT recipients to measure liver iron content by graphite furnace atomic absorption spectrometry. A significantly higher amount of liver iron was found in allo-BMT recipients during aGvHD than in syn-BMT recipients or mice receiving no transplant (Figure 3A). In this model, most allo-BMT recipients died from aGvHD. In the small subset of survivors, those who had clinically recovered from aGvHD, persistent high liver iron content at day +69 post-allo-BMT was found (Figure 3A). Furthermore, in histological sections, iron deposits in spleen (red and white pulpa) and kidney were seen in allo-BMT recipients but not in syn-BMT recipients (data not shown). No iron deposits were seen in the hearts of the mice. We conclude that aGvHD is associated with organsiderosis in the liver, kidneys and spleen.
Figure 3.
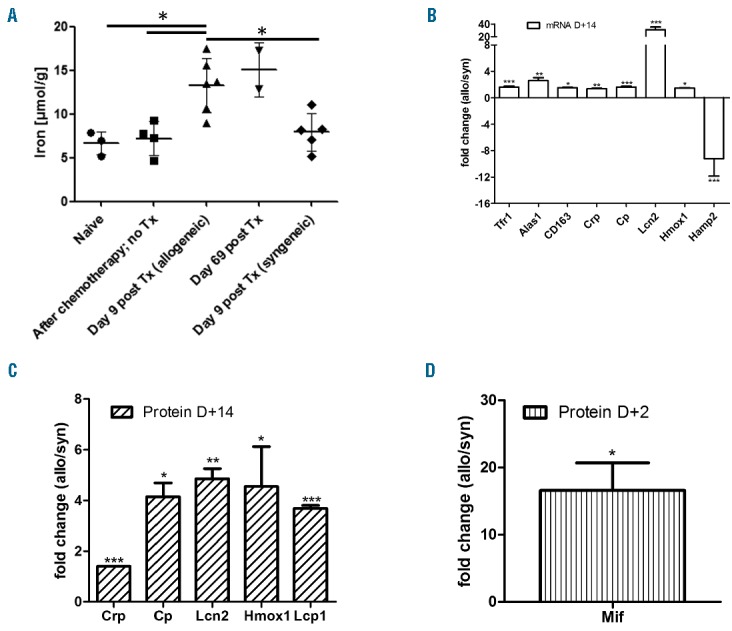
Effects of GvHD on ferritin and iron metabolism in mice. In C57BL/10 mice transplanted with Balb/c splenocytes and GvHD, a significantly higher amount of liver iron was found on day+9 (A); *P<0.05; unpaired t-test, two tailored). In accordance, mRNA expression of several genes related to iron metabolism and macrophage activation was changed during hepatic GvHD at day +14 (Balb/c →C57B6/N) (B); data are shown as mean±s.e.m; *P<0.05 and ***P<0.001; t-test; 4 mice per group. The expression of some proteins related to iron balance (Cp, Lcn2 and Hmox1) and others related to macrophage activation (Crp and Lcp1) was also significantly up regulated in allogeneic animals (LP/J→C57B6/N) (C); data are shown as mean±s.e.m; *P<0.05 and **P<0.01 and ***P<0.001; t-test; 4 mice per group. The proteomics analysis indicated that 2 days after BMT, compared to the syngeneic mice, macrophage migration inhibitory factor (Mif) was highly upregulated in the allogeneic livers (LP/J→C57B6/N) (D).
In a next step we focused on the molecular mechanisms; we analyzed mRNA expression by gene array and protein expression by proteomic profiling (Online Supplementary Methods) during hepatic aGvHD vs. syn. controls at day +14. We found that Ceruloplasmin (Cp), Lipocalin-2 (LCN2) and Heme oxygenase 1 (Hmox1) were significantly upregulated on an mRNA level as well as on a protein level during hepatic aGvHD (Figure 3B and Figure 3C). In addition, transferrin receptor 1 (Tfr1) and delta-aminolevulinate synthase 1 (Alas1) were significantly upregulated, and hepcidin 2 (Hamp2) was significantly downregulated on an mRNA level but not on a protein level (Figure 3B). Due to the hyperferritinemia and BM hemophagocytes that we observed during aGvHD, we were specifically interested in gene expression and protein level changes related to macrophage activation. On an mRNA level, we found a significant upregulation of the hemoglobin scavenger receptor (CD163, also related to iron metabolism), and of the c-reactive protein (CRP), at day+14 (Figure 3B). On a protein level, we confirmed the significant elevation of CRP, and found that lymphocyte cytosolic protein 1 (Lcp1) was significantly upregulated during aGvHD at day+14 (Figure 3C). In addition, we detected early, high upregulation (day +2) of macrophage migration inhibitory factor (Mif) on a protein level during hepatic aGvHD (Figure 3D)
In our patient population, very high ferritin levels (>10000μg/l) occurred exclusively during GvHD and were associated with reduced overall survival. Ferritin is a key protein in iron metabolism and iron storage, which may be used to estimate body iron content under noninflammatory conditions. However, its use to measure iron overload during allo-HSCT has been criticized for often being inadequate.1,2 In fact, serum ferritin is primarily derived from macrophages, leading to very high ferritin concentrations in severe medical conditions associated with macrophage activation.8–10 It is a well-known fact that macrophage activation can lead to hemophagocytosis in the bone marrow (BM); the hallmark of macrophage activation syndrome and hemophagocytic lymphohistiocytosis.11 In allo-HSCT recipients with very high ferritin levels, we detected hemophagocytes in the BM as well as clinical and laboratory signs of macrophage activation. However, in our cohort, formal diagnosis of hemophagocytic lymphohistiocytosis was not possible in most patients due to the retrospective data collection (clinical criteria are summarized in the Online Supplementary Data section). Of note, allogeneic macrophage activation has previously been described in experimental GvHD models.12 During GvHD, the activation of macrophages may ultimately result in the release of intracellular iron, underlining a possible connection between GvHD and organsiderosis.13 Gene and protein level changes related to macrophage activation during GvHD were indentified in this study, including c-reactive protein (CRP), macrophage migration inhibitory factor (Mif), lymphocyte cytosolic protein 1 (Lcp1), and the hemoglobin scavenger receptor (CD163). Of note, CD163 may connect macrophage activation with iron metabolism because it is involved in cellular haemoglobin uptake, and it is also expressed on tissue resident macrophages, such as Kupffer cells in the liver.14 The association of macrophage activation and GvHD-related mortality is particularly interesting because the level of CD163+macrophage infiltration in the skin, but not the level of T-cell infiltration, has been described to be predictive for mortality during GvHD.15 Due to the observation of the devastating course of patients with this presentation, alternative therapeutic strategies might be warranted, such as the potential use of etoposide.
In summary, we found that hemophagocytosis in the BM and hyperferritinemia, likely due to systemic macrophage activation, are associated with high mortality in patients with GvHD. In preclinical models, we found that GvHD leads to profound changes in the hepatic iron metabolism, eventually resulting in organsiderosis.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Armand P, Kim HT, Virtanen JM, et al. Iron overload in allogeneic hematopoietic cell transplantation outcome: a meta-analysis. Biol Blood Marrow Transplant. 2014;20(8):1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wermke M, Schmidt A, Middeke JM, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Cancer Res. 2012;18(23):6460–6468. [DOI] [PubMed] [Google Scholar]
- 3.Ali S, Pimentel JD, Munoz J, et al. Iron overload in allogeneic hematopoietic stem cell transplant recipients. Arch Pathol Lab Med. 2012;136(5):532–538. [DOI] [PubMed] [Google Scholar]
- 4.Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008;14(7):790–794. [DOI] [PubMed] [Google Scholar]
- 5.Meyer SC, O’Meara A, Buser AS, Tichelli A, Passweg JR, Stern M. Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(3):440–444. [DOI] [PubMed] [Google Scholar]
- 6.Sucak GT, Yegin ZA, Ozkurt ZN, Aki SZ, Karakan T, Akyol G. The role of liver biopsy in the workup of liver dysfunction late after SCT: is the role of iron overload underestimated¿ Bone Marrow Transplant. 2008;42(7):461–467. [DOI] [PubMed] [Google Scholar]
- 7.Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59(8):1079–1087. [DOI] [PubMed] [Google Scholar]
- 8.Rosario C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11(185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116(9):1574–1584. [DOI] [PubMed] [Google Scholar]
- 10.Schaer DJ, Schaer CA, Schoedon G, Imhof A, Kurrer MO. Hemophagocytic macrophages constitute a major compartment of heme oxygenase expression in sepsis. Eur J Haematol. 2006; 77(5):432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janka GE, Lehmberg K. Hemophagocytic syndromes–an update. Blood Rev. 2014;28(4):135–142. [DOI] [PubMed] [Google Scholar]
- 12.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175(2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestel FP, Greene RN, Kichian K, Ponka P, Lapp WS. Activation of macrophage cytostatic effector mechanisms during acute graft-versus-host disease: release of intracellular iron and nitric oxide-mediated cytostasis. Blood. 2000;96(5):1836–1843. [PubMed] [Google Scholar]
- 14.Schaer DJ, Boretti FS, Hongegger A, et al. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics. 2001;53(2):170–177. [DOI] [PubMed] [Google Scholar]
- 15.Nishiwaki S, Terakura S, Ito M, et al. Impact of macrophage infiltration of skin lesions on survival after allogeneic stem cell transplantation: a clue to refractory graft-versus-host disease. Blood. 2009; 114(14):3113–3116. [DOI] [PubMed] [Google Scholar]


